Abstract
Background:
Cirrhosis with acute decompensation (AD) and acute-on-chronic liver disease (ACLF) are characterized by high morbidity and mortality. Cytolysin, a toxin from Enterococcus faecalis (E. faecalis), is associated with mortality in alcohol-associated hepatitis (AH). It is unclear whether cytolysin also contributes to disease in AD and ACLF.
Methods:
We studied the role of fecal cytolysin in 78 patients with AD/ACLF.
Results:
Fecal cytolysin and E. faecalis abundance do not predict chronic liver failure (CLIF-C) AD and ACLF scores in AD and ACLF patients, respectively. Presence of fecal cytolysin is not associated with other liver disease markers, including Fibrosis-4 (FIB-4) index, ‘Age, serum Bilirubin, INR, and serum Creatinine’ (ABIC) score, Child-Pugh score, Model for End-Stage Liver Disease (MELD) or MELD-Na scores in AD and/or ACLF patients (including in patients with alcohol-associated etiology only).
Conclusions:
In conclusion, fecal cytolysin does not predict disease severity in AD and ACLF patients, including patients with alcohol-associated cirrhosis with AD or ACLF. The predictive value of fecal cytolysin positivity for mortality appears to be restricted to AH.
Keywords: Liver disease, acute decompensation, ACLF, microbiome, MELD
Background
Intestinal dysbiosis, or an imbalance of beneficial and potentially pathogenic microbes [1, 2], is present in several hepatic diseases [3, 4]. Besides fungal [5-8] and viral dysbiosis[9, 10], bacterial dysbiosis plays a central role in the pathogenesis of liver disease [11, 12]. Particularly liver cirrhosis is associated with dysbiosis and increased gut permeability [13, 14]. We recently found that cytolysin, a toxin from Enterococcus faecalis (E. faecalis), directly lyses hepatocytes in ethanol-induced liver disease (ALD) models [15]. Cytolysin-positive E. faecalis is a significant predictor for mortality in alcohol-associated hepatitis (AH) with 89% 180-day mortality in cytolysin-positive AH patients compared with only 3.8% in cytolysin-negative AH patients [15]. It is unclear whether its predictive value is confined to ALD, or AH specifically. Cytolysin-positive E. faecalis does not associate with disease severity in nonalcoholic fatty liver disease (NAFLD) [16]. The role of cytolysin-positive E. faecalis is unknown in other liver diseases, including cirrhosis with acute decompensation (AD) or acute-on-chronic liver failure (ACLF). The pathophysiology of ACLF is still incompletely understood and it is a difficult-to-treat condition with a very high transplant-free 90-day mortality up to 50% [17]. AH is one of the most common triggers for the development of ACLF and, together with bacterial infection, is present in almost all patients who develop ACLF [18, 19].
We therefore aimed to investigate the role and predictive value of cytolysin in cirrhosis with AD and ACLF. We hypothesized that cytolysin predicts disease severity in AD and ACLF.
Material and methods
Patients
Fecal samples were collected when patients were evaluated for a transjugular intrahepatic portosystemic shunt (TIPS). Inclusion criteria were over 18 years of age with clinical signs of liver cirrhosis and an indication for TIPS insertion. Exclusion criteria were the presence of systemic infection, severe hepatic encephalopathy of unknown cause, severe hyperbilirubinemia, pulmonary hypertension or pregnancy as previously described [20].
Ethics approval and consent to participate
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the human research and ethical committee of the University of Bonn (121/14). Written informed consent was obtained from all patients.
Bacterial DNA extraction and real-time quantitative PCR
Human stool samples were stored at −80°C, DNA from fecal samples was extracted and qPCR performed as described [15]. Primer sequences: E. faecalis forward: 5′-CGCTTCTTTCCTCCCGAGT-3′, reverse: 5'-GCCATGCGGCATAAACTG-3'; E. faecalis cylLL forward: 5′-CTGTTGCGGCGACAGCT-3′, reverse: 5′-CCACCAACCCAGCCACAA-3′; E. faecalis cylLS forward: 5'-GTAAAATAAGTAAAATCAAGAAAACTATTACTC-3', reverse: 5′-CAAAAGAAGGACCAACAAGTTCTAATT-3′ [15, 16].
Statistical analysis
Results are expressed as median and interquartile range in brackets for each continuous outcome and as number and percentage for factor variables, if not stated otherwise. Area under the curve (AUC), best threshold to maximize the Youden Index, sensitivity, specificity, accuracy, precision, positive predictive value, negative predictive value, and p value between two AUCs of receiver operating characteristic (ROC) curves per Delong method were calculated using the pROC library in R. Kaplan-Meier curves to visualize survival were created employing the survival library in R. Mean decrease Gini score and mean decrease accuracy were calculated with the randomForest library, and odds ratios with the questionr library in R. Continuous variables were compared using the Mann-Whitney test. Categorical variables were compared using the Pearson's Chi-squared test. A value of p < 0.05 was considered to be statistically significant. Statistical analyses were performed using R statistical software, R version 1.3.1093, 2020 the R Foundation for Statistical Computing
Results
Study population with cirrhosis and acute decompensation or acute-on-chronic liver failure
The study population consisted of 78 patients with acutely decompensated cirrhosis (AD, n=63) or acute-on-chronic liver failure (ACLF, n=15) (Table 1). All subjects were enrolled when they received a transjugular intrahepatic portosystemic shunt (TIPS) to treat complications of portal hypertension, most frequently for ascites (47.4%) and variceal bleeding (26.3%) (Table 1). Half of the subjects were male (52.6%), the average age was 56.5 years. 57.7% of all subjects had alcohol-associated liver disease (ALD). Liver cirrhosis was cryptogenic in 9%, due to Hepatitis C virus (HCV) in 6.4%, and mixed in 6.4%. The ACLF patients had a significantly higher Model for End-Stage Liver Disease (MELD) score (medians 16.2 vs 10.2), MELD-Na score (18.2 vs 10.2), European Foundation for the study of chronic liver failure (CLIF-C) organ failure score (8 vs 6), higher grade of hepatic encephalopathy, significantly more frequently renal failure (46.7% vs 0%), circulatory failure (33.3% vs 1.6%), and respiratory failure (13.3% vs 0%) than AD patients. The CLIF-C AD score median was 48.08 in the AD group and the CLIF-C ACLF score median was 43.83 in the ACLF group. The white blood count (WBC) and serum neutrophils were significantly higher in the ACLF group compared with the AD group (7.55 vs 5.39 leukocytes/mL and 5.47 vs 3.67 neutrophils/mL, respectively). Both cohorts had a similar rate of antibiotic use but ACLF patients had a significantly higher use of rifaximin (40.0% vs 12.7%). However, the groups did not significantly differ regarding fecal cytolysin positivity (ACLF 6.67% vs AD 15.9%) (Table 1). Only one of the 78 patients died within 90 days after enrollment.
Table 1.
Baseline demographic and laboratory data of the study population.
| N | ALL SUBJECTS |
ACUTE DECOMPEN- SATION |
ACLF |
P VALUE |
|
|---|---|---|---|---|---|
| N=78 | N=63 | N=15 | |||
| Gender: | 78 | 0.353 | |||
| Male | 41 (52.6%) | 31 (49.2%) | 10 (66.7%) | ||
| Age | 78 | 56.5 [51.0;65.0] | 56.0 [51.0;65.0] | 57.0 [48.5;69.5] | 0.770 |
| Etiology: | 78 | 0.127 | |||
| Alcohol-associated | 45 (57.7%) | 36 (57.1%) | 9 (60.0%) | ||
| Budd-Chiari-Syndrome | 2 (2.56%) | 0 (0.00%) | 2 (13.3%) | ||
| Cryptogenic | 7 (8.97%) | 6 (9.52%) | 1 (6.67%) | ||
| Drug-Induced Liver Injury | 2 (2.56%) | 1 (1.59%) | 1 (6.67%) | ||
| HCV | 5 (6.41%) | 4 (6.35%) | 1 (6.67%) | ||
| Hepatic Sinusoidal Obstruction Syndrome | 2 (2.56%) | 1 (1.59%) | 1 (6.67%) | ||
| Mixed | 5 (6.41%) | 5 (7.94%) | 0 (0.00%) | ||
| NASH | 1 (1.28%) | 1 (1.59%) | 0 (0.00%) | ||
| Other | 7 (8.97%) | 7 (11.1%) | 0 (0.00%) | ||
| PBC/PSC | 2 (2.56%) | 2 (3.17%) | 0 (0.00%) | ||
| Child-Pugh | 74 | 7.00 [6.00;8.00] | 7.00 [6.00;8.00] | 8.00 [6.00;9.50] | 0.292 |
| MELD | 77 | 11.0 [8.53;14.6] | 10.2 [7.99;12.7] | 16.2 [12.6;17.8] | 0.001 |
| MELD-Na | 77 | 11.0 [8.53;16.0] | 10.2 [7.99;13.6] | 18.2 [15.9;22.0] | <0.001 |
| CLIF-C OF Score | 78 | 6.00 [6.00;7.00] | 6.00 [6.00;6.00] | 8.00 [7.50;8.50] | <0.001 |
| ACLF Grade: | 78 | <0.001 | |||
| 0 | 63 (80.8%) | 63 (100%) | 0 (0.00%) | ||
| 1 | 12 (15.4%) | 0 (0.00%) | 12 (80.0%) | ||
| 2 | 3 (3.85%) | 0 (0.00%) | 3 (20.0%) | ||
| CLIF-C AD Score | 63 | 48.08 [43.52;51.42] | 48.08 [43.52;51.42] | - | |
| CLIF-C ACLF Score | 15 | 43.83 [38.80;46.18] | - | 43.83 [38.80;46.18] | |
| HE Grade: | 78 | 0.003 | |||
| 0 | 68 (87.2%) | 59 (93.7%) | 9 (60.0%) | ||
| 1 | 7 (8.97%) | 3 (4.76%) | 4 (26.7%) | ||
| 2 | 2 (2.56%) | 1 (1.59%) | 1 (6.67%) | ||
| 3 | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| 4 | 1 (1.28%) | 0 (0.00%) | 1 (6.67%) | ||
| Ascites Grade: | 78 | 0.568 | |||
| 0 | 22 (28.2%) | 17 (27.0%) | 5 (33.3%) | ||
| 1 | 20 (25.6%) | 18 (28.6%) | 2 (13.3%) | ||
| 2 | 33 (42.3%) | 25 (39.7%) | 8 (53.3%) | ||
| 3 | 3 (3.85%) | 3 (4.76%) | 0 (0.00%) | ||
| SBP: | 78 | 0.826 | |||
| In past | 6 (7.69%) | 5 (7.94%) | 1 (6.67%) | ||
| No | 67 (85.9%) | 53 (84.1%) | 14 (93.3%) | ||
| Yes | 5 (6.41%) | 5 (7.94%) | 0 (0.00%) | ||
| Liver Failure: | 78 | 0.192 | |||
| Yes | 1 (1.28%) | 0 (0.00%) | 1 (6.67%) | ||
| Renal Failure: | 78 | <0.001 | |||
| Yes | 7 (8.97%) | 0 (0.00%) | 7 (46.7%) | ||
| Cerebral Failure: | 78 | 0.350 | |||
| Yes | 2 (2.56%) | 1 (1.59%) | 1 (6.67%) | ||
| Coagulation Failure: | 78 | - | |||
| Yes | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Circulatory Failure: | 78 | 0.001 | |||
| Yes | 6 (7.69%) | 1 (1.59%) | 5 (33.3%) | ||
| Respiratory Failure: | 78 | 0.035 | |||
| Yes | 2 (2.56%) | 0 (0.00%) | 2 (13.3%) | ||
| TIPS indication: | 57 | 0.499 | |||
| Ascites | 27 (47.4%) | 23 (51.1%) | 4 (33.3%) | ||
| Ascites + Hydropic decompen-sation | 4 (7.02%) | 2 (4.44%) | 2 (16.7%) | ||
| Ascites + Pleural effusion | 1 (1.75%) | 1 (2.22%) | 0 (0.00%) | ||
| Ascites + Portal vein thrombosis | 1 (1.75%) | 1 (2.22%) | 0 (0.00%) | ||
| Hydropic decompen-sation | 1 (1.75%) | 1 (2.22%) | 0 (0.00%) | ||
| Other | 1 (1.75%) | 0 (0.00%) | 1 (8.33%) | ||
| Portal vein thrombosis | 2 (3.51%) | 2 (4.44%) | 0 (0.00%) | ||
| Variceal bleeding | 15 (26.3%) | 11 (24.4%) | 4 (33.3%) | ||
| Variceal bleeding + Ascites | 4 (7.02%) | 3 (6.67%) | 1 (8.33%) | ||
| Variceal bleeding + Pleural effusion | 1 (1.75%) | 1 (2.22%) | 0 (0.00%) | ||
| Liver Transplantation | 77 | 2 (2.60%) | 2 (3.23%) | 0 (0.00%) | 1.000 |
| 90-day Survival: | 78 | 1.000 | |||
| Alive | 77 (98.7%) | 62 (98.4%) | 15 (100%) | ||
| WBC [/mL] | 78 | 6.17 [4.52;8.78] | 5.39 [4.20;7.94] | 7.55 [6.38;11.6] | 0.019 |
| Neutrophils [/mL] | 57 | 4.05 [3.04;6.41] | 3.67 [2.79;5.65] | 5.47 [4.56;7.22] | 0.045 |
| CRP [mg/dL] | 67 | 1.00 [0.57;2.70] | 0.96 [0.53;2.70] | 1.38 [0.78;2.10] | 0.601 |
| Hemoglobin [g/dL] | 78 | 10.6 [9.10;12.2] | 10.5 [9.35;12.2] | 11.1 [8.35;12.2] | 0.800 |
| Platelets [109/L] | 77 | 134 [90.0;184] | 134 [90.8;195] | 116 [65.5;158] | 0.348 |
| ALT [U/L] | 78 | 29.5 [21.0;40.8] | 29.0 [21.0;39.0] | 30.0 [22.5;44.0] | 0.590 |
| AST [U/L] | 75 | 47.0 [34.5;62.0] | 47.0 [35.0;61.0] | 47.5 [36.0;71.2] | 0.892 |
| GGT [U/L] | 77 | 120 [75.0;199] | 115 [73.0;180] | 150 [102;240] | 0.232 |
| AP [U/L] | 68 | 118 [88.0;162] | 117 [88.0;157] | 133 [106;169] | 0.553 |
| Bilirubin [mg/dL] | 78 | 1.14 [0.82;1.93] | 1.10 [0.78;1.66] | 1.58 [0.97;2.50] | 0.085 |
| Albumin [g/dL] | 71 | 3.10 [2.72;3.90] | 3.06 [2.76;3.69] | 3.61 [2.65;4.02] | 0.508 |
| INR | 78 | 1.20 [1.10;1.30] | 1.20 [1.10;1.30] | 1.20 [1.10;1.30] | 0.781 |
| Sodium [mmol/L] | 78 | 138 [136;141] | 139 [136;141] | 138 [132;142] | 0.554 |
| Creatinine [mg/L] | 77 | 0.99 [0.79;1.38] | 0.96 [0.78;1.30] | 1.56 [0.82;2.30] | 0.082 |
| Dialysis | 78 | 1 (1.28%) | 0 (0.00%) | 1 (6.67%) | 0.192 |
| Anticoagulation | 58 | 48 (82.8%) | 37 (80.4%) | 11 (91.7%) | 0.670 |
| Statins | 58 | 4 (6.90%) | 1 (2.17%) | 3 (25.0%) | 0.025 |
| Beta-Blockers | 58 | 32 (55.2%) | 29 (63.0%) | 3 (25.0%) | 0.042 |
| Spirono-lactone | 58 | 38 (65.5%) | 31 (67.4%) | 7 (58.3%) | 0.734 |
| Furosemide | 58 | 11 (19.0%) | 10 (21.7%) | 1 (8.33%) | 0.429 |
| ACE Inhibitors | 58 | 4 (6.90%) | 4 (8.70%) | 0 (0.00%) | 0.571 |
| AT2 Inhibitors | 58 | 2 (3.45%) | 1 (2.17%) | 1 (8.33%) | 0.374 |
| Ca Inhibitors | 58 | 3 (5.17%) | 1 (2.17%) | 2 (16.7%) | 0.106 |
| Antibiotics | 78 | 31 (39.7%) | 22 (34.9%) | 9 (60.0%) | 0.136 |
| Rifaximin | 78 | 14 (17.9%) | 8 (12.7%) | 6 (40.0%) | 0.023 |
| Lactulose | 58 | 34 (58.6%) | 28 (60.9%) | 6 (50.0%) | 0.527 |
| Terlipressin | 57 | 7 (12.3%) | 5 (11.1%) | 2 (16.7%) | 0.630 |
| Human Albumin | 58 | 27 (46.6%) | 21 (45.7%) | 6 (50.0%) | 1.000 |
| Vaso-pressors | 78 | 7 (8.97%) | 2 (3.17%) | 5 (33.3%) | 0.002 |
| Cytolysin: | 78 | 0.681 | |||
| Positive | 11 (14.1%) | 10 (15.9%) | 1 (6.67%) |
Values are presented as median and interquartile range in brackets. The number of subjects for which data were available is indicated in the second column. Continuous variables were compared using the Mann-Whitney test. Categorical variables were compared using the Pearson's Chi-squared test. Level for statistical significance is P<0.05. Significant p values are indicated in bold. ACE, angiotensin-converting enzyme, ACLF, acute-on-chronic liver failure; AD, acute decompensation; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; AT2, angiotensin 2; Ca, calcium; CLIF-C, European Foundation for the study of chronic liver failure; CRP, C-reactive protein; FIB-4, Fibrosis-4 Index; GGT, gamma-glutamyltransferase; HCV, hepatitis C virus; HE, hepatic encephalopathy; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, sodium-adjusted model for end-stage liver disease; NASH, nonalcoholic steatohepatitis; OF, organ failure; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; SBP, spontaneous bacterial peritonitis; TIPS, transjugular intrahepatic portosystemic shunt; WBC, white blood count.
Presence of fecal cytolysin does not predict liver disease severity in cirrhosis with acute decompensation or acute-on-chronic liver failure per CLIF-C AD and ACLF scores
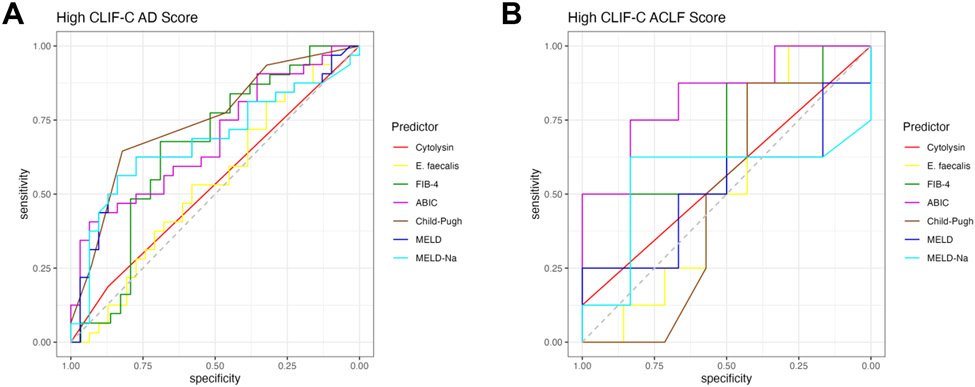
Since fecal cytolysin positivity predicts mortality in patients with alcohol-associated hepatitis (AH) [15, 21], we aimed to examine its role in patients with cirrhosis and AD or ACLF. To determine disease severity in patients with acutely decompensated cirrhosis, the CLIF-C AD score was employed, which has a significantly better performance for predicting mortality in AD patients than Child-Pugh score (CPS), MELD, and MELD-Na scores [22]. Similarly, disease severity in patients with ACLF was quantified using the CLIF-C ACLF score, which also has better predictive value in ACLF patients than CPS, MELD, and MELD-Na [23, 24]. To create binary disease measures for the AD and ACLF groups, “High CLIF-C AD” and “Low CLIF-C AD” as well as “High CLIF-C ACLF” and “Low CLIF-C ACLF” were designated using the respective medians as cutoffs (Table 1). In our cohort, fecal cytolysin had a lower performance than all other tested liver disease markers (including Fibrosis-4 index [FIB-4], ‘Age, serum Bilirubin, INR, and serum Creatinine’ [ABIC] score, CPS, MELD, MELD-Na) in patients with AD with a low area under the curve (AUC) of 0.53 and low best Youden Index of 1.06 compared with the best performer CPS with an AUC of 0.75 and best Youden Index 1.47 (Figure 1A, Table 2). The performance of fecal cytolysin was similar to fecal E. faecalis abundance (Figure 1A, Table 2). Likewise, its predictive performance was poor in the ACLF group with an AUC of 0.56 and best Youden Index of 1.12, whereas the ABIC score had a high AUC of 0.83 and best Youden Index of 1.58 (Figure 1B, Table 3).
Figure 1. Presence of fecal cytolysin does not predict liver disease severity in cirrhosis with acute decompensation or acute-on-chronic liver failure per CLIF-C AD and ACLF scores.
(A) ROC curves of liver disease markers for high CLIF-C AD score in AD patients (Cytolysin n=63, E. faecalis n=63, FIB-4 n=60, ABIC n=63, Child-Pugh=59, MELD score n=63, and MELD-Na score n=63). (B) ROC curves of liver disease markers for high CLIF-C ACLF score in ACLF patients (Cytolysin n=15, E. faecalis n=15, FIB-4 n=14, ABIC n=14, Child-Pugh=15, MELD score n=14, and MELD-Na score n=14). ABIC, ‘Age, serum bilirubin, INR, and serum creatinine score’; ACLF, acute-on-chronic liver failure; AD, acute decompensation; CLIF-C, European Foundation for the study of chronic liver failure; FIB-4, Fibrosis-4 Index; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, sodium-adjusted model for end-stage liver disease; ROC, receiver operating characteristic.
Table 2.
High CLIF-C AD Score Predictors
| Marker | AUC | Threshold | Youden | Sens | Spec | Acc | Prec | PPV | NPV |
p value vs Cyt. AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| Cytolysin | 0.53 | positive | 1.06 | 0.19 | 0.87 | 0.52 | 0.60 | 0.60 | 0.51 | 1.000 |
| E. faecalis | 0.54 | 0.51 | 1.14 | 0.81 | 0.32 | 0.57 | 0.55 | 0.55 | 0.62 | 0.899 |
| FIB-4 | 0.66 | 3.99 | 1.37 | 0.68 | 0.69 | 0.68 | 0.70 | 0.70 | 0.67 | 0.167 |
| ABIC | 0.69 | 7.95 | 1.34 | 0.41 | 0.94 | 0.67 | 0.87 | 0.87 | 0.60 | 0.061 |
| Child-Pugh | 0.75 | 7.50 | 1.47 | 0.65 | 0.82 | 0.73 | 0.80 | 0.80 | 0.68 | 0.003 |
| MELD | 0.68 | 11.64 | 1.40 | 0.56 | 0.84 | 0.70 | 0.78 | 0.78 | 0.65 | 0.059 |
| MELD-Na | 0.68 | 11.81 | 1.40 | 0.56 | 0.84 | 0.70 | 0.78 | 0.78 | 0.65 | 0.074 |
The best threshold was determined to maximize the youden index (= sensitivity + specificity) for each marker. Cytolysin n=63, E. faecalis n=63, FIB-4 n=60, ABIC n=63, Child-Pugh=59, MELD score n=63, and MELD-Na score n=63. ABIC, ‘Age, serum bilirubin, INR, and serum creatinine score’; acc, accuracy; AD, acute decompensation; AUC, area under the curve; CLIF-C, European Foundation for the study of chronic liver failure; Cyt, cytolysin; FIB-4, Fibrosis-4 Index; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, sodium-adjusted model for end-stage liver disease; NPV, negative predictive value; PPV, positive predictive value; pre, precision; sens, sensitivity; spec, specificity.
Table 3.
High CLIF-C ACLF Score Predictors
| Marker | AUC | Threshold | Youden | Sens | Spec | Acc | Prec | PPV | NPV |
p value vs Cyt. AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| Cytolysin | 0.56 | positive | 1.12 | 0.12 | 1.00 | 0.53 | 1.00 | 1.00 | 0.50 | 1.000 |
| E. faecalis | 0.54 | 7.69 | 1.30 | 0.88 | 0.43 | 0.67 | 0.64 | 0.64 | 0.75 | 0.536 |
| FIB-4 | 0.65 | 3.10 | 1.38 | 0.88 | 0.50 | 0.71 | 0.70 | 0.70 | 0.75 | 0.646 |
| ABIC | 0.83 | 7.42 | 1.58 | 0.75 | 0.83 | 0.79 | 0.86 | 0.86 | 0.71 | 0.833 |
| Child-Pugh | 0.47 | 9.50 | 1.30 | 0.88 | 0.43 | 0.67 | 0.64 | 0.64 | 0.75 | 0.473 |
| MELD | 0.52 | 21.22 | 1.25 | 0.25 | 1.00 | 0.57 | 1.00 | 1.00 | 0.50 | 0.521 |
| MELD-Na | 0.52 | 17.35 | 1.46 | 0.62 | 0.83 | 0.71 | 0.83 | 0.83 | 0.62 | 0.552 |
The best threshold was determined to maximize the youden index (= sensitivity + specificity) for each marker. Cytolysin n=15, E. faecalis n=15, FIB-4 n=14, ABIC n=14, Child-Pugh=15, MELD score n=14, and MELD-Na score n=14. ABIC, ‘Age, serum bilirubin, INR, and serum creatinine score’; acc, accuracy; ACLF, acute-on-chronic liver failure; AUC, area under the curve; CLIF-C, European Foundation for the study of chronic liver failure; Cyt, cytolysin; FIB-4, Fibrosis-4 Index; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, sodium-adjusted model for end-stage liver disease; NPV, negative predictive value; PPV, positive predictive value; pre, precision; sens, sensitivity; spec, specificity.
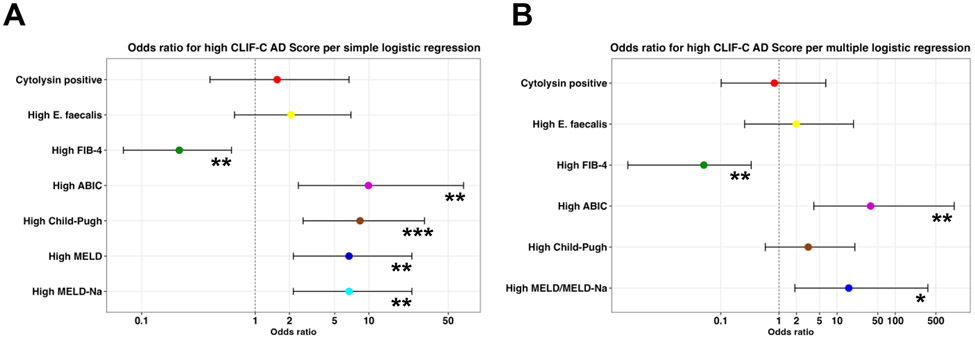
Next, we used the best threshold that maximized the Youden Index in AD (Table 2) and calculated the odds ratios (ORs) for a high CLIF-C AD score for values exceeding the best threshold for the respective marker. All ORs for high liver disease markers – but not for cytolysin positivity or high E. faecalis abundance – were significant per simple logistic regression: cytolysin positivity OR=1.56 (95% confidence interval 0.40-6.69; p=0.53), high E. faecalis OR=2.06 (0.66-6.95; p=0.22), high FIB-4 OR=0.21 (0.07-0.62; p=0.006), high ABIC OR=9.92 (2.39-68.18; p=0.005), high CPS OR=8.36 (2.63-30.85; p<0.001), high MELD OR=6.68 (2.16-23.91; p=0.002), and high MELD-Na OR=6.68 (2.16-23.91; p=0.002) (Figure 2A). As high MELD and high MELD-Na had the same values for OR and significance, the MELD/MELD-Na score was only used once for multiple logistic regression. After multiple logistic regression using all markers, high FIB-4 OR=0.05 (95% confidence interval 0.003-0.33; p=0.009), high ABIC OR=37.73 (3.98-1033.18; p=0.007), and high MELD/MELD-Na OR=15.88 (1.88-364.24; p=0.026) retained significance, whereas cytolysin positivity OR=0.83 (0.10-6.40; p=0.86), high E. faecalis OR=1.99 (0.26-19.15; p=0.52), and high CPS OR=3.19 (0.58-20.22; p=0.19) were not significant (Figure 2B). Odds ratios for high CLIF-C ACLF for the ACLF group using the aforementioned markers could not be calculated or were not significant (not shown).
Figure 2. Cytolysin positivity does not confer higher odds ratio for high CLIF-C AD score.
(A-B) Odds ratios for high CLIF-C AD score per (A) simple logistic regression and (B) multiple logistic regression were calculated for cytolysin positivity and various liver disease markers for values that exceeded the best thresholds per Table 2 (95% confidence interval indicated by black brackets for each marker). Cytolysin n=63, E. faecalis n=63, FIB-4 n=60, ABIC n=63, Child-Pugh=59, MELD score n=63, and MELD-Na score n=63. ABIC, ‘Age, serum bilirubin, INR, and serum creatinine score’; AD, acute decompensation; CLIF-C, European Foundation for the study of chronic liver failure; FIB-4, Fibrosis-4 Index; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, sodium-adjusted model for end-stage liver disease. Significance is indicated by *P<0.05, **P<0.01, and ***P<0.001.
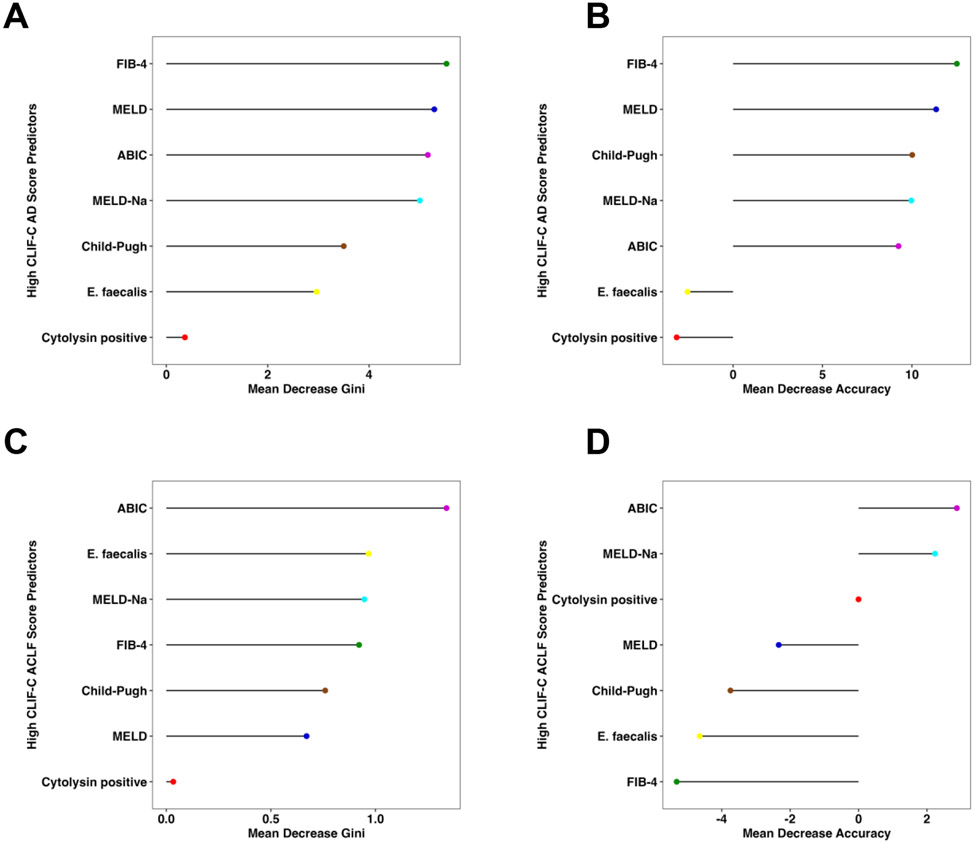
Consistent with the findings above, presence of fecal cytolysin had a low feature importance for high CLIF-C AD and ACLF scores per random forest analysis with a very low mean decrease Gini score and mean decrease accuracy in relation to the other markers in AD (Figure 3A-B) and ACLF (Figure 3C-D).
Figure 3. Fecal cytolysin is not an important feature for liver disease severity in cirrhosis with acute decompensation and acute-on-chronic liver failure per random forest analysis.
(A) Mean decrease Gini score and (B) mean decrease accuracy by random forest analysis were quantitated for presence of cytolysin and multiple liver disease markers to determine their respective feature importance for high CLIF-C AD score (Cytolysin n=63, E. faecalis n=63, FIB-4 n=60, ABIC n=63, Child-Pugh=59, MELD score n=63, and MELD-Na score n=63). (C) Mean decrease Gini score and (D) mean decrease accuracy for high CLIF-C AD score (Cytolysin n=15, E. faecalis n=15, FIB-4 n=14, ABIC n=14, Child-Pugh=15, MELD score n=14, and MELD-Na score n=14). ABIC, ‘Age, serum bilirubin, INR, and serum creatinine score’; ACLF, acute-on-chronic liver failure; AD, acute decompensation; CLIF-C, European Foundation for the study of chronic liver failure; FIB-4, Fibrosis-4 Index; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, sodium-adjusted model for end-stage liver disease.
Other liver disease markers do not depend on fecal cytolysin positivity in cirrhosis with acute decompensation or acute-on-chronic liver failure
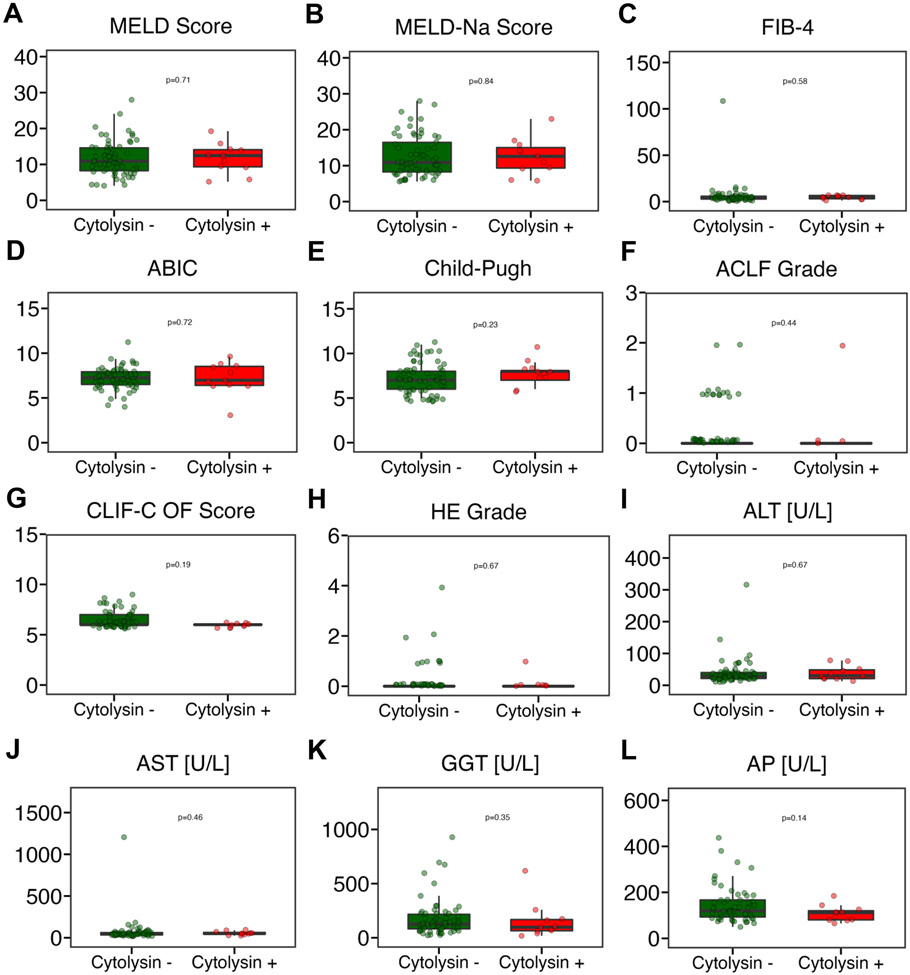
Next, we performed simple linear regression with fecal cytolysin as the independent variable and the liver disease markers FIB-4, ABIC, CPS, MELD, and MELD-Na as the dependent outcome variables. None of the liver disease markers were significantly associated with presence of fecal cytolysin in the combined AD and ACLF cohort (Table 4) nor for the AD (Supplementary Table S1) or ACLF cohorts separately (Supplementary Table S2). As fecal cytolysin positivity predicts mortality in patients with AH [15, 21], we further assessed the predictive value of fecal cytolysin in patients with AD or ACLF of our cohort in whom liver disease was alcohol-associated. However, also in this subgroup, none of the liver disease markers were significantly associated with fecal cytolysin (Supplementary Table S3). The cytolysin positivity in that subgroup was 13.3%, similar to 14.1% overall (Table 1). Finally, various liver disease indicators (e.g. MELD score, CPS, but also laboratory markers ALT, AST, GGT, or AP) were not significantly different between fecal cytolysin positive vs fecal cytolysin negative subjects in the combined AD and ACLF cohort (Figure 4), the AD cohort only (Supplementary Figure S1), or in the alcohol-associated combined AD and ACLF cohort (Supplementary Figure S2). Rifaximin use was significantly higher in the ACLF group compared with the AD group (Table 1). To account for possible confounding by Rifaximin, we excluded all patients that were given Rifaximin and evaluated for an association between fecal cytolysin and the various liver disease markers, and again none of the disease measures depended on fecal cytolysin (not shown).
Table 4.
Simple linear regression of fecal cytolysin with liver disease markers for combined cohort
| Outcome | Variable | Estimate | 2.5 % | 97.5 % | p value |
|---|---|---|---|---|---|
| FIB-4 | Cytolysin | −1.68 | −9.82 | 6.46 | 0.68 |
| ABIC | Cytolysin | 0.03 | −0.81 | 0.88 | 0.93 |
| Child-Pugh | Cytolysin | 0.58 | −0.52 | 1.68 | 0.30 |
| MELD | Cytolysin | 0.11 | −2.95 | 3.16 | 0.95 |
| MELD-Na | Cytolysin | −0.02 | −3.63 | 3.59 | 0.99 |
Cytolysin n=78, FIB-4 n=74, ABIC n=77, Child-Pugh=74, MELD score n=77, and MELD-Na score n=77. ABIC, ‘Age, serum bilirubin, INR, and serum creatinine score’; FIB-4, Fibrosis-4 Index; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, sodium-adjusted model for end-stage liver disease.
Figure 4. Fecal cytolysin-positive patients with cirrhosis and acute decompensation or acute-on-chronic liver disease do not have more severe liver disease than cytolysin-negative patients.
(A) MELD score (n=77). (B) MELD-Na score (n=77). (C) FIB-4 (n=74). (D) ABIC score (n=77). (E) Child-Pugh score (n=74). (F) ACLF grade (n=78). (G) CLIF-C organ failure score (n=78). (H) Hepatic encephalopathy (n=78). (I) ALT (n=78). (J) AST (n=75). (K) GGT (n=77). (L) AP (n=68). ABIC, ‘Age, serum bilirubin, INR, and serum creatinine score’; ACLF, acute-on-chronic liver failure; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; CLIF-C, European Foundation for the study of chronic liver failure; FIB-4, Fibrosis-4 Index; GGT, gamma-glutamyltransferase; HE, hepatic encephalopathy; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, sodium-adjusted model for end-stage liver disease; OF, organ failure. P value of equal or less than 0.05 was considered as statistically significant.
Discussion
We have shown previously that fecal cytolysin positivity predicts mortality in patients with AH [15, 21]. We therefore aimed to investigate its role in patients with cirrhosis and AD or ACLF. However, we found that fecal cytolysin does not predict disease severity in acutely decompensated cirrhosis or ACLF. We have also demonstrated previously that cytolysin positivity is not associated with disease severity in NAFLD [16], which indicates that cytolysin might only confer risk in ALD. However, even if we select subjects with alcohol-associated cirrhosis and AD or ACLF in the current cohort, no liver disease markers were associated with presence of fecal cytolysin. This further supports the idea that cytolysin positivity indicates a higher risk for worse outcome in AH specifically. Even though AH is a major trigger of AD and ACLF, the colonization with E. faecalis associated with fecal cytolysin does not appear to be an important mechanism in AD and ACLF. This study shows once again that AD and ACLF are multifactorial, and besides AH as one of the triggers, other infections are also relevant inducers of AD and ACLF.
Strengths of this study include that this is a well-characterized AD and ACLF cohort. Moreover, the predictive value of fecal cytolysin for disease severity in this cohort was evaluated comprehensively from various angles and the results have been consistent throughout the different methodologies. Limitations of the study include that the studied cohort had overall mild disease with only one dead subject in the first 90 days after enrollment, which appears low in the context of cirrhosis with AD or ACLF, which oftentimes has transplant-free 90-day mortality up to 50% [17]. The low cytolysin positivity of only 14.1% of the overall study population (compared with up to 30% in AH) [15] likely did not contribute to the relatively low mortality, as cytolysin did not associate with any liver disease severity measures in our study cohort. It is possible that higher rifaximin use in the ACLF group might have contributed to a better survival in that group. Rifaximin is known to kill E. faecalis [25] and reduces its translocation into the blood stream [26]. However, even after exclusion of Rifaximin-exposed subjects, there was no association between fecal cytolysin and liver disease measures in our cohort.
Conclusion
In conclusion, fecal cytolysin positivity does not indicate higher disease severity in patients with AD and ACLF. Fecal cytolysin appears to exclusively predict worse outcome in AH.
Supplementary Material
Financial support:
This work was supported by National Institutes of Health (NIH) grant K12 HD85036, University of California San Diego Altman Clinical and Translational Research Institute (ACTRI)/NIH grant KL2TR001444, and Pinnacle Research Award in Liver Diseases Grant #PNC22-159963 from the American Association for the Study of Liver Diseases Foundation (to PH), Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) fellowship (LA 4286/1-1) and the “Clinical and Translational Research Fellowship in Liver Disease” by the American Association for the Study of Liver Diseases (AASLD) Foundation (to SL), by National Institutes of Health grants R01 AA24726, R01 AA020703, U01 AA026939, by Award Number BX004594 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development, and a Biocodex Microbiota Foundation Grant (to BS) and services provided by NIH centers P30 DK120515 and P50 AA011999. This study was supported by the German Research Foundation (DFG) project ID 403224013 – SFB 1382 (to JT), by the German Federal Ministry of Education and Research (BMBF) for the DEEP-HCC project (to JT), by the Hessian Ministry of Higher Education, Research and the Arts (HMWK) for the ENABLE and ACLF-I cluster projects (to JT). The MICROB-PREDICT (project ID 825694), DECISION (project ID 847949), GALAXY (project ID 668031), LIVERHOPE (project ID 731875), and IHMCSA (project ID 964590) projects (all to JT) have received funding from the European Union’s Horizon 2020 research and innovation program. The manuscript reflects only the authors’ views, and the European Commission is not responsible for any use that may be made of the information it contains. The funders had no influence on study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- ACE
angiotensin-converting enzyme
- ACLF
acute-on-chronic liver failure
- AD
acute decompensation
- ALT
alanine aminotransferase
- AP
alkaline phosphatase
- AST
aspartate aminotransferase
- AT2
angiotensin 2
- Ca
calcium
- CLIF-C
European Foundation for the study of chronic liver failure
- CRP
C-reactive protein
- FIB-4
Fibrosis-4 Index
- GGT
gamma-glutamyltransferase
- HCV
hepatitis C virus
- HE
hepatic encephalopathy
- INR
international normalized ratio
- MELD
model for end-stage liver disease
- MELD-Na
sodium-adjusted model for end-stage liver disease
- NASH
nonalcoholic steatohepatitis
- OF
organ failure
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
- SBP
spontaneous bacterial peritonitis
- TIPS
transjugular intrahepatic portosystemic shunt
- WBC
white blood count
Footnotes
Potential competing interests: BS has been consulting for Ferring Research Institute, HOST Therabiomics, Intercept Pharmaceuticals, Mabwell Therapeutics, Patara Pharmaceuticals and Takeda. BS’s institution UC San Diego has received grant support from Axial Biotherapeutics, BiomX, CymaBay Therapeutics, NGM Biopharmaceuticals, Prodigy Biotech and Synlogic Operating Company. BS is founder of Nterica Bio. UC San Diego has filed several patents with BS and SL as inventors related to this work.
IRB/Ethics approval and consent to participate: The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the human research and ethical committee of the University of Bonn (121/14). Written informed consent was obtained from all patients.
Availability of data and material: Raw data can be obtained from the authors upon request.
References:
- 1.Hartmann P, Schnabl B. Risk factors for progression of and treatment options for NAFLD in children. Clin Liver Dis (Hoboken) 2018;11:11–15. doi: 10.1002/cld.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartmann P, Schnabl B. New Developments in Microbiome in Alcohol-Associated and Nonalcoholic Fatty Liver Disease. Semin Liver Dis 2021;41:87–102. doi: 10.1055/s-0040-1719174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann P. Editorial: The Microbiome in Hepatobiliary and Intestinal Disease. Front. Physiol 2022;13.893074. doi: 10.3389/fphys.2022.893074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann P, Chu H, Duan Y, Schnabl B. Gut microbiota in liver disease: too much is harmful, nothing at all is not helpful either. Am J Physiol Gastrointest Liver Physiol 2019;316:G563–G573. doi: 10.1152/ajpgi.00370.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demir M, Lang S, Hartmann P, Duan Y, Martin A, Miyamoto Y, et al. The fecal mycobiome in non-alcoholic fatty liver disease. J Hepatol 2022;76:788–799. doi: 10.1016/j.jhep.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann P, Schnabl B. Fungal infections and the fungal microbiome in hepatobiliary disorders. J Hepatol 2022. doi: 10.1016/j.jhep.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng S, Hartmann P, Park M, Duan Y, Lang S, Llorente C, et al. Malassezia restricta promotes alcohol-induced liver injury. Hepatology Communications 2022;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann P, Lang S, Zeng S, Duan Y, Zhang X, Wang Y, et al. Dynamic Changes of the Fungal Microbiome in Alcohol Use Disorder. Front Physiol 2021;12:699253. doi: 10.3389/fphys.2021.699253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CL, Zhang X, Jiang L, Lang S, Hartmann P, Pride D, et al. Intestinal virome in patients with alcohol use disorder and after abstinence. Hepatol Commun 2022;6:2058–2069. doi: 10.1002/hep4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang S, Demir M, Martin A, Jiang L, Zhang X, Duan Y, et al. Intestinal Virome Signature Associated With Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology 2020;159:1839–1852. doi: 10.1053/j.gastro.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu CL, Wang Y, Duan Y, Chu H, Hartmann P, Llorente C, et al. Differences in Bacterial Translocation and Liver Injury in Ethanol Versus Diet-Induced Liver Disease. Dig Dis Sci 2023. doi: 10.1007/s10620-023-07860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res 2015;39:763–775. doi: 10.1111/acer.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann P, Chen WC, Schnabl B. The intestinal microbiome and the leaky gut as therapeutic targets in alcoholic liver disease. Front Physiol 2012;3:402. doi: 10.3389/fphys.2012.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasa E, Hartmann P, Schnabl B. Liver cirrhosis and immune dysfunction. Int Immunol 2022. doi: 10.1093/intimm/dxac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang S, Demir M, Duan Y, Martin A, Schnabl B. Cytolysin-positive Enterococcus faecalis is not increased in patients with non-alcoholic steatohepatitis. Liver Int 2020;40:860–865. doi: 10.1111/liv.14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426-1437, 1437.e1421–1429. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 18.Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol 2021;74:1097–1108. doi: 10.1016/j.jhep.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 2018;67:1870–1880. doi: 10.1136/gutjnl-2017-314240. [DOI] [PubMed] [Google Scholar]
- 20.Queck A, Uschner FE, Ferstl PG, Schulz M, Brol MJ, Praktiknjo M, et al. Role of circulating angiogenin levels in portal hypertension and TIPS. PLoS One 2021;16:e0256473. doi: 10.1371/journal.pone.0256473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabré N, Hartmann P, Llorente C, Kouno T, Wang Y, Zeng S, et al. Immunoglobulin Y antibodies against cytolysin reduce ethanol-induced liver disease in mice. Hepatology Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P, et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol 2015;62:831–840. doi: 10.1016/j.jhep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 2014;61:1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Engelmann C, Thomsen KL, Zakeri N, Sheikh M, Agarwal B, Jalan R, et al. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care 2018;22:254. doi: 10.1186/s13054-018-2156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pistiki A, Galani I, Pyleris E, Barbatzas C, Pimentel M, Giamarellos-Bourboulis EJ. In vitro activity of rifaximin against isolates from patients with small intestinal bacterial overgrowth. Int J Antimicrob Agents 2014;43:236–241. doi: 10.1016/j.ijantimicag.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Tamai Y, Iwasa M, Eguchi A, Shigefuku R, Kamada Y, Miyoshi E, et al. Rifaximin ameliorates intestinal inflammation in cirrhotic patients with hepatic encephalopathy. JGH Open 2021;5:827–830. doi: 10.1002/jgh3.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.