Abstract
The intestinal barrier is composed of several essential elements including luminal enzymes, bile acids, water layer, epithelial layer, and enterocyte layer. It acts as a dynamic interface between the luminal contents of food, commensal and pathogenic bacteria, and the gastrointestinal tract. The role of barrier dysfunction is of significant research interest in the development and targeted treatment of chronic inflammatory gastrointestinal conditions, such as inflammatory bowel disease. This review aims to examine the role of intestinal barrier dysfunction in the development of inflammatory bowel disease, the pathophysiology of increased barrier permeability in inflammatory bowel disease, and to explore potential treatment targets and clinical applications.
Keywords: Intestinal permeability, Inflammatory bowel disease, Mucus, Tight junctions
Introduction
The objectives of this review are to examine the potential role of barrier dysfunction as a risk factor for the development of inflammatory bowel diseases (IBD), the molecular mechanisms involved in the leakiness of the barrier, and opportunities for treatment of the abnormal barrier to aid in the management of IBD. The assessment of human intestinal barrier function in vitro and in vivo and the interaction of microbiota with intestinal permeability have been addressed in prior reviews [1, 2]. The importance of the diverse components of the barrier is evident through barrier effects of fecal metabolites, and the role of cellular mechanisms in maintaining the barrier integrity. Recent data have documented deficiencies in the commonly used disaccharide to monosaccharide ratios to measure intestinal permeability. In order to appraise intestinal barrier in human disease states, it is essential to use more valid endpoints for in vivo measurements, and refining these measurements is necessary to (further elucidate the altered barrier function in IBD and its potential treatment through targeting the cellular and molecular mechanisms putatively involved in IBD [3].
Intestinal Barrier
There are several important elements of the intestinal barrier (Fig. 1). In the lumen, there is degradation of bacteria and antigens by bile, gastric acid, and pancreatic enzymes, as well as commensal bacteria that produce antimicrobial substances and inhibit colonization by pathogens. Adjacent to the epithelial surface, the microclimate is composed of the unstirred water layer, glycocalyx, and mucus layers, which prevent bacterial adhesion through their physical properties and by the effects of immunoglobulin (Ig) A secreted by immunocytes in the lamina propria. The epithelial cells (enterocytes) are connected by junctional complexes; the enterocytes transport luminal content and react to noxious intraluminal stimuli by secretion of chloride and antimicrobial peptides including lysozyme, and α defensins from Paneth cells. Beneath the epithelium, the lamina propria includes innate and acquired immune cells secreting Ig and cytokines. Products of the enteroendocrine cells and the enteric nervous system can stimulate intestinal propulsive motility to clear potentially injurious elements downstream [4].
Fig. 1.
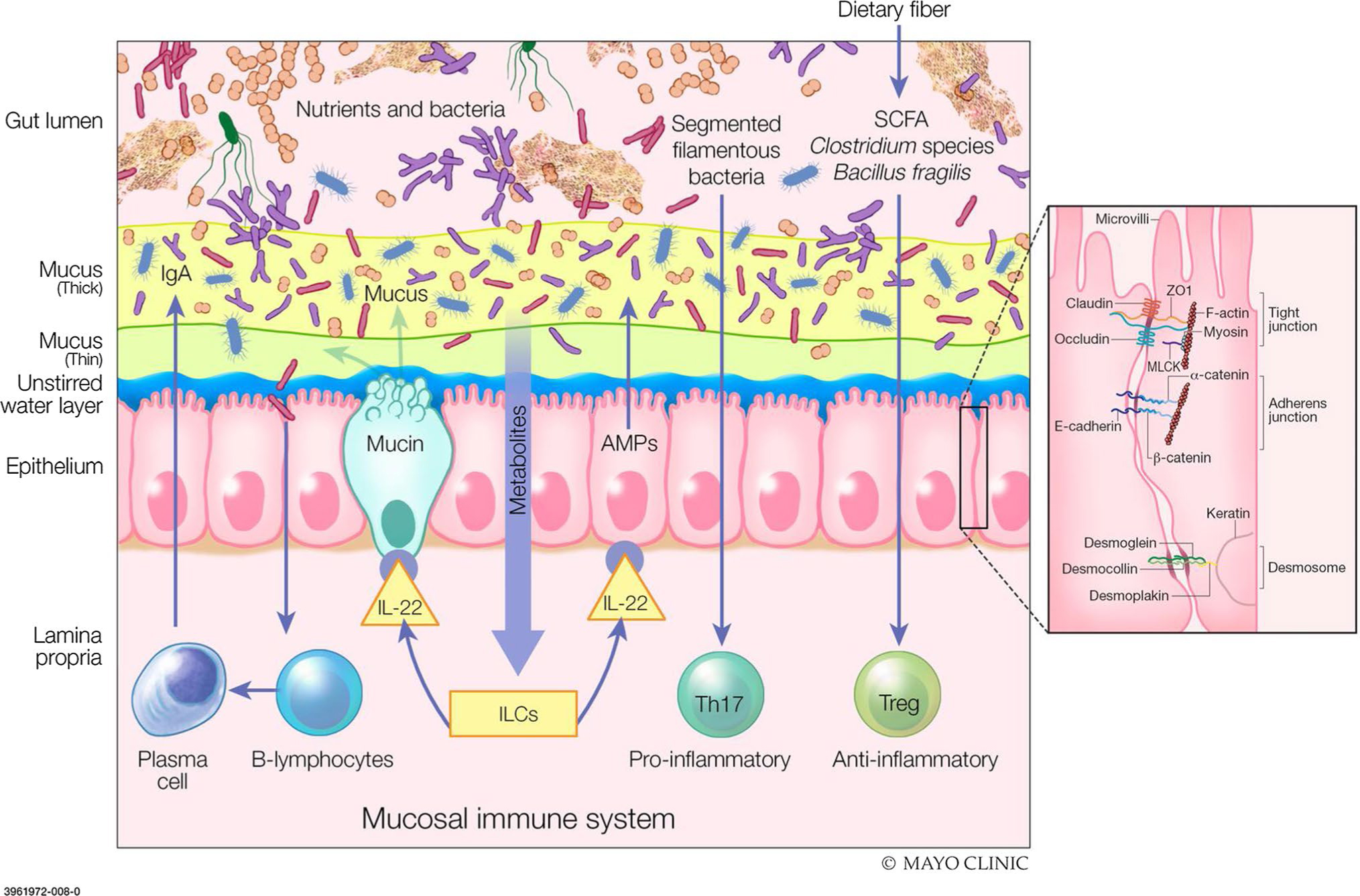
Components of intestinal barrier function. The gut lumen is where the degradation of bacteria and antigens by bile, gastric acid and pancreatic juice occurs. Commensal bacteria inhibit the colonization of pathogens through production of defense antimicrobial substances. The unstirred water layer, glycocalyx and thick/thin mucus layer works to prevent bacterial adhesion by immunoglobulin A (IgA) secretion. Within the epithelium, tight junctions (TJs) connect the epithelial cells to transport luminal contents. TJ-associated proteins (occludin, zonula occludens-1, claudin-1/4, junctional adhesion molecules) are vital to barrier integrity. Myosin light chain activation regulates the contraction and tension of actin which allows for opening of the paracellular pathways. The lamina propria is home to innate and acquired immune cells which secrete immunoglobulins and cytokines for use within the endocrine and enteric nervous system. Disruption of the intestinal barrier with permeation of noxious molecules to the lamina propria, induces mucosal immune system activation and inflammation. SCFA short chain fatty acids, IgA immunoglobulin A, IL interleukin, ILCs innate lymphoid cells, Th t helper cells, Treg T regulatory cells, ZO-1 zonula occludens-1, MLCK myosin light-chain kinase (From: Camilleri M, Lyle BJ, Madsen KL, Sonnenburg J, Verbeke K & Wu GD. Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. Am J Physiol Gastrointest Liver Physiol. 2019 Jul 1;317(1):G17-G39; used with permission of Mayo Foundation for Medical Education and Research, all rights reserved)
Although the enterocyte layer constitutes only one component of the barrier, it has been extensively studied and includes elements that are relevant to the potential role of barrier dysfunction in IBD. The main components of epithelial intercellular junctions are the apical junctional complex which includes adherens junctions and desmosomes [5–7]. First, the apical junctional complex is characterized by interactions between F-actin, myosin II, zonula occludens-1 (ZO-1), claudins, tight junction–associated marvel proteins (TAMPs), and immunoglobulin superfamily members such as junctional adhesion molecules (JAM) and the coxsackie adenovirus receptor (CAR) [8]. Claudin-2 expression is limited to crypt, rather than surface, epithelial cells in the normal colon [9]. In fact, claudin-2 expression is greater in the Brunner’s glands of the small bowel where its effects may be more important [9–11]. Claudins 1 and 2 are associated with increased permeability, and claudin-2 expression is increased by IL-6 in inflammatory states [12]. Claudin-1 and claudin-2 expression may even be involved in early stages of IBD-associated neoplasia[11]. Second, the adherens junctions, specifically catenins (intracellular components) and e-cadherins which are calcium-dependent transmembrane proteins, are responsible for cell–cell adhesion and the maintenance of the cell layer structure. Third, the desmosomes including desmoglein, desmoplakin and desmosomal cadherins, represent the strongest link between epithelial cells, maintaining epithelial structural integrity in case of mechanical stress [5].
Transport Across the Intestinal Barrier
Paracellular transport or permeability is the passage of molecules between adjacent epithelial cells. Drugs and other molecules pass through the tight junctions (TJ) via two distinct pathways based on different sizes and selectivities. The high capacity, charge-selective pore pathway allows for passage of small ions and uncharged molecules (typically < 8 Å). The paracellular pathway of absorption involves simple passive diffusion along a concentration gradient. The pathways can also be distinguished based on their capacity, as the pore pathway can allow for absorption of large quantities of small uncharged solutes and ions. Paracellular permeability is largely determined by claudin proteins and their role in the pore pathway, as the pore is formed by interactions between β-sheets within extracellular loop 1 of claudins on adjacent cells. Claudins are a group of > 25 types of proteins that are involved in TJ formation, with molecular weights ranging from 21 to 34 kDa (207–305 amino acids) [5]. The permeability of these pathways can be measured using several complementary methods such as the in vitro measurement of Trans-Epithelial Electrical Resistance (TEER), which measures the flux of all ions especially Na+ and Cl− across the epithelium and is most useful as a measure of permeability when ion conductance across the junction is far greater than the conductance across apical and basolateral membranes, as in leaky epithelia.
The low capacity, leak pathway allows flux of larger ions and molecules (typically < 100 Å), regardless of charge, though to a lesser capacity than the pore pathway [13]. Active (energy-dependent) uptake at epithelia involves several mechanisms including brush border transmembrane proteins, membrane fusion, transcytosis via vesicle carriers, or drug absorption into the cell. After entry into the cell, absorbed molecules are secreted on the basolateral side of the enterocyte into the systemic circulation [5]. For example, sodium-glucose cotransport-1 (SLGT-1) is a well-known transmembrane protein, which mediates most sodium-dependent glucose uptake in the small intestine [14]. Leak pathway permeability can be assessed by directly measuring macromolecular flux of tracers of relatively low molecular weight and molecular diameter across the epithelium. Commonly used tracers are mannitol, sucrose, lactulose, sucralose, inulin, ora polydisperse of individual molecules of varying sizes as in polyethylene glycol (PEG) 400) [8]. Larger molecules, such as dextran 4 or 10 kDa, tend to be used to assess the leak pathway in studies of mucosa evaluated in vitro. However, it is important to note that several of these probe molecules have estimated diameters of < 8 Å (especially the monosaccharides) and, therefore, they also reflect pore pathway permeability (e.g., erythritol 3.2–6.0 Å and mannitol 6.7–7.2 Å) due to their reported diameters or mathematically estimated diameters based on molecular mass [13, 15]. Leak pathways are more permeable with downregulation of occludin, non-muscle myosin II and ZO-1 [16–19]. Occludin is a 65 kDa protein whose expression is regulated by different kinases, and localization follows that of ZO protein because of its linkage.
Considerations in Measuring “Leaky Gut” in vivo in Humans
Although there are in vitro methods to measure mucosal permeability, histological measurements of barrier proteins in mucosal biopsies, and confocal endomicroscopy of the duodenum, all these methods are invasive, and cannot be easily repeated longitudinally on large patient cohorts. Thus, most observations on GI barrier function use administration of oral probes, monosaccharides and disaccharides, with measured urinary excretion. However, criteria for non-invasive methods to measure permeability are not established. The urinary excretion of orally ingested probe molecules for the in vivo measurement of intestinal permeability includes probes of diverse molecular weight and measurement of mathematically estimated molecular diameters [15, 20]. In relation to studies without overt ulceration or epithelial cell apoptosis (e.g., functional diarrhea, or relatives of patients with IBD), it is important to note that the absorption of the disaccharide probes (lactulose, sucralose), EDTA, DTPA, and PEG is limited from the perspective of % of mass administered. Thus, even though studies typically administer 2.5–5 times as much lactulose as mannitol, there is usually a higher amount of mannitol than lactulose excreted with concomitant administration of the two sugar probes. In fact, studying the individual sugar excretions (rather than the ratio) shows more mannitol excreted during 0–2 h after oral administration in the fasting state, than after 4 h; this suggests that the small bowel is more permeable than the colon, as has been documented in many studies [21].
The timing of urine collection is also critical to assess the relative contributions of small intestine and colon to measurements of permeability. By conducting simultaneous gamma-radiolabeled imaging and timed urine collections (in 2-h aliquots for the first 8 h, followed by a collection from 8 to 24 h), it was demonstrated that urine collections at 0–2 h reflect predominantly small bowel, 2–8 h reflect both small bowel and colon, and 8–24 h exclusively colon [21, 22].
Another pitfall in measurements of permeability stems from contamination with the oral probes from baseline or during the studies. The prototypical contamination is with sucralose and mannitol which is identified in a range of fruits and vegetables (e.g. watermelon, clingstone peaches, button mushrooms, cauliflower, celery, snow peas, butternut squash, and sweet potato), ‘sugar free’ products (listed as mannitol, or under its food additive number, e421), and in cosmetics and beauty products where they are used primarily as a humectant or as a binder, masking agent, moisturizing agent, flavoring agent (lip balms, etc.) and skin conditioner.
The potential confounders with oral probe molecules were extensively documented in 60 adults studied on three occasions, with significant quantities of mannitol in 70% of participants, and rhamnose and sucralose in 6–7% [23]. Evidence of contamination with rhamnose was also documented in a pediatric study of celiac disease [24]. This contamination may also impact the estimated ratios of lactulose to mannitol. For example, in the same healthy individuals, there was higher LMR beyond 4 h after oral ingestion, suggesting colonic permeability is greater than small bowel permeability, which is clearly incorrect [21]. Moreover, given the relatively small mass of disaccharide absorbed normally, a very small increase in lactulose absorption has a marked impact on the calculated ratio of disaccharide to monosaccharide.
Mucus
The gut mucus layer consists of mucins, a family of large, complex, glycosylated proteins vital for maintaining intestinal health, which creates a coat that covers the intestinal cells protecting them from contact with external and toxic substances, digestive enzymes, and bacteria. The mucins consist of a protein core composed of sequences containing the amino acid residues, proline (Pro), threonine (Thr), and serine (Ser), called PTS-rich sequences. These sequences are often repeated in tandem, in which the Ser and Thr are extensively O-glycosylated and confer a ‘bottle brush’-like conformation. More than 80% of the mucin mass is made up of O-glycans. This leads to the creation of a glycan coat, hiding the protein core of the mucins and protecting it from endogenous protease degradation, in addition to conferring the capacity to bind and be soluble in water and to form a gel [25].
The regulation of the intestinal mucus barrier is complex, and there is bidirectional interaction between host glycans and gut microbes whose composition contributes to the regulation of the intestinal mucus barrier function. Mucin glycans bind water, conferring moisturizing and lubricant properties, protecting the epithelial cells from dehydration and mechanical stress during the passage of luminal content and peristalsis forces. Mucus thickness and types of mucins in the intestinal tract in mice and humans have been documented [25].
Transmembrane mucins form the glycocalyx, whereas secreted mucins from sentinel goblet cells form the gel overlying the epithelium. When sentinel goblet cellsfinish emptying mucus, they are expelled from the colonic crypt and are often replaced by bacteria [26]. In humans, the goblet cell-to-enterocyte ratio changes along the intestinal tract: 4% in the duodenum, 6% in the jejunum, 12% in the ileum, and 16% in the distal colon, reflecting the proportion of goblet cells which increase proportionally to the increase in the number of microorganisms in each region (103 in the duodenum, 104 in the jejunum, 107 in the ileum, and 1012 in the colon) [25]. The mucus of the small intestine is non-attached, easily removed, discontinuous, and relatively porous and penetrable to different components such as bacteria. In the small intestine, peristalsis leads to decreased contact time for luminal contents and the epithelium, compared to the colon. In comparison, the colon has both an inner adherent and outer non-attached mucus layer accounting for differences in mucus thickness [27, 28]. The combination of transit time, and structural differences in the mucus layers of the small intestine and colon contribute to regional differences in permeability [25].
The proportion of mucus-degrading bacteria increases when the diet is deprived of specific dietary fibers, since mucus then becomes an energy source for the gut microbiota [23]. In addition to supplying attachment sites for the microbiota, mucin glycans also serve as nutrients for microorganisms called ‘mucolytic bacteria’, favoring their replication. Bacteria digest glycans through their glycosidase enzymes. Table 1 summarizes mucus regulation by specific pathogenic or commensal microorganisms and microbial metabolites [25].
Table 1.
Specific pathogenic or commensal microorganisms and microbial metabolites regulating mucus [25]
| Microorganism or metabolite class | Specific microorganism or metabolite | Effect on mucins or mucus | Citations |
|---|---|---|---|
|
| |||
| Pathogenic | Vibrio cholerae Giardia lamblia Entamoeba histolytica Trichuris muris | Induce mucins degradation | [111, 112] |
| Listeria monocytogenes Entamoeba histolytica Nippostrongylus brasiliensis Trichinella spiralis | Inhibit mucus production Regulate Goblet cell function Regulate mucin expression | [113, 114] | |
| Commensals | Lactobacillus spp. | Stimulate MUC3 expression and MUC2 production and secretion | [115] |
| Bifidobacterium longum | Restore mucus growth | [116] | |
| Lactobacillus reuteri | Increase mucus layer thickness | [117] | |
| Akkermansia muciniphila | Restore/increase mucus layer thickness Increase Goblet cell number | [34] | |
| Microbial metabolites | Short chain fatty acids: acetate and butyrate | Stimulate MUC2 expression Increase mucus production Increase mucus secretion | [118] |
Modified from Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–2243
MUC mucins
Dietary Perturbations of Mucin and Microbiota Drive Intestinal Inflammation
Among the most important dietary components capable of perturbing the intestinal barrier, emulsifiers and fat have been shown to drive intestinal inflammation, especially if there is a genetic predisposition as shown in interleukin-10 (IL-10) knockout mice [29, 30].
First, exposure of the microbiome and mucus to food additive emulsifiers in the intestinal lumen leads to decreased diversity of the microbiome and increased pro-inflammatory potential. Some emulsifiers increase bacterial expression of flagellin and lipopolysaccharide (enhancing the ability of bacteria to translocate through the mucus layer to the epithelial cell) and thinning of the mucus, thereby increasing penetrability through alterations in membrane-associated proteins such as ZO-1. However, this is controversial as data of intestinal epithelial specific ZO-1 knockout mice showed intact barrier function [31]. Bacterial translocation activates inflammatory pathways through the B-cell lymphoma/leukaemia-10 (Bcl-10) and Toll-like receptor-4 (TLR-4), which in turn activate the nuclear factor kappa light-chain-enhancer of activated β cells (NF-kβ) cascade, secretion of pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α) and IL-6, and the development of colitis [29].
Second, a high fat diet is associated with changes in the microbiota such as increases in sulfur-reducing bacteria (SRB), with increased production of hydrogen sulfide (H2S) leading to decreased butyrate oxidation or reduced Bifidobacteria leading to increased inflammation and barrier dysfunction [30, 32].
The type of fat in the diet may exert different effects on microbiota and permeability, as shown by Devkota et al. [30] Thus, a diet high in saturated fat (milk) exerted different effects on microbiota compared with a diet high in polyunsaturated fat (safflower oil) [30]. A high milk fat diet promoted an increase in Bilophila wadsworthia, a SRB, that is present in inflammatory conditions where by it promotes changes in the host bile acid composition resulting in dysbiosis and possibly leading to intestinal damage in genetically susceptible hosts. Another fat, corn oil [a source of omega-6-polyunsaturated fatty acids (PUFA)] decreased integrin-linked kinase which is indispensable for barrier function, and decreased expression of several TJ proteins of the intestinal barrier [33]. Paradoxically, a high fat diet is associated with lower abundance of the mucin-degrading bacteria A. muciniphila in healthy subjects, and it was associated with restoration of mucus thickness, suggesting that fat may disrupt epithelial components while enhancing the contribution of mucus to barrier function [34].
Gastrointestinal Disease Impairs Barrier Function
Diseases and disorders associated with altered intestinal barrier function and natural surfactants such as bile acids, in addition to food emulsifiers, can dissolve mucus and increase mucosal permeability [35]. Effects of non-steroidal anti-inflammatory drugs (NSAIDs) provide an example of an inflammatory diathesis leading to a range of disorders, from enteropathy or erosions to altered barrier function [36]. Traditionally, NSAIDs have been used to study a perturbed barrier in experimental models. The increased permeability due to NSAIDs has been measured by urinary excretion of orally administered 51Chromium-EDTA or saccharide probes [37–39]. In an experimental model, the presence of ischemia and cytokines increased permeability, which were reversible pharmacologically with lubiprostone [40].
Barrier defects have been described in association with intestinal diseases including Crohn’s disease (CD) and ulcerative colitis (UC) (Table 2) [41–46]. Fecal metabolomics in IBD have also been extensively reviewed; based on current knowledge, the most significant factors affecting intestinal epithelial barrier are short chain fatty acids, tryptophan metabolites, and bile acids [47]. The biological effects of metabolites are summarized in Table 3, based on the extensive review by Iyer and Corr [47]. As researchers gain a greater understanding of the role of metabolites in controlling immune metabolism, this may become a significant therapeutic target for autoimmune diseases such as IBD.
Table 2.
Barrier defects associated with inflammatory diseases of the intestine [41]
| Disease | Cause of barrier defect | Timing of barrier defect | Effects on Tight Junction proteins and cytoskeleton | Citations |
|---|---|---|---|---|
|
| ||||
| Crohn’s disease | Unknown; Association with frameshift insertion at nucleotide 3020 of NOD2 | Before clinical onset or clinical relapse | Claudin-2 upregulation; MLCK activation; | [61, 119, 120] |
| Ulcerative colitis | Unknown | Unknown | Occludin downregulation | [61, 119, 120] |
| C. difficile colitis | Actomyosin disruption; glycosylation of Rho proteins | Toxin release; disease onset | Loss of ZO-1 and ZO-2 | [121] |
| GVHD | Associated t TNF production | After clinical onset | Unknown | [122] |
Modified from Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809.
GVHD graft-versus host disease, MLCK myosin light chain kinase, ZO zonula occludens
Table 3.
Effects of metabolites on immune, tight junction, and cellular elements of intestinal barrier [47]
| Metabolite | Microbial sources of metabolites | Receptors | Effects of metabolites |
Citations | ||
|---|---|---|---|---|---|---|
| Immune | TJ / cellular | Microbial | ||||
|
| ||||||
| Butyrate | Clostridium clusters | GPR41, GPR109A, GPR65 (?) | ↑ TGF-β | ↓ proliferation of crypt stem cells ↑ TJ proteins (occlu- din, ZO-1) |
[123] | |
| Acetate | Bacteroides spp. Prevotella spp. | GPR43 | [124] | |||
| Propionate | Bacteroides spp., Veillonella spp., Dialister spp. Ruminococcus spp. | GPR41, GPR43 | [125] | |||
| Bile acids | Bacteroides spp., Eubacterium spp., Lactobacillus spp. Clostridium spp. | FXR, TGR5, pregnane X receptor (PXR), vitamin D (VDR) | ↓ by UDCA, LCA | Permeability ↑ by DCA, CDCA ↓ IL-8 production by DCA, LCA |
[126] | |
| Tryptophan metabolites: kynurenic acid, HT, indole derivatives | Bacteroides spp., Lactobacillus spp. Clostridium spp. | GPR35 (?), Aryl hydrocarbon receptor (AHR), PXR | Indoles ↑ IL-10 to ↑ goblet cell differentiation | Indoles regulate epithelial repair and differentiation and reduce disassembly of adherens junction | Indole derivatives promote expression of anti-micro-bials | [127] |
Modified from Iyer N, Corr SC. Gut Microbial Metabolite-Mediated Regulation of the Intestinal Barrier in the Pathogenesis of Inflammatory Bowel Disease. Nutrients. 2021;13[12]
GPR G protein receptors, FXR Farnesoid X receptor, TGR Triterpene glycoside receptor, TGF-B Transforming growth factor beta, UDCA Ursodeoxycholic acid, LCA Lithocholic acid, IL interleukin, ZO Zonula occludens, DCA Deoxycholic acid, CDCA Chenodeoxycholic acid, HT hydroxytryptamine
Interestingly, prior meta-analyses in IBD have shown that achieving endoscopic remission can lead to improved outcomes in both CD and UC, including steroid-free clinical remission, colectomy-free survival and hospitalizations [48, 49]. This has led to consensus of the therapeutic goal of mucosal healing as outlined in the STRIDE-II initiative [50]. Additionally, intestinal barrier healing has been associated with disease risk of progression of IBD using confocal endoscopy, when compared to standard of endoscopic and histologic remission [51, 52]. Despite immune modulating treatments, over 30% of patients with IBD still have persistent symptoms in the absence of endoscopic inflammation [53]. Prior research alludes to the barrier as key in understanding this cohort of patients and makes the intestinal barrier an appealing target for future therapeutic modalities.
Permeability as a Risk Factor in Development of IBD
Intestinal barrier dysfunction has been proposed as a risk factor for the development of IBD. It is hypothesized that prior to the onset of IBD, barrier dysfunction and resultant increased intestinal permeability leads to an ingress of luminal contents into the lamina propria resulting in innate and adaptive immune cell activation, eventually leading to intestinal inflammation (Fig. 2) [54]. Several earlier studies had documented altered intestinal permeability in both CD and UC, even as a marker of clinical course of disease [45, 55–58]. Cytokines have been shown to modify the expression of TJ proteins [59]. In active IBD, claudin-2 expression and myosin light chain kinase (MLCK) activity are both increased, suggesting that both pore and leak pathways are involved in disease-associated impairment of barrier function [11, 60, 61]. Additionally, in UC, mucus barrier structural weakening can be found prior to the onset of inflammation which may account for the sentinel goblet cells weakened response to pathogens, and the histological hallmark of goblet cell and mucin depletion in colonic mucosal biopsies in patients with UC [62].
Fig. 2.
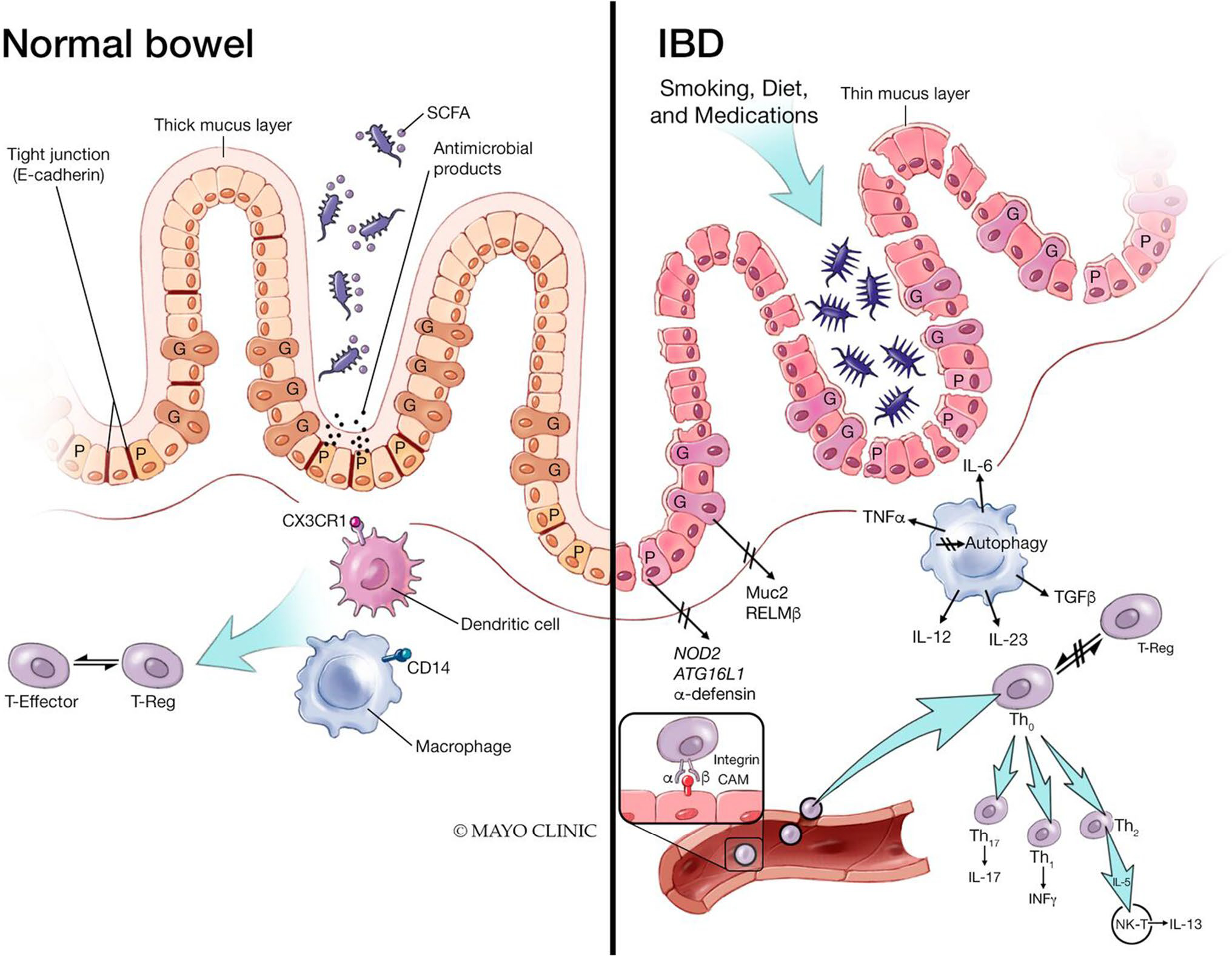
Impact of inflammatory bowel disease on the intestinal barrier. The pathogenesis of inflammatory bowel disease (IBD) is multifactorial due to genetic susceptibility, environmental factors, and immune dysregulation. The cytokine-mediated dysfunction of the TJ barrier via IL-6 and TNFα results in immune activation and mucosal inflammation, an essential component of IBD. Additionally, patients with IBD have increased intestinal permeability reflecting decreased epithelial barrier function. SCFA short chain fatty acids, CXCr1 C-X-C motif chemokine receptor 1, CD14 cluster of differentiation 14, IL interleukin, TNFα tumor necrosis factor alpha, Muc2 Mucin 2, RELMβ resistin-like molecule beta, ATG16L1 autophagy related 16 like 1, Th T helper cells, INFy interferon gamma, NK-T natural killer cell (From: Ramos GP & Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019 Jan;94(1):155–165; used with permission of Mayo Foundation for Medical Education and Research, all rights reserved)
More recent large studies conducted in first-degree relatives of patients with CD suggest that increased intestinal permeability is a factor predisposing to the development of CD [54]. In a study of 1,420 asymptomatic, first-degree relatives (aged 6 to 35 years) of patients with CD, followed over an average of 7.8 years, urine lactulose to mannitol ratio (LMR) at baseline was significantly higher in the individuals who went on to develop CD and this effect was independent of subclinical intestinal inflammation (measured by fecal calprotectin > 100 μg/g) [63]. Among the 50 relatives who developed CD, the median time to diagnosis during follow-up was 2.7 years. The test of intestinal permeability involved ingestion of lactulose (5 g), mannitol (2 g), sucrose (100 g), and flavored drink crystals (1.5 g) in 500 mL of tap water before bed, and then urine collection the following morning. Abnormal LMR was associated with diagnosis of CD during follow-up [63].
In a subsequent extension of the study, the cohort was divided into a discovery cohort (n = 2,472) and a validation cohort (n = 655). A regression model used to assess microbial associations with abnormal LMR (> 0.025) showed reduced alpha-diversity (Chao1 index) and altered beta-diversity (Bray–Curtis dissimilarity index) compared to subjects with LMR ≤ 0.025. Random forest approach revealed that bacterial community was associated with gut barrier function: 4 genera (decreased prevalence of Adlercreutzia, Clostridia UCG 014, and Clostridium sensu stricto 1 and increased abundance of Colidextribacter) and eight pathways (including decreased biosynthesis of glutamate, tryptophan and threonine) were replicated in the validation cohort [64].
Mechanisms to Protect or Modulate the Intestinal Barrier in Inflammatory Bowel Disease
Several mechanisms may protect or modulate the intestinal barrier in IBD. These mechanisms are summarized below.
A. Urokinase Interaction
Urokinase-type plasminogen activator (uPA) interacts with its receptor uPAR to mediate cell migration and activation. Recent human/mouse intestinal organoid studies have investigated the role of uPA-uPAR interaction which may be upregulated in biopsies of IBD mucosa [65]. Through simulated barrier damage using pathogenic cytokines, this study evaluated how the inhibition of uPA-uPAR impacted permeability, which was measured using TEER. The findings suggest that pharmacologic inhibition of the uPA-uPAR interaction may protect the epithelial barrier from intestinal damage associated with inflammation. Further investigation is needed to determine if this is a potential therapeutic target for IBD.
B. Cytokines
B1. Exogenous tumour necrosis factor (TNF) increases MLCK1 activity in cultured intestinal epithelial cells, which causes perijunctional myosin II regulatory light chain phosphorylation (pMLC) and condensation, and triggers occludin endocytosis [66, 67]. In this sequence of reactions, MLCK1 requires binding to the tacrolimus-sensitive protein FK506 binding protein 8 (FKBP8) to be recruited to the perijunctional actomyosin ring. MLCK1 can then induce phosphorylation of MLC (pMLC), which drives internalization of the transmembrane TJ protein occludin. This increases flux across the TJ leak pathway and enhances paracellular permeability to large solutes because of immune-mediated barrier loss. Tacrolimus has no effect on TNF induction of MLCK1 expression, but it causes dissociation of FKBP8 from MLCK1 thereby inhibiting MLCK1 recruitment to the perijunctional actomyosin ring, inhibition of MLCK1-mediated p-MLC, occludin internalization, and TNF-induced increased permeability [68]. In summary, FKBP8 can be blocked by tacrolimus, which therefore prevents TNF-induced perijunctional MLCK1 recruitment, MLC phosphorylation, and FKBP8 interactions. These actions appear to counter perijunctional MLCK1 recruitment and MLCK1-FKBP8 interactions that are increased in CD [69]. Another approach to targeted inhibition of MLCK-mediated TJ barrier regulation was tested to inhibit the specific induction of long MLCK1 and its recruitment to the perijunctional actomyosin ring. Such specific targeting of long MLCK1 (by divertin) prevents TNF-induced barrier loss and reverses immune colitis without the potential of unacceptable toxicities resulting from loss of MLCK enzymatic activity in smooth muscle and non-muscle cells [70]. These exciting data require further replication in IBD models to show impact on disease.
B2. Interleukin-8 is a cytokine used for recruitment and activation of neutrophils acting as a pro-inflammatory role. In situ hybridization of IBD patients’ mucosa showed strong signals with IL-8 anti-sense RNA probes [71]. It was found that quantity of cells expressing IL-8 was proportional to severity of inflammation. Prior studies have also shown an association with adherent E coli in IBD triggering mitogen-activated protein kinase IL-8 release, which may be inhibited by mesalamine. The role of IL-8 may play a role in the pathogenesis of IBD, though it is unclear the distinct role in permeability of the membrane [71].
B3. Interkeukin-10 works by suppressing cytokine synthesis by Th1 helper cells, through impaired macrophage dependent stimulation of antigen reactive Th1 cells [72]. This was elucidated in IL-10 deficient mice models which developed chronic enterocolitis [73]. Further studies have no demonstrated successful decrease in established inflammation when IL-10 is administered systemically. Though it is considered that IL-10 downregulation may prevent the development of IBD [74].
B4. Interleukin-13 can stimulate claudin-2 expression in surface epithelial cells (where the TJ protein is not normally expressed). This increases flux across small TJ pores, thereby enhancing paracellular cation permeability [41]. There is evidence that glutamine is able to reverse the deleterious effect of IL-13 on intestinal permeability by increasing claudin-1 expression via disruption of the phosphatidylinositol-3-kinase/Akt signaling pathway in Caco-2 cells [75].
B5. Interleukin-22 critically regulates epithelial homeostasis through regulation of epithelial cell permeability and mucus production. IL-22 is expressed in response to pro-inflammatory cytokines and is required to activate the DNA damage response of the intestine [76]. Paradoxically, IL-22 has also been shown to promote inflammation [77]. Through induced expression of mucin genes via STAT3-dependent signaling and increase in number of intestinal goblet cells, IL-22 helps with the clearance of pathogens penetrating the barrier [78, 79]. In IBD, the role of IL-22 is complex and dependent on context. Without a preclinical model, investigating the role of IL-22 has been based on studies of the effects of pharmacologic IL-22 agonists in patients with active IBD [76]. For example, UTTR1147A is a IL-22 IgG4 Fc fusion protein which is a IL-22 pathway agonist. It has been shown to safely promote tissue regeneration in phase I studies of healthy patients and UC [80]. UTTR1147A is currently being tested in patients with active IBD [81].
B6. Interferon-gamma—Gene expression of NOD2 in human intestinal epithelial cells is upregulated synergistically by TNF-α and interferon-gamma (IFN-γ) [82]. IFN-γ increases paracellular permeability in intestinal epithelial cells through the redistribution and expression of TJ proteins and the rearrangement of the actin cytoskeleton [83]. There is evidence that the deleterious effect of IFN-γ on intestinal barrier function can be reduced by the product of protein tyrosine phosphatase non-receptor type 2 (PTPN2) gene, called T cell protein tyrosine phosphatase (TCPTP). Thus, TCPTP protected against intestinal barrier dysfunction induced by IFN-γ by two mechanisms: It maintained localization of ZO-1 and occludin at apical TJs and restricted the expression and insertion of the cation pore-forming transmembrane protein, claudin-2, at TJ through upregulation of the inhibitory cysteine protease, matriptase [84]. A consistent barrier-protective effect of TCPTP was documented in other studies: Cells expressing TCPTP effectively dampen immune responses to limit changes in flux across the pore and the leak pathways, whereas TCPTP loss in intestinal epithelial cells leads to upregulation of claudin-2 expression, triggering of excessive cytokine secretion in TCPTP-deficient immune cells, activation of MLCK to cause occludin internalization, and increased leak pathway permeability triggering further immune activation and creating a vicious cycle that leads to disease [85]. Similarly, TCPTP deficiency increases intestinal expression of claudin-2 in vitro, in vivo, and in PTPN2-genotyped patient with CD [84].
B7. T regulatory (Treg) cell dysfunction is found in patients with IBD, as these cells play a critical role in limiting an autoimmune response and may be a future therapeutic target using antigen-directed Treg therapy [86]. Th17 cells are thought to be closely related to the development of IBD and other autoimmune conditions. The presence of mucosal Th17 helps regulate barrier integrity by stimulating the formation of tight junctions and antimicrobial peptides. In contrast, the role of Th17 is dichotomous and can also be pro-inflammatory under pathological conditions [87]. Innate lymphoid cells (ILCs) remain understudied in IBD, though are a distinct immune cell different from T or C cells. Studies show that ILCs can contribute to host defense via epithelial barrier function by integrating both pro and anti-inflammatory signals. Additionally, consumption of ILCs can allow for influx of commensal bacteria leading to infection and systemic inflammation [88].
Potential of Dietary Modulation to Impact Intestinal Permeability
Patients may appreciate efforts to study how specific key dietary factors influence the intestinal barrier such as a high fat diet, emulsifiers, and alcohol, or specific dietary supplementations (Table 4) [3, 89, 90].
Table 4.
Key dietary components associated with potential to have positive effects on overall gut integrity [3]
| Componenta | Common food sources |
|---|---|
|
| |
| Prebiotic fiber | |
| Beta Glucan | Barley, mushroom, oat |
| Fructans | Asparagus, banana, barley, chicory root, garlic, honey, Jerusalem artichoke, leek, nectarine, onion, scallion, rye, wheat |
| Galacto-oligosaccharide | Cashew, legume (chickpea, red kidney bean, soybean, split pea), milk, pistachio, squash (butternut, pumpkin) |
| Pectin | Apple, banana, broccoli, carrot, dried pea, grapefruit, lemon, orange, potato, tomato |
| Resistant starch | banana, legume (black bean, dried pea, fava bean, lentil, pinto bean, soybean), whole Grain (barley, oat) |
| Xylo-oligosaccharide | Bamboo shoots and other vegetables, fruit, honey, milk |
| Polyphenol: subclass | |
| Flavonoid: Anthocyanin | Black bean, blackberry, black currant, black raspberry, blueberry, cherry, cranberry, eggplant, pecan, purple sweet potato, red cabbage, red grape, red (or blood) orange, red radish, red raspberry |
| Tannin: Ellagitannin | Almond, blackberry, blueberry, cranberry, pecan, pomegranate, raspberry, strawberry, walnut |
| Probiotic: bacteria/yeast | |
| Bifidobacterium Escherichia coli Lactobacilli Saccharomyces | Fermented dairy and nondairy sources: kefir, kimchi, kombucha, miso, sauerkraut, tempeh, yogurt |
| Amino Acid | |
| Glutamine |
Animal-based source: dairy (cheese, milk, yogurt), egg, meat, poultry, seafood Plant-based source: almond, cashew, kale, legume (chickpea, kidney bean, lentil, peanut, soybean), mushroom (shiitake), pistachio, seed (pumpkin, sunflower), red cabbage, spinach, tomato, whole grain (oat, quinoa, wheat) L-glutamine used as a food additive and nutritional supplement |
| Mineral | |
| Zinc |
Animal-based source: dairy (cheese, milk, yogurt), egg, meat (red), poultry (dark), shellfish (crab, lobster, oyster) Plant-based source: almond, legume (bean, lentil, pea), potato, seed (chia, pumpkin, sunflower), walnut, whole grain (oat, quinoa, wheat) Zinc-L-carnosine used as a nutritional supplement |
| Macronutrient | |
| Fat | Modify a typical Western-style diet high in fat (e.g. saturated fat). A Mediterranean diet eating pattern with focus on healthy unsaturated fats (olive oil, nuts), plant- based choices (fruits, vegetables, whole grains) and lean protein sources (fish, legumes) may favorably impact intestinal gut function |
The U.S. Department of Agriculture (USDA) food composition data resource at https://fdc.nal.usda.gov/about-us.html was accessed to obtain information on food sources
Commercially produced components (e.g., inulin, oligosaccharides, pectin, resistant starch, bacteria/yeast strains, l-glutamine, zinc-l-carnosine) are used as food additives/nutritional supplements
Fiber has been found to improve intestinal barrier health, through alteration of the gut microbiota and mucus layer through fermentation products, mainly short chain fatty acids (SCFAs) [91]. The SCFAs, acetate, propionate and butyrate, regulate neuroimmune functions as a protective measure for the intestinal barrier. For example, butyrate regulates hypoxia-inducible factor-1 to modulate the efficiency of genes coding for claudin-1 [92]. Butyrate can enhance TEER and affect relocation of ZO-1 and occludin. A study of fermentable fiber in dextran sulfate sodium (DSS)-induced colitis mice, showed a protective effect on TJs leading to suppression of colitis [93]. Paradoxically, when Kaiko et al. administered mice with DSS, they found an increased exposure of the stem cells to luminal butyrate, which did not allow for healing of the ulceration [93]. Emulsifiers are food additives such as carboxymethylcellulose and polysorbate-80, found in most processed food items to extend shelf stability. When emulsifiers were given to mice, the microbiota composition became more pro-inflammatory thereby increasing permeability [29, 94]. In humans, data should be interpreted with caution as permeability was not directly measured and prior studies restricted emulsifiers in combination with other dietary interventions making it difficult to attribute inflammatory affects to emulsifiers alone [95]. Fat-soluble vitamins may also impact permeability. In CD, vitamin D supplementation was shown to improve gastroduodenal permeability compared to placebo, though small bowel and colonic permeability was not impacted [96]. Whereas, a high fat diet is known to alter TJ proteins and microbiota, thereby inducing inflammation in rat studies leading to increased permeability; prior UC cohorts have failed to show clear associations [97, 98]. Polyphenols are secondary metabolites found in fruits and vegetables, and mice studies have shown a reduction in colonic damage in the setting of indomethacin through the assembly of ZO-2, occludin and claudin-1; human studies have not yet been completed [3, 99]. Glutamine, an l-alpha amino acid abundant in human blood, has been shown to reduce intestinal permeability in patients with CD when given enterally [100]. Acute and chronic alcohol use in patients with concomitant liver disease can lead to increased permeability via cell damage and TJ protein alteration [101, 102]. Further research is needed to understand how dietary interventions impact patients with IBD.
Summary of Potential Pharmacologic Restoration of Normal Barrier Function in IBD
Given the pivotal role of TNF-α and MLCK as the main regulators of the leak paracellular pathway, there is evidence for:
Adalimumab was tested in two in vitro models of mucosal inflammation, stimulation of Caco-2 and T-84 cells with interferon-γ and TNF-α; it had beneficial effects on TJ proteins, reduced MLCK phosphorylation as well as reversing the decreased transepithelial resistance [103]. Based on studies of endoscopic biopsies from non-inflamed colon of seven patients with CD before and after infliximab infusions, 51Cr-EDTA permeability was increased in CD and restored to control levels by infliximab. In addition, transmucosal uptake of adherent-invasive E. coli strain was reduced through a mechanism that involves blocking lipid rafts in epithelial cells [104]. Treatment of patients with infliximab resulted in a marked decrease of intestinal permeability as measured by the LMR, and mucosal mRNA expression associated with increased intestinal permeability (e.g., claudin, collagen, and laminin genes) was reversed by infliximab in patients with UC [105, 106]. These data suggest that barrier protection might therefore constitute a novel mechanism of how anti-TNF-α therapy contributes to epithelial restitution and tissue repair in IBD.
A small molecule, divertin, prevents MLCK1 recruitment by proinflammatory cytokines including TNF-α, interleukin-1β, and several related molecules to the perijunctional actomyosin ring [70]. Such recruitment of MLCK1 leads to molecular reorganization of TJs’ structure and composition, including occludin endocytosis. Ultimately, divertin restores barrier function after TNF-induced barrier loss and prevents disease progression in IBD [70].
Older studies found some anti-inflammatory effects of phosphatidyl choline phospholipids on the colonic barrier [107]. More recently, a double-blinded, placebo-controlled randomized controlled trial found an improvement in Simple Clinical Colitis Activity Index in patients who received a newly designed phosphatidylcholine formula [108]. Further studies have yet to be completed to evaluate this unique therapeutic target in IBD patients with permeability measures.
Specific drugs and targets may regulate intestinal permeability and are under investigation. These include monoclonal antibodies to IL-13, IL-22 and IFNγ, and drugs targeting receptors (e.g., α2-adrenergic receptors with dexmedetomidine, glucagon-like peptide 2, glucocorticoids), or ion channels (e.g., chloride channel with lubiprostone), or mast cell stabilizers [89]. MicroRNAs are small non-coding RNAs that regulate several pathways in IBD including dysregulation of autophagy and Th17 signalling. In the future, microRNAs may show promise in treating IBD through mechanisms to fix pathology instead of targeting symptomology [109, 110].
Conclusion
There is compelling evidence of altered barrier function in both established IBD and as a risk factor in first-degree relatives of patients with IBD, particularly CD. Mechanistic studies have allowed for early investigations of unique IBD treatments by restoring the barrier function. However, it is essential to consider the complexity and multidimensional nature of the intestinal barrier that cannot be fully characterized using in vitro measurements or morphological description of TJ elements in the epithelial layer. It is important to apply valid measurements to document breaching of the barrier and responses to experimental approaches to treatment. The demonstration of environmental exposures to probe molecules in the diet and cosmetic preparations (e.g., mannitol, rhamnose, sucralose) suggests significant measurement bias may have occurred in prior studies, and thus there is the need for replication and validation of observations on barrier function in IBD. The validated methods are now available and should promote further research on the potential role of barrier function in the etiopathogenesis and management of IBD.
Funding
National Institute for Health Care Management Foundation [R01-DK115950 (MC)].
Footnotes
Conflict of interest KAD has no conflicts of interest. LER is on the Advisory Board for Fresenius Kabi Gastroenterology and Janssen Pharmaceuticals. MC has stock options for consulting with Thelium Therapeutics (epithelial barrier function) re: divertin.
Data availability
The data that support the findings of this review are available on request from the corresponding author. The data are not publically available due to privacy or ethical restrictions.
References
- 1.Camilleri M Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145:16–27. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Vella A. What to do about the leaky gut. Gut. 2022;71:424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718–733. [DOI] [PubMed] [Google Scholar]
- 5.Brunner J, Ragupathy S, Borchard G. Target specific tight junction modulators. Adv Drug Deliv Rev. 2021;171:266–288. [DOI] [PubMed] [Google Scholar]
- 6.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. [DOI] [PubMed] [Google Scholar]
- 7.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22:85–89. [DOI] [PubMed] [Google Scholar]
- 8.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong M, Yeruva S, Sailer A, Nilsen SP, Turner JR. Differential regulation of claudin-2 and claudin-15 expression in children and adults with malabsorptive disease. Lab Invest. 2020;100:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3:e977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollander D, Kaunitz JD. The “Leaky Gut”: Tight Junctions but Loose Associations? Dig Dis Sci. 2020;65:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulsen SB, Fenton RA, Rieg T. Sodium-glucose cotransport. Curr Opin Nephrol Hypertens. 2015;24:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camilleri M, Lyle BJ, Madsen KL, Sonnenburg J, Verbeke K, Wu GD. Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. Am J Physiol Gastrointest Liver Physiol. 2019;317:G17–g39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naydenov NG, Feygin A, Wang D, Kuemmerle JF, Harris G, Conti MA et al. Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis. Scientific Reports. 2016;6:24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokuda S, Higashi T, Furuse M. ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape. PLoS One. 2014;9:e104994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monaco A, Ovryn B, Axis J, Amsler K. The Epithelial Cell Leak Pathway. Int J Mol Sci. 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Nadeau A, Lamsam J, Nord SL, Ryks M, Burton D et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil. 2010;22:e15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao AS, Camilleri M, Eckert DJ, Busciglio I, Burton DD, Ryks M et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol. 2011;301:G919–G928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoshbin K, Khanna L, Maselli D, Atieh J, Breen-Lyles M, Arndt K, et al. Development and Validation of Test for “Leaky Gut” Small Intestinal and Colonic Permeability Using Sugars in Healthy Adults. Gastroenterology. 2021;161:463–75 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtz LR, Hoffmann J, Linneman L, He M, Smyrk TC, Liu TC et al. Rhamnose Is Superior to Mannitol as a Monosaccharide in the Dual Sugar Absorption Test: A Prospective Randomized Study in Children With Treatment-Naïve Celiac Disease. Front Pediatr. 2022;10:874116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69:2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridler C Sentinel goblet cells flush out bacteria from crypts. Nature Reviews Gastroenterology & Hepatology. 2016;13:438-. [DOI] [PubMed] [Google Scholar]
- 27.Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GMH, Schütte A et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunological Reviews. 2014;260:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birchenough GMH, Johansson ME, Gustafsson JK, Bergström JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunology. 2015;8:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bancil AS, Sandall AM, Rossi M, Chassaing B, Lindsay JO, Whelan K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J Crohns Colitis. 2021;15:1068–1079. [DOI] [PubMed] [Google Scholar]
- 30.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB et al. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology. 2021;161:1924–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol. 2012;3:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abulizi N, Quin C, Brown K, Chan YK, Gill SK, Gibson DL. Gut Mucosal Proteins and Bacteriome Are Shaped by the Saturation Index of Dietary Lipids. Nutrients. 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quigley EM. Leaky gut - concept or clinical entity? Curr Opin Gastroenterol. 2016;32:74–79. [DOI] [PubMed] [Google Scholar]
- 36.Endo H, Sakai E, Kato T, Umezawa S, Higurashi T, Ohkubo H et al. Small bowel injury in low-dose aspirin users. J Gastroenterol. 2015;50:378–386. [DOI] [PubMed] [Google Scholar]
- 37.Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology. 2018;154:500–514. [DOI] [PubMed] [Google Scholar]
- 38.Moore A, Bjarnason I, Cryer B, Garcia-Rodriguez L, Goldkind L, Lanas A et al. Evidence for endoscopic ulcers as meaningful surrogate endpoint for clinically significant upper gastrointestinal harm. Clin Gastroenterol Hepatol. 2009;7:1156–1163. [DOI] [PubMed] [Google Scholar]
- 39.Sigthorsson G, Tibble J, Hayllar J, Menzies I, Macpherson A, Moots R et al. Intestinal permeability and inflammation in patients on NSAIDs. Gut. 1998;43:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuppoletti J, Blikslager AT, Chakrabarti J, Nighot PK, Malinowska DH. Contrasting effects of linaclotide and lubiprostone on restitution of epithelial cell barrier properties and cellular homeostasis after exposure to cell stressors. BMC Pharmacol. 2012;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- 42.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noguchi E, Homma Y, Kang X, Netea MG, Ma X. A Crohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat Immunol. 2009;10:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. [DOI] [PubMed] [Google Scholar]
- 45.Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437–1439. [DOI] [PubMed] [Google Scholar]
- 46.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. [DOI] [PubMed] [Google Scholar]
- 47.Iyer N, Corr SC. Gut Microbial Metabolite-Mediated Regulation of the Intestinal Barrier in the Pathogenesis of Inflammatory Bowel Disease. Nutrients. 2021;13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah SC, Colombel J-F, Sands BE, Narula N. Mucosal Healing Is Associated With Improved Long-term Outcomes of Patients With Ulcerative Colitis: A Systematic Review and Meta-analysis. Clinical Gastroenterology and Hepatology. 2016;14:1245–55.e8. [DOI] [PubMed] [Google Scholar]
- 49.Shah SC, Colombel J-F, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Alimentary Pharmacology & Therapeutics. 2016;43:317–333. [DOI] [PubMed] [Google Scholar]
- 50.Turner D STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. 2021. [DOI] [PubMed]
- 51.Rath T, Atreya R, Bodenschatz J, Uter W, Geppert CE, Vitali F et al. Intestinal Barrier Healing Is Superior to Endoscopic and Histologic Remission for Predicting Major Adverse Outcomes in Inflammatory Bowel Disease: The Prospective ERIca Trial. Gastroenterology. 2023;164:241–255. [DOI] [PubMed] [Google Scholar]
- 52.Chang J, Leong RW, Wasinger VC, Ip M, Yang M, Phan TG. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients With Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology. 2017;153:723–31.e1. [DOI] [PubMed] [Google Scholar]
- 53.Fairbrass KM, Selinger CP, Gracie DJ, Ford AC. Prevalence and impact of Rome IV versus Rome III irritable bowel syndrome in patients with inflammatory bowel disease. Neurogastroenterology & Motility. 2022;34:e14256. [DOI] [PubMed] [Google Scholar]
- 54.Mehandru S, Colombel JF. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat Rev Gastroenterol Hepatol. 2021;18:83–84. [DOI] [PubMed] [Google Scholar]
- 55.Pearson AD, Eastham EJ, Laker MF, Craft AW, Nelson R. Intestinal permeability in children with Crohn’s disease and coeliac disease. Br Med J (Clin Res Ed). 1982;285:20–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. [DOI] [PubMed] [Google Scholar]
- 57.D’Incà R, Di Leo V, Corrao G, Martines D, D’Odorico A, Mestriner C et al. Intestinal permeability test as a predictor of clinical course in Crohn’s disease. Am J Gastroenterol. 1999;94:2956–2960. [DOI] [PubMed] [Google Scholar]
- 58.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–5. [DOI] [PubMed] [Google Scholar]
- 59.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrett KE. Claudin-2 pore causes leak that breaches the dam in intestinal inflammation. J Clin Invest. 2020;130:5100–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. [DOI] [PubMed] [Google Scholar]
- 62.van der Post S, Jabbar KS, Birchenough G, Arike L, Akhtar N, Sjovall H et al. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019;68:2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turpin W, Lee SH, Raygoza Garay JA, Madsen KL, Meddings JB, Bedrani L et al. Increased Intestinal Permeability Is Associated With Later Development of Crohn’s Disease. Gastroenterology. 2020;159:2092–100.e5. [DOI] [PubMed] [Google Scholar]
- 64.Leibovitzh H, Lee SH, Xue M, Raygoza Garay JA, Hernandez-Rocha C, Madsen KL, et al. Altered Gut Microbiome Composition and Function Are Associated With Gut Barrier Dysfunction in Healthy Relatives of Patients With Crohn’s Disease. Gastroenterology. 2022. [DOI] [PubMed] [Google Scholar]
- 65.Cheng Y, Hall TR, Xu X, Yung I, Souza D, Zheng J, et al. Targeting uPA-uPAR interaction to improve intestinal epithelial barrier integrity in inflammatory bowel disease. eBioMedicine. 2022;75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR 2nd et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo L, Kuo WT, Turner JR. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell Mol Gastroenterol Hepatol. 2020;10:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCole DF. Finding a mate for MLCK: improving the potential for therapeutic targeting of gut permeability. Gut. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuo L, Kuo WT, Cao F, Chanez-Paredes SD, Zeve D, Mannam P, et al. Tacrolimus-binding protein FKBP8 directs myosin light chain kinase-dependent barrier regulation and is a potential therapeutic target in Crohn’s disease. Gut. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He WQ, Wang J, Sheng JY, Zha JM, Graham WV, Turner JR. Contributions of Myosin Light Chain Kinase to Regulation of Epithelial Paracellular Permeability and Mucosal Homeostasis. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue JA et al. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- 72.Herfarth H, Schölmerich J. IL-10 therapy in Crohn’s disease: at the crossroads. Treatment of Crohn’s disease with the anti-inflammatory cytokine interleukin 10. Gut. 2002;50:146–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G829–33. [DOI] [PubMed] [Google Scholar]
- 74.Tilg H, van Montfrans C, van den Ende A, Kaser A, van Deventer SJ, Schreiber S et al. Treatment of Crohn’s disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut. 2002;50:191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M, Oshima T, Ito C, Yamada M, Tomita T, Fukui H et al. Glutamine Blocks Interleukin-13-Induced Intestinal Epithelial Barrier Dysfunction. Digestion. 2021;102:170–179. [DOI] [PubMed] [Google Scholar]
- 76.Keir ME, Yi T, Lu TT, Ghilardi N. The role of IL-22 in intestinal health and disease. Journal of Experimental Medicine. 2020;217(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He C, Chen Z, Huang J, Gan R, Wang J, Wang L et al. Interleukin-22 Ameliorates Dextran Sulfate Sodium-Induced Colitis through the Upregulation of lncRNA-UCL to Accelerate Claudin-1 Expression via Sequestering miR-568 in Mice. Oxid Med Cell Longev. 2022;2022:8543720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner J-E, Stockinger B, Helmby H. IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection. PLOS Pathogens. 2013;9:e1003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rothenberg ME, Wang Y, Lekkerkerker A, Danilenko DM, Maciuca R, Erickson R et al. Randomized Phase I Healthy Volunteer Study of UTTR1147A (IL-22Fc): A Potential Therapy for Epithelial Injury. Clinical Pharmacology & Therapeutics. 2019;105:177–189. [DOI] [PubMed] [Google Scholar]
- 81.Wagner F, Mansfield J, Geier C, Dash A, Wang Y, Li C, et al. P420 A randomised, observer-blinded phase Ib multiple, ascending dose study of UTTR1147A, an IL-22Fc fusion protein, in healthy volunteers and ulcerative colitis patients. Journal of Crohn’s and Colitis. 2020;14:S382–S3. [Google Scholar]
- 82.Rosenstiel P, Fantini M, Bräutigam K, Kühbacher T, Waetzig GH, Seegert D et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–1009. [DOI] [PubMed] [Google Scholar]
- 83.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. Faseb j. 2005;19:923–933. [DOI] [PubMed] [Google Scholar]
- 84.Marchelletta RR, Krishnan M, Spalinger MR, Placone TW, Alvarez R, Sayoc-Becerra A, et al. T cell protein tyrosine phosphatase protects intestinal barrier function by restricting epithelial tight junction remodeling. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sweat YY, Turner JR. PTPN2 mutations cause epithelium-intrinsic barrier loss that synergizes with mucosal immune hyperactivation. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Himmel ME, Yao Y, Orban PC, Steiner TS, Levings MK. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology. 2012;136:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao J, Lu Q, Liu Y, Shi Z, Hu L, Zeng Z et al. Th17 Cells in Inflammatory Bowel Disease: Cytokines, Plasticity, and Therapies. J Immunol Res. 2021;2021:8816041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Y, Shen J. Innate Lymphoid Cells in Crohn’s Disease. Front Immunol. 2020;11:554880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fortea M, Albert-Bayo M, Abril-Gil M, Ganda Mall JP, Serra-Ruiz X, Henao-Paez A et al. Present and Future Therapeutic Approaches to Barrier Dysfunction. Front Nutr. 2021;8:718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Effects of dietary components on intestinal permeability in health and disease. 2020. [DOI] [PMC free article] [PubMed]
- 91.Khoshbin K, Camilleri M. Effects of dietary components on intestinal permeability in health and disease. Am J Physiol Gastrointest Liver Physiol. 2020;319:G589–g608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell. 2015;26:2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vancamelbeke M, Laeremans T, Vanhove W, Arnauts K, Ramalho AS, Farré R et al. Butyrate Does Not Protect Against Inflammation-induced Loss of Epithelial Barrier Function and Cytokine Production in Primary Cell Monolayers From Patients With Ulcerative Colitis. J Crohns Colitis. 2019;13:1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.What to do about the leaky gut. 2021.
- 96.Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: Results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G et al. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion. 2008;77:57–64. [DOI] [PubMed] [Google Scholar]
- 98.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao R, Long X, Yang J, Du L, Zhang X, Li J et al. Pomegranate peel polyphenols reduce chronic low-grade inflammatory responses by modulating gut microbiota and decreasing colonic tissue damage in rats fed a high-fat diet. Food Funct. 2019;10:8273–8285. [DOI] [PubMed] [Google Scholar]
- 100.Benjamin J, Makharia G, Ahuja V, Anand Rajan KD, Kalaivani M, Gupta SD et al. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: a randomized controlled trial. Dig Dis Sci. 2012;57:1000–1012. [DOI] [PubMed] [Google Scholar]
- 101.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. [DOI] [PubMed] [Google Scholar]
- 102.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. [DOI] [PubMed] [Google Scholar]
- 103.Fischer A, Gluth M, Pape UF, Wiedenmann B, Theuring F, Baumgart DC. Adalimumab prevents barrier dysfunction and antagonizes distinct effects of TNF-α on tight junction proteins and signaling pathways in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;304:G970–G979. [DOI] [PubMed] [Google Scholar]
- 104.Yakymenko O, Schoultz I, Gullberg E, Ström M, Almer S, Wallon C, et al. Infliximab restores colonic barrier to adherent-invasive E. coli in Crohn’s disease via effects on epithelial lipid rafts. Scand J Gastroenterol. 2018;53:677–84. [DOI] [PubMed] [Google Scholar]
- 105.Noth R, Stüber E, Häsler R, Nikolaus S, Kühbacher T, Hampe J et al. Anti-TNF-α antibodies improve intestinal barrier function in Crohn’s disease. J Crohns Colitis. 2012;6:464–469. [DOI] [PubMed] [Google Scholar]
- 106.Toedter G, Li K, Sague S, Ma K, Marano C, Macoritto M et al. Genes associated with intestinal permeability in ulcerative colitis: changes in expression following infliximab therapy. Inflamm Bowel Dis. 2012;18:1399–1410. [DOI] [PubMed] [Google Scholar]
- 107.Stremmel W, Merle U, Zahn A, Autschbach F, Hinz U, Ehehalt R. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005;54:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karner M, Kocjan A, Stein J, Schreiber S, von Boyen G, Uebel P et al. First multicenter study of modified release phosphatidylcholine “LT-02” in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses. Am J Gastroenterol. 2014;109:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalla R, Ventham NT, Kennedy NA, Quintana JF, Nimmo ER, Buck AH et al. MicroRNAs: new players in IBD. Gut. 2015;64:504–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiao L, Ma XX, Luo J, Chung HK, Kwon MS, Yu TX et al. Circular RNA CircHIPK3 Promotes Homeostasis of the Intestinal Epithelium by Reducing MicroRNA 29b Function. Gastroenterology. 2021;161:1303–17.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16:457–470. [DOI] [PubMed] [Google Scholar]
- 113.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3:e982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017;117:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schroeder BO, Birchenough GMH, Ståhlman M, Arike L, Johansson MEV, Hansson GC et al. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe. 2018;23:27–40.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahl D, Liu H, Schreiber O, Roos S, Phillipson M, Holm L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol (Oxf). 2016;217:300–310. [DOI] [PubMed] [Google Scholar]
- 118.Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. [DOI] [PubMed] [Google Scholar]
- 120.Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown GR, Lindberg G, Meddings J, Silva M, Beutler B, Thiele D. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999;116:593–601. [DOI] [PubMed] [Google Scholar]
- 123.van der Hee B, Wells JM. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021;29:700–712. [DOI] [PubMed] [Google Scholar]
- 124.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mayorgas A, Dotti I, Salas A. Microbial Metabolites, Postbiotics, and Intestinal Epithelial Function. Mol Nutr Food Res. 2021;65:e2000188. [DOI] [PubMed] [Google Scholar]
- 126.Fiorucci S, Carino A, Baldoni M, Santucci L, Costanzi E, Graziosi L et al. Bile Acid Signaling in Inflammatory Bowel Diseases. Dig Dis Sci. 2021;66:674–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am J Pathol. 2018;188:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this review are available on request from the corresponding author. The data are not publically available due to privacy or ethical restrictions.


