Abstract
Background:
Delays in COVID-19 testing may increase the risk of secondary household and community transmission. Little is known about what patient characteristics and symptom profiles are associated with delays in test seeking.
Methods:
We conducted a retrospective cohort study of all symptomatic patients diagnosed with COVID-19 and assessed in a COVID Expansion to Outpatients (COVIDEO) virtual care program between March 2020 and June 2021. The primary outcome was later test seeking more than 3 days from symptom onset. Multivariable logistic regression was used to examine predictors of later testing including patient characteristics and symptoms (30 individual symptoms or 7 symptom clusters).
Results:
Of 5,363 COVIDEO patients, 4,607 were eligible and 2,155/4,607 (46.8%) underwent later testing. Older age was associated with increased odds of late testing (adjusted odds ratio [aOR] 1.007/year; 95% CI 1.00 to 1.01), as was history of recent travel (aOR 1.4; 95% CI 1.01 to 1.95). Health care workers had lower odds of late testing (aOR 0.50; 95% CI 0.39 to 0.62). Late testing was associated with symptoms in the cardiorespiratory (aOR 1.2; 95% CI 1.05, 1.36), gastrointestinal (aOR = 1.2; 95% CI 1.04, 1.4), neurological (aOR 1.1; 95% CI 1.003, 1.3) and psychiatric (aOR 1.3; 95% CI 1.1, 1.5) symptom clusters. Among individual symptoms, dyspnea, anosmia, dysgeusia, sputum, and anorexia were associated with late testing; pharyngitis, myalgia, and headache were associated with early testing.
Conclusion:
Certain patient characteristics and symptoms are associated with later testing, and warrant further efforts to encourage earlier testing to minimize transmission.
Keywords: COVID-19, diagnostic testing, patient characteristics, SARS-CoV-2, symptoms
Abstract
Historique :
Les retards à effectuer les tests de dépistage de la COVID-19 peuvent accroître le risque de transmission secondaire dans la famille et la communauté. On ne sait pas vraiment quels sont les caractéristiques des patients et leurs profils de symptômes associés aux retards à se faire dépister.
Méthodologie :
Les chercheurs ont réalisé une étude de cohorte auprès de tous les patients symptomatiques ayant obtenu un diagnostic de COVID-19 évalués dans le cadre du programme de soins virtuels COVID Expansion to Outpatients (COVIDEO, ou expansion de la COVID aux patients ambulatoires) entre mars 2020 et juin 2021. Le résultat primaire était une demande de dépistage plus de trois jours après l’apparition des symptômes. Les chercheurs ont utilisé la régression logistique multivariable pour examiner les prédicteurs d’un dépistage tardif, y compris les caractéristiques et les symptômes des patients (30 symptômes individuels ou sept grappes de symptômes).
Résultats :
Des 5 363 patients ayant participé au programme COVIDEO, 4 607 étaient admissibles et 2 155 de ces 4 607 (46,8 %) se sont soumis à un dépistage tardif. Une plus grande probabilité de dépistage tardif était liée à un âge avancé (rapport de cotes corrigé [RCc] 1,007/année, IC à 95 %, 1,00 à 1,01), de même qu’à un voyage récent (RCc = 1,4, IC à 95 %, 1,01 à 1,95). Les travailleurs de la santé étaient moins susceptibles de se faire dépister tardivement (RCc = 0,50, IC à 95 %, 0,39 à 0,62). Le dépistage tardif était associé à des symptômes de la grappe cardiorespiratoire (RCc = 1,2, IC à 95 %, [1,05, 1,36]), gastrointestinale (RCc = 1,2, IC à 95 %, [1,04, 1,4]), neurologique (RCc = 1,1, IC à 95 %, [1,003, 1,3]) et psychiatrique (RCc = 1,3, IC à 95 %, [1,1, 1,5]). Parmi les symptômes individuels, la dyspnée, l’anosmie, la dysgueusie, les expectorations et l’anorexie étaient associées à un dépistage tardif, et la pharyngite, les myalgies et les céphalées, à un dépistage précoce.
Conclusion :
Certaines caractéristiques des patients et certains symptômes étaient associés à un dépistage tardif, ce qui justifie des efforts supplémentaires pour favoriser un dépistage plus rapide afin de limiter la transmission.
Summary:
This study of more than 4,000 patients with COVID-19 identified predictors of later test seeking, including older age, recent travel, non-health care worker occupation, cardiorespiratory, gastrointestinal, neurologic and psychiatric symptom clusters, and dyspnea, anosmia, dysgeusia, sputum, and anorexia.
Mots-Clés : COVID-19, test diagnostique, caractéristiques des patients, SRAS-CoV-2, symptômes
Introduction
Testing is an essential component of the public health response to the COVID-19 pandemic. Earlier testing is likely to decrease the risk of onward transmission to household members and the wider community (1). Considering that viral shedding peaks early in SARS-CoV-2 infection, time is of the essence. Studies have found that effective contract tracing relies on earlier COVID-19 testing and reporting (2). Earlier testing also has the potential to improve individual patient outcomes, in the context of emerging antiviral treatments that must be administered early in the disease course. Delays in testing can be attributed to systemic barriers to test access, as well as individual and household level factors.
There is limited research on the predictors of later testing. Some of the social science literature has explored how psychological factors such as risk perception influence an individual's willingness to undergo testing (3). Disparities in COVID-19 prevalence, testing and vaccination are also driven by social determinants of health (4), but among those that undergo testing little is known about predictors of time to testing. Prior work has identified female sex, health worker status, and age ≥80 years as being associated with earlier test seeking, but such population-wide analyses do not include detailed aspects of clinical presentation (5).
The primary objective of this study was to determine the factors that impact the timeliness of COVID-19 test seeking among adult patients assessed by a comprehensive COVID Expansion to Outpatients (COVIDEO) virtual care program. We hypothesized that later test seeking would be associated with upper respiratory tract symptoms, younger age, and no baseline comorbidities, while known exposures and cardiorespiratory or neurologic symptoms may drive early test seeking.
Methods
General study design
This retrospective cohort study analyzed data from patients seen by the COVIDEO program at Sunnybrook Health Sciences Centre (SHSC) between March 1, 2020 and June 30, 2021, corresponding to the first three waves of the COVID-19 pandemic in the province of Ontario, in order to determine demographic and clinical predictors of late versus early test seeking in this cohort.
Setting
Launched in March 2020, COVIDEO was a virtual COVID-19 care program run by SHSC (6). It was designed to enable a clinical team, led by Infectious Diseases (ID) physicians, to assess and monitor individuals diagnosed with COVID-19. Most cases were ambulatory outpatients diagnosed at the SHSC Assessment Centre, a COVID-19 mass testing site, and isolating at home. There were, however, some inpatients included in the program, both those who were hospitalized due to COVID and patients who were admitted for other reasons.
When a patient was referred to COVIDEO, an initial assessment was done primarily by telephone. The clinical assessment consisted of patient demographics, history of presenting illness (symptom onset and progression), past medical history, medications, exposures and contacts and investigation results. Follow-up assessments were conducted based on the patient's clinical status and likelihood for deterioration.
Patient selection criteria
We included adult patients testing positive for COVID-19 in an outpatient or inpatient setting, who had ≥1 assessment conducted by the COVIDEO team. We excluded patients that were asymptomatic at time of initial COVIDEO assessment, had date of symptom onset after date of specimen collection, or had a delay in testing exceeding 30 days from diagnosis. The main rationale for excluding asymptomatic patients is that they lack a date of symptom onset which was required for calculation of the primary outcome; an additional rationale is that this removed patients that were tested as part of pre-operative screening, pre-chemotherapy screening, occupational health investigations, and other asymptomatic screening programs in which patients did not actively seek testing. We excluded patients seeking testing more than 30 days from diagnosis, given that these tests likely represented resolved infection, with testing sought for other reasons such as clearance for work or travel.
Primary outcome: timeliness of test seeking
The primary outcome of this study was the timeliness of test seeking. We calculated time from symptom onset to diagnosis of COVID-19 as follows: (date of specimen collection – date of symptom onset) + 1 day. In the rare cases that specimen collection dates were not available, we substituted with test finalization dates. Next, we dichotomized time from symptom onset to diagnosis as ‘earlier presentation’ or ‘later presentation’. Early was defined as ≤3 days; later was defined as >3 days. This threshold was pre-specified given that it was expected to represent the approximate median of the distribution.
Clinical outcomes
We described downstream clinical outcomes including hospitalization within 30 days of diagnosis, days from diagnosis to hospital admission, duration of hospital admission, ICU admission within 30 days of diagnosis, mechanical ventilation within 30 days of diagnosis, duration of mechanical ventilation, and death within 90 days of diagnosis.
Predictors of later test seeking
We examined predictors of later test seeking categorized as: baseline patient characteristics (demographics and comorbidities), COVID-19 exposure and testing, and symptoms (individual symptoms and symptom clusters).
Baseline patient characteristics: age, sex, pregnant, health care worker, self-reported smoker.
Comorbidities: cardiac disease, hypertension, diabetes, chronic lung disease, asthma, chronic kidney disease, liver disease, malignancy, chronic hematological disease, HIV, chronic neurological disease, self-reported obesity.
Exposure and testing: travel outside of Canada, known exposure to COVID-19, location of COVID-19 test (Assessment Centre, Emergency Department, other).
Symptom clusters (and individual symptoms): constitutional symptoms (fever, chills, lymphadenopathy, fatigue), upper respiratory (pharyngitis, rhinorrhea, otalgia, conjunctivitis), cardiorespiratory (chest pain, cough, sputum, hemoptysis, dyspnea), gastrointestinal (abdominal pain, nausea, vomiting, diarrhea), rheumatologic (myalgia, arthralgia, rash, hair loss), neurologic (anosmia, dysgeusia, headache, confusion), and psychiatric symptoms (depression, anxiety, insomnia, anorexia).
Statistical analysis
Descriptive statistics were performed on the predictors described above. For continuous variables, the median (inter-quartile range) was calculated. Categorical variables were analyzed in terms of proportions. We examined the relationship between predictor variables and the primary outcome (earlier versus later presentation) by performing chi-square testing for categorical dependent variables and t-tests for continuous variables. In the primary analysis, we performed multivariable logistic regression analysis incorporating seven symptom clusters, and in a secondary analysis we incorporated 30 individual symptoms. These multivariable models also included age, gender, location of testing, calendar month, pregnancy, health care worker occupation, known exposure to COVID-19, and presence of any cardiorespiratory comorbidity. We conducted two post-hoc sensitivity analyses to test the robustness of our findings: (1) excluding health care workers, and (2) redefining earlier versus later testing as ≤2 days versus ≥6 days (and excluding those with testing at 3–5 days).
Sample size calculation
For the primary analysis, we estimated that we would be able to detect an increase from 50% to 60% later test seeking associated with a given patient characteristic (such as presence of upper respiratory tract symptoms) with a sample size of 776 patients (power 0.8, alpha 0.05). To detect a smaller increase from 50% to 55% later test seeking we would require 3130 patients (sample size calculation performed at https://www.stat.ubc.ca/∼rollin/stats/ssize/b2.html).
Results
Cohort characteristics
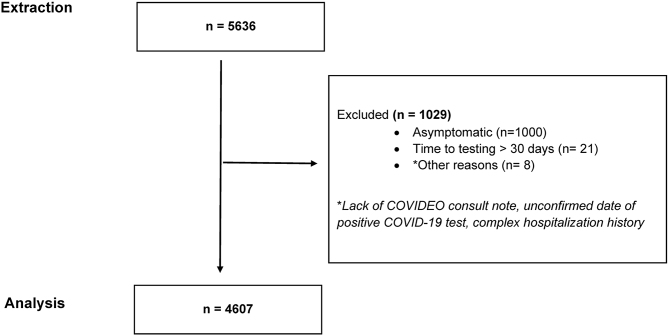
Of the 5,636 COVIDEO patients assessed in the first three waves of the COVID-19 pandemic (March 1, 2020 to June 30, 2021), 4,607 individuals were ultimately included in this analysis (Figure 1). The remaining 1,029 patients were excluded largely due to asymptomatic status. The median (interquartile range [IQR]) age of this cohort was 38.0 (26.0, 53.0) and there was close to an even split in terms of patient sex (50.9% female; 49.1% male) (Table 1). Health care workers accounted for 9.6% of the sample.
Figure 1:
Consort Flow Diagram
Table 1:
Baseline patient characteristics
| Variable | Overall (n = 4,607) | Among patients with early testing (≤3d; n = 2452) | Among patients with late testing (>3d; n = 2,155) | p-value |
|---|---|---|---|---|
| Age, median (interquartile range) | 38 (26, 53) | 36 (26, 51.0) | 39 (27,56) | <0.0001 |
| Female sex | 2,347 (50.9%) | 1.234 (50.3%) | 1,113 (51.6%) | 0.37 |
| Pregnant | 74 (1.6%) | 38 (1.5%) | 36 (1.7%) | 0.74 |
| Health care worker | 440 (9.6%) | 294 (12.0%) | 146 (6.8%) | <0.0001 |
| Smoker | 316 (6.9%) | 178 (7.3%) | 138 (6.4%) | 0.25 |
| Comorbidities | ||||
| Cardiac disease | 236 (5.1%) | 119 (4.9%) | 117 (5.4%) | 0.38 |
| Hypertension | 584 (12.7%) | 272 (11.1%) | 312 (14.5%) | 0.001 |
| Diabetes | 316 (6.9%) | 152 (6.2%) | 164 (7.6%) | 0.06 |
| Lung disease | 95 (2.1%) | 54 (2.2%) | 41 (1.9%) | 0.48 |
| Asthma | 309 (6.7%) | 169 (6.9%) | 140 (6.5%) | 0.59 |
| Kidney disease | 110 (2.4%) | 59 (2.4%) | 51 (2.4%) | 0.93 |
| Liver disease | 37 (0.80%) | 21 (0.89%) | 16 (0.74%) | 0.67 |
| Malignancy | 174 (3.8%) | 87 (3.5%) | 8 (4.0%) | 0.39 |
| Hematologic disease | 65 (0.2%) | 37 (0.3%) | 28 (0.2%) | 0.55 |
| HIV | 11 (0.2%) | 7 (0.3%) | 4 (0.2%) | 0.49 |
| Neurological disease | 16 (3.6%) | 104 (4.2%) | 61 (2.8%) | 0.01 |
| Obesity | 248 (5.4%) | 127 (5.2%) | 121 (5.6%) | 0.51 |
The most prevalent comorbidities were hypertension (12.7%), diabetes (6.9%), and asthma (6.7%). Just over half (53.6%) of patients had a known exposure to the virus; most patients were tested at SHSC's Assessment Centre (86.5%), with a minority tested in the Emergency Department or other settings (Table 2). The symptom clusters experienced by the highest proportion of patients were constitutional (61.9%), cardiorespiratory (55.4%), neurologic (54.7%), and upper respiratory (51.3%) (Table 3). Approximately 10% of patients were hospitalized within 30 days of their COVID-19 diagnosis; the median (IQR) duration of hospitalization was 7.0 days (3.0, 13.0) (Supplemental Table 1).
Table 2:
COVID-19 exposure and testing
| Variable | Overall (n = 4,607) | Among patients with early testing (≤3d; n = 2,452) | Among patients with late testing (>3d; n = 2,155) | p-value |
|---|---|---|---|---|
| Number of days from symptom onset to COVID-19 Test median, IQR) | 3 (2, 6) | 2 (1, 3) | 6 (4, 8) | |
| Travel outside Canada | 161 (3.5%) | 76 (3.1%) | 85 (3.9%) | 0.12 |
| Known exposure to COVID-19 | 2,471 (53.6%) | 1,339 (54.6%) | 1,132 (52.5%) | 0.16 |
| Location of COVID-19 test | ||||
| Assessment Centre | 3,986 (86.5%) | 2,131 (86.9%) | 1,855 (86.1%) | 0.41 |
| Emergency Department | 303 (6.6%) | 128 (5.2%) | 175 (8.1%) | 0.0001 |
| Other | 319 (6.9%) | 193 (7.9%) | 125 (5.8%) | 0.006 |
IQR = Interquartile range
Table 3:
COVID-19 symptoms overall and among those with early and late testing
| Variable | Overall (n = 4,607) | Among patients with early testing (≤3d; n = 2,452) | Among patients with late testing (>3d; n = 2,155) | p-value |
|---|---|---|---|---|
| Symptom clusters | ||||
| Upper respiratory | 2,364 (51.3%) | 1,305 (53.2%) | 1,059 (49.1%) | 0.01 |
| Cardiorespiratory | 2,550 (55.4%) | 1,298 (52.9%) | 1,252 (58.1%) | 0.0004 |
| Constitutional | 2,851 (61.9%) | 1,512 (61.7%) | 1,339 (62.1%) | 0.74 |
| Gastrointestinal | 973 (21.1%) | 473 (19.3%) | 500 (23.2%) | 0.001 |
| Neurologic | 2,521 (54.7%) | 1,307 (53.3%) | 1,214 (56.3%) | 0.04 |
| Psychiatric | 1,024 (22.2%) | 489 (19.9%) | 535 (24.8%) | 0.0001 |
| Rheumatologic | 1,676 (36.4%) | 920 (37.5%) | 756 (35.1%) | 0.17 |
| Symptoms | ||||
| Fever | 1,461 (31.7%) | 779 (31.8%) | 682 (31.6%) | 0.93 |
| Pharyngitis | 1,337 (29.0%) | 787 (32.1%) | 550 (25.5%) | <0.0001 |
| Rhinorrhea | 1,497 (32.5%) | 770 (31.4%) | 727 (33.7%) | 0.09 |
| Cough | 2,360 (51.2%) | 1,214 (49.5%) | 1,146 (53.2%) | 0.01 |
| Sputum | 176 (3.8%) | 79 (3.2%) | 97 (4.5%) | 0.02 |
| Hemoptysis | 14 (0.3%) | 6 (0.2%) | 8 (0.4%) | 0.44 |
| Dyspnea | 455 (9.9%) | 173 (7.1%) | 282 (13.1%) | <0.0001 |
| Chills | 987 (21.4%) | 530 (21.6%) | 457 (21.2%) | 0.74 |
| Chest pain | 353 (7.7%) | 179 (7.3%) | 174 (8.1%) | 0.32 |
| Otalgia | 130 (2.8%) | 63 (2.6%) | 67 (3.1%) | 0.27 |
| Conjunctivitis | 158 (3.4%) | 90 (3.7%) | 68 (3.2%) | 0.34 |
| Anosmia | 991 (21.5%) | 388 (15.8%) | 603 (28.0%) | <0.0001 |
| Dysgeusia | 984 (21.4%) | 400 (16.3%) | 584 (27.1%) | <0.0001 |
| Myalgia | 1,553 (33.7%) | 850 (34.7%) | 703 (32.6%) | 0.14 |
| Arthralgia | 443 (9.6%) | 223 (9.1%) | 220 (10.2%) | 0.20 |
| Abdominal pain | 251 (5.4%) | 125 (5.1%) | 126 (5.8%) | 0.26 |
| Nausea/Vomiting | 450 (9.8%) | 214 (8.7%) | 236 (11.0%) | 0.01 |
| Diarrhea | 541 (11.7%) | 252 (10.3%) | 289 (13.4%) | 0.001 |
| Lymphadenopathy | 14 (0.3%) | 8 (0.3%) | 6 (0.3%) | 0.77 |
| Rash | 67 (1.5%) | 34 (1.4%) | 33 (1.5%) | 0.68 |
| Fatigue | 2,005 (43.5%) | 1,034 (42.2%) | 971 (45.1%) | 0.05 |
| Headache | 1,767 (38.4%) | 987 (40.3%) | 780 (36.2%) | 0.005 |
| Confusion | 83 (1.8%) | 42 (1.7%) | 41 (1.9%) | 0.63 |
| Depression/Anxiety | 227 (4.9%) | 113 (4.6%) | 114 (5.3%) | 0.29 |
| Insomnia | 320 (6.9%) | 166 (6.8%) | 154 (7.1%) | 0.62 |
| Anorexia | 780 (16.9%) | 355 (14.5%) | 425 (19.7%) | <0.0001 |
Time to testing
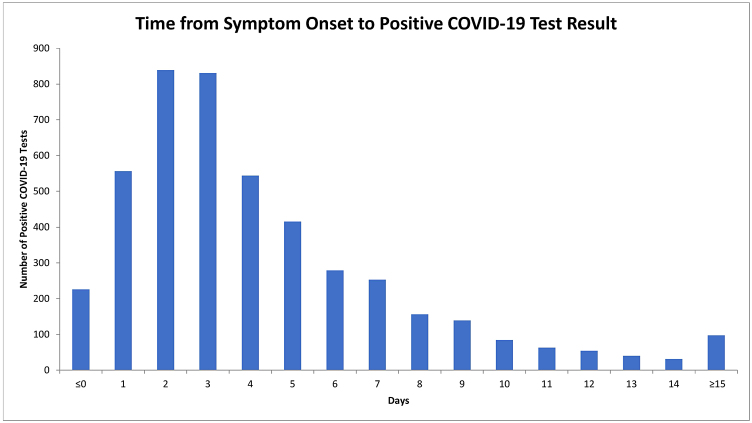
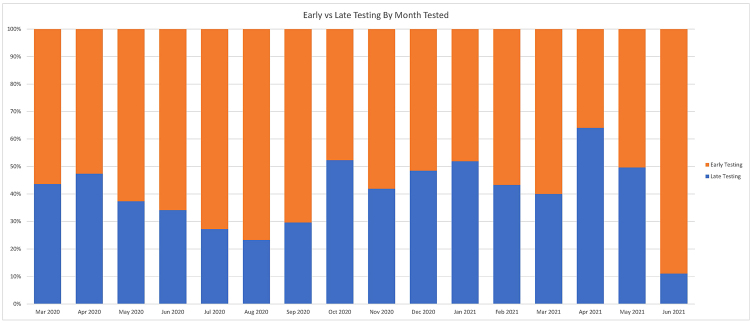
The time from symptom onset to a positive COVID-19 test result was normally distributed (Figure 2), and the median (IQR) time to testing was 3.0 days (2.0, 6.0). The primary outcome of later testing of more than 3 days occurred in 2,155 (46.8%) patients. Later testing varied over the course of the pandemic, from as high as 64.1% in April 2021 to as low as 11.1% in June 2021 (Figure 3).
Figure 2:
Time from symptom onset to positive test result
Figure 3:
Early versus late testing by month tested
Predictors of late testing
Patients undergoing later testing were older than those undergoing early testing (median 39 versus 36 years, p = 0.0001), and less likely to be health care workers (6.8% versus 12%, p < 0.0001) (Table 1). In unadjusted analyses, patients with late testing were more likely to have cardiorespiratory, gastrointestinal, neurologic, and psychiatric symptoms than those with early testing; they were less likely to have upper respiratory tract symptoms (Table 3).
In multivariable analyses, each additional year of age was associated with 1.007 times higher adjusted odds of late testing (95% CI 1.003 to 1.011). Health care workers had lower odds of late testing than non–health care workers (adjusted odds ratio [aOR] 0.50; 95% CI 0.394 to 0.623). Patients who had traveled within 14 days of symptom onset had higher odds of later testing compared to those who had not traveled (aOR 1.4; 95% CI 1.006 to 1.948). Those with symptom onset in January, February, March, May, June, August, September, and November had lower odds of late testing as compared to the average month of October (Table 4).
Table 4:
Multivariable analysis examining predictors of testing delays
| Patient characteristic | Adjusted odds ratio | 95% CI |
|---|---|---|
| Age (per year) | 1.007 | 1.003–1.011 |
| Female sex | 1.077 | 0.952–1.219 |
| Calendar month | ||
| January | 0.730 | 0.567–0.939 |
| February | 0.655 | 0.493–0.871 |
| March | 0.603 | 0.460–0.790 |
| April | 1.298 | 0.982–1.716 |
| May | 0.291 | 0.185–0.459 |
| June | 0.231 | 0.098–0.546 |
| July | 0.406 | 0.119–1.391 |
| August | 0.200 | 0.079–0.504 |
| September | 0.486 | 0.338–0.699 |
| October | --Referent category-- | --Referent category-- |
| November | 0.735 | 0.563–0.959 |
| December | 1.137 | 0.891–1.451 |
| Pregnant | 1.277 | 0.784–2.079 |
| Healthcare worker | 0.495 | 0.394–0.623 |
| Travel within 14 days | 1.400 | 1.006–1.948 |
| Known exposure to COVID-19 | 0.958 | 0.848–1.082 |
| Comorbidity | 0.926 | 0.805–1.065 |
| Symptom cluster | ||
| Upper respiratory | 0.893 | 0.789–1.011 |
| Cardiorespiratory | 1.198 | 1.054–1.363 |
| Constitutional | 0.940 | 0.822–1.076 |
| Gastrointestinal | 1.215 | 1.037–1.423 |
| Neurological | 1.139 | 1.003–1.293 |
| Psychiatric | 1.299 | 1.105–1.526 |
| Rheumatologic | 0.823 | 0.719–0.942 |
Late testing was associated with having symptoms within the cardiorespiratory cluster (aOR 1.2; 95% CI 1.05, 1.36), as well as gastrointestinal (aOR 1.2; 95% CI 1.04, 1.4), neurological (aOR 1.1; 95% CI 1.003, 1.3), and psychiatric (aOR 1.3; 95% CI 1.1, 1.5) symptom clusters. Having at least one rheumatological symptom was associated lower odds of late testing (aOR 0.82; 95% CI 0.7 to 0.9). In contrast, upper respiratory and constitutional symptom clusters were not significantly associated with timing of testing (Table 4).
In secondary multivariable analyses incorporating individual symptoms rather than symptom clusters, dyspnea (aOR 2.26; 95% CI 1.73 to 2.95) and anosmia (aOR 1.74; 95% CI 1.40 to 2.16) were found to have the greatest strength of association with later testing. Other symptoms associated with higher odds of later testing were: dysgeusia (aOR 1.3; 95% CI 1.1 to 1.7), sputum (aOR 1.4; 95% CI 1.01 to 2.1), and anorexia (aOR 1.3; 95% CI 1.1 to 1.6). Patients who reported pharyngitis (aOR 0.78; 95% CI 0.68 to 0.90), myalgia (aOR 0.85, 95% CI [0.73 to 0.98]), and headache (aOR 0.82; 95% CI 0.71 to 0.94) had lower odds of late testing than those without these symptoms (Supplemental Table 2).
Sensitivity analyses excluding health care workers or re-defining earlier versus later testing as ≤2 days versus ≥6 days yielded results in line with the primary analysis (data not shown).
Discussion
Later testing for COVID-19 is likely associated with greater risk of viral transmission, and our study of more than 4,600 patients has identified several important predictors of this phenomenon. Later testing was associated with older age, recent travel, four symptom clusters (cardiorespiratory, gastrointestinal, neurological, and psychiatric) and five individual symptoms (dyspnea, anosmia, dysgeusia, sputum, and anorexia). Conversely, health care workers and patients who reported rheumatological symptoms, pharyngitis, myalgia, and headache had lower odds of testing late.
A prior population-level study examined demographic predictors of earlier COVID-19 test seeking in Ontario during the first wave (5). This study corroborates our finding that health care workers have significantly lower odds of late testing compared to non–health care workers. However, the findings of this Ontario-wide study differed with respect to age-associated testing delays; our analysis detected a small but significant increase in late testing with each additional year of age, whereas this prior study detected a reduction in testing with older age. It is difficult to make a direct comparison because age was analysed as a categorical variable. Our study built upon this prior work by providing access to detailed symptom and comorbidity data.
Our hypothesis was that upper respiratory tract symptoms, younger age and fewer baseline comorbidities would be predictors of later test seeking, whereas known exposures, and cardiorespiratory and neurologic symptoms would be predictors of earlier testing. We reasoned that upper respiratory tract symptoms could easily be misattributed to allergies or a ‘common cold’, leading to later patient presentations to testing sites. However, our data suggest that the upper respiratory syndrome cluster was not significantly associated with test timing, and in fact exhibited a trend towards being more common among those with early testing. This may have been driven by pharyngitis, the one upper respiratory symptom which was strongly associated with early testing, and which would not be confused for typical allergysymptoms.
We hypothesized that cardiorespiratory and neurologic symptoms would be predictors of early rather than late test seeking, because these symptoms would either be more concerning (eg, dyspnea) or specific for SARS-CoV-2 infection (eg, anosmia). This was not borne out by our analysis. One possible explanation for this finding is that symptoms such as dyspnea and anosmia are more likely to present later in the course of illness. A meta-analysis found that anosmia and ageusia typically presented around 5 days after the onset of other COVID-19 symptoms (7). The time to testing variable is defined in relation to the onset of the first symptom(s) and there was no documentation of when other individual symptoms began. Due to this limitation in study design, the association of anosmia with late testing could reflect reverse causality.
Our retrospective cohort design was also subject to other limitations. We are only able to study delays in testing among those that were ultimately tested; we are unable to study predictors of never being tested. Our analysis could not incorporate unmeasured household factors such as the presence of other at-risk family members. This dimension is important in the context of a highly transmissible infectious disease. Further research might also consider how testing behaviours changed across the different waves of the pandemic, as new variants emerged, vaccines were rolled-out and public health measures evolved. Both the criteria and capacity for COVID-19 testing changed over the course of the study period. It is worth noting that the shift from mass PCR testing to targeted testing for individuals belonging to high-risk groups occurred after the end of our study period in December 2021 during the 5th wave of the pandemic. Another limitation of this study was the lack of information on social determinants of health (eg, race/ethnicity, occupation, and household income) and other individual and temporal barriers to testing. Research has shown that patients experiencing social and economic marginalization were more likely to acquire COVID-19 and have worse clinical outcomes in terms of hospitalization and mortality (8,9). They were also less likely to be tested and vaccinated early in the pandemic prior to the introduction of health equity initiatives (10). We examined multiple patient predictors without explicit correction for multiple hypothesis testing.
In summary, this retrospective cohort study analyzed predictors of later COVID-19 testing in a large cohort of patients assessed by a virtual COVID-19 care program in Toronto. Having detailed clinical assessments as part of this program was an advantage that allowed for multivariable analysis of the relationships between patients’ baseline comorbidities, presenting symptoms and later testing. Of note, recent travel, cardiorespiratory and neurologic symptom clusters, as well as dyspnea, anosmia, and dysgeusia individually were associated with late testing. Therefore, further research is needed to confirm potential causal associations, and to elucidate methods to encourage earlier testing in these patient subgroups. Later testing carries even greater consequences now that there are available therapies which must be administered soon after symptom onset.
Acknowledgements:
This study was made possible by a grant from the Sunnybrook Foundation & Sunnybrook Research Institute COVID-19 Research Initiative. The authors would like to acknowledge all of the many dedicated, multidisciplinary health care workers, and team members that made COVIDEO care possible.
Data accessibility:
Nick Daneman has full access to data.
Contributors:
Conceptualization, N Daneman, P Lam, A Chan, N Andany, A Ga’al, A Kapsack, A Mahmud; Data Curation, J Estrada-Codecido; Formal Analysis, A Ga’al, A Kiss; Funding Acquisition, N Andany, P Lam, A Chan, N Andany; Investigation, A Ga’al, A Kapsack, A Mahmud, J Estrada-Codecido, P Lam, A Chan, N Andany, A Simor, A Kiss, N Daneman; Methodology, A Ga’al, A Kapsack, A Mahmud, J Estrada-Codecido, P Lam, A Chan, N Andany, A Simor, A Kiss, N Daneman; Project Administration, N Daneman, A Chan, N Andany, P Lam, J Estrada-Codecido; Writing original draft, A Ga’al; Writing – Reviewing/Editing, A Ga’al, A Kapsack, A Mahmud, J Estrada-Codecido, P Lam, A Chan, N Andany, A Simor, A Kiss, N Daneman
Accessed and verified all data: J Estrada-Codecido, N Daneman, A Ga’al
Ethics Approval:
Ethics approval was not required.
Informed Consent:
N/A
Registry and the Registration No. of the Study/Trial:
N/A
Funding:
No funding was received for this work.
Disclosures:
The authors have nothing to disclose.
Animal Studies:
N/A
Supplemental Material
References
- 1.Kretzschmar ME, Rozhnova G, Bootsma MCJ, van Boven M, van de Wijgert JHHM, Bonten MJM. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020; 5 8:e452–9. 10.1016/S2468-2667(20)30157-2. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020; 26 5:672–5. 10.1038/s41591-020-0869-5. PMID: [DOI] [PubMed] [Google Scholar]
- 3.Bruine de Bruin W, Bennett D. Relationships between initial COVID-19 risk perceptions and protective health behaviors: a national survey. Am J Prev Med. 2020; 59 2:157–67. 10.1016/j.amepre.2020.05.001. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020; 17 9:e1003379. 10.1371/journal.pmed.1003379. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joh E, Buchan SA, Daneman N, Paul LA, Brown KA. Factors associated with timely test seeking, test turnaround, and public reporting of COVID-19: a retrospective analysis in Ontario, Canada (p. 2021.02.22.21252219). medRxiv; 2021. 10.1101/2021.02.22.21252219 [DOI]
- 6.Lam PW, Sehgal P, Andany N, et al. A virtual care program for outpatients diagnosed with COVID-19: a feasibility study. CMAJ Open. 2020; 8 2:E407–13. 10.9778/cmajo.20200069. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos REA, da Silva MG, do Monte Silva MCB, et al. Onset and duration of symptoms of loss of smell/taste in patients with COVID-19: a systematic review. Am J Otolaryngol. 2021; 42 2:102889. 10.1016/j.amjoto.2020.102889. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundaram ME, Calzavara A, Mishra S, et al. Individual and social determinants of SARS-CoV-2 testing and positivity in Ontario, Canada: a population-wide study. CMAJ. 2021; 193 20:E723–34. 10.1503/cmaj.202608. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KH, Denice P, Haan M, Zajacova A. Studying the social determinants of COVID-19 in a data vacuum. Can Rev Sociol. 2021; 58 2:146–64. 10.1111/cars.12336. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenzie K, Dube S, Peterson S. (2021). (rep.). Tracking COVID-19 through race-based data. Wellesley Institute and Ontario Health; 2021 [cited 2022]. Available from: https://www.ontariohealth.ca/about-us/our-programs/provincial-equity-indigenous-health/equity-inclusion-diversity-anti-racism/report-tracking-covid-19-through-race-based-data#:∼:text=This%20report%20examines%20race%2Dbased,infection%20compared%20with%20white%20Ontarians.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Joh E, Buchan SA, Daneman N, Paul LA, Brown KA. Factors associated with timely test seeking, test turnaround, and public reporting of COVID-19: a retrospective analysis in Ontario, Canada (p. 2021.02.22.21252219). medRxiv; 2021. 10.1101/2021.02.22.21252219 [DOI]
Supplementary Materials
Data Availability Statement
Nick Daneman has full access to data.