Abstract
The role of CD8 T cells in controlling Mycobacterium tuberculosis infections in mice was confirmed by comparing the levels of growth of the organism in control, major histocompatibility complex class II knockout, and athymic mice and by transferring T-cell populations into athymic mice. By using donor mice which were incapable of making gamma interferon (IFN-γ), it was shown that IFN-γ production was essential for CD8 cell mediation of protective immunity against M. tuberculosis.
Cell-mediated immunity is crucial for the control of mycobacterial infections. Athymic mice (4) and mice whose T cells have been depleted (22, 23) are much more susceptible to infection with mycobacteria than euthymic or unmanipulated mice. However, the contributions of the different components of the T-cell response are unclear. CD4 T cells are thought to play a major role in controlling infections with the primary human tubercle bacillus, Mycobacterium tuberculosis; individuals with reduced CD4 counts, from infection with human immunodeficiency virus, for example, are known to be more susceptible to M. tuberculosis infections (12). Activation of CD4 cells by antigen in association with major histocompatibility complex (MHC) class II molecules results in clonal expansion and the production of cytokines, most notably gamma interferon (IFN-γ), which activate macrophages so that they become mycobactericidal. Mice with deletions of the IFN-γ gene are much more susceptible to M. tuberculosis infection than wild-type mice (5, 9). However, in addition to CD4 cells, other components of the cell-mediated response are thought to play roles in controlling infection with M. tuberculosis. For example, CD8 T cells have been shown to be involved (20, 24): β2 microglobulin-deficient knockout mice, which lack an effective CD8 response, show increased susceptibility to M. tuberculosis infection (10). Other cell types, such as T cells bearing the γ/δ T-cell receptor (19) and NK cells (1), are also thought to have roles in protection against intracellular bacteria, while a number of T cells with novel phenotypes and unknown functions have been shown to recognize mycobacterial antigens (2, 28).
CD8 T cells are known to contribute to the protective response against M. tuberculosis, but the mechanism(s) by which they exert this protective effect is unknown. CD8 T cells produce a range of cytokines, including IFN-γ (11, 17, 25, 26), but their primary role is thought to be cytotoxic. However, it has recently been shown that mice with a targeted disruption in either the perforin gene or the granzyme gene and mice which are Fas receptor defective are no more susceptible to infection with M. tuberculosis than are wild-type mice (6, 16). Since perforin (13, 18) and Fas-Fas ligand interactions (21, 27, 31) are thought to be the primary mechanisms of cytotoxicity mediated by CD8 T cells, such cells may contribute their antimycobacterial activity through noncytotoxic pathways.
In this study, we have used MHC class II-deficient mice and athymic mice to confirm the role of non-CD4 T-cell-mediated mechanisms in protection against M. tuberculosis infection. Using transfer of purified CD4 and CD8 cells into athymic mice, we have demonstrated that these cells contribute equally to protective immunity in this system. However, by using mice with deletions of the IFN-γ gene as T-cell donors, we have shown that production of IFN-γ is required in order for CD8 T cells to exert their antimycobacterial effect.
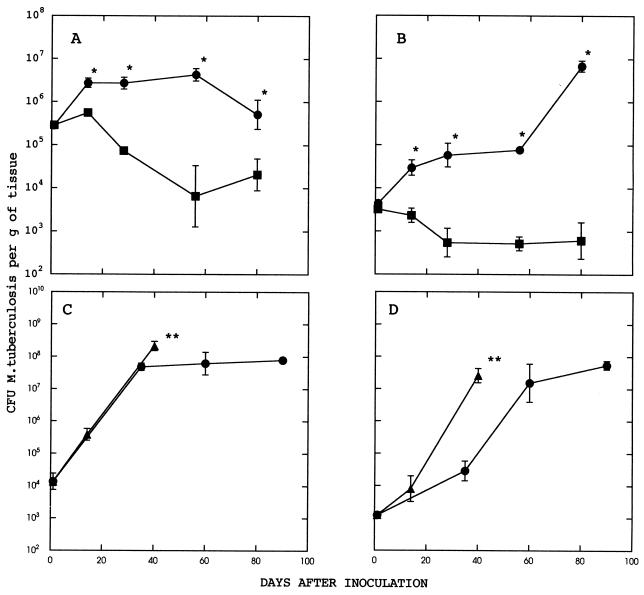
In preliminary experiments, the levels of growth of M. tuberculosis in MHC class II knockout, athymic, and normal mice were compared. MHC class II knockout (Aβ−/−) mice were obtained as a breeding nucleus (kindly provided by D. Gray, Hammersmith Hospital, London, United Kingdom, with permission from D. Mathis, Institut National de la Santé et de la Recherche Médicale). These mice were bred from heterozygous (Aβ+/−) parents and genotyped as described previously (7). Heterozygous littermates were used as controls. Stock cultures of M. tuberculosis H37Rv were grown in Dubos 7H9 broth for 14 days, and then they were aliquoted and stored in liquid nitrogen. For infection, aliquots were thawed, diluted in phosphate-buffered saline, and inoculated intraperitoneally into mice. The infection was monitored by removing the lungs and spleens of infected mice at various intervals; the baseline level of infection of each tissue was estimated by harvesting organs from the mice 18 h after infection and determining viable counts. The tissues were weighed and homogenized by shaking with 2-mm-diameter glass beads in chilled saline with a Mini-Bead Beater (Biospec Products, Bartlesville, Okla.), and 10-fold dilutions of the suspension were plated onto Dubos 7H11 agar with Dubos oleic albumic complex supplement (Difco Laboratories, Surrey, United Kingdom). Numbers of CFU were determined after the plates had been incubated at 37°C for approximately 20 days. The results are shown in Fig. 1A and B. In control mice, there was a transient increase in bacterial counts in the spleen, followed by a steady decline over 60 days and then by a levelling out of the infection at approximately 104 CFU per g of tissue. In MHC class II knockout mice, there was an initial growth of the infection over the first 60 days, followed by a plateau phase during which the infection appeared to be controlled but was significantly more severe than in wild-type mice (Fig. 1A). In lung tissue (Fig. 1B), a similar pattern emerged, except that in the MHC class II knockout mice, control of the infection broke down in some of the mice after about 60 days, when there was a sudden increase in bacterial counts. By day 80, counts had reached approximately 107 CFU per g of tissue, a 10,000-fold increase over the counts seen in wild-type mice.
FIG. 1.
Growth of M. tuberculosis in the tissues of MHC class II knockout, control, and athymic mice. (A and B) Growth in spleens and lungs, respectively, of MHC class II knockout mice (•) and their wild-type littermates (▪). (C and D) Growth in spleens and lungs, respectively, of MHC class II knockout (•) and athymic (▴) mice. Data are the geometric means ± the standard errors of the means for three to five mice. An asterisk indicates a significant difference between values for MHC class II knockout and control mice (P < 0.05 by Students’ t test). A double asterisk denotes that at the indicated time, all remaining mice in the group were killed because of the widely disseminated nature of the infection.
These results emphasize the importance of the MHC class II-CD4 T-cell pathway in controlling M. tuberculosis infection. However, in spite of the fact that after the first few days of infection there was always a highly significant difference between the level of viable M. tuberculosis organisms in MHC class II knockout mice and the level in control mice, some control of bacterial multiplication did appear to occur in the MHC class II knockout mice. In order to demonstrate that this apparent partial control of the infection in MHC class II knockout mice was mediated by T cells, we compared growth in these mice with growth in athymic mice. Athymic (nude) BALB/c mice were obtained from a breeding colony at the National Institute for Medical Research. Athymic and MHC class II knockout mice were infected intraperitoneally, and the infections were monitored as described above. Whereas the MHC class II knockout mice were again able to control the infection to some degree, growth in athymic mice was unchecked and the mice had to be killed at 40 days because of overwhelming infection (Fig. 1C and D).
These results confirm the importance of CD4 cells in controlling M. tuberculosis infections but also suggest that a contribution is made by non-CD4-mediated mechanisms. It has previously been shown that depletion of CD8 cell populations in mice with anti-CD8 antibodies (20) or abolition of a CD8 response by disruption of the β2 microglobulin gene (10) renders mice highly susceptible to infection with M. tuberculosis. CD8 T cells have also been implicated in human tuberculosis; CD8+ T cells with specificity for mycobacterium-pulsed target cells have been described (14, 32), and an individual with recurrent tuberculosis was found to have a specific reduction in CD8 T cells (3).
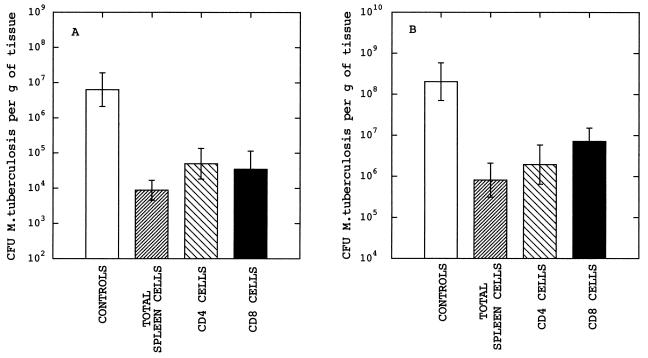
In order to investigate the contribution of CD8 T cells to the control of M. tuberculosis infections in mice, total spleen cells, CD4 T cells, and CD8 T cells were transferred from control BALB/c mice into infected athymic BALB/c mice. Splenocytes were incubated in hypotonic medium to lyse erythrocytes and washed twice. To obtain highly purified populations of CD4 and CD8 cells, cell suspensions were enriched by negative selection with T-cell-subset columns (R & D Systems Inc., Minneapolis, Minn.) according to the manufacturer’s instructions. The resulting populations were >90% CD4 or CD8 T cells, as determined by flow cytometric analysis. The cells were washed, resuspended in sterile saline, and injected intravenously such that recipient mice received 5 × 106 cells. The mice were then infected with M. tuberculosis, and organs were harvested 21 days later for CFU counts. The results of a typical experiment are shown in Fig. 2. In athymic mice which had not received any transferred cells, the infection reached approximately 107 CFU per g in the lung (Fig. 2A) and 108 CFU per g in the spleen (Fig. 2B). Transfer of total spleen cells from naive BALB/c mice reduced the number of CFU 100- to 1,000-fold in both tissues. It appeared that CD4 and CD8 T cells contributed approximately equally to the observed protection.
FIG. 2.
Infection of athymic mice with M. tuberculosis following transfer of splenocytes from euthymic mice. (A and B) Results for the lungs and spleen, respectively, of mice infected intravenously with approximately 106 CFU 21 days prior to harvest. Transfer of cells was carried out 24 h before infection. Data are the means ± the standard errors of the means for three to five mice. Mice received either no cells, total spleen cells, CD4 cells, or CD8 cells. All three groups of mice which received cells showed significantly reduced CFU counts compared to controls (P < 0.05 by Student’s t test).
The mechanism by which CD8 T cells exert this antimycobacterial response is not understood. It has been suggested that the cytotoxicity of mycobacterium-laden target cells could be involved, perhaps through the release of M. tuberculosis bacilli from ineffective macrophages to cells with greater antimycobacterial potential (15). However, perforin or granzyme knockout mice and Fas receptor-defective mice, when infected, did not display any increased susceptibility to infection, compared to wild-type controls (6, 16). Interestingly, both the perforin knockout mice and the Fas receptor-defective mice had elevated levels of cytokines, including IFN-γ, in the absence of infection, and levels in infected mice were similar to those seen in wild-type mice (16). Thus, neither perforin-, granzyme-, nor Fas-mediated cytotoxicity appeared to be involved in the control of these experimental infections (6, 16). Conversely, however, Silva and colleagues (29) produced CD8+ T-cell clones which were capable of conferring protection against M. tuberculosis in recipient mice, and the level of protection correlated with the level of cytotoxic activity rather than with the level of IFN-γ secretion.
In a recent study of human cytotoxic cells with mycobacterial specificity, it was found that CD4− CD8− T cells lysed macrophages through a Fas-Fas ligand interaction but the lysis was not associated with mycobacterial killing, whereas CD8+ T-cells lysed macrophages by a Fas-independent pathway and the lysis resulted in the killing of mycobacteria (30). The human T-cell lines used for these experiments were unusual in that they were CD1 restricted.
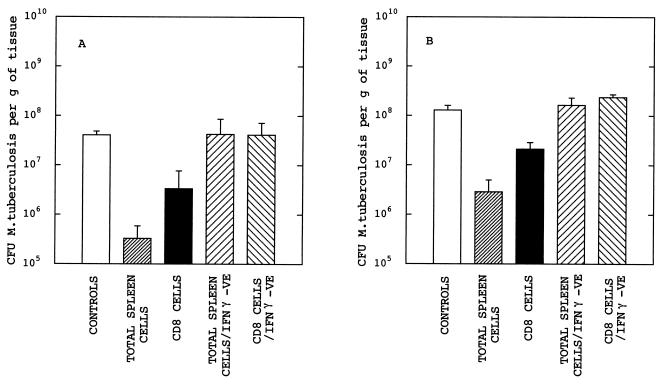
Since CD8 T cells were clearly able to confer significant levels of protection against M. tuberculosis in our cell transfer model, we next investigated the role of IFN-γ in this protection. Again athymic mice were recipients of either total spleen cells or CD8 cells. This time, however, donor mice were either normal BALB/c mice or IFN-γ knockout mice (8) and recipient mice received 3 × 106 cells. The results (Fig. 3) clearly demonstrate the requirement for IFN-γ. Transfer of total spleen cells or CD8 T cells from normal mice gave protection, although the level of protection was slightly lower than that seen in the previous experiment (Fig. 2). This was probably because the number of cells transferred was lower (3 × 106 rather than 5 × 106). However, the protection seen in both organs was significant (P < 0.05). Importantly, transfer of cells from IFN-γ knockout mice gave no protection.
FIG. 3.
Infection of athymic mice with M. tuberculosis following transfer of splenocytes from control BALB/c and IFN-γ knockout (IFN-γ −VE) BALB/c mice. (A and B) Results for the lungs and spleen, respectively. The experimental design was identical to that for Fig. 2. Mice received either no cells, total spleen cells from wild-type BALB/c mice, CD8 cells from BALB/c mice, total spleen cells from IFN-γ knockout mice, or CD8 cells from IFN-γ knockout mice. Mice which received cells from control BALB/c mice (total spleen or CD8 cells) showed significantly reduced CFU counts compared to naive athymic mice (P < 0.05); there were no significant differences between values for naive athymic mice and mice which received either total spleen cells or CD8 cells from IFN-γ knockout BALB/c mice.
Thus, the results reported in this study confirm the role of CD8 T cells in the control of M. tuberculosis infections in mice. We have also demonstrated that this control requires the ability of the CD8 cells to produce IFN-γ, suggesting that such cells may exert their effects through classical cytokine-mediated macrophage activation rather than through a cytotoxic mechanism. The recent demonstration that human CD1-restricted CD8 T cells were able to kill mycobacteria in vitro through a cytotoxicity-mediated pathway (30) suggests that different subpopulations of CD8 cells may have different effector mechanisms; since no murine equivalent of the CD1-restricted CD8 T cell has been described, this mechanism may be absent in mice. Alternatively, the results reported earlier for murine CD8 T-cell lines (29) or human CD8, CD1-restricted T-cell lines (30) may reflect the activity of primed or memory T cells, whereas the results reported in the present study reflect the activity of unprimed cells. Primed CD8 T cells have been shown to be hyperreactive to antigenic challenge in vitro and may employ different effector mechanisms. That production of IFN-γ by CD8 T cells is required in order to control infection has also been reported for viral infections (11, 26), where cytotoxicity has long been thought to be the major mechanism of CD8-mediated antiviral activity. IFN-γ and other cytokines have been shown to be major components of the mechanism by which hepatitis B virus is controlled in mice by CD8 cells without the killing of hepatocytes (11). The results reported in this study demonstrate that IFN-γ is essential for CD8-mediated protection against M. tuberculosis infection in mice.
REFERENCES
- 1.Bancroft G J, Schreiber R D, Unanue E R. Natural immunity: a T-cell-independent pathway of macrophage activation defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 2.Beckman E M, Porcelli S A, Morlta C T, Behar S M, Furlong S T, Brenner M B. Recognition of a lipid antigen by CD1-restricted αβ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 3.Bothamley G H, Festenstein G H, Newland A. Protective role for CD8+ cells in tuberculosis. Lancet. 1992;339:315–316. doi: 10.1016/0140-6736(92)91397-q. [DOI] [PubMed] [Google Scholar]
- 4.Colston M J, Hilson G R F. Growth of Mycobacterium leprae and M. marinum in congenitally athymic (nude) mice. Nature. 1976;262:399–401. doi: 10.1038/262399a0. [DOI] [PubMed] [Google Scholar]
- 5.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A M, D’Souza C, Frank A A, Orme I M. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 8.Dalton D K, Pitts-Meek S, Keskav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 9.Flynn J-A L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidotti L G, Chisari F V. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 12.Hopewell P C. Impact of human immunodeficiency virus infection on the epidemiology, clinical features, management, and control of tuberculosis. Clin Infect Dis. 1992;15:540–547. doi: 10.1093/clind/15.3.540. [DOI] [PubMed] [Google Scholar]
- 13.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and porin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 14.Kaleab B, Ottenoff T, Converse P, Halapi E, Tadesse G, Rottenberg M, Kiessling R. Mycobacterial-induced cytotoxic T cells as well as nonspecific killer cells derived from healthy individuals and leprosy patients. Eur J Immunol. 1990;20:2651. doi: 10.1002/eji.1830201219. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann S H E. Immunity to intracellular microbial pathogens. Immunol Today. 1995;16:338–342. doi: 10.1016/0167-5699(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 16.Laochumroonvorapong P, Wang J, Liu C-C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Gros G, Erard G. Non-cytotoxic, IL-4, IL-5, IL-10 producing CD8+ T cells: their activation and effector functions. Curr Opin Immunol. 1994;6:453–457. doi: 10.1016/0952-7915(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 18.Liu C-C, Walsh C M, Young J D-E. Perforin: structure and function. Immunol Today. 1995;16:194–201. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 19.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann S H E. Different roles of α/β and γ/δ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 20.Müller I, Cobbold S P, Waldmann H, Kaufmann S H E. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 22.North R J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973;7:166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- 23.Orme I M, Furney S K, Roberts A D. Dissemination of enteric Mycobacterium avium infections in mice rendered immunodeficient by thymectomy and CD4 depletion or by prior infection with murine AIDS retroviruses. Infect Immun. 1992;60:4747–4753. doi: 10.1128/iai.60.11.4747-4753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 25.Orme I M, Miller E S, Roberts A D, Furney S K, Griffin J P, Dobos K M, Chi D, Rivoire B, Brennan P J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection: evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–196. [PubMed] [Google Scholar]
- 26.Ramsay A J, Ruby J, Ramshaw I A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 27.Rouvier E, Luciani M-F, Golstein P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, Modlin R L. CD-1 restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 29.Silva C L, Silva M F, Pietro R C L R, Lowrie D B. Characterization of T cells that confer a high degree of protective immunity against tuberculosis in mice after vaccination with tumor cells expressing mycobacterial hsp65. Infect Immun. 1996;64:2400–2407. doi: 10.1128/iai.64.7.2400-2407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenger S, Mazzaccaro R J, Uyemura K, Cho S, Barnes P F, Rosat R-P, Sette A, Brenner M B, Porcelli S A, Bloom B R, Modlin R L. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 31.Suda T, Nagata S. Purification and characterisation of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner J, Dockrell H M. Stimulation of human peripheral blood mononuclear cells with live Mycobacterium tuberculosis BCG activates cytolytic CD8+ T cells in vitro. Immunology. 1996;87:339–342. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]