Abstract
Pneumolysin-deficient mutant strains of Streptococcus pneumoniae are known to cause less-severe sepsis than wild-type pneumococcal strains that produce pneumolysin. This difference is associated with greater host resistance in mice infected with the pneumolysin-deficient strains. These studies show that the host resistance developed during the first 1 to 2 days after infection with a pneumolysin-deficient mutant strain is dependent on tumor necrosis factor alpha but is apparently independent of interleukin 1β (IL-1β) or IL-6. Survival beyond 5 days appeared to depend on the ability of the mice to produce IL-1β.
We previously reported that the capsular type 2 pneumococcal strain D39 and its pneumolysin-deficient mutant, PLN, exhibit different growth patterns in the blood of CBA/N-XID mice after intravenous (i.v.) challenge (3). The number of CFU of D39 increases exponentially from inoculation until the death of the mouse, generally within 24 to 36 h of challenge, when the level of CFU in the blood becomes greater than 109/ml. The number of CFU of PLN in the blood increases in parallel with that of D39 until reaching 106 to 107 per ml, at which point the number of CFU of PLN ceases to increase and is maintained at a relatively constant level for several days. Once the number of PLN pneumococci in the blood reaches 106 CFU/ml, a subsequent infection with D39 is no longer able to cause rapid death. We hypothesized that the host response generated in the absence of pneumolysin was partially protective and accounted for the control of bacteremia at 106 CFU/ml. In contrast, the host response generated in the presence of pneumolysin was not protective, thus allowing the numbers of pneumococci in the blood to increase exponentially (3). Using derivatives of D39 which express point mutations in pneumolysin (6), we showed that the hemolytic and complement-activating properties of the toxin did not account for the difference in bacteremia associated with D39 and PLN infections (5).
Our previous results also demonstrated that mice actively infected with D39 produced more interleukin 6 (IL-6) per CFU in plasma than mice infected with comparable levels of PLN, exactly the opposite of what would have been expected if the host resistance that developed during PLN infections was dependent on IL-6 (3). Gamma interferon (IFN-γ) also did not appear to be responsible for the host resistance of PLN-infected mice; IFN-γ in plasma was detected only in mice infected with D39 (not PLN), and then only when the mice were extremely septic and near death (3). Thus, neither of these cytokines appeared to be responsible for mediating the host resistance of PLN-infected mice. Other studies have shown that tumor necrosis factor alpha (TNF-α) may be important for host resistance to lung infections with wild-type pneumococci (15) and that purified pneumolysin induces the production of TNF-α and IL-1β in isolated human monocytes and a human monocyte cell line (11).
To investigate further the nature of the host response to pneumococcal bacteremia caused by strains D39 and PLN, we examined the levels of proinflammatory cytokines in blood, such as TNF-α, IL-1β, and IL-6; cytokines with potential regulatory functions in mediating inflammation, such as IL-4 and IL-10; and cytokines which are not believed to play a central role in mediating inflammation, such as IL-2 and IL-5 (9, 12). We also reexamined the induction of IFN-γ, which is the best-known activator of macrophages (12). We examined the roles of TNF-α and IL-1β in the pathogenesis of pneumococcal bacteremia through in vivo neutralization via administration of polyclonal antibody to TNF-α or IL-1β.
Pneumococcal bacteremia model.
CBA/CAHN-XID/J mice (Jackson Laboratories, Bar Harbor, Maine), at 8 weeks of age, were challenged i.v. as previously described (4) with approximately 5 × 104 CFU of strain D39 (2) or strain PLN (7). The numbers of CFU per milliliter of blood of PLN and D39 were determined at selected time points postinfection by quantitative plating on blood agar (4). For quantitation of circulating cytokine levels, individual blood samples were diluted with 4 to 9 volumes of Ringer’s solution, centrifuged to remove the cells, and the plasma was stored at −20°C until cytokine enzyme-linked immunosorbent assays were performed. TNF-α, IL-1β, and IL-6 concentrations in the plasma of infected mice were measured with mouse Quantikine M Immunoassay kits (R&D Systems, Inc., Minneapolis, Minn.) according to the manufacturer’s instructions. Numbers of CFU per milliliter of blood and cytokine levels for groups of mice were expressed as geometric means with standard errors. Statistical differences between treated and control groups were calculated with the Wilcoxon two-sample rank test.
Effect of administration of antibody to TNF-α on levels of TNF-α, IL-1β, and IL-6 in plasma.
Mice were placed into four experimental groups consisting of six mice each. Two groups received 103 U of polyclonal rabbit anti-mouse TNF-α antibody (Genzyme, Cambridge, Mass.), diluted with Ringer’s solution to a volume of 200 μl, by intraperitoneal injection, and two control groups received injections of equivalent volumes of Ringer’s solution. One hour later, one group that received anti-TNF-α antibody and one control group (untreated mice) were infected i.v. with strain D39. The other anti-TNF-α antibody-treated group and untreated group were infected with strain PLN. Samples of blood for determination of CFU and cytokine levels were obtained over a period of 42 h postchallenge (Fig. 1).
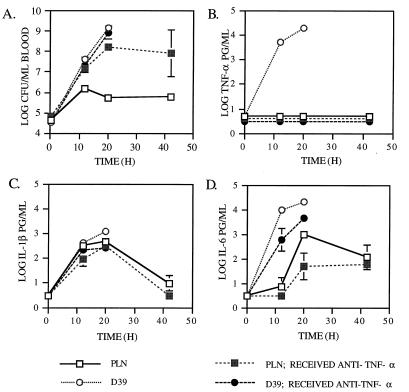
FIG. 1.
Effects of anti-TNF-α antibody on infection with D39 and PLN. The geometric means (±standard errors) of CFU per ml of blood (A) and TNF-α (B), IL-1β (C), and IL-6 (D) per ml of plasma were determined for groups of six mice, which either were untreated or received 103 U of anti-TNF-α antibody 1 h prior to i.v. challenge with 5 × 104 CFU of D39 or PLN. The absence of standard error bars indicates that the standard error was too small to be depicted. The 20-h time point for untreated mice infected with D39 includes data from the five surviving mice. All mice infected with D39 died prior to the 42-h time point. The 42-h time point for mice infected with PLN that received anti-TNF-α antibody includes data from the two survivors. The lower limit of detection of each cytokine was 0.5 log pg/ml. Data points plotted at this level indicate that the plasma samples contained no detectable cytokine. The lower limit of detection of pneumococci was 2.0 log CFU per ml.
Mice challenged with PLN did not produce detectable levels of TNF-α in plasma at any time point. However, levels of TNF-α in plasma were detected in untreated mice at 12 and 20 h after challenge with D39 (Fig. 1B). The fact that levels of TNF-α in plasma were observed only in mice infected with D39 may have been due to the much higher CFU levels in mice infected with D39 than in those infected with PLN. Measurable levels of TNF-α in plasma were observed only in mice infected with D39 once the CFU levels had reached 5 × 107. In mice infected with PLN, the numbers of CFU in the blood did not exceed 1.3 × 106 during the same period of observation (Fig. 1A).
In mice infected with D39, the increase in levels of TNF-α in plasma between 12 and 20 h was significant at P = 0.0087. The administration of antibody to TNF-α was shown to effectively eliminate the appearance of TNF-α in the plasma of mice challenged with D39. This finding demonstrated that the anti-TNF-α antibody treatment used was able to neutralize TNF-α in vivo as expected (15).
Effect of administration of antibody to TNF-α on the levels of bacteremia and mouse survival times postinfection with D39 and PLN.
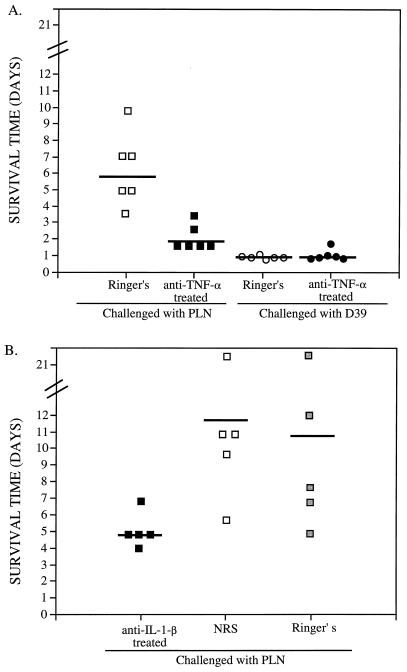
In Fig. 1A, it is apparent that D39 caused exponential sepsis and rapid death, whereas the concentration of PLN in the blood of untreated mice leveled off around 106 CFU/ml of blood as expected (3). The mean CFU levels of PLN in the blood of anti-TNF-α-treated mice were 10-fold higher at 12 h, 295-fold higher at 20 h, and 140-fold higher at 42 h than those of untreated mice. The differences at 12 and 20 h were significant at P = 0.0022. A valid statistical comparison of CFU levels could not be made at the 42-h time point, because there were only two survivors in the anti-TNF-α-treated group challenged with PLN. Mice infected with PLN died significantly (P = 0.0022) sooner if they were treated with anti-TNF-α antibody (1.7 mean days of survival) than if they were untreated (5.8 mean days of survival) (Fig. 2A).
FIG. 2.
Effect of anti-TNF-α or anti-IL-1β antibody on mouse survival time. The survival times for individual mice are shown for untreated (Ringer’s) mice and mice treated with anti-TNF-α antibody (A) and mice treated with anti-IL-1β antibody, NRS, or Ringer’s solution (B). Mice were infected i.v. with 5 × 104 CFU of either D39 or PLN 1 h posttreatment. The mean survival times for each group are indicated by horizontal bars. CFU-per-milliliter and cytokine levels in the plasma of these mice are shown in Fig. 1 and 3.
In contrast, treatment of D39-infected mice with anti-TNF-α antibody had essentially no effect on the level of bacteremia or survival time of mice (Fig. 1A and 2A). The CFU levels of D39 did not differ between the untreated and anti-TNF-α antibody-treated mice. The median survival time of mice challenged with D39 was 0.9 days for both the anti-TNF-α antibody-treated and untreated groups of mice. Thus, although the treatment of mice with antibody to TNF-α abolished their ability to limit the growth of PLN pneumococci in the blood and resulted in decreased survival times of mice, it had no effect on infections with D39. The inability of anti-TNF-α treatment to affect the level of CFU or survival time of D39-infected mice may be an indication that D39 is already growing at its maximum in vivo rate in untreated mice.
The observation that anti-TNF-α antibody treatment enhanced bacteremia in mice infected with PLN demonstrates that the protective host response generated during infection with PLN is dependent on TNF-α. This result was particularly interesting, since mice infected with PLN did not have measurable (<3 pg/ml) levels of TNF-α in plasma at 12, 24, or 40 h. One interpretation of this observation is that the effect of TNF-α on host resistance may be systemic but occurs at levels below our experimental limit of detection. A more likely interpretation is that TNF-α has local effects in the spleen and other sites of blood filtration without leading to detectable levels in plasma.
Effect of administration of antibody to TNF-α on levels of IL-1β and IL-6 in plasma.
Levels of IL-1β in plasma were observed in all mice infected with PLN or D39 at the 12- and 20-h time points, regardless of whether they received antibody to TNF-α (Fig. 1C). At the 12- and 20-h time points, the levels of IL-1β observed in untreated mice infected with D39 were higher (P = 0.015 and P = 0.0043, respectively) than those of the anti-TNF-α antibody-treated mice, a finding that is consistent with earlier studies indicating that elevations in TNF-α can lead to elevated IL-1β levels (9). Among mice challenged with PLN, there was no significant difference in the levels of IL-1β in plasma between the anti-TNF-α antibody-treated and untreated groups at any time point. This result indicates that inducers other than TNF-α were probably responsible for IL-1β production in the PLN-infected mice, which, unlike D39-infected mice, had no detectable TNF-α in their blood.
A significant increase in the level of IL-6 in plasma was not observed for mice infected with PLN until at 20 h (Fig. 1D). At this point, all of the untreated mice, but only half of the anti-TNF-α antibody-treated mice, had measurable IL-6 levels. This difference in IL-6 levels was not statistically significant. In mice infected with D39, the untreated group exhibited significantly higher levels of IL-6 in plasma than the anti-TNF-α antibody-treated group at the 12- and 20-h time points (P = 0.0022 and P = 0.0043, respectively). These effects of anti-TNF-α antibody on IL-6 levels are consistent with past results showing that elevations in TNF-α can lead to elevated levels of IL-6 (1, 9).
Interpretation of the effect of treatment with anti-TNF-α antibody on IL-1β and IL-6 levels is complicated by the fact that anti-TNF-α antibody leads to increased levels of infection at the same time it probably reduces the local TNF-α levels. The failure of anti-TNF-α antibody to lead to low levels of IL-1β and IL-6 may thus be due to a compensating TNF-α-independent stimulation resulting from high levels of infection. When the levels of IL-1β and IL-6 were normalized for CFU levels, both cytokines were significantly higher in untreated mice than in mice that received anti-TNF-α antibody (data not shown). In any case, IL-1β and IL-6 are probably not important in the TNF-α-mediated host resistance to infection seen in PLN mice, because the levels of IL-1β and IL-6 are approximately the same with or without TNF-α treatment.
Effect of administration of antibody to IL-1β on levels of IL-1β and IL-6 in plasma in mice infected with PLN.
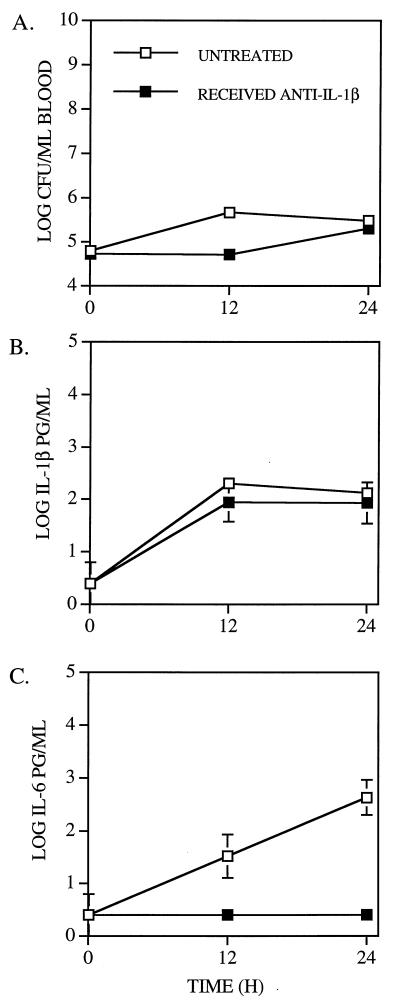
To further test the possible role of IL-1β and IL-6 in host immunity elicited by infection with PLN, five CBA/N mice received 300 μg of polyclonal rabbit anti-mouse IL-1β (Genzyme, Cambridge, Mass.) diluted in 200 μl of normal rabbit serum (NRS), by intraperitoneal injection 1 h prior to challenge with PLN. Control mice received an equal volume of NRS or Ringer’s lactate solution. The levels of IL-1β in plasma of infected mice treated with anti-IL-1β antibody did not differ significantly from those of mice not treated with anti-IL-1β antibody (Fig. 3B). In Fig. 3, the untreated controls represent pooled data from mice that received NRS or Ringer’s solution, since these two treatments did not yield statistically different results. Although levels of IL-1β in plasma were not significantly affected, anti-IL-1β treatment blocked the production of measurable levels of IL-6 in plasma (Fig. 3C). Thus, it appeared that the antibody to IL-1β may have interfered with the biologic activity of IL-1β, even though it did not affect its level in plasma as detected by ELISA.
FIG. 3.
Effects of anti-IL-1β antibody on infection with PLN. The geometric means (± standard errors) of CFU per milliliter of blood (A) and IL-1β (B) and IL-6 (C) per milliliter of plasma were determined for groups of five mice, which received either 300 μg of anti-IL-1β antibody or equivalent volumes of NRS or Ringer’s solution 1 h prior to i.v. challenge with 5 × 104 CFU of PLN. The data from the groups which received NRS and Ringer’s solution were pooled, since the results in these two groups did not differ significantly. The absence of standard error bars indicates that the standard error was too small to be depicted. The lower limit of detection of each cytokine was 0.5 log pg/ml. Data points plotted at this level indicate that the plasma samples contained no detectable cytokine. The lower limit of detection of pneumococci was 2.0 log CFU per ml.
Effect of administration of antibody to IL-1β on bacteremia and survival times of mice infected with PLN.
The CFU levels of strain PLN in the blood of anti-IL-1β antibody-treated mice did not differ significantly over the first 24 h postchallenge from those of mice not treated with anti-IL-1β antibody (Fig. 3A). However, the survival times of anti-IL-1β antibody-treated mice (4.8 mean days) were significantly shorter than those of untreated mice that received NRS (11.6 mean days, P = 0.016) or Ringer’s solution (10.4 mean days, P = 0.031) (Fig. 2B). Thus, although IL-1β did not appear to play a role in controlling bacteremia during the first 24 h, it did appear to be important in the survival of mice beyond 5 days postinfection. These data suggest that the mechanisms involved in regulating bacteremia in mice infected with PLN during the first 24 h may be different from those affecting eventual survival. Moreover, the failure of IL-6 to play a role in bacteremia during the first 24 h is also consistent with the fact that it does not reach maximal levels until at least 20 h, whereas the difference in bacteremia caused by PLN between untreated mice and mice that received anti-TNF-α antibody is apparent by 12 h.
In other studies, antibody to IL-1β has been used successfully to block the biologic effects of IL-1β in mouse models of meningitis and collagen-induced arthritis (10, 13, 14, 17), although its effect on levels of IL-1β in plasma in those studies was not reported.
Measurement of other cytokines.
In a separate experiment, we examined the levels of IL-2, IL-4, IL-5, IL-10, and IFN-γ in plasma at 12 and 20 h postinfection with D39 or PLN in untreated mice. Assays were conducted by previously described methods (16). The cytokines IL-2, IL-4, IL-5, and IL-10 were detected in only a small minority of the mice and showed no relationship to the disease process. As expected from our previous study (3), IFN-γ was observed only in mice infected with D39, and then only when they were extremely septic (5 × 107 CFU/ml of blood or greater). IFN-γ was not observed in mice infected with PLN, but we did not obtain blood samples just prior to death, since these mice survive for variable lengths of time and the exact time of death is difficult to predict. Thus, while the data from these surveys do not rule out roles for IL-2, IL-4, IL-5, IL-10, or IFN-γ, they do fail to provide any evidence implicating these cytokines in host immunity elicited by infections with either D39 or PLN pneumococci.
Relationship of these results to those obtained in a pulmonary infection model.
Our observation that the administration of antibody to TNF-α increases the virulence of PLN is consistent with a recent report on the effect of TNF-α antibody on bacteremic pneumonia in mice (15). Systemic administration of antibody to TNF-α to mice infected intranasally with capsular type 19 pneumococci resulted in the reduction of TNF-α in plasma to undetectable levels, increased the levels of CFU in the blood, and decreased the survival time of the mice (15). Our study and that of Takashima et al. (15) point to the importance of TNF-α-mediated host responses in resistance to pneumococcal infection. Our studies used capsular type 2 wild-type strain D39, which is much more virulent in mice than strains of capsular type 19 (8), which were used by Takashima et al. (15). Differences in the genetic backgrounds of the pneumococci and the inbred strains of mice used, as well as differences in the route of challenge, may have contributed to the fact that we failed to see a role for TNF-α in the resistance to wild-type pneumococci, whereas we saw a strong role for TNF-α in resistance that developed during infections with a less-virulent pneumolysin-deficient strain.
Acknowledgments
This work was supported by NIH grant AI21548 to D.E.B. K.A.B. was supported by NIH training grant AI07051.
REFERENCES
- 1.Abbas A K, Lichtman A H, Pober J S. Cytokines. In: Wonsiewicz M, editor. Cellular and molecular immunology. Philadelphia, Pa: The W. B. Saunders Co.; 1991. pp. 226–242. [Google Scholar]
- 2.Avery O T, MacLeod C M, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a deoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton K A, Everson M P, Briles D E. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect Immun. 1995;63:448–455. doi: 10.1128/iai.63.2.448-455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton K A, Paton J C, Briles D E. Differences in virulence for mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect Immun. 1997;65:1237–1244. doi: 10.1128/iai.65.4.1237-1244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benton K A, Paton J C, Briles D E. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb Pathog. 1997;23:201–209. doi: 10.1006/mpat.1997.0150. [DOI] [PubMed] [Google Scholar]
- 6.Berry A M, Alexander J E, Mitchell T J, Andrew P W, Hansman D, Paton J C. Effect of defined point mutations in the pneumolysin gene on the virulence of Streptococcus pneumoniae. Infect Immun. 1995;63:1969–1974. doi: 10.1128/iai.63.5.1969-1974.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry A M, Yother J, Briles D E, Hansman D, Paton J C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989;57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briles D E, Crain M J, Gray B M, Forman C, Yother J. A strong association between capsular type and mouse virulence among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durum S K, Oppenheim J J. Proinflammatory cytokines and immunity. In: Paul W E, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press, Ltd.; 1993. pp. 801–835. [Google Scholar]
- 10.Geiger T, Towbin H, Cosenti-Vargas A, Zingel O, Arnold J, Rordorf C, Glatt M, Vosbeck K. Neutralization of interleukin-1 beta activity in vivo with a monclonal antibody alleviates collagen-induced arthritis in DBA/1 mice and prevents the associated acute phase response. Clin Exp Rheumatol. 1993;11:515–522. [PubMed] [Google Scholar]
- 11.Houldsworth S, Andrew P W, Mitchell T J. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard M C, Miyajima A, Coffman R. T cell derived cytokines and their receptors. In: Paul W E, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press, Ltd.; 1993. pp. 763–800. [Google Scholar]
- 13.Joosten L A, Helsen M M, van de Loo F A, van den Berg W B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996;35:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 14.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997;65:257–260. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 17.van den Berg W B, Joosten L A, Helsen M, van de Loo F A. Amelioration of established murine collagen-induced arthritis with anti-Il-1 treatment. Clin Exp Immunol. 1994;95:237–243. doi: 10.1111/j.1365-2249.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]