Abstract
Background:
Severe gram-positive infections are frequent in people who inject drugs, and successful completion of treatment presents unique challenges in this population.
Objectives:
We aimed to evaluate the feasibility of a long-acting antibiotic, dalbavancin, as an alternative to standard-of-care antibiotics for severe infections due to vancomycin-susceptible pathogens requiring ⩾2 weeks of therapy.
Design:
We designed an investigator-initiated single-arm unblinded prospective cohort study to evaluate the safety and efficacy of an early switch to dalbavancin in two doses administered 1 week apart.
Methods:
We screened patients admitted with bloodstream infection, osteomyelitis, septic arthritis, infective endocarditis or deep abscesses, and comorbid substance use disorder (SUD) for eligibility. Consenting patients were switched to dalbavancin within 7 days from their index culture. They were monitored in the hospital for efficacy and safety of the treatment until the second dose of dalbavancin 7 days later and then discharged if stable. Study participants were evaluated with a decision support engine for a hypothetical appropriate level of care regarding their SUD after discharge. Their follow-up was planned for 12 months from the index culture, either in-person or via telehealth/telephone.
Results:
The enrollment was terminated early due to significant loss-to-follow-up. In all, 11 patients were enrolled, 4 completed 12 months of follow-up, 2 completed 8 months of follow-up, and 1 was seen once after discharge. The remaining five patients were lost to follow-up immediately after discharge. All 11 patients continued to improve after switching to dalbavancin between the first and second doses. There were two per-protocol failures of treatment. Dalbavancin was well tolerated, though some adverse events were reported.
Conclusion:
Dalbavancin may be a safe and effective alternative for an early switch in treating severe gram-positive infections.
Trial registration:
The trial was registered as NCT04847921 with clinicaltrials.gov.
Keywords: bacteremia, bloodstream infection, dalbavancin, gram-positive bacteria, infective endocarditis, long-acting lipoglycopeptide, osteomyelitis, septic arthritis, Staphylococcus, Streptococcus, substance use disorder
Introduction
Conditions requiring 2 weeks or more of targeted gram-positive therapy include uncomplicated Staphylococcus aureus bacteremia in patients with immunocompromising conditions or in those with persistent bacteremia as well as any gram-positive bloodstream infections (BSIs) complicated by deep-seated invasive infection [i.e. infective endocarditis (IE), osteomyelitis (OM), septic arthritis (SA), or deep abscess (DA)].1,2 After initial stabilization, many patients can be discharged to outpatient rehabilitation facilities or homes with outpatient parenteral antimicrobial therapy (OPAT) for continuation of intravenous (IV) therapy. OPAT is a safe and effective way of delivering prolonged IV antimicrobial therapy to patients who no longer require inpatient monitoring. This currently requires maintaining IV access for the duration of therapy,3,4 which contributes to treatment-emergent complications. 5 Unsupervised OPAT at home cannot be safely administered to those without relatively stable housing and those who cannot understand the care needed to maintain the indwelling line and apply required infusions without compromising sterility. Patients with housing insecurity, significant mental health disorders, or substance use disorders (SUD) are often considered poor OPAT candidates, often due to misperception or stigma.
Opioids, as well as other misused substances, are frequently injected for greater effect. Many patients present to healthcare facilities with life-threatening infections directly related to injection drug use (IDU). Treatment of serious infections that require IV access for antibiotics, including IE, OM, and DAs, is therefore complicated in people who use drugs (PWUDs).
Safety concerns regarding line contamination or overdose through the line are the main provider concerns in PWUD. PWUDs are thus frequently kept in the hospital for the duration of therapy, though alternatives exist. 6 Due to the stigma or under-treatment of SUD, many of these patients leave the hospital against medical advice (AMA) before completion of therapy.7,8 Leaving AMA leads to poor outcomes and frequent readmissions. In addition to improved outcomes, the administration of full-scale SUD treatment has been shown to improve inpatient treatment retention.9,10
Long-acting lipoglycopeptide – dalbavancin
Dalbavancin (DALVANCE, ABBVIE Inc.) is an intravenous (IV) lipoglycopeptide antibiotic similar to teicoplanin and can maintain prolonged therapeutic concentrations in blood and various other tissues, including bone (terminal half-life ranging 250–350 h or 10–14 days). 11 A human pharmacokinetic study to evaluate dalbavancin penetration to bone found that either weekly administration or a consolidated two-dose regimen provided sustained drug concentrations above the minimum inhibitory concentration (MIC) for susceptible gram-positive pathogens for 8 weeks. 12 Dalbavancin has only been FDA approved for acute bacterial skin and skin structure infections caused by selected vancomycin-susceptible gram-positive organisms. Prospective trials for IE and other serious infections are lacking. Only one smaller prospective randomized trial has been completed, suggesting initial efficacy in OM. 12 However, phase I/II and in vitro susceptibility data are available to support the feasibility of successful dalbavancin use for these indications.13–15 Other studies projected significant cost savings and reported promising results with dalbavancin in various settings.16–22 These retrospective studies document the use of long-acting lipoglycopeptides (e.g. dalbavancin), in various conditions, including severe infections, infective IE, and BSI, often complicated by bone and joint and other deep-seated infections.
Alternatives to standard-of-care (SOC) treatment strategies have not been sufficiently studied, and treatment outcome comparisons and caveats are not well established. We set out to study a treatment approach that focuses on achieving the most flexible alternative for serious infections to provide a safe and effective alternative to current SOC and accommodate patient preferences. 23
Methods
We designed an investigator-initiated single-arm unblinded prospective cohort study to evaluate the safety and efficacy of an early switch to dalbavancin in two doses administered 1 week apart. The study was conducted at the University of Colorado Hospital. We planned to use historical cohorts as a comparison for efficacy.24–26
Based on investigational drug availability, up to 60 adult patients with severe infections (BSI, IE, OM, SA, and DA) due to vancomycin-susceptible gram-positive pathogens were screened and were eligible for enrollment if they reported a history of active or recent SUD (excluding tobacco or alcohol alone) or IDU posing as a potential barrier to OPAT enrollment (i.e. their infection was directly linked with IDU; they reported active psychoactive substance without evidence of remission before hospitalization; or their toxicology screen showed misused substances, including prescription medications they have not been authorized to use by any prescribing physician).27,28
Patients were excluded if they had a history of an allergy to glycopeptide antibiotics, if they had infected foreign materials without a plan for removal, or if their BSI complication involved compartments not known to be sufficiently penetrated by dalbavancin (e.g. central nervous system infections). Patients with left-sided endocarditis meeting criteria for surgery, pregnancy, creatinine clearance <30 ml/min, or significant psychiatric conditions rendering early discharge unsafe or follow-up as infeasible, were also ineligible.
Primary outcomes included clinical, laboratory, and imaging criteria of improvement in fever, lesions/wounds, purulence, pain, warmth, erythema, swelling, or organ function, clearance of bacteremia, and improvement in inflammatory markers (C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)). Treatment success was defined as the resolution of bacteremia or clinical, imaging, and laboratory-supported improvement in the primary infection without the need for additional antimicrobial therapy during the typical course of treatment, and treatment failure was defined as a failure to achieve treatment success, the use of additional antimicrobials effective for gram-positive pathogens causing the index infection, or a relapse of the same infection with the same organism (based on microbiological and antimicrobial resistance testing), assessed as likely failure to clear the organism from the infected site with two doses of dalbavancin.
Other than the early switch to dalbavancin, all other treatments provided were consistent with current standards of care for infections with vancomycin-susceptible gram-positive pathogens. Patients had to be enrolled within 7 days from the first qualifying culture (blood or deep tissue/abscess or body fluid or abscess), and prior antibiotics were replaced by dalbavancin alone.
Prior to dalbavancin, the patients were on typical broad-spectrum empirical regimens until the spectrum of pathogens involved was narrowed by cultures, which enabled some initial narrowing of the regimen to broader gram-positive agents (e.g. daptomycin or vancomycin) alone. These were further narrowed where appropriate after the pathogen susceptibility was clarified by testing. After the first dose of dalbavancin 1500 mg IV, patients were monitored in the hospital for 7 days until the second 1500 mg dose and subsequently discharged if stable. Outpatient follow-up was planned based on OPAT principles and could be in-person or via telehealth with an extension to 12 months post-enrollment. 3
American Society of Addiction Medicine (ASAM) Criteria and its electronic decision engine ASAM Continuum is a standardized format for evaluation and appropriate treatment-level placement for individuals suffering from all forms of SUD.29,30 The evaluation follows a complex routine assessing patients based on six dimensions related to their SUD and adds recommendations based on their co-occurring mental health disorders and medical conditions. The six dimensions are as follows: (1) acute intoxication or withdrawal potential; (2) biomedical conditions and complications; (3) emotional, behavioral, or cognitive conditions and complications; (4) readiness to change; (5) relapse, continued use, or continued problem potential; and (6) recovery/living environment. The placement recommendation follows a numeric scale in which level 0.5 indicates a need for an early intervention alone (e.g. counseling, motivational interventions, screening, brief intervention and referral to treatment but also longer programs, such as driving under the influence education); level 1 indicates a need for regular outpatient services, including cognitive-behavioral therapy techniques, ‘stages of change’ work and often includes opioid agonist therapy, etc.; level 2 recommends intensive outpatient services typically at least three times/week, 9 h minimum (2.1) and partial hospitalization which provides services approximately 5 days/week (20 h minimum) and hospital level consultations and laboratory work up are typically available in person or via telephone/telemedicine (2.5); level 3 represent a gradation in residential treatment, from clinically managed low-intensity (3.1), medium- to high-intensity (3.3 and 3.5) through medically monitored inpatient services (3.7), toward fully medically managed intensive inpatient services (level 4). Depending on patients’ availability and interest, they were offered an evaluation via ASAM Continuum by a volunteering licensed addiction counselor (coauthor VC) at enrollment and the end of follow-up.
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the University of Colorado Anschutz Medical Campus. 31 REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources. Statistical analysis was performed using R statistical software (v4.1.2; R Core Team 2021, R Founfation for Statistical Computing, Vienna, Austria - https://www.R-project.org/).
The study was approved by the Colorado Multi-Institutional Review Board (COMIRB #19-0650), registered as NCT04847921 with clinicaltrials.gov, and the FDA approved the investigational use of dalbavancin for off-label indication under IND 151822.
AbbVie Inc. provided dalbavancin powder for injection, and all investigational tasks were performed by the principal investigator and volunteering co-investigators.
Results
We terminated enrollment early due to a significant loss to follow-up (LTF). In all, 14 patients were approached, and 11 (79%) consented to enrollment. One patient was female (9%), one Hispanic (9%), and two patients were black (18%) without overlap in those categories. The mean age was 40 years (30–61). Three patients had their index culture collected in the intensive care unit. Eight patients (73%) reported IDU, with the rest using other routes of administration. The majority of patients reported the use of multiple substances. Table 1 lists the demographic characteristics of the cohort. The mean duration of other antibiotics prior to dalbavancin was 5.9 days (range: 3–7 days).
Table 1.
Demographic characteristics.
| Age: median (range) | 37 (30–61) |
| Female (%) | 1 (9%) |
| Race (%) | |
| White | 8 (73%) |
| Black | 2 (18%) |
| Hispanic | 1 (9%) |
| Insurance: Medicaid (%) | 11 (100%) |
| Index culture while admitted to ICU (%) | 3 (27%) |
| Charlson Comorbidity Index: median (range) | 1 (0–3) |
| Immunocompromising factors: median (range) | 0 (0) |
| Body mass index, kg/m2, mean (range) | 24 (18–29) |
| Psychiatric disorders, n (%) | |
| None | 2 (18%) |
| Major depressive disorder | 5 (46%) |
| Generalized anxiety disorder | 3 (27%) |
| Bipolar disorder | 4 (36%) |
| Psychotic disorder | 2 (18%) |
| PTSD | 2 (18%) |
| ADHD | 1 (9%) |
| OCD | 1 (9%) |
| Insomnia | 1 (9%) |
| IDU, n (%) | 8 (73%) |
| Polysubstance use, n (%) | 8 (73%) |
| Drugs of use, n (%) | |
| Opioids | 6 (55%) |
| Amphetamines | 7 (64%) |
| Cocaine | 4 (36%) |
| Benzodiazepines | 1 (9%) |
| Alcohol | 2 (18%) |
| Cannabinoids | 2 (18%) |
| Tobacco | 1 (9%) |
| Hepatitis C, n (%) | |
| Current | 1 (9%) |
| History | 5 (46%) – 3 cleared spontaneously |
| HIV, n (%) | 0 (0%) |
| Sites of infection, n (%) | |
| Bacteremia (%) | 9 (82%) (only 1 without additional sites of infection) |
| Septic embolism into the lungs/pleural space complicated by pneumonia (%) | 2 (18%) |
| Thrombophlebitis (%) | 2 (18%) |
| OM (%) | 2 (18%) |
| Pyomyositis (%) | 1 (9%) |
| SA (%) | 2 (18%) |
| Skin and soft tissue infection | 4 (36%) (2 with bacteremia, 1 with pyomyositis and deep abscess, and 1 with OM and SA) |
| Bacterial pathogens, n (%) | |
| MSSA (%) | 7 (64%) |
| MRSA (%) | 3 (27%) |
| Streptococcus pyogenes | 1 (9%) |
| Vancomycin MIC (S. pyogenes not tested) | |
| MSSA mean (range) | 0.93 (0.5–1) |
| MRSA mean (range) | 1 (1) |
| Duration of antibiotics prior to dalbavancin, mean (range) | 5.9 (3–7) days |
ADHD, attention-deficit disorder; ICU, intensive care unit; IDU, injection drug use; MIC, minimum inhibitory concentration; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; OCD, obsessive-compulsive disorder; OM, osteomyelitis; PTSD, post-traumatic stress disorder; SA, septic arthritis.
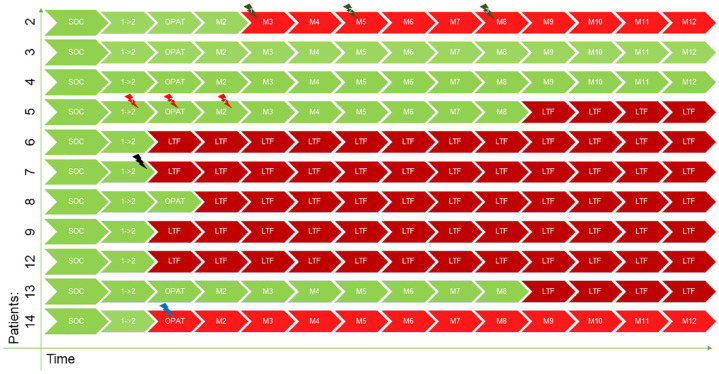
Of the 11 patients, only 4 completed 12 months of follow-up (Table 2 and Figure 1). Two patients had an uneventful and successful course [one with an uncomplicated methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia and one with MSSA bacteremia complicated by SA of the sacroiliac joint]. The other two experienced a failure, as defined by the protocol. One experienced a relapse of IDU, which prompted a cessation of home health services, including wound care, and he reported that his wound in the sternoclavicular area never healed. He was readmitted with a worsening wound during month 3 of the follow-up period and was started on broad-spectrum antibiotics. His follow-up bone biopsy with culture showed a different pathogen (methicillin-resistant Staphylococcus epidermidis instead of MSSA). However, we conservatively assessed this as a treatment failure due to a 6-day delay prior to the culture and the preceding failure to heal the wound. The other per-protocol failure involved a patient who was admitted with MSSA bacteremia and thrombophlebitis at the IDU site. He was also experiencing an upper gastrointestinal bleed, which was severe enough to require a splenic artery embolization only 3 days after the index MSSA blood culture. Four days later, he received the first dose of dalbavancin (second dose 7 days later), and 12 days after the second dose, he was readmitted and ultimately underwent splenectomy for a splenic abscess or necrosis, from which no cultures or pathology were sent to confirm the diagnosis. This patient was considered a per-protocol failure, likely due to a lack of source control. Two additional patients completed the follow-up until we could no longer reach them from month 9 onward. A total of four patients (36%) stopped following up immediately after discharge from the hospital, and one additional patient came for a successful OPAT (month 1) visit but stopped following up thereafter. The follow-up course and outcomes are listed in Table 2 and Figure 1.
Table 2.
Clinical outcomes.
| ID# | Index culture | Index infection | Organism | Failure | AE #1 | AE #2 | AE #3 | ASAM Continuum placement recommendation |
|---|---|---|---|---|---|---|---|---|
| 2 | BCx – MSSA 5/12/21 (sternum MSSA 5/13/21) | BSI + sterno/manubrial SA (native)/OM and associated SSTI | MSSA | Month 3: sternum MRSE | Month 3: sternum MRSE (same as the reason for failure; grade 3; not dalbavancin related) | Month 5: GNR CLABSI (grade 4; not dalbavancin related) | Month 8: MSSA BSI/spinal infection – (grade 4; not dalbavancin related) | Level 4 => patient indicated this to be unacceptable |
| 3 | BCx – MSSA 5/26/21 | BSI | MSSA | n/a | n/a | Level 2.1 | ||
| 4 | BCx – MSSA 5/28/21 | BSI + L SI SA (native) | MSSA | n/a | n/a | |||
| 5 | BCx – MSSA 6/3/21 (neck MSSA 6/4/21) | BSI + neck SSTI abscess + pyomyositis | MSSA | LTF from month 9 | Between first and second dose: rash to cefepime or clindamycin => resolved in 7 days with oral antihistamine and topical hydrocortisone (grade 2; unlikely to be dalbavancin related) | OPAT (month 1): headache – likely due to high BP (grade 2; unlikely to be dalbavancin related | Month 2: headache – likely due to high BP (grade 2; unlikely to be dalbavancin related | Level 3.7; with co-occurring capable program and biomedical enhanced program => patient indicated this to be unacceptable |
| 6 | Pleural fluid – MRSA 6/12/21 | PNA + empyema + septic embolism? | MRSA | LTF immediately after discharge – though the boyfriend’s mother always said he was doing well and that she’d ask them to call back | Unable to assess due to LTF | Level 3.7; with co-occurring capable program and biomedical enhanced program => patient indicated this to be unacceptable | ||
| 7 | BCx – MRSA 6/12/21 | BSI + PNA + septic embolism | MRSA | LTF immediately after discharge – though mother always said she was doing well and that she’d ask them to call back | Nausea/vomiting during the second dalbavancin infusion (patient believed due to withdrawal and then completed the infusion without additional problems) (grade 1; possibly dalbavancin related) | Level 2.1; preferably with OTP (methadone) and co-occurring mental health disorder enhanced program | ||
| 8 | BCx – MRSA 7/8/21 | SSTI + BSI | MRSA | LTF from month 2 – though the mother always stated no knowledge of additional infectious problems on the phone | Unable to assess due to LTF | |||
| 9 | BCx – MSSA 7/27/21 | BSI + thrombophlebitis | MSSA | LTF from month 2 | Unable to assess due to LTF | |||
| 12 | BCx – GAS 2/5/22 | SSTI + BSI | GAS | LTF from month 2 | Unable to assess due to LTF | |||
| 13 | Spine abscess/tissue – MSSA 2/8/22 | Spine infection/OM | MSSA | LTF from month 9 | Unable to assess due to LTF | |||
| 14 | BCx – MSSA 6/20/22 => 6/23/22 splenic artery embolization | BSI + thrombophlebitis | MSSA | OPAT (month 1): splenic abscess/necrosis – never cultured | OPAT (month 1): splenic abscess/necrosis (grade 3; not dalbavancin related) |
AE, adverse events; ASAM, American Society of Addiction Medicine; BCx, blood culture; BSI, bloodstream infection; CLABSI, catheter-associated bloodstream infection; GAS, group A Streptococcus (Streptococcus pyogenes); GNR, gram-negative rods; LTF, loss-to-follow up; MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis; MSSA, methicillin-susceptible S. aureus; OM, osteomyelitis; OPAT, outpatient parenteral antimicrobial therapy; OTP, outpatient treatment program; PNA, pneumonia; SA, septic arthritis; SI, sacroiliac; SSTI, skin and soft structure infection.
Figure 1.
Follow-up timeline.
 No evidence of treatment failure.
No evidence of treatment failure.
 Treatment failure.
Treatment failure.
 Treatment failure due to loss-to-follow-up alone.
Treatment failure due to loss-to-follow-up alone.
1 → 2, 7 days from first to second dose of dalbavancin; LTF, loss-to-follow up; lightning bolts represent AEs and are color coded to match respective patients; M with numbers, consecutive month of follow up; OPAT, period similar to standard of care OPAT (weeks 3–6); SOC, up to 7 days standard of care (green bolt 1, wound infection; green bolt 2, gram-negative rod catheter-associated bloodstream infection; green bolt 3, methicillin-susceptible Staphylococcus aureus bacteremia and spine infection; red bolt 1, allergic rash; red bolt 2, headache; black bolt, nausea/vomiting; blue bolt, splenic abscess/necrosis).
Four patients experienced eight adverse events (AEs) in total (Table 3). In total, 3/6 AEs that occurred later during follow-up were diagnosed at a time when dalbavancin would no longer have been present [>5 half-lives (or >10 weeks) after the last dose]. Only the splenic abscess was diagnosed 12 days after the second dose, though this was in the setting of splenic artery embolization performed 3 days after the diagnosis of MSSA BSI. Only three AEs were thus truly treatment-emergent. Of those, the nausea occurred during the infusion of the second dose, though the patient believed it to be due to opioid withdrawal and proceeded to complete the infusion without further issues. The allergic rash, which occurred 6 days after the first infusion of dalbavancin, was likely brought on by 2 days of added clindamycin and cefepime in a patient with recorded anaphylaxis to penicillin but previous tolerance of ceftriaxone, cephalexin, and cefazolin, and who was 10 days after extensive incisions and drainage (2 consecutive days of surgeries) for concern regarding necrotizing soft tissue infection in the neck and shoulder area and whose wounds were still open at the time. A workup of fever revealed no additional pathology. The cefepime and clindamycin were stopped 1 day later when the patient received his second dose of dalbavancin, and his rash resolved with a brief course of topical hydrocortisone and oral diphenhydramine.
Table 3.
Adverse events.
| Event | During/post-dose 1 | During/post-dose 2 | Any time during follow-up |
|---|---|---|---|
| Nausea (with or without vomiting) | 1 | ||
| Headache | 2 | ||
| GNR CLABSI | 1 | ||
| MSSA bacteremia/spinal infection | 1 | ||
| Splenic abscess | 1 | ||
| MRSE wound infection | 1 | ||
| Allergic rash | 1 |
The same color numbers represent the same patient.
GNR CLABSI, gram-negative rod catheter-associated bloodstream infection; MRSE, methicillin-resistant Staphylococcus epidermidis; MSSA, methicillin-resistant Staphylococcus aureus.
Five patients (46%) agreed to participate in the initial ASAM Continuum facilitated evaluation shortly after enrollment into the study (Table 2). Of these, only two were evaluated as eligible for intensive outpatient management (level 2.1), while the other three were thought to need high-level inpatient management (two at level 3.7 and one at level 4). The patient who was recommended for level 2.1 without co-occurring mental health disorder completed the entire follow-up and experienced no additional infections, AEs, or other problems. The other level 2.1 recommendation, though with a need for co-occurring mental health services, went to a patient who stopped follow-up immediately after discharge. One patient with a level 3.7 management recommendation and additional co-occurring mental health and biomedical needs successfully followed up until month 9. By contrast, the other level 3.7 patient was LTF after discharge. Interestingly, the only patient with level 4 needs was the patient who relapsed with IDU shortly after discharge and experienced a failure in wound healing and treatment per-protocol.
Discussion
In this prospective study of dalbavancin as an early-transition agent for the treatment of vancomycin-susceptible gram-positive pathogens causing BSI and related deep-seated infections in patients with SUD, we were unable to evaluate enough subjects and secure sufficient follow-up to determine the feasibility of the agent conclusively. The results of a concurrent trial may further clarify this for Staphylococcus aureus: ‘DOTS: Dalbavancin as an Option for Treatment of Staphylococcus aureus Bacteremia’ (NCT04775953).
Hospitalizations for acute and frequently life-threatening conditions, including serious infections, present a unique opportunity to engage PWUD in SUD treatment. At the same time, their motivation is high and fueled by the immediate need to improve their often painful and temporarily disabling conditions directly linked to IDU. 32 However, engagement and retention in care are frequent limiting factors33,34 because many PWUDs are suspicious of authority due to stigma and the risk of incarceration. SUD treatment in such circumstances has improved mortality and other important outcomes.35,36 Despite this opportunity, few hospitals currently offer full SUD evaluation and treatment for patients hospitalized with SUD-related medical complications, and most SUD evaluation and treatment take place in specialized independent facilities whose expert providers most often do not have consultation privileges in acute care hospitals.37–39 The practice of deferred referrals to specialized SUD treatment centers only after the patients are fully treated for their acute medical conditions frequently leads to diminished motivation to seek additional help with SUD once the acute problem has been resolved.
An early evaluation with a decision support algorithm, such as ASAM Criteria, with or without the software engine, ASAM Continuum, may be a beneficial tool for post-discharge placement decisions and coordination for patients with SUD. Our study was unfunded, and ASAM Continuum evaluation was thus only exploratory as we could not ensure recommended placement.
A high LTF, such as in our study, is thus not unexpected in this population, though it was likely further exacerbated by the COVID-19-related disruptions. Some LTF may have been prevented by offering a small gratuity for attendance of follow-up appointments (contingency management), though this would have required formal study funding.
Still, a few things could be conclusively stated:
1. Dalbavancin was well tolerated despite an early transition from SOC IV antibiotics (⩽7 days).
2. None of the enrolled subjects experienced worsening and continued to improve clinically after the early transition to dalbavancin for a week until discharge.
3. The two clearly documented failures per-protocol were never confirmed to be due to the same pathogen and were confounded by factors that could have represented a reinfection in one and a source control issue versus a complication of splenic artery embolization in the other case. Source control is a known problem in treatment with SOC antibiotics and can occur even without the additional risk presented by proximal artery embolization.
4. All patients were discharged in a stable and improved condition in 14 or fewer days.
5. An early ASAM Continuum enhanced evaluation for patients correctly identified the highest-risk patient. However, the most freedom-restrictive placement treatment recommendations were perceived as unacceptable by the patients, which could potentially be offset by facilitating the initiation of level 3.7 or 4 early in the course or an acute medical illness.
Studying SUD-related outcomes was beyond the scope and capacity of this feasibility pilot. However, the facilitation of more appropriate treatment for SUD (the true cause of many of these infections) afforded by the use of an antibiotic without the need for frequent dosing and monitoring can be the most beneficial feature of this approach in PWUD.
Residential SUD treatments/therapies require a substantial time commitment, which is impractical in traditional daily operations within an acute care hospital setting. Moreover, patients without IV access could continue their treatment/recovery from infection at a specialized residential SUD treatment facility, and at least a part of such treatment can be covered by saving from prolonged acute care hospitalization. 20 In addition, the use of long-acting antibiotics could serve as a harm reduction tool for people who are not ready to commit to treatment in residential facilities but who do not wish to remain hospitalized for daily IV antibiotics alone.
Preventive services, diagnosis, and management of emergent conditions in PWUD are often challenging for numerous reasons, including established guidelines, hospital protocols, and healthcare practitioners’ perceptions, and clinical research in this population is not different. Paradoxically, extrapolated healthcare guidelines and hospital protocols from the general population to PWUD result in worse outcomes and additional high utilization costs.40,41 Intoxication, illicit substance use, and associated behaviors lead to disconnect and reciprocal distrust between PWUD and medical professionals, which creates a need for alternative approaches to care focused on harm reduction.
Additional groups of patients able to benefit from alternatives to conventional OPAT include the following:
1. Patients who cannot comply with follow-up or daily antibiotics.42–44
2. Patients from rural communities who are/have transportation barriers.
3. Patients under extenuating social circumstances (i.e. homelessness, living out of state, living in correctional facilities) or when a systemic presence of antimicrobials may not always be reliably achieved with oral agents.
4. Patients with financial barriers to OPAT, which is not entirely covered by Medicare (or other insurers).45,46
Conclusion
The efficacy and cost-effectiveness of healthcare in PWUD can be improved with existing tools. However, implementation lags due to pending evidence, which is particularly difficult to collect in this population. In other words, oral antibiotics, long-acting antibiotics, as well as SUD treatment options that have been shown to improve outcomes are available already. Putting these interventions together via proper implementation is the missing part.
Perhaps counterintuitively, research in this population should be invested in preferentially, because a wise investment in research and implementation will offset the cost of high utilization at a fraction of the price while also significantly improving the quality of care and its delivery. Importantly, LTF will continue to be more prevalent in this population, as will leaving AMA, if we try to continue coercive measures only. Despite that, unlike in clinical trials – LTF does not automatically mean treatment failure in the real world – especially if a systemic presence of effective antimicrobials for the required duration is ensured with long-acting medications.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361231223889 for Substance use disorder-associated infections’ treatment with dalbavancin enabling outpatient transition (SUDDEN OUT) – an investigator-initiated single-arm unblinded prospective cohort study by Martin Krsak, Sias Scherger, Matthew A. Miller, Vincent Cobb, Brian T. Montague, Andrés F. Henao-Martínez and Kyle C. Molina in Therapeutic Advances in Infectious Disease
Acknowledgments
We would like to thank AbbVie, Inc. for providing dalbavancin powder for injection and a small grant for help with initial administrative processing. We would also like to thank the University of Colorado Hospital for their support of our unfunded study, without which we would not have the help of the Investigational/Research Pharmacy staff or able to monitor the patients in the hospital in between the doses. Finally, we would also like to thank the American Society of Addiction Medicine (ASAM) and their business partner, FEI Systems, for providing access to ASAM Continuum for the duration of the study.
Footnotes
ORCID iDs: Martin Krsak  https://orcid.org/0000-0002-1746-7462
https://orcid.org/0000-0002-1746-7462
Andrés F. Henao-Martínez  https://orcid.org/0000-0001-7363-8652
https://orcid.org/0000-0001-7363-8652
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Martin Krsak, Division of Infectious Diseases, Department of Medicine, University of Colorado School of Medicine, Mail Stop B163, Anschutz Outpatient Pavilion, 1635 Aurora Court, Aurora, CO 80045-2581, USA.
Sias Scherger, Division of Infectious Diseases, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, USA; Division of Infectious Diseases, University of Nebraska School of Medicine, Omaha, NE, USA.
Matthew A. Miller, Department of Pharmacy, Children’s Hospital Colorado, Aurora, CO, USA
Vincent Cobb, Division of Infectious Diseases, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, USA.
Brian T. Montague, Division of Infectious Diseases, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, USA
Andrés F. Henao-Martínez, Division of Infectious Diseases, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, USA
Kyle C. Molina, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora, CO, USA
Declarations
Ethics approval and consent to participate: All patients voluntarily provided written informed consent and obtained a copy for their records. The study was approved by the Colorado Multi-Institutional Review Board (COMIRB #19-0650), registered as NCT04847921, and the investigational use of dalbavancin for off-label indication was approved by the FDA under IND 151822.
Consent for publication: Not applicable.
Author contributions: Martin Krsak: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Sias Scherger: Investigation; Writing – review & editing.
Matthew A. Miller: Conceptualization; Investigation; Writing – review & editing.
Vincent Cobb: Investigation; Writing – review & editing.
Brian T. Montague: Conceptualization; Software; Writing – review & editing.
Andrés F. Henao-Martínez: Investigation; Writing – review & editing.
Kyle C. Molina: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We received dalbavancin powder for injection and a $7500 grant for help with initial administrative processing. The study was otherwise unfunded, and the investigators volunteered all the associated work.
The authors declare that there is no conflict of interest. The Editor in Chief of Therapeutic Advances in Infectious Disease is an author on this paper. Therefore, the review process was managed by alternative members of the Editorial Board and the submitting Editor had no involvement in the decision-making process.
Availability of data and materials: Data and materials are not freely available outside of formal regulatory inquiries.
References
- 1. Holland TL, Arnold C, Fowler VG. Clinical management of Staphylococcus aureus bacteremia. JAMA 2014; 312: 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52: e18–e55. [DOI] [PubMed] [Google Scholar]
- 3. Norris AH, Shrestha NK, Allison GM, et al. 2018. Infectious Diseases Society of America Clinical Practice Guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis 2019; 68: e1–e35. [DOI] [PubMed] [Google Scholar]
- 4. Williams DN, Baker CA, Kind CA, et al. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents 2015; 46: 307–312. [DOI] [PubMed] [Google Scholar]
- 5. Shrestha NK, Shrestha J, Everett A, et al. Vascular access complications during outpatient parenteral antimicrobial therapy at home: a retrospective cohort study. J Antimicrob Chemother 2016; 71: 506–512. [DOI] [PubMed] [Google Scholar]
- 6. Fanucchi L, Leedy N, Li J, et al. Perception and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med 2016; 11: 581–582. [DOI] [PubMed] [Google Scholar]
- 7. Garland A, Ramsey CD, Fransoo R, et al. Rates of readmission and death associated with leaving hospital against medical advice: a population-based study. CMAJ 2013; 185: 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health 2015; 105: e53–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi M, Kim H, Qian H, et al. Readmission rates of patients discharged against medical advice: a matched cohort study. PLoS ONE 2011; 6: e24459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan ACH, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice – the mitigating role of methadone and social support. J Acquir Immune Defic Syndr 2004; 35: 56–59. [DOI] [PubMed] [Google Scholar]
- 11. Manufacturer’s highlights of prescribing information, https://www.allergan.com/assets/pdf/dalvance_pi (accessed 2 October 2023).
- 12. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis 2019; 6: ofy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunne MW, Puttagunta S, Sprenger CR, et al. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 2015; 59: 1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005; 40: 374. [DOI] [PubMed] [Google Scholar]
- 15. Citron DM, Tyrrell KL, Goldstein EJC. Comparative in vitro activities of dalbavancin and seven comparator agents against 41 Staphylococcus species cultured from osteomyelitis infections and 18 VISA and hVISA strains. Diagn Microbiol Infect Dis 2014; 79: 438–440. [DOI] [PubMed] [Google Scholar]
- 16. Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for Gram-positive infective endocarditis: 2-year experience at the general hospital of Vienna. Clin Infect Dis 2018; 67: 795–798. [DOI] [PubMed] [Google Scholar]
- 17. Ektare V, Khachatryan A, Xue M, et al. Assessing the economic value of avoiding hospital admissions by shifting the management of gram+ acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ 2015; 18: 1092–1101. [DOI] [PubMed] [Google Scholar]
- 18. Bouza E, Valerio M, Soriano A, et al.; DALBUSE Study Group (Dalbavancina: Estudio de su uso clinico en España). Dalbavancin in the treatment of different gram-positive infections: a real-life experience. Int J Antimicrob Agents 2018; 51: 571–577. [DOI] [PubMed] [Google Scholar]
- 19. Morrisette T, Miller MA, Montague BT, et al. On- and off-label utilization of dalbavancin and oritavancin for gram-positive infections. J Antimicrob Chemother 2019; 74: 2405–2416. [DOI] [PubMed] [Google Scholar]
- 20. Morrisette T, Miller MA, Montague BT, et al. Long-acting lipoglycopeptides: ‘lineless antibiotics’ for serious infections in persons who use drugs. Open Forum Infect Dis 2019; 6: ofz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molina KC, Lunowa C, Lebin M, et al. Comparison of sequential dalbavancin with standard-of-care treatment for Staphylococcus aureus bloodstream infections. Open Forum Infect Dis 2022; 9: ofac335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krsak M, Morrisette T, Miller MA, et al. Advantages of outpatient treatment with long-acting lipoglycopeptides for serious gram-positive infections: a review. Pharmacotherapy 2020; 40: 469–478. [DOI] [PubMed] [Google Scholar]
- 23. Marra CA, Frighetto L, Goodfellow AF, et al. Willingness to pay to assess patient preferences for therapy in a Canadian setting. BMC Health Serv Res 2005; 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arbeit RD, Maki D, Tally FP, et al. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 2004; 38: 1673–1681. [DOI] [PubMed] [Google Scholar]
- 25. Fowler VG, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355: 653–665. [DOI] [PubMed] [Google Scholar]
- 26. Rehm S, Campion M, Katz DE, et al. Community-based outpatient parenteral antimicrobial therapy (CoPAT) for Staphylococcus aureus bacteraemia with or without infective endocarditis: analysis of the randomized trial comparing daptomycin with standard therapy. J Antimicrob Chemother 2009; 63: 1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dunne MW, Sahm D, Puttagunta S. Use of vancomycin as a surrogate for dalbavancin in vitro susceptibility testing: results from the DISCOVER studies. Ann Clin Microbiol Antimicrob 2015; 14: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones RN, Farrell DJ, Flamm RK, et al. Surrogate analysis of vancomycin to predict susceptible categorization of dalbavancin. Diagn Microbiol Infect Dis 2015; 18: 73–77. [DOI] [PubMed] [Google Scholar]
- 29. American Society of Addiction Medicine, Inc. ASAM criteria. 3rd ed. Carson City, NV: The Change Companies, 2013. [Google Scholar]
- 30. ASAM Continuum™, https://www.asamcontinuum.org/ (accessed 26 November 2018).
- 31. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Toole TP, Conde-Martel A, Hunter Young J, et al. Managing acutely ill substance-abusing patients in an integrated day hospital outpatient program: medical therapies, complications and overall treatment outcomes. J Gen Intern Med 2006; 21: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woody GE, Bruce D, Krthius PT, et al. HIV risk reduction with buprenorphine-naloxone or methadone: findings from a randomized trial. J Acquir Immune Defic Syndr 2014; 66: 288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruce RD. Perspective: opioid addiction, opioid addiction treatment, and HIV infection. Top Antivir Med 2018; 26: 89–92. [PMC free article] [PubMed] [Google Scholar]
- 35. Rodger L, Glockler-Lauf SD, Shojaei E, et al. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open 2018; 1: e185220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marks LR, Munigala S, Warren DK, et al. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis 2019; 68: 1935–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services – linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat 2017; 75: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service, expertise for hospitalized patients with complex addiction problems. Med Clin North Am 2018; 102: 587–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fanucchi L, Lofwall MR. Putting parity into practice – integrating opioid-use disorder treatment into the hospital setting. N Engl J Med 2016; 357: 811–813. [DOI] [PubMed] [Google Scholar]
- 40. Merchant E, Burke D, Shaw L, et al. Hospitalization outcomes of people who use drugs: one size does not fit all. J Subst Abuse Treat 2020; 112: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewer D, Freer J, King E, et al. Frequency of healthcare utilization by adults who use illicit drugs: a systematic review and meta-analysis. Addiction 2020; 115: 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J Hosp Med 2016; 11: 531–535. [DOI] [PubMed] [Google Scholar]
- 43. Salit S, Kuhn EM, Hartz AJ, et al. Hospitalization costs associated with homelessness in New York City. N Engl J Med 1998; 338: 1734–1740. [DOI] [PubMed] [Google Scholar]
- 44. Kushel MB, Vittinghoff E, Haas JS. Factors associated with the health care utilization of homeless persons. JAMA 2001; 285: 200–206. [DOI] [PubMed] [Google Scholar]
- 45. Keller S, Pronovost P, Cosgrove S. What Medicare is missing. Clin Infect Dis 2015; 61: 1890–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drozd EM, Hoban N. Impact on Medicare expenditures from expanding coverage of infusion therapy of anti-infective drugs to the home setting, http://www.nhia.org/resource/legislative/documents/AvalereFinalHomeInfusionReport.pdf (2014, accessed 3 September 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361231223889 for Substance use disorder-associated infections’ treatment with dalbavancin enabling outpatient transition (SUDDEN OUT) – an investigator-initiated single-arm unblinded prospective cohort study by Martin Krsak, Sias Scherger, Matthew A. Miller, Vincent Cobb, Brian T. Montague, Andrés F. Henao-Martínez and Kyle C. Molina in Therapeutic Advances in Infectious Disease