Abstract
Purpose
To evaluate the effects of chromium (Cr) and magnesium (Mg) ions on metabolic profiles, inflammation, and oxidative stress with impaired glucose tolerance (IGT) and insulin resistance (IR).
Methods
120 individuals with IGT and IR were randomly divided into four groups treated with (1) chromium, (2) magnesium, (3) chromium and magnesium or (4) placebo. Metabolic and inflammatory indicators were measured at baseline and after 3 months intervention.
Results
Comparison among groups showed that fasting plasma glucose (FPG), 2 h post glucose (2hPPG), fasting insulin (FINS) and homeostatic model assessment for insulin resistance (HOMA-IR) in Cr + Mg group were significantly decreased compared with the other three groups (p < .05), and high density lipoprotein (HDL-c) levels were higher. 8-iso prostaglandin F2 alpha (8-iso-PGF2a) decreased in Cr, Mg, and Cr + Mg groups compared with placebo (p < .05), and 8-iso-PGF2a decreased in Cr + Mg groups compared with Cr group and Mg groups (p > .05). Intra-group comparison showed that the levels of FPG, 2hPPG and FINS in Cr + Mg group were significantly decreased after intervention (p < .05), and FINS in Mg group was significantly decreased (p < .01). The levels of HDL-c and triacylglycerol (TG) in Cr + Mg group were significantly improved (p < .05). The level of HDL-c in Mg group was significantly improved compared with baseline (p < .05). Compared with baseline, high-sensitivity C-reactive protein (hsCRP) levels in Cr + Mg group and Mg group were significantly decreased (p < .05).
Conclusions
The co-supplementation of Cr and Mg improves glycemic and lipid levels and reduces the inflammatory response and oxidative stress profiles of individuals with impaired glucose tolerance and insulin resistance.
Keywords: Chromium, magnesium, impaired glucose tolerance, insulin resistance
Introduction
Impaired glucose tolerance (IGT) is a risk factor for the development of both diabetes and cardiovascular disease, which has dramatically increased in recent years.1,2 Currently some 6.9% of the global population (316 million people) have IGT, a figure expected to rise to more than 470 million by 2035. 3 More than 9.7% of Chinese adults have diabetes. 4 Studies have shown that the prevalence of isolated IGT (19.98%) is approximately twice that of isolated impaired fasting glucose (IFG) (8.14%). The increased costs associated with managing the consequences of IGT and Type 2 Diabetes mellitus (T2DM) emphasise the need to reduce the conversion rate of IGT to T2DM.5,6
Chromium (Cr) is a ubiquitous metal which naturally occurs in water, soil and biological systems. Trivalent chromium is an important nutrient element in both animals and human nutrition. 7 As a micronutrient, chromium has the potential to improve glucose tolerance due to effects on insulin resistance, glucose metabolism, insulin secretion, muscle mass and, ultimately, the prevention of diabetes.8,9 Magnesium (Mg) is an abundant intracellular ion which has an important role in regulating insulin action and sensitivity. 10 Hypomagnesemia has been independently relevant to the development of IGT. 11 In randomized controlled trials (RCTs), Mg supplementation improved glycemic control and insulin resistance (IR) in both frank diabetes, and healthy, overweight adults.12,13
Although some studies have focussed on supplementation with either Cr or Mg alone to reduce glucose, there have been no studies to assess the co-supplementation of Cr and Mg on glucose and lipid metabolism in IGT, and such treatment on inflammation, and oxidative stress. Hence, we hypothesized that Cr and Mg co-supplementation would significantly improve glucose and lipid metabolism through improving inflammation and oxidative stress, compare to either Cr or Mg alone. Therefore the influence of Cr and Mg alone or in combination on glucose homeostasis and lipid levels, and markers of inflammation and oxidative stress were evaluated at baseline and after 3 months intervention in 120 IGT individuals with IR.
Materials and methods
Participants
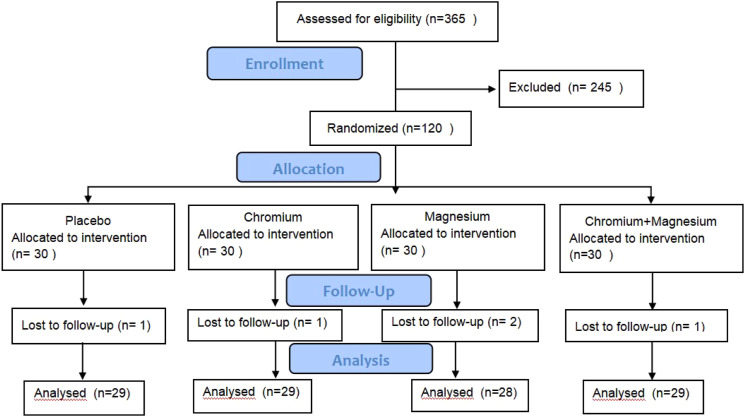
A total of 120 participants were recruited from the Second Affiliated Medical College of Qingdao University between February 2012 and February 2015. A total of 365 subjects were initially screened, and after excluding individuals with renal failure, inflammatory diseases, cancer, liver and thyroid diseases, and those who were pregnant or lactating, 120 eligible participants were enrolled in the study (Figure 1). The inclusion criteria for the study were as follows: individuals aged between 18 and 65 years, with BMI ranging from 19 kg/m2 to 28 kg/m2, fasting plasma glucose (FPG) levels below 7.0 mmol/l (126 mg/dl), 2-h post glucose load levels between 7.8 mmol/l (140 mg/dl) and 11.1 mmol/l (200 mg/dl), and homeostatic model assessment for insulin resistance (HOMA-IR) levels greater than 1. Exclusion criteria included individuals with hypermagnesemia, high Cr levels, or those taking Cr and Mg supplements. All participants provided written informed consent.
Figure 1.
Flow chart of participants through the study.
Study design
This study was designed as a double-blind randomized placebo-controlled trial. A total of 120 individuals who met the inclusion criteria were randomly assigned to one of four groups: the Cr group, Mg group, and Cr + Mg group and placebo group, .In the Cr group (n = 30) received 160 µg of Cr yeast capsules and 400 mg of vitamin C per day. The Mg group (n = 30) received 200 mg of Mg and 400 mg of vitamin C per day. The placebo group (n = 30), participants were given 400 mg of vitamin C daily. Lastly, the Cr + Mg group (n = 30) received 160 µg of Cr yeast capsules, 200 mg of Mg, and 400 mg of vitamin C per day. This treatment regimen was followed for a period of 3 months. All participants were advised to receive a total caloric intake of 30 kcal/kg of ideal body weight per day and advised to consume a diet with 40% carbohydrates, 40% lipids, and 20% proteins. Additionally, it was suggested that they engage in physical activity at least three times a week for a minimum of 30 min.
Blood sample collection
To quantify metabolic profiles, blood samples were taken during the study’s baseline (month 0) and again after 3 months of intervention (month 3). These samples were collected in the early morning following an overnight fast. Additionally, the participants’ liver and kidney functions were assessed every 2 weeks. If a participant’s kidney creatinine level exceeded the normal level on 3 occasions, they were excluded from further participation in the trial. This trial was registered at Chinese Clinical Trials Registry (chictr.org.cn) under the registration number chiCTR-TRC-14004863.
Assessment of anthropometric variables
Body weight and height were measured at baseline and body weight again at 3 months. BMI was calculated as the weight in kg divided by the height in meters squared. Waist circumferences were measured at the minimum circumference between the iliac crest and the last rib. Hip circumferences were measured at the maximum protuberance of the buttocks. Waist to hip ratios (WHR) were calculated as the waist divided by hip circumferences.
Assessment of blood biochemical parameters
Blood samples were centrifuged (Thermo Scientific Sorvall ST 8R, America) at 3000 r/min for 10 min to collect serum. Then, the samples were stored at −80 C before analyses. The following parameters were assayed using an OLYMPUS AU2700 automatic biochemical analyzer:FPG,triglycerides cholesterol (TC), triacylglycerol (TG), high density lipoprotein-cholesterol (HDL-c) and high-sensitivity C-reactive protein (hsCRP). Serum insulin was assessed by a Cobase601 fully automatic immune analyzer. Serum 8-iso prostaglandin F2 alpha (8-iso-PGF2a) was assayed using an ELISA kit (Wuhan Cloud Clone Corp. Chemical Technology). Beta cell function and IR were evaluated from the HOMA-IR values calculated using a previously described equation. 14
Measurement of Cr and Mg levels in RBCs
Total Cr and Mg levels in RBCs were measured after microwave digestion. Briefly, 6 mL nitric acid were added to 0.05 g RBCs, and soaked for 1 h. The resulting solutions were analysis by ICP-MS (Agilent 7500cx, Santa Clara, CA, USA). 15
Statistical analysis
The data analysis was conducted using the Statistical Package of Social Sciences (SPSS) program (version 27.0). The distribution of variables was tested by the Shapiro-Wilk test. In the data statistical analysis, FPG, TC, WHR, low density lipoprotein cholesterol (LDL-c), HDL-c, TG, and BMI follow a normal distribution, while HOMA-IR, 2 h post glucose (2hPPG), hsCRP, 8-iso-PGF2a, and fasting insulin (FINS) do not follow a normal distribution. Values with normal distribution were expressed as mean ± standard deviation, while those without normal distribution were shown as median and interquartile range. Depending on the distribution of variables, a comparison between the groups was performed by the ANOVA test, and Kruskal–Wallis test followed by Mann-Whitney U-test. Paired t test is used for intra-group comparison. Additionally, since variables were not normally distributed, correlations were assessed by Spearman’s test.
Results
This study initially selected 120 research subjects who met the inclusion criteria. After 12 weeks of intervention, a total of 115 participants successfully completed the study. One participant from the placebo group, one from the Cr group, two from the Mg group, and one from the Cr + Mg group were excluded due to lost contact or being diagnosed with cancer. As shown in Table 1, the baseline characteristics including age, weight, metabolism, inflammatory response, oxidative stress, and other indicators did not differ among the four groups.
Table 1.
Baseline characteristics of the study participants.
| Placebo (n = 30) | Chromium (n = 30) | Magnesium (n = 30) | Chromium + Magnesium (n = 30) | |
|---|---|---|---|---|
| Age (years) | 50.89 ± 8.06 | 53.87 ± 8.73 | 52 ± 7.75 | 51 ± 9.05 |
| Gender, male (%) | 12 (40%) | 18 (60%) | 27 (90%) | 21 (70%) |
| BMI(kg/m2) | 23.67 ± 3.09 | 23.80 ± 1.54 | 24.37 ± 1.89 | 24.40 ± 1.80 |
| Fasting glucose (mmol/L) | 5.85 ± 0.50 | 5.85 ± 0.77 | 5.77 ± 0.98 | 5.77 ± 0.56 |
| 2 h glucose (mmol/L) | 9.28 ± 0.53 | 9.41 ± 0.79 | 9.45 ± 0.81 | 9.51 ± 1.03 |
| Fasting Insulin (uU/ml) | 12.40 ± 5.77 | 13.44 ± 8.35 | 13.99 ± 7.95 | 12.48 ± 8.75 |
| HOMA-IR | 3.27 ± 1.76 | 3.35 ± 2.70 | 3.00 ± 3.00 | 2.18 ± 1.67 |
| Triglyceride (mg/dl) | 2.15 ± 1.03 | 2.08 ± 0.66 | 2.11 ± 1.12 | 2.13 ± 0.79 |
| Total cholesterol (mg/dl) | 5.08 ± 0.89 | 5.11 ± 0.95 | 4.93 ± 0.76 | 5.12 ± 0.96 |
| LDL-cholesterol (mg/dl) | 3.21 ± 0.86 | 3.26 ± 0.82 | 3.42 ± 0.95 | 3.23 ± 1.31 |
| HDL-cholesterol (mg/dl) | 1.33 ± 0.32 | 1.33 ± 0.31 | 1.28 ± 0.34 | 1.21 ± 0.21 |
| Waist hip ratio | 0.88 ± 0.06 | 0.89 ± 0.06 | 0.89 ± 0.06 | 0.89 ± 0.05 |
| hsCRP (mg/L) | 2.43 ± 1.46 | 2.50 ± 1.49 | 2.42 ± 1.46 | 2.36 ± 1.13 |
| 8-iso-PGF2a (pg/ml) | 67.89 ± 11.03 | 66.71 ± 8.72 | 68.03 ± 6.88 | 67.51 ± 8.95 |
*Where applicable, values are expressed as Mean ± SEM and median and interquartile range. No significant difference was observed among the groups (p > .05).
Effects of Cr and Mg combination on glucose and IR
The Cr + Mg group showed a significant decrease in FPG (p < .05), 2hPPG and HOMA-IR compared to placebo, and the Cr and Mg groups (p < .05);The Mg group and Cr + Mg group had lower FINS compared to the placebo group and Cr group (p < .05) (Table 2).
Table 2.
Effects of chromium, magnesium and their combination on anthropometric measurements and hsCRP,8-iso-PGF2a in impaired glucose tolerance people after 3 months of intervention.
| n | BMI (kg/m2) | Fasting glucose (mmol/L) | Fasting Insulin (uU/ml) | 2 h Glucose (mmol/L) | HOMA-IR | Waist hip ratio | |
|---|---|---|---|---|---|---|---|
| Placebo | 29 | 24.29 ± 3.43 | 6.38 ± 1.32 | 12.29 ± 6.48 | 9.00 ± 1.83 | 3.17 ± 2.24 | 0.90 ± 0.07 |
| Chromium | 29 | 23.37 ± 2.27 | 5.66 ± 2.30 | 10.95 ± 4.90 | 8.88 ± 2.91 | 3.25 ± 2.56 | 0.88 ± 0.05 |
| Magnesium | 28 | 23.50 ± 2.45 | 5.33 ± 0.63 | 9.63 ± 5.51* | 8.52 ± 2.51 | 2.91 ± 1.34 | 0.88 ± 0.05 |
| Chromium + Magnesium | 29 | 23.40 ± 2.66 | 5.02 ± 1.40* | 8.99 ± 5.36* | 7.00 ± 2.60* | 1.48 ± 0.86* | 0.84 ± 0.06* |
| p | 0.52 | 0.024 | 0.156 | 0.027 | 0.043 | 0.017 | |
| n | Triglyceride (mmol/L) | Total cholesterol (mmol/L) | LDL-cholesterol (mmol/L) | HDL-cholesterol (mmol/L) | hsCRP (mg/L) | 8-iso-PGF2a (pg/ml) | |
| Placebo | 29 | 1.98 ± 1.13 | 4.90 ± 0.98 | 3.13 ± 0.90 | 1.38 ± 0.34 | 2.59 ± 1.21 | 61.71 ± 7.96 |
| Chromium | 29 | 1.55 ± 0.78 | 5.27 ± 1.23 | 3.51 ± 1.24 | 1.46 ± 0.30 | 2.50 ± 1.34 | 52.83 ± 5.72* |
| Magnesium | 28 | 1.53 ± 1.21 | 5.59 ± 1.28 | 3.80 ± 1.17 | 1.49 ± 0.35 | 1.74 ± 0.88* | 56.33 ± 6.46* |
| Chromium + Magnesium | 29 | 1.57 ± 0.48 | 4.81 ± 0.75 | 3.23 ± 0.61 | 1.67 ± 0.51* | 1.47 ± 0.65* | 40.37 ± 6.12* |
| p | 0.215 | 0.059 | 0.105 | 0.048 | 0.000 | 0.000 |
Data as mean ± SEM and as median and interquartile range.
HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; OGTT, oral glucose tolerance test;HOMA-IR = fasting plasma insulin (µIU/mL) × fasting plasma glucose(mmol/L) × 22.5.
*p < .05 was considered to be statistically significant.
In the Cr + Mg group, there were significant reductions in FPG, 2hPPG, and FINS levels after intervention (p < .05). Compared with baseline,the FINS index in the Mg group evidently decreased (p < .01), while FPG and 2hPPG revealed no statistically significant difference (p > .05);The Cr and placebo groups also showed no changes in FPG, FINS, and 2hPPG levels (p > .05);There were non significant minor reductions in HOMA-IR in all four groups.
Effects of Cr and Mg combination on lipids
The Cr + Mg group had higher HDL-c levels compared to the placebo group (1.67 vs 1.38) (p < .05). No significant differences were observed in the comparison between the other groups (p > .05) as shown in Table 2. The WHR in the Cr + Mg group was also significantly lower than that in the control group at 3 months (p < .05).
Compared with baseline, the HDL-c and TG levels in the Cr + Mg group were significantly improved (p < .05). However, there were no significant differences in TC or LDL-c levels (p > .05). We noted a significant improvement in HDL-c levels over the baseline level in the Mg group (p < .05), TG exhibited a decrease relative to baseline P > 0.05), the levels of TC and LDL were elevated compared to baseline levels. There were no differences in lipids between the placebo group and the Cr group (p > .05). The study also found a significant reduction in the WHR in the Cr + Mg group (0.89 [SD 0.05] vs 0.84 [SD 0.06]) (p < .05)compared to baseline. There were no differences in the other three groups (p > .05).
Effects of Cr and Mg combination on inflammation and oxidative stress
Compared with placebo, the Cr group, Mg group, and Cr + Mg group showed a decrease in 8-iso-PGF2a (p < .05). The Cr + Mg group displayed a fall in 8-iso-PGF2a contrasted with Cr group and Mg group (p < .05).
A significant improvement in hsCRP levels occurred in both the Cr + Mg group and the Mg group compared to baseline (p < .05). Levels of hsCRP in the placebo and Cr groups were unchanged,the levels of 8-iso-PGF2a in the four groups had a slightly greater. There were overall differences in 8-iso-PGF2a between all four interventions (P < 0.05).
Supplementation of Cr or/and Mg has no effect on Cr and Mg levels
There are no significant differences in four groups between baseline and after 3 months treatment in Cr And Mg levels.
Discussion
In this double blind, randomized, placebo-controlled clinical trial, individuals with IGT and IR were treated with co-supplementations of Cr and Mg. The study found that co-supplementation of Cr and Mg resulted in significant reductions in hsCRP and 8-iso-PGF2α levels, as well as FBG, 2hPPG, FINS, WHR, and HOMA-IR levels. Additionally, co-supplementation significantly improved serum HDL-c levels.
Previous studies have shown positive effects of Cr alone in lowering blood glucose and serum lipids levels thereby delaying the onset of diabetes. 16 However, most of these studies were not conducted in individuals with IGT and IR. Our findings suggest that combined Cr and Mg co-supplementation may improve both the glycemic status of individuals with IGT and IR and measures of inflammation and oxidative stress. In a previous study by Whitfield the addition of cinnamon, Cr and Mg to kanuka honey were not associated with significant improvements in glucose metabolism or glycemic control in individuals with T2DM. 17 However, other studies have shown a significant improvement in body weight and blood lipid parameters. 18 It is important to note that the previous studies involved individuals with T2DM, while our study focused on individuals with IGT and IR. This provides an opportunity to study the effects of combined Cr and Mg supplementation during the specific period before the onset of T2DM. Based on our findings, combined Cr and Mg co-supplementation of individuals with IGT and IR may be beneficial for controlling glycemic and IR profiles.
Our study found that co-supplementation of Cr and Mg resulted in a significant increase in serum HDL-c levels. This is consistent with the findings of previous studies by Whitfield and Parry-Strong, which also reported improved lipid parameters in groups receiving combined supplementation compared to placebo groups. 17 However, Guimaraes et al. did not find a significant effect of Cr or Mg supplementation on lipid levels. 19 The role of co-supplementation in preventing obesity and weight loss has been debated in previous studies, with conflicting findings. In our study, combined Cr and Mg supplementation improved WHR, potentially indicating a positive influence on metabolic profiles.
Several mechanisms may explain the influence of Cr and Mg co-supplementation on metabolic profiles. Cr enhances insulin sensitivity by promoting insulin binding to cells, increasing insulin receptor numbers, and enhancing insulin receptor kinase activity. 20 Research by Martin has shown that Cr picolinate may enhance insulin sensitivity, improve glucose control, and facilitate weight loss. 21 Glucose loads have been associated with transient endothelial dysfunction in individuals with diabetes and IGT. Endothelial dysfunction is directly related to IR and hyperglycemia and is also related to an increased risk of developing diabetes. 22 Studies have indicated that Cr supplementation significantly improved endothelial functions. 23 The positive effects of Cr on endothelial functions may have been subtle and not fully captured by our relatively crude metabolic measurements. Hyperglycemia has been shown to deplete intracellular free Mg and elevate intracellular calcium levels. A recent meta-analysis has suggested that higher Mg intake may lead to reduced FPG and circulating insulin concentrations. 24 Mg supplementation could increase insulin secretion and improve the ability of beta-cells to compensate for changes in insulin sensitivity in healthy individuals. 25 Mg intake has also been strongly and negatively correlated with metabolic syndromes. Hypomagnesemia has been independently associated with the development of IGT. 26 Our study further demonstrated that co-supplementation of Cr and Mg can improve the inflammatory response and reduce oxidative stress in individuals with IGT.
Oxidative stress plays a significant role in the development and progression of IGT and T2DM. A reliable biomarker of oxidative stress is 8-iso-PGF2α, which forms through free radical-catalyzed attacks on arachidonate. 27 Elevated levels of 8-iso-PGF2α indicate lipid peroxidation, further underscoring the presence of oxidative stress in IGT. 28 Hyperglycemic conditions stimulate the generation of reactive oxygen species (ROS) through various enzymatic and non-enzymatic pathways, including glucose oxidative phosphorylation. 29 The measurement of 8-iso-PGF2α can serve as a dependable marker for assessing the extent of oxidative stress in individuals with IGT. Given the role of oxidative stress in the pathogenesis of IGT, exploring mechanism-based therapeutic approaches holds promise for future research.
The relationship between chronic inflammation and IGT has also been extensively established. HsCRP is commonly employed as an indicator of inflammation. Studies consistently demonstrate significantly elevated hsCRP levels in individuals with IGT, with a strong association between increased hsCRP levels and the presence of IGT. 30 This finding supports the concept that chronic low-grade inflammation is a risk factor for the development of T2DM. Similar results have been reported by Simental-Mendia et al., who found an association between elevated hsCRP levels and prediabetes. 31 Some studies have indicated that oral Mg supplementation significantly decreases hsCRP levels in apparently healthy subjects with prediabetic indicators. Furthermore, joint Cr-Mg supplementation might improve the inflammatory and oxidative stress status of individuals with insulin resistance and IGT. In our study, co-supplementation of Cr and Mg resulted in a significant reduction in hsCRP and 8-iso-PGF2α levels, while no significant effects were observed with Cr or Mg supplementation alone. Therefore, the reduction of ROS and chronic inflammation may contribute to the underlying mechanisms of treating IGT.
However, this study has some limitations. The use of the HOMA-IR method for assessing β cell insulin resistance may have limitations in certain populations. The relatively short duration of the study limits our understanding of the long-term effects of Cr and Mg co-supplementation. Nevertheless, it’s important to note that no toxic effects were observed with the supplements, and the daily supplementation of 400 micrograms of Cr was found to be safe and well-tolerated.
In conclusion, our study suggests that co-supplementation of Cr and Mg can improve glycemic status and lipid profiles in individuals with IGT and IR. This combined treatment was more effective than receiving Cr or Mg alone and was associated with reduced inflammation and oxidative stress. Co-supplementation of Cr and Mg may serve as an adjunct therapy for individuals with IGT, and it may also hold potential for preventing the development of diabetes in individuals with IR or prediabetes. Further research at the molecular level is needed to confirm the roles of Cr and Mg in correcting hyperglycemia, insulin resistance, inflammation, and oxidative stress.
Acknowledgements
The authors alone are responsible for the content and writing of the paper.
Footnotes
Author Contributions: Yang Zhao, Mengmeng Zhou, Yongfang Shang, Mei Dou, Shan Gao, Hai Yang, Fanghua Zhang contributed to the design of the present study.Yang Zhao and Fanghua Zhang performed the experiments and analyzed the data.Yang Zhao, Mengmeng Zhou drafted the manuscript.Yang Zhao, Mengmeng Zhou, Yongfang Shang, Mei Dou, Shan Gao, Hai Yang, Fanghua Zhang confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the National Natural Science Foundation of China Grants 81370990, 30901637 and 81001296.
Ethical statement
Ethical approval
The present study was approved by the Ethics Committee of Affiliated Qingdao Central Hospital, Qingdao University.
Informed consent
Written informed consent was obtained from all participants.
ORCID iD
Yang Zhao https://orcid.org/0009-0006-9229-1942
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
References
- 1.Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ 2020; 369: m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishii H. Cardiovascular events and atherosclerosis in patients with type 2 diabetes and impaired glucose tolerance: what are the medical treatments to prevent cardiovascular events in such patients? J Diabetes Investig 2022; 13(7): 1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman M, Abdul-Ghani M, DeFronzo RA, et al. Review of methods for detecting glycemic disorders. Diabetes Res Clin Pract 2020; 165: 108233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Su S, McCall WV, et al. Rest-activity circadian rhythm and impaired glucose tolerance in adults: an analysis of NHANES 2011-2014. BMJ Open Diabetes Res Care 2022; 10(2): e002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Li J, Wu Y, et al. Evidence from a systematic review and meta-analysis: classical impaired glucose tolerance should Be divided into subgroups of isolated impaired glucose tolerance and impaired glucose tolerance combined with impaired fasting glucose, according to the risk of progression to diabetes. Front Endocrinol 2022; 13: 835460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta M, Agrawal RP, Meena BL, et al. Association between long term exposure to air pollution, impaired fasting glucose, impaired glucose tolerance and prevalence of diabetes. J Assoc Physicians India 2022; 70(4): 11. [Google Scholar]
- 7.Wiesner A, Gajewska D, Pasko P. Levothyroxine interactions with food and dietary supplements-A systematic review. Pharmaceuticals 2021; 14(3): 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey P, Thakur V, Chattopadhyay M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients 2020; 12(6): 1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorklund G, Dadar M, Pivina L, et al. The role of zinc and copper in insulin resistance and diabetes mellitus. Curr Med Chem 2020; 27(39): 6643. [DOI] [PubMed] [Google Scholar]
- 10.Donnarumma M, Marasca C, Palma M, et al. An oral supplementation based on myo-inositol, folic acid and liposomal magnesium may act synergistically with antibiotic therapy and can improve metabolic profile in patients affected by Hidradenitis suppurativa: our experience. G Ital Dermatol Venereol 2020; 155(6): 749. [DOI] [PubMed] [Google Scholar]
- 11.Verma H, Garg R. Evaluation of synergistic combination comprising magnesium orotate, menaquinone-7, and cholecalciferol for management of type 2 diabetes and dyslipidemia. Magnes Res 2020; 33(4): 88–105. [DOI] [PubMed] [Google Scholar]
- 12.He G, Gang X, Sun Z, et al. Type 2 diabetes mellitus caused by Gitelman syndrome-related hypokalemia: a case report. Medicine (Baltim) 2020; 99(29): e21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mooren FC, Kruger K, Volker K, et al. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects - a double-blind, placebo-controlled, randomized trial. Diabetes Obes Metab 2011; 13(3): 281. [DOI] [PubMed] [Google Scholar]
- 14.Abdesselam A, Zidoum H, Zadjali F, et al. Estimate of the HOMA-IR cut-off value for identifying subjects at risk of insulin resistance using a machine learning approach. Sultan Qaboos Univ Med J 2021; 21(4): 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neff C, Becker P, Hattendorf B, et al. LA-ICP-MS using a nitrogen plasma source. J Anal At Spectrom 2021; 36(8): 1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao F, Pan D, Wang N, et al. Effect of chromium supplementation on blood glucose and lipid levels in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Biol Trace Elem Res 2022; 200(2): 516. [DOI] [PubMed] [Google Scholar]
- 17.Whitfield P, Parry-Strong A, Walsh E, et al. The effect of a cinnamon-chromium- and magnesium-formulated honey on glycaemic control, weight loss and lipid parameters in type 2 diabetes: an open-label cross-over randomised controlled trial. Eur J Nutr 2016; 55(3): 1123. [DOI] [PubMed] [Google Scholar]
- 18.Chang GR, Hou PH, Chen WK, et al. Exercise affects blood glucose levels and tissue chromium distribution in high-fat diet-fed C57BL6 mice. Molecules 2020; 25(7): 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guimaraes MM, Martins Silva Carvalho AC, Silva MS. Chromium nicotinate has no effect on insulin sensitivity, glycemic control, and lipid profile in subjects with type 2 diabetes. J Am Coll Nutr 2013; 32(4): 243. [DOI] [PubMed] [Google Scholar]
- 20.Yao X, Liu R, Li X, et al. Zinc, selenium and chromium co-supplementation improves insulin resistance by preventing hepatic endoplasmic reticulum stress in diet-induced gestational diabetes rats. J Nutr Biochem 2021; 96: 108810. [DOI] [PubMed] [Google Scholar]
- 21.Jamilian M, Foroozanfard F, Kavossian E, et al. Effects of chromium and carnitine Co-supplementation on body weight and metabolic profiles in overweight and obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 2020; 193(2): 334. [DOI] [PubMed] [Google Scholar]
- 22.Xu S, Ilyas I, Little PJ, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev 2021; 73(3): 924. [DOI] [PubMed] [Google Scholar]
- 23.Imanparast F, Mashayekhi FJ, Kamankesh F, et al. Improving the endothelial dysfunction in type 2 diabetes with chromium and vitamin D(3) byreducing homocysteine and oxidative stress: a randomized placebo-controlled trial. J Trace Elem Med Biol 2020; 62: 126639. [DOI] [PubMed] [Google Scholar]
- 24.Fong C, Alesi S, Mousa A, et al. Efficacy and safety of nutrient supplements for glycaemic control and insulin resistance in type 2 diabetes: an umbrella review and hierarchical evidence synthesis. Nutrients 2022; 14(11): 2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosseini Dastgerdi A, Ghanbari Rad M, Soltani N. The therapeutic effects of magnesium in insulin secretion and insulin resistance. Adv Biomed Res 2022; 11: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Sousa Melo SR, Dos Santos LR, da Cunha Soares T, et al. Participation of magnesium in the secretion and signaling pathways of insulin: an updated review. Biol Trace Elem Res 2022; 200(8): 3545. [DOI] [PubMed] [Google Scholar]
- 27.Di Minno A, Aveta A, Gelzo M, et al. 8-Hydroxy-2-Deoxyguanosine and 8-iso-prostaglandin F2alpha: putative biomarkers to assess oxidative stress damage following robot-assisted radical prostatectomy (RARP). J Clin Med 2022; 11(20): 6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin CH, Lin YA, Chen SL, et al. American ginseng attenuates eccentric exercise-induced muscle damage via the modulation of lipid peroxidation and inflammatory adaptation in males. Nutrients 2021; 14(1): 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black HS. A synopsis of the associations of oxidative stress, ROS, and antioxidants with diabetes mellitus. Antioxidants 2022; 11(10): 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong X, Ao Q, Xing F. Serum levels of HCY, MIF, and hs-CRP correlate with glycolipid metabolism in adults with never-medicated first-episode schizophrenia. Evid Based Complement Alternat Med 2021; 2021: 7394699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation decreases C-reactive protein levels in subjects with prediabetes and hypomagnesemia: a clinical randomized double-blind placebo-controlled trial. Arch Med Res 2014; 45(4): 325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.