Abstract
Representational difference analysis (RDA) is a recently developed technique used for amplifying genetic differences between two closely related genomes. We compared RDA and a modified version of RDA to examine genomic differences between the two Vibrio cholerae serogroups that cause epidemic cholera, O1 and O139, and between the two biotypes of the O1 serogroup. With both techniques, we recovered several sequences known to be found only in V. cholerae O139 but absent in its presumed progenitor, V. cholerae O1 El Tor. A greater number of unique fragments were generated in comparing the two V. cholerae O1 biotypes, consistent with the probable greater genetic differences between the two biotypes.
Epidemic cholera is caused by Vibrio cholerae serogroup O1 and a single other serogroup, V. cholerae O139, which emerged in 1992. V. cholerae O1 comprises two distinct biotypes, classical and El Tor, which differ in several biochemical traits. Data from many investigators suggests that V. cholerae O139 is likely to have derived from a V. cholerae O1 El Tor organism which underwent a recombinational event resulting in the substitution of the cluster of genes encoding the O139 serogroup antigen for the cluster of genes encoding the O1 serogroup antigen (1, 2, 4, 5, 11–13). In addition to changes in the cell surface structure of V. cholerae O139, two potentially mobile genetic elements have been found in this organism that are not present in V. cholerae O1 of the El Tor biotype. Waldor et al. (14) have described the presence in V. cholerae O139 of a conjugative transposon-like transmissible element that mediates resistance to trimethoprim, sulfamethoxazole, and streptomycin. A κ-type vibriophage is also present in V. cholerae O139, the K139 phage, and encodes a protein related to G proteins (9). It is unclear how many separate genetic events underlie the evolution of V. cholerae O139 from V. cholerae O1 of the El Tor biotype, but it is postulated that the organism arose by stepwise acquisition and loss of distinct regions of the chromosome.
The presence of chromosomal “pathogenicity islands” in several bacterial pathogens suggests that evolution of bacteria may commonly involve horizontal acquisition of substantial blocks of chromosomal DNA encoding a series of related gene products which convey a new set of properties on the recipient (6). A variety of techniques have been developed to detect regions of DNA that differ between two closely related genomes and that may be involved in genomic evolution. One such method, representational difference analysis (RDA), was developed as a tool in eukaryotic organisms to identify genetic polymorphisms in human neoplasia (7, 8). This technique has also been used to demonstrate the presence of human herpesvirus 8 in the tissues of patients with Kaposi’s sarcoma (3). Recently, a procedure related to RDA was used to detect and clone genomic differences between two closely related bacterial species, Neisseria meningitidis and N. gonorrhoeae (10). We were interested in applying the technique of RDA to examining genomic evolution in pathogenic strains of V. cholerae.
Bacterial strains were grown with antibiotics as needed (ampicillin, 100 μg/ml; streptomycin, 100 μg/ml; trimethoprim, 32 μg/ml; sulfamethoxazole, 160 μg/ml). V. cholerae O1 strain O395 of the classical biotype and strain C6709 of the El Tor biotype, as well as V. cholerae O139 strain MO10 have been previously described (2). K139 phage was obtained from V. cholerae MO10 and purified on the nonlysogenic strain C6709 as described by Reidl and Mekalanos (9). Cosmid pO139, which carries a large insert that comprises nearly all of the O139 capsular biosynthetic region, has been previously described (14). Cosmids pSXT1, pSXT3, and pSXT7, which contain overlapping regions of the transmissible element conferring resistance to trimethoprim, sulfamethoxazole, and streptomycin from V. cholerae MO10 cloned into the vector pSuperCos1 (Stratagene, La Jolla, Calif.), have been previously described (14).
The protocol of Lisitsyn et al. was used to examine differences between several V. cholerae strains (7). In RDA, the organism in which unique sequences are being sought is designated the tester and the organism whose genome is being subtracted from the tester is referred to as the driver. In the standard RDA approach, chromosomal DNAs of both tester and driver strains were digested to completion with BglII. Following phenol-chloroform extraction, fragments of chromosomal DNA were precipitated in ethanol in the presence of a large excess of yeast tRNA. Three sets of complementary oligonucleotide pairs, designated RBgl, JBgl, and NBgl, are available for RDA commercially from Operon Technologies, Inc. (Alameda, Calif.); each of these pairs consists of a 24-mer and a 12-mer which, when annealed, generate a BglII-compatible overhang. The RDA procedure begins with generation of a “representation” of each genome being compared, using PCR to amplify BglII-digested fragments of chromosomal DNA from each strain. To make this representation, the pair of RBgl oligonucleotides were ligated onto the ends of BglII-digested chromosomal DNA fragments, such that only the 24-mer was covalently attached to the chromosomal fragment. After an initial extension reaction at 72°C for 5 min to fill in the complementary strand to the ligated 24-mer, PCR was used to amplify these fragments with the 24-mer oligonucleotide as primer. Following amplification, the RBgl oligonucleotides were digested off of the amplified fragments with BglII and the fragments from the tester strain were gel purified on NuSieve low-melting-point agarose (FMC Bioproducts, Rockland, Maine). The second set of oligonucleotides, JBgl, was then ligated onto the purified fragments of tester DNA as above. Fragments of tester DNA were mixed with a 100-fold excess of DNA fragments from the driver strain, extracted with phenol-chloroform, precipitated in ethanol, and resuspended in a very small volume of 3× EPPS (N-[2-hydroxyethyl][piperazine-N′-[3-propanesulfonic acid]])–EDTA at pH 8.0. The mixture of DNA fragments was denatured in a PCR machine in a thick-wall PCR tube, NaCl was added to a final concentration of 1 M, and the DNA was allowed to anneal at 67°C for 20 h. The DNA was diluted with Tris-EDTA, and 10 rounds of subtractive/kinetic enrichment PCR were performed with the JBgl 24-mer as the PCR primer. Exponential amplification occurs only with reannealed fragments of tester DNA, which contain the 24-mer JBgl sequence at either end. Single-stranded pieces of tester DNA which anneal with a complementary strand from the driver population contain only the 24-mer JBgl sequence at one end and amplify arithmetically, and reannealed driver sequences fail to amplify. After 10 rounds of PCR, remaining single-stranded template was eliminated by treatment with mung bean nuclease (New England Biolabs, Beverly, Mass.) and 20 more cycles of PCR were performed to complete the amplification. The JBgl oligonucleotide ends of these “difference products” were digested off with BglII, and the fragments were purified as above. The third pair of oligonucleotides, NBgl, was ligated onto the pool of amplified difference product fragments, and this pool was mixed with a large excess of driver DNA fragments. A second round of subtractive/kinetic enrichment PCR was carried out, with the NBgl 24-mer oligonucleotide as a primer. For the third and final round of subtractive/kinetic enrichment, the NBgl pair of oligonucleotides was digested off, the JBgl oligonucleotide pair was ligated back onto the tester DNA fragments, and subtractive/kinetic enrichment PCR was repeated. Fragments generated after three successive rounds of RDA were ligated into the PCR cloning vector pGEM-T (Promega, Inc., Madison, Wis.) either directly from the PCR mixture or after isolation and purification of the fragments using low-melting-temperature agarose. These inserts were recovered from individual plasmids by restriction endonuclease digestion with BglII and used as probes in Southern hybridizations.
We also utilized a modification of the RDA procedure, partially based on a protocol recently described by Tinsley and Nassif (10). In this procedure, 2-μg aliquots of tester DNA were digested to completion with Sau3A (New England Biolabs) to generate a pool of smaller fragments than those generated by BglII digestion. These fragments were extracted with phenol-chloroform and precipitated in isopropanol with tRNA as a carrier. Chromosomal DNA from the driver strain, at a concentration of 0.1 μg/μl, was sheared mechanically in 800-μl aliquots by 60 passes through a 30-gauge needle to generate fragments in the 3- to 4-kbp range. Sau3A-digested tester DNA was ligated directly to the second pair of oligonucleotides, JBgl, used in the RDA protocol above. Ligated tester DNA and sheared driver DNA were mixed together with approximately a 100:1 excess of driver DNA relative to tester DNA, precipitated, and annealed as above. Subtractive/kinetic enrichment PCR was performed with the JBgl 24-mer oligonucleotide as a primer. Following amplification, the JBgl oligonucleotides were digested off the first-round difference products with Sau3A and the pair of NBgl oligonucleotides was ligated on as above. This pool of amplified fragments was again annealed to an excess of driver DNA, and subtractive/kinetic enrichment was performed a second time, with mung bean nuclease treatment as described above. Following digestion of the NBgl oligonucleotides from the amplified products, a third round of RDA was performed. These third-round difference products were visualized by gel electrophoresis and were radiolabeled en masse and used as probe in Southern hybridization experiments to ensure that the amplified fragments were unique to the tester DNA and not present in the driver strain.
We initially compared V. cholerae O139 isolate MO10 as tester and V. cholerae O1 El Tor strain C6709 as driver, utilizing the standard RDA procedure. Following three rounds of subtractive/kinetic enrichment, agarose gel electrophoresis of the amplified difference products revealed a small number of DNA fragments between 500 and 1,000 bp, with two bands in the 700- to 800-bp range present in greatest abundance. These two fragments were gel purified and used individually as probes in Southern hybridization experiments to confirm that they were unique to the tester strain MO10 and not present in the driver C6709 (Fig. 1). In addition, neither probe cross-hybridized with the other or with a panel of chromosomal DNAs from other non-O1 V. cholerae strains, several noncholera Vibrio species, including V. fluvialis, V. vulnificus, V. parahaemolyticus, V. alginolyticus, and V. mimicus, or Aeromonas hydrophila (data not shown).
FIG. 1.
Southern blot analysis of BglII-digested DNA. Chromosomal DNA is from V. cholerae O139 strain MO10 (tester) and V. cholerae O1 El Tor strain C6709 (driver). Cosmids are derived from the commercial vector pSuperCos1. Phage DNA is from the O139-specific K139 vibriophage. The blot on the left was probed with the RDA-generated 750-bp BglII fragment unique to MO10. The blot on the right was probed with the 720-bp RDA-generated fragment unique to MO10. Molecular weight markers are indicated in kilobase pairs at the right of each blot.
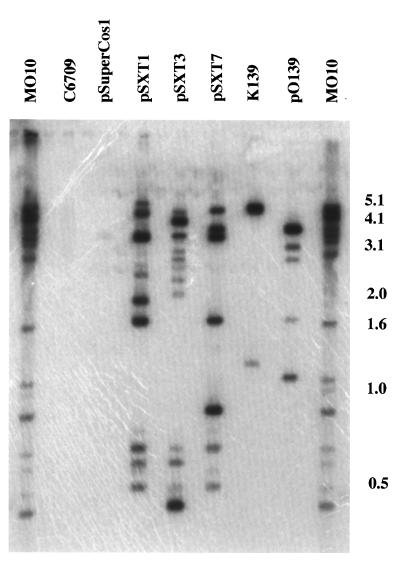
We utilized Southern hybridization to determine whether the two major difference products from the RDA protocol were homologous to one or more of the three previously described regions unique to V. cholerae O139. Neither fragment hybridized to DNA from bacteriophage K139 or to the cosmid pO139 containing nearly all of the capsular biosynthetic region (Fig. 1). Both of the recovered difference products did hybridize with cosmids containing overlapping regions of the transmissible genetic element conferring resistance to trimethoprim, sulfamethoxazole, and streptomycin in V. cholerae O139 (Fig. 1). The high-molecular-weight band in pSXT1 hybridizing with the 750-bp fragment suggested hybridization to a junctional fragment between the cosmid vector and the cloned insert.
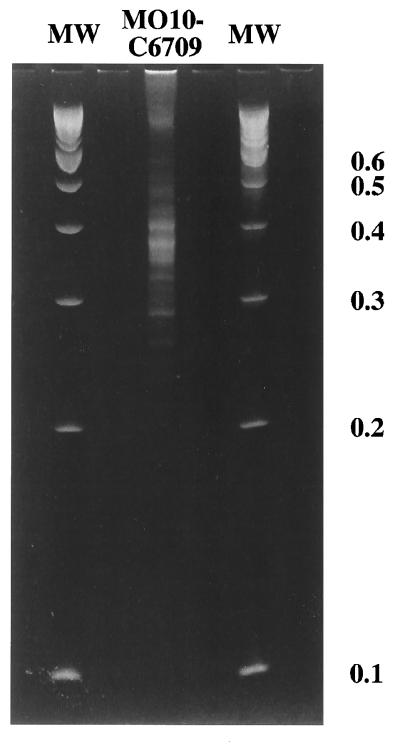
Since the standard RDA protocol produced only a small number of genomic differences between V. cholerae O139 and El Tor O1 strains, we examined a modification of the RDA protocol, using complete Sau3A digestion of tester DNA and shearing of driver DNA. Following three rounds of subtractive/kinetic enrichment, this modification of the procedure yielded a much larger number of difference products, mostly in the range of 300 to 500 bp (Fig. 2). When this pool of fragments was radiolabeled and used as a probe in Southern hybridization, each of the fragments was unique to strain MO10 and not present in strain C6709 (Fig. 3). This pool of difference products also hybridized with all three of the previously described genomic differences between strain MO10 and strain C6709, including the K139 bacteriophage, the cosmid encoding the O139 capsular biosynthetic region, and the pSXT cosmids. The majority of the fragments that were unique to strain MO10 hybridized to one of the three previously described genomic differences, suggesting that there are few, if any, additional genomic differences between these two strains (Fig. 3).
FIG. 2.
Ethidium bromide-stained 6% polyacrylamide gel of amplified difference products from the Sau3A variant of RDA, with V. cholerae O139 strain MO10 used as tester and V. cholerae O1 El Tor strain C6709 used as driver (MO10-C6709). Molecular weight (MW) marker sizes are indicated on the right in kilobase pairs.
FIG. 3.
Southern blot analysis of chromosomal, phage, and cosmid DNAs digested with BglII and probed with pooled radiolabeled fragments of the Sau3A variant RDA third-round difference product of O139 strain MO10 and O1 El Tor strain C6709. Molecular weight markers are indicated in kilobase pairs on the right.
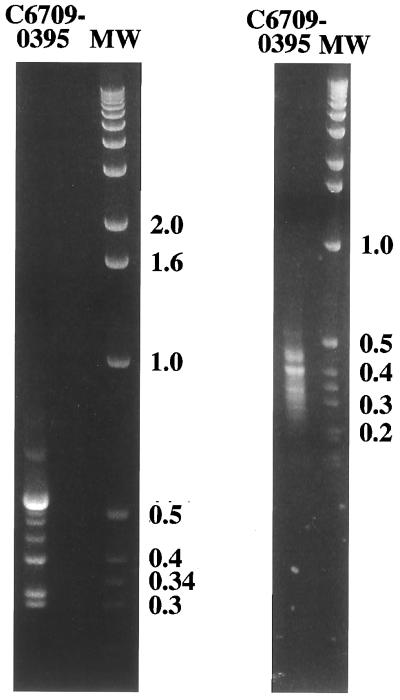
We next utilized the two RDA procedures to compare genomic differences between two V. cholerae O1 strains that may be more distantly related, the classical-biotype strain O395 and the El Tor biotype strain C6709. For both of the RDA procedures, strain C6709 was used as tester and strain O395 was used as driver. The standard RDA protocol generated at least eight distinct bands in the 300- to 700-bp range as difference products between the El Tor and classical biotype (Fig. 4). The modified RDA procedure, utilizing Sau3A digestion, yielded a much larger number of difference products, in the size range of 250 to 500 bp (Fig. 4). Each of these pools of difference products was radiolabeled and used as a probe to demonstrate that they were indeed unique to tester chromosomal DNA and not found in the driver strain (data not shown).
FIG. 4.
Ethidium bromide-stained 2% agarose gels of difference products between V. cholerae O1 El Tor C6709 and V. cholerae O1 classical O395. The gel on the left shows the difference products after three rounds of standard RDA; the gel on the right shows difference products after three rounds of the Sau3A modification. Molecular weight (MW) marker sizes are indicated in kilobase pairs.
We have shown that two versions of RDA can be used to generate a pool of DNA difference products which are unique to one of a pair of similar organisms. The BglII-based procedure, which utilizes a 6-bp cutter and a representation of the chromosome prior to the subtractive/kinetic enrichment, generates a simpler and somewhat more restricted subset of all possible unique fragments. However, these fragments are easily recovered by gel electrophoresis and can be used as probes to identify potential chromosomal pathogenicity islands. The modification of RDA utilizing Sau3A digestion yields a larger number of smaller fragments. As shown in Fig. 3, this approach identified DNA sequences that constitute parts of all of the three previously described genomic differences between strain O139 and El Tor O1 strains, suggesting that this approach may yield a more complete set of difference products for analyzing genomic differences between two closely related strains. RDA between V. cholerae O1 of the two different biotypes yielded a substantially larger number of difference products than the comparison between V. cholerae O1 of the El Tor biotype and V. cholerae O139. This suggests that the two biotypes of V. cholerae O1 have a larger number of genomic differences than does the El Tor biotype compared with V. cholerae O139. Several of these difference products are currently being isolated for use as probes to recover the genomic differences which define the different biotypes of V. cholerae O1.
Acknowledgments
We thank the following individuals for helpful discussions and suggestions: Brian Seed, Ramnik Xavier, Yuan Chang, Joseph Hendrick, Edward Ryan, Jonathan Bogan, Nikolai Lisitsyn, Xavier Nassif, and Brendan Cormack.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (NIAID) (AI34968, to S.B.C.) and a grant from Procter and Gamble under the University Exploratory Research Program. K.E.C. was supported by a National Research Service Award from NIAID and a Searle Scholar’s Fellowship through the Infectious Diseases Society of America.
REFERENCES
- 1.Bik E M, Bunschoten A E, Willems R J L, Chang A C Y, Mooi F R. Genetic organization and functional analysis of the otn DNA essential for cell-wall polysaccharide synthesis in Vibrio cholerae O139. Mol Microbiol. 1996;20:799–811. doi: 10.1111/j.1365-2958.1996.tb02518.x. [DOI] [PubMed] [Google Scholar]
- 2.Calia K E, Murtagh M, Ferraro M J, Calderwood S B. Comparison of Vibrio cholerae O139 with Vibrio cholerae O1 classical and El Tor biotypes. Infect Immun. 1994;62:1504–1506. doi: 10.1128/iai.62.4.1504-1506.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 4.Comstock L E, Johnson J A, Michalski J M, Morris J G J, Kaper J B. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol. 1996;19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x. [DOI] [PubMed] [Google Scholar]
- 5.Comstock L E, Maneval D J, Panigrahi P, Joseph A, Levine M M, Kaper J B, Morris J G J, Johnson J A. The capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in Vibrio cholerae O1. Infect Immun. 1995;63:317–323. doi: 10.1128/iai.63.1.317-323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacker J, Blum-Oehler G, Mühldorfer I, Tschäpe H. Pathogenicity islands of virulent bacteria: structure, function, and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 7.Lisitsyn N, Lisitsyn N, Wigler M. Cloning the differences between two complex genomes. Science. 1993;259:946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- 8.Lisitsyn N, Wigler M. Representational difference analysis in detection of genetic lesions in cancer. Methods Enzymol. 1995;254:291–304. doi: 10.1016/0076-6879(95)54021-0. [DOI] [PubMed] [Google Scholar]
- 9.Reidl J, Mekalanos J J. Characterization of Vibrio cholerae bacteriophage K139 and use of a novel mini-transposon to identify a phage-encoded virulence factor. Mol Microbiol. 1995;18:685–701. doi: 10.1111/j.1365-2958.1995.mmi_18040685.x. [DOI] [PubMed] [Google Scholar]
- 10.Tinsley C R, Nassif X. Analysis of the genetic differences between Neisseria meningitidis and Neisseria gonorrhoeae: two closely related bacteria expressing two different pathogenicities. Proc Natl Acad Sci USA. 1996;91:11109–11114. doi: 10.1073/pnas.93.20.11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldor M K, Colwell R R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldor M K, Mekalanos J J. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 13.Waldor M K, Mekalanos J J. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect Immun. 1994;62:72–78. doi: 10.1128/iai.62.1.72-78.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldor M K, Tschape H K, Mekalanos J J. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J Bacteriol. 1996;178:4157–4165. doi: 10.1128/jb.178.14.4157-4165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]