Abstract
Background
Paired sampling of acute (aST) and basal (bST) serum tryptase has been recommended when investigating patients with a suspected perioperative hypersensitivity (POH) reaction. In the current consensus formula, an aST value exceeding (1.2×bST+2) confirms mast cell activation. The current consensus formula has been validated in adults but not in children.
Methods
We prospectively included 96 children who underwent uneventful anaesthesia and sampled serum tryptase at baseline and 60–90 min after induction. Tryptase changes were then compared with those in 94 children with suspected POH who were retrospectively included from four reference centres in Belgium, France, and Denmark.
Results
We observed a median decrease in serum tryptase during uneventful anaesthesia of 0.41 μg L−1 (–15.9%; P<0.001). The current consensus formula identified mast cell activation in 31.9% of paediatric POH patients. After generating receiver operating characteristic curves through 100 repeated five-fold cross-validation, aST>bST+0.71 was identified as the optimal cut-off point to identify mast cell activation. This new paediatric formula has higher sensitivity than the current consensus formula (53.2% vs 31.9%, P<0.001) with a specificity of 96.9%. Analysis in the subpopulation where a culprit was identified and in grade 3–4 reactions similarly yielded higher sensitivity for the new paediatric formula when compared with the current consensus formula (85.3% vs 61.8%; P=0.008 and 78.0% vs 48.8%; P<0.001, respectively). Internally cross-validated sensitivity and specificity were 53.3% and 93.3%, respectively.
Conclusions
This is the first study suggesting the need for an adjusted formula in children to identify perioperative mast cell activation as tryptase is significantly lowered during uneventful anaesthesia. We propose a new formula (aST>bST+0.71) which performs significantly better than the current consensus formula in our multicentric paediatric population.
Keywords: Formula for mast cell activation in children, Paediatric POH, Paired serum tryptase, Performance of consensus formula, Perioperative anaphylaxis, Perioperative hypersensitivity, POH diagnosis, Uneventful anaesthesia
Perioperative hypersensitivity (POH) reactions can be life-threatening, and correct recognition and diagnosis can pose a serious challenge. The pharmacological effects of the anaesthetic drugs and the anaesthetic and surgical procedure can mimic hypersensitivity reactions (including anaphylaxis). Furthermore, simultaneous exposure to several drugs and other (often hidden) compounds often complicates correct identification of the culprit.1,2
As reviewed elsewhere, the pathophysiology of a POH reaction generally relies upon the activation and degranulation of mast cells and basophils.3 Tryptase is a trypsin-like protease mainly stored in secretory granules of mast cells, and at a 200-fold lower level, in those of basophils.4,5 α- and β-protryptase monomers are released spontaneously at baseline by resting mast cells, as observed by unstimulated mast cells in vitro.6 This accounts for nearly all of the tryptase measured in baseline samples of serum or plasma, a concentration that remains relatively constant for a given individual over time. However, baseline serum tryptase (bST) concentrations vary significantly between individuals, depending primarily on genetic factors.7, 8, 9 Another portion of α- and β-protryptases are converted to mature tryptase, in the form of β-homotetramers or α-/β-heterotetramers. These forms are stored in secretory granules and released upon mast cell degranulation.10
Therefore, even though an elevated serum tryptase does not necessarily reflect an IgE-mediated allergic reaction,11 an increase in acute serum tryptase (aST) over bST is supportive for mast cell activation even if aST is within the normal reference range.12 Consequently, paired sampling of aST and bST has been recommended when investigating patients with a suspected POH reaction.12, 13, 14, 15 aST should be obtained between 30 and 120 min after initiation of symptoms, whereas bST should be measured in a sample collected before the event or at least 24 h after all signs and symptoms have resolved.16
The optimal threshold to predict mast cell-related POH has long been debated. In 2012, Valent and colleagues14 proposed that an aST value exceeding (1.2×bST+2) confirmed mast cell degranulation. This consensus formula has been validated in POH, with sensitivity and specificity varying at 75–78% and 86–95%, respectively.17, 18, 19 However, some researchers have also examined the use of delta tryptase (ΔT=aST–bST) or the percentage of tryptase increase (ΔT%=[aST–bST]/bST) to confirm mast cell activation,20,21 whereas others have focused on defining the variability of bST to improve accuracy in identifying anaphylaxis,8 a phenomenon that could be particularly relevant in children, such as young infants who have higher bST.22
Another important element that has only been explored to a limited extent is the effect of uncomplicated anaesthesia and surgery per se on serum tryptase, especially in children. Studies in adults generally have shown no significant change in serum tryptase during coronary artery bypass surgery using extracorporeal circulation,23 cardiac defibrillation after suxamethonium administration,24 or in the 75 adult control individuals included in our study on mast cell activation during suspected POH.17 In contrast, in a study on tryptase before and after uneventful orthopaedic surgery in 120 subjects aged 18 yr or older, a statistically significant decrease in serum tryptase concentration of 0.45 μg L−1 was observed, which the authors attributed to dilution from i.v. fluids.12 We are not aware of any literature about the effect of uncomplicated anaesthesia on serum tryptase concentrations in children and the potential consequences for the interpretation of aST in this specific population.
Therefore, the aim of our current study is dual. First, to explore the effect of uncomplicated general anaesthesia and surgery on serum tryptase concentration in children. Next, we attempted to validate and optimise the current consensus formula to identify perioperative mast cell activation in children.
Methods
Population
From January 2021 to May 2022, control children under 18 yr old were included prospectively via the Department of Anaesthesia of the Antwerp University Hospital. Children and their legal guardians were asked to participate if they were to undergo general anaesthesia that would last longer than 90 min. In all children, a bST sample was obtained immediately after venous cannulation and before any i.v. drugs or fluids were administered. An intraoperative serum tryptase sample was obtained 60–90 min after induction (ioST). The ioST samples were included only if the children had uncomplicated anaesthesia, that is, did not show any sign of a potential POH reaction as described in the international hypersensitivity clinical scoring scheme (IHCSS).25
Next, 94 paediatric patients who had undergone investigation for suspected POH were included retrospectively through datasets of the Allergy department of the Antwerp University Hospital, Belgium (January 2006 to January 2022; n=19), the University Hospital of Leuven, Belgium (2021–2; n=5), the University Hospital of Marseille, France (March 2013 to December 2020; n=22), and the Danish Anaesthesia Allergy Centre, Gentofte Hospital, Denmark (2000–21; n=48). All referred children within the respective timeframes were included if they were <18 yr old at the time of their reaction and if the referring anaesthetist and the treating allergist considered them POH patients based on the signs, symptoms, and test results. Patients were excluded if there was no paired tryptase sampling available. All patients had a diagnostic work-up according to international consensus recommendations.12
To compare our findings with adult controls, we re-analysed paired aST and bST measurements from 75 adult control patients who had undergone uneventful general anaesthesia at the Antwerp University Hospital. Part of these adult data were previously published.17
Approval for this study was obtained from the local ethics committee (reference no. B300201316408). All prospectively included participants, their representatives, or both signed an informed consent in accordance with the Declaration of Helsinki. Samples were registered and stored in the Antwerp Biobank (ID: BE 71030031000). Anonymised retrospective data collection for patients from the University Hospitals of Marseille was approved by the institutional ethics committee (reference 2021-059). Anonymised retrospective data collection for patients from the Danish Anaesthesia Allergy Centre, Gentofte, Denmark, was approved by the Capital Region under the research project journal number P-2022-317. Anonymised retrospective data collection for patients from the University Hospitals of Leuven was approved by the research ethics committee UZ/KU Leuven (reference MP0126642021-059).
Tryptase
Tryptase was measured by the FEIA ImmunoCAP technique (Phadia Thermo Fisher Scientific, Uppsala, Sweden). Samples were obtained as described above and tryptase concentrations were expressed in μg L−1. The lower limit of quantitation for the Thermo Fisher Scientific total tryptase assay is 1 μg L−1 and coefficients of variation are 3–7% as reported by the manufacturer. Reported values lower than 1 μg L−1 were set to 1 μg L−1 in concordance with the lower limit of quantitation.
Statistical analyses
Statistical analysis was performed using SPSS® statistics 27 (IBM®, New York, NY, USA) and R Statistical Software (version 4.1.3; R Core Team 2021). Data were checked for normality in the overall group and subgroups with the Kolmogorov–Smirnov test. Skewed data were transformed using log scale, square root, and Box-Cox transformation. As normality was not achieved, untransformed data were used and analysed with non-parametric tests.
Results are expressed as median (range). The non-parametric Mann–Whitney U-test was used to compare unpaired continuous variables, and the Wilcoxon matched-pairs signed-rank test was used to compare paired continuous variables. Categorical variables were expressed as frequencies, and differences were evaluated using χ2 test and a Fisher exact test when appropriate (frequency of variable <5). Correlation significance and coefficients were calculated using Kendall's Tau correlation analysis.
To explore potential alternatives to the consensus formula to predict POH, receiver operating characteristics (ROC) analyses were used to analyse sensitivity and specificity for different cut-off points for ΔT and ΔT% to discriminate between patients and control individuals. This was done by using 100 repeated five-fold cross-validation. In each run, the dataset was split into five subsets, using four sets for training and the remaining data as a test set. This process was repeated 100 times.
Statistically significant differences between the performance of different decision thresholds were obtained by comparing the ROC areas under the curve. McNemar's test was used to compare the sensitivity of a potential alternative formula with that of the current consensus formula. P-values ≤0.05 were considered significant.
Results
Variation in tryptase in children during uneventful anaesthesia
A total of 100 children who had uncomplicated anaesthesia were included as controls. In four of these 100 control children, the exact timing of the sampling was uncertain, and samples were excluded from further analysis. Thus, paired bST and ioST from 96 control children (49 pre-school children up to 6 yr old, 47 school children aged 6–16 yr old) were available for further analyses (Fig 1). The types of surgery were: abdominal (n=7), maxillofacial (n=17), neurosurgery (n=2), ear nose throat (n=22), ophthalmic (n=4), orthopaedic (n=9), urological (n=34), and thoracovascular surgery (n=1).
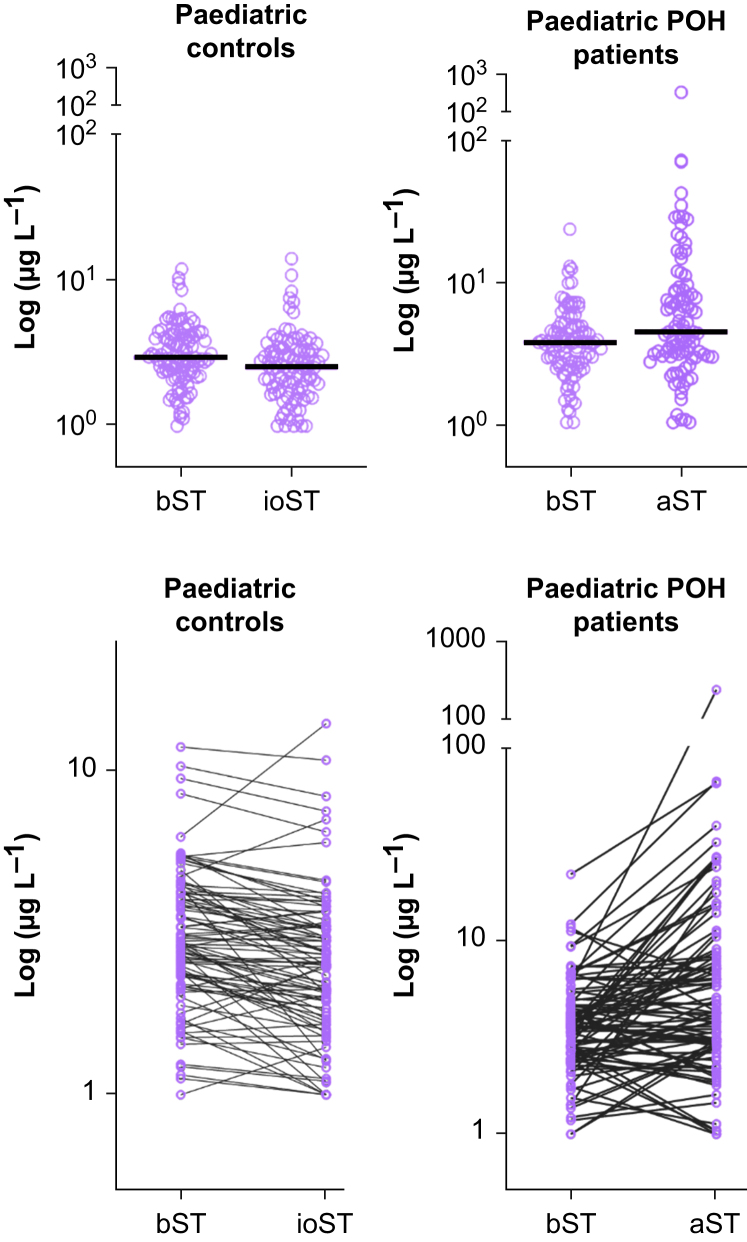
Fig. 1.
Tryptase concentrations for controls and patients with perioperative hypersensitivity (POH). The upper panels show individual tryptase values with the median concentration shown as a horizontal line. The lower panels show the tryptase concentration changes in individual patients. aST, acute serum tryptase; bST, basal serum tryptase; ioST, intraoperative serum tryptase.
In the control children, the median bST was 2.92 μg L−1 (range 1.00–11.75), with the concentration significantly higher in pre-school children than in school children (3.29 vs 2.67 μg L−1; range 1.43–11.75 vs 1.00–10.25; P=0.038; Table 1). The median ioST was 2.52 μg L−1 (range 1.00–13.87), which was significantly lower than the corresponding bST. This statistically significant reduction was observed in all age groups. The median change between bST and ioST was –0.41 μg L−1 (range –3.09–7.66; Table 2).
Table 1.
Baseline serum tryptase concentration in control children undergoing uneventful general anaesthesia. bST, baseline serum tryptase; NS, not significant; Pre-school, 0–6 yr; School, 6–18 yr.
| n (%) | Control children |
p-value | |
|---|---|---|---|
| bST (ng ml−1) median (range) | |||
| Total | 96 (100) | 2.92 (1.00–11.75) | |
| Age group Pre-school School |
49 (51.04) 47 (48.96) |
3.29 (1.43–11.75) 2.67 (1.00–10.25) |
0.038 |
| Sex Female Male |
28 (29.17) 68 (70.83) |
2.95 (1.00–9.40) 2.89 (1.15–11.75) |
NS |
Table 2.
Variation in serum tryptase concentration in control children undergoing uneventful general anaesthesia. ΔT, intraoperative serum tryptase – baseline serum tryptase; ΔT%, % variation in serum tryptase; bST, baseline serum tryptase; Pre-school, 0–6 yr; School, 6–18 yr. P-values were calculated to compare bST and intraoperative serum tryptase in each subgroup.
| ΔT absolute value (ng ml−1) median (range) | ΔT as a % of bST median (range) |
p-value | Total (n) | |
|---|---|---|---|---|
| Total | –0.41 (–3.09 to 7.66) | –15.33 (–70.81 to 123.23) | <0.001 | 96 |
| Age group Pre-school School |
–0.51 (–3.09 to 2.33) –0.41 (–1.97 to 7.66) |
–14.50 (–70.81 to 52.36) –15.53 (–60.23 to 123.23) |
<0.001 <0.001 |
49 47 |
| Sex Female Male |
–0.37 (–2.33 to 0.40) –0.46 (–3.09 to 7.66) |
–15.36 (–60.23 to 40.38) –15.33 (–70.81 to 123.23) |
<0.001 0.004 |
28 68 |
Overall, in 76 out of 96 (79.2%) children ioST was lower than bST, one patient's serum tryptase remained unchanged, and in 19 out of 96 (19.8%) children the ioST was higher than the bST. One of these fulfilled the current consensus formula of mast cell activation even though no symptoms or signs were reported.
In 51 control children, the infusion rates (ml kg−1 h−1) of the administered i.v. fluids were available. In these 51 children, there was a correlation between the fluid infusion rate (ml kg−1 h−1) and the decrease in ioST (P=0.028, correlation coefficient –0.215). Fluid administration is shown in the Supplementary Figure S1.
In our previously published study with 75 paired samples of adult controls, the median ioST was lower than—but did not differ significantly from—bST.17 The `median bST in adults was 2.28 μg L−1 (range 0.04–19.42), which is significantly lower than that in children (2.93 μg L−1; range 1.00–11.75; P<0.001).
Variation in tryptase in paediatric patients with perioperative hypersensitivity
A total of 94 paediatric POH patients with paired bST and aST were retrospectively included (Fig 1, Table 3). Of these, 49 (52.1%) patients experienced grade 1–2 reactions according to the modified Ring and Messmer classification,26 41 (43.6%) patients experienced grade 3–4 reactions, with no severity grading available in the remaining four (4.3%) patients. The severity grades did not differ significantly between the different participating centres (P=0.300). A culprit drug was identified in 34 out of 94 (36.2%) patients.
Table 3.
Paediatric perioperative hypersensitivity (POH) patients. ΔT, intraoperative serum tryptase – baseline serum tryptase; ΔT%, % variation in serum tryptase; aST, acute serum tryptase; bST, baseline serum tryptase; F, female; GRADE, grade of the hypersensitivity reaction according to the Modified Ring and Messmer classification; NA, not applicable; NS, not statistically significant. P-values were calculated for the comparison between females and males, grades 1 and 2 compared with grades 3 and 4 and for bST compared with aST. P-values in the upper right column were to compare control vs POH population.
| Paediatric POH patients |
Control vs POH p-value | ||
|---|---|---|---|
| p-value | |||
| Total | 94 | NA | |
| Age (months), median (range) | 178 (1–213) | NA | |
| Sex (F, %) | 31 (32.98) | <0.001 | NA |
| Grade 1 or 2, n (%) | 49 (52.13) | NS | NA |
| Grade 3 or 4, n (%) | 41 (43.62) | NS | NA |
| Grade NG, n (%) | 4 (4.26) | NA | |
| bST (μg L−1), median (range) | 3.60 (1.00–22.10) | <0.001 | 0.035 |
| aST (μg L−1), median (range) | 4.24 (1.00–228) | <0.001 | |
| ΔT (μg L−1), median (range) | 0.78 (–7.41 to 222.12) | <0.001 | |
| ΔT% (%), median (range) | 23.72 (–63.64 to 37777.55) | <0.001 | |
The median bST was 3.60 μg L−1 (range 1.00–22.10), which is significantly higher than the 2.92 μg L−1 found in the control children (P=0.042). The median aST was 4.24 μg L−1 (range 1.00–228.00), which is significantly higher than the median bST of 3.60 μg L−1 (range 1.00–22.10) in this group of patients (P<0.001). The median ΔT was 0.79 μg L−1 and the median ΔT% was 23.72%. We observed an increase in serum tryptase in 59 out of 94 (62.77%) patients, whereas 33 (35.11%) showed a decrease in serum tryptase. The ΔT, ΔT%, and number of patients showing an increase in serum tryptase did not differ significantly among the participating centres.
Performance of different methods to confirm mast cell activation
The current consensus formula identified mast cell activation in 31.91% (30 out of 94) of POH patients (sensitivity), with a specificity of 99%. Next, we adjusted the consensus formula by subtracting 0.41 μg L−1, that is, the median decrease in ioST during uneventful anaesthesia. By doing this, mast cell activation, now defined as an aST > 1.2bST + 1.59, was observed in 35.1% of the cases, without reducing specificity.
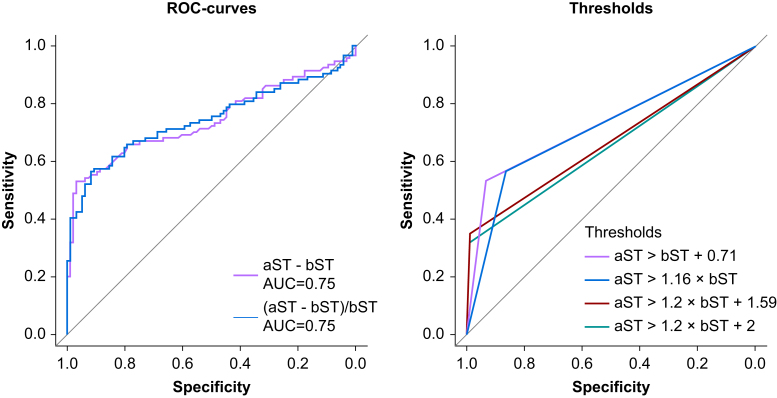
Using the paired tryptase samples from patients and controls and using 100 repeated five-fold cross-validation, ROC curves were generated for ΔT% and ΔT to analyse different cut-off points to distinguish between patients and controls (Fig 2). The optimal cut-off for ΔT% as generated by the Youden index was 15.9%, generating a sensitivity of 56.6% and a specificity of 86.4% through cross-validation. The optimal cut-off point for ΔT as generated by the Youden index was 0.71 μg L−1, associated with a sensitivity of 53.3% and specificity of 93.3% through cross-validation. As both methods have a higher area under the curve than the current and amended consensus formulas, a new improved formula to identify mast cell activation in paediatric POH patients could be:
| aST>bST×1.16 |
| or aST>bST+0.71 |
Fig. 2.
Receiver operating characteristic (ROC) curves for different methods of determination of mast cell activation. aST, acute serum tryptase; AUC, area under the curve; bST, basal serum tryptase.
The ROC curves for the different methods are shown in Fig 2.
As the aST>bST+0.71 formula retained the highest specificity, further analysis was performed with this formula. This new paediatric formula had statistically higher sensitivity than the current consensus formula in our population (53.2% vs 31.9%; P<0.001), with a specificity of 96.9%. Subgroup analysis in the population where a culprit was identified yielded higher sensitivity for both the current consensus formula (61.67% vs 15.00%; P<0.001) and the aST>bST + 0.71 formula (85.3% vs 35.0%; P<0.001) compared with the group where no culprit was identified. Similarly, subgroup analysis in grade 3 and 4 reactions yielded higher sensitivity for both the current consensus formula (48.8% vs 18.4%; P=0.002) and the aST>bST+0.71 formula (78.0% vs 34.7%; P<0.001) compared with grade 1 and 2 reactions. In both subgroups, the sensitivity of the aST>bST+0.71 formula was significantly higher than the current consensus formula (85.3% vs 61.7%; P=0.008 and 78.0% vs 48.8%; P<0.001, respectively).
Discussion
Given the multiple differential diagnoses and difficulties with correct diagnosis of POH, the current consensus formula identifying mast cell activation is an important advance in the clinical assessment of a suspected POH reaction.27 In order to validate or optimise this formula in the paediatric population, it is of utmost importance to explore the effect of uneventful anaesthesia and surgery on serum tryptase concentrations. To date, this has only been studied to a limited extent and only in adults.12,17,23,24
We found that baseline tryptase concentrations in control children (median 2.92 μg L−1) were lower than those in POH children (median 3.60 μg L−1) and interestingly also lower than those in control children reported by Komarow and colleagues28 (median 3.44 μg L−1). This might reflect geographical or ethnic differences as reported baseline tryptase concentrations vary based on location.29,30 Our data are also consistent with the lower baseline tryptase concentrations in adults in our region that we reported earlier (median 2.28 μg L−1).17 The current study included patients from Belgium, France, and Denmark, but larger multinational studies are required to examine the effect of geographical variation in paediatric tryptase concentration on dynamic tryptase performance in POH.
Our data show that during the first 60–90 min, nearly 80% of children who underwent uncomplicated general anaesthesia had a lower ioST than their bST. This statistically significant decrease (median ΔT –0.41 μg L−1) was not observed in the 75 adult controls as reported in our previous publication,17 but a very similar median decrease of 0.45 μg L−1 was reported by Garvey and colleagues.12 As mentioned above, we hypothesise that this decrease reflects dilution from i.v. fluids. Although adults are also administered fluids during surgery, the presumed dilutionary effect could be more pronounced in children if they receive larger amounts of fluids than their body surface area and blood volume.
Although the exact volume of fluid administered by the time of measuring the ioST could not be reliably determined from the anaesthetic charts, we were able to determine infusion rates in about half of the control children. Among these patients, there was a clear correlation between the infusion rate and the decrease in tryptase concentration. We should also consider that, as fluid administration is a key therapy in treating anaphylaxis,1 POH patients were likely to have received a larger amount of fluid in the period immediately before aST sampling, potentially lowering this value. Indeed, the poor sensitivity of the consensus formula (31.91%) among 94 POH patients from four centres in three countries and the fact that one-third of our paediatric POH patients also showed a decrease in aST seem to support this and suggests that a very sensitive algorithm is needed to detect discrete changes in tryptase.
Although the new formula derived in this study (aST>bST+0.71) performs significantly better in our population than the current consensus formula, it does not yield similar sensitivity as observed in adults.17 This might relate to the fact that children experience less severe reactions than adults. Only about 43% of the paediatric POH patients experienced severe anaphylaxis (grade 3 or 4) compared with 60% in adults.27,31, 32, 33
Another possibility that could explain the lower sensitivity of tryptase changes is a difference in the underlying mechanisms of POH in children compared with adults. Indeed, the mechanism of POH can be either allergic (IgE-mediated) or non-allergic (e.g. Mas-related G-protein coupled receptor member X2 [MRGPRX2] agonism by neuromuscular blocking agents, COX-1/2 inhibition by non-steroidal anti-inflammatory drugs). Moreover, in a study by Mertes and colleagues34 higher tryptase changes were observed in children who presented an IgE-mediated reaction than in those in whom no clear IgE-mediated process was demonstrable. In line with these findings, our data show a higher sensitivity of tryptase in reactions where a culprit was identified suggesting an allergic (antigen-specific) mechanism in these patients. However, even in this population, the new formula performs significantly better than the current consensus formula (85.29% vs 61.67%; P=0.008). Also, nonspecific mast cell activation by the MRGPRX2 receptor has been proposed as one of the main mechanisms of non-allergic POH and we have demonstrated that, as tryptase is released in both reactions, it does not discriminate between IgE-mediated and MRGPRX2-mediated reactions.35,36 This remains an area that needs further study.
Similarly, we need to look critically at the inclusion criteria of the POH cohort. Because the reference test in POH, the drug challenge, is not commonly performed,37 especially in children, there remains the risk of false-positive diagnoses. We included all children in our reference centres that were considered POH patients by the allergists, but no formal scoring system was used. Hopkins and colleagues25 have introduced such a scoring system, the IHCSS, which was since validated by Sadleir and colleagues38 in adults. By using a formal scoring system to identify almost certain POH cases, more objectivity and confidence in the true positive cohort can be obtained. Unfortunately, as our POH cohort was retrospectively recruited, we did not have sufficient information on all patients to accurately calculate the IHCSS. However, looking at the IHCSS, it seems that it might also not optimally apply to children, as the clinical presentation of POH in children and adults differs.27,34 In any case, the inclusion criteria of the true positive cohort are a limitation of this study and this will remain so for all diagnostic accuracy studies as long as a reference test is not practically feasible. In the meanwhile, the implementation of a formal scoring system such as the IHCSS can and should be used in future prospective studies to improve certainty in the true positive cohort of such studies. We would stress, however, that all patients in this study were referred to specialised POH reference centres and only received a work-up after careful consideration and a compelling history. Moreover, in all four participating centres, the percentage of severe reactions and reactions in which a culprit was found, was almost identical. This consistency in the data from several specialised reference centres in different countries leads us to believe our data to be representative and valid.
Another remark is that the new formula achieves a greater sensitivity at the cost of a slightly lower specificity (aST>bST+0.71 has a cross-validated specificity of 93.3% compared with 99% in the current consensus formula). A high threshold with consequently an almost perfect specificity is certainly useful as a positive result allows us to confidently make the diagnosis. However, this comes at the cost of lower sensitivity with the current consensus formula identifying just 32% of POH patients. This poses significant risks as a false-negative diagnosis can lead to future re-exposures to the culprit. It is also important to consider the pretest odds in the population where tryptase would be measured. In uneventful anaesthesia, tryptase would not be routinely measured. Only in situations where an anaesthetist suspects POH will they measure it. A somewhat lower specificity (93% vs 99%) with a significantly higher sensitivity (53% vs 32%) in a situation with high pretest odds means many more patients will be correctly diagnosed without many false positives. We therefore consider this a worthwhile trade-off as fewer cases of POH will be missed and further investigations can invalidate any false-positives results, especially through the increased use of direct drug provocation tests with anaesthetics.37 Alternatively, both the current consensus formula and the paediatric formula could be used in a modified paediatric clinical scoring system corresponding to different points to be added analogous to the different cut-off points in the current IHCSS. The main utilisation of biological evidence for mast cell activation is to supplement the score which is most important in less severe cases or patients with ‘single organ’ involvement where clinical diagnosis might be less straightforward.
From the technical viewpoint of total tryptase determination, confidently assessing slight tryptase variations, such as of 13% on average, requires sound quality assessment procedures in the clinical laboratory.39 Analytical intra-laboratory performance of repeated ImmunoCAP total tryptase measurements displayed median coefficients of variation of 1.9–2.6% on the same day and 5.7–6.1% if measurements were done on different days, similar to data provided by the manufacturer.40 However, inter-laboratory variability ranged from –24% to +21%. These data suggest that optimal analytical performance as required for POH tryptase measurements is more likely to be achieved when aST and bST samples are processed in the same clinical laboratory and in the same run, which was the case in the control arm of our study.
In conclusion, this is the first study suggesting the need for an adjusted formula in children to identify perioperative mast cell activation as tryptase is significantly lowered during uneventful anaesthesia. This decrease might be caused by dilution, but further studies that closely examine total amounts of administered fluids and perhaps the concurrent changes in concentration of other biomarkers are needed. In order to correct for this effect, we propose a new formula: aST>bST+0.71. This formula performs significantly better in our multicentric paediatric population than the current consensus formula and was internally validated through 100 repeated five-fold cross-validation. However, the proposed paediatric formula does not perform as well in children as the current consensus formula does in adults. This may be because children experience less severe reactions, a difference in the underlying mechanisms of POH in children compared with adults, difficulties in identifying true POH patients without a reference test, or both. We therefore call for further studies in order to confirm this new formula for identifying mast cell activation during paediatric POH and for a modified paediatric clinical scoring system to identify paediatric POH patients.
Funding
Fonds Wetenschappelijk Onderzoek - Vlaanderen National Fund for Scientific Research (FWO) senior clinical investigator fellowship (1805518N, 1804518N, and 1800614N to DGE and VSO). Fonds Wetenschappelijk Onderzoek - Vlaanderen National Fund for Scientific Research (FWO) senior clinical investigator fellowship (1805523N to RS).
Authors’ contributions
Drafting of manuscript: NV, MVP, DGE.
Reviewing and editing the manuscript: all authors.
Study design: NV, DGE, VSO
Data collection: NV, CM, JV, LHG
Creation of figures: MVP
Approval of the final text: all authors
Acknowledgements
Dr Tim Stals contributed to the data collection for patients from UZ/KU Leuven (MP012664).
Handling editor: Phil Hopkins
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2023.100254.
Declaration of interest
JV reports speaker and consultancy fees and travel support in the past 5 yr from ALK, AstraZeneca, HpVac, Novartis, L’Oréal, Sanofi, Stallergenes Greer, Thermo Fisher Scientific, outside the submitted work. The other authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Garvey L.H., Dewachter P., Hepner D.L., et al. Management of suspected immediate perioperative allergic reactions: an international overview and consensus recommendations. Br J Anaesth. 2019;123:e50–e64. doi: 10.1016/j.bja.2019.04.044. [DOI] [PubMed] [Google Scholar]
- 2.Garvey L.H., Ebo D.G., Mertes P.M., et al. An EAACI position paper on the investigation of perioperative immediate hypersensitivity reactions. Allergy. 2019;74:1872–1884. doi: 10.1111/all.13820. [DOI] [PubMed] [Google Scholar]
- 3.Ebo D.G., Clarke R.C., Mertes P.M., Platt P.R., Sabato V., Sadleir P.H.M. Molecular mechanisms and pathophysiology of perioperative hypersensitivity and anaphylaxis: a narrative review. Br J Anaesth. 2019;123:e38–e49. doi: 10.1016/j.bja.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Castells M.C., Irani A.M., Schwartz L.B. Evaluation of human peripheral blood leukocytes for mast cell tryptase. J Immunol. 1987;138:2184–2189. [PubMed] [Google Scholar]
- 5.Jogie-Brahim S., Min H.K., Fukuoka Y., Xia H.Z., Schwartz L.B. Expression of alpha-tryptase and beta-tryptase by human basophils. J Allergy Clin Immunol. 2004;113:1086–1092. doi: 10.1016/j.jaci.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz L.B., Min H.K., Ren S., et al. Tryptase precursors are preferentially and spontaneously released, whereas mature tryptase is retained by HMC-1 cells, Mono-Mac-6 cells, and human skin-derived mast cells. J Immunol. 2003;170:5667–5673. doi: 10.4049/jimmunol.170.11.5667. [DOI] [PubMed] [Google Scholar]
- 7.Sverrild A., van der Sluis S., Kyvik K.O., et al. Genetic factors account for most of the variation in serum tryptase--a twin study. Ann Allergy Asthma Immunol. 2013;111:286–289. doi: 10.1016/j.anai.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Mateja A., Wang Q., Chovanec J., et al. Defining baseline variability of serum tryptase levels improves accuracy in identifying anaphylaxis. J Allergy Clin Immunol. 2022;149:1010–1017. doi: 10.1016/j.jaci.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waters A.M., Park H.J., Weskamp A.L., et al. Elevated basal serum tryptase: disease distribution and variability in a regional health system. J Allergy Clin Immunol Pract. 2022;10:2424–2435. doi: 10.1016/j.jaip.2021.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Q.T., Lyons J.J., Naranjo A.N., et al. Impact of naturally forming human alpha/beta-tryptase heterotetramers in the pathogenesis of hereditary alpha-tryptasemia. J Exp Med. 2019;216:2348–2361. doi: 10.1084/jem.20190701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabato V., Ebo D.G., Van Der Poorten M.M., et al. Allergenic and MRGPRX2-activating properties of drugs: resolving the two. J Allergy Clin Immunol Pract. 2023;11:395–404. doi: 10.1016/j.jaip.2022.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Garvey L.H., Bech B., Mosbech H., et al. Effect of general anesthesia and orthopedic surgery on serum tryptase. Anesthesiology. 2010;112:1184–1189. doi: 10.1097/ALN.0b013e3181d40383. [DOI] [PubMed] [Google Scholar]
- 13.Ebo D.G., Faber M., Elst J., et al. In vitro diagnosis of immediate drug hypersensitivity during anesthesia: a review of the literature. J Allergy Clin Immunol Pract. 2018;6:1176–1184. doi: 10.1016/j.jaip.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Valent P., Akin C., Arock M., et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–225. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valent P., Bonadonna P., Hartmann K., et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe systemic mast cell activation and mast cell activation syndrome. Int Arch Allergy Immunol. 2019;180:44–51. doi: 10.1159/000501079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitte J., Sabato V., Tacquard C., et al. Use and interpretation of acute and baseline tryptase in perioperative hypersensitivity and anaphylaxis. J Allergy Clin Immunol Pract. 2021;9:2994–3005. doi: 10.1016/j.jaip.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Ebo D.G., De Puysseleyr L.P., Van Gasse A.L., et al. Mast cell activation during suspected perioperative hypersensitivity: a need for paired samples analysis. J Allergy Clin Immunol Pract. 2021;9:3051–3059. doi: 10.1016/j.jaip.2021.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Baretto R.L., Beck S., Heslegrave J., et al. Validation of international consensus equation for acute serum total tryptase in mast cell activation: a perioperative perspective. Allergy. 2017;72:2031–2034. doi: 10.1111/all.13226. [DOI] [PubMed] [Google Scholar]
- 19.Vitte J., Amadei L., Gouitaa M., et al. Paired acute-baseline serum tryptase levels in perioperative anaphylaxis: an observational study. Allergy. 2019;74:1157–1165. doi: 10.1111/all.13752. [DOI] [PubMed] [Google Scholar]
- 20.Egner W., Sargur R., Shrimpton A., York M., Green K. A 17-year experience in perioperative anaphylaxis 1998-2015: harmonizing optimal detection of mast cell mediator release. Clin Exp Allergy. 2016;46:1465–1473. doi: 10.1111/cea.12785. [DOI] [PubMed] [Google Scholar]
- 21.Borer-Reinhold M., Haeberli G., Bitzenhofer M., et al. An increase in serum tryptase even below 11.4 ng/mL may indicate a mast cell-mediated hypersensitivity reaction: a prospective study in Hymenoptera venom allergic patients. Clin Exp Allergy. 2011;41:1777–1783. doi: 10.1111/j.1365-2222.2011.03848.x. [DOI] [PubMed] [Google Scholar]
- 22.Belhocine W., Ibrahim Z., Grandné V., et al. Total serum tryptase levels are higher in young infants. Pediatr Allergy Immunol. 2011;22:600–607. doi: 10.1111/j.1399-3038.2011.01166.x. [DOI] [PubMed] [Google Scholar]
- 23.Fayaz K.M., Pugh S., Balachandran S., Sudheer P.S., Hall J.E. Histamine release during adult cardiopulmonary bypass. Anaesthesia. 2005;60:1179–1184. doi: 10.1111/j.1365-2044.2005.04368.x. [DOI] [PubMed] [Google Scholar]
- 24.Storjord E., Nielsen E.W. Tryptase levels after suxamethonium administration and defibrillation. Acta Anaesthesiol Scand. 2008;52:838–840. doi: 10.1111/j.1399-6576.2007.01508.x. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins P.M., Cooke P.J., Clarke R.C., et al. Consensus clinical scoring for suspected perioperative immediate hypersensitivity reactions. Br J Anaesth. 2019;123:e29–e37. doi: 10.1016/j.bja.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Ring J., Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466–469. doi: 10.1016/s0140-6736(77)91953-5. [DOI] [PubMed] [Google Scholar]
- 27.Ebo D.G., Van Gasse A.L., Decuyper, et al. Acute management, diagnosis, and follow-up of suspected perioperative hypersensitivity reactions in Flanders 2001-2018. J Allergy Clin Immunol Pract. 2019;7:2194–2204. doi: 10.1016/j.jaip.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 28.Komarow H.D., Hu Z., Brittain E., Uzzaman A., Gaskins D., Metcalfe D.D. Serum tryptase levels in atopic and nonatopic children. J Allergy Clin Immunol. 2009;124:845–848. doi: 10.1016/j.jaci.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slot M.C., Claessen L.H.J., Bons J.A.P., Menheere P.P.C.A., Nieuwhof C.M.G., de Boer D. Tryptase reference ranges are age-dependent in a large population-based cohort. Allergy. 2022;77:2833–2834. doi: 10.1111/all.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitte J., Sjölander A., Rydell N., et al. Tryptase reference values in a Swedish middle-aged general population and association with diabetes mellitus. Clin Exp Allergy. 2022;52:1330–1333. doi: 10.1111/cea.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garvey L.H., Roed-Petersen J., Menné T., Husum B. Danish anaesthesia Allergy centre - preliminary results. Acta Anaesthesiol Scand. 2001;45:1204–1209. doi: 10.1034/j.1399-6576.2001.451005.x. [DOI] [PubMed] [Google Scholar]
- 32.Antunes J., Kochuyt A.M., Ceuppens J.L. Perioperative allergic reactions: experience in a Flemish referral centre. Allergol Immunopathol (Madr) 2014;42:348–354. doi: 10.1016/j.aller.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Tacquard C., Collange O., Gomis P., et al. Anaesthetic hypersensitivity reactions in France between 2011 and 2012: the 10th GERAP epidemiologic survey. Acta Anaesthesiol Scand. 2017;61:290–299. doi: 10.1111/aas.12855. [DOI] [PubMed] [Google Scholar]
- 34.Mertes P.M., Alla F., Tréchot P., Auroy Y., Jougla E. Groupe d'Etudes des Réactions Anaphylactoïdes Peranesthésiques. Anaphylaxis during anesthesia in France: an 8-year national survey. J Allergy Clin Immunol. 2011;128:366–373. doi: 10.1016/j.jaci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Elst J., van der Poorten M.M., Van Gasse A.L., et al. Tryptase release does not discriminate between IgE- and MRGPRX2-mediated activation in human mast cells. Clin Exp Allergy. 2022;52:797–800. doi: 10.1111/cea.14110. [DOI] [PubMed] [Google Scholar]
- 36.Ebo D.G., Van der Poorten M.L., Elst J., et al. Immunoglobulin E cross-linking or MRGPRX2 activation: clinical insights from rocuronium hypersensitivity. Br J Anaesth. 2021;126:e27–e29. doi: 10.1016/j.bja.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Garvey L.H., Ebo D.G., Krøigaard M., et al. The use of drug provocation testing in the investigation of suspected immediate perioperative allergic reactions: current status. Br J Anaesth. 2019;123:e126–e134. doi: 10.1016/j.bja.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Sadleir P.H.M., Clarke R.C., Goddard C.E., Mickle P., Platt P.R. Agreement of a clinical scoring system with allergic anaphylaxis in suspected perioperative hypersensitivity reactions: prospective validation of a new tool. Br J Anaesth. 2022;129:670–678. doi: 10.1016/j.bja.2022.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Michel M., Klingebiel C., Vitte J. Tryptase in type I hypersensitivity. Ann Allergy Asthma Immunol. 2023;130:169–177. doi: 10.1016/j.anai.2022.08.996. [DOI] [PubMed] [Google Scholar]
- 40.Sarrat A., Couderc R., Alyanakian M.A., et al. [Performance criteria for the verification of IgE and tryptase assay methods: recommendations from the AllergoBioNet network] Ann Biol Clin (Paris) 2020;78:329–342. doi: 10.1684/abc.2020.1549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.