Abstract
Leishmania major and Leishmania braziliensis both cause cutaneous leishmaniasis, but the former kills BALB/c mice while the latter is killed by the mice. This killing of L. braziliensis occurred by a gamma interferon-dependent mechanism, potentially made possible by the observed lack of high interleukin-4 production.
The most widely studied model for cutaneous leishmaniasis is infection of mice with Leishmania major, wherein it has been observed that certain mice (e.g., C3H) develop a parasite-specific Th1 response (high levels of gamma interferon [IFN-γ] and low levels of interleukin-4 [IL-4]), which often is associated with cure, while other mice (BALB/c) develop a Th2 response (low levels of IFN-γ and high levels of IL-4), which leads to disease progression (reviewed in references 4, 8, 13, 14, and 21). In contrast, although Leishmania braziliensis induces a disease that is a serious health problem in South America, relatively little experimental work has been done to characterize the immune response to this parasite, probably because the parasite is weakly infectious for mice (6, 12). To examine why L. braziliensis is weakly infectious, we infected the same mouse strain (BALB/c) with either L. major (which kills BALB/c mice) or L. braziliensis (which BALB/c mice kill) and compared the development of cutaneous lesions and the immune responses to the two species of parasites.
The course of cutaneous lesion development following infection with L. major or L. braziliensis in BALB/c mice.
BALB/cBy mice (National Cancer Institute, Frederick, Md.) were injected with either 106 L. major (R/SU/59/Neal P) (22) or 107 L. braziliensis (MAN/BR/LTB-111) (10) organisms in a hind footpad. Because L. braziliensis is weakly infectious for mice, a dose of 107 parasites was required to obtain consistent results in the assay systems used. On the other hand, because L. major is quite infectious for BALB/c mice, a lower dose of L. major (106 organisms) was used; a dose of 107 L. major organisms would have overwhelmed the mice so rapidly that there would not have been sufficient time to compare the courses of infection with the two species of parasites. Using this approach, we found that the majority of both species were killed within the first 3 days of infection. At this time the numbers of parasites remaining in the developing footpad lesions were similar for the two species (parasites were enumerated by limiting-dilution analysis; for techniques, see reference 11): 1.4 × 104 L. major organisms (95% confidence limits, 0.2 × 104 to 2.6 × 104) and 5.6 × 104 L. braziliensis organisms (confidence limits, 0.4 × 104 to 10.7 × 104). Such massive destruction of L. major within the first few days of infection has been reported before (22). Beyond day 3 postinfection, L. major replicated to achieve a 712-fold expansion by day 42 postinfection. In contrast, L. braziliensis doubled its numbers by day 7 postinfection and thereafter was gradually destroyed, so that beyond day 42 postinfection the parasite could not be detected. Cutaneous lesion development directly correlated with lesion (footpad) parasite burden. L. major induced a rapid increase in footpad size, such that by day 42 postinfection, footpad thickness had tripled and lesions had become ulcerated and necrotic (at which point the animals were sacrificed). L. braziliensis induced modest (never more than a 50% increase in footpad thickness) lesions that were nodular and never ulcerated.
Production of Th1- and Th2-associated cytokines by BALB/c mice infected with L. major or L. braziliensis.
Cytokines play counteracting roles in the control (e.g., IFN-γ) or exacerbation (e.g., IL-4) of L. major infection. Therefore, we determined the cytokines produced by lymph node cells taken from BALB/c mice infected with either L. major or L. braziliensis. Three to five mice from each group were killed for evaluation at various times postinfection. Single-cell suspensions were prepared from the lymph nodes draining the lesion, and the cells were placed into culture as described elsewhere (20). To assess IL-4, IL-10, and IFN-γ production, culture supernatants were harvested 72 h later (a time when peak production of the cytokines was achieved) and analyzed for the presence of the cytokines by using enzyme-linked immunosorbent assays (ELISA) described elsewhere (5, 20). For tumor necrosis factor alpha (TNF-α) production, culture supernatants were harvested 6, 24, 48, and 72 h later and analyzed for TNF by ELISA (capture and detection antibodies and recombinant TNF standard were obtained from PharMingen [San Diego, Calif.]).
Because IFN-γ plays a protective role in leishmaniasis and because BALB/c mice cure an infection with L. braziliensis, we anticipated that these mice would produce more IFN-γ than BALB/c mice infected with L. major. BALB/c mice were infected with L. braziliensis or L. major, and at varying times after infection (day 3, 7, 21, or 42), the lymph node cells draining the lesion were assessed for IFN-γ production. The levels of IFN-γ produced were not different. L. braziliensis elicited 27.8 ± 13.5 ng of IFN-γ/ml of culture supernatant, while L. major elicited 17.7 ± 7.3 (the numbers were obtained by averaging the amounts of IFN-γ produced at the four time points ± standard deviation [SD]). Using the same approach, we also found that C3H mice infected with L. braziliensis produced an average of 47.9 ± 32.2 ng of IFN-γ/ml of culture supernatant. Analysis of IL-10 production yielded results similar to those for IFN-γ; L. braziliensis elicited 2.1 ± 1.5 ng of IL-10/ml of culture supernatant, and L. major elicited 2.9 ± 1.8 ng/ml. Finally, TNF-α production in response to infection with either species of parasite was not detected. This inability to detect TNF-α production was not due to technical failure, since C3H mice produced substantial levels of TNF-α following infection with L. braziliensis (e.g., 257.7 pg/ml of culture supernatant at day 3 postinfection).
IL-4 plays a central role in the susceptibility of BALB/c mice to infection with L. major. Therefore, since BALB/c mice heal an infection with L. braziliensis, we predicted that less IL-4 would be produced by these mice. Significantly (by analysis by nonpaired t test; a P value of <0.05 was considered significant) less IL-4 (10- to 15-fold) was produced in response to infection with L. braziliensis than in response to infection with L. major, and by day 42 postinfection, L. braziliensis-infected mice did not produce detectable levels of IL-4 (Table 1). It should also be noted that when C3H mice are infected with L. braziliensis, the mice develop barely perceptible cutaneous lesions and never make a detectable IL-4 response (data not shown).
TABLE 1.
Production of IL-4 by BALB/c mice infected with L. major or L. braziliensis
| Day postinfection | Response (pg of IL-4/ml of culture supernatant ± SD)a to:
|
|
|---|---|---|
| L. major | L. braziliensis | |
| 3 | ND | ND |
| 7 | 751.6 ± 250.6* | 53.9 ± 15.3 |
| 21 | 326.6 ± 27.9* | 38.8 ± 4.3 |
| 42 | 484.1 ± 22.6* | ND |
Average ± SD of cytokine levels measured in three independent experiments. ∗, statistically significant difference (P < 0.05) by nonpaired t test. ND, none detected.
The course of cutaneous lesion development following infection with L. braziliensis in BALB/c mice treated with either anti-IFN-γ or anti-IL-4 antibody.
Because IL-4 can inhibit the protective effects of IFN-γ in mice infected with L. major (7, 9), and because L. braziliensis-infected BALB/c mice produced significantly less IL-4 than L. major-infected BALB/c mice (Table 1), we hypothesized that the amount of IFN-γ produced by L. braziliensis-infected mice might be sufficient to control infection. Therefore, we treated L. braziliensis-infected mice with a neutralizing anti-IFN-γ.
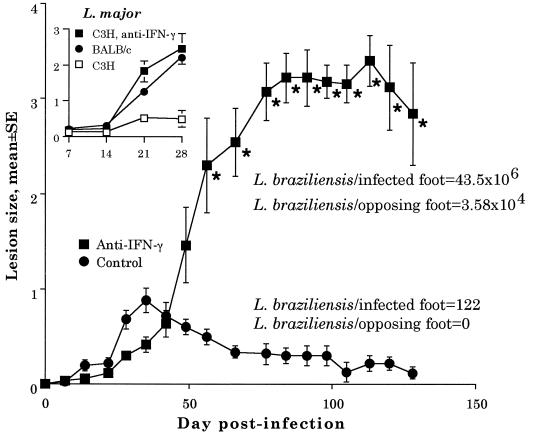
First, we tested the potency of our neutralizing anti-IFN-γ preparation (anti-IFN-γ was purified from the ascites fluid of the anti-IFN-γ-producing hybridoma XMG1.2 [a gift from R. Coffman, DNAX, Palo Alto, Calif.] by salt precipitation [17, 18;]) by determining whether it would prevent C3H/HeJ mice (National Cancer Institute) from healing an infection with L. major as reported by others (3). Our anti-IFN-γ preparation converted C3H mice into animals completely susceptible to infection with L. major (see Fig. 1, inset). Since C3H mice produce considerably more IFN-γ than BALB/c mice following infection with L. major (16), and since BALB/c mice produce equivalent amounts of the cytokine following infection with either L. braziliensis or L. major, our preparation of anti-IFN-γ would be more than sufficient to neutralize IFN-γ in BALB/c mice infected with L. braziliensis.
FIG. 1.
Course of cutaneous lesion development following infection with L. braziliensis in BALB/c mice treated with anti-IFN-γ antibody. Each value represents the mean (± standard error of the mean) lesion size of five animals per group. The results are representative of two independent experiments. Control animals were treated with an isotype-matched antibody (1.0 mg of anti-β-galactosidase [GL113; DNAX]/week) prepared in a fashion identical to that for the anti-IFN-γ antibody. The asterisk indicates that from day 56 of infection and beyond, there was a significant difference (P < 0.05) in lesion size between the groups as determined by analysis of variance followed by Student-Newman-Keuls posthoc t tests. Numbers of parasites per foot in control and IFN-γ-treated animals are given for day 144. (Inset) BALB/c and C3H mice were infected with 106 L. major organisms and left untreated or treated with anti-IFN-γ, as indicated.
Treating L. braziliensis-infected BALB/c mice with anti-IFN-γ (by intraperitoneal injections of 1.0 mg of XMG1.2 on the day of infection and at weekly intervals thereafter until the completion of the experiment) significantly enhanced lesion size and prevented the mice from resolving their infection (Fig. 1). In addition, anti-IFN-γ treatment caused mice to produce more IL-4 in response to infection with L. braziliensis. For instance, at 2 weeks following infection, lymph node cells draining the lesion in treated mice produced 58.5 ± 1.6 pg of IL-4/ml (mean ± SD) when the cells were restimulated in culture with L. braziliensis. In contrast, untreated control mice produced 10.8 ± 1.6 pg of IL-4/ml, or 5.4-fold less IL-4 (in cultures not restimulated with L. braziliensis, no IL-4 was detected). Finally, by day 144 of infection, anti-IFN-γ treatment had markedly enhanced parasite burden in the lesions (122 parasites/lesion in control mice versus 43.5 × 106 in treated mice, which is a 356,557-fold difference), which resulted in systemic infection with L. braziliensis, as evidenced by the fact that large numbers of the parasite (3.58 × 104) could be detected in the opposing (uninfected) footpad (Fig. 1). It is possible that parasites could be isolated from the opposing footpad because the L. braziliensis strain used (LTB-111) was originally isolated from a cutaneous lesion. Therefore, the parasite may prefer the lower temperature of cutaneous sites. Taken together, these data suggest that an IFN-γ-dependent mechanism is responsible for the killing of L. braziliensis by BALB/c mice.
IL-4 exacerbates disease in L. major-infected BALB/c mice (15). This predicts that neutralizing IL-4 in mice infected with L. braziliensis would lessen disease severity. Lesions on anti-IL-4-treated mice (for techniques, see reference 10) infected with L. braziliensis resolved in half the time and never were greater than 20% of the size of lesions on control mice (data not shown).
These data suggest that the weak infectivity of L. braziliensis for mice may be due to the inability of the parasite to elicit strong and sustained IL-4 production in the animals. Alternatively, it is possible that L. braziliensis is unable to elicit the production of other cytokines that inhibit the development of a Th1 response. IL-10 inhibits Th1 development; however, BALB/c mice produced equivalent amounts of IL-10 following infection with either L. braziliensis or L. major (see above). Transforming growth factor β (TGF-β) also inhibits Th1 responses. TGF-β correlates with susceptibility to infection with both L. braziliensis (2; reviewed in reference 13) and L. major (19). However, we have been unable to detect TGF-β (protein or mRNA, in vitro or in vivo) following infection with L. braziliensis. Since different isolates of L. braziliensis vary in their ability to induce TGF-β production (1), it is possible that LTB-111 is a poor inducer of TGF-β.
In conclusion, the data presented here extend the Th1 (protective)/Th2 (exacerbative) paradigm in leishmaniasis established by injection of different mouse strains with L. major. However, the approach taken here is unique. The Th1/Th2 paradigm with L. major was formulated by injecting different mouse strains with the parasite or by injecting the same mouse strain with the parasite followed by intervention with neutralizing anti-cytokines (anti-IL-4 or anti-IFN-γ) (reviewed in references 4, 8, 13, 14, and 21). Here, the same mouse strain (BALB/c) was injected with two leishmanial species that cause cutaneous disease; parasites that elicited a strong Th2 (IL-4) response survived (L. major), while those that did not (L. braziliensis) were killed.
Acknowledgments
This work was supported by NIH grant AI-29955. H.C.L. received partial support from a World Health Organization TDR–WHO scholarship (M8/181/4/L.238).
The technical assistance of Monica Estay and Julie Bleyenberg is gratefully acknowledged.
REFERENCES
- 1.Barral A, Barral-Netto M, Yong E C, Brownell C E, Twardzik D R, Reed S G. Transforming growth factor β as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci USA. 1993;90:3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barral-Netto M, Barral A, Brownell C E, Skeiky Y A W, Ellingsworth L R, Twardzik D R, Reed S G. Transforming growth factor-β in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 3.Belosevic M, Finbloom D S, van der Meide P, Slayter M V, Nacy C A. Administration of monoclonal anti-IFN-γ antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989;143:266–274. [PubMed] [Google Scholar]
- 4.Bogdan C, Gessner A, Rollinghoff M. Cytokines in leishmaniasis: a complex network of stimulatory and inhibitory interactions. Immunobiology. 1993;189:356–396. doi: 10.1016/S0171-2985(11)80366-9. [DOI] [PubMed] [Google Scholar]
- 5.Chakkalath H R, Titus R G. Leishmania major-parasitized macrophages augment Th2-type T cell activation. J Immunol. 1994;153:4378–4387. [PubMed] [Google Scholar]
- 6.Childs G E, Lighther L K, McKinney L A, Groves M, Price E, Hendricks L. Inbred mice as model hosts for cutaneous leishmaniasis. I. Resistance and susceptibility to infection with Leishmania braziliensis, L. mexicana and L. aethiopica. Ann Trop Med Parasitol. 1984;78:25–34. doi: 10.1080/00034983.1984.11811769. [DOI] [PubMed] [Google Scholar]
- 7.Lehn M, Weiser W Y, Engelhorn S, Gillis S, Remold H G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-γ. J Immunol. 1989;143:3020–3024. [PubMed] [Google Scholar]
- 8.Liew F Y, O’Donnell C A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 9.Liew F Y, Millott S, Li Y, Lelchuk R, Chan W L, Ziltener H. Macrophage activation by interferon-γ from host-protective T cells is inhibited by interleukin (IL) 3 and IL-4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989;19:1227–1232. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- 10.Lima H C, Titus R G. Effects of sand fly vector saliva on development of cutaneous lesions and the immune response to Leishmania braziliensis in BALB/c mice. Infect Immun. 1996;64:5442–5445. doi: 10.1128/iai.64.12.5442-5445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima H C, Bleyenberg J A, Titus R G. A simple method for quantifying Leishmania in tissues of infected animals. Parasitol Today. 1997;13:80–82. doi: 10.1016/s0169-4758(96)40010-2. [DOI] [PubMed] [Google Scholar]
- 12.Neal R A, Hale C. A comparative study of susceptibility of inbred and outbred mouse strains compared with hamsters to infection with New World cutaneous leishmaniases. Parasitology. 1983;87:7–13. doi: 10.1017/s0031182000052379. [DOI] [PubMed] [Google Scholar]
- 13.Reed S G, Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993;5:524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- 14.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 15.Sadick M D, Heinzel F P, Holaday B J, Pu R T, Dawkins R S, Locksley R M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon γ-independent mechanism. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scharton-Kersten T, Scott P. The role of the innate immune response in Th1 cell development following Leishmania major infection. J Leukocyte Biol. 1995;57:515–522. doi: 10.1002/jlb.57.4.515. [DOI] [PubMed] [Google Scholar]
- 17.Shankar A, Titus R G. Leishmania major-specific, CD4+, MHC class-II-restricted T cells derived in vitro from lymphoid tissues of naive mice. J Exp Med. 1993;178:101–111. doi: 10.1084/jem.178.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shankar A H, Titus R G. The influence of antigen presenting cell type and IFN-γ on priming and cytokine secretion of Leishmania major-specific T cells. J Infect Dis. 1997;175:151–157. doi: 10.1093/infdis/175.1.151. [DOI] [PubMed] [Google Scholar]
- 19.Stenger S, Thuring H, Rollinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994;180:783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theodos C M, Shankar A, Glasebrook A L, Roeder W D, Titus R G. The effect of treating with anti-interleukin-1 receptor antibody on the course of experimental murine cutaneous leishmaniasis. Parasite Immunol. 1994;16:571–577. doi: 10.1111/j.1365-3024.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 21.Titus R G, Theodos C M, Shankar A, Hall L R. Interactions between Leishmania major and macrophages. Immunol Ser. 1994;60:437–459. [PubMed] [Google Scholar]
- 22.Titus R G, Marchand M, Boon T, Louis J A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]