Abstract
Aim
Postoperative small bowel obstruction (SBO) is one of the major complications that is mainly caused by postoperative adhesion. Recently, the antiadhesion membrane has become popular for postoperative SBO prevention. However, its efficacy is yet to be confirmed in the gastric cancer surgery field. Here, we conducted the supplemental analysis of the randomized controlled trial JCOG1001 to investigate the efficacy of the antiadhesion membrane on SBO prevention in patients with open gastrectomy for gastric cancer.
Methods
Of the 1204 patients enrolled in JCOG1001, 1200 patients were included. The development of SBO of Grade ≥ IIIa according to the Clavien–Dindo classification was recorded. Univariable and multivariable analyses were performed using the Fine and Gray model to determine the risk factors for SBO.
Results
Fifty‐one patients developed SBO (median follow‐up duration: 5.6 years). Total gastrectomy, combined resection, and blood loss significantly increased the risk for SBO development in the univariable analysis. Large amount of blood loss was independently associated with SBO development in the multivariable analysis (hazard ratio [HR], 3.089; 95% confidence interval [CI], 1.562–6.109, p = 0.0012). Antiadhesion membrane did not reduce the risk for SBO (HR, 1.299; 95% CI 0.683–2.470; p = 0.4246). In the patients belonging to subgroup analyses who received distal and total gastrectomy, the antiadhesion membrane was not associated with the incidence of SBO.
Conclusions
Antiadhesion membrane did not decrease SBO occurrence rate after open gastrectomy. Therefore, the use of antiadhesion membrane would not be effective for preventing SBO in gastric cancer surgery.
Keywords: antiadhesion membrane, gastrectomy, gastric cancer, roux‐en‐Y reconstruction, small bowel obstruction
Antiadhesion membrane did not decrease small bowel obstruction occurrence rate after open gastrectomy. Therefore, the use of antiadhesion membrane would not be effective for preventing SBO in gastric cancer surgery.
1. INTRODUCTION
Small bowel obstruction (SBO) is one of the major complications after gastric surgery. It is associated with a deterioration of patients' quality of life, with even fatal consequences. A large meta‐analysis revealed that 9% of patients develop postoperative SBO. 1 Among the several causes of SBO, adhesive SBO was the most frequent pattern, 1 and preventing adhesion postoperatively is important to prevent the development of postoperative SBO. Therefore, the antiadhesion membrane has recently become popular for postoperative SBO prevention. 2 , 3
Gastrectomy is an important treatment strategy for gastric cancer. As with other surgeries, SBO is a major concern after gastrectomy, 4 , 5 , 6 , 7 , 8 and an antiadhesion membrane is gradually accepted in the gastric surgery field as well. Several studies have investigated the efficacy of antiadhesion membranes in this context. 5 , 6 , 8 Although some studies reported the efficacy of the antiadhesion membrane, 6 , 8 a randomized controlled trial (RCT) with a relatively small sample size failed to show this efficacy. 5 Therefore, the benefit of antiadhesion membranes on SBO prevention in gastric cancer surgery has not been clear.
The Japan Clinical Oncology Group (JCOG) trial JCOG1001 9 is a multicenter RCT designed to test the superiority of bursectomy to conventional omentectomy in patients with cT3–4 advanced gastric cancer. 9 This RCT failed to show the superiority of bursectomy on long‐term survival. In this follow‐up analysis using the data from the JCOG1001 trial, we investigated the association between the antiadhesion membrane and the SBO occurrence in patients undergoing open gastrectomy for gastric cancer, as the antiadhesion membrane was not applied in approximately one‐third of the enrolled patients. Additionally, we examined the correlation of antiadhesion membrane with SBO occurrence classified by types of surgery in the study.
2. METHODS
2.1. Patients
This supplemental study was conducted using data from a multicenter RCT (JCOG1001 9 ). The JCOG1001 trial was conducted at 57 hospitals in Japan, and the patients who participated in the JCOG1001 trial were enrolled between June 1, 2010, and March 30, 2015. The final follow‐up was made on April 17, 2020, and the data analysis for the present study was performed on March 7, 2022. The eligibility criteria were detailed elsewhere. 9 In brief, patients aged 20–80 years with cT3 (SS)‐T4a (SE) gastric cancer according to the 14th Japanese Classification of Gastric Carcinoma, Eastern Cooperative Oncology Group performance status of 0–1, and a body mass index (BMI) of <30 kg/m2, and without a history of digestive surgery (except staging laparoscopy, appendectomy for appendicitis, and laparoscopic cholecystectomy for cholelithiasis) were enrolled. We cannot provide the patients' ethnic distribution as the race was not recorded in case report forms. Of the 1204 patients enrolled in JCOG1001, one with exploratory laparotomy, one with Billroth‐II (B‐II) reconstruction, and two who did not undergo protocol treatment were excluded. As a result, 1200 patients were included in this supplemental analysis. All data used in this study were obtained from the case report forms of JCOG1001. JCOG1001 was registered with UMIN‐CTR (number UMIN000003688). The ethics committee of all participating institutions approved the study protocol and written informed consent, including permission for the secondary use of the trial data, was obtained from all patients upon enrollment in JCOG1001. All procedures followed were in accordance with the ethical standards on human experimentation and with the Declaration of Helsinki of 1924 and later versions.
2.2. Surgical procedure and follow‐up
All patients included in this study underwent either open distal gastrectomy followed by Billroth I (DG–BI) or Roux‐en‐Y reconstruction (DG–RY), or open total gastrectomy followed by Roux‐en‐Y reconstruction (TG–RY) with radical lymph node dissection. All patients underwent complete omentectomy, among whom nearly half underwent complete bursectomy as well. Seprafilm (Baxter, Deerfield, IL, USA) was employed as antiadhesion membrane in this study. It is a synthetic absorbable adhesion barrier and has been reported to reduce adhesion. 2 , 10 Its use was selected at the physicians' discretion and recorded according to the study protocol. The conditions or restrictions of its use were not regulated. In the Roux‐en‐Y (RY) reconstruction, closure of the Petersen's and jejunojejunostomy defects was not mandatory. Each attending surgeon decided on these procedures.
2.3. Definition of SBO
As part of the study protocol, details of early (until the first discharge after surgery) and late (after first discharge from surgery) complications, including SBO, were collected, and the dates of complications according to Clavien–Dindo classification 11 were recorded. Grade ≥III SBOs were regarded as complications. The diagnosis of SBOs also depended on the attending surgeon. In this study, only the initial SBO occurrences were evaluated. To avoid contamination of SBO caused by peritoneal dissemination, (1) patients diagnosed with peritoneal recurrence before SBO development and (2) those diagnosed with peritoneal recurrence within 90 days after SBO development were not included as SBO events in this study.
2.4. Statistical analysis
Univariable and multivariable analyses were performed to explore if the antiadhesion membrane was associated with the SBO occurrence. Fisher's exact and chi‐squared tests were used to compare the patient characteristics between the groups with and without antiadhesion membrane for categorical values. The medians and ranges were determined for continuous values and Wilcoxon rank sum test was performed for their comparisons. Bursectomy (Yes/No), preoperative BMI (≥25/<25 kg/m2), types of surgery (DG/TG), types of reconstruction (B‐I/RY), antiadhesion membrane use (Yes/No), combined resection (Yes/No), operative time (<238/≥238 min), and blood loss (<285/≥285 mL) were included as factors for the univariable and multivariable analyses in the overall population. The efficacy of antiadhesion membrane in the subgroups according to the types of surgery (i.e., DG, DG–BI, DG–RY, and TG–RY) was also investigated. In addition to the abovementioned variables, the route of the Roux limb (antecolic/retrocolic) was also included in the case of RY reconstruction. The time to SBO occurrence from surgery was estimated using the cumulative incidence approach. Deceased patients (321 patients) or those who developed peritoneal recurrence without SBO (five patients) were considered a competing risk for the analyses. Hazard ratios (HRs) and 95% confidence intervals (95% CI) were estimated by using the Fine–Gray model. Since this study was not an RCT, we also conducted a post hoc propensity score matching (PSM) analysis to minimize bias and verify the efficacy results of the antiadhesion membrane. Patients with or without Seprafilm groups were matched 1:1 using the greedy nearest propensity score on the logit scale with a 0.2 × standard deviation caliper. The balance of the matched cohort was assessed by calculating the standardized difference, and the cut off value of the meaningful imbalance was defined as an absolute standardized mean difference of 0.25. Two‐sided p values were calculated, and p values of <0.05 were considered statistically significant. SAS ver. 9.4 (SAS Institute Inc. Cary, NC, USA) software package was used for all statistical analyses.
3. RESULTS
3.1. Patients' characteristics
Antiadhesive membrane was used in 821 patients (68.4%). Table 1 shows the characteristics of the patients. Patients with antiadhesion membrane were significantly older (p = 0.001). Sex, type of surgery, surgical history, and bursectomy status were not different between the groups with and without antiadhesion membrane. Antiadhesion membrane was used more frequently in patients with RY reconstruction through the antecolic route (p < 0.001) and those who underwent gastrectomy with combined resection (p = 0.003). The amount of blood loss during surgery was larger in the group with antiadhesion membrane (p = 0.036). The other factors, including pathological stage and postoperative adjuvant chemotherapy were not different between the groups.
TABLE 1.
Patient characteristics.
| Without Seprafilm n = 379 | With Seprafilm n = 821 | p value | |
|---|---|---|---|
| Age, years, median (range) | 64 (29–80) | 66 (29–80) | 0.001 |
| Sex, No. (%) | |||
| Male | 269 (71.0) | 574 (69.9) | 0.734 |
| Female | 110 (29.0) | 247 (30.1) | |
| BMI, kg/m2, median (range) | 22.9 (15–29.6) | 22.7 (14.9–29.9) | 0.805 |
| Surgical history b , No. (%) | |||
| No | 278 (73.4) | 630 (76.7) | 0.219 |
| Yes | 101 (26.6) | 191 (23.3) | |
| Surgical procedure, No. (%) | |||
| Distal gastrectomy | 258 (68.1) | 527 (64.2) | 0.192 |
| Total gastrectomy | 121 (31.9) | 294 (35.8) | |
| Bursectomy, No. (%) | |||
| No | 200 (52.8) | 407 (49.6) | 0.321 |
| Yes | 179 (47.2) | 414 (50.4) | |
| Combined resection, No. (%) | |||
| No | 230 (60.7) | 422 (51.4) | 0.003 |
| Yes | 149 (39.3) | 399 (48.6) | |
| Type of reconstruction, No. (%) | |||
| RY (antecolic route) | 65 (17.2) | 237 (28.9) | |
| RY (retrocolic route) | 217 (57.3) | 355 (43.2) | <0.001 |
| BI | 97 (25.6) | 229 (27.9) | |
| Blood loss, mL, median (range) | 260 (6–3068) | 294 (0–2140) | 0.036 |
| Operative time, min, median (range) | 239 (80–453) | 238 (83–630) | 0.845 |
| Adjuvant chemotherapy, No. (%) | |||
| No | 148 (39.1) | 323 (39.3) | 0.949 |
| Yes | 231 (60.9) | 498 (60.7) | |
| Pathological stage, No. (%) | |||
| IA | 16 (4.2) | 41 (5.0) | |
| IB | 24 (6.3) | 74 (9.0) | |
| IIA | 77 (20.3) | 129 (15.7) | |
| IIB | 74 (19.5) | 149 (18.1) | 0.178 a |
| IIIA | 54 (14.2) | 121 (14.7) | |
| IIIB | 69 (18.2) | 148 (18.0) | |
| IIIC | 46 (12.1) | 91 (11.1) | |
| IV | 19 (5.0) | 68 (8.3) | |
Abbreviations: BI, Billroth I; BMI, body mass index, RY, Roux‐en‐Y.
Chi‐squared test.
Surgery other than digestive surgery, appendectomy for appendicitis, staging laparoscopy, or laparoscopic cholecystectomy.
3.2. Risk factors for SBO development and the efficacy of antiadhesion membrane
In total, 51 patients (4.3%) developed SBO; of these, 14, 32, three, and two patients were of Grade IIIa, IIIb, IVa, and IVb, respectively. The results of the univariable and multivariable analyses are shown in Table 2. Although TG (HR, 1.841; 95% CI, 1.063–3.189; p = 0.0295), combined resection (HR, 2.035; 95% CI, 1.152–3.593; p = 0.0144), and blood loss (HR, 2.710; 95% CI, 1.467–5.006; p = 0.0015) were significantly associated with SBO development in the univariable analyses, only blood loss was found to be associated with SBO development in the multivariable analysis (HR, 3.089; 95% CI, 1.562–6.109; p = 0.0012). In this study, patients with major abdominal surgery were excluded from the protocol. Therefore, intraabdominal surgical history included only staging laparoscopy, appendectomy, and laparoscopic cholecystectomy. A history of these surgeries was not associated with SBO development. Figure 1 shows the effect of antiadhesion membrane on SBO occurrence. Antiadhesion membrane use did not influence the SBO occurrence rate (HR, 1.299; 95% CI 0.683–2.470; p = 0.4246). The risk factors for SBO in DG, and TG–RY cases are shown in Tables 3 and 4, respectively. The types of reconstruction (i.e., DG–BI and DG–RY) further classified patients with DG as demonstrated in Tables 5 and 6. In patients with DG, and DG–RY, no factor influenced SBO occurrence. In patients with DG–BI, large amount of blood loss was associated with increased risk of SBO (HR, 12.881; 95% CI, 1.191–139.243; p = 0.0354). Shorter operative time was associated with the risk of SBO (HR, 0.292; 95% CI, 0.132–0.643; p = 0.0022) in addition to large amount of blood loss (HR, 7.593; 95% CI, 2.280–25.285; p = 0.0010) in TG–RY patients. Antiadhesion membrane did not influence SBO development in any type of surgery.
TABLE 2.
Factors associated with SBO in the overall population.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Events/N | 5‐year cumulative incidence rates (95% CI) | HR (95% CI) | p value | HR (95% CI) | p value | |
| Bursectomy | ||||||
| No | 27/607 | 4.3 (2.9–6.1) | 1 | 1 | ||
| Yes | 24/593 | 3.7 (2.4–5.5) | 0.904 (0.522–1.565) | 0.7176 | 0.838 (0.477–1.475) | 0.5407 |
| BMI, kg/m2 | ||||||
| <25 | 42/930 | 4.3 (3.1–5.8) | 1 | 1 | ||
| ≥25 | 9/270 | 3.0 (1.4–5.6) | 0.730 (0.357–1.492) | 0.3875 | 0.630 (0.296–1.340) | 0.2302 |
| Surgical procedure | ||||||
| DG | 26/785 | 3.1 (2.0–4.4) | 1 | 1 | ||
| TG | 25/415 | 5.8 (3.8–8.4) | 1.841 (1.063–3.189) | 0.0295 | 1.106 (0.579–2.112) | 0.7603 |
| Type of reconstruction | ||||||
| BI | 8/326 | 2.2 (1.0–4.2) | 1 | 1 | ||
| RY | 43/874 | 4.7 (3.4–6.3) | 2.019 (0.949–4.296) | 0.0682 | 1.684 (0.710–3.994) | 0.2369 |
| Seprafilm | ||||||
| No | 13/379 | 3.2 (1.7–5.3) | 1 | 1 | ||
| Yes | 38/821 | 4.4 (3.2–6.0) | 1.362 (0.725–2.556) | 0.3368 | 1.299 (0.683–2.470) | 0.4246 |
| Combined resection | ||||||
| No | 19/652 | 2.6 (1.6–4.1) | 1 | 1 | ||
| Yes | 32/548 | 5.7 (4.0–7.9) | 2.035 (1.152–3.593) | 0.0144 | 1.516 (0.803–2.861) | 0.1998 |
| Operative time, min | ||||||
| <238 | 26/593 | 4.4 (3.0–6.3) | 1 | 1 | ||
| ≥238 | 25/607 | 3.6 (2.4–5.4) | 0.929 (0.538–1.606) | 0.7926 | 0.555 (0.294–1.047) | 0.0692 |
| Blood loss, mL | ||||||
| <285 | 14/600 | 2.2 (1.2–3.6) | 1 | 1 | ||
| ≥285 | 37/600 | 5.9 (4.2–8.0) | 2.710 (1.467–5.006) | 0.0015 | 3.089 (1.562–6.109) | 0.0012 |
| Surgical history | ||||||
| No | 34/908 | 3.7 (2.6–5.0) | 1 | |||
| Yes | 17/292 | 5.1 (3.0–8.1) | 1.582 (0.884–2.831) | 0.1225 | ||
Abbreviations: 95% CI, 95% confidence interval; BI, Billroth I; BMI, body mass index; DG, distal gastrectomy; HR, hazard ratio; RY, Roux‐en‐Y; TG, total gastrectomy.
FIGURE 1.
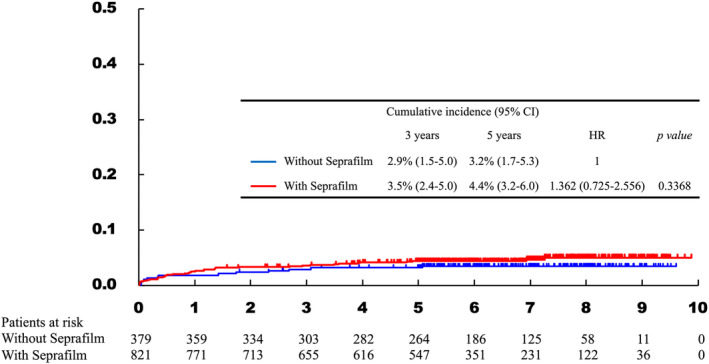
Cumulative incidence curve of patients with and without antiadhesion membrane. The incidence of small bowel obstruction was not significantly different between patients with and without antiadhesion membrane.
TABLE 3.
Factors associated with SBO in patients with DG.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Events/N | 5‐year cumulative incidence rates (95% CI) | HR (95% CI) | p value | HR (95% CI) | p value | |
| Bursectomy | ||||||
| No | 10/400 | 2.3 (1.1–4.1) | 1 | 1 | ||
| Yes | 16/385 | 3.9 (2.3–6.2) | 1.664 (0.756–3.665) | 0.2061 | 1.498 (0.637–3.521) | 0.3539 |
| BMI, kg/m2 | ||||||
| <25 | 20/604 | 3.2 (2.0–4.8) | 1 | 1 | ||
| ≥25 | 6/181 | 2.8 (1.0–6.0) | 0.991 (0.401–2.451) | 0.9844 | 0.867 (0.321–2.346) | 0.7794 |
| Type of reconstruction | ||||||
| BI | 8/326 | 2.2 (1.0–4.2) | 1 | 1 | ||
| RY | 18/459 | 3.7 (2.2–5.7) | 1.592 (0.692–3.663) | 0.2739 | 1.452 (0.592–3.564) | 0.4153 |
| Seprafilm | ||||||
| No | 9/258 | 3.5 (1.7–6.3) | 1 | 1 | ||
| Yes | 17/527 | 2.9 (1.7–4.6) | 0.917 (0.408–2.062) | 0.8341 | 0.882 (0.365–2.132) | 0.7809 |
| Combined resection | ||||||
| No | 18/593 | 2.7 (1.6–4.3) | 1 | 1 | ||
| Yes | 8/192 | 4.2 (2.0–7.7) | 1.385 (0.602–3.189) | 0.4439 | 1.248 (0.517–3.013) | 0.6222 |
| Operative time, min | ||||||
| <238 | 12/439 | 2.7 (1.5–4.6) | 1 | 1 | ||
| ≥238 | 14/346 | 3.5 (1.9–5.8) | 1.472 (0.683–3.171) | 0.3234 | 1.025 (0.408–2.576) | 0.9574 |
| Blood loss, mL | ||||||
| <285 | 11/443 | 2.3 (1.2–4.0) | 1 | 1 | ||
| ≥285 | 15/342 | 4.1 (2.4–6.6) | 1.792 (0.824–3.896) | 0.1412 | 1.623 (0.647–4.075) | 0.3021 |
Abbreviations: 95% CI, 95% confidence interval; BI, Billroth I; BMI, body mass index; DG, distal gastrectomy; HR, hazard ratio; RY, Roux‐en‐Y; TG, total gastrectomy.
TABLE 4.
Factors associated with SBO in patients with TG–RY.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Events/N | 5‐year cumulative incidence rates (95% CI) | HR (95% CI) | p value | HR (95% CI) | p value | |
| Bursectomy | ||||||
| No | 17/207 | 8.2 (5.0–12.5) | 1 | 1 | ||
| Yes | 8/208 | 3.4 (1.5–6.6) | 0.459 (0.198–1.060) | 0.0682 | 0.439 (0.187–1.030) | 0.0586 |
| BMI, kg/m2 | ||||||
| <25 | 22/326 | 6.5 (4.1–9.5) | 1 | 1 | ||
| ≥25 | 3/89 | 3.5 (0.9–9.1) | 0.487 (0.148–1.602) | 0.2363 | 0.416 (0.127–1.364) | 0.1479 |
| Seprafilm | ||||||
| No | 4/121 | 2.5 (0.7–6.6) | 1 | 1 | ||
| Yes | 21/294 | 7.2 (4.6–10.6) | 2.245 (0.782–6.451) | 0.1330 | 2.390 (0.822–6.947) | 0.1094 |
| Combined resection | ||||||
| No | 1/59 | 1.7 (0.1–8.1) | 1 | 1 | ||
| Yes | 24/356 | 6.5 (4.3–9.4) | 4.108 (0.564–29.921) | 0.1631 | 3.987 (0.576–27.581) | 0.1610 |
| Operative time, min | ||||||
| <238 | 14/154 | 9.2 (5.2–14.4) | 1 | 1 | ||
| ≥238 | 11/261 | 3.9 (2.0–6.7) | 0.448 (0.204–0.983) | 0.0453 | 0.292 (0.132–0.643) | 0.0022 |
| Blood loss, mL | ||||||
| <285 | 3/157 | 1.9 (0.5–5.1) | 1 | 1 | ||
| ≥285 | 22/258 | 8.2 (5.3–12.0) | 4.690 (1.415–15.548) | 0.0115 | 7.593 (2.280–25.285) | 0.0010 |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; HR, hazard ratio.
TABLE 5.
Factors associated with SBO in patients with DG–B‐I.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Events/N | 5‐year cumulative incidence rates (95% CI) | HR (95% CI) | p value | HR (95% CI) | p‐value | |
| Bursectomy | ||||||
| No | 4/171 | 1.8 (0.5–4.7) | 1 | 1 | ||
| Yes | 4/155 | 2.6 (0.9–6.1) | 1.119 (0.284–4.412) | 0.8721 | 0.691 (0.116–4.128) | 0.6851 |
| BMI, kg/m2 | ||||||
| <25 | 5/257 | 1.6 (0.5–3.7) | 1 | 1 | ||
| ≥25 | 3/69 | 4.3 (1.1–11.1) | 2.235 (0.540–9.255) | 0.2673 | 1.517 (0.266–8.666) | 0.6392 |
| Seprafilm | ||||||
| No | 3/97 | 3.1 (0.8–8.1) | 1 | 1 | ||
| Yes | 5/229 | 1.8 (0.6–4.2) | 0.677 (0.164–2.801) | 0.5903 | 0.518 (0.114–2.347) | 0.3935 |
| Combined resection | ||||||
| No | 7/275 | 2.2 (0.9–4.5) | 1 | 1 | ||
| Yes | 1/51 | 2.0 (0.2–9.2) | 0.748 (0.090–6.193) | 0.7877 | 0.584 (0.073–4.670) | 0.6119 |
| Operative time, min | ||||||
| <238 | 5/244 | 2.1 (0.8–4.5) | 1 | 1 | ||
| ≥238 | 3/82 | 2.4 (0.5–7.7) | 1.790 (0.430–7.448) | 0.4236 | 0.973 (0.126–7.531) | 0.9788 |
| Blood loss, mL | ||||||
| <285 | 1/199 | 0.5 (0.0–2.6) | 1 | 1 | ||
| ≥285 | 7/127 | 4.7 (1.9–9.5) | 11.259 (1.391–91.130) | 0.0232 | 12.881 (1.191–139.243) | 0.0354 |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; HR, hazard ratio.
TABLE 6.
Factors associated with SBO in patients with DG–RY.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Events/N | 5‐year cumulative incidence rates (95% CI) | HR (95% CI) | p value | HR (95% CI) | p value | |
| Bursectomy | ||||||
| No | 6/229 | 2.6 (1.1–5.3) | 1 | 1 | ||
| Yes | 12/230 | 4.8 (2.5–8.1) | 1.988 (0.747–5.290) | 0.1689 | 1.971 (0.753–5.160) | 0.1668 |
| BMI, kg/m2 | ||||||
| <25 | 15/347 | 4.3 (2.5–6.9) | 1 | 1 | ||
| ≥25 | 3/112 | 1.8 (0.3–5.7) | 0.610 (0.178–2.092) | 0.4322 | 0.627 (0.168–2.348) | 0.4885 |
| Seprafilm | ||||||
| No | 6/161 | 3.7 (1.5–7.5) | 1 | 1 | ||
| Yes | 12/298 | 3.7 (2.0–6.3) | 1.080 (0.405–2.879) | 0.8784 | 1.004 (0.306–3.299) | 0.9947 |
| Combined resection | ||||||
| No | 11/318 | 3.2 (1.6–5.5) | 1 | 1 | ||
| Yes | 7/141 | 5.0 (2.2–9.5) | 1.455 (0.565–3.748) | 0.4377 | 1.468 (0.520–4.142) | 0.4686 |
| Route of Roux limb | ||||||
| Antecolic | 6/166 | 3.6 (1.5–7.3) | 1 | 1 | ||
| Retrocloic | 12/293 | 3.8 (2.0–6.4) | 1.127 (0.423–3.003) | 0.8105 | 1.051 (0.348–3.172) | 0.9301 |
| Operative time, min | ||||||
| <238 | 7/195 | 3.6 (1.6–6.9) | 1 | 1 | ||
| ≥238 | 11/264 | 3.8 (1.9–6.6) | 1.151 (0.449–2.955) | 0.7697 | 1.083 (0.405–2.896) | 0.8745 |
| Blood loss, mL | ||||||
| <285 | 10/244 | 3.7 (1.8–6.6) | 1 | 1 | ||
| ≥285 | 8/215 | 3.7 (1.7–6.9) | 0.913 (0.362–2.303) | 0.8472 | 0.848 (0.306–2.345) | 0.7502 |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; HR, hazard ratio.
3.3. Results of propensity score matching
Patient characteristics after PSM are demonstrated in Table S1. After PSM, there were 366 patients in each group. The absolute standardized mean differences of all factors were within 0.25. The results of the cumulative incidence curve of patients with and without antiadhesion membrane are shown in Figure S1. The antiadhesion membrane did not decrease the incidence of SBO (HR, 1.538; 95% CI, 0.929–2.549; p = 0.0944); on the contrary, it tended to increase SBO.
3.4. Relationship between SBO and other complications
Table S2 shows the relationship between SBO and other intraabdominal complications. No other complications were associated with SBO.
4. DISCUSSION
In this study, we used data from the multicenter RCT JCOG1001, and large amount of blood loss was identified as an independent risk factor for SBO after open gastrectomy for gastric cancer. Moreover, we also observed that the antiadhesion membrane did not influence the incidence of SBO. We also analyzed the risk factors for SBO in each operative procedure, which was not reported in previous studies. The antiadhesion membrane was not associated with the incidence of SBO in any type of gastrectomy. It has been previously reported that the antiadhesion membrane Seprafilm could reduce adhesion, 2 , 10 and might have some benefits during reoperation. 12 , 13 Thus, Seprafilm may benefit patients who are expected to undergo future surgery, such as colon cancer patients with liver metastasis or constructing diverting stoma. However, these situations are extremely rare in the context of gastric cancer treatment.
Antiadhesion membrane did not prevent SBO development. Several reasons might explain these unexpected results. First, an antiadhesion membrane can only prevent adhesion between the intestine and abdominal wall. The area where antiadhesion membrane can be used is limited since the Japanese national health system coverage sets the upper limit of its use. SBO can occur due to adhesion other than between the intestine and abdominal wall, i.e., intestine–intestine, intestine–mesenteric fat, or intestine–other organs. In the previous reports, studies using many sheets of antiadhesion agent tended to reduce the risk of severe SBO requiring surgery. 2 , 3 , 5 In this study, the conditions or restrictions of antiadhesion membrane use were not regulated. If an antiadhesion membrane is used not only in the space between the abdominal wall and intestine but also in other places, the antiadhesion agent might reduce the risk of adhesive SBO development. Unfortunately, this is impractical for economic reasons. Based on this, the use of spray‐type adhesion barrier might be beneficial. The efficacy of such adhesion barriers would be worth investigating in future. Another possible explanation for the unforeseen results is the SBO caused by an internal hernia (IH). The incidence of IH after open gastrectomy has been reported to be 0.2%–4.1%. 14 , 15 , 16 , 17 In this study, 37 patients received surgical treatment for SBO. Although the details of the surgery have not been recorded, some of the patients might have undergone surgery for IH. In RY reconstruction cases, IH can be caused by artificial mesenteric defects (Petersen's hernia) and gastric cancer surgery is different from other general surgeries in this aspect. Laparoscopic surgery is reported to be highly associated with IH as it leads to less adhesion formation. 14 , 18 , 19 , 20 In other words, an antiadhesion membrane can increase the risk for SBO caused by IH after open gastrectomy. Significant blood loss was associated with SBO occurrence. As autologous blood is used in pleurodesis, the blood loss is associated with the adhesions, and the association between blood loss and an increase in SBO incidence is reasonable.
The efficacy of the antiadhesion membrane was not consistent across previous studies. Two RCTs presented opposite results. 5 , 6 Hayashi et al. 5 concluded that Seprafilm did not show benefit in terms of SBO prevention, whereas Kim et al. 6 presented the efficacy of the antiadhesion membrane. This discrepancy might be due to the variation of reconstruction. B‐I reconstruction was performed in 61.8% of the patients and no patient underwent Billroth‐II (B‐II) reconstruction in the former study, whereas only 8.4% and 40.7% of the patients underwent B‐I and B‐II reconstructions, respectively, in the latter. B‐I reconstruction is reported to be associated with a lower incidence of SBO, whereas B‐II reconstruction has the highest incidence. 4 As a result, the incidence of SBO in the group without antiadhesion membrane was much higher in the latter study, although the incidence of SBO in patients with antiadhesion membrane differed only slightly between these studies. Additionally, the difference in the antiadhesion membrane used (Seprafilm in the former study, and Guardix‐SG [Genewel, Dongsung Company, Seongnam, Korea] in the latter) is also indispensable. Our study was similar to Hayashi et al.'s study in that Seprafilm was used as an antiadhesion membrane, only patients with B‐I and RY reconstructions were included, and ultimately the inefficacy of the antiadhesion membrane was shown. The incidence of SBO in the patients without antiadhesion membrane was lower in our study than in these previous studies (3.2% in our study, and 12.2%–20% in the previous study), which we consider as an important difference. First, the definition of SBO might have differed between this study and previous studies. Our study included only severe SBO (Clavien–Dindo Grade IIIa or more) and patients with mild SBO might have been included in other studies. Secondly, although these studies were RCTs, the number of patients included in each study was much smaller (144 and 214 patients) than our study, and the follow‐up periods were short (3 and 1 year). Thus, the incidence of SBO could be imprecise. This study used data from a multicenter RCT, and accordingly, the number of patients was much larger (1200 patients) and the follow‐up period was longer (median follow‐up duration: 5.6 years), which is more likely to reflect the real world‐expected proportion of SBO occurrence. Therefore, based on our study results, Seprafilm is not effective in the prevention of SBO after gastrectomy with B‐I and RY reconstructions.
Regarding detailed gastrectomy, Kawamura et al. 8 reported the efficacy of Seprafilm on SBO prevention in DG–BI patients. This is inconsistent with our results showing Seprafilm's irrelevance to SBO prevention in this population. Kawamura et al.'s study was a single‐institution retrospective study. Although both the previous and present studies included only some patients who developed SBO after DG–BI, they investigated the efficacy of Seprafilm by using a historical control, which is a major problem. Thus, our cohort would be more appropriate for the evaluations.
There are a few limitations to our study. First, although we included a large number of patients, this study is not a randomized comparison. Parameters, such as age, amount of blood loss, combined resection, and types of reconstruction, significantly differed in the background between the groups with and without antiadhesion membrane. Since the use of the antiadhesion membrane was not unified by the protocol, and whether or not to use the antiadhesion membrane was decided by each surgeon or institution, heterogeneity in the decisions of surgeons or institutions is the most plausible explanation for these differences. We conducted PSM to verify the primary results of this study and showed that antiadhesion membrane is not effective for SBO prevention. Another study limitation is that the causes of SBO were not recorded. It is possible that the antiadhesion membrane works oppositely on adhesion and IH‐related SBOs. The actual causes of SBO were unclear and may include peritoneal recurrence. Nevertheless, precisely diagnosing the cause of SBO is sometimes difficult without surgery, and it was important to include all SBO types, which was the case of this study. Another limitation was that, in the evaluation of the cumulative incidence of SBO, only the first event of SBO was recorded and repeated cases were not evaluated in this study.
In conclusion, an antiadhesion membrane did not decrease the SBO occurrence rate after open gastrectomy. Therefore, the use of antiadhesion membrane would not be effective for prevention of SBO in gastric cancer surgery.
AUTHOR CONTRIBUTIONS
Tetsuro Toriumi, Masanori Terashima, Junki Mizusawa, Kohei Uemura, Yukinori Kurokawa, and Seiji Ito conceived and designed this article. Masanori Terashima, Junki Mizusawa, Kohei Uemura, Yukinori Kurokawa, Shuji Takiguchi, Yuichiro Doki, Jun Hihara, Hiroshi Imamura, Akinori Takagane, Seiji Ito, Takaki Yoshikawa, Takeshi Sano, and Mitsuru Sasako participated in the acquisition of data. Junki Mizusawa and Kohei Uemura performed the statistical analysis. Tetsuro Toriumi and Masanori Terashima interpreted the data and drafted the article. Junki Mizusawa, Kohei Uemura, Yukinori Kurokawa, Shuji Takiguchi, Yuichiro Doki, Jun Hihara, Hiroshi Imamura, Akinori Takagane, Seiji Ito, Takaki Yoshikawa, Takeshi Sano, and Mitsuru Sasako revised the manuscript critically. All authors approved the final version of this manuscript to be published.
FUNDING INFORMATION
This study was supported by the National Cancer Center Research and Development Funds (2020‐J‐3, 2023‐J‐03), and by AMED under Grant Number JP16ck0106048.
CONFLICT OF INTEREST STATEMENT
Yuichiro Doki and Yukinori Kurokawa are editorial board members of Annals of Gastroenterological Surgery. Junki Mizusawa has received personal fees from Taiho Pharmaceutical outside the submitted work, and his spouse is employed by Pfizer. Takeshi Sano has received personal fees from Taiho, Chugai, Ono, Lilly, and Daiichi‐Sankyo, outside the submitted work. Mitsuru Sasako has received personal fees from Chugai, Taiho, and Daiichi‐Sankyo outside the submitted work. For the remaining authors (Tetsuro Toriumi, Masanori Terashima, Kohei Uemura, Yukinori Kurokawa, Shuji Takiguchi, Yuichiro Doki, Jun Hihara, Hiroshi Imamura, Akinori Takagane, Seiji Ito, and Takaki Yoshikawa), none were declared.
ETHICAL APPROVAL
All procedures followed were performed according to the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later versions.
Approval of the research protocol; N/A.
Informed consent: Written informed consent, including permission for the secondary use of the trial data, was obtained from all patients upon enrollment in JCOG1001.
Registry and Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Figure S1
Table S1
ACKNOWLEDGMENTS
None.
Toriumi T, Terashima M, Mizusawa J, Uemura K, Kurokawa Y, Takiguchi S, et al. Association between the antiadhesion membrane and small bowel obstruction after open gastrectomy: A supplemental analysis of the randomized controlled JCOG1001 trial. Ann Gastroenterol Surg. 2024;8:30–39. 10.1002/ags3.12722
DATA AVAILABILITY STATEMENT
Individual participant data that support the results after de‐identification will be shared with investigators whose proposed use of the data has been approved by the investigators from the JCOG Stomach Cancer Study Group. Proposals should be directed to m.terashima@scchr.jp.
REFERENCES
- 1. ten Broek RP, Issa Y, van Santbrink EJ, Bouvy ND, Kruitwagen RF, Jeekel J, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met‐analysis. BMJ. 2013;347:f5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Becker JM, Dayton MT, Fazio VW, Beck DE, Stryker SJ, Wexner SD, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate‐based bioresorbable membrane: a prospective, randomized, double‐blind multicenter study. J Am Coll Surg. 1996;183(4):297–306. [PubMed] [Google Scholar]
- 3. Fazio VW, Cohen Z, Fleshman JW, van Goor H, Bauer JJ, Wolff BG, et al. Reduction in adhesive small‐bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Dis Colon Rectum. 2006;49(1):1–11. [DOI] [PubMed] [Google Scholar]
- 4. Pan T, Galiullin D, Chen XL, Zhang WH, Yang K, Liu K, et al. Incidence of adhesive small bowel obstruction after gastrectomy for gastric cancer and its risk factors: a long‐term retrospective cohort study from a high‐volume institution in China. Updates Surg. 2021;73(2):615–626. [DOI] [PubMed] [Google Scholar]
- 5. Hayashi S, Takayama T, Masuda H, Kochi M, Ishii Y, Matsuda M, et al. Bioresorbable membrane to reduce postoperative small bowel obstruction in patients with gastric cancer: a randomized clinical trial. Ann Surg. 2008;247(5):766–770. [DOI] [PubMed] [Google Scholar]
- 6. Kim SG, Song KY, Lee HH, Kim EY, Lee JH, Jeon HM, et al. Efficacy of an antiadhesive agent for the prevention of intra‐abdominal adhesions after radical gastrectomy: a prospective randomized, multicenter trial. Med (Baltim). 2019;98(19):e15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohri Y, Tanaka K, Uchida K, Ohi M, Inoue M, Araki T, et al. Oncologic outcome with use of sodium hyaluronate‐carboxymethylcellulose barrier in gastric cancer. Int Surg. 2013;98(3):271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawamura H, Yokota R, Yokota K, Watarai H, Tsunoda Y, Yamagami H, et al. A sodium hyaluronate carboxymethylcellulose bioresorbable membrane prevents postoperative small‐bowel adhesive obstruction after distal gastrectomy. Surg Today. 2010;40(3):223–227. [DOI] [PubMed] [Google Scholar]
- 9. Kurokawa Y, Doki Y, Mizusawa J, Terashima M, Katai H, Yoshikawa T, et al. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): a phase 3, open‐label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3(7):460–468. [DOI] [PubMed] [Google Scholar]
- 10. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL‐F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm adhesion study group. Fertil Steril. 1996;66(6):904–910. [PubMed] [Google Scholar]
- 11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Krabben AA, Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, Schaapveld M, Van Goor H. Morbidity and mortality of inadvertent enterotomy during adhesiotomy. Br J Surg. 2000;87(4):467–471. [DOI] [PubMed] [Google Scholar]
- 13. Baiocchi GL, D'Ugo D, Coit D, Hardwick R, Kassab P, Nashimoto A, et al. Follow‐up after gastrectomy for cancer: the charter Scaligero consensus conference. Gastric Cancer. 2016;19(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly KJ, Allen PJ, Brennan MF, Gollub MJ, Coit DG, Strong VE. Internal hernia after gastrectomy for cancer with roux‐Y reconstruction. Surgery. 2013;154(2):305–311. [DOI] [PubMed] [Google Scholar]
- 15. Miyagaki H, Takiguchi S, Kurokawa Y, Hirao M, Tamura S, Nishida T, et al. Recent trend of internal hernia occurrence after gastrectomy for gastric cancer. World J Surg. 2012;36(4):851–857. [DOI] [PubMed] [Google Scholar]
- 16. Yoshikawa K, Shimada M, Kurita N, Sato H, Iwata T, Higashijima J, et al. Characteristics of internal hernia after gastrectomy with roux‐en‐Y reconstruction for gastric cancer. Surg Endosc. 2014;28(6):1774–1778. [DOI] [PubMed] [Google Scholar]
- 17. Han WH, Eom BW, Yoon HM, Kim YW, Ryu KW. Clinical characteristics and surgical outcomes of internal hernia after gastrectomy in gastric cancer patients: retrospective case control study. Surg Endosc. 2019;33(9):2873–2879. [DOI] [PubMed] [Google Scholar]
- 18. Hosoya Y, Lefor A, Ui T, Haruta H, Kurashina K, Saito S, et al. Internal hernia after laparoscopic gastric resection with antecolic roux‐en‐Y reconstruction for gastric cancer. Surg Endosc. 2011;25(10):3400–3404. [DOI] [PubMed] [Google Scholar]
- 19. Toriumi T, Makuuchi R, Kamiya S, Tanizawa Y, Bando E, Terashima M. Obesity is a risk factor for internal hernia after laparoscopic or robot‐assisted gastrectomy with mesenteric defect closure for gastric cancer. Surg Endosc. 2020;34(1):436–442. [DOI] [PubMed] [Google Scholar]
- 20. Kojima K, Inokuchi M, Kato K, Motoyama K, Sugihara K. Petersen's hernia after laparoscopic distal gastrectomy with roux‐en‐Y reconstruction for gastric cancer. Gastric Cancer. 2014;17(1):146–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Data Availability Statement
Individual participant data that support the results after de‐identification will be shared with investigators whose proposed use of the data has been approved by the investigators from the JCOG Stomach Cancer Study Group. Proposals should be directed to m.terashima@scchr.jp.


