Abstract
Development of transcatheter mitral valve interventions has ushered a significant need for large animal models of secondary mitral regurgitation. Though currently used heart failure models that chronically develop secondary mitral regurgitation are viable, the severity is lower than patients, the incubation time is long, and mortality is high. We sought to develop a swine model of acute secondary mitral regurgitation that uses image-guided placement of snares around the mitral chordae. Twenty-seven adult swine (n = 27) were assigned to secondary mitral regurgitation induced by valve tethering with image-guided chordal encircling snares (group 1, n = 7, tether MR (tMR)); secondary mitral regurgitation by percutaneous posterolateral myocardial infarction causing ventricular dysfunction and regurgitation (group 2, n = 6, functional MR (fMR)); and control animals (group 3, n = 14). Regurgitant fraction in tMR was 42.1 ± 14.2%, in fMR was 22 ± 9.6%, and in controls was 5.3 ± 3.8%. Mitral tenting height was 9.6 ± 1.3 mm in tMR, 10.1 ± 1.5 mm in fMR, and 5.8 ± 1.2 mm in controls. Chordal encircling tethers reproducibly induce clinically relevant levels of secondary mitral regurgitation, providing a new animal model for use in translational research.
Keywords: Heart failure, Secondary mitral regurgitation, Transcatheter mitral valve repair, Transcatheter mitral valve replacement
Introduction
Treating secondary mitral regurgitation (SMR) as a therapeutic target in heart failure (HF) patients is a topic of current interest in cardiology, since the COAPT trial using the MitraClip® system reported reduced hospitalizations and improved survival after correction of SMR [1]. This data has now spurred the development of variety of new approaches for transcatheter correction of SMR [2], especially since MitraClip® does not always yield a satisfactory hemodynamic outcome, may be challenging to use in some anatomies, and carries risk of causing functional mitral stenosis [3, 4]. Several technologies are in development, yet most devices have failed to achieve a hemodynamically optimal result in early clinical trials. Lack of appropriate preclinical animal models of SMR, to the severity and valve tenting patterns seen in patients, has contributed to these technology failures. New devices are often tested in healthy animals, confirming their safety but not their efficacy. A significant need exists for animal models of SMR, which are reproducible and can be reliably used to study investigative devices.
Several in vitro models of SMR exist in literature [5–19], but none mimics the dynamic pathogenic interaction between the mitral valve and the left ventricle that is central to this lesion. We and others have reported isolated mitral valve models with mechanical systems to induce annular dilatation and papillary muscle displacement, which are very useful, but lack dynamism [5–10, 12]. Recently, we and others have reported whole heart models where ventricular dilatation was induced by muscular thinning or by excessive ventricular chamber pressurization, which imposes passive tethering of the valve, yet does not have the dynamic interaction between the valve and the ventricle [18, 19]. Thus, animal models of SMR are warranted. Traditionally, SMR was induced in animals by infracting the posterolateral myocardial wall that causes asymmetric left ventricular dilatation, papillary muscle dysfunction, systolic valve tenting, and mitral insufficiency [20–23] (Fig. 1A, B). Our group and others have confirmed the validity of this model, but the regurgitation developed over a 2–3-month period is moderate in most animals and mild sometimes. The severity of regurgitation can be worsened by increasing the myocardial infarct size, but the risk of fatal arrhythmias, heart failure, and mortality are high. Recently, a pig model that occluded the entire left circumflex was reported, which caused higher secondary mitral regurgitation (SMR) severity after 3 months of incubation [20]. Rapid pacing of the heart is also used to induce SMR, but its relevance to the human lesion is limited [24]. Continuous rapid pacing is necessary to sustain SMR, as ceasing rapid pacing causes reversal of ventricular dilatation and dysfunction. Altogether, all of these models have several attributes that are equivalent to those seen in patients, but are plagued by drawbacks as well.
Fig. 1.
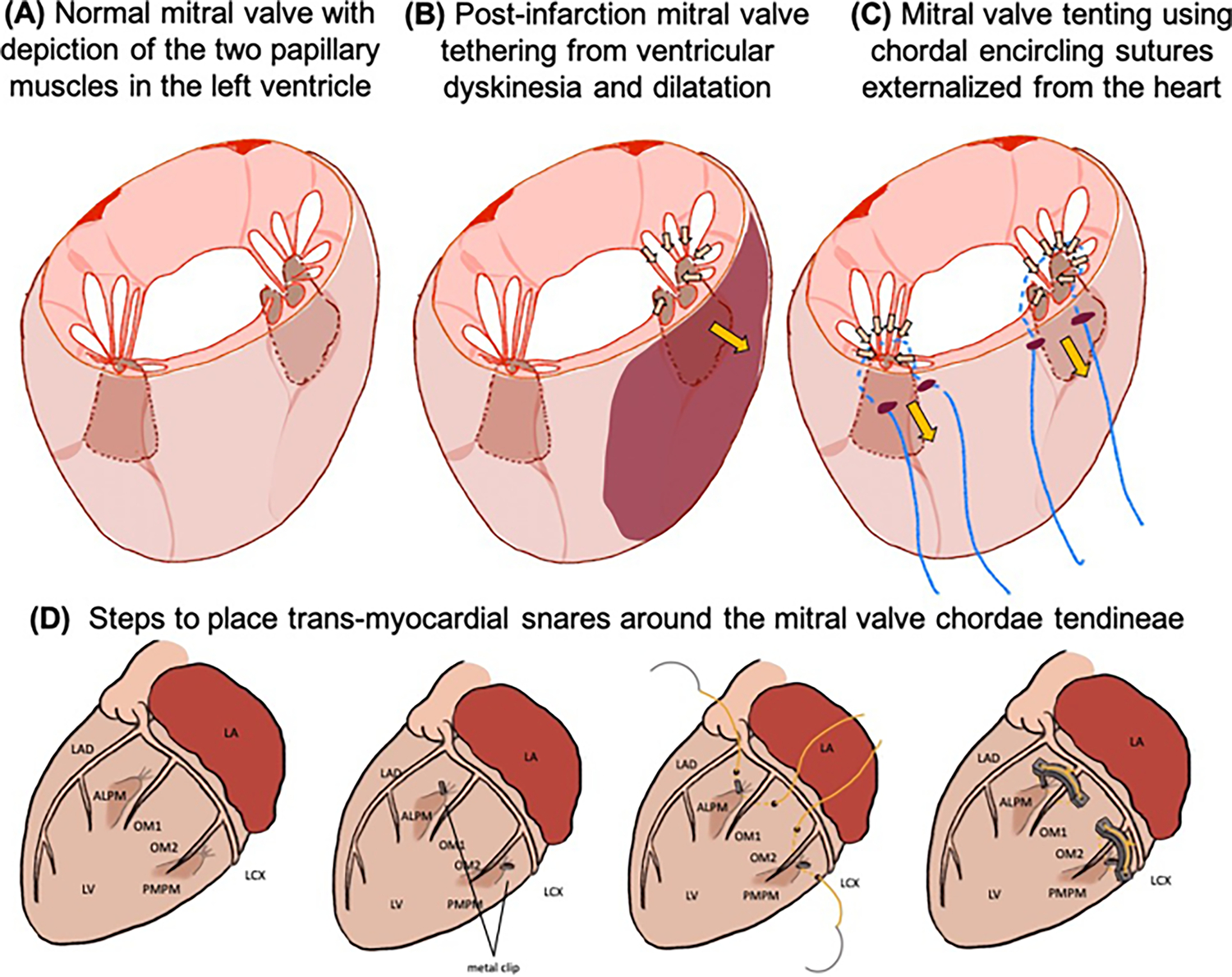
A Physiological configuration of the mitral valve in the normal heart. B Mechanism of secondary mitral regurgitation in post-myocardial infarction heart failure, wherein the dilated and regionally dyskinetic left ventricle(LV) tethers the mitral valve and restricts its coaptation. C Mechanism of mitral regurgitation in the new tether model, wherein snares are placed around the chordae tendineae through the ventricular myocardium and tethered to induce mitral regurgitation. D Steps to place transmyocardial snares around the chordae tendineae of the mitral valve, to tether the valve and induce mitral regurgitation. The antero lateral papillary muscle (ALPM) and the postero medial papillary muscle (PMPM) are identified in relation to the coronary arteries (LAD: left anterior descending; LCX: left circumflex; OM1: obtuse marginal branch 1; OM 2: obtuse marginal branch 2) using imaging. Snares are then placed through the LV, and the valve is imaged using an ultrasound probe placed on the left atrium (LA).
To overcome these challenges, we sought to develop a model of acute SMR that does not depend on left ventricular dysfunction and focuses on direct tethering of the mitral valve (Fig. 1C, D). Since the mechanism of secondary mitral regurgitation is tenting of the mitral leaflets due to their restriction in systole by the dysfunctional left ventricle [25], we mimicked such a scenario in healthy animals by encircling the chordae from each papillary muscle with snares, in a beating heart with image guidance. These snares were tightened onto two epicardial bridges that protected the coronaries from compression, leading to chordal restriction, causing tenting of the mitral valve and SMR.
In this report, we compare the regurgitant fraction and mitral valve kinematics in this new tether SMR model (tMR), to traditional post-myocardial infarction model over an 8–10-week period (functional MR (fMR)) and to age- and weight-matched control animals.
Methods
Ethics Statement and Use of Animals in This Research
This study was reviewed by the Institutional Animal Care and Use committee (IACUC) at Emory University, and procedures were performed according to the National Institutes of Health guidelines for use and care of animals in research. Swine were chosen as the appropriate model due to anatomical similarities, and the ease of animal care and imaging, compared to ruminants [21, 26, 27].
Animal Acquisition and Husbandry
Swine were acquired from Palmetto farms (Galivants Ferry, SC) and transported by road in a climate-controlled truck to our laboratory. After health checkup by the veterinarians, swine were accepted into our facility and quarantined for 3–5 days prior to use in this study. Swine were socially housed prior to the procedure and single housed after the procedure. Cages had continuous access to drinking water, and the animals were fed standard pig chow daily.
Experimental Design
Twenty-seven swine were assigned to the following groups: group 1: tMR, n = 7—tether model of SMR in which transventricular snares that encircle the chordae tendineae were implanted, to tether the mitral valve; group 2: fMR, n = 7—received a percutaneous posterolateral myocardial infarction resulting in ventricular dilatation, mitral valve tenting, and regurgitation; group 3: control, n = 14—no cardiac procedures, but a small left thoracotomy for epicardial echocardiographic imaging. 2D and 3D echocardiography was performed to assess mitral valve and left ventricular geometry and hemodynamics in each model.
Operative Methods
Anesthesia and Preoperative Medications
All study animals were pretreated with 600 mg of amiodarone, 81 mg of aspirin, and 75 mg of plavix for 3 days prior to any procedure. The animals were fasted overnight, and on the day of the procedure, telazol (2–8.8 mg/kg, IM) was used for sedation. After endotracheal intubation and onset of mechanical ventilation, the animal was maintained on a surgical plane of anesthesia with 1–2% isoflurane in 100% oxygen. Animals in the fMR group also received preoperative cefazolin (22–25 mg/kg, IV), rimadyl (2 mg/kg, IM), and buprenex (0.0075–0.01 mg/kg, IM). Propofol (1–3 mg/kg, IV) was used for additional anesthetic support. Amiodarone (0.5 mg/min, IV) was administrated as a prophylactic measure to avoid ventricular arrhythmias, and required dosages of phenylephrine and dobutamine were administered to maintain mean arterial pressure at 60–70 mmHg. Body temperature, heart rate, and cuff blood pressures were monitored continuously, and an auricular vein catheter was used to infuse drugs and fluids.
tMR Group
With the pig in right lateral recumbent position, a left thoracotomy was performed. The fourth intercostal rib space was opened; the pleura was incised. The left lung was gently packed with a laparotomy sponge, exposing the left atrium and the lateral surface of the left ventricle within the pericardium. The pericardium was incised to expose the left atrium and the basal aspect of the LV (Fig. 2A). A transesophageal echocardiography probe (Z6Ms, Siemens Healthineers, WA) was tunneled onto the left atrium, and 2D, bi-plane, and 3D echocardiographic images of the left heart were obtained (Fig. 2B). With the same probe placed on the epicardial surface of the left ventricle, using short-axis images of the left heart, the location of the mitral annulus, the tips, and bases of the anterolateral papillary muscle (ALPM) and posteromedial papillary muscle (PMPM) were identified and marked with epicardial metal clips (Fig. 2C). To encircle maximum number of chordae with the suture snare, it was necessary to insert the needle in a short-axis plane closer to the papillary muscle tip and not close to the leaflet edge. The chordae tendineae are unbranched, single elements closer to the papillary muscle, but expand into a web of thinner chordae closer to the leaflet, where capturing all the chordae may not be possible. Chromic suture (size 0, Ethibond) with a blunt-tip needle of 65 mm diameter was inserted through the left ventricular myocardium adjacent to the epicardial clip location (Fig. 2D) and externalized on the opposite side of the papillary muscle (Fig. 2E). Chromic suture was used as it was available and the needle was large curved and blunt tipped. Any such suture with similar needle dimensions can be used. Using non-absorbable suture may be useful in chronic survival animals, but this was not relevant to this acute study. This suture placement encircled the chordae tendineae and exited the myocardium at a 50–60-mm lateral location to the initial needle insertion site (Video 1). Some anatomical landmarks were useful when placing these snares around the papillary muscles. To capture the chordae emerging from the ALPM, the needle was inserted below the atrioventricular groove and to the right of the first obtuse marginal branch, angled towards left anterior descending artery (LAD). To tether the chordae originating from the PMPM, the needle was inserted from left of the second obtuse marginal branch to encircle the chordae and exit the heart to the right of the third obtuse marginal branch. Once both sutures were placed to encircle the chordae tendineae, their free ends were inserted into a 3D-printed polymeric epicardial brace and tied down (Fig. 2F). The suture knots were tensioned to the maximum extent possible and when further tensioning was not possible due to the chordae being pulled to the endocardial surface of the adjacent left ventricular wall. The brace raised the suture knot from the epicardial surface and avoided coronary compression and causing a myocardial infarction. In all cases, ultrasound imaging was performed after tightening one of the suture loops, and then the second one, causing different tethering patterns and regurgitation jet directions and severity. Both suture loops were tethered to the maximum extent possible, which was defined as the suture loop touching the endocardial surface within the left ventricle on ultrasound imaging.
Fig. 2.
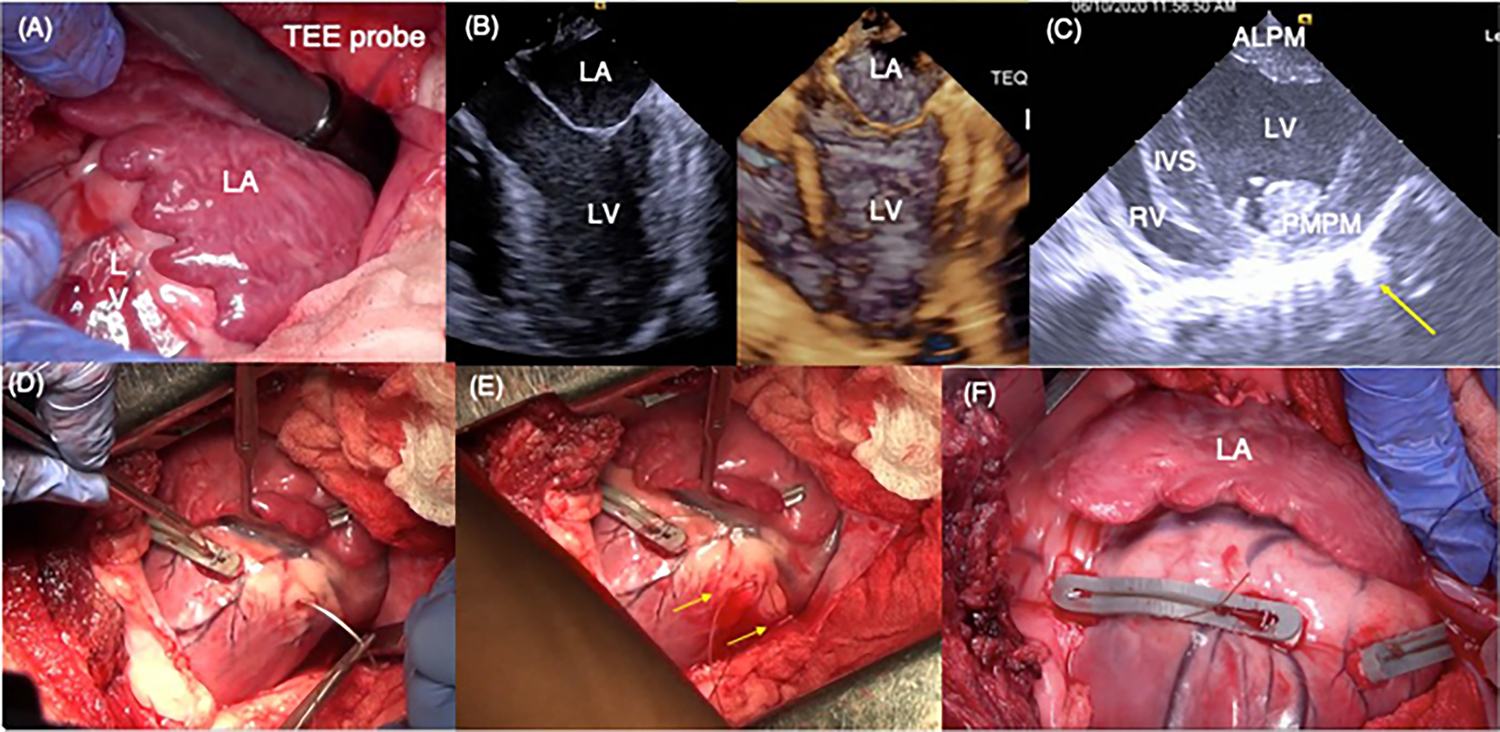
A Intra-operative view of the left atrium and the basal aspect of the left ventricle through a left thoracotomy. The transesophageal echo probe was tunneled onto the roof of the left atrium. B 2D and 3D echocardiographic views of the left atrium, mitral valve, and the left ventricle are shown. The papillary muscle position is identified in this view by palpating the epicardium. C Short-axis view of the left ventricle depicting the two papillary muscles in the left ventricle. Epicardial vascular clips were placed at regions where the papillary muscles were identified upon palpation. Yellow arrow indicates reflection from a clip on the epicardium. D Representative photograph depicting placement of the posterior snare, by inserting the needle through the myocardium on the lateral side of the papillary muscle and externalizing on the medial side. E Two ends of the suture externalized through the myocardium, encircling the chordae of the posteromedial papillary muscle in the left ventricle. F Free ends of the snares were tethered maximally and then tied onto a polymer bridge that lifted the snares off the epicardium to avoid coronary compression
fMR Group
Animals in group 2 were induced with a posterolateral myocardial infarction using percutaneous coronary catheterization techniques that were reported earlier [21, 26]. All animals were pretreated with 600 mg amiodarone for 3 days prior to the procedure, and continuous infusion of amiodarone (0.5 kg/min) during the entire procedure helped reduce the risk of arrhythmias. With the animal under anesthesia, an 8-Fr arterial sheath (SuperSheath®, Boston Scientific, Natick, MA) was placed in the right femoral artery for the catheter insertion and monitoring blood pressure during the procedure. A 6-Fr pig-tail catheter (SiteSeer®, Medtronic, Minneapolis, MN) was inserted through the sheath and advanced into the left ventricle to perform a ventriculogram and identify the location of PMPM. A 6- or 7-Fr hockey stick catheter (VistaBrite ®, Cordis, Miami Lakes, FL) was inserted via the femoral sheath and positioned at the ostium of the left and right coronary artery for coronary angiography. Ventriculogram and angiogram were performed with a GE Innova 2000 single-plane fluoroscopy camera (70° LAO and 15° cranial view), and the vessels perfusing the PMPM were identified (Fig. 3A, B1, C1). Those branches of LCX that supplied blood to PMPM (usually the second obtuse marginal (OM) artery onwards) were occluded (Fig. 3B2). When part of posterolateral wall was perfused by the posterior descending artery (PDA) branch from RCA, it was also occluded (Fig. 3C2). These vessels were occluded by inflating an angioplasty balloon (2.5 mm × 8 mm, Trek over the wire, Abbott Vascular®) in the selected arteries and injecting 100%, 200-proof ethanol (0.5–2 ml in each vessel, E7023, Sigma-Aldrich, USA). The balloon was left inflated for 10 min, during which time the static blood mixed with ethanol and formed a clot. After occluding all the target vessels, a final coronary angiogram was performed to confirm loss of perfusion into these vessels. The catheters and sheath were removed, and the vascular access site was compressed until hemostasis was achieved. The animals were then weaned from ventilation and anesthesia and survived to 2 months after which imaging was performed.
Fig. 3.
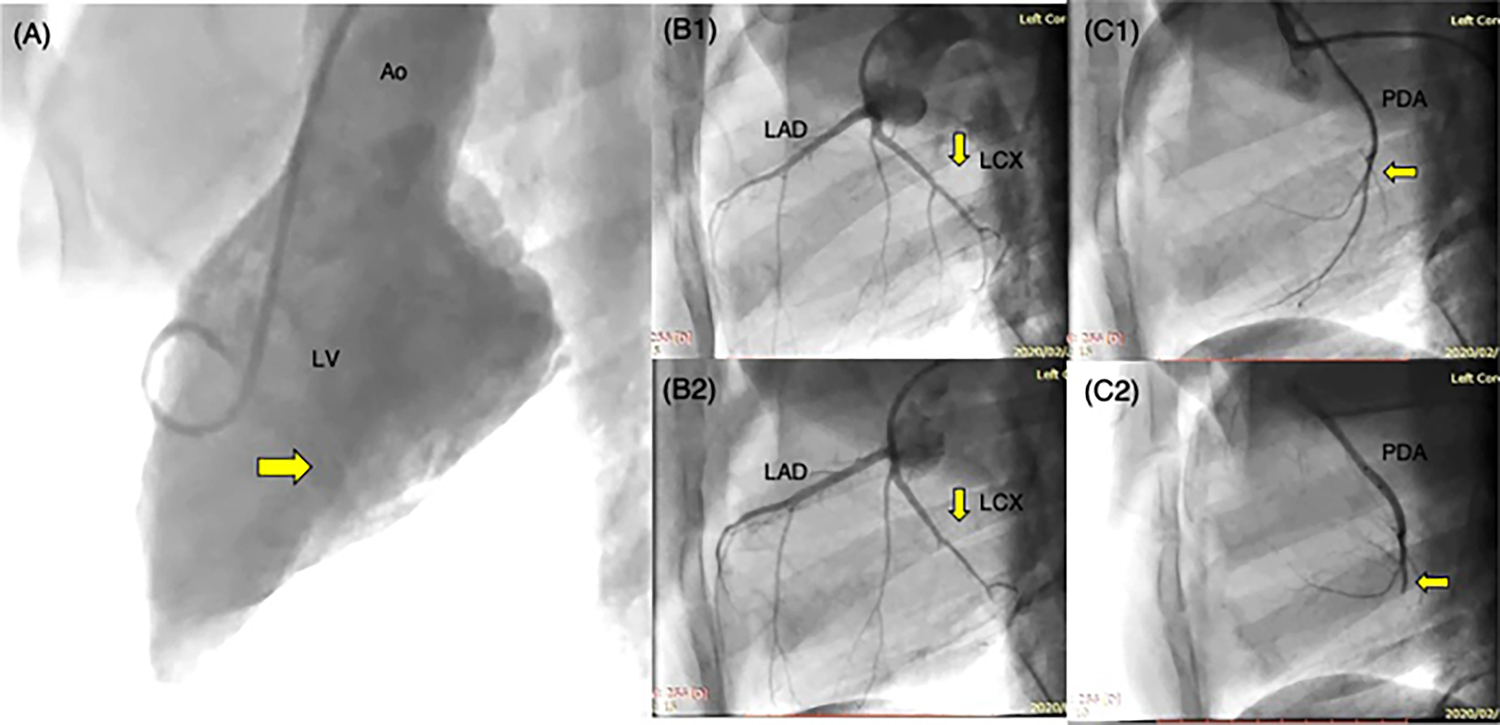
A Left ventriculogram with the camera angled at 70° LAO and 15° CRA, to identify the location and level of the papillary muscles in the left ventricle. B1 Angiogram of the left coronary artery tree, depicting the left anterior descending artery (LAD) and the left circumflex artery (LCX). The left circumflex artery further branches into the obtuse marginal branches. B2 Angiogram of the same left coronary artery tree upon thrombosing the obtuse marginal branches with ethanol. C1 Angiogram of the right coronary artery, with only the posterior descending artery (PDA) depicted in the photograph. C2 Angiogram after occluding the posterior descending artery (PDA) with ethanol
Control
Animals in this group underwent a left thoracotomy for epicardial echocardiography. No other cardiac procedures beyond a pericardiotomy were performed.
Imaging and Analysis
Echocardiography was performed from the left atrium, by tunneling a transesophageal ultrasound probe (Z6Ms, 3–6.3 MHz, Siemens SC2000 PRIME, Munich, Germany). This probe position was most optimal to image the MV, as neither the parasternal transthoracic nor transesophageal images were adequate to obtain both B-mode and Doppler data (without excessive angle correction) (Fig. 4A–D). Gated B-mode long-axis images were obtained, from which the MV leaflet kinematics and geometry were quantified. In a three-chamber long-axis view of the left heart in systole, the septal-lateral annular diameter, coaptation length, tenting height, tenting area, systolic anterior and posterior leaflet angle, and diastolic anterior and posterior leaflet angle were measured. Septal-lateral diameter was measured by obtaining a long-axis plane through the A2 and P2 cusps of the mitral valve and measuring the distance from the anterior leaflet-annular hinge to the posterior leaflet-annular hinge. Coaptation length was measured in the same plane at peak systole, by tracing the length from where the anterior and posterior leaflets overlap to the edge of the longest leaflet below the coaptation. In most cases, the edge of the leaflet coincided with where both leaflets ended, whereas in some, one leaflet would extend beyond the end of coaptation. In later cases, we measured the overlap region and the residual leaflet length below the overlap. Tenting height was measured as the distance from the mitral annular plane to the first point of coaptation in peak systole, and tenting area was measured as the area captured between the mitral annular plane and the two leaflets at peak systole. All of these measurements were performed on 2D echo images. Leaflet kinematics were quantified using excursion angle of each leaflet. For each leaflet, in the A2-P2 plane, the angles between the leaflet and the mitral annular plane were measured in systole and diastole. Excursion angle of anterior and posterior leaflet was calculated by subtracting systolic leaflet angle from diastolic leaflet angle, quantifying the mobility of each leaflet. 3D echocardiographic images were obtained and used to measure the commissure-commissure (CC) diameter; mitral annular area; A1, A2, and A3 leaflet lengths and areas in diastole; P1, P2, and P3 leaflet lengths and areas in diastole; total anterior leaflet area in diastole; and posterior leaflet area in diastole with eSie valve analysis tool (Siemens Healthineers, NJ). The leaflets were semiautomatically tracked and segmented in each phase of the cardiac cycle, and the resulting point cloud was fitted with a surface. Leaflet lengths were then computed by fitting a spline from the annulus to the edge of the leaflets. For the anterior leaflet, this was performed by connecting the midpoint between the two trigones, to a point on the edge of the corresponding leaflet. For the posterior leaflet, the annular spline was divided into corresponding cusp regions, and the midpoint of the corresponding spline and the farthest edge of the leaflet were connected and measured. Color Doppler imaging was performed to quantify the mitral regurgitation as follows: (1) vena contracta width, and (2) regurgitant volume and fraction were calculated by proximal isovelocity surface area (PISA) method and stroke volume method as follows:
Fig. 4.
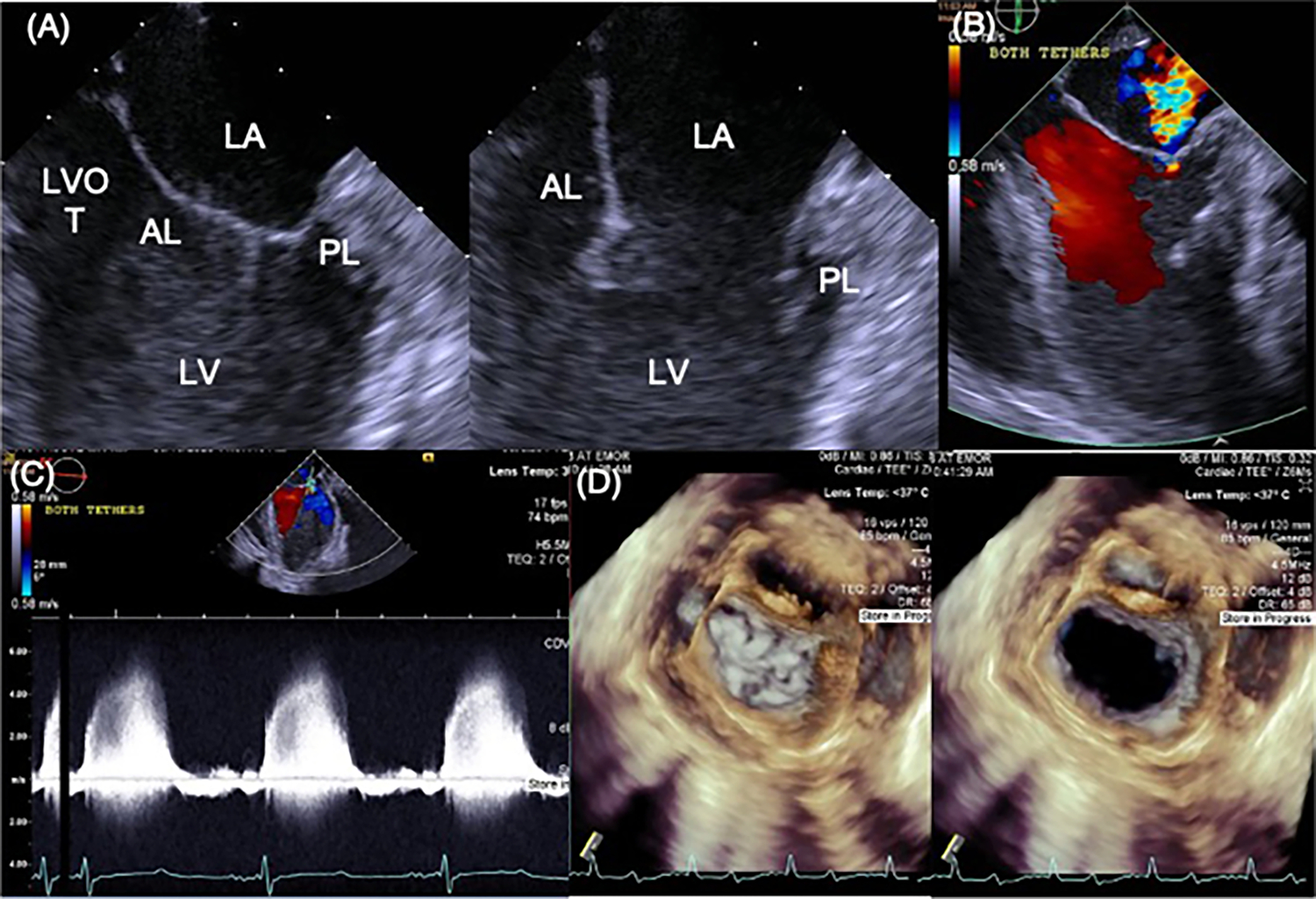
A 2D B-mode view of the mitral valve in systole and diastole with the transesophageal probe placed on the roof of the left atrium. B A representative image of mitral regurgitation in this same view. C Continuous wave imaging of the mitral regurgitation, without the need for angle correction, providing accurate assessment of the severity of regurgitation. D 3D views of the mitral valve from the same probe position
Ejection fraction (EF), end-diastolic volume (EDV), and end-systolic volume (ESV) of the left ventricle were measured using 2D long-axis view of the ventricle with eSie left ventricular analysis tool.
Statistical Methods
Normality was tested with the D’Agostino and Pearson test. Normally distributed data are presented as mean ± standard deviation and others as median and interquartile range. One-way ANOVA with Tukey’s multiple comparison test was performed for normally distributed data, and the Kruskal–Wallis test with Dunn’s multiple comparisons was performed for skewed data. p < 0.05 was considered to be statistically significant. All graphs and analyses were performed with Prism 8.0 (GraphPad Software Inc, San Diego, CA).
Results
Procedural Success and Operative Notes
Procedures in the tMR group were all acute and were performed without mortality. Encircling the chordae tendineae was possible in all the animals, and the chordae did not rupture in any of the animals. Regurgitant volume, severity, and jet characteristics differed based on tethering of a single loop or both loops, shown in Fig. 5A–C. When the PMPM tether alone was tensioned, the regurgitation was milder and between P2 and P3 cusps, mimicking a type IIIb-like lesion. When both the ALPM and PMPM tethers were tensioned, the regurgitation at the P2-P3 worsened, but an equivalently large jet between P1 and P2 had opened, depicting cross swords like jets that are observed in patients (Fig. 5B). Regurgitation was quantified after tensioning both the chordal loops. In some pigs, these two gaps would merge and form a single central gap (Fig. 5C). In the fMR group, infarction was possible in all the animals, and they survived the procedure. When fibrillation occurred, defibrillation was successfully used. One animal in the fMR group died suddenly at 4 weeks after the myocardial infarction, which necropsy revealed to be from ileus that led to colon rupture. The control group of animals was only used for imaging purposes after which they were assigned to other studies.
Fig. 5.
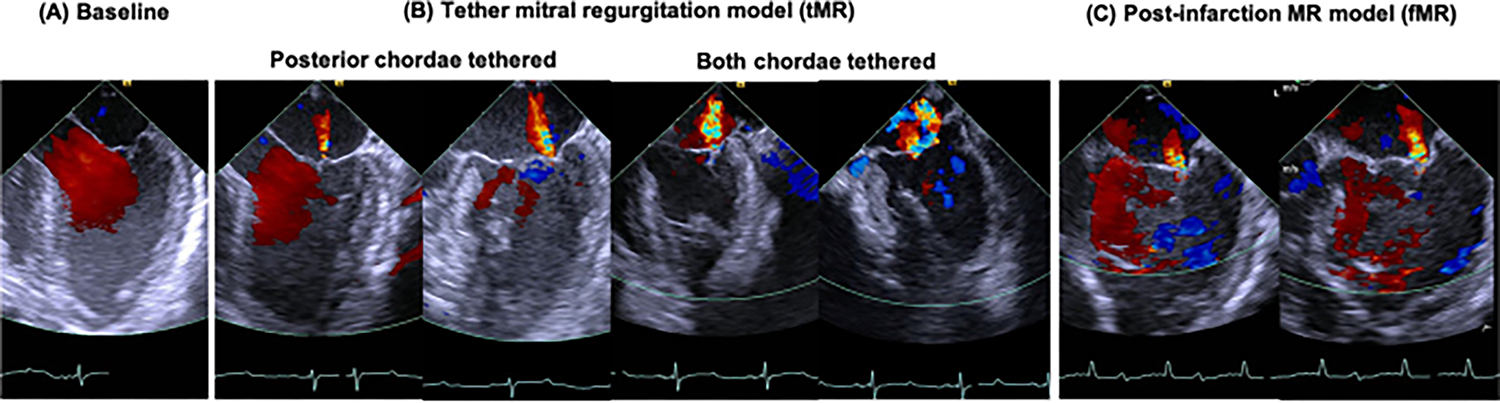
Representative peak systolic echocardiography images of the left heart, depicting the mitral valve and transmitral flow, in a pig in the tMR group. A Normal mitral valve function without any regurgitation and excellent coaptation. B Mitral regurgitation with the chordal encircling tethers in two configurations: when only the posterior chordae are tethered, some regurgitation and tenting of the leaflets are observed. The regurgitant jet is focal to the P2-P3 cusps, mimicking a type IIIb-like lesion; when both the posterior and anterior chordae are tethered, significant regurgitation with valve tenting is observed. The regurgitation was central in the anterior–posterior plane, but two jets were observed in the commissure-commissure plane, with a jet emanating from the P1-P2 cusps and another from the P2-P3 cusps. C Mitral regurgitation with ventricular dilatation several weeks after a myocardial infarction. The regurgitation is focal at P2-P3 cusps as expected due to the posterolateral location of the infarction
Mitral Regurgitation Severity
Regurgitant volume in the control group was 4.1 ± 2.7 ml, was 55.0 ± 33.2 ml in the tMR group after chordal tethering (control: p < 0.0001, fMR: p = 0.0331), and was 25.4 ± 13.3 ml in the fMR group at 10 weeks after the myocardial infarction (vs control: p = 0.11). Regurgitant fraction was 5.3 ± 3.8% in the control group, 42.1 ± 14.2% in the tMR group (p < 0.001), and 22.0 ± 9.6% in the fMR group (p = 0.0132). EROA was 0 (IQR 0–0.01) cm2 in the control group, 0.26 (IQR 0.24–0.34) cm2 in the tMR group (p < 0.0001), and 0.23 (IQR 0.17–0.24) cm2 in the fMR group (p = 0.0132). Vena contracta width in the control group was 0 (IQR 0–0.19) mm, 4.76 (IQR 3.23–6.58) mm in the tMR group (p = 0.0001), and 3.48 (IQR 2.60–5.54) mm in the fMR group (p = 0.0032). Mean stroke volume in the control group was 57.1 ± 17.1 ml which was relatively smaller than those of an adult human. Considering this discrepancy between swine and human heart function, it can be deduced that animals in the fMR group developed moderate MR, and the tMR group developed moderate to severe MR based on regurgitant fraction, but moderate MR based on EROA and vena contracta width. Since tMR group animals are healthy with preserved ventricular contractility, for the same EROA and vena contracta as the fMR group, higher regurgitant volumes and fractions are seen. This data is graphically depicted in Fig. 6A1–4, with individual data points represented in the graphs.
Fig. 6.
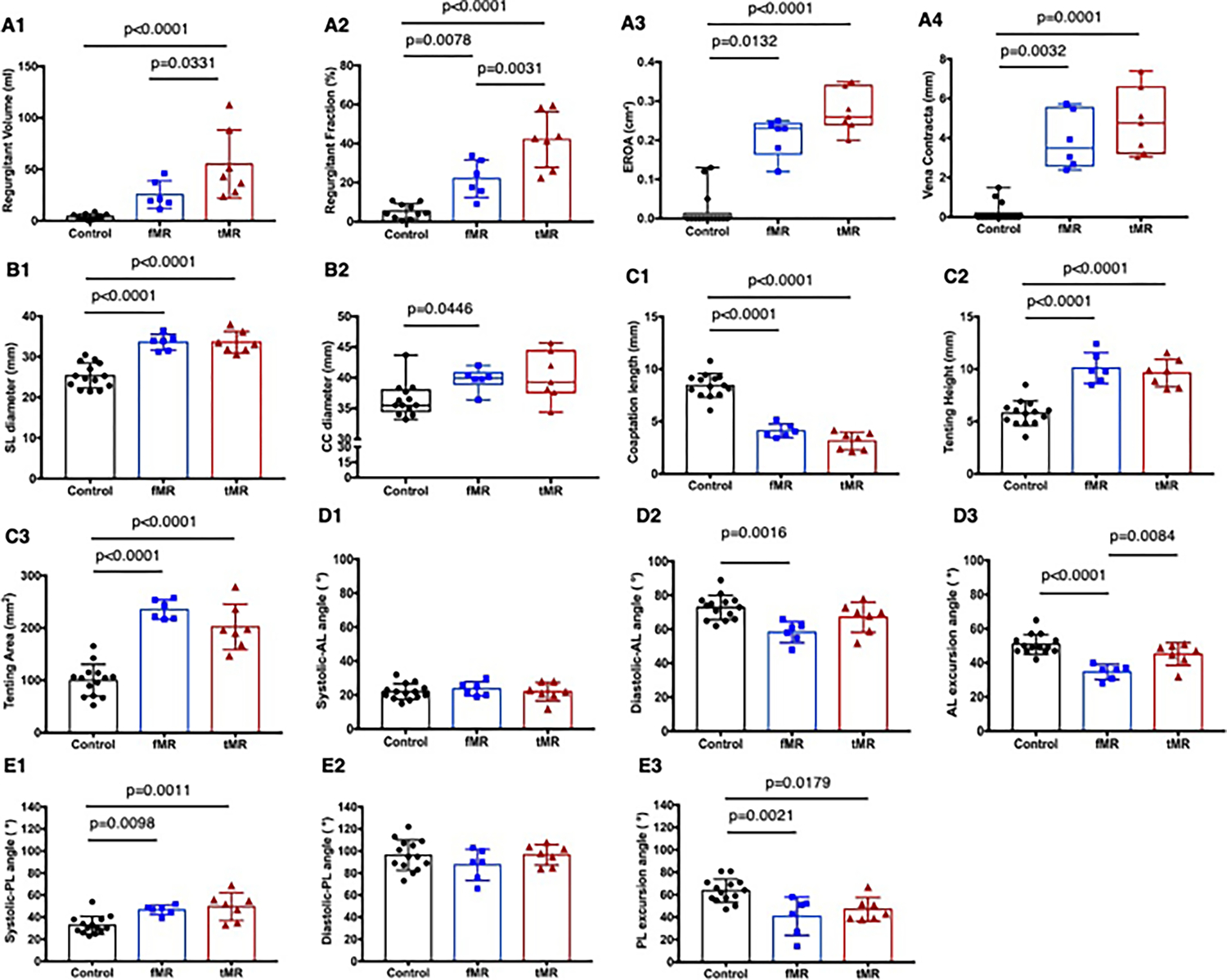
Mitral valve function, geometry, and kinematics in each experimental group. A1–4 Mitral regurgitation severity measured using clinical guidelines for assessment of mitral valve function. B1–2 Septal-lateral and commissure-commissure diameters of the mitral annulus. C1–3 Coaptation length and tenting characteristics of the valve at peak systolic closure. D1–3 Anterior leaflet mobility assessed as systolic anterior angle, diastolic anterior angle, and the excursion angle. E1–3 Posterior leaflet mobility assessed as systolic anterior angle, diastolic anterior angle, and the excursion angle
Mitral Valve Annular Geometry and Systolic Coaptation Indices
Septal-lateral (SL) mitral annular diameter was 23.6 ± 3.1 mm in the control group, 33.6 ± 2.6 mm in the tMR group (p < 0.0001 vs control), and 33.6 ± 2.0 mm in the fMR group (p < 0.0001 vs control). Commissure-commissure diameter was 35.5 mm (IQR 34.5–38.0) in the control group, 39.3 mm (IQR 37.6–44.4) in the tMR group (p = 0.0775), and 39.75 mm (IQR 38.95–40.8) in the fMR group (p = 0.0446). Tenting height in the control group was 5.8 ± 1.2 mm, was higher at 10.1 ± 1.5 mm in the tMR group (p < 0.0001), and 9.6 ± 1.3 mm in the fMR group (p < 0.0001). Similarly, tenting areas in both groups were significantly greater than the control group (fMR: 235.2 ± 18.5 mm2, p < 0.0001; tMR: 202.2 ± 43.6 mm2, p < 0.0001; and control: 100.1 ± 30.5 mm2). In the tMR group, A1, A2, and A3 leaflet lengths were longer than the control (15.8 ± 2.2 mm vs 13.1 ± 1.6, p = 0.0089; 24.8 ± 3.2 mm vs 20.7 ± 2.3, p = 0.0060; and 15.3 ± 1.6 mm vs 13.4 ± 1.8, p = 0.0511, respectively), but in the fMR group, only A3 length was significantly longer than the control group (15.5 ± 1.5 mm vs 13.4 ± 1.8, p = 0.0430). Similarly, A2 and A3 leaflet areas in the tMR group were significantly larger than the control group, but only A3 leaflet area in the fMR group was larger than the control. On the contrary, there were no differences in the P1, P2, and P3 lengths among the groups. However, P3 area in the fMR group was significantly larger than the control. Posterior leaflets in the fMR and the tMR were tethered, and it increased anterior leaflet length and area evenly and decreased posterior length and area evenly in all three segments in the tMR group (symmetric mitral annular dilatation); however, both A3 and P3 length and area increased in the fMR group indicative of deformation of P3 annulus due to inferolateral infarction which can create type IIIb like lesion (asymmetric mitral annular dilatation). Overall, anterior areas in the fMR and tMR were significantly greater compared to the control group, but there were no differences in the posterior area among the groups. Coaptation length was 4.1 ± 0.7 mm in the fMR group and 3.1 ± 0.8 mm in the tMR group. There was no difference between the fMR and the tMR group; however, they were significantly smaller than the control group (8.4 ± 1.1 mm, p < 0.0001). There were no differences in systolic anterior leaflet angles among the groups; however, the diastolic anterior leaflet angle in the fMR group was significantly lower than in the control group (58.3 ± 6.3° vs 72.9 ± 7.3°, p = 0.0016). As a result, anterior leaflet excursion angle in the fMR group was significantly lower compared to both the tMR and the control groups, indicating the restricted mobility of anterior leaflet in the fMR group, but not in the tMR group. Systolic posterior leaflet angles in the fMR and the tMR were significantly higher than the control group (46.7 ± 4.4°, 49.6 ± 12.6°, and 32.7 ± 8.1°, respectively); however, there were no differences in the diastolic posterior leaflet angle among the groups. Therefore, posterior excursion angle in the fMR and the tMR were significantly lower than the control group, indicating the increased tethering of posterior leaflets in both experimental groups in mid-systole. Mitral valve kinematic data are summarized in Fig. 6B1–2, C1–3, D1–3, E1–3, depicting individual data points and the data spread. Table 1 summarizes the group-wise comparisons for the different indices depicting mitral valve geometry and kinematics.
Table 1.
Mitral valve geometry and kinematics in each experimental group
| Control | fMR | tMR | Control vs fMR | Control vs tMR | fMR vs tMR | |
|---|---|---|---|---|---|---|
|
| ||||||
| Mitral annular and leaflet geometry and areas | ||||||
| SL diameter (mm) | 25.3 ± 3.1 | 33.6 ± 2.0 | 33.6 ± 2.6 | p < 0.0001 | p < 0.0001 | p > 0.9999 |
| CC diameter (mm) | 35.5 (34.5–38.0) | 40.0 (39.0–40.8) | 39.3 (37.6–44.4) | p = 0.0446 | p = 0.0775 | p > 0.9999 |
| A1 length (mm) | 13.1 ± 1.6 | 14.8 ± 1.3 | 15.8 ± 2.2 | p = 0.1409 | p = 0.0089 | p = 0.5783 |
| A2 length (mm) | 20.7 ± 2.3 | 22.7 ± 2.4 | 24.8 ± 3.2 | p = 0.2687 | p = 0.0060 | p = 0.3136 |
| A3 length (mm) | 13.4 ± 1.8 | 15.5 ± 1.5 | 15.3 ± 1.6 | p = 0.0430 | p = 0.0551 | p = 0.9752 |
| A1 area (mm2) | 123.9 ± 27.6 | 161.4 ± 26.7 | 163.3 ± 47.2 | p = 0.0831 | p = 0.0510 | p = 0.9942 |
| A2 area (mm2) | 240.2 ± 51.7 | 267.4 ± 49.5 | 337.6 ± 86.3 | p = 0.6548 | p = 0.0077 | p = 0.1278 |
| A3 area (mm2) | 136.1 ± 22.5 | 167.4 ± 26.5 | 167.3 ± 21.6 | p = 0.0302 | p = 0.0226 | p > 0.9999 |
| P1 length (mm) | 14.4 ± 2.3 | 16.8 ± 1.6 | 14.5 ± 2.3 | p = 0.0931 | p = 0.9961 | p = 0.1679 |
| P2 length (mm) | 13.1 ± 1.6 | 14.8 ± 1.3 | 15.8 ± 2.2 | p = 0.1409 | p = 0.0089 | p = 0.5783 |
| P3 length (mm) | 14.4 ± 1.6 | 13.9 ± 1.3 | 13.4 ± 1.3 | p = 0.8016 | p = 0.3803 | p = 0.8336 |
| P1 area (mm2) | 191.1 ± 30.3 | 193.3 ± 24.6 | 190.2 ± 36.9 | p = 0.9889 | p = 0.9979 | p = 0.9826 |
| P2 area (mm2) | 184.8 ± 28.4 | 205.3 ± 42.0 | 204.8 ± 26.1 | p = 0.3951 | p = 0.3769 | p = 0.9995 |
| P3 area (mm2) | 198.4 ± 44.6 | 260.1 ± 36.0 | 228.2 ± 72.8 | p = 0.0459 | p > 0.9999 | p = 0.2797 |
| AL area (mm2) | 500.1 ± 85.0 | 596.1 ± 39.3 | 668.1 ± 122.8 | p = 0.0981 | p = 0.0016 | p = 0.3361 |
| PL area (mm2) | 574.3 ± 84.2 | 658.7 ± 57.6 | 623.2 ± 110.8 | p = 0.1455 | p = 0.4677 | p = 0.7487 |
| Mitral valve systolic geometric indices | ||||||
| Coaptation length (mm) | 8.4 ± 1.1 | 4.1 ± 0.7 | 3.1 ± 0.8 | p < 0.0001 | p < 0.0001 | p = 0.2089 |
| Tenting height (mm) | 5.8 ± 1.2 | 10.1 ± 1.5 | 9.6 ± 1.3 | p < 0.0001 | p < 0.0001 | p = 0.7837 |
| Tenting area (mm2) | 100.1 ± 30.5 | 235.2 ± 18.5 | 202.2 ± 43.6 | p < 0.0001 | p < 0.0001 | p = 0.1815 |
| Mitral valve kinematics | ||||||
| Systolic-AL angle (°) | 22.0 ± 4.6 | 23.7 ± 4.1 | 22.0 ± 5.4 | p = 0.7531 | p > 0.9999 | p = 0.8035 |
| Diastolic-AL angle (°) | 72.9 ± 7.3 | 58.3 ± 6.3 | 67.1 ± 8.8 | p = 0.0016 | p = 0.2455 | p = 0.1080 |
| AL excursion angle (°) | 50.9 ± 5.8 | 34.7 ± 4.4 | 45.1 ± 6.6 | p < 0.0001 | p = 0.1000 | p = 0.0084 |
| Systolic-PL angle (°) | 32.7 ± 8.1 | 46.7 ± 4.4 | 49.6 ± 12.6 | p = 0.0098 | p = 0.0011 | p = 0.8276 |
| Diastolic-PL angle (°) | 96.3 ± 13.9 | 87.5 ± 14.2 | 96.6 ± 9.2 | p = 0.3603 | p = 0.9987 | p = 0.4300 |
| PL excursion angle (°) | 63.6 ± 10.4 | 40.8 ± 17.0 | 47.0 ± 10.5 | p = 0.0021 | p = 0.0179 | p = 0.6345 |
SL septal-lateral, CC commissure-commissure, A anterior, P posterior, AL anterior leaflet, PL posterior leaflet
LV Geometry and Function
EDV in the fMR group was significantly greater than the control group (140.8 ± 28.8 ml vs 102.1 ± 29.7 ml, p = 0.0443). EDV in the tMR group was also larger than the control, but there was no significance (136.6 ± 35.3 ml vs 102.1 ± 29.7 ml, p = 0.0613). ESV in the fMR group was the largest (89.9 ± 23.2 ml vs control: 44.9 ± 15.9 ml, p = 0.0002, vs tMR: 63.5 ± 21.4 ml, p = 0.0511), indicating tMR group developed dilated LV due to surgically induced acute volume overload; however, still able to maintain cardiac function since there was no infarction. EF in the fMR was 36.5 ± 4.9% and it was 34.6% lower compared to the control group (55.8 ± 6.5%, p < 0.0001), whereas EF in the tMR was maintained (53.1 ± 9.0%, p = 0.6918) (Fig. 7). Group-wise comparisons with p values are depicted in Table 2.
Fig. 7.
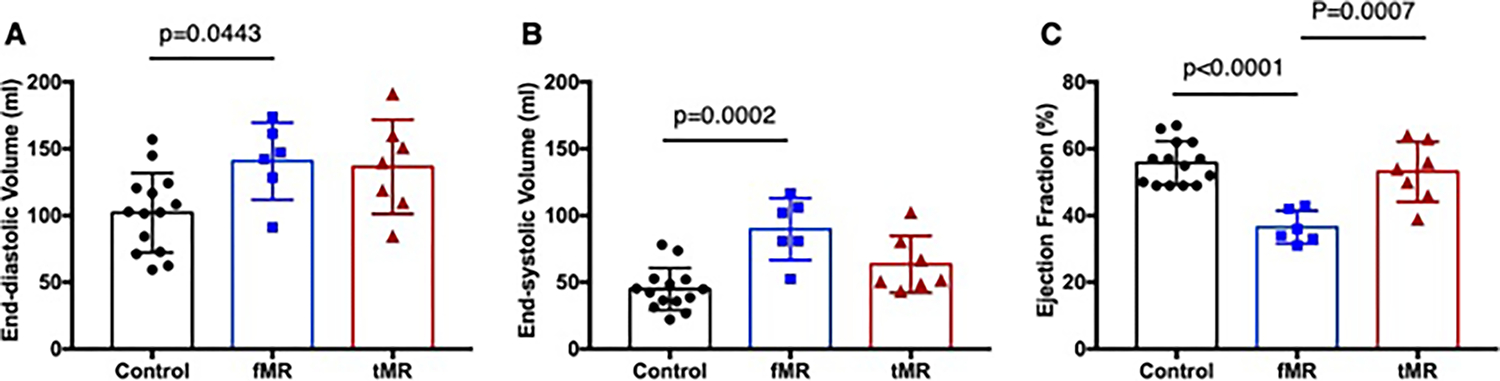
Left ventricular volumes and function in the three experimental groups—A end-diastolic volume, B end-systolic volume, and C ejection fraction
Table 2.
Left ventricular geometry and function
| EDV (ml) | ESV (ml) | EF (%) | |
|---|---|---|---|
|
| |||
| Control | 102.1 ± 29.7 | 44.9 ± 15.9 | 55.8 ± 6.5 |
| fMR | 140.8 ± 28.8 | 89.9 ± 23.2 | 36.5 ± 4.9 |
| tMR | 136.6 ± 35.3 | 63.5 ± 21.4 | 53.1 ± 9.0 |
| p value | |||
| Control vs fMR | p = 0.0443 | p = 0.0002 | p < 0.0001 |
| Control vs tMR | p = 0.0613 | p = 0.1104 | p = 0.6918 |
| fMR vs tMR | p = 0.9678 | p = 0.0511 | p = 0.0007 |
EDV end-diastolic volume, ESV end-systolic volume, EF ejection fraction
Discussion
We successfully induced SMR in swine by tethering the mitral valve using transmyocardial, image-guided chordal encircling tethers. Regurgitant severity was significantly higher in this acute tether model, than regurgitation developing secondary to a chronic myocardial infarction (regurgitant fraction: 42.1 ± 14.2% vs 22 ± 9.6%), with regurgitant severity characterized per clinically used imaging techniques [28]. In the tMR group, regurgitant volume was 55.0 ± 33.2 ml and regurgitant fraction was 42.1 ± 14.2%, which can be characterized using clinical scales as grade III (moderate to severe) regurgitation. EROA was 0.27 ± 0.05 cm2 and vena contracta width in the tMR group was 4.82 ± 1.68 mm. Considering the range of the weight and the heart size, we believe that using regurgitant fraction could be the most reliable parameter in a large animal model, especially if juvenile large animals are used for the experiment. In this model, the extent of mitral valve tenting in systole was also similar to humans. Based on this data, the tMR model corresponds well with clinical patients. Jaworek et al., in an ex vivo model, used a papillary muscle displacing approach with tethers to induce leaflet tenting [29]. Though they did not encircle the chordae as we report, tethering the papillary muscles led to their displacement and tenting of the valve.
Technically, the chordal encircling snares can be implanted reproducibly in the beating heart, via the left thoracotomy approach. For reproducibility, some technical aspects need to be considered. Firstly, implanting the chordae closer to the papillary muscle tip is useful, as the chordae in this region are still larger struts that have not yet diverged into multiple smaller chordae that insert into the leaflets. Thus, capturing the chordae at this level is easier and reproducible. Secondly, the largest needle should be used such that encircling the chordae is achieved with one attempt, and can help in avoiding the risk of bleeding through the needle holes. To maximize use of the entire curvature of the needle, we recommend inserting the needle tip vertically through the myocardium until half of the needle is advanced into the heart and then curving the needle around the chordae to ensnare them. This should be performed in smaller steps, rather than taking the entire needle bite in one attempt, which can often be challenging. If at first attempt, the chordae are not ensnared, i.e., lacking tethering and regurgitation, then the needle should be retracted and the procedure repeated through the same needle hole. Since most large needles are relatively thick, the needle entry hole can cause bleeding. Finally, making bridges of adequate offset from the epicardial surface is necessary to avoid introducing slack into the snare. In our study, we would pass the free ends of the two sutures through the bridge, upon ensnaring the chordae. The surgeon would then hold the two ends of the sutures and pull them away from the heart, while using the other hand to push the bridge towards the heart. When optimally tensioned, the first assist would place vascular clips on the suture at the bridge, so that the tension remains once the surgeon leaves the free ends of the suture. With these clips in place, the free ends are then knotted onto the bridge tightly. These technical details help reproduce the model consistently between animals.
The chordal ensnaring and tethering approach provides several advantages. Firstly, by imaging the chordae tendineae and encircling them based on the anatomy, the inter-animal variability and its impact on regurgitation are reduced. In preclinical laboratories using the myocardial infarction models to cause regurgitation, anatomical variability between animals has been a challenge, yielding heterogenous outcomes after a myocardial infarction. Secondly, by titrating the extent of tethering with the snares, the severity and location of the regurgitation along the mitral valve coaptation can be controlled. As shown in Fig. 5, when both snares were tethered equally, a wide central regurgitation jet was observed, which mimics a patient with regurgitation secondary to concentric, dilated cardiomyopathy or severe left ventricular dilatation. When the posterior snare was tethered more than the anterior snare, the regurgitant jet moved towards the posterior commissure, creating a type IIIb-like valve lesion that develops secondary to an inferior myocardial infarction. Such control over the severity and location of regurgitation is not possible in the traditional post-infarction models of that this model enables.
A significant methodological advancement in this study is that for the first time, regurgitant severity has been evaluated in animals using techniques being used in clinical trials. This is an important step forward for reproducibility in structural heart preclinical research. Most previous studies have used the ratio of the regurgitant jet area to the left atrial area as a semi-quantitative index [22]. A challenge with this approach is that accurately mapping the regurgitant jet in two dimensions is not reproducible, unless it is a central jet. Secondly, in response to regurgitation, the left atrium remodels and enlarges in a non-linear manner, despite the same regurgitant volume. This index is thus prone to errors. Another index in use is to measure the regurgitant flow velocities; however, since flow rate/volume is dependent not only on velocity, but also on the regurgitant gap area, this approach is prone to error as well [30]. Others have used a grading scale to describe regurgitant severity [31], which is highly subjective and does not lend itself to reproducibility. In this study, we assessed regurgitation with several parameters based on the guidelines from American Association of Echocardiography such as regurgitant volume, regurgitant fraction calculated by stroke volume methods, PISA radius, and vena contracta width [28].
The acute nature of this model provides several procedural and cost benefits. The tMR model has severe regurgitation, but a healthy LV, which reduces the risk of intraprocedural arrhythmias or cardiac arrest that is common when performing invasive procedures in chronic HF swine. The reproducibility of regurgitation in this model also helps quantitatively assess the extent of reduction that a new repair or replacement technology is able to provide, providing valuable information in assessing its efficacy. The cost savings with the tMR model are also significant, as regurgitation can be induced and corrected in an acute setting, and animals can be survived thereafter if chronic valve function is to be assessed. On the contrary, in the traditional fMR models, a 2–3-month wait period after the myocardial infarction is necessary so that the LV dilates adequately to tether the mitral valve and cause SMR. The attrition rate in swine during this post-infarction period is non-trivial and can add to project costs.
The tMR model is appropriate to study new transcatheter mitral repair and replacement devices. As the mechanism of regurgitation in this model is from pure leaflet tethering, it is applicable to study leaflet and chordal approaches, but not as much annular or ventricular approaches to repair mitral regurgitation. Since the field of transcatheter mitral valve repair for this lesion has largely percolated around leaflet approaches (MitraClip, PASCAL, cardiac leaflet enhancer (CARLEN), and half-moon medical), the model is very timely and useful. Transcatheter mitral valve replacement devices that anchor to the mitral leaflets can also be studied in this model, especially since the valve kinematics are restricted alike a human patient. This clinically relevant anatomy and interface with the replacement valve can be useful to study intra-leaflet thrombosis that was observed clinically. The traditional fMR models still have relevance, especially to investigate annular and ventricular approaches, and when the effect of the repair strategy on left ventricular reverse remodeling needs to be investigated.
Limitations
Despite these benefits, there are some limitations to this model. Firstly, the acute nature of SMR is a result of mitral valve geometric perturbations and not ventricular perturbations. Thus, this model is useful to study the efficacy of devices in correcting SMR, but is not appropriate to study its long-term impact on cardiac function or recovery of HF. However, most new valve technologies are assessed for their impact on cardiac function and recovery, in human trials and not in preclinical models. Secondly, due to the acute nature of the SMR, biological remodeling of the mitral valve has also not occurred, and thus, the tissue is alike healthy animals. This limitation may be overcome by allowing the animals in tMR some time to undergo tissue remodeling, before their use to study devices.
Conclusion
Chordae encircling snare to tether the mitral valve and induce mitral regurgitation is a reproducible animal model that mimics clinically significant severity of the valve lesion and the tethered mitral valve geometry seen in patients.
Supplementary Material
Acknowledgements
The authors acknowledge the veterinary staff that provided anesthesia support during the procedures.
Funding
This work was funded by grants from the National Institutes of Health HL135145, HL140325, and HL133667, and infrastructure support from the Carlyle Fraser Heart Center at Emory University Hospital Midtown to M. Padala.
Abbreviations
- SMR
Secondary mitral regurgitation
- HF
Heart failure
- MV
Mitral valve
- LV
Left atrium
- PMPM
Posteromedial papillary muscle
- ALPM
Anterolateral papillary muscle
- EDV
End-diastolic volume
- ESV
End-systolic volume
- EF
Ejection fraction
Footnotes
Competing Interests M. Padala received consulting fees from Heart Repair Technologies, Inc., and Boston Scientific, which did not have any role in this work, nor did they sponsor and review this submission. M. Padala is a founder of Nyra Medical Inc. and reports significant stock ownership in it, which did not have any role in this study. K. Suresh reports minority stock ownership in Nyra Medical. The other authors declare no competing interests.
Ethics Approval Human studies were not carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s12265-021-10177-x.
References
- 1.Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ, & Investigators C (2018). Transcatheter mitral-valve repair in patients with heart failure. New England Journal of Medicine, 379, 2307–2318. [DOI] [PubMed] [Google Scholar]
- 2.Espiritu D, Onohara D, Kalra K, Sarin EL, & Padala M (2017). Transcatheter mitral valve repair therapies: Evolution, status and challenges. Annals of Biomedical Engineering, 45, 332–359. [DOI] [PubMed] [Google Scholar]
- 3.Shim H, Harloff M, Percy E, Hirji S, Shah PB, & Kaneko T (2020). Prediction for residual regurgitation after MitraClip for functional mitral regurgitation using leaflet coaptation index. Journal of Cardiac Surgery, 35, 3555–3559. [DOI] [PubMed] [Google Scholar]
- 4.Reichart D, Kalbacher D, Rubsamen N, Tigges E, Thomas C, Schirmer J, Reichenspurner H, Blankenberg S, Conradi L, Schafer U, & Lubos E (2020). The impact of residual mitral regurgitation after MitraClip therapy in functional mitral regurgitation. European Journal of Heart Failure, 22, 1840–1848. [DOI] [PubMed] [Google Scholar]
- 5.Padala M, Sweet M, Hooson S, Thourani VH, & Yoganathan AP (2014). Hemodynamic comparison of mitral valve repair: Techniques for a flail anterior leaflet. Journal of Heart Valve Disease, 23, 171–176. [PubMed] [Google Scholar]
- 6.Padala M, Gyoneva LI, Thourani VH, & Yoganathan AP (2014). Impact of mitral valve geometry on hemodynamic efficacy of surgical repair in secondary mitral regurgitation. Journal of Heart Valve Disease, 23, 79–87. [PubMed] [Google Scholar]
- 7.Padala M, Cardinau B, Gyoneva LI, Thourani VH, & Yoganathan AP (2013). Comparison of artificial neochordae and native chordal transfer in the repair of a flail posterior mitral leaflet: An experimental study. Annals of Thoracic Surgery, 95, 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padala M, Gyoneva L, & Yoganathan AP (2012). Effect of anterior strut chordal transection on the force distribution on the marginal chordae of the mitral valve. J Thorac Cardiovasc Surg, 144, 624–633.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padala M, Sacks MS, Liou SW, Balachandran K, He Z, & Yoganathan AP (2010). Mechanics of the mitral valve strut chordae insertion region. J Biomech Eng, 132, 081004. [DOI] [PubMed] [Google Scholar]
- 10.Padala M, Powell SN, Croft LR, Thourani VH, Yoganathan AP, & Adams DH (2009). Mitral valve hemodynamics after repair of acute posterior leaflet prolapse: Quadrangular resection versus triangular resection versus neochordoplasty. Journal of Thoracic and Cardiovascular Surgery, 138, 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erek E, Padala M, Pekkan K, Jimenez J, Yalcinba YK, Salihoglu E, Sarioglu T, & Yoganathan AP (2009). Mitral web—A new concept for mitral valve repair: Improved engineering design and in-vitro studies. Journal of Heart Valve Disease, 18, 300–306. [PubMed] [Google Scholar]
- 12.Padala M, Vasilyev NV, Owen JW Jr., Jimenez JH, Dasi LP, del Nido PJ, & Yoganathan AP (2008). Cleft closure and undersizing annuloplasty improve mitral repair in atrioventricular canal defects. Journal of Thoracic and Cardiovascular Surgery, 136, 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imbrie-Moore AM, Paullin CC, Paulsen MJ, Grady F, Wang H, Hironaka CE, Farry JM, Lucian HJ, & Woo YJ (2020). A novel 3D-printed preferential posterior mitral annular dilation device delineates regurgitation onset threshold in an ex vivo heart simulator. Medical Engineering & Physics, 77, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imbrie-Moore AM, Paulsen MJ, Thakore AD, Wang H, Hironaka CE, Lucian HJ, Farry JM, Edwards BB, Bae JH, Cutkosky MR, & Woo YJ (2019). Ex vivo biomechanical study of apical versus papillary neochord anchoring for mitral regurgitation. Annals of Thoracic Surgery, 108, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukamachi K, Inoue M, Doi K, Schenk S, Nemeh H, Faber C, Navia JL, & McCarthy PM (2005). Reduction of mitral regurgitation using the Coapsys device: A novel ex vivo method using excised recipients’ hearts. ASAIO J, 51, 82–4. [DOI] [PubMed] [Google Scholar]
- 16.Croft LR, Jimenez JH, Gorman RC, Gorman JH 3rd., & Yoganathan AP (2007). Efficacy of the edge-to-edge repair in the setting of a dilated ventricle: An in vitro study. Annals of Thoracic Surgery, 84, 1578–1584. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya S, Pham T, He Z, & Sun W (2014). Tension to passively cinch the mitral annulus through coronary sinus access: An ex vivo study in ovine model. Journal of Biomechanics, 47, 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agra EJ, Suresh KS, He Q, Onohara D, Guyton RA, & Padala M (2020). Left ventricular thinning and distension in pig hearts as a reproducible ex vivo model of functional mitral regurgitation. ASAIO Journal, 66, 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaworek M, Mangini A, Maroncelli E, Lucherini F, Rosa R, Salurso E, Votta E, Antona C, Fiore GB, & Vismara R (2021). Ex vivo model of functional mitral regurgitation using deer hearts. Journal of Cardiovascular Translational Research, 14, 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasrija C, Quinn RW, Alkhatib H, Tran D, Bernstein D, Rice M, Kotloff E, Morales D, D’Ambra MN, Vesely MR, & Gammie JS (2021). Development of a reproducible swine model of chronic ischemic mitral regurgitation: Lessons learned. Annals of Thoracic Surgery, 111, 117–125. [DOI] [PubMed] [Google Scholar]
- 21.Shi W, McIver BV, Kalra K, Sarin EL, Schmarkey S, Duggan M, Thourani VH, Guyton RA, & Padala M (2017). A swine model of percutaneous intracoronary ethanol induced acute myocardial infarction and ischemic mitral regurgitation. Journal of Cardiovascular Translational Research, 10, 391–400. [DOI] [PubMed] [Google Scholar]
- 22.Llaneras MR, Nance ML, Streicher JT, Lima JA, Savino JS, Bogen DK, Deac RF, Ratcliffe MB, & Edmunds LH Jr. (1994). Large animal model of ischemic mitral regurgitation. Annals of Thoracic Surgery, 57, 432–439. [DOI] [PubMed] [Google Scholar]
- 23.Hamza O, Kiss A, Kramer AM, Tillmann KE, & Podesser BK (2020). A novel percutaneous closed chest swine model of ischaemic mitral regurgitation guided by contrast echocardiography. EuroIntervention, 16, e518–e522. [DOI] [PubMed] [Google Scholar]
- 24.Timek TA, Dagum P, Lai DT, Liang D, Daughters GT, Ingels NB Jr., & Miller DC (2001). Pathogenesis of mitral regurgitation in tachycardia-induced cardiomyopathy. Circulation, 104, I47–I53. [DOI] [PubMed] [Google Scholar]
- 25.Levine RA, Hagege AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia-Naji N, Bruneval P, Butcher JT, Carpentier A, Chaput M, Chester AH, Clusel C, Delling FN, Dietz HC, Dina C, Durst R, … Leducq Mitral Transatlantic N (2015). Yacoub MH Mitral valve disease—Morphology and mechanisms. Nat Rev Cardiol, 12, 689–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarin EL, Shi W, Duara R, Melone TA, Kalra K, Strong A, Girish A, McIver BV, Thourani VH, Guyton RA, & Padala M (2016). Swine (Sus scrofa) as a model of postinfarction mitral regurgitation and techniques to accommodate its effects during surgical repair. Comparative Medicine, 66, 290–299. [PMC free article] [PubMed] [Google Scholar]
- 27.Crick SJ, Sheppard MN, Ho SY, Gebstein L, & Anderson RH (1998). Anatomy of the pig heart: Comparisons with normal human cardiac structure. Journal of Anatomy, 193(Pt 1), 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, & Weissman NJ (2017). Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. Journal of the American Society of Echocardiography, 30, 303–371. [DOI] [PubMed] [Google Scholar]
- 29.Jaworek M, Lucherini F, Romagnoni C, Gelpi G, Contino M, Romitelli P, Antona C, Fiore GB, & Vismara R (2017). Modelling of lesions associated with functional mitral regurgitation in an ex vivo platform. Annals of Biomedical Engineering, 45, 2324–2334. [DOI] [PubMed] [Google Scholar]
- 30.Cui YC, Li K, Tian Y, Yuan WM, Peng P, Yang JZ, Zhang BJ, Zhang HD, Wu AL, & Tang Y (2014). A pig model of ischemic mitral regurgitation induced by mitral chordae tendinae rupture and implantation of an ameroid constrictor. PLoS One, 9, 111689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzaki K, Morita M, Hamamoto H, Noma M, Robb JD, Gillespie MJ, Gorman JH 3rd., & Gorman RC (2010). Elimination of ischemic mitral regurgitation does not alter long-term left ventricular remodeling in the ovine model. Annals of Thoracic Surgery, 90, 788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


