Abstract
We compared immune responses to intranasal and intramuscular DNA vaccinations against human immunodeficiency virus type 1 with monophosphoryl lipid A (MPL) used as an adjuvant. Both routes of vaccination resulted in similar levels of cell-mediated immunity, but the intestinal secretory immunoglobulin A response was higher following intranasal immunization than after intramuscular immunization. MPL demonstrated its adjuvanticity in vaccination by both routes.
DNA vaccination has come to be the preferred means of eliciting antigen-specific immunity against various pathogens in animal models (5, 15, 16, 24, 27, 29), and it is thought to have several advantages over more traditional vaccination with polypeptides (4, 9). Our group and others have tried a number of approaches to enhance DNA-derived immunity, including particle bombardment (5, 16) and the use of expression vectors of certain cytokines (3, 13, 22, 29) or other immunomodulatory molecules (21, 23); these investigations have all yielded promising results. Our recent study on human immunodeficiency virus type 1 (HIV-1) DNA vaccination showed that monophosphoryl lipid A (MPL), an immunologic adjuvant obtained from bacterial lipopolysaccharide (25), is also effective in enhancing antigen-specific immunity (19).
Although these studies have indicated that the route of DNA delivery and the coadministration of immunomodulators must both be considered in modifying DNA-derived immunity, the optimal inoculation approach has not yet been established. As a follow-up to an earlier study referred to above (19), we compared intranasal (i.n.) and intramuscular (i.m.) routes of immunization with an MPL adjuvant-DNA vaccine against HIV-1 in terms of their potential to induce serum and mucosal antibody responses and to enhance cell-mediated immunity.
BALB/c mice were used for vaccination, and the DNA vaccine constructs pCMV160IIIB and pcREV (hereafter referred to IIIB/REV) used have been described elsewhere (15). Although the IIIB/REV vaccine was designed to induce HIV-1 env-specific immune responses, a rev expression plasmid was included because an early study (12) demonstrated that expression of env protein is dependent on rev coexpression. The adjuvant used in the present study was MPL-SE (i.e., MPL in stable emulsion) (19, 25), which was generously provided by Ribi ImmunoChem Research Inc., Hamilton, Mont.
The procedures and vaccine formulation for i.m. immunization were identical to those used in our recent study (19). Briefly, 2 μg each of IIIB/REV (total, 4 μg) was dissolved in sterile phosphate-buffered saline with or without 50 μl of MPL-SE. One hundred microliters of vaccine preparation was then injected into the biceps femoris muscle. For i.n. immunization, the DNA dose was also 2 μg each of IIIB/REV (total, 4 μg), which was dissolved in phosphate-buffered saline containing MPL-SE. The dose of adjuvant is indicated in the figures and Table 1. Mice were anesthetized with diethyl ether, and 30 μl of the prepared vaccine was dropped into the nostril gradually to prevent suffocation. The mice were therefore able to inhale the vaccine preparation in a natural manner. Both i.m. and i.n. inoculations were performed once only. The immunological parameters included the following: titers of serum immunoglobulin G (IgG), IgG1, and IgG2a, as well as the titer of fecal secretory IgA, which were all measured by enzyme-linked immunosorbent assay (ELISA); the delayed-type hypersensitivity (DTH) reaction, which was assessed by a footpad swelling test; and antigen-specific cytotoxic-T-lymphocyte (CTL) activity of splenocytes harvested from the immunized animal, which was determined by the standard 51Cr release assay. The methods used for these assays are described elsewhere (19). Fecal extract samples were prepared and the secretory IgA (sIgA) titer was measured as described in an earlier report (2). Statistical analyses were conducted by using an unpaired two-tailed t test for comparison of two groups or a one-way factorial analysis of variance for distributed parameters. Significance for both analyses was defined as a P of <0.05.
TABLE 1.
Footpad swelling response induced by DNA vaccine plus MPL adjuvant administered via the i.n. or i.m. routea
| Immunization route | Vaccine formulation | Swelling response (10−2 mm)b
|
|
|---|---|---|---|
| V3 peptide | Myoglobin peptide | ||
| i.n. | IIIB/REV (4 μg) | 10.4 ± 2.1 | 2.4 ± 1.3 |
| IIIB/REV (4 μg) + MPL-SE (5 μl) | 17.2 ± 2.4c | NTd | |
| IIIB/REV (4 μg) + MPL-SE (20 μl) | 16.6 ± 1.9c | 1.8 ± 0.5 | |
| pCMV-emptye (4 μg) + MPL-SE (20 μl) | 1.9 ± 1.1 | 2.6 ± 2.1 | |
| i.m. | IIIB/REV (4 μg) | 9.8 ± 2.5 | NT |
| IIIB/REV (4 μg) + MPL-SE (50 μl) | 17.0 ± 3.2c | 3.1 ± 2.0 | |
Mice were immunized once via the i.m. or i.n. route with the DNA vaccine formulated as shown. Fourteen days after immunization, 10 μg of V3 peptide (helper epitope for HIV-1 strain IIIB) was injected into the rear footpads. The extent of footpad swelling was assayed 24 h after peptide injection. Control mice were injected with sperm whale myoglobin peptide.
Data are expressed as the mean ± standard error of the mean of four (control group) or five (experimental group) mice. Similar results were obtained in a separate experiment.
Significantly different from the mean value for mice immunized with IIIB/REV alone (P < 0.05).
NT, not tested.
pCMV-empty, empty vector.
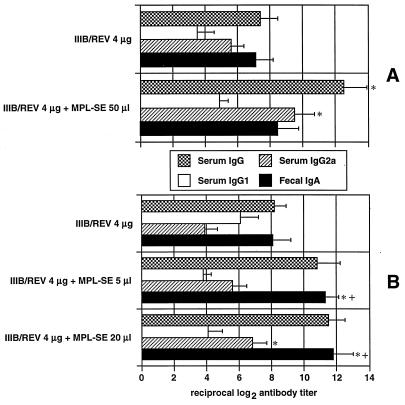
Serum and fecal antibody responses were determined by ELISA 4 weeks after immunization, as shown in Fig. 1. Both i.n. and i.m. immunizations consistently raised the titers of serum and fecal HIV-1-specific antibody, and MPL enhanced antibody production. Vaccination via both routes in the absence of adjuvant seemed to trigger a sIgA response. In the presence of the adjuvant, intestinal production of this antibody was significant with i.n. immunization although not with i.m. immunization. With regard to the serum IgG subclasses, i.m. immunization without the adjuvant resulted in an increase in the IgG2a titer, whereas i.n. immunization tended to raise the level of IgG1. In the presence of adjuvant, this profile was altered with respect to i.n. immunization in that the level of IgG2a increased and exceeded that of IgG1. In i.m. immunized mice, the IgG2a response was greater than the IgG1 response regardless of whether the adjuvant was used, and MPL enhanced titers of both antibodies. These results indicate that MPL is capable of acting as a mucosal adjuvant for i.n. DNA vaccination in terms of the intestinal sIgA response and that MPL preferentially elicits IgG2a antibody production when either route is used.
FIG. 1.
Humoral immune responses induced by MPL adjuvant-DNA vaccination through the i.m. (A) and i.n. (B) routes. Mice were immunized once with 4 μg of IIIB/REV formulated with the indicated doses of MPL-SE. HIV-1 principal neutralizing determinant-specific titers of serum IgG, IgG1, and IgG2a and fecal sIgA were measured by ELISA using sera collected 4 weeks after immunization. The results are expressed as the mean ± standard error of the mean of five (experimental group) or four (control group) mice. The symbols * and + indicate a significantly enhanced antibody response compared with either the response obtained with IIIB/REV alone delivered via the same route or the response of the group administered vaccine plus adjuvant i.m., respectively (P < 0.05). Similar results were obtained in another experiment. No antibody was detected in the group administered empty vector (pCMV-empty) and 20 μl of MPL-SE via the i.n. route.
Previous studies have demonstrated that pathogen-specific sIgA is able to protect a host against i.n. challenge with influenza virus (17) and herpes simplex virus (6) or against intestinal challenge with Vibrio cholerae (28). In an earlier study, we also found that HIV-1-specific fecal sIgA is capable of neutralizing HIV-1 in vitro (2). Therefore, HIV-specific mucosal sIgA in the gastrointestinal or urogenital tract may act effectively in helping to block HIV penetration of the mucosal membrane. This is an important consideration in designing a vaccine for AIDS prevention where the goal is to inhibit the sexual transmission of the virus, and the i.n. route of immunization may therefore be more suitable than the i.m. route in view of the stimulation of sIgA antibody production in the gastrointestinal tract observed with i.n. delivery.
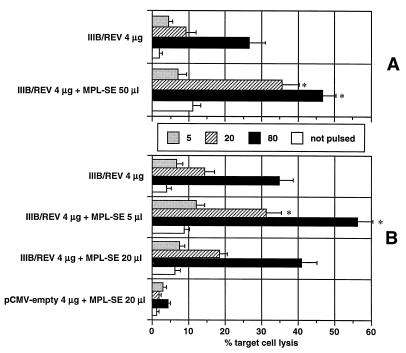
The HIV-1-specific DTH responses assayed 2 weeks after inoculation were similar for the i.n. and i.m. groups, regardless of whether the adjuvant was administered, and when MPL was employed, the DTH reactions obtained by both routes were enhanced and at almost the same levels (Table 1). These results suggest that i.n. DNA vaccination can induce a DTH reaction comparable to that of the i.m. route. HIV-1-specific cytolytic activity was determined 3 weeks after immunization (Fig. 2). The i.n. administered DNA vaccine formulated with 5 μl of MPL-SE produced the strongest cytolytic response, and increasing the dose of adjuvant to 20 μl resulted in no further enhancement, in spite of a consistent increase in the strength of the DTH reaction. The optimal MPL dose for activation of CD8+ CTL and CD4+ helper T cells, which are responsible for cytolytic and DTH responses, respectively, may be different with i.n. DNA vaccination. Although the difference was not statistically significant, the i.n. route appeared to elicit a stronger cytolytic response than did the i.m. route. Induction of strong HIV-1-specific CTL activity is an important aim in developing vaccine strategies against HIV-1 (1). In the murine model, the i.n. route may prove to be the preferred means of achieving this goal.
FIG. 2.
Antigen-specific cytolytic activity of bulk splenic mononuclear cells harvested from mice immunized with MPL adjuvant-DNA vaccine administered via the i.m. (A) or i.n. (B) route. Splenocytes were harvested and cultured for 5 days with V3 peptide (also known as a CTL epitope of HIV-1 strain IIIB). Syngeneic cells (P815 cells) pulsed with or without the same peptide were used as targets, and the percent target cell lysis was determined by means of a 5-h 51Cr release assay. The activity was titrated with effector/target (E/T) cell ratios of 5, 20, and 80. Nonspecific cytolytic activity (not pulsed) was measured at an E/T ratio of 80. The results were obtained in duplicate assays and are expressed as the mean ± standard error of the mean of the four to five mice in each group. The symbol * indicates a significantly enhanced cytolytic response compared with the response of mice which received IIIB/REV alone via the same immunization route.
Nasal or oral vaccination studies targeting mucosal immunity have commonly employed cholera toxin (CT) adjuvant (10, 20, 30). Although CT is reliable as a mucosal adjuvant, it is classified as a Th2 type (26) and is reported to induce a Th2-biased immune response to both polypeptide (20, 30) and DNA vaccination (10). Hence, CT is not considered suitable for inducing systemic Th1-derived cell-mediated immunity. On the other hand, MPL is a lipopolysaccharide-based Th1-type adjuvant (26), and our present results demonstrate that i.n. MPL administration results in the enhancement of not only the mucosal sIgA antibody response (Fig. 1) but also systemic cell-mediated immunity (Fig. 2; Table 1). Therefore, when administered by the i.n. route, a DNA vaccine which includes MPL as an adjuvant may be more useful than the vaccines which are administered by mucosal routes and are conventionally formulated with CT adjuvant because the former can induce a mucosal sIgA response as well as CTL activity, both of which are important in controlling HIV infection.
The mechanism for DNA-derived immunity by i.n. DNA administration, although important, remains an inadequately explored subject. It is known, however, that the surface of nasal mucosa has lymphoid tissues which are histologically similar in structure to Peyer’s patches (11). DNA-transfected antigen-presenting cells in nasal lymphoid tissue can migrate through lymph vessels and/or the bloodstream, which may result in their dissemination to remote lymphoid tissues such as those of the gut and spleen. Hypotheses similar to this have been advocated by other groups (5, 8). We consider that i.n. immunization is more likely to cause lymphoid cell dissemination to remote sites than i.m. immunization.
The adjuvanticity of MPL is associated with its capacity for activating macrophages and inducing interleukin 2 and gamma interferon (7, 18), activities which are thought to play a central role in eliciting DNA-derived immunity, as previously reported (3, 13, 22, 29). MPL enhancement of cell-mediated immunity, as observed in this study, is considered to depend upon these activities. In terms of the enhanced intestinal sIgA response, MPL mediation of macrophage activation alone may be important since the involvement of cytokines in the mechanism regulating mucosal sIgA production is different from the systemic antibody responses (14, 30).
Two major conclusions can be drawn from this study. The first is that DNA vaccinations via the i.n. and i.m. routes are comparable in terms of enhancing both mucosal antibody production and systemic cell-mediated immunity; however, the former route is more advantageous for eliciting mucosal antibody. The second is that MPL is an effective mucosal adjuvant for augmenting intestinal sIgA production in i.n. immunized mice. Further efforts to determine the optimal DNA vaccine formulation and inoculation procedure must continue. Moreover, examining the usefulness of immunogens other than strain IIIB is also important since macrophage-tropic strains are more likely to transmit infection.
Acknowledgments
We thank Ribi ImmunoChem Research Inc. for providing the MPL used in the present study. We also thank T. Kaneko, A. Honsho, and K. Niikura for their skillful technical assistance.
This work was partially supported by a Grant-in-Aid from the Yokohama Foundation of Medical Science Promotion.
REFERENCES
- 1.Ada G L, McElrath M J. HIV type 1 vaccine-induced cytotoxic T cell responses: potential role in vaccine efficacy. AIDS Res Hum Retroviruses. 1997;13:205–210. doi: 10.1089/aid.1997.13.205. [DOI] [PubMed] [Google Scholar]
- 2.Bukawa H, Sekigawa K, Hamajima K, Fukushima J, Yamada Y, Kiyono H, Okuda K. Neutralization of HIV-1 by secretory IgA induced by oral immunization with a new macromolecular multicomponent peptide vaccine candidate. Nat Med. 1995;1:681–685. doi: 10.1038/nm0795-681. [DOI] [PubMed] [Google Scholar]
- 3.Chow Y-H, Huang W-L, Chi W-K, Chu Y-D, Tao M-H. Improvement of hepatitis B virus DNA vaccine by plasmids coexpressing hepatitis B surface antigen and interleukin-2. J Virol. 1997;71:169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 5.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines—protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallichan W S, Johnson D C, Graham F L, Rosenthal K L. Mucosal immunity and protection after intranasal immunization with recombinant adenovirus expressing herpes simplex virus glycoprotein-b. J Infect Dis. 1993;168:622–629. doi: 10.1093/infdis/168.3.622. [DOI] [PubMed] [Google Scholar]
- 7.Gustafson G L, Rhodes M J. Bacterial cell wall products as adjuvants: early interferon gamma as a marker for adjuvants that enhance protective immunity. Res Immunol. 1992;143:483–488. doi: 10.1016/0923-2494(92)80058-s. [DOI] [PubMed] [Google Scholar]
- 8.Harokopakis E, Hajishengallis G, Greenway T E, Russell M W, Michalek S M. Mucosal immunogenicity of a recombinant Salmonella typhimurium-cloned heterologous antigen in the absence or presence of coexpressed cholera toxin A2 and B subunits. Infect Immun. 1997;65:1445–1454. doi: 10.1128/iai.65.4.1445-1454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassett D E, Whitton J L. DNA immunization. Trends Microbiol. 1996;4:307–312. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 10.Kuklin N, Daheshia M, Karem K, Manickan E, Rouse B T. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J Virol. 1997;71:3138–3145. doi: 10.1128/jvi.71.4.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuper C F, Koornstra P J, Hameleers D M, Biewenga J, Spit B J, Duijvestijn A M, van Breda V P, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 12.Malim M H, Hauber J, Fenrick R, Cullen B R. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988;335:181–183. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- 13.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Wahren B, Okuda K. Intranasal immunization of a DNA vaccine with interleukin 12 and granulocyte macrophage colony stimulating factor (GM-CSF) expressing plasmids in liposomes induce strong mucosal and cell-mediated immune responses against HIV-1 antigen. J Immunol. 1997;159:3638–3647. [PubMed] [Google Scholar]
- 14.Okahashi N, Yamamoto M, Vancott J L, Chatfield S N, Roberts M, Bluethmann H, Hiroi T, Kiyono H, McGhee J R. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuda K, Bukawa H, Hamajima K, Kawamoto S, Sekigawa K, Yamada Y, Tanaka S, Ishi N, Aoki I, Nakamura M, Yamamoto H, Cullen B R, Fukushima J. Induction of potent humoral and cell-mediated immune responses following direct injection of DNA encoding the HIV type 1 env and rev gene products. AIDS Res Hum Retroviruses. 1995;11:933–943. doi: 10.1089/aid.1995.11.933. [DOI] [PubMed] [Google Scholar]
- 16.Pertmer T M, Roberts T R, Haynes J R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renegar K B, Small P A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 18.Rickman L S, Gordon D M, Wistar R J, Krzych U, Gross M, Hollingdale M R, Egan J E, Chulay J D, Hoffman S L. Use of adjuvant containing mycobacterial cell-wall skeleton, monophosphoryl lipid A, and squalane in malaria circumsporozoite protein vaccine. Lancet. 1991;337:998–1001. doi: 10.1016/0140-6736(91)92659-p. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki S, Tsuji T, Hamajima K, Fukushima J, Ishii N, Kaneko T, Xin K-Q, Mohri H, Aoki I, Okubo T, Nishioka K, Okuda K. Monophosphoryl lipid A enhances both humoral and cell-mediated immune responses to DNA vaccination against human immunodeficiency virus type 1. Infect Immun. 1997;65:3520–3528. doi: 10.1128/iai.65.9.3520-3528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staats H F, Nichols W G, Palker T J. Mucosal immunity to HIV-1—systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN (A) J Immunol. 1996;157:462–472. [PubMed] [Google Scholar]
- 21.Tsuji T, Fukushima J, Hamajima K, Ishii N, Aoki I, Xin K-Q, Bukawa H, Ishigatsubo Y, Tani K, Okubo T, Dorf M E, Okuda K. HIV-1-specific cell-mediated immunity is enhanced by co-inoculation of TCA3 expression plasmid with DNA vaccine. Immunology. 1997;90:1–6. doi: 10.1046/j.1365-2567.1997.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji T, Hamajima K, Fukushima J, Xin K-Q, Ishii N, Aoki I, Ishigatsubo Y, Tani K, Kawamoto S, Nitta Y, Miyazaki J, Koff W C, Okubo T, Okuda K. Enhancement of cell-mediated immunity against HIV-1 induced by coinoculation of plasmid-encoded HIV-1 antigen with plasmid expressing IL-12. J Immunol. 1997;158:4008–4014. [PubMed] [Google Scholar]
- 23.Tsuji T, Hamajima K, Ishii N, Aoki I, Fukushima J, Xin K Q, Kawamoto S, Sasaki S, Matsunaga K, Ishigatsubo Y, Tani K, Okubo T, Okuda K. Immunomodulatory effects of a plasmid expressing B7-2 on human immunodeficiency virus-1-specific cell-mediated immunity induced by a plasmid encoding the viral antigen. Eur J Immunol. 1997;27:782–787. doi: 10.1002/eji.1830270329. [DOI] [PubMed] [Google Scholar]
- 24.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 25.Ulrich J T, Myers K R. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. In: Powell M F, Newman M J, editors. Vaccine design. New York, N.Y: Plenum Press; 1995. pp. 495–523. [PubMed] [Google Scholar]
- 26.Vogel F R. Immunologic adjuvants for modern vaccine formulations. Ann NY Acad Sci. 1995;754:153–160. doi: 10.1111/j.1749-6632.1995.tb44448.x. [DOI] [PubMed] [Google Scholar]
- 27.Whalen R G, Leclerc C, Deriaud E, Schirmbeck R, Reinmann J, Davis H L. DNA-mediated immunization to the hepatitis B surface antigen—activation and entrainment of the immune response. Ann NY Acad Sci. 1995;772:64–76. doi: 10.1111/j.1749-6632.1995.tb44732.x. [DOI] [PubMed] [Google Scholar]
- 28.Winner L, Mack J, Weltzin R, Mekalanos J J, Kraehenbuhl J P, Neutra M. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991;59:977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang Z Q, Ertl H C J. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M, Vancott J L, Okahashi N, Marinaro M, Kiyono H, Fujihashi K, Jackson R J, Chatfield S N, Bluethmann H, McGhee J R. The role of Th1 and Th2 cells for mucosal IgA responses. Ann NY Acad Sci. 1996;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]