Abstract
Background:
In China, traditional Chinese medicines (TCMs), as a complementary therapy combined with chemotherapy, is widely used in the treatment of gastric cancer (GC). In order to systematically evaluate and synthesize existing evidence to provide a scientific basis for the efficacy and safety of this complementary therapy, we present an overview of systematic reviews (SRs) and meta-analyses (MAs) on the topic of TCMs as a complementary therapy in combination with chemotherapy for the treatment of GC.
Methods:
SRs/MAs on TCMs combined with chemotherapy for GC were comprehensively searched in 8 databases. Methodological quality, risk of bias, reporting quality, and quality of evidence were assessed using the Assessment of Multiple Systematic Reviews 2 (AMSTAR-2), the Risk of Bias in Systematic (ROBIS) scale, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020), as well as the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
Results:
Thirteen published SRs/MAs were included in our study. In terms of methodology, all SRs/MAs were considered to be of very low quality. Only 3 SRs/MAs has been assessed as low risk of bias. None of the SRs/MAs has been fully reported on the checklist. A total of 97 outcome indicators extracted from the included SRs/MAs were evaluated, and only 1 item was assessed as high quality.
Conclusions:
TCMs may be an effective and safe complementary therapy in combination with chemotherapy for the treatment of GC. However, this conclusion must be treated with caution as the quality of the evidence provided by SRs/MAs is generally low.
Keywords: traditional Chinese medicines, complementary therapies, gastric cancer, Systematic Reviews and Meta-Analyses, overview
Introduction
Gastric cancer (GC) is a prevalent malignancy of the digestive system. It is the fifth most common cancer and the third leading cause of cancer-related mortality on a global scale, and the risk factors for it include Helicobacter pylori infection, Epstein-Barr virus infection, age, high salt intake, and a diet low in fruits and vegetables, among others.1,2 The initial symptoms and signs of GC are often unremarkable. Despite the rapid advancements in gastroscopic technology, the diagnosis rate of early-stage GC remains low, most GCs are diagnosed only at advanced stages, which also leads to a poor prognosis and high mortality, making it a thorny issue to confront for clinicians.3-5 In clinical practice, chemotherapy is the first-line treatment for a large number of patients with GC, barring those who are diagnosed early and can undergo radical surgery.6,7 Despite the global acknowledgment of chemotherapy’s efficacy in treating GC, no matter which chemotherapy regimen is used, it has suboptimal efficacy and excessive adverse effects in the clinical setting, posing challenges that conventional treatment methods struggle to address.8,9 Even the emerging combination of immunotherapy, radiotherapy, and chemotherapy has not completely solved the issue of suboptimal efficacy and excessive adverse reactions in treatment. Furthermore, the addition of these 2 therapies may lead to new adverse reactions.10,11 It will make patients unable to obtain the ideal antitumor effect and seriously affect their medical compliance, thus leading to a serious detrimental effect on survival, quality of life, and even the outcome of treatment failure. Not only that, severe adverse reactions will directly reduce the quality of life of patients, which is extremely unfavorable for weak patients with GC. In addition, poor efficacy and excessive adverse reactions can also lead to the decline in patients’ self-care abilities, damage to their dignity, and incur more treatment costs, causing great damage to their psychological and social levels. Therefore, there is an urgent need to find a new complementary therapy that can be combined with chemotherapy for the treatment of GC patients.
In ancient times, Chinese doctors had a clear understanding of tumor disease, which was called “Zhongyang,” and the use of traditional Chinese medicines (TCMs) under the guidance of syndrome differentiation and treatment theory was an effective treatment plan at that time. 12 With the progress of related research, the clinical value of TCMs has been more and more recognized worldwide. There are many studies reporting that many TCMs can enhance the antitumor therapeutic effect by inhibiting proliferative, proapoptotic, antimetastatic, antiangiogenic, modulating immune responses, reversing chemoresistance; and they can also reduce the adverse reactions caused by chemotherapeutic agents through multiple pathways.13-15 In practice, because of the high efficacy and safety, TCMs have also been recognized by many clinicians and patients as a complementary therapy combined with chemotherapy, and the positive effects provided by them are gratifying. 16 Therefore, the application of TCMs in this field deserves to be further explored to achieve better efficacy and fewer adverse effects in patients treated with chemotherapy for GC.
Nowadays, there are many systematic reviews (SRs)/meta-analyses (MAs) to evaluate the benefits of TCMs as complementary therapies in combination with chemotherapy for patients with GC. SR/MA is considered the gold standard for assessing the efficacy of clinical interventions. It can guide physicians’ clinical decisions and is also an important basis for researchers to conduct relevant studies or develop guidelines. However, the quality of these studies has not been assessed, which may mislead physicians and researchers into making practical decisions. The overview of SRs/MAs in this study is a new approach that combines multiple SRs/MAs to assess their quality and various findings, addressing inconsistencies between them, to determine the effects resulting from interventions. The purpose of this study is to objectively and comprehensively evaluate the scientific quality of SRs/MAs of TCMs as a complementary therapy combined with chemotherapy for GC, and to provide evidence for the application of this treatment in clinical practice.
Methods
This research was conducted according to the Cochrane Handbook and some high quality articles with scientific research methodologies.17-19 This overview protocol has been registered with the PROSPERO website (CRD42023423046).
Eligibility Criteria
Inclusion criteria
Type of research
SRs/MAs were based on randomized controlled trials (RCTs) about TCMs as a complementary therapy combined with chemotherapy for GC, and the language involved is limited to English and Chinese.
Types of participants
The participants were patients diagnosed with GC according to any national or international criteria without distinction of stage, age, sex, race, or nationality.20,21
Type of intervention
The control group received chemotherapy alone, while the intervention group received the same chemotherapy regimen combined with TCMs (including TCM formula, Chinese patent medicine, and TCM injection).
Types of outcomes
The following are the outcome measures: clinical objective response rate (ORR, including complete response and partial response of the tumor), disease control rate (DCR, including complete response, partial response, and stability of the tumor), Karnofsky performance score (KPS) and improvement rate of KPS, quality of life improved rate (QIR), pain relief rate (PRR), overall survival time, 1-year survival rate, 3-year survival rate, incidence of myelosuppression, incidence of leucopenia, incidence of anemia, incidence of thrombocytopenia, incidence of neutropenia, incidence of hepatorenal toxicity, incidence of hepatotoxicity, incidence of renal toxicity, incidence of neurotoxicity, incidence of gastrointestinal reaction, incidence of diarrhea, incidence of nausea and vomiting, incidence of hand-foot syndrome, incidence of oral mucositis, incidence of alopecia.
Exclusion Criteria
(1) Network meta-analyses, SRs without MAs, narrative reviews, conference abstracts, editorials, case reports, dissertations, and replication studies; (2) Publication with missing information; (3) Animal experiments; (4) Other traditional drugs not clearly defined as TCMs were used in the intervention group.
Publication Search Strategy
Two researchers independently searched a total of 8 literature databases, including PubMed, Cochrane Library, Embase, Web of Science, Wanfang Database, VIP Journal Database, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM). We used the search method of Medical Subject Headings (MeSH) terms or keywords combined with free words to search. The keywords included “traditional Chinese medicine,” “gastric cancer,” “systematic review,” and “meta-analysis.” The specific search strategy was adjusted according to different databases. And the search time range was from database establishment to June 28, 2023. The search strategy of PubMed is shown in Table 1. Search strategies for other databases are described in “Supplemental Materials.”
Table 1.
The Search Strategy of PubMed.
| Query | Search term |
|---|---|
| #1 | “TCM” OR “traditional Chinese medicine” OR “Chinese medicine” OR “Chinese herb” OR “traditional medicine” |
| #2 | Stomach Neoplasms [Mesh] |
| #3 | “Neoplasm, Stomach” OR “Stomach Neoplasm” OR “Neoplasms, Stomach” OR “Gastric Neoplasms” OR “Gastric Neoplasm” OR “Neoplasm, Gastric” OR “Neoplasms, Gastric” OR “Cancer of Stomach” OR “Stomach Cancers” OR “Gastric Cancer” OR “Cancer, Gastric” OR “Cancers, Gastric” OR “Gastric Cancers” OR “Stomach Cancer” OR “Cancer, Stomach” OR “Cancers, Stomach” OR “Cancer of the Stomach” OR “Gastric Cancer, Familial Diffuse” |
| #4 | #2 OR #3 |
| #5 | Meta-Analysis as Topic [Mesh] |
| #6 | “Systematic review” OR “meta-analysis” OR “meta analysis” OR “meta-analyses” OR “Review, Systematic” |
| #7 | #5 OR #6 |
| #8 | #1 AND #4 AND #7 |
Publications Screening and Data Extraction
Publications screening and data extraction were performed independently by 2 researchers. The Publications to be screened were imported into NoteExpress software for literature management to remove duplicate studies and then preliminarily screened by browsing the title, abstract, and key words of the publication according to the established criteria. Finally, the full text was scanned to identify the included publications. At the same time, we also reviewed the references in the retrieved publications to avoid omissions. After identifying the included publications, we extracted the following data from them: first author, publication year, country, number of included RCTs, therapeutic measures for intervention groups and control groups, RCT quality assessment tool, and main conclusions.
Quality Evaluation of SRs/MAs
Two researchers independently assessed the methodological quality, risk of bias, report quality, and evidence quality of the included SRs/MAs, and any disagreements were left to the third researcher to resolve.
Assessment of methodological quality
We assessed the methodological quality of the included SRs/MAs using Assessment System for Evaluating Methodological Quality-2 (AMSTAR-2), an internationally recognized systematic methodological quality assessment tool. 22 The tool contains 7 key items (2, 4, 7, 9, 11, 13, and 15). Each item was categorized as “no,” “partially yes,” or “yes” depending on their adherence to the criteria. The overall methodological quality was classified into 4 levels: high, medium, low, or very low.
Assessment of risk of bias
This overview used the Risk of Bias in Systematic Reviews (ROBIS) scale to assess the risk of bias for inclusion in SRs/MAs. 23 The evaluation was conducted in 3 phases, each consisting of one or more key items, which were rated as “yes,” “partial yes,” “partial no,” “no,” and “no information” according to the corresponding criteria, and the risk of bias in each phase was rated as “low,” “high,” and “unclear.”
Assessment of reporting quality
The quality of each SR/MA report included was assessed by the list of Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020). This list consists of 27 items, each rated “yes,” “partial yes,” and “no” according to the corresponding criteria, focusing on reporting methods and outcomes for the inclusion of SRs/MAs. 24
Assessment of quality of evidence
The quality of evidence for each SR/MA outcome was assessed by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE). 25 According to the criteria, 5 aspects will lead to a decrease in the quality of evidence, including risk of bias, inconsistency, indirectness, imprecision, and publication bias. RCTs were initially considered to be high-quality studies. During the evaluation, if any of the above problems were identified, the quality of the evidence in the publicationwas reduced. Ultimately, evidence quality ratings were determined as “high,” “medium,” “low,” or “very low.” The evidence with no degradation factor is rated as high quality, while the evidence with one degradation factor is rated as medium quality, 2 degradation factors are rated as low quality, and more than 3 (including 3) degradation factors are rated as extremely low quality.
Data Synthesis
Narrative descriptions were given for the included SRs/MAs. Dichotomous variables were expressed as risk ratios (RR), odds ratios (OR), or hazard ratios (HR) with 95% confidence intervals (CI), while continuous variables were expressed as mean differences (MD) with 95% CI. In addition, the results of the AMSTAR-2, ROBIS, PRISMA 2020, and GRADE assessments are shown in the tables.
Network of SRs/MAs and RCTs
We collated the included SRs/MAs and the RCTs included in these SRs/MAs, established the network relationships between them, and completed the visualization using Cytoscape software.
Results
Results on Publication Search and Selection
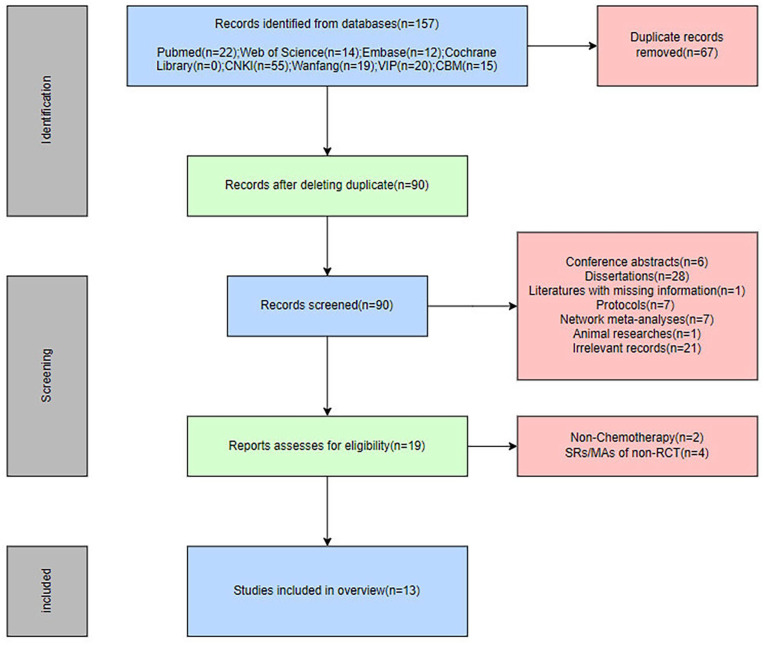
A total of 157 publications were obtained from 8 databases, of which 67 were duplicates. After removing duplicates, the remaining 90 publications were preliminarily screened by reading titles and abstracts, and a total of 71 publications were excluded as unqualified according to the established inclusion and exclusion criteria. Subsequently, the remaining 19 publications were read in full, and it was found that 2 publications did not use chemotherapy at the time of intervention,26,27 and the studies included in the 4 publications were not RCTs.28-31 Finally, we identified 13 SRs/MAs for inclusion in our study.32-44 The process of study selection is shown in Figure 1.
Figure 1.
The flowchart of the screening process.
Description of Included SRs/MAs
The characteristics of the 13 SRs/MAs included in the overview are shown in Table 2. These publicationswere published between 2013 and 2022, and all were published by Chinese researchers. Among them, 6 are in English,32-37 and the remaining 7 are in Chinese.38-44 The number of RCTs included in these SRs/MAs ranged from 6 to 40, with a minimum sample size of 608 and a maximum sample size of 3098. In terms of therapeutic measures, the control group was treated with chemotherapy regimens commonly used for GC, while the intervention group received TCMs in addition to the same chemotherapy regimen. TCMs included 3 types: TCM formula,32,35,36,38-44 Chinese patent medicine,32,34-40,42,43 and TCM injection.33-35,37,38,40 About quality evaluation scales, 12 SRs/MAs32-39,41-44 used the Cochrane criteria for risk of bias assessment of included RCTs, and 1 SR/MA 40 used the Jadad scale. All SRs/MAs were subjected to meta-analysis and all reported positive results.
Table 2.
Characteristics of the Included SRs/Mas.
| Author and Country | Trials (Samples) | Participant | Intervention group | Control group | Main TCM type | Quality assessment | Main results |
|---|---|---|---|---|---|---|---|
| Tan et al, 32 China | 40 (3029) | Advanced GC | TCM + oxaliplatin-based chemotherapy | Oxaliplatin-based chemotherapy | Formula, Chinese patent medicine | Cochrane Criteria | Overall, CHM has a positive effect on improving oxaliplatin-based chemotherapy for AGC, which can improve short-term efficacy and reduce the incidence of AEs. |
| Zhang et al, 33 China | 12 (853) | Advanced GC | TCM + Conventional chemotherapy | Conventional chemotherapy | TCM injection | Cochrane Criteria | Our study still provided helpful information for clinical practice that Cinobufacini injection could enhance the efficacy of other treatments in AGC patients, reduce the side-effects induced by chemotherapy, and help to relieve cancer pain, which might be helpful for clinical medication. |
| Sun et al, 34 China | 27 (1939) | Advanced GC | TCM + Conventional chemotherapy | Conventional chemotherapy | Chinese patent medicine, TCM injection | Cochrane Criteria | In summary, this meta-analysis indicated that cinobufotalin and chemotherapy combined therapy was effective in treating advanced GC. Clinical application of cinobufotalin not only evidently improved the therapeutic effects of chemotherapy but also effectively alleviated most of the side effects caused by chemotherapy. |
| Zhang et al, 35 China | 10 (761) | GC | TCM + Conventional chemotherapy | Conventional chemotherapy | Formula, TCM injection, Chinese patent medicine | Cochrane Criteria | In conclusion, traditional Chinese medicine combined with chemotherapy can improve the treatment efficiency and survival rate of patients with gastric cancer and reduce the incidence of nausea and vomiting after chemotherapy. |
| Chen et al, 36 China | 26 (3098) | GC | TCM + Conventional chemotherapy | Conventional chemotherapy | Formula, Chinese patent medicine | Cochrane Criteria | Traditional Chinese medicine Jianpi Bushen therapy combined with chemotherapy in the treatment of gastric cancer may really enhance the immunity of patients to improve the clinical efficacy and safety. |
| Li et al, 37 China | 14 (1109) | GC | TCM + paclitaxel-based chemotherapy | paclitaxel-based chemotherapy | TCM injection, Chinese patent medicine | Cochrane Criteria | The results indicate that paclitaxel + TCM is more effective and safer. |
| Qiao et al, 38 China | 20 (1735) | Advanced GC | TCM + Conventional chemotherapy | Conventional chemotherapy | Formula, TCM injections, Chinese patent medicine | Cochrane Criteria | In conclusion, Fuzheng Sanjie method of traditional Chinese medicine adjuvant chemotherapy may be better and safer than simple chemotherapy, and has certain clinical promotion and application value, but the evidence is weak. |
| Liang et al, 39 China | 6 (608) | GC | TCM + Conventional chemotherapy | Conventional chemotherapy | Formula, Chinese patent medicine | Cochrane Criteria | Traditional Chinese medicine combined with chemotherapy based on invigorating spleen and supplementing qi is superior to chemotherapy alone in improving the quality of life and inhibiting tumor metastasis, but it cannot significantly improve the survival rate. |
| Xu et al, 40 China | 15 (1052) | GC undergoing gastrectomy | TCM + Conventional chemotherapy | Conventional chemotherapy | Formula, TCM injection, Chinese patent medicine | Jadad | This study shows that traditional Chinese medicine combined with chemotherapy is better than chemotherapy alone in the side effects and survival time of postoperative patients with gastric cancer, suggesting that traditional Chinese medicine combined with chemotherapy is better than chemotherapy alone, which provides some evidence for clinical treatment. |
| Chen et al, 41 China | 21 (1728) | Advanced GC | TCM + Conventional chemotherapy | Conventional chemotherapy | Formula | Cochrane Criteria | Traditional Chinese medicine compound combined with chemotherapy is more helpful to improve the clinical symptoms and tumor conditions of elderly patients with gastric cancer, improve the quality of life, and reduce the occurrence of gastrointestinal reactions. |
| Wang et al, 42 China | 18 (1477) | Locally advanced GC undergoing gastrectomy | TCM + Conventional chemotherapy | Conventional chemotherapy | Formula, Chinese patent medicine | Cochrane Criteria | Traditional Chinese medicine combined with postoperative adjuvant chemotherapy is superior to chemotherapy alone in the prevention and treatment of postoperative recurrence and metastasis of LAGC, and has higher safety. Although the above conclusions are of low quality and the supporting evidence is relatively weak, clinicians should be cautious when applying the conclusions of this study in combination with clinical practice. |
| Wu et al, 43 China | 23 (1339) | Advanced GC | TCM + FOLFOX chemotherapy | FOLFOX chemotherapy | Formula, Chinese patent medicine | Cochrane Criteria | In conclusion, TCM decoction combined with chemotherapy can benefit patients with advanced gastric cancer from multiple aspects |
| Zhao et al, 44 China | 19 (1654) | Advanced GC | TCM + Conventional chemotherapy | Conventional chemotherapy | Formula | Cochrane Criteria | The results showed that the traditional Chinese medicine decoction combined with chemotherapy can effectively improve the clinical efficacy, and the safety is good, has the effect of reducing toxicity, increasing the efficacy, and has certain guiding significance for the clinical treatment of advanced gastric cancer. |
Methodological Quality Assessment
We conducted a methodological assessment of the included SRs/MAs using AMSTAR-2, and the specific results are shown in Table 3. All 13 SRs/MAs included were rated as very low quality due to multiple deficiencies in critical and non-critical items. The reasons for the reduction of methodological quality mainly come from the following items: item 2 (only 1 SR/MA 32 had a registered study protocol), item4 (only 2 SRs/MAs33,38 were adequately searched for the literatures), item 7 (only 1 SR/MA 37 provided a list of excluded articles), and item 10 (none of the SRs/MAs provided a list of funding for RCTs). Among them, item 2, item 4, and item 7 were key items, which directly led to the reduction of the methodological quality of these SRs/MAs.
Table 3.
Result of the AMSTAR-2 Assessments.
| Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Overall quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tan et al 32 | Y | Y | N | PY | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | VL |
| Zhang et al 33 | Y | PY | N | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | VL |
| Sun et al 34 | Y | PY | N | PY | N | Y | N | Y | Y | N | Y | Y | N | Y | Y | Y | VL |
| Zhang et al 35 | Y | PY | Y | PY | N | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | VL |
| Chen et al 36 | Y | PY | Y | PY | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | VL |
| Li et al 37 | Y | PY | Y | PY | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | VL |
| Qiao et al 38 | Y | PY | Y | Y | Y | Y | N | PY | Y | N | Y | Y | Y | Y | Y | N | VL |
| Liang et al 39 | Y | PY | N | PY | Y | Y | N | PY | Y | N | Y | Y | Y | Y | N | N | VL |
| Xu et al 40 | Y | PY | N | PY | N | Y | N | PY | Y | N | Y | Y | Y | Y | N | N | VL |
| Chen et al 41 | Y | PY | N | PY | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | N | VL |
| Wang et al 42 | Y | PY | N | PY | N | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | VL |
| Wu et al 43 | Y | PY | Y | PY | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | VL |
| Zhao et al 44 | Y | PY | Y | PY | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | VL |
Abbreviations: Y, yes; PY, partially yes; N, no; VL, very low.
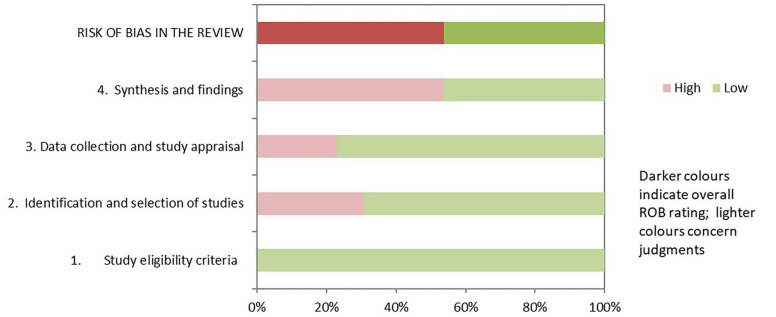
Risk of Bias of the Included SRs/MAs
The assessment details of SRs/MAs are shown in Table 4 and Figure 2. Regarding the results of the ROBIS assessment, both phase 1 (assessing relevance) and domain 1 (study eligibility criteria) of phase 2 rated all SRs/MAs as having low risk of bias. Nine of the SRs/MAs32,33,36-39,41,43,44 were rated as low risk in domain 2 (identification and selection of studies), 10 SRs/MAs32,34-39,41-44 were rated as low risk in domain 3 (collection and study appraisal), and 6 SRs/MAs33,35,36,38,42,43 were rated as low risk in domain 4 (synthesis and findings). In phase 3 (risk of bias in the review), only 6 SRs/MAs32,36-38,42,43 had a low risk of bias.
Table 4.
Results of the ROBIS Assessments.
| Tan et al 32 | Zhang et al 33 | Sun et al 34 | Zhang et al 35 | Chen et al 36 | Li et al 37 | Qiao et al 38 | Liang et al 39 | Xu et al 40 | Chen et al 41 | Wang et al 42 | Wu et al 43 | Zhao et al 44 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Assessing relevance | Q1 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| The risk | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | ||
| Phase 2 | Domain 1: Study eligibility criteria | Q1 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Q2 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Q3 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Q4 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Q5 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| The risk | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | ||
| Domain 2: Identification and selection of studies | Q1 | PY | Y | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | |
| Q2 | PY | Y | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | ||
| Q3 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Q4 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Q5 | Y | Y | N | N | Y | Y | Y | Y | N | Y | N | Y | Y | ||
| The risk | Low | Low | High | High | Low | Low | Low | Low | High | Low | High | Low | Low | ||
| Domain 3: Collection and study appraisal | Q1 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Q2 | Y | PN | PN | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Q3 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Q4 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | ||
| Q5 | PY | Y | PY | Y | PY | PY | PY | PY | PY | PY | PY | PY | PY | ||
| The risk | Low | High | High | Low | Low | Low | Low | Low | High | Low | Low | Low | Low | ||
| Domain 4: Synthesis and findings | Q1 | PN | PY | PN | Y | PY | PN | PY | PN | PN | PN | PY | PY | PN | |
| Q2 | Y | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | ||
| Q3 | Y | Y | Y | Y | PY | Y | Y | Y | Y | PY | Y | Y | Y | ||
| Q4 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Q5 | Y | Y | Y | Y | Y | Y | PY | PY | PY | Y | PY | PY | Y | ||
| Q6 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| The risk | High | Low | High | Low | Low | High | Low | HIGH | High | High | Low | Low | High | ||
| Phase 3 | Risk of bias in the review | Q1 | Y | N | N | N | Y | Y | Y | N | N | Y | N | Y | N |
| Q2 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Q3 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| The risk | Low | High | High | High | Low | Low | Low | High | High | Low | High | Low | High |
Abbreviations: Y, yes; PY, partially yes; PN, partially no.
Figure 2.
Results of the ROBIS assessments.
Report Quality
We used the PRISMA 2020 checklist to evaluate the report quality of SRs/MAs, details of which are provided in Table 5. The 13 included SRs/MAs were fully reported in the title, abstract, introduction, and discussion sections, but there were non-negligible defects in other sections. Some items had a response rate less than 50%, and some even had a response rate of 0%, which was the main reason for the reporting defect. In terms of method, item 7 had a response rate of 0%, none of the SRs/MAs could provide a complete search strategy; item 15 had a response rate of 0%, no SR/MA could provide a certainty assessment. In the results section, item 16 (b) response rate was 7.69%, only 1 SR/MA 37 provided a detailed exclusion list; item 20 (d) response rate was 46.15%, 6 SRs/MAs32-35,43,44 presented the results of the sensitivity analysis; and item 22 response rate was 0%, none of the SRs/MAs had certainty of evidence. In the other information section, only 1 SR/MA 32 registered the study protocol, but it did not describe or explain whether there were any changes or modifications to the information of registration content or the protocol. The response rate of item 24 (a, b, c) was 7.69%, 7.69%, and 0%, respectively. Moreover, the response rate of item 26 was 46.15%, only 6 SRs/MAs declared the conflict of interest of the authors.
Table 5.
Results of the PRISMA 2020 Checklist.
| Section/Topic | Items | Tan et al 32 | Zhang et al 33 | Sun et al 34 | Zhang et al 35 | Chen et al 36 | Li et al 37 | Qiao et al 38 | Liang et al 39 | Xu et al 40 | Chen et al 41 | Wang et al 42 | Wu et al 43 | Zhao et al 44 | Number of Yes or Partially Yes(%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Title | Q1 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% |
| Abstract | Abstract | Q2 | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | 100% |
| Introduction | Rationale | Q3 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% |
| Objectives | Q4 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Methods | Eligibility criteria | Q5 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% |
| Information sources | Q6 | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | 92.31% | |
| Search strategy | Q7 | N | N | N | N | N | N | N | N | N | N | N | N | N | 0% | |
| Selection process | Q8 | Y | Y | N | N | Y | Y | Y | Y | N | Y | N | Y | Y | 69.23% | |
| Data collection process | Q9 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Data items | Q10 (a) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q10 (b) | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | 100% | ||
| Study risk of biasassessment | Q11 | Y | Y | N | Y | N | Y | N | Y | N | Y | Y | Y | Y | 69.23% | |
| Effect measures | Q12 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Synthesis methods | Q13 (a) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q13 (b) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | ||
| Q13 (c) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | ||
| Q13 (d) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | ||
| Q13 (e) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | ||
| Q13 (f) | Y | Y | Y | Y | N | N | N | Y | N | N | N | Y | Y | 53.85% | ||
| Reporting bias assessment | Q14 | Y | Y | N | Y | Y | Y | N | Y | N | Y | Y | Y | N | 69.23% | |
| Certainty assessment | Q15 | N | N | N | N | N | N | N | N | N | N | N | N | N | 0% | |
| Results | Study selection | Q16 (a) | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 92.31% |
| Q16 (b) | N | N | N | N | N | Y | N | N | N | N | N | N | N | 7.69% | ||
| Study characteristics | Q17 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Risk of bias in studies | Q18 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | 92.31% | |
| Results of individual studies | Q19 (a) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q19 (b) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | ||
| Results of syntheses | Q20 (a) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | |
| Q20 (b) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | ||
| Q20 (c) | Y | Y | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 69.23% | ||
| Q20 (d) | Y | Y | Y | Y | N | N | N | N | N | N | N | Y | Y | 46.15% | ||
| Reporting biases | Q21 | Y | Y | N | Y | N | Y | N | Y | N | Y | Y | Y | Y | 69.23% | |
| Certainty of evidence | Q22 | N | N | N | N | N | N | N | N | N | N | N | N | N | 0% | |
| Discussion | Discussion | Q23 (a) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% |
| Q23 (b) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | ||
| Q23 (c) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | ||
| Q23 (d) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% | ||
| Other information | Registration and protocol | Q24 (a) | Y | N | N | N | N | N | N | N | N | N | N | N | N | 7.69% |
| Q24 (b) | Y | N | N | N | N | N | N | N | N | N | N | N | N | 7.69% | ||
| Q24 (c) | N | N | N | N | N | N | N | N | N | N | N | N | N | 0% | ||
| Support | Q25 | Y | Y | Y | Y | Y | Y | N | Y | N | N | Y | Y | Y | 76.92% | |
| Competing interests | Q26 | Y | Y | Y | Y | Y | Y | N | N | N | N | N | N | N | 46.15% | |
| Availability of data, code, and other materials | Q27 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100% |
Abbreviations: Y, yes; PY, partially yes; N, no.
Evidence Quality of the Included SRs/MAs
GRADE specific assessment details are shown in Table 6. In this study, there are 97 outcomes included in 13 SRs/MAs evaluated according to the GRADE guideline. We found that 1 item was rated as high quality, 23 items were rated as moderate quality, 43 items were rated as low quality, and the remaining 30 items were rated as very low quality. Among the downgrading factors, risk of bias (n = 82) was the most common downgrading factor, followed by imprecision (n = 52), publication bias (n = 41), inconsistency (n = 27), and indirectness (n = 0).
Table 6.
Results of Certainty of Quality.
| Author | Outcomes | Limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Quality |
|---|---|---|---|---|---|---|---|
| Tan et al 32 | Objective response rate (ORR) | −1 A | 0 | 0 | 0 | 0 | Moderate |
| Disease control rate (DCR) | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of myelosuppression | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of leucopenia | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of anemia | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of thrombocytopenia | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of gastrointestinal reaction | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of diarrhea | −1 A | 0 | 0 | −1 C | 0 | LOW | |
| Incidence of nausea and vomiting | −1 A | −1 B | 0 | 0 | 0 | Low | |
| Incidence of hepatorenal toxicity | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of hepatotoxicity | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of renal toxicity | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of neurotoxicity | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Zhang et al 33 | Objective response rate (ORR) | −1 A | 0 | 0 | 0 | −1 D | Low |
| Disease control rate (DCR) | −1 A | 0 | 0 | 0 | −1 D | Low | |
| Overall Survival Time | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Improvement rate of KPS | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Incidence of leucopenia | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Incidence of nausea and vomiting | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Incidence of hand-foot syndrome | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Pain relief rate (PRR) | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of anemia | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of diarrhea | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Incidence of neurotoxicity | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Incidence of oral mucositis | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Sun et al 34 | Objective response rate (ORR) | −1 A | 0 | 0 | 0 | 0 | Moderate |
| Disease control rate (DCR) | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Overall survival time | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Score of KPS | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Quality of life improved rate (QIR) | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Pain relief rate (PRR) | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of nausea and vomiting | −1 A | −1 B | 0 | 0 | 0 | Low | |
| Incidence of diarrhea | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of leucopenia | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of thrombocytopenia | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of hepatotoxicity | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Incidence of renal toxicity | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of oral mucositis | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Incidence of alopecia | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of hand-foot syndrome | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of anemia | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of gastrointestinal reaction | −1 A | −1 B | 0 | 0 | 0 | Low | |
| Incidence of neurotoxicity | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Incidence of neutropenia | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of myelosuppression | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Zhang et al 35 | Disease control rate (DCR) | −1 A | 0 | 0 | 0 | 0 | Moderate |
| 1-year survival rate | −1 A | 0 | 0 | −1 C | 0 | Low | |
| 3-year survival rate | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of nausea and vomiting | −1 A | 0 | 0 | 0 | 0 | Moderate | |
| Chen et al 36 | Disease control rate (DCR) | 0 | 0 | 0 | 0 | 0 | High |
| Improvement rate of KPS | 0 | 0 | 0 | 0 | −1 D | Moderate | |
| Incidence of leucopenia | 0 | 0 | 0 | 0 | −1 D | Moderate | |
| Incidence of thrombocytopenia | 0 | 0 | 0 | 0 | −1 D | Moderate | |
| Incidence of anemia | 0 | −1 B | 0 | 0 | −1 D | Low | |
| Incidence of gastrointestinal reaction | 0 | −1 B | 0 | 0 | −1 D | Low | |
| Incidence of neurotoxicity | 0 | 0 | 0 | −1 C | −1 D | Low | |
| Incidence of hand-foot syndrome | 0 | 0 | 0 | −1 C | −1 D | Low | |
| Incidence of myelosuppression | 0 | 0 | 0 | −1 C | −1 D | Low | |
| Li et al 37 | Objective response rate (ORR) | −1 A | 0 | 0 | 0 | −1 D | Low |
| Improvement rate of KPS | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of neutropenia | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Incidence of leucopenia | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Incidence of anemia | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of thrombocytopenia | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of nausea and vomiting | −1 A | −1 B | 0 | −1 C | 0 | Very low | |
| Incidence of hepatotoxicity | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Incidence of neurotoxicity | −1 A | 0 | 0 | −1 C | 0 | Low | |
| Qiao et al 38 | Disease control rate (DCR) | 0 | 0 | 0 | 0 | −1 D | Moderate |
| Overall survival time | 0 | −1 B | 0 | 0 | −1 D | Low | |
| Incidence of nausea and vomiting | 0 | −1 B | 0 | 0 | −1 D | Low | |
| Incidence of leucopenia | 0 | 0 | 0 | 0 | −1 D | Moderate | |
| Liang et al 39 | Quality of life improved rate (QIR) | −1 A | −1 B | 0 | −1 C | 0 | Very low |
| Overall survival time | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Xu et al 40 | 1-year survival rate | −1 A | 0 | 0 | 0 | −1 D | Low |
| 3-year survival rate | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Incidence of leucopenia | −1 A | 0 | 0 | 0 | −1 D | Low | |
| Incidence of nausea and vomiting | −1 A | 0 | 0 | 0 | −1 D | Low | |
| Chen et al 41 | Objective response rate (ORR) | −1 A | 0 | 0 | 0 | 0 | Moderate |
| Improvement rate of KPS | −1 A | −1 B | 0 | 0 | −1 D | Very low | |
| Incidence of nausea and vomiting | −1 A | −1 B | 0 | 0 | −1 D | Very low | |
| Wang et al 42 | Score of KPS | −1 A | −1 B | 0 | 0 | −1 D | Very low |
| Incidence of leucopenia | −1 A | −1 B | 0 | −1 C | −1 D | Very low | |
| Incidence of anemia | −1 A | −1 B | 0 | −1 C | −1 D | Very low | |
| Incidence of thrombocytopenia | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Incidence of hepatotoxicity | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Incidence of renal toxicity | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Incidence of nausea and vomiting | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Incidence of diarrhea | −1 A | −1 B | 0 | −1 C | −1 D | Very low | |
| Incidence of neurotoxicity | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Wu et al 43 | Disease control rate (DCR) | −1 A | 0 | 0 | 0 | −1 D | Low |
| Improvement rate of KPS | −1 A | 0 | 0 | 0 | −1 D | Low | |
| Incidence of nausea and vomiting | −1 A | 0 | 0 | 0 | −1 D | Low | |
| Incidence of diarrhea | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Incidence of leucopenia | −1 A | −1 B | 0 | 0 | −1 D | Very low | |
| Incidence of neurotoxicity | −1 A | 0 | 0 | −1 C | −1 D | Very low | |
| Zhao et al 44 | Disease control rate (DCR) | 0 | −1 B | 0 | 0 | −1 D | Low |
| Incidence of adverse reactions (Mainly including gastrointestinal reaction and myelosuppression) | 0 | 0 | 0 | −1 C | −1 D | Low |
A, The included studies have a large bias in methodology such as randomization, allocation concealment, and blinding. B, The confidence interval overlaps less or the I2 value of the combined results was larger. C, The sample size from the included studies does not meet the optimal sample size or the 95% confidence interval crosses the invalid line. D, The funnel chart is asymmetry.
SRs/MAs Outcomes of Intervention
In this overview, we have summarized and provided a narrative description of the outcomes for the quantitative evaluation of SRs/MAs. Complete information can be found in Table 7.
Table 7.
Summary of Evidence.
| Author | Outcomes | Studies (Participants) | Heterogeneity (%) | Relative effect (95% CI) | P-value | Quality |
|---|---|---|---|---|---|---|
| Tan et al 32 | Objective response rate (ORR) | 40 (3029) | 0 | RR = 1.35 (1.25, 1.45) | <.001 | Moderate |
| Disease control rate (DCR) | 40 (3029) | 0 | RR = 1.12 (1.08, 1.16) | <.001 | Moderate | |
| Incidence of myelosuppression | 11 (819) | 0 | RR = 0.50 (0.41, 0.61) | <.001 | Low | |
| Incidence of leucopenia | 23 (1717) | 0 | RR = 0.54 (0.48, 0.61) | <.001 | Moderate | |
| Incidence of anemia | 14 (926) | 3.25 | RR = 0.77 (0.64, 0.92) | <.001 | Low | |
| Incidence of thrombocytopenia | 21 (1484) | 0 | RR = 0.57 (0.47, 0.70) | <.001 | Moderate | |
| Incidence of gastrointestinal reaction | 14 (1017) | 11 | RR = 0.55 (0.47, 0.64) | <.001 | Moderate | |
| Incidence of diarrhea | 9 (620) | 0 | RR = 0.54 (0.42, 0.69) | <.001 | Low | |
| Incidence of nausea and vomiting | 20 (1506) | 39.16 | RR = 0.61 (0.51, 0.73) | <.001 | Low | |
| Incidence of hepatorenal toxicity | 9 (779) | 0 | RR = 0.71 (0.56, 0.89) | <.001 | Low | |
| Incidence of hepatotoxicity | 20 (1374) | 23.38 | RR = 0.65 (0.52, 0.81) | <.001 | Moderate | |
| Incidence of renal toxicity | 12 (802) | 0 | RR = 0.55, (0.40, 0.77) | <.001 | Low | |
| Incidence of neurotoxicity | 27 (1980) | 0 | RR = 0.70 (0.61, 0.80) | <.001 | Moderate | |
| Zhang et al 33 | Objective response rate (ORR) | 12 (853) | 0 | RR = 1.28 (1.10-1.48) | .001 | Low |
| Disease control rate (DCR) | 11 (796) | 9 | RR = 1.12 (1.04-1.20) | .003 | Low | |
| Overall Survival Time | 2 (205) | 0 | HR = 0.94 (0.75-1.18) | .59 | Low | |
| Improvement rate of KPS | 6 (NR) | 0 | RR = 1.83 (1.40-2.39) | <.001 | Very low | |
| Incidence of leucopenia | 6 (NR) | 50 | RR = 0.76 (0.58-0.99) | .04 | Very low | |
| Incidence of nausea and vomiting | 5 (NR) | 47 | RR = 0.68 (0.53-0.86) | .001 | Very low | |
| Incidence of hand-foot syndrome | 3 (NR) | 0 | RR = 0.55 (0.33-0.91) | .02 | Low | |
| Pain relief rate (PRR) | 2 (NR) | 0 | RR = 1.81 (1.30-2.54) | <.001 | Low | |
| Incidence of anemia | 3 (NR) | 0 | RR = 0.79 (0.58-1.08) | .14 | Low | |
| Incidence of diarrhea | 5 (NR) | 0 | RR = 0.77 (0.52-1.15) | .21 | Very low | |
| Incidence of neurotoxicity | 3 (NR) | 91 | RR = 0.57 (0.23-1.43) | .23 | Very low | |
| Incidence of oral mucositis | 2 (NR) | 88 | RR = 0.37 (0.04-3.47) | .39 | Very low | |
| Sun et al 34 | Objective response rate (ORR) | 26 (1898) | 0 | OR = 1.88 (1.54-2.31) | <.001 | Moderate |
| Disease control rate (DCR) | 25 (1841) | 0 | OR = 2.05 (1.63-2.58) | <.001 | Moderate | |
| Overall Survival Time | 3 (366) | 0 | OR = 1.43 (0.89-2.30) | .14 | Low | |
| Score of KPS | 3 (180) | 79 | MD = 7.00 (2.25-11.75) | .004 | Very low | |
| Quality of life improved rate (QIR) | 12 (947) | 5 | OR = 2.39 (1.81-3.15) | <.001 | Moderate | |
| Pain relief rate (PRR) | 3 (201) | 32 | OR = 4.06 (2.24-7.35) | <.001 | Low | |
| Incidence of nausea and vomiting | NR (889) | 37 | OR = 0.55 (0.41-0.74) | <.001 | Low | |
| Incidence of diarrhea | NR (774) | 0 | OR = 0.65 (0.46-0.90) | .01 | Moderate | |
| Incidence of leucopenia | NR (849) | 34 | OR = 0.62 (0.47-0.82) | <.001 | Moderate | |
| Incidence of thrombocytopenia | NR (356) | 0 | OR = 0.69 (0.44-1.11) | .13 | Low | |
| Incidence of hepatotoxicity | NR (386) | 56 | OR = 0.53 (0.24-1.16) | .11 | Very low | |
| Incidence of renal toxicity | NR (224) | 0 | OR = 0.56 (0.16-1.95) | .36 | Low | |
| Incidence of oral mucositis | NR (468) | 64 | OR = 0.62 (0.28-1.34) | .22 | Very low | |
| Incidence of alopecia | NR (263) | 0 | OR = 0.61 (0.24-1.56) | .3 | Low | |
| Incidence of hand-foot syndrome | NR (690) | 0 | OR = 0.57 (0.41-0.79) | <.001 | Moderate | |
| Incidence of anemia | NR (583) | 0 | OR = 0.69 (0.48-0.99) | .05 | Moderate | |
| Incidence of gastrointestinal reaction | NR (572) | 57 | OR = 0.56 (0.32-1.00) | .05 | Low | |
| Incidence of neurotoxicity | NR (528) | 0 | OR = 0.32 (0.20-0.50) | <.001 | Moderate | |
| Incidence of neutropenia | NR (110) | 0 | OR = 0.45 (0.14-1.42) | .17 | Low | |
| Incidence of myelosuppression | NR (184) | 80 | OR = 0.38 (0.08-1.84) | .23 | Very low | |
| Zhang et al 35 | Disease control rate (DCR) | 9 (664) | 0 | OR = 1.96 (1.39, 2.78) | <.001 | Moderate |
| 1-year survival rate | 4 (311) | 0 | OR = 3.25 (1.90, 5.54) | <.001 | Low | |
| 3-year survival rate | 4 (311) | 0 | OR = 1.71 (1.06, 2.78) | .03 | Low | |
| Incidence of nausea and vomiting | 10 (760) | 0 | OR = 0.47 (0.34, 0.64) | <.001 | Moderate | |
| Chen et al 36 | Disease control rate (DCR) | 8 (890) | 0 | OR = 1.44 (1.09, 1.90) | .01 | High |
| Improvement rate of KPS | 10 (1011) | 0 | OR = 2.86, (2.11, 3.86) | <.001 | Moderate | |
| Incidence of leucopenia | 15 (2218) | 6 | OR = 0.21 (0.16, 0.26) | <.001 | Moderate | |
| Incidence of thrombocytopenia | 9 (1173) | 29 | OR = 0.30 (0.19, 0.48) | <.001 | Moderate | |
| Incidence of anemia | 7 (648) | 54 | OR = 0.33 (0.19, 0.59) | <.001 | Low | |
| Incidence of gastrointestinal reaction | 12 (1919) | 37 | OR = 0.31 (0.24, 0.40) | <.001 | Low | |
| Incidence of neurotoxicity | 5 (356) | 0 | OR = 0.33, (0.20, 0.55) | <.001 | Low | |
| Incidence of hand-foot syndrome | 5 (495) | 0 | OR = 0.31 (0.21, 0.45) | <.001 | Low | |
| Incidence of myelosuppression | 3 (196) | 0 | OR = 0.31 (0.17, 0.56) | <.001 | Low | |
| Li et al 37 | Objective response rate (ORR) | 13 (948) | 12 | RR = 1.39 (1.24, 1.57) | <.001 | Low |
| Improvement rate of KPS | 4 (246) | 0 | RR = 1.53 (1.19, 1.96) | <.001 | Low | |
| Incidence of neutropenia | 5 (392) | 44 | RR = 0.68 (0.55, 0.84) | <.001 | Very low | |
| Incidence of leucopenia | 4 (292) | 40 | RR = 0.69 (0.54, 0.90) | .006 | Very low | |
| Incidence of anemia | 5 (373) | 0 | RR = 0.65 (0.40, 1.04) | .07 | Low | |
| Incidence of thrombocytopenia | 6 (414) | 32 | RR = 0.66 (0.46, 0.96) | .03 | Low | |
| Incidence of nausea and vomiting | 8 (562) | 85 | RR = 0.50 (0.32, 0.80) | .004 | Very low | |
| Incidence of hepatotoxicity | 3 (260) | 0 | RR = 0.63 (0.33, 1.20) | .16 | Low | |
| Incidence of neurotoxicity | 3 (188) | 0 | RR = 0.64 (0.26, 1.55) | .32 | Low | |
| Qiao et al 38 | Disease control rate (DCR) | 19 (1673) | 26 | RR = 1.77 (1.12, 1.22) | <.001 | Moderate |
| Overall survival time | 6 (469) | 50 | RR = 0.57 (0.44, 0.73) | <.001 | Low | |
| Incidence of nausea and vomiting | 14 (1228) | 48 | RR = 0.49 (0.41, 0.57) | <.001 | Low | |
| Incidence of leucopenia | 14 (1228) | 34 | RR = 0.49 (0.44, 0.55) | <.001 | Moderate | |
| Liang et al 39 | Quality of life improved rate (QIR) | 3 (360) | 83 | OR = 12.88 (2.30, 71.99) | .004 | Very low |
| Overall survival time | 2 (188) | 0 | OR = 1.80 (0.98, 3.28) | .06 | Very low | |
| Xu et al 40 | 1-year survival rate | 4 (399) | 0 | OR = 2.17 (1.15, 4.08) | .02 | Low |
| 3-year survival rate | 4 (407) | 0 | OR = 2.26 (1.51, 3.99) | <.001 | Very low | |
| Incidence of leucopenia | 10 (666) | 0 | OR = 0.16 (0.11, 0.23) | <.001 | Low | |
| Incidence of nausea and vomiting | 10 (728) | 9 | OR = 0.20 (0.14, 0.28) | <.001 | Low | |
| Chen et al 41 | Objective response rate (ORR) | 17 (1447) | 0 | OR = 1.90 (1.53, 2.36) | <.001 | Moderate |
| Improvement rate of KPS | 9 (869) | 53 | OR = 2.35 (1.77, 3.13) | <.001 | Very low | |
| Incidence of nausea and vomiting | 11 (958) | 56 | OR = 0.29 (0.19, 0.45) | <.001 | Very low | |
| Wang et al 42 | Score of KPS | 6 (455) | 74 | MD = 7.24 (5.17, 9.31) | <.001 | Very low |
| Incidence of leucopenia | 6 (404) | 79 | RR = 0.64 (0.46, 0.89) | .007 | Very low | |
| Incidence of anemia | 5 (344) | 83 | RR = 0.60 (0.39, 0.93) | .02 | Very low | |
| Incidence of thrombocytopenia | 5 (347) | 34 | RR = 0.72 (0.54, 0.97) | .03 | Very low | |
| Incidence of hepatotoxicity | 5 (359) | 0 | RR = 0.69 (0.29, 1.64) | .41 | Very low | |
| Incidence of renal toxicity | 5 (359) | 0 | RR = 0.59 (0.18, 2.00) | .4 | Very low | |
| Incidence of nausea and vomiting | 4 (267) | 0 | RR = 0.54 (0.41, 0.70) | <.001 | Very low | |
| Incidence of diarrhea | 4 (267) | 38 | RR = 0.61 (0.39, 0.95) | .03 | Very low | |
| Incidence of neurotoxicity | 3 (207) | 0 | RR = 0.47 (0.21, 1.06) | .07 | Very low | |
| Wu et al 43 | Disease control rate (DCR) | 15 (797) | 0 | OR = 1.67 (1.24, 2.26) | <.001 | Low |
| Improvement rate of KPS | 8 (466) | 0 | OR = 4.75 (2.87, 7.86) | <.001 | Low | |
| Incidence of nausea and vomiting | 10 (640) | 0 | OR = 0.33 (0.22, 0.48) | <.001 | Low | |
| Incidence of diarrhea | 8 (389) | 0 | OR = 0.30 (0.19, 0.48) | <.001 | Very low | |
| Incidence of leucopenia | 12 (679) | 41 | OR = 0.52 (0.36, 0.74) | <.001 | Very low | |
| Incidence of neurotoxicity | 9 (437) | 19 | OR = 0.48 (0.31, 0.75) | .001 | Very low | |
| Zhao et al 44 | Disease control rate (DCR) | 13 (1215) | 45 | RR = 1.51 (1.34, 1.70) | <.001 | Low |
| Incidence of adverse reactions (Mainly including gastrointestinal reaction and myelosuppression) | 5 (505) | 12 | RR = 0.51 (0.38, 0.69) | <.001 | Low |
Abbreviations: NR, not report.
Effectiveness Assessment
In terms of clinical remission and control of the tumor, ORR was reported in 5 SRs/MAs32-34,37,41 (evidence quality: 3 moderate, 2 low), and the meta-analysis of these 5 showed that TCMs in combination with chemotherapy had a more significant therapeutic effect than chemotherapy alone and could significantly improve patients’ ORR. Additionally, 8 SRs/MAs32-36,38,43,44 reported DCR (evidence quality: 1 high, 4 moderate, 3 low), all of these studies demonstrated that the efficacy of TCMs combined with chemotherapy was considerably superior to that of chemotherapy alone, which might greatly increase patients’ DCR. Seven SRs/MAs reported KPS (evidence quality: 1moderate, 2 low, 4 very low), of which 5 were KPS improvement rate33,36,37,41,43 and 2 were the score,34,42 all showed that TCMs combined with chemotherapy could improve KPS more than chemotherapy alone. Two SRs/MAs34,39 reported the QIR (evidence quality: 1 moderate, 1 very low), which showed that TCMs combined with chemotherapy could significantly improve the quality of life of patients. Four SRs/MAs33,34,38,39 reported overall survival time (evidence quality: 3 low, 1 very low), only 1 showed TCMs combined with chemotherapy can improve in overall survival time. Besides, 2 SRs/MAs35,40 reported 1-year survival rate and 3-year survival rate (evidence quality: 3 low, 1 very low), they all proved that TCMs combined with chemotherapy is more effective. Two SRs/MAs33,34 reported PRR (evidence quality: 2 low), both of which showed that the combination of TCMs and chemotherapy could increase PRR in patients.
Safety Assessment
The reported adverse reactions of the included SRs/MAs were very comprehensive and diverse. In the hematology section, there are 3 SRs/MAs reported incidence of myelosuppression, 9 SRs/MAs reported incidence of leucopenia, 6 SRs/MAs reported incidence of anemia, 5 SRs/MAs reported incidence of thrombocytopenia, and 2 SRs/MAs reported incidence of neutropenia. In the digestive system section, 3 SRs/MAs reported incidence of gastrointestinal reaction, 10 SRs/MAs reported incidence of nausea and vomiting, 5 SRs/MAs reported incidence of diarrhea. In terms of liver and kidney toxicity, 1 SR/MA reported incidence of hepatorenal toxicity, 4 SRs/MAs reported incidence of hepatotoxicity, 3 SRs/MAs reported incidence of renal toxicity. In other areas, 7 SRs/MAs reported incidence of neurotoxicity, 3 SRs/MAs reported incidence of hand-foot syndrome, 2 SRs/MAs reported incidence of oral mucositis, 1 SR/MA reported incidence of alopecia. There are many contradictions among these results, and the level of quality is generally poor, but some results are worth noting that the addition of TCMs could reduce incidence of leucopenia32-34,36-38,40,42,43 (evidence quality: 4 moderate, 1 low, 4 very low), incidence of gastrointestinal reaction32,34,36 (evidence quality: 1 moderate, 2 low), incidence of nausea and vomiting32-35,37,38,40-43 (evidence quality: 1 moderate, 5 low, 4 very low), and incidence of hand-foot syndrome33,34,36 (evidence quality: 1 moderate, 2 low).
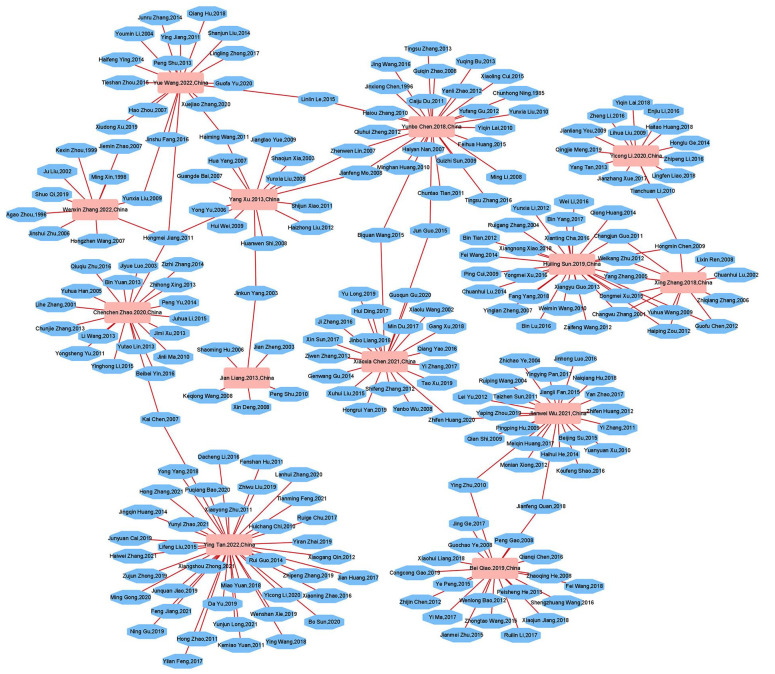
Network Relationships Between SRs/MAs and RCTs
The relationships between the SRs/MAs and the RCTs are shown in Figure 3. This network graph has 238 nodes and 250 edges, representing the 13 SRs/MAs included as well as the 225 RCTs included in these SRs/MAs. Because the number of nodes and edges is relatively similar, there were not too many identical RCTs included in different SRs/MAs. It shows the original researches that can support us in carrying out this overview are relatively sufficient, and there are no significant limitations due to the inclusion of a large number of similar studies.
Figure 3.
Network diagram incorporating SRs/MAs with RCTs.
Discussion
As one of the main treatment methods for GC, chemotherapy is of great significance for the health and quality of life for patients with GC. However, there are still nonnegligible problems associated with either one of the related chemotherapy regimens, mainly in poor efficacy and significant adverse reactions.45,46 In China, TCMs are widely used in combination with chemotherapy in the treatment of GC to achieve more significant efficacy and fewer adverse reactions for patients.47,48 The number of SRs/MAs of TCMs combined with chemotherapy for GC has been increasing in recent years. However, currently, there is no research to integrate them for a comprehensive and systematic review. Therefore, it is necessary to conduct research on this topic. To our knowledge, this study is the first to provide evidence on the efficacy and safety of TCMs as a complementary therapy combined with chemotherapy in the treatment of GC through a comprehensive systematic evaluation of existing SRs/MAs which based on RCTs.
Quality Issues of the Included SRs/MAs
According to the specific circumstances assessed by AMSTAR-2, the main reasons for the low methodological quality of the included publications come from 4 aspects: protocol registration (completion rate 1/13), adequate search of the literatures (completion rate 2/13), exclusion list (completion rate 1/13), and funding sources (completion rate 0/13). First, it is important to register research protocols after researchers have identified the research topic, which can help to increase transparency in the research process and minimize selective reporting bias, improve the rigor and credibility of research reports. 49 Apparently, the included SRs/MAs are deficient in this regard. Similarly, the vast majority of SRs/MAs did not provide an exclusion list of the literature, which also makes the transparency of the studies impaired, and there may be situations in which the studies cannot be replicated, leading to decreased reliability of these studies. Most SRs/MAs are insufficient in the retrieval of RCTs, which may lead to missing RCTs that meet the inclusion criteria, increase the risk of bias in research, and may lead to some deviations in the results. Therefore, a more perfect retrieval strategy should be developed to ensure the reliability of the results. In addition, there are no studies reporting the funding sources of the included RCTs, which may also increase the bias in reporting of clinical trials, as commercially funded research results may be biased by the interest of supporting relevant agencies.
According to the results of the ROBIS assessment, only 6 SRs/MAs were found to have a low risk of bias, while the main reasons leading to high risk of bias were inadequate assessment of publication bias in phase 2 and inadequate interpretation of risk of bias in phase 3.
In terms of reporting quality, after PRISMA 2020 evaluation, the results of which are more similar to those of AMSTAR-2. The 13 SRs/MAs included have considerable shortfalls in reporting on study protocol registration, literature search, exclusion lists and RCTs’ funding sources. In addition, all SRs/MAs’ assessments of the quality of outcome evidence are missing, which can reduce the support for their conclusions. And more than half of SRs/MAs did not conduct sensitivity analysis, which is not conducive to ensuring the stability of the judgment assessment. The aforementioned defects will lead to a decrease in the credibility of the research results and conclusions, and cannot provide strong support for their views.
Regarding evidence quality, the evaluation of 97 results included show that only 1 result is of high quality, 23 results are of medium quality, and the rest are of low or very low quality. The overall quality of evidence is not satisfactory. The most important factor leading to the reduction of quality is the risk of bias (82/97). The main problem is that the included RCTs had methodological defects, most of them did not clearly describe the methods of the random sequence generation method, the allocation concealment method, or the blinding method, which are also common defects of related studies. 50 Other factors contributing to the degradation of the quality of evidence were imprecision (52/97), publication bias (41/97), inconsistency (27/92), while reasons for these issues included insufficient size of included RCTs, lack of publication bias assessment, unjustified study design leading to high heterogeneity in relevant outcome measures.
Regarding the Efficacy and Safety of Traditional Chinese Medicine as a Complementary Therapy in Combination With Chemotherapy for Gastric Cancer
According to the results of our study, the reports of ORR and DCR all show that the effect of TCMs combined with chemotherapy is more therapeutic than chemotherapy alone, and more than half of them have high or moderate quality of evidence, which also indicates that these results are quite valuable, further supporting the conclusion that TCMs as a complementary therapy combined with chemotherapy can improve antitumor efficacy. In terms of patient quality of life, whether the KPS or the QIR or the PRR, and the effect of the intervention group is better than that of the control group, indicating that the addition of TCMs can indeed enable patients to obtain a higher quality of life, which is beneficial for the treatment of patients with GC. However, the quality of outcome evidence in this area is relatively poor compared to that in antitumor, so the support for the combination of TCMs and chemotherapy in improving the survival quality of GC patients is somewhat weakened. On survival time, because many results showed that TCMs combined with chemotherapy cannot confer longer survival than chemotherapy alone, and the quality of available evidence on the outcomes of TCMs combined with chemotherapy improving patient survival time was low, we cannot be sure of the conclusion that the addition of TCMs can prolong patient survival time.
Although these SRs/MAs reported results on various adverse reactions, due to inconsistencies in some of the results and insufficient evidence quality, we can only make the judgment that the treatment plan of TCMs combined with chemotherapy has advantages in only some aspects. It can reduce the occurrence of leucopenia, gastrointestinal reactions, nausea and vomiting, and hand-foot syndrome, but it cannot seem to be helpful for myelosuppression, anemia, thrombocytopenia, neutropenia, hepatotoxicity, renal toxicity, neurotoxicity, oral mucositis, or alopecia.
Overall, narrative analysis indicates that TCMs is an effective complementary treatment for GC chemotherapy patients, which can enhance disease control and relief, improve patients’ quality of life, and reduce the occurrence of some adverse reactions. However, the overall poor quality of the included SRs/MAs may hinder its use as a scientific guide for clinical practice, so it is still necessary to be cautious when recommending TCMs as complementary therapy combined with chemotherapy for GC. In this current situation, medication can be guided based on Chinese medicine theories with a long history, such as syndrome differentiation and corresponding prescriptions, to ensure efficacy and safety.
Implications for Future Practice and Research
As an important means of Chinese medicine in the treatment of GC, TCMs has the characteristics of multiple targets, significant efficacy and few adverse reactions; TCM formula, Chinese patent medicine and TCM injection all have played a positive role in the treatment process.51,52 At present, the anti-tumor mechanism of TCMs cannot be fully revealed, but there are many studies have reported multiple molecules and several pathways involved in the treatment of GC by TCMs.53-55 In clinical practice, we also found that the regimens of TCMs combined with chemotherapy were superior to chemotherapy alone both in terms of efficacy in treating tumors, as well as in the incidence of adverse reactions. Patients could achieve better clinical remission and survival for the control of the disease, higher quality of life and dignity of life, fewer adverse reactions, a relatively better treatment experience with TCM treatment, and this aspect was also confirmed by a large number of studies.56,57 Therefore, we expect that TCMs can be more widely and standardized used with chemotherapy for GC, which also requires a large number of SRs/MAs with the highest evidence-based level on this issue to support it. However, the quality of available relevant studies is unsatisfactory, leading to an inability to robustly support the widespread and standardized combination of TCMs into the chemotherapeutic treatment of GC, which requires a higher quality and level of relevant SRs/MAs in the future. We strongly recommend that future SRs/MAs be refined and improved on multiple fronts. Before the start of the SRs/MAs, researchers should register or publish the study protocol in advance to minimize the risk of bias, make the study transparent, and ensure the reliability of the results. In order to increase the reproducibility of the study and reduce the publication bias, researchers should comprehensively search the literature as much as possible, including the gray literature, provide a complete search strategy list of each electronic database, a list of excluded literatures, and the funding source of the RCTs. Moreover, researchers should also conduct sensitivity analysis during the course of the study to improve the reliability of the study. For the high risk of bias in SRs/MAs, investigators should provide reasonable explanations to ensure the quality of conclusion. Furthermore, conducting a complete publication bias assessment will also improve the accuracy of meta-analysis results. In addition, more high-quality RCTs with good design, rigorous implementation and complete reporting are the cornerstone of research in this topic, which can well avoid the risk of bias. 58 It cannot be ignored that TCMs have the characteristics of a large variety of drugs and formulas as well as a flexible type of preparation, the difference of them may cause different results. Only a few of the SRs/MAs we included tried to solve this problem, which is far from enough. So, we suggest that future researchers can adopt more standardized medication regimens and more meticulous grouping studies when conducting RCTs and SRs/MAs, in order to more specifically study the effects of TCMs as complementary therapy in GC chemotherapy. Besides, the current indicators for efficacy evaluation are mostly focused on the rate of clinical control and remission, and we suggest that increased attention be paid to more objective and quantitative indicators such as tumor markers and biochemical indicators in subsequent studies to better investigate the underlying mechanisms of TCMs in the treatment process and to make clinical research more scientific.
Strengths and Limitations
Our study is the first to evaluate SRs/MAs based on RCTs which regarding TCMs as complementary therapy in combine with chemotherapy for GC according to AMSTAR-2, ROBIS, PRISMA 2020, and GRADE. It can give clinicians helpful advice on developing treatment options. This study also revealed obvious limitations and defect of current SRs/MAs and RCTs, which can help guide the design and conduct of future high-quality clinical research. However, at the same time, the overview has certain limitations because the assessment is subjective. Although our assessments were assessed and reviewed by 2 independent assessors, it is possible that the assessors had their own personal judgments and cognitive biases for each factor and thus the results may have bias. And we found that many of the included SRs/MAs were of poor quality, which might result in reduced confidence in the final conclusions.
Conclusion
TCMs may be an effective and safe complementary therapy in combination with chemotherapy for the treatment of GC, especially in enhancing control and remission of tumors. However, due to the generally poor quality of the existing SRs/MAs and RCTs, which reduce the reliability of the conclusions, physicians should treat these findings with caution in the clinical treatment process. And in order to provide convincing evidence for relevant researchers and clinicians, more high-quality clinical studies of TCMs as a complementary therapy in combine with chemotherapy for GC should be conducted, and the quality of relevant SRs/MAs should be improved.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354231225961 for Efficacy and Safety of Traditional Chinese Medicines as a Complementary Therapy Combined With Chemotherapy in the Treatment of Gastric Cancer: An Overview of Systematic Reviews and Meta-Analyses by Weijian Xie, Yunsong Zhang, Jingyun Tang, Xiaolin Zhu, Shijun Wang and Meiqi Lu in Integrative Cancer Therapies
Footnotes
Author Contributions: Weijian Xie designed the study. Weijian Xie, Meiqi Lu, Yunsong Zhang contributed to the publications search and data extraction. Weijian Xie and Shijun Wang performed data assessment. Weijian Xie, Yunsong Zhang, Meiqi Lu, and Shijun Wang wrote sections of the manuscript. Jingyun Tang and Xiaolin Zhu helped with manuscript revision. All authors reviewed the manuscript. All authors read and approved the final version of the manuscript. Weijian Xie is the first author. Both Meiqi Lu and Shijun Wang are the leaders of this study, and they jointly played a guiding and coordinating role in the research process. Meiqi Lu and Shijun Wang are both corresponding authors.
Data Availability Statement: The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Shandong Provincial Natural Science Foundation of China (ZR2020MH355).
Trial Registration Number: This overview protocol has been registered with the PROSPERO website (CRD42023423046).
ORCID iDs: Weijian Xie  https://orcid.org/0000-0002-4496-4835
https://orcid.org/0000-0002-4496-4835
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. doi: 10.1016/S0140-6736(20)31288-5 [DOI] [PubMed] [Google Scholar]
- 2. Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179-1203. doi: 10.1007/s10555-020-09925-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ajani J, D℉Amico T, Bentrem D, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:167-192. doi: 10.6004/jnccn.2022.0008 [DOI] [PubMed] [Google Scholar]
- 4. Wu H, Fu M, Liu J, et al. The role and application of small extracellular vesicles in gastric cancer. Mol Cancer. 2021;20:71. doi: 10.1186/s12943-021-01365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma Y, Wang B, Maswikiti EP, et al. Pathological complete remission of a locally advanced gastric cancer by neoadjuvant therapy “sandwich” regimen as SOXAP+ fluorescence laparoscopic surgery +SOXAP: case report. Front Pharmacol. 2022;13:1008755. doi: 10.3389/fphar.2022.1008755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. doi: 10.3322/caac.21657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim R, An M, Lee H, et al. Early tumor-immune microenvironmental remodeling and response to first-line fluoropyrimidine and platinum chemotherapy in advanced gastric cancer. Cancer Discov. 2022;12:984-1001. doi: 10.1158/2159-8290.CD-21-0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wei L, Sun J, Zhang N, et al. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. 2020;19:62. doi: 10.1186/s12943-020-01185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colapietro A, Mancini A, D℉Alessandro AM, Festuccia C. Crocetin and crocin from saffron in cancer chemotherapy and chemoprevention. Anticancer Agents Med Chem. 2019;19:38-47. doi: 10.2174/1871520619666181231112453 [DOI] [PubMed] [Google Scholar]
- 10. Thompson J, Schneider B, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw. 2019;17:255-289. doi: 10.6004/jnccn.2019.0013 [DOI] [PubMed] [Google Scholar]
- 11. Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor microenvironment as a “game changer” in cancer radiotherapy. Int J Mol Sci. 2019;20:3212. doi: 10.3390/ijms20133212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su Y, Liu N, Lai Y, Zhou L, Zhang Y. Progress in traditional Chinese medicine syndrome types research of gastric cancer. Shanxi Zhong Yi. 2023;44:262-266. doi: 10.3969/j.issn.1000-7369.2023.02.029 [DOI] [Google Scholar]
- 13. Luo H, Vong CT, Chen H, et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med. 2019;14:48. doi: 10.1186/s13020-019-0270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Zhang Q, Chen Y, et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 2020;121:109570. doi: 10.1016/j.biopha.2019.109570 [DOI] [PubMed] [Google Scholar]
- 15. Wei J, Liu Z, He J, et al. Traditional Chinese medicine reverses cancer multidrug resistance and its mechanism. Clin Transl Oncol. 2022;24:471-482. doi: 10.1007/s12094-021-02716-4 [DOI] [PubMed] [Google Scholar]
- 16. Wang L, Chen L, Wang Q, et al. Clinical observation on Sanleng Xiaoliu mixture combined with chemotherapy in treatment of advanced gastric cancer of intermingled phlegm and stasis syndrome. Shanghai Zhong Yi Yao Za Zhi. 2023;57:36-40. doi: 10.16305/j.1007-1334.2023.2209101 [DOI] [Google Scholar]
- 17. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J, Chen S, Zhou Y, Wang S, Wu W. Efficacy and safety of Huaier granule as an adjuvant therapy for cancer: an overview of systematic reviews and meta-analyses. Integr Cancer Ther. 2022;21:15347354221083910. doi: 10.1177/15347354221083910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C, Zheng Y, Niu D, et al. Effects of traditional Chinese medicine injections for anthracyclines-induced cardiotoxicity: an overview of systematic reviews and meta-analyses. Integr Cancer Ther. 2023;22:15347354231164753. doi: 10.1177/15347354231164753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang F, Zhang X, Li Y, et al. The Chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021;41:747-795. doi: 10.1002/cac2.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. doi: 10.1016/j.annonc.2022.07.004 [DOI] [PubMed] [Google Scholar]
- 22. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whiting P, Savović J, Higgins JP, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225-234. doi: 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383-394. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 26. Hu M, Wang Y, Tu X, Zhang T. Meta-analysis of the efficacy and safety of traditional Chinese medicine combined with apatinib in the treatment of advanced gastric cancer. Zhejiang Zhong Xi Yi Jie Za Zhi. 2022;32:277-282. [Google Scholar]
- 27. Zhang J, Liu Y, Cheng M, Yan R. Systematic review of efficacy of TCM combined with early enteral nutrition in treatment of postoperative patients with gastric cancer. Zhong Guo Zhong Yi Yao Xin Xi Za Zhi. 2019;26:99-103. doi: 10.3969/j.issn.1005-5304.2019.07.022 [DOI] [Google Scholar]
- 28. Cheng M, Hu J, Zhao Y, et al. Efficacy and safety of astragalus-containing traditional Chinese medicine combined with platinum-based chemotherapy in advanced gastric cancer: a systematic review and meta-analysis. Front Oncol. 2021;11:632168. doi: 10.3389/fonc.2021.632168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie X, Huang X, Li J, et al. Efficacy and safety of huachansu combined with chemotherapy in advanced gastric cancer: a meta-analysis. Med Hypotheses. 2013;81:243-250. doi: 10.1016/j.mehy.2013.04.038 [DOI] [PubMed] [Google Scholar]
- 30. Zhang J, Liu Y, Xu Y, Li S, Yan R. Traditional Chinese medicine combined with chemotherapy in treatment of postoperative gastric cancer: a meta-analysis. Zhong Hua Zhong Yi Yao Xue Kan. 2019;37:1819-1825. doi: 10.13193/j.issn.1673-7717.2019.08.005 [DOI] [Google Scholar]
- 31. Shi G, Shang G, Zhou Y, Yang H. Meta-analysis of traditional Chinese medicine plus chemotherapy in treatment of postoperative gastric cancer. Zhong Guo Shi Yan Fang Ji Xue Za Zhi. 2012;18:261-266. doi: 10.13422/j.cnki.syfjx.2012.01.071 [DOI] [Google Scholar]
- 32. Tan Y, Wang H, Xu B, et al. Chinese herbal medicine combined with oxaliplatin-based chemotherapy for advanced gastric cancer: a systematic review and meta-analysis of contributions of specific medicinal materials to tumor response. Front Pharmacol. 2022;13:977708. doi: 10.3389/fphar.2022.977708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Yuan Y, Xi Y, et al. Cinobufacini injection improves the efficacy of chemotherapy on advanced stage gastric cancer: a systemic review and meta-analysis. Evid Based Complement Alternat Med. 2018;2018:7362340. doi: 10.1155/2018/7362340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun H, Wang W, Bai M, Liu D. Cinobufotalin as an effective adjuvant therapy for advanced gastric cancer: a meta-analysis of randomized controlled trials. Onco Targets Ther. 2019;12:3139-3160. doi: 10.2147/OTT.S196684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang W, Zhao Y, Liu H, Jing C. Efficacy of traditional Chinese medicine combined with chemotherapy in the treatment of gastric cancer: a meta-analysis. Comput Math Methods Med. 2022;2022:8497084. doi: 10.1155/2022/8497084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Y, Zhang G, Chen X, et al. Jianpi Bushen, a traditional Chinese medicine therapy, combined with chemotherapy for gastric cancer treatment: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:4924279. doi: 10.1155/2018/4924279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Y, Sui X, Su Z, et al. Meta-analysis of paclitaxel-based chemotherapy combined with traditional Chinese medicines for gastric cancer treatment. Front Pharmacol. 2020;11:132. doi: 10.3389/fphar.2020.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qiao B, Luo S, Liu Z, Zhao Y, Yang W. Meta-analysis of efficacy and safety of Fuzheng Sanjie TCM combined with chemotherapy in the treatment of advanced gastric cancer. Xi Zang Ke Ji. 2019;44-48. [Google Scholar]
- 39. Liang J, Jiang M, Deng X, Wu F. Systematic review of Jianpi Yiqi TCM combined with chemotherapy for gastric cancer. Liao Ning Zhong Yi Za Zhi. 2013;40:630-633. doi: 10.13192/j.ljtcm.2013.04.28.liangj.021 [DOI] [Google Scholar]
- 40. Xu Y, Wang Y, Wang W. Meta-analysis of traditional Chinese medicine combined with chemotherapy in postoperative patients with gastric cancer. Zhong Guo Yi Yao Dao Bao. 2013;10:67-70. [Google Scholar]
- 41. Chen X, Chen G, Liang Y, et al. Meta-analysis of efficacy and safety of TCM compound combined with chemotherapy in the treatment of advanced gastric cancer in the elderly. Hu Nan Zhong Yi Za Zhi. 2021;37:137-141. doi: 10.16808/j.cnki.issn1003-7705.2021.08.049 [DOI] [Google Scholar]
- 42. Wang Y, Zhao W, Li X, et al. Traditional Chinese medicine combined with chemotherapy in prevention and treatment of recurrence and metastasis of locally advanced gastric cancer after radical resection: a meta-analysis. Zhong Liu Fang Zhi Yan Jiu. 2022;49:913-922. [Google Scholar]
- 43. Wu J, Gong H, Qin D, et al. Meta-analysis of clinical efficacy and safety of Chinese medicine decoction combined with FOLFOX chemotherapy in the treatment of stage Ⅲ-Ⅳ gastric cancer. Zhong Yi Zhong Liu Xue Za Zhi. 2021;3:67-77. doi: 10.19811/j.cnki.ISSN2096-6628.2021.05.015 [DOI] [Google Scholar]
- 44. Zhao C, Pang W, Yang P, et al. Systematic review of randomized controlled trial of TCM decoction combined with chemotherapy in the treatment of advanced gastric cancer. Shi Zhen Guo Yi Guo Yao. 2020;31:896-899. [Google Scholar]
- 45. Yuan L, Xu ZY, Ruan SM, et al. Long non-coding RNAs towards precision medicine in gastric cancer: early diagnosis, treatment, and drug resistance. Mol Cancer. 2020;19:96. doi: 10.1186/s12943-020-01219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nie S, Yang G, Lu H. Current molecular targeted agents for advanced gastric cancer. Onco Targets Ther. 2020;13:4075-4088. doi: 10.2147/OTT.S246412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang WJ, Ruan S, Wen F, et al. Multidrug resistance of gastric cancer: the mechanisms and Chinese medicine reversal agents. Cancer Manag Res. 2020;12:12385-12394. doi: 10.2147/CMAR.S274599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15:283-298. doi: 10.5582/bst.2021.01318 [DOI] [PubMed] [Google Scholar]
- 49. Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. 2012;1:7. doi: 10.1186/2046-4053-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shi H, Wang S, Zhang Y, et al. The effects of tai chi exercise for patients with type 2 diabetes mellitus: an overview of systematic reviews and meta-analyses. J Diabetes Res. 2022;2022:6587221. doi: 10.1155/2022/6587221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang K, Chen Q, Shao Y, et al. Anticancer activities of TCM and their active components against tumor metastasis. Biomed Pharmacother. 2021;133:111044. doi: 10.1016/j.biopha.2020.111044 [DOI] [PubMed] [Google Scholar]
- 52. Wang CY, Bai XY, Wang CH. Traditional Chinese medicine: a treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014;42:543-559. doi: 10.1142/S0192415X14500359 [DOI] [PubMed] [Google Scholar]
- 53. Khan M, Shamim S. Anisi Stellati fructus, a significant traditional Chinese medicine (TCM) herb and its bioactivity against gastric cancer. Evid Based Complement Alternat Med. 2022;2022:4071489. doi: 10.1155/2022/4071489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu Q, Tang J, Chen S, et al. Berberine for gastric cancer prevention and treatment: multi-step actions on the Correa℉s cascade underlie its therapeutic effects. Pharmacol Res. 2022;184:106440. doi: 10.1016/j.phrs.2022.106440 [DOI] [PubMed] [Google Scholar]
- 55. Gao Z, Deng G, Li Y, et al. Actinidia chinensis planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression. Biomed Pharmacother. 2020;126:110092. doi: 10.1016/j.biopha.2020.110092 [DOI] [PubMed] [Google Scholar]
- 56. Liu X, Xiu LJ, Jiao JP, et al. Traditional Chinese medicine integrated with chemotherapy for stage IV non-surgical gastric cancer: a retrospective clinical analysis. J Integr Med. 2017;15:469-475. doi: 10.1016/S2095-4964(17)60377-7 [DOI] [PubMed] [Google Scholar]
- 57. Ye HN, Liu XY, Qin BL. Research progress of integrated traditional Chinese and Western medicine in the treatment of advanced gastric cancer. World J Gastrointest Oncol. 2023;15:69-75. doi: 10.4251/wjgo.v15.i1.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354231225961 for Efficacy and Safety of Traditional Chinese Medicines as a Complementary Therapy Combined With Chemotherapy in the Treatment of Gastric Cancer: An Overview of Systematic Reviews and Meta-Analyses by Weijian Xie, Yunsong Zhang, Jingyun Tang, Xiaolin Zhu, Shijun Wang and Meiqi Lu in Integrative Cancer Therapies