Abstract
Gastrointestinal clear cell sarcoma (GICCS)/malignant gastrointestinal neuroectodermal tumor (GNET) is an extremely rare form of cancer with aggressive clinical behavior. It has distinct pathological, immunohistochemical, ultrastructural, and molecular features. Herein, we present the case of a 20-year-old woman with no notable medical history who presented to the outpatient department with complaints of abdominal pain and vomiting. Symptoms had been evolving for 3 months. The physical examination revealed slight abdominal tenderness and melena. Biological investigations revealed iron-deficiency anemia. The upper and lower endoscopies showed no abnormalities. Magnetic resonance enterography revealed small bowel wall thickening of 15 mm × 2 mm. Exploratory laparotomy revealed an ileal mass with mesenteric lymphadenopathy. A wide resection of the mass was then performed. The final pathological report confirmed the diagnosis of small bowel GICCS/GNET. After 11 months of follow-up, the patient presented with mesenteric lymph node metastases.
Keywords: malignant gastrointestinal neuroectodermal tumor, gastrointestinal clear cell sarcoma, EWSR1, small bowel, surgical pathology
Introduction
Gastrointestinal clear cell sarcoma (GICCS) also referred as malignant gastrointestinal neuroectodermal tumor (GNET) is an extremely rare malignant primary gastrointestinal mesenchymal tumor with highly aggressive behavior.1,2
Only a few cases have been reported in the existing literature till now.3,4
Herein, we report the case of a 20-year-old woman with small intestine GICCS.
Case Presentation
A young woman of 20 years presented to the outpatient department with complaints of enduring abdominal pain and vomiting. Symptoms had been evolving for 3 months. Her previous medical records did not reveal any noteworthy medical history. There was no record of cancer in her family history.
Physical examination revealed slight abdominal tenderness and melena. The gynecological examination was normal. Standard blood analysis showed iron-deficiency anemia with a normal count of white blood cells and platelets. No lesions were found during the upper and lower endoscopies. Magnetic resonance enterography revealed small bowel wall thickening of 15 mm × 2 mm (Figure 1).
Figure 1.
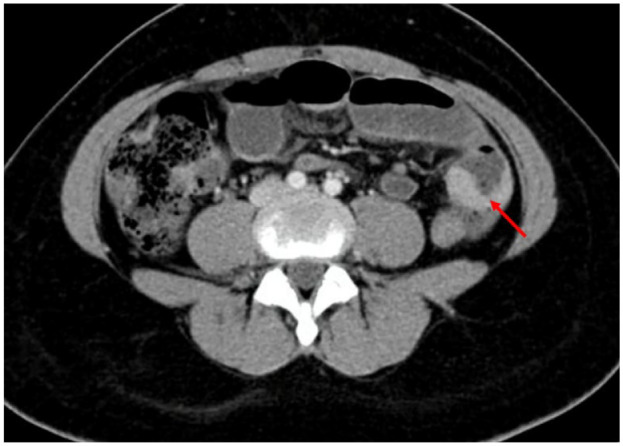
Magnetic resonance enterography revealed small bowel wall thickening (arrow).
Exploratory laparotomy was subsequently undertaken, and it revealed an ileal mass with mesenteric lymphadenopathy. No signs of peritoneal spread or liver metastasis were observed. A wide resection of the mass was then performed (Figure 2A). The postoperative course was uneventful.
Figure 2.
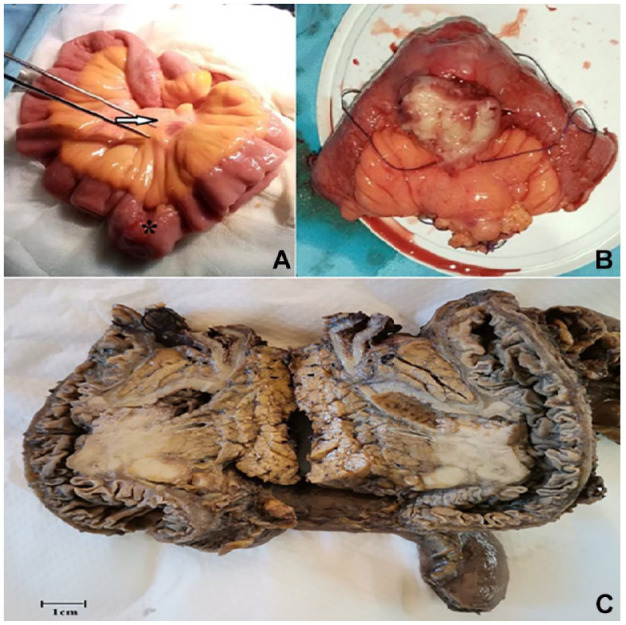
(A) Intraoperative views showing the tumor (arrow) with mesenteric lymphadenopathy (asterisk). (B) Gross examination revealed a polypoid mass within the small intestine wall. (C) On the cut section, the mass had a tan-white lobulated appearance.
The macroscopic examination of the resected specimen revealed a 30 mm × 25 mm exophytic mass within the small intestine wall. On the cut section, the mass displayed a lobulated appearance with a tan-white color (Figure 2B and C).
Microscopically, there was an infiltrative proliferation composed of epithelioid and spindle cells with nested and fascicular patterns of growth. The tumor cells were monomorphous with a varying abundance of either eosinophilic or clear cytoplasm. The nuclei were round to oval with vesicular chromatin and inconspicuous nucleoli. The mitotic count indicated the presence of up to 10 mitoses per 10 high-power field (HPF). The tumor was transmural extending from the submuscosa to the subserosa with mucosal ulceration. There was no evidence of melanin pigment. Lymphovascular and perineural invasions were absent. Three mesenteric lymph nodes were found to be free from malignancy (Figure 3).
Figure 3.
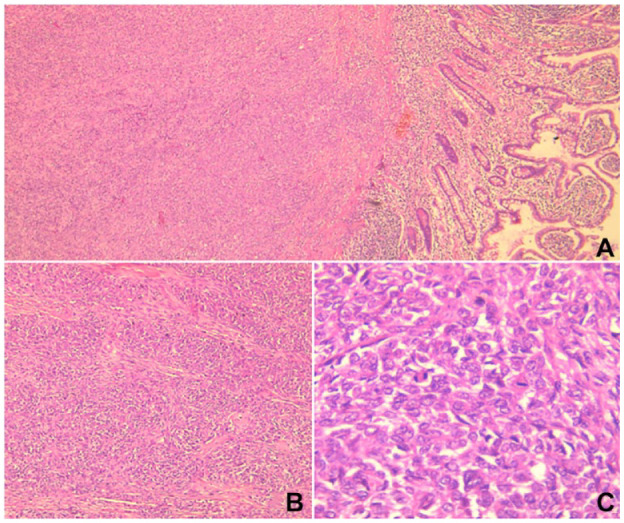
(A) Microscopic examination revealed an infiltrative proliferation centered in the submucosa of the ileum (H&E stain, ×40). (B) The proliferation displayed a nested pattern of growth (H&E stain, ×100). (C) The tumor was composed of uniform epithelioid and spindle cells with variable amounts of eosinophilic cytoplasm. The nuclei were vesicular with inconspicuous nucleoli and mitotic figures. (H&E stain, ×400).
Abbreviation: H&E, hematoxylin and eosin.
In the immunohistochemical analysis, the tumor cells exhibited diffuse and strong expression of S100 protein and SOX10. Melanocytic markers, including HMB45 and Melan A, yielded negative results. There was a patchy expression of synaptophysin. CD117, DOG1, cytokeratin, CD34, smooth muscle actin (SMA), desmin, chromogranin, and CD56 showed all negative results (Figure 4).
Figure 4.
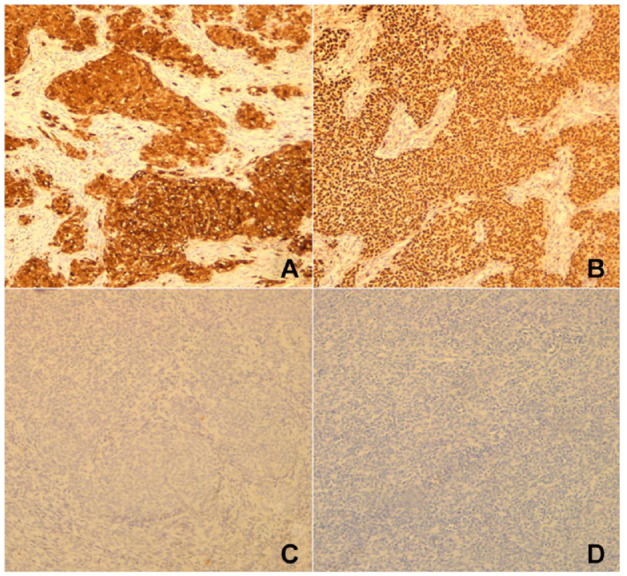
(A) Diffuse and strong staining with S100. (B) Diffuse and strong staining with SOX10. (C) Staining with HMB45 was negative. (D) The tumor cells did not express Melan A.
Histological and immunohistochemical findings were consistent with small bowel GICCS/GNET.
Unfortunately, fluorescence in situ hybridization (FISH) analysis to identify EWSR1 gene rearrangement was not conducted for technical reasons.
The multidisciplinary team decided not to prescribe adjuvant chemotherapy and recommended active regular surveillance using thoraco-abdominal computed tomography (CT) scan. Follow-up imaging conducted 6 months after the surgery revealed the absence of both local recurrence and distant metastasis. At 11 months after undergoing surgical resection, surveillance CT scan revealed suspicious mesenteric lymph node swelling. Laparoscopic lymphadenectomy was then performed. Microscopic examination proved nodal metastases.
Discussion
Gastrointestinal clear cell sarcoma/GNET is an uncommon form of cancer.1,5 The largest series of 62 cases were described by Mishra et al 4 in 2022. In 2003, Zambrano et al first recognized the malignancy as a distinct entity in a series of 6 cases. In that review, authors described the neoplasm as “osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts.” 6 Recently, in 2012, Stockman et al 7 designated the tumor as GNET based on characteristic ultrastructural features.
Gastrointestinal clear cell sarcoma typically affects young to middle-aged patients with an average age of 42 years. 8 There is no sex predilection.5,8
Malignant gastrointestinal neuroectodermal tumor occurs predominantly in the small intestine and less frequently in the stomach and the colon.1,9 The esophagus and the anal canal are rarely affected. 1
Clinically, patients usually present with abdominal pain, intestinal obstruction, or the detection of an abdominal mass through incidental findings on imaging.5,7 Other symptoms include anorexia, weight loss, weakness, anemia, and fever.5,7
Radiologic findings of GICCS include an irregular mass, intestinal obstruction, bowel wall thickening, enlarged lymph nodes, and liver metastases. 3 ,9-12
Molecularly, GICCS/GNET is characterized by rearrangement of the Ewing sarcoma breakpoint region 1 gene (EWSR1) including EWSR1-ATF1 (t(12;22)(q13;q12)) and EWSR1-CREB1 (t(2;22)(q34;q12)) fusion genes.1,5,7,8 EWSR1 rearrangement is also observed in various tumors, including clear cell sarcoma of tendons and aponeuroses, Ewing sarcoma, desmoplastic small round cell tumor, and angiomatoid fibrous histiocytoma.1,5
In a cohort of 20 GNET, comprehensive genomic profiling utilizing both DNA and RNA sequencing, unveiled recurrent EWSR1 chromosomal rearrangements with a scarcity of other recurrent or driver genomic alterations. All these cases displayed a low tumor mutational burden and were microsatellite stable. Interestingly, 2 cases within this cohort exhibited a EWSR1-FLI1 fusion, which is the hallmark feature of Ewing sarcoma. The authors, in this cohort, reached the conclusion that GNET exhibits biological heterogeneity. They asserted that, while EWSR1 rearrangement is the hallmark feature, it is not an absolute diagnostic criterion for GNET and accurate diagnosis requires substantial pathological expertise. 13
Moreover, Yagi et al 14 reported the case of small intestine GNET with BRAF mutation and 2 subtypes of EWSR1-ATF1 fusion genes in a woman with a history of desmoplastic melanoma exhibiting BRAF mutation.
Grossly, tumors have usually solid and firm consistency.7,8 They may develop as exophytic polypoid masses that project into the lumen or present as circumferential lesions. 7 Mucosal ulceration may be observed. 7 The median size is 3.8 cm (2.4-15 cm). 7 The cut-surface is frequently tan-white, with lobulated appearance, focal areas of hemorrhage, and necrosis. 7 Cystic changes may be occasionally seen.5,8
Morphologically, GNET typically involves the submucosa and the muscularis propria with occasional extension into the mucosa. 8 The tumors display various growth patterns encompassing sheets, nests, pseudoalveolar structures, pseudopapillary formations, microcystic pattern, fascicular arrangement, and cord-like architecture. 7 Extensive tumor necrosis may be observed. 7 The neoplastic population consists of uniform ovoid to epithelioid and/or spindle tumor cells with variable amounts of pale eosinophilic or, rarely, clear cytoplasm.5,7,12 The nuclei are round or oval-shaped, characterized by vesicular chromatin, and typically exhibit indistinct to small nucleoli.1,7 The mitotic activity can range from 0 to 20 mitoses per 10 HPF. 7 Approximately half of the cases exhibit dispersed osteoclast-like giant cells. 8 Melanin pigment is typically absent which is a characteristic feature of GICCS/GNET.5,12 Lymphovascular and perineural invasions may be present. 12
Immunohistochemically, GICCS/GNET typically exhibits positivity for S-100 protein and SOX10 which is a neuroectodermal marker.1,8,7 The melanocytic markers including HMB45, melan A, mircophthalmia transcription factor, and tyrosinase consistently demonstrate negative results.1,5,8 Around 50% of cases exhibit positive staining for synaptophysin and neuron-specific enolase. 8 KIT, DOG1, CD34, cytokeratin, desmin, and SMA are typically negative. 8 Moreover, osteoclast-like giant cells express CD68.5,9
By electron microscopy, ultrastructural features of GICCS/GNET showed signs of neural differentiation including multiple interdigitating cell processes, dense-core granules, and clear vesicles resembling synaptic bulbs. 7 There is no evidence of melanocytic differentiation. 7 These findings have led to the suggestion that such tumors may arise from precursor cells originating from neural crest derivation, which exhibit neural differentiation but lack melanocytic features. 7
The histological differential diagnosis for GICCS/GNET includes metastatic clear cell sarcoma (CCS) of tendons and aponeuroses, gastrointestinal stromal tumor (GIST), primary or metastatic melanoma, and monophasic synovial sarcoma. 7
Metastatic CCS of tendons and aponeuroses is the main differential diagnosis of GICCS/GNET. 12 Both tumors display similar morphological features, share positivity for S100 protein and SOX10, and they both can harbor similar genetic abnormalities (EWSR1 rearrangements).1,5,8 However, in contrast to conventional CCS, GICCS/GNET may feature osteoclast-like giant cells and, notably, it does not show signs of melanocytic differentiation. 8 GIST is the most common mesenchymal tumor occurring in the gastrointestinal tract and may represent a differential diagnosis given that it also can display epithelioid and spindled cell morphology. 1 Negativity for CD34, CD117, and DOG-1 support the diagnosis of GICCS/GNET. 1
Melanoma can be differentiated from GNET by the expression of HMB-45 or Melan-A and the absence of EWSR1 rearrangements. 5
Monophasic synovial sarcoma is infrequently observed in the gastrointestinal tract, particularly in the stomach, and may be difficult to distinguish from GICCS/GNET.8,15 Expression of cytokeratin, EMA (Epithelial Membrane Antigen), TLE1 (Transducin-Like Enhancer of split 1), and detection of SS18 rearrangement support the diagnosis of synovial sarcoma.8,15
Due to its rarity, treatment protocols are not well established. 13 The standard therapeutic strategy is radical resection of the tumor followed by close monitoring of the patient. 1 Efficacy of chemotherapy and targeted therapies including, mammalian target of rapamycin (mTOR) inhibitors and tyrosine kinase inhibitors (TKI), remains unknown and seems to be variable. 13
Gastrointestinal clear cell sarcoma/GNET is reported to have an aggressive behavior with tendency for local recurrence and dismal prognosis.2,4,7-9, 12 Liver and lymph nodes metastases are frequently observed at the time of diagnosis.5,16 The time to distant recurrence varies from 2 weeks to 109 months. 4 The median survival is about 18.5 months. 5
Conclusion
Gastrointestinal clear cell sarcoma is a rare malignancy with poor prognosis. The disease has unique pathological, immunohistochemical, ultrastructural, and molecular features. This form of malignancy presents considerable difficulties in diagnosis and management because of its rarity. Moreover, treatment protocols are not well established. Malignant gastrointestinal neuroectodermal tumor is often misdiagnosed and inappropriately treated. Therefore, pathologists should be aware of this form of cancer when considering a gastrointestinal mass with spindle and/or epithelioid morphology. Reporting more such cases is needed to better understand this diagnostically challenging entity.
Footnotes
Author Contributions: Drafting of the manuscript: Manel Njima and Bahaeddine Lahbacha.
Acquisition of data: Sadok Ben Jabra, Amani Moussa, Seifeddine Ben Hammouda, and Ahlem Bellalah.
Analysis and interpretation of data: Bahaeddine Lahbacha, Nouha Ben Abdeljelil, Seifeddine Ben Hammouda, Ahlem Bellalah, and Amani Moussa.
Critical revision and literature review for important intellectual content: Leila Njim, Rim Hadhri, Nouha Ben Abdeljelil, Manel Njima, and Abdelfattah Zakhama.
Study conception and design: Manel Njima, Leila Njim, Rim Hadhri, and Abdelfattah Zakhama.
All authors approved the final version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
ORCID iD: Lahbacha Bahaeddine  https://orcid.org/0000-0003-0012-6693
https://orcid.org/0000-0003-0012-6693
Data Availability Statement: The data that support the findings of this work are available from the corresponding author upon reasonable request.
References
- 1. Chang B, Yu L, Guo WW, et al. Malignant gastrointestinal neuroectodermal tumor. Am J Surg Pathol. 2020;44(4): 456-466. [DOI] [PubMed] [Google Scholar]
- 2. Sivasubramaniam P, Tiegs-Heiden CA, Sturgis CD, Hagen CE, Hartley CP, Thangaiah JJ. Malignant gastrointestinal neuroectodermal tumor: cytologic, histologic, immunohistochemical, and molecular pitfalls. Ann Diagn Pathol. 2021;55:151813. [DOI] [PubMed] [Google Scholar]
- 3. Sasaki M, Tanaka M, Asukai K, et al. Malignant gastrointestinal neuroectodermal tumor presenting with small intestinal obstruction: a case report. DEN Open. 2022;2(1):e119. Accessed January 12, 2023. https://onlinelibrary.wiley.com/doi/10.1002/deo2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mishra P, Biswas D, Pattnaik SA, et al. Malignant gastrointestinal neuroectodermal tumor: a case-based review of literature. J Cancer Res Ther. 2022;18(4):885-897. [DOI] [PubMed] [Google Scholar]
- 5. Wang J, Thway K. Clear cell sarcoma–like tumor of the gastrointestinal tract: an evolving entity. Arch Pathol Lab Med. 2015;139(3):407-412. [DOI] [PubMed] [Google Scholar]
- 6. Zambrano E, Reyes-Mugica M, Franchi A, Rosai J. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol. 2003;11(2):75-81. [DOI] [PubMed] [Google Scholar]
- 7. Stockman DL, Miettinen M, Suster S, et al. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol. 2012;36(6):857-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doyle LA, Hornick JL. Mesenchymal tumors of the gastrointestinal tract other than GIST. Surg Pathol Clin. 2013;6(3): 425-473. [DOI] [PubMed] [Google Scholar]
- 9. Gahanbani Ardakani A, Boyle DJ, Elton C. Gastrointestinal clear cell sarcoma-like tumour of the ascending colon. Ann R Coll Surg Engl. 2016;98(3):e37-e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damle A, Sreenija Y, Mathews NR, et al. Malignant gastrointestinal neuroectodermal tumour—case report with review of literature. J Gastrointest Cancer. 2021;52(3):1125-1130. [DOI] [PubMed] [Google Scholar]
- 11. Alyousef MJ, Alratroot JA, ElSharkawy T, et al. Malignant gastrointestinal neuroectodermal tumor: a case report and review of the literature. Diagn Pathol. 2017;12(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Askan G, Kombak FE, Seven IE, et al. Clear cell sarcoma-like tumor of the gastrointestinal tract. J Gastrointest Cancer. 2019;50(3):651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kandler T, Cortez E, Clinton L, et al. A case series of metastatic malignant gastrointestinal neuroectodermal tumors and comprehensive genomic profiling analysis of 20 cases. Curr Oncol. 2022;29(2):1279-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yagi T, Nagata S, Yamamoto T, et al. Malignant gastrointestinal neuroectodermal tumor with BRAF mutation and a history of malignant melanoma: a case report. Mol Clin Oncol. 2020;14(2):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romeo S, Rossi S, Acosta Marín M, et al. Primary Synovial Sarcoma (SS) of the digestive system: a molecular and clinicopathological study of fifteen cases. Clin Sarcoma Res. 2015;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Washimi K, Takagi M, Hisaoka M, et al. Clear cell sarcoma-like tumor of the gastrointestinal tract: a clinicopathological review: letter to the editor. Pathol Int. 2017;67(10):534-536. [DOI] [PubMed] [Google Scholar]


