Abstract
Chylous ascites (CA) is a rare complication following renal surgery. Here we present the case of a 28-year-old female who developed CA after a robotic left partial nephrectomy. After failing conservative management, she underwent successful robotic-assisted diagnostic laparoscopy and ligation of lymphoperitoneal fistulae. The higher incidence of CA after left versus right-sided renal surgery may be explained by the para-aortic drainage of the intestinal lymphatic channels. Surgical intervention should be considered when conservative management fails.
Keywords: Robotic surgery, Chylous ascites, Lymphocele, Partial nephrectomy, Donor nephrectomy
Funding
No funding was received for this project.
1. Introduction
CA, or the accumulation of chyle within the peritoneal cavity, is a rare, but potentially debilitating complication following renal surgery. In a postoperative patient, CA often results in extended hospital length of stay, readmission, and re-intervention. The literature describing CA following renal surgery is scant and mostly limited to case reports and donor nephrectomies. In this review, we present our case of CA following robotic partial nephrectomy. We will also discuss the anatomical foundation for the left-sided predominance of this rare complication as well as management strategies following renal surgery.
2. Case presentation
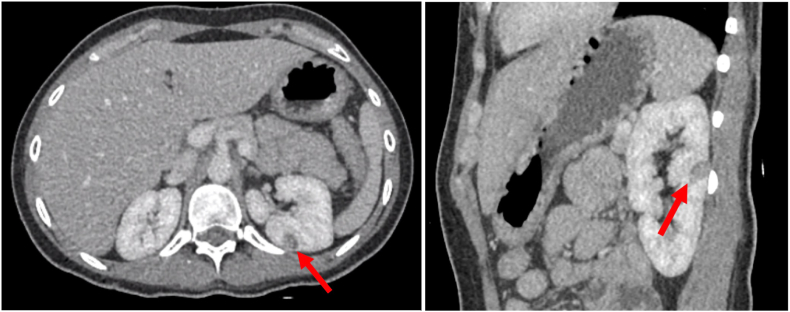
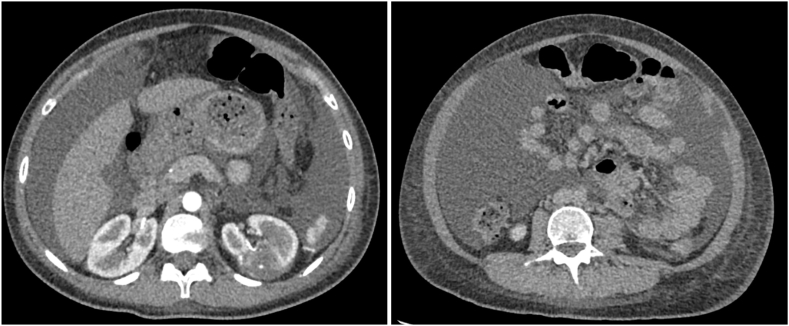
Our patient was a 28-year-old female with a history of poorly controlled type 1 diabetes, diabetic gastroparesis, and prior cholecystectomy with a 1.3 cm × 1.1 cm left posterior renal mass, as seen on contrasted CT scan (Fig. 1). It was not determined to be a lipid-poor angiomyolipoma. She had a family history of renal cell carcinoma. She elected against genetic testing. After discussing all options, she underwent uneventful robotic left partial nephrectomy. Notably, prominent lymphatic vessels were encountered and disrupted during left renal hilar dissection, which were clipped. She had a prolonged hospital course because of difficulty optimizing blood glucose as well as urinary retention. She was discharged post-operative day 5 following drain removal. Final pathology returned angiomyolipoma. On post-operative day 13 she was readmitted with abdominal pain, distention, and milky drainage from two of her incision sites. CT scan obtained during re-admission demonstrated a large volume ascites with no urine leak on delayed imaging (Fig. 2). A percutaneous drain was placed with initial aspiration of 3 L of milky white fluid consistent with chyle (Fig. 3). MCT diet was initiated, however drain output remained high. She was placed on complete bowel rest with total parenteral nutrition and octreotide. She failed these interventions and had persistently elevated drain ouput (averaged 1–3 L per 24 hours). Interventional radiology at our institution determined she was not a candidate for lymphangiogram. After discussing conservative management versus surgical re-exploration, she elected for robotic-assisted diagnostic laparoscopy on post-operative day 25.
Fig. 1.
Axial (A) and coronal CT (B) of our patient's 1.3 cm × 1.1 cm left renal mass.
Fig. 2.
(A) Cystic fluid collection associated with renorrhaphy. (B) Large volume CA.
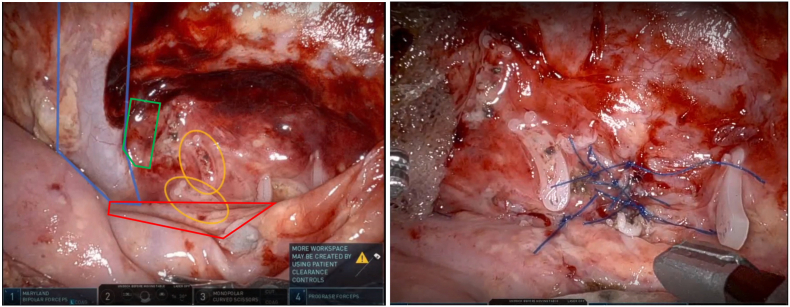
Fig. 3.
(A)Left renal hilum during surgical re-exploration. Blue outline is left renal vein. Orange ovals highlight previously placed hem-o-lok clips. Chylous leakage was seen coming from behind these clips. (B)Suture ligation of multiple lymphatic defects identified surrounding and beneath previously placed hem-o-lok clips. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The chylous fluid collection maintained separation of previously dissected anatomic planes, facilitating exposure of the renal hilum (Fig. 3A). We encountered chyle leakage from beneath and between our previously placed Hem-o-lok clips from the initial surgery, suggesting inadequate ligation of the ymphatics by the clips. We excised select clips in an effort to better expose the lymphatic fistulae. Because of the flush opening of the fistulae and adjacent inflammatory tissue quality, we decided to ligated the fistulae with 4-0 prolene suture instead of using clips (Fig. 3B). We then covered the repair with Vistaseal fibrin sealant and oxidized cellulose gauze followed by placement of a drain.
Following the repair, our patient's drain output declined with the fluid changing from chlye to more serous in appearance. She was discharged four days later. Two weeks after readmission, she continued to do well with low drain output while resuming a regular diet and her drain was removed.
3. Discussion
CA is a rare complication following renal surgery, and much of the available reports are in the donor nephrectomy literature and almost all occurring folling left-sided procedures. In studies that included donor nephrectomy, laparoscopic partial nephrectomy, simple nephrectomy, and radical nephrectomy, the incidence ranges between 0.6 and 5.9 %.1 In our literature review, only three cases of CA after robotic partial nephrectomy were found.2,3 Interestingly, one of these cases occurred on the right side, while the other three occurred on the left.
Understanding the regional intestinal lymphatic system anatomy helps to better understand the cause of CA ascites following renal surgery and the predominance of occurrence following left-sided procedures. Right and left ascending lumbar lymphatic trunks run along either side the aorta and drain lymph from the lower extremities, genitalia, and pelvis. Ascendinng intestinal lymphatics also travel along the aorta and coalesce at the inferior and superior mesenteric arteries before joining the lumbar lyphatics to form the cisterna chyli, marking the end of the retroperitoneal lymphatic vessels and beginning of the thoracic duct.4,5
Because of its close proximity to the para-aortic lymphatic trunks, dissection of specifically the left renal hilar vessels along the para-aortic location can cause disruption of these ascending lymphatic channels resulting in chylous leakage. This may explain the higher incidence of CA specifically following left donor nephrectomy where skeletenozation of the left renal artery is performed down to the origin of the aorta in efforts to optimize renal artery length and facilitate future allograft vascular implantation. This is opposed to dissection of the right renal hilar vessels that takes place immediately lateral to the vena cava where intestinal lymphatics are generally not present.
Because they do not contract and thrombose like blood vessels do, disrupted lymphatic vessels that are not properly ligated may then persistently leak and form lymphoperitoneal fistulae.5 Disruption of these lymphatic vessels may be difficult to recognize at the time of surgery. Pneumoperitoneum can mask disruption of these low-pressure vessels.
Most patients are initially managed conservatively with dietary intervention (MCT diet or bowel rest), TPN, and a somatostat-analog. However, spontaneous resolution is less likely in the face of persistent, severe leakage of greater than 1000 mL per day and/or failure of conservative management.4 These cases often require procedural intervention. Lymphangiogram with embolization is an emerging minimally invasive approach. As described above, surgical exploration with suture ligation is a feasible and effective solution for patients with persistent, high volume CA. While any ligation strategy may be employed, including bioplar sealing devices, clipping, or suture ligating, tissue quality and the flush opening of the fistulae may favor suture ligation.
4. Conclusion
Chylous leakage is a rare but debilitating complication following renal surgery. This complication is more commonly reported following left versus right-sided procedures, likely due to the proximity of the ascending para-aortic intestinal lymphatic trunks to the left renal hilum. Understanding anatomy, as well as meticulous ligation may help prevent this complication. Prompt diagnosis and stepwise, algorithmic management can help optimize resolution of this challenging complication.
CRediT authorship contribution statement
Kevin Morgan: Writing – review & editing, Writing – original draft, Investigation. Marc Abboud: Writing – review & editing, Investigation. Brett Friedman: Writing – review & editing, Investigation, Data curation. Li-Ming Su: Writing – review & editing, Investigation, Conceptualization.
Declaration of competing interest
None of the authors listed have any conflicts of interest to disclose.
Abbreviations
- CA
Chylous ascites
- MCT
Medium chain triglyceride
Contributor Information
Kevin Morgan, Email: kevin.morgan@urology.ufl.edu.
Marc Abboud, Email: marc.abboud@urology.ufl.edu.
Brett Friedman, Email: brett.friedman@urology.ufl.edu.
Li-Ming Su, Email: li-ming.su@urology.ufl.edu.
References
- 1.Kim B.S., Yoo E.S., Kim T.-H., Kwon T.G. Chylous ascites as a complication of laparoscopic nephrectomy. J Urol. 2010;184(2):570–574. doi: 10.1016/j.juro.2010.03.128. [DOI] [PubMed] [Google Scholar]
- 2.Pahouja G., Patel K., Ricchiuti D.J. Chylous ascites as a complication of left sided robot-assisted laparoscopic partial nephrectomy. Arch Ital Urol Androl. 2016;88(3):217–222. doi: 10.4081/aiua.2016.3.217. [DOI] [PubMed] [Google Scholar]
- 3.Roberson D., Chelluri R., Wein A.J., Mucksavage P. Chylous ascites following a right robotic assissted laparoscopic partial nephrectomy. Urol Case Rep. 2020;32 doi: 10.1016/j.eucr.2020.101207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leibovitch I., Mor Y., Golomb J., Ramon J. The diagnosis and management of postoperative chylous ascites. J Urol. 2002;167(2 Pt 1):449–457. doi: 10.1016/S0022-5347(01)69064-5. [DOI] [PubMed] [Google Scholar]
- 5.Meulen S.T., van Donselaar-van der Pant K.A., Bemelman F.J., Idu M.M. Chylous ascites after laparoscopic hand-assisted donor nephrectomy: is it specific for the left-side? Urol Ann. 2013;5(1):45–46. doi: 10.4103/0974-7796.106967. [DOI] [PMC free article] [PubMed] [Google Scholar]