Abstract
Enterococci have emerged as leading agents of nosocomial infection, yet relatively little is known about the pathogenesis of enterococcal disease. In previous studies, we developed an Enterococcus faecalis endophthalmitis infection model which provides unique opportunities to study the evolution of enterococcal disease by direct observation, as well as through sensitive electrophysiologic measures of organ function. The present study was designed to determine whether E. faecalis possesses traits that permit its attachment to mammalian tissues during infection. It was also of interest to determine whether a plasmid-encoded adhesin, aggregation substance, contributes to enterococcal localization or otherwise mediates adherence to alternate sites. These studies found that, in this model, enterococci attach to membranous structures occurring within the vitreous but that this attachment or the course or severity of disease is unaffected by the aggregation substance phenotype.
Enterococci have emerged as important nosocomial pathogens and are among the most frequently isolated organisms in hospital-acquired infections (14, 35, 38). Although enterococci exist as commensal inhabitants of the mammalian gastrointestinal tract, serious enterococcal infection (i.e., bacteremia) is associated with a high mortality rate (25, 42). Increasing interest in enterococcal virulence has been prompted by concerns that many antimicrobial regimens are inadequate in treating serious infection caused by multiply resistant strains. Since enterococcal strains possess the capacity to resist virtually all currently available antimicrobial agents (20), improvement in treating serious enterococcal infections will likely depend upon a clearer understanding of virulence traits and host-parasite interactions (25, 36).
Bacterial adherence to host tissues is an important first step in establishment of infection (2). The mechanisms by which enterococci progress from a state of commensal colonization to cause infection are unknown. Studies have begun to identify and test the roles of potential enterococcal adherence/colonization factors, including (i) cell surface carbohydrates (18, 19); (ii) EfaA, a homolog of cell surface adhesins found on a number of streptococcal species (30); and (iii) aggregation substance, an RGD-containing, inducible cell surface protein (6, 29, 37) encoded by pheromone-responsive plasmids typified by pAD1 (8–10).
Since enterococci are commensal organisms, virulence traits are typically subtle. For example, Ike et al. (22) were the first to ascribe a direct, dose-dependent correlation between the Enterococcus faecalis plasmid pAD1-encoded cytolysin and toxicity by using a murine peritonitis model and isogenic E. faecalis strains with specifically attenuated cytolysin expression. However, differential effects between isogenic strains were observed only after injections of greater than 108 CFU. By using a rabbit endocarditis model, Chow et al. (6) observed that heart valve vegetation size was significantly increased in animals infected with E. faecalis strains expressing aggregation substance, compared to isogenic aggregation substance-negative strains, but mortality was increased only in animals infected with strains expressing both aggregation substance and the pAD1-encoded cytolysin together. Although the endocarditis model suggested a role for an enterococcal adhesin in vivo, the adherence of enterococci, in either the wild type or isogenic variants, to specific animal tissues was not examined beyond measuring the mass of vegetations.
In previous studies, we developed an experimental endophthalmitis model to more precisely assess the role of an enterococcal virulence factor (cytolysin) in vivo (24, 39). As with other nosocomial infections, enterococci rank as a leading cause of postoperative endophthalmitis, which is usually associated with significant loss of vision (11, 15, 33), providing additional relevance to human infection. A strength of this model is that as few as 10 E. faecalis CFU can be used to establish an infection, which then evolves under in vivo conditions (24, 39). Further, endophthalmitis provides an exquisitely sensitive infection system in which organ function can be directly assessed by electroretinography (ERG; an electrophysiologic measure of neuroretinal function), by parameters of ophthalmologic observation (slit lamp direct/indirect ophthalmoscopy), or by histopathological assessment. Moreover, organ function can be compromised without triggering a confounding cascade of end stage events. Taking advantage of transposon Tn917 insertional mutants that were either wild type or specifically attenuated with respect to cytolysin expression, the role of cytolysin as an enterococcal virulence factor was unambiguously demonstrated (24).
In addition to destructive intraocular changes caused by cytolytic strains, histopathological analysis demonstrated E. faecalis cells in close association with retinal inner limiting membranes in eyes infected with either cytolytic or noncytolytic strains (24), suggesting expression of one or more enterococcal adhesins. We hypothesized that the apparent attachment of enterococci to internal ocular structures may be a function of aggregation substance (24), since both of the strains used in the study expressed aggregation substance and since intraocular tissues are known to contain cell surface integrins, potential mammalian ligands for aggregation substance (5, 7, 13, 28). However, since the strains used in our previous endophthalmitis study were all aggregation substance producers, it was impossible to ascribe a role for aggregation substance in intraocular enterococcal localization.
Because in vitro data suggest that enterococci may express general colonization and/or invasion factors (18, 19, 30, 37, 43) and aggregation substance makes a contribution in the endocarditis model (6), it was of interest to use endophthalmitis as a sensitive model to evaluate the ability of enterococci to adhere to mammalian tissue during the course of infection and to assess the role of aggregation substance in that binding.
Three isogenic, pAD1-containing, cytolytic E. faecalis strains were selected for analysis in the rabbit experimental endophthalmitis model. E. faecalis JH2SS(pAM714) is a wild-type strain harboring a transposon Tn917 insertion in a region of pAD1 not affecting cytolysin or aggregation substance expression (23). The course and severity of the endophthalmitis caused by this strain have been characterized (24, 39). E. faecalis JH2SS(pAM944) is an isogenic, cytolysin-positive, aggregation substance-deficient strain harboring a Tn917 insertional mutation in the aggregation substance structural gene of pAD1 and is nonresponsive to pheromone-induced clumping (29). E. faecalis JH2SS(pAM2120) is also an isogenic, cytolysin-positive strain but is constitutive with respect to aggregation substance expression as a result of Tn917 insertion in the regulatory gene traA (41). Inclusion of the aggregation substance-constitutive strain was prompted for several reasons. (i) A previous study indicated that aggregation substance expression in wild-type strains [such as JH2SS(pAM714)] is induced by serum components (29). (ii) Strains used in the endocarditis model (6) were either wild type or negative with respect to aggregation substance expression, and therefore the actual level of aggregation substance expression in that in vivo model remained unknown. (iii) In the present study, the potential effects of ocular fluids on induction of aggregation substance expression could not be predicted. Strains JH2SS(pAM944) and JH2SS(pAM2120) were generously provided by Don Clewell, University of Michigan. Aggregation substance phenotypes were confirmed by using the pheromone-induced bacterial clumping assay (12).
Preparation, enumeration, and injection of E. faecalis cells (100 CFU/0.1 ml of phosphate-buffered saline) into the mid-vitreous of New Zealand White rabbit eyes were performed as previously described (24, 39) and in accordance with the Association for Research in Vision and Ophthalmology Statement on the Use and Care of Animals in Ophthalmic Research. Contralateral eyes served as surgical controls and received only sterile saline. Infection course was monitored by (i) scotopic ERG, a measure of neuroretinal responsiveness, on postinfection days 1, 2, and 3 as previously described (3, 4, 24, 39); (ii) clinical evaluation (slit lamp biomicroscopy) of ocular inflammatory changes according to a quantitative grading scale (34) on postinfection days 1, 2, and 3; and (iii) whole-globe histopathological analysis (hematoxylin and eosin and tissue Gram staining) of 7-μm sections at postinfection days 1 and 3 according to published procedures (31).
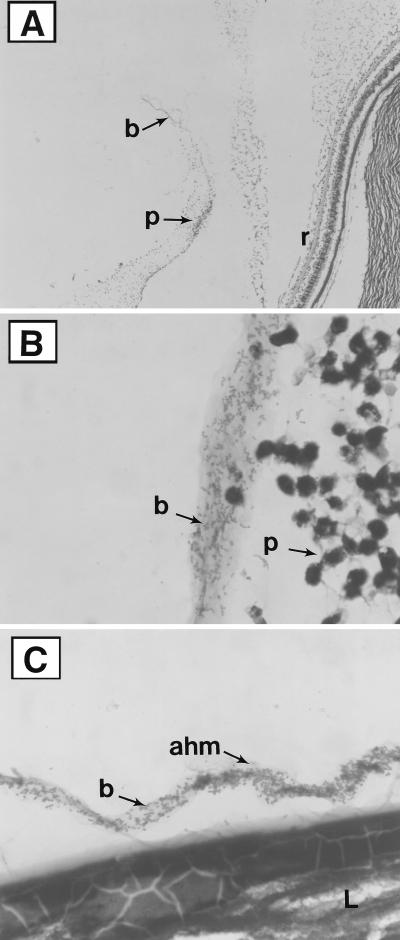
At 24 h postinfection with wild-type (cytolysin- and aggregation substance-positive) E. faecalis JH2SS(pAM714), distinct patterns of intraocular localization were observed by histopathological examination. Rather than random and diffuse distribution of the organisms within the vitreous body, enterococci were observed predominantly in relatively dense packets adjacent to, or in contact with, a number of intraocular structures. Enterococci were observed in large clusters along the posterior hyaloid membrane, the Cloquet’s Canal vitreous interface, and the anterior hyaloid membrane (Fig. 1). Unexpectedly, similar patterns of localization were observed in eyes infected with aggregation substance-negative strain JH2SS(pAM944). Moreover, eyes infected with the aggregation substance-constitutive strain revealed no discernible differences in enterococcal localization patterns, compared to wild-type and aggregation substance-negative strains. These results demonstrate a discrete in vivo localization but one that is independent of the aggregation substance phenotype, suggesting a role for other, uncharacterized, and perhaps nonspecific, enterococcal adhesins. Massive destructive and inflammatory changes at day 3 precluded clear visualization of bacterial cells by tissue Gram staining (data not shown).
FIG. 1.
Histologic analysis of representative infected eyes at postinfection day 1 (Brown and Hopps tissue Gram stain). (A) Low magnification of a posterior segment, demonstrating bacteria [E. faecalis JH2SS(pAM714)] and inflammatory cells along the posterior vitreous hyaloid membrane (8.8 times the original magnification). (B) High magnification of a posterior segment, demonstrating bacteria [E. faecalis JH2SS(pAM2120)] associated with a vitreous strand (88 times the original magnification). (C) High magnification of a posterior segment, demonstrating bacteria [E. faecalis JH2SS(pAM944)] associated with the anterior hyaloid membrane (88 times the original magnification). r, retina; p, inflammatory cells (predominantly PMNs); ahm, anterior hyaloid membrane; L, lens; b, bacteria (E. faecalis). Similar patterns of intraocular localization were observed for all three enterococcal strains, regardless of aggregation substance expression.
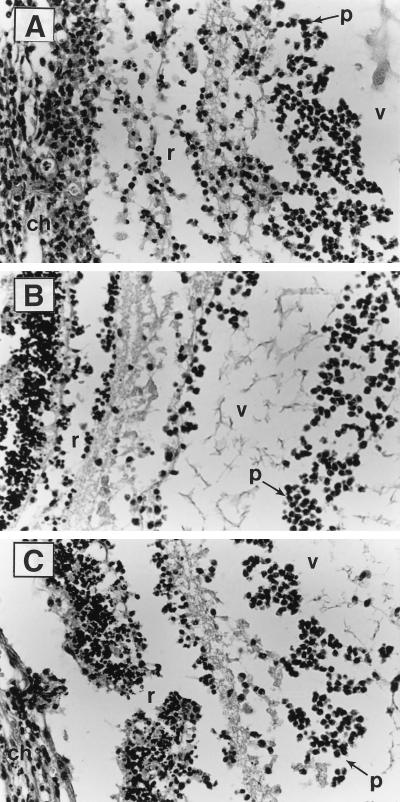
Histopathological analysis of intraocular inflammation and tissue damage revealed identical findings among groups varying only in aggregation substance expression at postoperative days 1 and 3. Thin sections at day 1 (Fig. 1) revealed destructive retinal changes and moderate infiltration of the vitreous chamber with inflammatory cells (predominantly polymorphonuclear leukocytes [PMNs]) similar to that previously observed in studies assessing the role of the E. faecalis cytolysin (24, 39) but in which all strains were phenotypically aggregation substance positive (24). At postinfection day 3, thin sections from all infected eyes demonstrated marked anterior chamber fibrin clots and inflammatory cell infiltration, massive vitritis with nearly complete filling of the vitreous chamber with inflammatory cells and fibrin, and nearly total destruction and disorganization of retinal architecture and underlying choroidal tissues, even in eyes infected with aggregation substance-negative strains (Fig. 2).
FIG. 2.
Histologic analysis of representative infected eyes at postinfection day 3 (hematoxylin and eosin staining; about 30 times the original magnification). (A) Retina-vitreous interface from eyes infected with 100 CFU of E. faecalis JH2SS(pAM714). (B) Retina-vitreous interface from eyes infected with 100 CFU of E. faecalis JH2SS(pAM944). (C) Retina-vitreous interface from eyes infected with 100 CFU of E. faecalis JH2SS(pAM2120). r, retina; v, vitreous; ch, choroid; p, inflammatory cells (predominantly PMNs). Control eyes receiving saline injections appeared normal and are not shown.
Results from the endocarditis model suggested that aggregation substance could enhance the virulence of cytolytic E. faecalis (6); however, mortality measurements are relatively insensitive and are not designed to directly assess the effects of bacterial factors on host tissue physiology. We therefore assessed the ability of aggregation substance to potentiate pathological changes and organ function loss in endophthalmitis by using electrophysiologic measures as well as direct ophthalmologic observation of affected tissues. In contrast to the enhanced virulence ascribed to cytolysin-positive, aggregation substance-positive strains in the endocarditis model, the course of cytolytic E. faecalis endophthalmitis, by clinical parameters, was aggregation substance independent. Eyes from all infection groups varying in aggregation substance expression demonstrated mild to moderate inflammatory changes by 24 h postinfection, consisting of 1+ to 2+ anterior chamber flare, 0 to 2+ anterior chamber cell, 0 to 1+ vitreous cell, and slightly reduced red reflex. At 48 and 72 h, all infected eyes demonstrated marked inflammatory changes: 3+ to 4+ anterior chamber cell and flare, severe iritis and limbal blood vessel engorgement, a markedly diminished or absent red reflex, and the presence of large masses of anterior chamber fibrin which obscured posterior vitreous chamber visualization. Control eyes remained normal throughout the experimental course.
Similarly, organ function losses were identical in all eyes, regardless of the aggregation substance phenotype. Baseline ERG B-wave amplitudes decreased approximately 30% by 24 h postinfection and declined to nearly undetectable levels by postinfection days 2 and 3 (Fig. 3). Statistical analysis (Student’s t test) of ERG results revealed no statistically significant differences among infection groups at postinfection days 1 (P > 0.7), 2 (P > 0.4), and 3 (P > 0.2). Furthermore, we were able to attribute the extent of ERG decline to cytolysin rather than aggregation substance, since an additional group of eyes infected with an isogenic cytolysin-negative, aggregation substance-positive strain demonstrated significant (P < 0.05) retention of neuroretinal function compared to cytolytic strains (Fig. 3). These results agree with those of previous studies on the role of cytolysin in endophthalmitis (24).
FIG. 3.
ERG results from rabbit eyes infected with isogenic E. faecalis strains differing in the expression of aggregation substance (Asa-1). Approximately 100 CFU of each strain were injected into the right eyes of New Zealand White rabbits at day 0. Surgical control left eyes were injected with sterile saline. Data at each postinfection day are presented as percentages of the B-wave amplitude retained compared to the baseline. Values and error bars represent means ± the standard errors of the means. Cyl+, cytolysin positive; Asa1+, aggregation substance wild type inducible; Asa1−, aggregation substance negative; Asa1c, aggregation substance constitutive. Eyes infected with isogenic, noncytolytic E. faecalis (•) were included in the present study for ERG comparisons only.
To ensure that the lack of observable differences between the strains used in this study was not due to in vivo loss of their respective Tn917 insertions and aggregation substance phenotypes, additional animals were infected and intraocular enterococci were retrieved at postinfection day 3 by plating serial dilutions of vitreous onto brain heart infusion agar. Ten of ten randomly selected colonies from each infection group displayed the anticipated phenotypes as determined by growth and hemolysis on brain heart infusion agar supplemented with erythromycin at 50 μg/ml and 5% rabbit erythrocytes and a pheromone-induced clumping assay, indicating that the infecting organisms were not revertants.
This study is the first, to our knowledge, to examine adherence patterns of enterococci in vivo. The results indicate that enterococci localize to and adhere to discrete tissues and structures but that localization patterns and the infection course are unaffected by expression of aggregation substance, suggesting in vivo expression of other enterococcal adhesins. While the importance of investigating the mechanisms by which enterococci colonize and/or invade host tissues is highlighted by the emergence of strains resistant to virtually all currently available antimicrobial agents (20, 36), the roles of enterococcal adhesins in disease pathogenesis remain unclear. Guzmàn et al. (18, 19) observed that E. faecalis strains isolated from urinary tract infections efficiently adhered to urinary tract epithelial cells, while endocarditis isolates adhered efficiently to Girardi heart cells in vitro. Competitive inhibition assays implicated enterococcal cell surface carbohydrates in mediating binding to cultured cell lines. Exposure of urinary tract infection isolates to serum resulted in enhanced binding to heart cells, suggesting that the adhesin(s) is inducible. However, the identity of this adhesin(s) has yet to be determined. In 1995, Lowe et al. (30) reported the nucleotide sequence of an E. faecalis cell surface antigen which was reactive with antisera from endocarditis patients. The deduced amino acid sequence of this antigen, termed EfaA, revealed a high degree of homology with adhesins from streptococcal species, including FimA from Streptococcus parasanguis, SsaB from S. sanguis, PsaA from S. pneumoniae, and ScaA from S. gordonii (30). Although EfaA was hypothesized to function as an adhesin in endocarditis, its role in enterococcal disease has not been tested.
Among the enterococcal surface moieties hypothesized to promote adhesion to mammalian tissues, the most thoroughly studied is E. faecalis aggregation substance. Aggregation substance is a surface-bound protein encoded by pheromone-responsive plasmids and is expressed upon induction by small peptide pheromones (12, 17, 21, 40). Aggregation substance participates in the formation of mating aggregates during enterococcal genetic exchange. Its hypothesized role in mediating adherence to mammalian tissues stems from nucleotide sequence analysis, indicating the presence of Arg-Gly-Asp (RGD) motifs believed to confer adhesive properties via direct interaction with host cell surface integrins (17, 29). Kreft et al. (29) observed that isogenic variants of E. faecalis OG1X harboring pAD1 bound cultured renal tubular cells at modestly higher levels than did aggregation substance-deficient mutants and that binding was partially inhibited by synthetic RGDS peptides. However, it is difficult to extrapolate an in vivo role for aggregation substance from these in vitro data, since unidentified components from serum used in the assays were found to induce aggregation substance expression (29).
Wells and colleagues expanded upon these studies by assessing both binding to and internalization by cultured intestinal epithelial cells (37). It was observed that uptake of E. faecalis OG1RF strains expressing aggregation substance was enhanced compared to that of aggregation substance-deficient derivative strains. However, they observed that the murine E. faecalis isolate M20 used in previous gastrointestinal translocation studies (43) was taken up less efficiently by cultured intestinal epithelial cells than were isogenic OG1RF strains not expressing aggregation substance. These results suggest that other enterococcal surface components are likely important in adherence and/or invasion (37).
A number of enterococcal phenotypic properties were assessed by Arduino et al. (1) for their contribution to PMN-mediated killing. It was observed that surface proteins involved in the aggregative response to pheromones played no discernible role in PMN-mediated killing of test strains. However, since isogenic strains with specifically attenuated aggregation substance gene expression were not tested, the specific contribution of aggregation substance to interactions with host clearance mechanisms could not be determined.
The only study to suggest a potential role for aggregation substance in a disease model was that of Chow et al. (6), who compared three isogenic E. faecalis strains which expressed both aggregation substance and cytolysin, cytolysin alone, or aggregation substance alone with a plasmid-free strain for the ability to cause endocarditis in rabbits. Those researchers observed that rabbits infected with strains expressing aggregation substance exhibited increased heart valve vegetation weight regardless of cytolysin expression (6). Although larger vegetations were noted in animals infected with cytolysin-negative, aggregation substance-positive strains, these strains were no more virulent with respect to mortality than was the plasmid-free strain. The role of aggregation substance in E. faecalis infectivity in the experimental endocarditis model remained unknown, since infective doses were not determined. Virulence in the endocarditis model was reported in terms of animal mortality and vegetation size (6), but adherence patterns of enterococcal strains to specific heart tissues were not examined.
To the best of our knowledge, there have been no well-controlled studies of enterococcal adherence in mammalian tissues during acute infection. Because (i) conclusions on the role of enterococcal adhesins have been largely derived from in vitro data and (ii) aggregation substance was suggested to play a role in experimental endocarditis, it was of interest to employ a sensitive system to evaluate the ability of enterococci to adhere to host tissues in vivo and to assess the role of aggregation substance in that binding. Previous studies on the E. faecalis cytolysin demonstrated experimental endophthalmitis to be a useful model for studying host-parasite interactions during the infection process. Experimental endophthalmitis was chosen as an exquisitely sensitive infection system for several reasons. First, experimental endophthalmitis is the only model system of enterococcal infection that allows introduction of low numbers of organisms, which are then capable of expanding in an in vivo environment. Second, because of the demonstrated importance of host environmental signals in affecting bacterial virulence (16, 32), it was important to choose a model that allows for exposure of organisms to host signals during in vivo growth. Third, because of immune deviation (27), the eye is slow to respond to, and clear, infectious insults and is therefore useful in studying subtle differences in bacterial pathogenicity. Last, compared to other infection sites, the eye is amenable to objective measures of organ function, direct observation of the host response (due, in part, to the transparency of the intraocular vitreous and aqueous fluids), and whole-organ histological analysis of bacterial adhesion patterns during the course of infection. Results from this study cannot necessarily be extrapolated to other infections (e.g., endocarditis) because of the unique physiology of the intraocular spaces, the tissue specificity of bacterial adherence factors, and a relative absence of humoral factors and mechanical clearance mechanisms within the intraocular spaces. Demonstrated contributions of bacterial adhesins in the endophthalmitis model may therefore require additional confirmatory testing in other in vitro and in vivo systems.
In a previous study on the E. faecalis cytolysin, we speculated that the apparent attachment of enterococci may play a role in affecting the course of experimental endophthalmitis (24). We hypothesized that aggregation substance-mediated adhesion would bring cytolysin-producing organisms in close contact with, and thereby concentrate cytolytic activity near, its target. However, this notion was not supported by the findings of the present study. The distinct localization patterns of E. faecalis within the eye, which were independent of the aggregation substance phenotype, may shed light on the reasons for the relative inadequacy of current endophthalmitis therapy for treating cytolytic E. faecalis endophthalmitis (26). Treatment of bacterial endophthalmitis frequently involves removal of vitreous fluids (vitrectomy) and direct intraocular instillation of antimicrobial agents. The findings from this study suggest that organisms sequestered in pockets within the eye may, in fact, be relatively inaccessible to surgical debridement and antibiotics, which may then relate to the uniformly poor outcome of these infections, as observed in a recent, large, multicenter study (15). It is anticipated that the experimental endophthalmitis model will prove to be a sensitive model for future assessment of other enterococcal adhesins.
Acknowledgments
This study was supported in part by an OCAST award for project HN6-040 from the Oklahoma Center for the Advancement of Science and Technology (B.D.J.), U.S. Public Health Service grant EY-08289 (M.S.G.), and unrestricted funds from Research to Prevent Blindness, Inc.
We thank Kenneth Hatter, Department of Ophthalmology, for valuable assistance in animal handling and ERG and Robert Shaver (Dean A. McGee Eye Institute Ocular Pathology Laboratory) and James Tomasek (University of Oklahoma HSC Department of Anatomical Sciences) for valuable assistance in histopathological analyses.
REFERENCES
- 1.Arduino R C, Murray B E, Rakita R M. Roles of antibodies and complement in phagocytic killing of enterococci. Infect Immun. 1994;62:987–993. doi: 10.1128/iai.62.3.987-993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddour L M, Christensen G D, Simpson W A, Beachy E H. Microbial adherence. In: Mandell G L, Douglas R G Jr, Bennett L E, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1990. pp. 9–25. [Google Scholar]
- 3.Booth M C, Atkuri R V, Nanda S K, Iandolo J J, Gilmore M S. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Invest Ophthalmol Vis Sci. 1995;36:1828–1836. [PubMed] [Google Scholar]
- 4.Booth M C, Cheung A L, Hatter K L, Jett B D, Callegan M C, Gilmore M S. Staphylococcal accessory regulator (sar) contributes to Staphylococcus aureus virulence in endophthalmitis only in conjunction with agr. Infect Immun. 1997;65:1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brem R B, Robbins S G, Wilson D J, O’Rourke L M, Mixon R N, Robertson J E, Planck S R, Rosenbaum J T. Immunolocalization of integrins in the human retina. Invest Ophthalmol Vis Sci. 1994;35:3466–3474. [PubMed] [Google Scholar]
- 6.Chow J W, Thal L A, Perri M B, Vazquez J A, Donabedian S M, Clewell D B, Zervos M J. Plasmid-encoded hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1993;37:2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciarrocchi A, Rieber M S, Rieber M. Extracellular RGD-binding proteins modulate cell adhesion. Biochem Biophys Res Commun. 1992;183:544–552. doi: 10.1016/0006-291x(92)90516-n. [DOI] [PubMed] [Google Scholar]
- 8.Clewell D B, Weaver K E. Sex pheromones and plasmid transfer in Enterococcus faecalis. Plasmid. 1989;21:175–184. doi: 10.1016/0147-619x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- 9.Clewell D B, Tomich P K, Gawron-Burke M C, Franke A E, Yagi Y, An F Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982;152:1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clewell D B. Bacterial sex pheromone-induced plasmid transfer. Cell. 1993;73:9–12. doi: 10.1016/0092-8674(93)90153-h. [DOI] [PubMed] [Google Scholar]
- 11.Driebe W T, Mandelbaum S, Forster R K, Schwartz L K, Culbertson W W. Pseudophakic endophthalmitis: diagnosis and management. Ophthalmology. 1986;93:442–448. doi: 10.1016/s0161-6420(86)33722-9. [DOI] [PubMed] [Google Scholar]
- 12.Dunny G M. Genetic functions and cell-cell interactions in the pheromone-inducible plasmid transfer system of Enterococcus faecalis. Plasmid. 1990;4:689–696. doi: 10.1111/j.1365-2958.1990.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 13.Elner S G, Elner V M. The integrin superfamily and the eye. Invest Ophthalmol Vis Sci. 1996;37:696–701. [PubMed] [Google Scholar]
- 14.Emori T G, Gaynes R P. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endophthalmitis Vitrectomy Study Group. Microbiologic factors and visual outcome in the endophthalmitis vitrectomy study. Am J Ophthalmol. 1996;122:830–846. doi: 10.1016/s0002-9394(14)70380-0. [DOI] [PubMed] [Google Scholar]
- 16.Falkow S. What is a pathogen? ASM News. 1997;63:359–365. [Google Scholar]
- 17.Galli D, Lottspeich F, Wirth R. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol Microbiol. 1990;4:895–904. doi: 10.1111/j.1365-2958.1990.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 18.Guzmàn C A, Pruzzo C, Lipira G, Calegari L. Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infections and endocarditis. Infect Immun. 1989;57:1834–1838. doi: 10.1128/iai.57.6.1834-1838.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzmàn C A, Pruzzo C, Platè M, Guardati M C, Calegari L. Serum dependent expression of Enterococcus faecalis adhesins involved in the colonization of heart cells. Microb Pathog. 1991;11:399–409. doi: 10.1016/0882-4010(91)90036-a. [DOI] [PubMed] [Google Scholar]
- 20.Handwerger S, Perlman D C, Altarac D, McAuliffe V. Concomitant high-level vancomycin and penicillin resistance in clinical isolates of enterococci. Clin Infect Dis. 1992;14:655–661. doi: 10.1093/clinids/14.3.655. [DOI] [PubMed] [Google Scholar]
- 21.Hirt H, Wanner G, Galli D, Wirth R. Biochemical, immunological and ultrastructural characterization of aggregation substances encoded by Enterococcus faecalis sex-pheromone plasmids. Eur J Biochem. 1993;211:711–716. doi: 10.1111/j.1432-1033.1993.tb17600.x. [DOI] [PubMed] [Google Scholar]
- 22.Ike Y, Hashimoto H, Clewell D B. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984;45:528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ike Y, Clewell D B. Genetic analysis of pAD1 pheromone response in Streptococcus faecalis using transposon Tn917 as an insertional mutagen. J Bacteriol. 1984;158:777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jett B D, Jensen H G, Nordquist R E, Gilmore M S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jett B D, Huycke M M, Gilmore M S. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jett B D, Jensen H G, Atkuri R V, Gilmore M S. Evaluation of therapeutic measures for treating endophthalmitis caused by isogenic toxin-producing and toxin-nonproducing Enterococcus faecalis strains. Invest Ophthalmol Vis Sci. 1995;36:9–15. [PubMed] [Google Scholar]
- 27.Jett B D, Parke II D W, Booth M C, Gilmore M S. Host-parasite interactions in bacterial endophthalmitis. Zentralbl Bakteriol. 1997;285:341–367. doi: 10.1016/s0934-8840(97)80002-3. [DOI] [PubMed] [Google Scholar]
- 28.Kohno T, Sorgente N, Ishibashi T, Goodnight R, Ryan S J. Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci. 1987;28:506–514. [PubMed] [Google Scholar]
- 29.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe A M, Lambert P A, Smith A W. Cloning of an Enterococcus faecalis endocarditis antigen: homology with adhesins from some oral streptococci. Infect Immun. 1995;63:703–706. doi: 10.1128/iai.63.2.703-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luna L G, editor. Manual of histologic staining methods of the Armed Forces Institute of Pathology. New York, N.Y: McGraw-Hill Book Co.; 1968. pp. 224–225. [Google Scholar]
- 32.Mahan M J, Slauch J M, Mekalanos J J. Environmental regulation of virulence gene expression in Escherichia, Salmonella, and Shigella. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1995. pp. 2803–2815. [Google Scholar]
- 33.Mandelbaum S, Forster R K, Gelender H, Culbertson W. Late-onset endophthalmitis associated with filtering blebs. Ophthalmology. 1985;92:964–972. doi: 10.1016/s0161-6420(85)33947-7. [DOI] [PubMed] [Google Scholar]
- 34.Meredith T A, Aguilar H E, Miller M J, Gardner S K, Trabelsi A, Wilson L A. Comparative treatment of experimental Staphylococcus epidermidis endophthalmitis. Arch Ophthalmol. 1990;108:857–860. doi: 10.1001/archopht.1990.01070080101043. [DOI] [PubMed] [Google Scholar]
- 35.Moellering R C., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992;14:1173–1178. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- 36.Murray B E. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olmstead S B, Dunny G M, Erlandsen S L, Wells C L. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured intestinal epithelial cells. J Infect Dis. 1994;170:1549–1556. doi: 10.1093/infdis/170.6.1549. [DOI] [PubMed] [Google Scholar]
- 38.Schaberg, D. R., D. H. Culver, and R. P. Gaynes. 1991. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 91(Suppl. 3B):72S–75S. [DOI] [PubMed]
- 39.Stevens S X, Jensen H G, Jett B D, Gilmore M S. A hemolysin-encoding plasmid contributes to bacterial virulence in experimental Enterococcus faecalis endophthalmitis. Invest Ophthalmol Vis Sci. 1992;33:1650–1656. [PubMed] [Google Scholar]
- 40.Wanner G, Formanek H, Galli D, Wirth R. Localization of aggregation substances of Enterococcus faecalis after induction by sex pheromones—an ultrastructural comparison using immunolabeling, transmission and high-resolution scanning electron microscopic techniques. Arch Microbiol. 1989;151:491–497. doi: 10.1007/BF00454864. [DOI] [PubMed] [Google Scholar]
- 41.Weaver K E, Clewell D B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J Bacteriol. 1988;170:4343–4353. doi: 10.1128/jb.170.9.4343-4352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinstein M P, Murphy J R, Reller L B, Lichtenstein K A. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983;5:54–69. doi: 10.1093/clinids/5.1.54. [DOI] [PubMed] [Google Scholar]
- 43.Wells C L, Jechorek R P, Erlandsen S L. Evidence for the translocation of Enterococcus faecalis across the mouse intestinal tract. J Infect Dis. 1990;162:82–90. doi: 10.1093/infdis/162.1.82. [DOI] [PubMed] [Google Scholar]