Abstract
Telehealth has the potential to improve the efficiency of healthcare while reducing the burden on patients and caregivers. Encounters can be synchronous or asynchronous. When used for care of those with amyotrophic lateral sclerosis (ALS) by individual health care providers or by a multidisciplinary team, synchronous telehealth is feasible, acceptable, may produce outcomes comparable to those of in-person care, and is cost effective. Individuals with ALS who utilize telehealth tend to have lower physical and respiratory function and to live farther from an ALS clinic than those who exclusively attend in-person clinic visits. Asynchronous telehealth can be utilized as a substitute full multidisciplinary visits, or for remote monitoring of pulmonary function, gait/falls, and speech. Barriers to implementing telehealth on a wider scale include disparities in access to technology and challenges surrounding medical licensure and billing, but these are being addressed.
Keywords: telehealth, telemedicine, ALS, mHealth, mobile health
Introduction to Telemedicine and Telehealth
Today’s healthcare landscape contains many references to, and examples of, telemedicine and telehealth. Sometimes, these terms are used interchangeably. Generally, telemedicine is defined as a remote interaction between a clinician and a patient. This is the traditional way most people think of telemedicine. Telehealth is the provision of a broader range of services remotely1. For patients with neuromuscular diseases, this can include collection of data on clinical measures such as respiratory function, gait, falls, muscle strength, and the rate and quality of speech.
Telehealth encounters can be synchronous or asynchronous. In synchronous encounters, information is transmitted in both directions during the same period of time. These can be binary transmissions between patients or caregivers and healthcare providers, or multi-person encounters with other healthcare providers or family members interacting simultaneously. A live videoconference between a patient, his or her caregiver, and a healthcare provider is an example of synchronous telehealth. Asynchronous encounters, also sometimes called the “store and forward” method, occur when information is collected at one time and then forwarded for review at another time. Examples of this include taking photographs, filming videos, or recording medical data (respiratory, cardiac, gait, etc.) at a location remote from the healthcare provider, and transmitting them to the provider for later review.
Patients can participate in telehealth encounters from their homes, from other healthcare providers’ offices, or from other healthcare facilities such as emergency departments. Equipment needed may include a smartphone or tablet paired with an application, or a computer with a webcam, internet connection, and web-based application or system.
Health Care Barriers and Burdens
Physician visits may pose time and travel burdens to patients and their caregivers. In the United States, there is on average a 20 day delay to obtain a 20-minute physician appointment. Including travel and wait times, appointments take an average of 2 hours2. At large referral centers for rare diseases such as amyotrophic lateral sclerosis (ALS), these times may be far longer due to delays in obtaining an appointment, long travel times due to the distance to the centers, and substantial times spent in waiting rooms. Telehealth has the potential to improve the efficiency of healthcare while reducing patient and caregiver burden. This has led in studies of the feasibility and acceptability of telehealth as a model for care, and to studies of telehealth outcomes relative to in-person care.
Telestroke was the earliest application of telehealth to neurological care. It typically involves acute assessment and coordination of care for a patient in an emergency department by a neurologist at another location3–6. Since then, telehealth has been used in several chronic neurological disorders, including multiple sclerosis, migraine and other headaches, Parkinson’s disease, epilepsy, and dementia7–12.
Standard of Care in ALS: Multidisciplinary Clinic
To understand the role that telehealth may play in ALS care, it is essential to understand the current standard-of-care multidisciplinary clinic model used by most large ALS centers. Patients are seen approximately every 3 months for evaluation by a multidisciplinary team, including an ALS neurologist, a nurse, social worker, speech language pathologist, dietician, occupational therapist, physical therapist, and mental health professional. Pulmonary function tests (PFTs) are typically performed at these visits by a respiratory therapist. Studies have shown that multidisciplinary clinics extend survival and likely improve quality of life of those with ALS13–18. Thus, mechanisms of care that preserve the multidisciplinary approach would be of value.
Beyond these relatively objective measures, patients perceive multidisciplinary clinic as a source of integrated care, a sort of “one-stop shopping,” where they receive quality care from healthcare providers who have expertise in ALS and where they have access to cutting edge research and clinical trials. Patient-reported negative factors associated with multidisciplinary clinic are related to time, travel, and fatigue; travel distances are often long, there are multiple providers to see, and there may be substantial wait times between those providers19. There is additional data that travel and time pose barriers to ALS clinic attendance. Nearly half of patients with ALS in the United States live more than 50 miles from an ALS center, and a quarter live more than 100 miles away. Family caregivers spend an average of 11 hours per day caring for an individual with ALS, and the physical and emotional burden increases with disease progression20–22.
Ideal ALS care would maintain the benefits of multidisciplinary clinic while reducing burdens. One option is to establish more multidisciplinary clinics, but this produces challenges. There is a limited supply of healthcare providers with expertise in ALS. Smaller centers would see fewer patients, leading to less experience and expertise by providers. Additionally, some areas are simply too sparsely populated to support ALS clinics. Telehealth has the potential to fill this gap by preserving multidisciplinary care and reducing time and travel.
Synchronous Telehealth in ALS
Feasibility and Acceptability
Several studies of synchronous telehealth visits have demonstrated the feasibility and acceptability of using telehealth in the ALS patient population. In Brisbane, Australia, 2 tertiary hospitals with multidisciplinary ALS clinics reported their experiences with telehealth. Visits were reported for 38 patients who were seen an average of 3 times each at intervals of 3–4 months. Telehealth visits were conducted from local hospitals or community health services via synchronous videoconferencing to a variety of health care providers at the ALS clinics: neurologist, nurse, palliative care physician, sleep physician, and sleep nurse. The average driving distance that would have occurred if visits were performed in-person was 612 km (386 miles), with a range of 158–1824 km (98–1133 miles). Major problems that were addressed via telehealth were symptom-based, consisting mainly of respiratory and palliative concerns. There was no assessment of health outcomes in the study, but the feasibility of such visits was clear23.
Massachusetts General Hospital in Boston, Massachusetts reported a retrospective chart review of 97 ALS patients who participated in synchronous videoconferencing from their homes with a physician or nurse practitioner from their ALS clinic. Patients lived a median distance of 211 miles from the clinic, and telehealth visits lasted an average of 32 minutes. Many of these patients had advanced disease. Half were ambulatory; one-third were using non-invasive ventilation or gastrostomy; 23% had a tracheostomy; 12% were receiving hospice services. The most commonly addressed problems were medication management, goals of care, research, and equipment concerns. The study concluded that in-home telehealth visits are feasible in this population24.
The Penn State Health Hershey ALS Center in Hershey, Pennsylvania conducted a prospective study of their telehealth program from March 2016 to February 2017. Patients included in the study all met El Escorial criteria for clinically definite, probable, probable laboratory-supported, or possible ALS, had attended in-person ALS multidisciplinary clinic visit at least once, and had the necessary equipment and technological capacity for a telehealth visit. Thirty-three visits were reported from 20 patients who were seen in their homes via synchronous telehealth by a multidisciplinary clinic team. Travel time from clinic was about equally divided into 3 groups among the sample: less than 60 minutes, 60–120 minutes, and more than 120 minutes. Visit length was approximately 15–30 minutes per healthcare provider, with multiple providers per visit. Patients and caregivers reported benefits such as the absence of travel, being in their home, time savings, reduction in fatigue, and increased comfort. The cons reported by patients and caregivers included problems with video/audio, lack of privacy if only the patient or the caregiver wished to speak with the provider, not getting out of the home, and the impersonal nature of telehealth. Four caregivers expressed concerns about a lack of physical examination, although no patient did so. Notably, the total number of responses by patients and caregivers identifying positive aspects of telehealth (66) was far greater than the number identifying negatives (17). Healthcare providers reported benefits such as patients appearing more comfortable and less stressed, the ability to see patients who would otherwise not be able to be seen, patients being more open and talkative, and that patients could be observed in their homes. The providers also reported some negatives, such as problems with video/audio, no physical examination, no cues from body language, and less of an emotional connection. In 93% of encounters, providers were able to understand the concerns described by the patient and their caregiver, and in 92% they were able to provide appropriate recommendations. During 88% of encounters, they found the communication to be adequate but rated the service to be equal in quality to in-person care only 58% of the time. Overall, feasibility and satisfaction were rated highly by patients, caregivers, and healthcare providers, although healthcare providers noted more shortcomings of telehealth in comparison to in-person care than did patients and caregivers25.
Outcomes
Research on clinical outcomes is very limited for ALS synchronous telehealth. A study conducted by the Veterans Affairs ALS Center in Cleveland, Ohio reported on outcomes of survival, disease progression, and malnutrition. Patients in the study were given a choice of receiving care in-person or via telehealth in their homes. Evaluations always included a physician and a nurse, with involvement of other providers based on an assessment of patients’ needs. PFTs and modified barium swallows were performed locally prior to telehealth visits if deemed necessary. Quality of care provided was based on the American Academy of Neurology (AAN) quality measures26. The study found that care delivered via telehealth was of the same quality and had similar patient clinical outcomes as in-person care27.
Roles of Synchronous Telehealth in ALS Care
Because synchronous videoconferencing appears to be feasible and acceptable, and may lead to outcomes equivalent to in-person care, it could play a number of roles in the care of individuals with ALS. First, it could be used as a substitute for regularly scheduled in-person visits with the multidisciplinary team, a model aimed primarily at those who cannot travel to clinic due to physical weakness or lack of resources or both. Secondly, 1-on-1 telehealth visits could be held between the patient in their home and selected multidisciplinary clinic team members prior to an in-person visit, in order to decrease the length of the in-person visit. This is most suitable for those patients who fatigue easily and cannot comfortably tolerate a full multidisciplinary in-person visit, particularly if the in-person visit is limited to those providers requiring physical contact (physical and occupational therapists, for example) and the telehealth visits are primarily “talk” visits (such as the social worker or mental health counselor). A third way in which telehealth could be used is for addressing urgent matters. In such instances, 1-on-1 telehealth visits between patients and selected team members would be triggered by messages from patients or caregivers. Examples of such visits include: communicating about a psychological crisis with the mental health professional; visualizing and discussing transfer or personal care techniques with the physical or occupational therapist; visualizing and discussing a bedsore or malfunctioning feeding tube with a nurse; or discussing goals of care or genetic test results with a physician25. The goal is not to substitute telehealth for all in-person visits, but to provide a wider menu of care options for patients.
Telehealth Utilization in an ALS Clinic
Based on the concepts of team and 1-on-1 telehealth visits described above, we set out to determine how telehealth was used in practice when offered to patients as part of routine care. Based on input from the ALS nursing staff regarding the burden to patients and caregivers of in-person visits, selected ALS patients were offered 2 types of telehealth visits into their homes using our institutional telehealth service, Penn State Health OnDemand (American Well, Boston, MA): 1) full multidisciplinary visits with members of the ALS team on ALS clinic days; or 2) 1-on-1 visits with individuals ALS healthcare providers on other days. Metrics were recorded by the telehealth platform from October 2018 through November 2019.
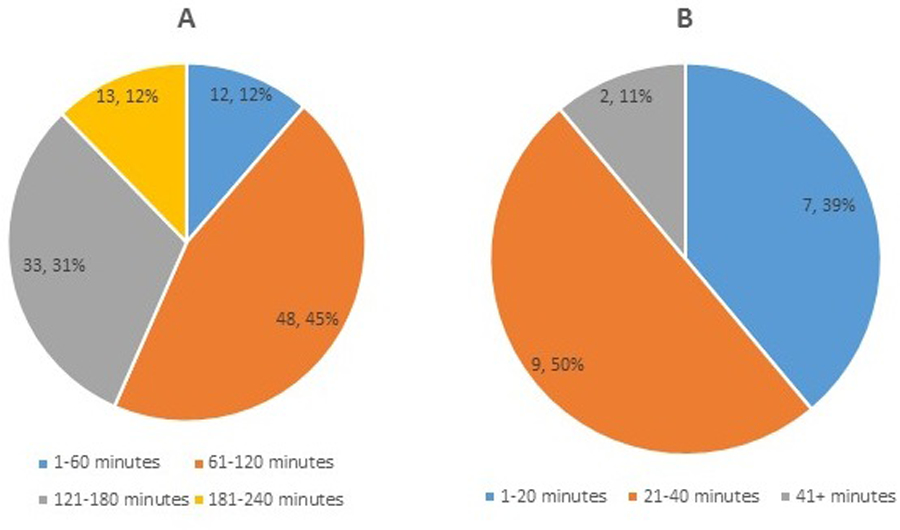
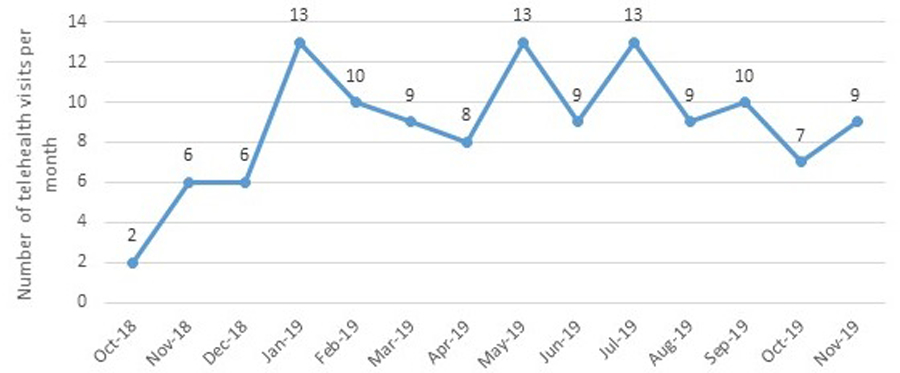
Sixty-one patients and caregivers participated in 1 to 7 telehealth encounters for a total of 124 telehealth visits (Figure 1A). Average visit length was 1 hour and 43 minutes, with a range of 5 minutes to nearly 4 hours (Figure 1B). The number of telehealth visits per month ranged from 2 to 13 (Figure 2). In 2019, 18.6% of all multidisciplinary ALS clinic visits were conducted via telehealth.
Figure 1. All telehealth visits, October 2018 through November 2019.
(A) Number of visits per patient. (B) Length of each visit. Numbers within each pie section indicate number of patients and percentage of total telehealth visits.
Figure 2.
Number of telehealth visits per month
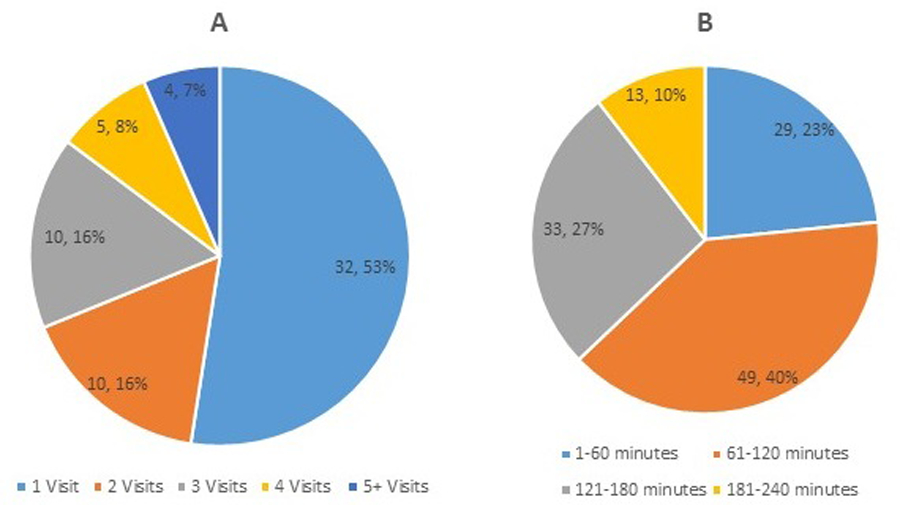
Eighty-five percent of these visits replaced in-person multidisciplinary clinic visits. The average length for these types of telehealth visits was 1 hour and 56 minutes, with a range of 7 minutes to 3 hours and 51 minutes (Figure 3A). The remaining 15% were individual visits with a healthcare provider. The average length for these types of telehealth visits was 27 minutes, with a range of 5 minutes to 1 hour and 50 minutes (Figure 3B).
Figure 3. Length of multidisciplinary telehealth visits and of individual healthcare provider telehealth visits.
(A) Visits involving the ALS multidisciplinary team. (B) 1-on-1 visits with individual healthcare providers. Numbers within each pie section indicate number of patients and percentage of total telehealth visits.
Telehealth patients lived, on average, 52 miles from the ALS clinic, with a range of 6–188 miles. Patients who had in-person visits over the same period lived an average of 35 miles from the clinic. Over 9,100 miles were saved by using telehealth. Patients who lived farthest away appeared to be overrepresented in the telehealth group versus the in-person group (Figure 4), supporting the use of this platform for those for whom travel would be the greatest burden.
Figure 4. Distance of patients from ALS clinic.
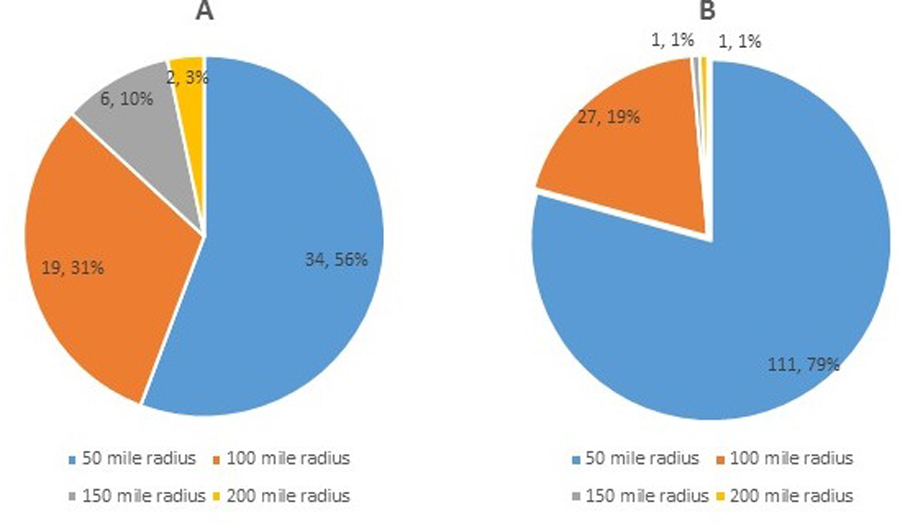
(A) Patients seen via telehealth. (B) Patients seen in-person.
Predictors of Telehealth Utilization for ALS Clinic Patients
We wished to perform a more detailed comparison of patients seen via telehealth to those seen via in-person visits. Beginning in November 2017, all patients at our ALS Center were offered participation in a study designed to assess their use of telehealth, but they were not required to utilize it for care. Patient characteristics describing clinical factors and technological access and comfort were gathered at the time of enrollment and, for patients utilizing telehealth, also at the time of each telehealth visit. For patients who never had a telehealth visit, the most recent clinical data was taken as a second time point. Comparisons were made between enrolled patients who had at least one telehealth visit and those who did not. Descriptive statistics were employed to describe variables within each group. Data was determined to be from a non-Gaussian population; therefore, Mann-Whitney tests were utilized to compare groups. Fisher’s exact tests and chi-square tests, where the sample was large enough, were utilized to compare binomial outcomes. The study was approved by our Institutional Review Board, and all participants provided informed consent.
One hundred fourteen patients were enrolled, one-third of whom (38) participated in at least 1 telehealth visit (Table 1). Patients with at least 1 telehealth visit had poorer physical and respiratory function and were more likely to use noninvasive ventilation than patients who never had a telehealth visit. A larger percentage of enrolled men than women participated in telehealth visits. Distance from clinic appeared to play a role in the decision to choose telehealth. A larger percentage of patients with at least 1 telehealth visit lived 2 or more hours from the clinic. Fifty-two percent of those living 2 or more hours from clinic had a telehealth visit, compared to only 25% of those living less than 30 minutes away. All patients with 4 or more telehealth visits lived at least an hour away from the clinic. Thus, patients with lower physical and respiratory function seen at our center were more likely to utilize telehealth, as were those living farthest from clinic.
Table 1.
Comparison of patients with telehealth visits to those with no telehealth visits
| At least one telehealth visit (n=38) | No telehealth visits (n=76) | p-value | |
|---|---|---|---|
| ALSFRS-R score (mean) | 21.4 | 28.1 | < 0.001 |
| FVC % predicted (mean) | 52.9 | 64.5 | 0.012 |
| Age in years (mean) | 62.0 | 61.9 | 0.672 |
| Disease duration in years (mean) | 1.6 | 1.9 | 0.255 |
| NIV use | 21/35 (60.0%) | 15/60 (25%) | 0.001 |
| Gender | 68.4% male | 42.1% male | 0.008 |
| Hospice | 7.38 (18.4%) | 12/63 (19%) | 0.938 |
| Gastrostomy/PEG | 10/38 (26.3%) | 16/63 (25.4%) | 0.918 |
| Tracheostomy | 3/38 (7.9%) | 3/63 (4.8%) | 0.405 |
| Needing assistance to travel | 29/36 (80.6%) | 48/75 (64.0%) | 0.077 |
| Comfortable or very comfortable with technology | 30/38 (78.9%) | 49/75 (65.4%) | 0.136 |
| Travel time to ALS clinic | |||
| < 30 minutes | 3 (7.9%) | 9 (12%) | 0.748 |
| 30–59 minutes | 8 (21.1%) | 19 (25.3%) | 0.640 |
| 60–89 minutes | 11 (28.9%) | 25 (33.3%) | 0.669 |
| 90–119 minutes | 3 (7.9%) | 10 (13.3%) | 0.539 |
| ≥ 120 minutes | 13 (34.2%) | 12 (16.0%) | 0.028 |
ALSFRS-R: ALS Functional Rating Scale – Revised; FVC: Forced vital capacity; NIV: noninvasive ventilation. p-values in bold are statistically significant
Cost Effectiveness Research
Researchers at Massachusetts General Hospital conducted a comparison of the cost of telehealth visits for patients in their homes and the cost of in-person multidisciplinary clinic visits. For in-person visits, the group took into account the costs of travel, lodging, time away from work for the patient and caregiver, and any out of pocket medical costs. It also included physician time, other healthcare personnel time, and room costs. For telehealth visits, the cost estimate included physician time, video infrastructure costs, follow-up time by multidisciplinary clinic team members, and the time of information technology personnel. Costs of telehealth visits were adjusted for their medical usefulness (MU) relative to in-person visits as rated by ALS healthcare providers and patients. The study found that, assuming all visits in a year were telehealth visits, patients would save 89% for a total annual savings of nearly $4,000, and institutions would save 41% for a total annual savings of $1,310 per patient, compared with the annual cost of in-person visits. A combination of 2 in-person visits and 2 telehealth visits per year would lead to nearly $2,000 per year in savings to the patient (45%), and $655 (20%) to the institution. If patients lived closer to the institution than in the original model, savings were less, but there were still savings by using telehealth visits. The study concluded that telehealth visits provide marked adjusted cost savings for both patients and institutions under a variety of assumptions28.
Asynchronous Telehealth in ALS
The University of Florida in Jacksonville conducted a study of asynchronous telehealth in ALS, often referred to as the “store and forward” model. In this study, a nurse was trained by other members of the ALS multidisciplinary clinic team, including the neurologist and physical, occupational, speech, and respiratory therapists, to perform an assessment of the patient in their home. The nurse then traveled to patients’ homes and performed a multidisciplinary assessment. A video and audio recording was made of the assessment and was sent to the multidisciplinary team providers at a later time for review. After review, the team members made recommendations for each patient’s care and provided them to the clinic director, who then formulated a plan and conveyed this back to the patient via the nurse using a videoconference or telephone call. This method resulted in high patient satisfaction and generally high, although more variable, provider satisfaction29.
Home Monitoring of Respiratory Function: Remote Pulmonary Function Tests
AAN guidelines recommend considering initiation of non-invasive ventilation (NIV) when maximum inspiratory pressure (MIP) is weaker than −60 cmH2O or when forced vital capacity (FVC) is less than 50% of predicted30. NIV in ALS prolongs survival, slows the rate of respiratory decline, and positively impacts health-related quality of life for sleep, physical fatigue, and depression30–32. Pulmonary function tests (PFTs) approximately every 3 months are a routine part of the ALS clinic evaluation and part of the ALS quality measures of the AAN33. When ALS clinic visits are performed via telehealth, patients must typically be transported to a facility for PFTs. This increases the burden of telehealth visits and undercuts the goal of providing care in the home.
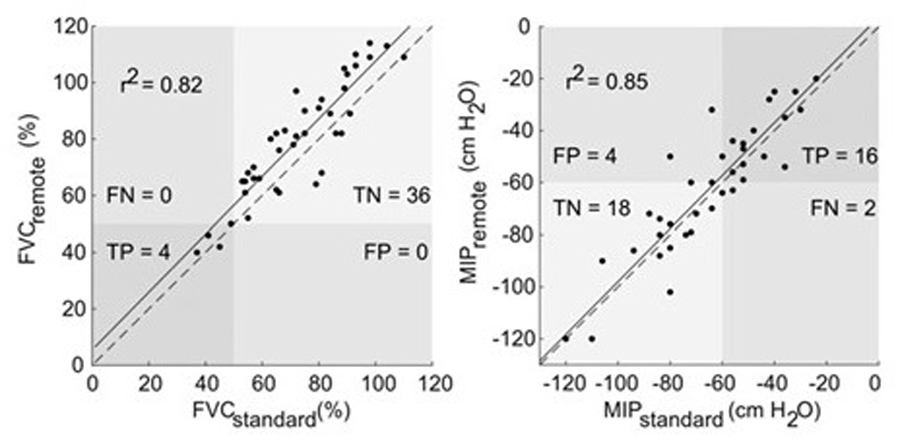
A study of remote PFTs was conducted by the Penn State Health Hershey ALS Center, following approval of the Institutional Review Board and provision of informed consent by participants. Inclusion criteria were a diagnosis of ALS, primary lateral sclerosis (PLS), or progressive muscular atrophy (PMA), ALS Functional Rating Scale (ALSFRS-R) scores of 2 or higher on all items for speech, swallowing, and saliva, and lack of cognitive impairment. Patients performed PFTs, specifically forced vital capacity (FVC) and maximum inspiratory pressure (MIP), with a respiratory therapist while in the clinic. On the same day, in another room, they performed a simulated remote PFT using a computer and equipment selected for performing PFTs at home. At the other end of the telehealth encounter was a respiratory therapist coaching the patient. Analysis was performed on the best of 3 maneuvers with results demonstrating a strong correlation between remote PFTs and standard assessments (Figure 5). Due to the fact that different reference systems were used internally by the standard respiratory therapy equipment and the remote PFT devices to calculate FVC as a percent of predicted, volumes measured rather than percent predicted resulted in an even stronger correlation between remote PFTs and standard assessments (r2=0.92, p<0.001). Patients, caregivers, and respiratory therapists reported high acceptability of this process34.
Figure 5. Scatterplots for standard and remote forced vital capacity (FVC, left) and maximal inspiratory pressure (MIP, right) assessments.
A solid line of best fit and associated correlation coefficient are shown, as well as dashed line showing the ideal fit. Labels in four quadrants show the relative proportions of true positive (TP), false positive (FP), true negative (TN), and false negative (FN) events based on an FVC and MIP thresholds of 50% and −60 cm water. From Geronimo A, Simmons Z. Evaluation of remote pulmonary function testing in motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degener 2019; 20:348–355, reprinted by permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com).
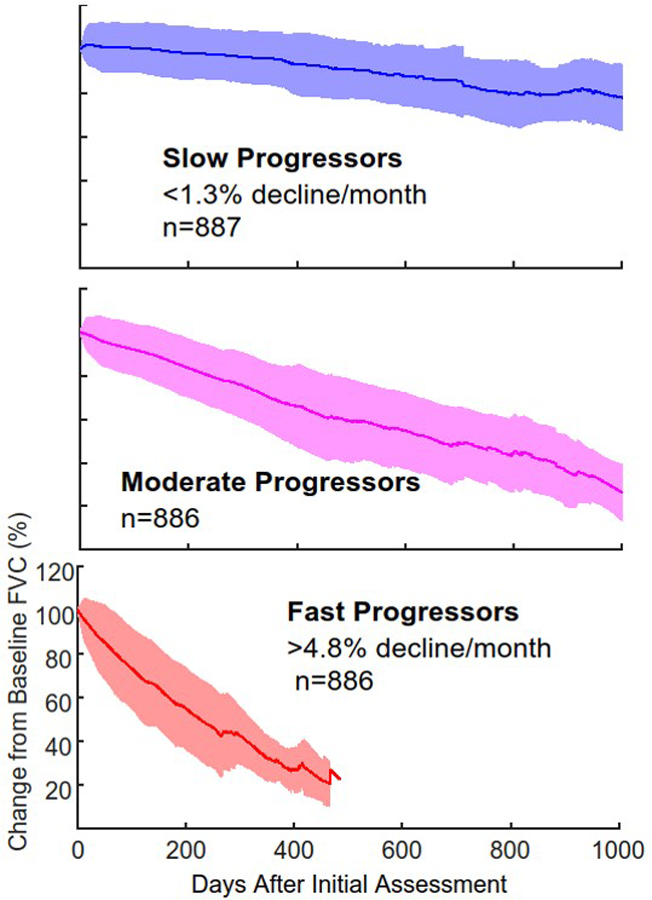
ALS disease progression is markedly heterogeneous. Data from the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database, containing over 10,700 ALS patient records from multiple completed clinical trials, can be used to stratify patients, based on rate of FVC decline over time, into those with slow, moderate, or fast progression (Figure 6)35. In contrast to disease progression, the clinical model for care is fairly homogeneous. Administering PFTs every 3 months is not based on data, but on the accepted model of ALS multidisciplinary care. More frequent PFTs may permit earlier initiation of NIV. The burden associated with such an increased frequency of assessments could potentially be lessened by performing them at home. Of 217 patients identified in our ALS clinic who had serial PTFs, 144 were found to have FVCs that declined below 50% of predicted, the threshold for initiating NIV. Using the data from clinic visits with PFTs every 3 months permits an estimation for each patient of the time when FVC would fall below 50% of predicted. The interval can then be calculated between this estimated threshold crossing and the first in-clinic FVC following that point for any single individual. Of the 144 patients, 100 had an interval of more than 30 days, indicating they would have received an earlier recommendation for the initiation of NIV if they had undergone monthly remote PFTs. A study is now underway to explore outcomes of these more frequent PFT assessments.
Figure 6. Rate of change over time of forced vital capacity as calculated from the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database.
Patients can be stratified into slow, moderate, and fast progressors, shown below as the mean and standard deviation for each group.
Remote Monitoring of Gait and Falls
It is well known that patients with ALS fall and that the consequences may be profound. Assessment of fall risk, with the goal of preventing or at least reducing the frequency of falls, is an important part of ALS care. In fact, one of the ALS quality measures published by the AAN is querying patients for falls occurring in the past 12 months33. The rate of falls in ALS has not been clearly established, with rates ranging from 0.05 to 0.2 to 1.8 falls per patient-month26. Self-reporting of falls is compromised by recall bias, resulting in underreporting of fall events. Individuals with ALS have gait measures that differ from healthy controls, including increased and highly variable gait cycle time (the time it takes to complete a full walking cycle), reduced stride length, and increased variability in stride length, compared to healthy controls36–38. A gait assessment of patients with ALS under the traditional model of care occurs once every 3 months by the physical therapist in multidisciplinary clinic. Telehealth has the potential to provide more frequent and more quantitative assessments.
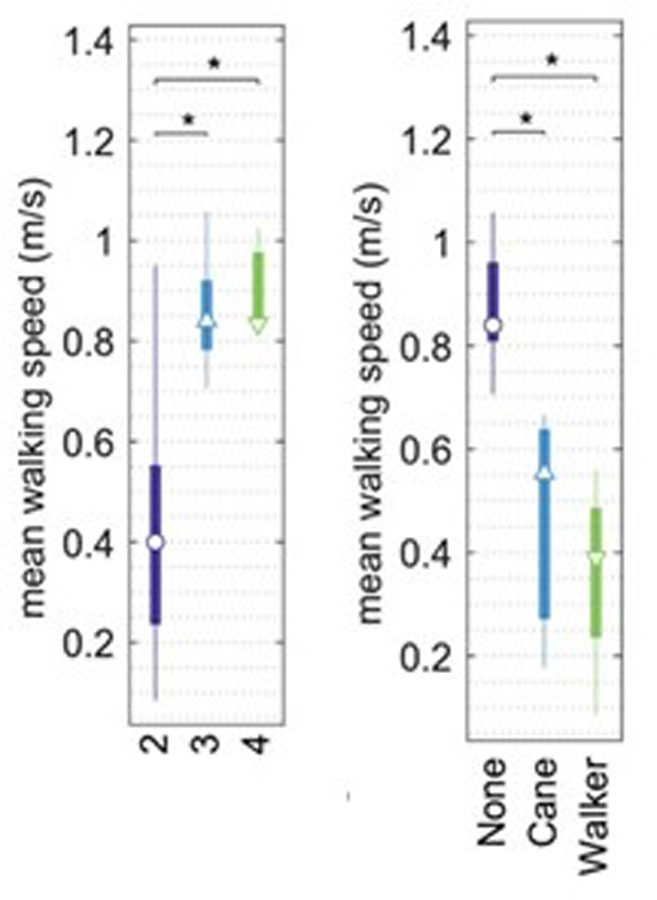
The Penn State Health Hershey ALS Center piloted the use of a wearable sensor as a way to provide regular estimates of stride length, duration, and walking speed. Institutional Review Board approval was obtained, and all participants signed informed consent. Thirty patients were included in the evaluation, most with ALS, but a few with PLS or PMA. All evaluations were performed during in-person ALS clinic visits. Patients wore sensors to detect accelerations and rotations of the body during therapist-guided walking sessions. ALSFRS-R walking sub-score (FRSw, item 8 on the ALSFRS-R) was collected. Results showed that walking speed derived from stride length and duration was able to distinguish those walking with assistance from those without, as well as those with FRSw of 2 vs. 3 and 2 vs. 4 (Figure 7). This pilot study suggests that stride length and stride duration measured from a short period of normal walking correlated with functional measures of ambulatory health. When implemented in the home, gait tracking could potentially help to reduce fall risk by allowing for evidence-based and timely decision support regarding safe mobility.
Figure 7. Relationship between walking speed, function, and need for assistive devices.
Median and interquartile ranges are shown for walking speed for subjects stratified by the walking subscale of the ALS Functional Rating Scale – Revised (FRSw, left) and by walking assistance (right). *significant differences between items by pairwise Tukey test following ANOVA.
A study is now underway to assess gait from home in patients with ALS using wearable devices. One aim of this study is to assess patient movement in relationship to falls. This will be done by monitoring general activity via a pendant monitor, arm activity via wrist sensors, and gait via sensors worn on the legs or feet. A second aim of this study is to conduct an accurate assessment of fall frequency via an automated fall detection algorithm.
Electronic Collection of Clinical Data without In-Person Visits
Developed at the University of Sheffield, the Telehealth in Motor Neuron Disease (TiM) system is a telehealth system that utilizes an application through which patients and caregivers are prompted to answer weekly questions regarding limb, bulbar, and respiratory function, nutrition, and wellbeing. Caregivers also communicate about strain, depression, and anxiety. Wi-Fi enabled scales are able to send information on patient weight measurements. The information entered into the application is uploaded for immediate review by the multidisciplinary team. Information is displayed for the provider in a clinically useful way, tracking trends in weight, caregiver strain, and other measures over time39.
The Barrow Neurological Institute developed the ALS AT HOME application with a goal of decreasing burden on patients and caregivers when participating in clinical trials. Outcome measures were performed at home and transmitted electronically via the application, including handgrip dynamometry, spirometry, electrical impedance myography, and ALSFRS-R. Speech was recorded using a separate application via which patients were asked to speak specific phrases into the microphone of their device. Recordings were uploaded to a separate cloud-based repository for analysis. Participation in the subsequent ALS AT HOME natural history study involved measuring each data point daily for 3 months and then twice a week for 6 months. Patients were recruited online through the Centers for Disease Control and Prevention (CDC) ALS patient registry, ALS Association, Muscular Dystrophy Association, and via social media. Diagnosis was confirmed by review of records by the study team. Patients were consented, enrolled, and trained entirely online40. The study demonstrated the potential value of electronic processes to reduce patient burden and increase enrollment into clinical trials.
Using Social Media to Create a Virtual Support Group
Support groups are a traditional way for patients with rare or life-threatening diseases and their caregivers to meet and provide friendship and support to one another. Barriers to attendance are the same as those that create barriers to ALS clinic attendance and can include travel, time, and fatigue. Paradoxically, those individuals for whom these barriers are most likely to be present, such as those whose disease is advancing most rapidly, or has created the greatest physical and psychological burdens, are often those who are likely to benefit most from a support group. The ALS Association Greater Philadelphia Chapter supports the Penn State Health Hershey ALS Center, among others, and hosts support groups throughout south central and eastern Pennsylvania, southern New Jersey, and Delaware. The support group in Hershey is facilitated by the ALS clinic psychologist.
Patients and caregivers from the Hershey ALS support group began tending a community garden. In June 2018, a Facebook group was founded to help manage the garden. The psychologist regularly reviews group content and consults with the page administrator (an elected member of the group) to answer questions and address concerns. The Facebook group remained active through the winter when the garden was closed. One year after creation, there were 70 members of the Facebook group, comprised of patients, family members of living and deceased patients, and ALS Association staff. There have been 582 posts, 747 comments, and 1,863 reactions. Pictures, videos, and updates of themselves, tips for one another, inspirational quotes, and pictures from get-togethers are posted by members regularly. Despite a decrease in the number of attendees at in-person support groups across the ALS Association Greater Philadelphia Chapter, the Hershey group has experienced an increase in attendance since the creation of the Facebook group. The use of social media has increased socialization and connection among patients and their caregivers, and has allowed caregivers of deceased patients to preserve their relationship with the ALS community. Next steps include implementing similar groups at other sites.
Barriers to Telehealth
Access to Technology
Access to technology may prevent 30% of rural populations from participating in telehealth. In 2018, only 69% of people living in rural areas had access to high-speed internet that met the minimum benchmark set by the Federal Communications Commission. People living in rural areas are also less likely to have a smartphone, with 71% owning a smartphone in 2019 compared to 83% of suburban and urban residents41. This number is expected to increase.
Medical Licensure
Some states require practitioners providing services via telehealth to be licensed in the state where the patient is located and a few states have additional licensing requirements42. The Interstate Medical Licensure Compact (IMLC) is an agreement between 29 states, the District of Columbia, and the Territory of Guam that allows for an expedited pathway to licensure for qualified physicians who wish to practice in multiple states. The mission of the IMLC is to increase access to healthcare for patients in underserved or rural areas and to allow them to more easily connect with medical experts through the use of telehealth technologies43.
Medical Billing
Medicare and other insurers now reimburse for some telehealth visits. With Medicare, out of pocket costs to patients typically remain the same as they are for in-person visits. In many cases, patients are required to be present in another healthcare facility in order to allow for billing to occur. It is still uncommon for synchronous telehealth encounters with established patients in their homes to be covered by insurance44. This is likely to change in 2020.
Conclusions
Traditional in-clinic ALS multidisciplinary care is valuable, but poses a substantial burden for many patients and caregivers due to time and travel requirements. Synchronous videoconferencing appears to be a viable adjunct to in-clinic care for patients with ALS. It is feasible, acceptable, and may be associated with outcomes at least equivalent to traditional multidisciplinary care. Telehealth can be incorporated into a busy ALS clinic. It is most likely to be used by those with lower physical and respiratory function, and by those who live farther from a clinic. Telehealth is cost effective. It can be used for the remote collection of clinical and research data, including respiratory function, gait, falls, handgrip strength, weight, and speech. Physician licensure, billing, and access to technology remain barriers, but are being addressed. Further development of in-home data collection should enhance the ability of ALS healthcare providers to care for individuals with ALS.
Acknowledgements:
We gratefully acknowledge financial support from the Paul and Harriett Campbell Fund for ALS Research, the ALS Association Greater Philadelphia Chapter, and many private donations to the Penn State Health Hershey ALS Center. Some of the projects described were supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR002014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- AAN
American Academy of Neurology
- FRSw
ALS Function Rating Scale – walking sub-score
- FVC
forced vital capacity
- IMLC
Interstate Medical Licensure Compact
- MIP
maximum inspiratory pressure
- MU
medical usefulness
- NIV
non-invasive ventilation
- PFTs
pulmonary function tests
Footnotes
Presented in part at the Annual Meeting of the American Association of Neuromuscular and Electrodiagnostic Medicine, Austin, Texas, October 2019.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures of Conflicts of Interest: Zachary Simmons has received consulting fees from Biogen and Cytokinetics, research support from Biogen, Biohaven, and Cytokinetics, and stipends from Wiley, Inc. None of the other authors has any conflict of interest to disclose.
References
- 1.Howard IM, Kaufman MS. Telehealth applications for outpatients with neuromuscular or musculoskeletal disorders. Muscle Nerve 2018; 58:475–485. [DOI] [PubMed] [Google Scholar]
- 2.Ray KN, Chari AV., Engberg J, Bertolet M, Mehrotra A. Disparities in time spent seeking medical care in the United States. JAMA Intern Med 2015; 175:1983–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handschu R, Scibor M, Willaczek B, Nückel M, Heckmann JG, Asshoff D, et al. Telemedicine in acute stroke: Remote video-examination compared to simple telephone consultation. J Neurol 2008; 255:1792–1797. Published online: 2008. DOI: 10.1007/s00415-008-0066-9. [DOI] [PubMed] [Google Scholar]
- 4.Amorim E, Shih MM, Koehler SA, Massaro LL, Zaidi SF, Jumaa MA, et al. Impact of telemedicine implementation in thrombolytic use for acute ischemic stroke: The university of pittsburgh medical center telestroke network experience. Journal of Stroke and Cerebrovascular Diseases 2013. Published online: 2013. DOI: 10.1016/j.jstrokecerebrovasdis.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Müller-Barna P, Hubert GJ, Boy S, Bogdahn U, Wiedmann S, Heuschmann PU, et al. Telestroke units serving as a model of care in rural areas: 10-year experience of the telemedical project for integrative stroke care. Stroke 2014; 45:2739–2744. Published online: 2014. DOI: 10.1161/STROKEAHA.114.006141. [DOI] [PubMed] [Google Scholar]
- 6.Zaidi SF, Jumma MA, Urra XN, Hammer M, Massaro L, Reddy V, et al. Telestroke-guided intravenous tissue-type plasminogen activator treatment achieves a similar clinical outcome as thrombolysis at a comprehensive stroke center. Stroke 2011; 42:3291–3293. Published online: 2011. DOI: 10.1161/STROKEAHA.111.625046. [DOI] [PubMed] [Google Scholar]
- 7.Robb JF, Hyland MH, Goodman AD. Comparison of telemedicine versus in-person visits for persons with multiple sclerosis: A randomized crossover study of feasibility, cost, and satisfaction. Mult Scler Relat Disord 2019. Published online: 2019. DOI: 10.1016/j.msard.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Friedman DI, Rajan B, Seidmann A. A randomized trial of telemedicine for migraine management. Cephalalgia 2019; 39:1577–1585. Published online: 2019. DOI: 10.1177/0333102419868250. [DOI] [PubMed] [Google Scholar]
- 9.Bekkelund SI, Müller KI. Video consultations in medication overuse headache. A randomized controlled trial. Brain Behav 2019; 9:1–8. Published online: 2019. DOI: 10.1002/brb3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spear K, Auinger P, Simone R, Dorsey E, Francis J. Patient Views on Telemedicine for Parkinson Disease. J Parkinsons Dis 2019; 9:401. Published online: 2019. DOI: 10.3233/JPD-181557. [DOI] [PubMed] [Google Scholar]
- 11.De Vries NM, Smilowska K, Hummelink J, Abramiuc B, Van Gilst MM, Bloem BR, et al. Exploring the Parkinson patients’ perspective on home-based video recording for movement analysis: A qualitative study. BMC Neuro 2019; 19:1–6. Published online: 2019. DOI: 10.1186/s12883-019-1301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers J, Buckner J. Reaching Out to Rural Caregivers and Veterans with Dementia Utilizing Clinical Video-Telehealth. Geriatrics 2018; 3:29. Published online: 2018. DOI: 10.3390/geriatrics3020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traynor BJ, Alexander M, Corr B, Frost E, Hardiman O. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: A population based study, 1996–2000. J Neurol Neurosurg Psychiatry 2003; 74:1258–1261. Published online: 2003. DOI: 10.1136/jnnp.74.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiò A, Bottacchi E, Buffa C, Mutani R, Mora G. Positive effects of tertiary centres for amyotrophic lateral sclerosis on outcome and use of hospital facilities. J Neurol Neurosurg Psychiatry 2006; 77:948–950. Published online: 2006. DOI: 10.1136/jnnp.2005.083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooney J, Byrne S, Heverin M, Tobin K, Dick A, Donaghy C, et al. A multidisciplinary clinic approach improves survival in ALS:A comparative study of ALS in Ireland and Northern Ireland. J Neurol Neurosurg Psychiatry 2015; 86:496–501. Published online: 2015. DOI: 10.1136/jnnp-2014-309601. [DOI] [PubMed] [Google Scholar]
- 16.Aridegbe T, Kandler R, Walters SJ, Walsh T, Shaw PJ, McDermott CJ. The natural history of motor neuron disease: Assessing the impact of specialist care. Amyotroph Lateral Scler Frontotemporal Degener 2013; 14:13–19. Published online: 2013. DOI: 10.3109/17482968.2012.690419. [DOI] [PubMed] [Google Scholar]
- 17.Van Den Berg JP, Kalmijn S, Lindeman E, Veldink JH, De Visser M, Van Der Graaff MM, et al. Multidisciplinary ALS care improves quality of life in patients with ALS. Neurology 2005; 65:1264–1267. Published online: 2005. DOI: 10.1212/01.wnl.0000180717.29273.12. [DOI] [PubMed] [Google Scholar]
- 18.Stephens HE, Felgoise S, Young J, Simmons Z. Multidisciplinary ALS clinics in the USA: A comparison of those who attend and those who do not. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16:196–201. Published online: 2015. DOI: 10.3109/21678421.2014.994530. [DOI] [PubMed] [Google Scholar]
- 19.Stephens HE, Young J, Felgoise SH, Simmons Z. A qualitative study of multidisciplinary ALS clinic use in the United States. Amyotroph Lateral Scler Frontotemporal Degener 2016; 17:55–61. Published online: 2016. DOI: 10.3109/21678421.2015.1069851. [DOI] [PubMed] [Google Scholar]
- 20.Horton DK, Graham S, Punjani R, Wilt G, Kaye W, Maginnis K, et al. A spatial analysis of amyotrophic lateral sclerosis (ALS) cases in the United States and their proximity to multidisciplinary ALS clinics, 2013. Amyotroph Lateral Scler Frontotemporal Degener 2018; 19:126–133. Published online: 2018. DOI: 10.1080/21678421.2017.1406953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy V, Felgoise SH, Walsh SM, Simmons Z. Problem solving skills predict quality of life and psychological morbidity in ALS caregivers. Amyotroph Lateral Scler 2009; 10:147–153. Published online: 2009. DOI: 10.1080/17482960802245007. [DOI] [PubMed] [Google Scholar]
- 22.Hecht MJ, Graesel E, Tigges S, Hillemacher T, Winterholler M, Hilz MJ, et al. Burden of care in amyotrophic lateral sclerosis. Palliat Med 2003; 17:327–333. Published online: 2003. DOI: 10.1191/0269216303pm754oa. [DOI] [PubMed] [Google Scholar]
- 23.Henderson RD, Hutchinson N, Douglas JA, Douglas C. Telehealth for motor neurone disease. Med J Aust 2014; 201:31. Published online: 2014. DOI: 10.5694/mja14.00170. [DOI] [PubMed] [Google Scholar]
- 24.Van De Rijn M, Paganoni S, Levine-Weinberg M, Campbell K, Swartz Ellrodt A, Estrada J, et al. Experience with telemedicine in a multi-disciplinary ALS clinic. Amyotroph Lateral Scler Frontotemporal Degener 2018; 19:143–148. Published online: January 2, 2018. DOI: 10.1080/21678421.2017.1392577. [DOI] [PubMed] [Google Scholar]
- 25.Geronimo A, Wright C, Morris A, Walsh S, Snyder B, Simmons Z. Incorporation of telehealth into a multidisciplinary ALS Clinic: feasibility and acceptability. Amyotroph Lateral Scler Frontotemporal Degener 2017; 18:555–561. Published online: October 2, 2017. DOI: 10.1080/21678421.2017.1338298. [DOI] [PubMed] [Google Scholar]
- 26.Miller RG, Brooks BR, Swain-Eng RJ, Basner RC, Carter GT, Casey P, et al. Quality improvement in neurology: Amyotrophic lateral sclerosis quality measures: Report of the quality measurement and reporting subcommittee of the American academy of neurology. Neurology 2013; 81:2136–2140. Published online: 2013. DOI: 10.1212/01.wnl.0000437305.37850.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selkirk SM, Washington MO, McClellan F, Flynn B, Seton JM, Strozewski R. Delivering tertiary centre specialty care to ALS patients via telemedicine: a retrospective cohort analysis. Amyotroph Lateral Scler Frontotemporal Degener 2017; 18:324–332. Published online: July 3, 2017. DOI: 10.1080/21678421.2017.1313867. [DOI] [PubMed] [Google Scholar]
- 28.Paganoni S, van de Rijn M, Drake K, Burke K, Doyle M, Ellrodt AS, et al. Adjusted cost analysis of video televisits for the care of people with amyotrophic lateral sclerosis. Muscle Nerve 2019; 60:147–154. Published online: August 1, 2019. DOI: 10.1002/mus.26606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulley MT, Brittain R, Hodges W, Frazier C, Miller L, Matyjasik-Liggett M, et al. Multidisciplinary amyotrophic lateral sclerosis telemedicine care: The store and forward method. Muscle Nerve 2019; 59:34–39. [DOI] [PubMed] [Google Scholar]
- 30.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice Parameter update: The care ofthe patient with amyotrophic lateral sclerosis: Drug, nutritional, and respiratory therapies (an evidence-based review): Report ofthe Quality Standards Subcommittee ofthe American Academy ofNeurology. Neurology 2009; 73:1218–1226. Published online: 2009. DOI: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: A randomised controlled trial. Lancet Neurol 2006; 5:140–147. Published online: 2006. DOI: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 32.Butz M, Wollinsky KH, Wiedemuth-Catrinescu U, Sperfeld A, Winter S, Mehrkens HH, et al. Longitudinal Effects of Noninvasive Positive-Pressure Ventilation in Patients with Amyotrophic Lateral Sclerosis. Am J Phys Med Rehabil 2003; 82:597–604. Published online: 2003. DOI: 10.1097/01.phm.0000078239.83545.d0. [DOI] [PubMed] [Google Scholar]
- 33.Miller RG, Brooks BR, Swain-Eng RJ, Basner RC, Carter GT, Casey P, et al. Quality improvement in neurology: Amyotrophic Lateral Sclerosis Quality Measures. Amyotroph Lateral Scler Frontotemporal Degener 2014; 15:165–168. Published online: 2014. DOI: 10.3109/21678421.2013.875706. [DOI] [PubMed] [Google Scholar]
- 34.Geronimo A, Simmons Z. Evaluation of remote pulmonary function testing in motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degener 2019; 20:348–355. Published online: July 3, 2019. DOI: 10.1080/21678421.2019.1587633. [DOI] [PubMed] [Google Scholar]
- 35.Prize4Life, Neurological Clinical Research Institute. PRO-ACT https://nctu.partners.org/ProACT/Document/DisplayLatest/5. Published 2020. Accessed February 7, 2020.
- 36.Brooks BR, Li J, Huang W, Anderson FA, Miller RG, Mitsumoto H. Epidemiology of falls in amyotrophic lateral sclerosis patients: analysis of cross-sectional and prospective cohorts in the ALS CARE registry. Ann Neuro 2007; 62(sup.11):S23–S24. Published online: 2007. [Google Scholar]
- 37.Pieterse AJ, Luttikhold TB, de Laat K, Bloem BR, van Engelen BG, Munneke M. Falls in patients with neuromuscular disorders. J Neurol Sci 2006; 251:87–90. Published online: 2006. DOI: 10.1016/j.jns.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Schell WE, Mar VS, Da Silva CP. Correlation of falls in patients with Amyotrophic Lateral Sclerosis with objective measures of balance, strength, and spasticity. NeuroRehabilitation 2019; 44:85–93. Published online: 2019. DOI: 10.3233/NRE-182531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hobson E V, Baird WO, Partridge R, Cooper CL, Mawson S, Quinn A, et al. The TiM system: developing a novel telehealth service to improve access to specialist care in motor neurone disease using user-centered design. Amyotroph Lateral Scler Frontotemporal Degener 2018; 19:351–361. Published online: July 3, 2018. DOI: 10.1080/21678421.2018.1440408. [DOI] [PubMed] [Google Scholar]
- 40.Rutkove SB, Qi K, Shelton K, Liss J, Berisha V, Shefner JM. ALS longitudinal studies with frequent data collection at home: study design and baseline data. Amyotroph Lateral Scler Frontotemporal Degener 2019; 20:61–67. Published online: January 2, 2019. DOI: 10.1080/21678421.2018.1541095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rural Health Information Hub. Barriers to Telehealth in Rural Areas https://www.ruralhealthinfo.org/toolkits/telehealth/1/barriers. Accessed October 12, 2019.
- 42.The Office of the National Coordinator for Health Information Technology (ONC). Are there state licensing issues related to telehealth? https://www.healthit.gov/faq/are-there-state-licensing-issues-related-telehealth. Published 2020.
- 43.Interstate Medical Licensure Compact. The IMLC https://imlcc.org/. Published 2020. Accessed February 7, 2020.
- 44.U.S. Centers for Medicare & Medicaid Services. Telehealth https://www.medicare.gov/coverage/telehealth. Published 2020. Accessed February 7, 2020.


