Abstract
In Saccharomyces cerevisiae, a single homolog of the tRNA methyltransferase Trm10 performs m1G9 modification on 13 different tRNAs. Here we provide evidence that the m1G9 modification catalyzed by S. cerevisiae Trm10 plays a biologically important role for one of these tRNA substrates, tRNATrp. Overexpression of tRNATrp (and not any of 38 other elongator tRNAs) rescues growth hypersensitivity of the trm10Δ strain in the presence of the antitumor drug 5-fluorouracil (5FU). Mature tRNATrp is depleted in trm10Δ cells, and its levels are further decreased upon growth in 5FU, while another Trm10 substrate (tRNAGly) is not affected under these conditions. Thus, m1G9 in S. cerevisiae is another example of a tRNA modification that is present on multiple tRNAs but is only essential for the biological function of one of those species. In addition to the effects of m1G9 on mature tRNATrp, precursor tRNATrp species accumulate in the same strains, an effect that is due to at least two distinct mechanisms. The levels of mature tRNATrp are rescued in the trm10Δmet22Δ strain, consistent with the known role of Met22 in tRNA quality control, where deletion of met22 causes inhibition of 5′–3′ exonucleases that catalyze tRNA decay. However, none of the known Met22-associated exonucleases appear to be responsible for the decay of hypomodified tRNATrp, based on the inability of mutants of each enzyme to rescue the growth of the trm10Δ strain in the presence of 5FU. Thus, the surveillance of tRNATrp appears to constitute a distinct tRNA quality control pathway in S. cerevisiae.
Keywords: tRNA modification, m1G9, 5-fluorouracil, rapid tRNA decay, tRNATrp
INTRODUCTION
Transfer ribonucleic acid (tRNA) molecules are the most highly modified RNA molecules in the cell. Cytoplasmic tRNAs in Saccharomyces cerevisiae have known modifications at 36 of the ∼75 nt positions and each tRNA has an average of 12.6 modifications (Juhling et al. 2009; Machnicka et al. 2013; Phizicky and Hopper 2023). tRNA modifications to the anticodon stem–loop (ACL) are often performed by genes that, when deleted, exhibit translation-related phenotypic defects due to their important roles in the efficiency or fidelity of translation (Phizicky and Hopper 2023). tRNA modifications found in the body of the tRNA, however, are performed by enzymes that often display mild to no obvious phenotypes when their genes are deleted in model systems such as S. cerevisiae. Recently, many of these genes have been implicated in more subtle roles that are often specific to unique tRNA species (Phizicky and Alfonzo 2010; Jackman and Alfonzo 2013; Howell and Jackman 2019; Phizicky and Hopper 2023). In cases studied so far, tRNA body modifications are generally thought to improve overall tRNA folding and stability and thus aid the tRNA in evading degradation by quality control pathways that remove low-quality tRNA from the pool of molecules available for translation (Whipple et al. 2011; Hopper and Huang 2015; Phizicky and Hopper 2023).
Multiple tRNA decay pathways in S. cerevisiae act to degrade specific tRNA species upon the loss of certain tRNA modifications or in the case of aberrant processing (Kadaba et al. 2004; Alexandrov et al. 2006; Hopper and Huang 2015; Payea et al. 2020; Tasak and Phizicky 2022). If the tRNA does not maintain its correct structure, including due to improper modification, or is mislocalized in the nucleus due to improper processing or trafficking, the tRNA is probably not sufficiently fit for its role in translation, and its removal helps to maintain the overall efficiency of protein synthesis. The tRNA quality control pathways so far identified in S. cerevisiae include: (i) the rapid tRNA decay (RTD) pathway that acts on mature hypomodified tRNA in both the nucleus and cytoplasm (Alexandrov et al. 2006; Chernyakov et al. 2008), (ii) the Met22-dependent decay pathway (MPD) that targets pre-tRNA with an aberrant intron–exon junction (Payea et al. 2020), (iii) the retrograde import pathway that targets improperly processed or modified tRNA in the nucleus (Kramer and Hopper 2013; Chatterjee et al. 2022) and (iv) the nuclear TRAMP complex that acts on aberrant initiator tRNAiMet (Kadaba et al. 2004). Interestingly, RTD was also recently shown to contribute together with the TRAMP pathway to the surveillance of initiator tRNA (Tasak and Phizicky 2022). In all cases where RTD has been identified in connection with the loss of a tRNA-modifying enzyme to date, only a subset of the tRNAs normally modified by the enzyme are substantially degraded upon the loss of the modification (Alexandrov et al. 2006; Kotelawala et al. 2008; Han et al. 2015). The stability of each tRNA species varies and each tRNA requires different modifications to differing degrees, which appears to be sensed distinctly by the exonucleases associated with each degradation pathway.
The tRNA methyltransferase 10 (Trm10) was first identified in S. cerevisiae as an m1G9 methyltransferase but is conserved in Archaea and Eukarya (Jackman et al. 2003). Trm10 modifies the core of tRNA and homologs from some species have been identified that act as m1G9 and/or m1A9 methyltransferases, despite all of these enzymes sharing a similar protein fold as members of the SPOUT superfamily (Kempenaers et al. 2010; Vilardo et al. 2012, 2020; Howell et al. 2019; Strassler et al. 2022). Trm10 has three paralogs in humans, with TRMT10A acting as an m1G9 methyltransferase on multiple tRNAs and TRMT10B performing m1A9 on only tRNAAsp, while TRMT10C is a bifunctional m1A9/m1G9 methyltransferase that functions in human mitochondria as part of an unusual protein-only RNaseP complex (Holzmann et al. 2008; Vilardo et al. 2012, 2020; Howell et al. 2019). tRNA modification has proved increasingly important to human health (Torres et al. 2014; Suzuki 2021). Nine different homozygous loss-of-function mutations in TRMT10A have been identified in patients that are associated with a syndrome that primarily manifests in metabolic and neurological disorders in the affected patients (Igoillo-Esteve et al. 2013; Gillis et al. 2014; Narayanan et al. 2015; Zung et al. 2015; Yew et al. 2016; Lin et al. 2020; Stern et al. 2022; Siklar et al. 2023). Interestingly, for one of these patients, having a premature stop codon mutation of TRMT10A leads human TRMT10A substrate tRNAGln lacking m1G9 to become susceptible to tRNA fragmentation (Igoillo-Esteve et al. 2013; Cosentino et al. 2018). The accumulation of 5′-tRFs derived from tRNAGln, while 3′-tRF levels remained largely the same in patient and control cells, suggests a role for these fragments in human disease. Nonetheless, while the presence of the m1G9 modification is clearly implicated in human health, the molecular basis for the specific disorders experienced by humans with disease-associated Trm10 mutations and how human tRNAs are impacted by the lack of m1G9 are not fully understood. A better understanding of this enzyme can help in identifying the molecular basis for the human health impacts of Trm10 deficiency and may also reveal principles that are applicable to other tRNA modification-related diseases.
In S. cerevisiae, where the activity of Trm10 was first characterized, there is only one homolog of Trm10, and this enzyme performs m1G9 modification on 13 of the 24 G9-containing tRNA species whose modification status is known (three G9-containing tRNA species remain uncharacterized in terms of modifications to date) (Jackman et al. 2003; Swinehart et al. 2013). TRM10 in S. cerevisiae is not an essential gene, but deletion of trm10 causes growth hypersensitivity to low concentrations of the antitumor drug 5-fluorouracil (5FU), which was revealed by a screen of the yeast deletion collection for strains that exhibit hypersensitivity to growth in the presence of 5FU (Gustavsson and Ronne 2008). Interestingly, in the genome-wide survey, the most 5FU sensitive strains were a group of rRNA- and tRNA-modifying enzyme gene deletions, adding to evidence suggesting that the effect of 5FU on structure and/or function of noncoding RNAs may be associated with the toxicity of this commonly used drug for treatment of solid cancer tumors (Hoskins and Butler 2008; Bash-Imam et al. 2017; Ge et al. 2017). 5FU is known to be directly incorporated into RNA, but also inhibits pseudouridine modification, which is normally abundant and functionally important in tRNA and other RNA molecules (Hoskins and Butler 2008; Borchardt et al. 2020). Thus, the loss of pseudouridine is likely to account for some of the growth sensitivity to the presence of the drug. However, only a subset of tRNA modification enzymes were identified to have the 5FU hypersensitive phenotype when deleted, suggesting that hypersensitivity is not due to a global effect on the tRNA pool, but rather that there may be specifically targeted tRNA species or structures that are uniquely sensitive to the presence of 5FU in the context of some modification enzyme deletions (Gustavsson and Ronne 2008). Among the 5FU hypersensitive deletion strains, the trm10Δ strain exhibited the most severe growth defect in the presence of 5FU, suggesting that the presence of Trm10 must be important for withstanding 5FU toxicity. But, the impact of the Trm10 m1G9 modification on any of its substrate tRNAs in S. cerevisiae has not been demonstrated to date. Here, we sought to define the biological importance of the highly conserved m1G9 modification by taking advantage of the 5FU hypersensitive phenotype in S. cerevisiae.
In this study, we demonstrate that the hypersensitivity of the trm10Δ strain to 5FU is due to severely depleted levels of a single Trm10 substrate, tRNATrp. Moreover, levels of mature tRNATrp are significantly decreased in the trm10Δ strain even in the absence of 5FU, despite no obvious growth defect of the trm10Δ strain. We also observed that levels of pre-tRNATrp increase significantly upon deletion of trm10 compared to wild-type cells. We demonstrated that trm10Δ hypersensitivity to 5FU and decreased levels of hypomodified mature tRNATrp are rescued by the deletion of met22, an enzyme that has been previously associated with at least two tRNA quality control pathways (RTD and MPD). The double mutant trm10Δ met22Δ strain grows normally in the presence of 5FU, and its levels of mature tRNATrp are rescued to wild-type abundance. We sought to define the specific MET22-dependent pathway that was being used to degrade tRNATrp lacking the m1G9 modification by creating double mutants of trm10Δ with several known exonucleases, including XRN1, RAT1, and RRP6, yet surprisingly none of these rescued the 5FU growth hypersensitive phenotype. Taken together, our results suggest that unknown nuclease(s) associated with MET22 sense tRNATrp lacking the m1G9 modification in S. cerevisiae, providing insight into how the cell recognizes and accounts for aberrant tRNA, while providing another example of a tRNA-modifying enzyme that modifies numerous substrates in the cell but is only important for the function of one tRNA.
RESULTS
Overexpression of tRNATrp rescues growth hypersensitivity of trm10Δ to 5-fluorouracil
We took advantage of the strong 5FU hypersensitive phenotype of trm10Δ strains to study the significance of the m1G9 modification in vivo in S. cerevisiae. We used an approach that has been used to study other (mainly temperature-sensitive) phenotypes associated with loss of tRNA modifications, and individually overexpressed 39 different elongator tRNAs from high copy number (2μ) plasmids in the trm10Δ strain to determine whether they could complement the 5FU hypersensitive phenotype (Chernyakov et al. 2008; Han et al. 2015). These tRNAs include all 12 elongator tRNA substrates of Trm10, as well as 27 other A9 and G9-containing nonsubstrate tRNAs, with the idea that if the abundance of a specific tRNA is impacted by the loss of m1G9 modification, adding more of the affected tRNA (despite its lack of modification) back to the cell will rescue growth. Of the 12 elongator tRNAs that are substrates for Trm10, only overexpression of tRNATrp was able to successfully rescue growth of the trm10Δ strain in the presence of increasing concentrations of 5FU, including at the highest tested concentration (5 μg/mL) where there is no detectable growth of the trm10Δ cells (Fig. 1A). Four tRNAs (tRNAAsn(GUU), tRNACys(GCA), tRNAThr(AGU), and tRNAVal(UAC)) are not substrates for Trm10 in wild-type yeast, but are capable of being modified by purified Trm10 enzyme in vitro (Swinehart et al. 2013). However, none of these four tRNAs rescued the 5FU phenotype (Fig. 1A). As expected, overexpression of any of the G9-containing tRNAs that are unmodified by Trm10 had no detectable effect on the ability of the trm10Δ strain to grow in the presence of 5FU (Fig. 1A). As a control, a selected group of substrate and nonsubstrate tRNAs were overexpressed in the wild-type TRM10 background (Fig. 1B). Here, none of the overexpressed tRNAs (including tRNATrp) conferred any detectable growth advantage at the highest concentrations of 5FU tested. These results indicate that there is no general ability of individual tRNAs (including tRNATrp) to confer 5FU resistance when tRNAs contain the m1G9 modification. Likewise, no increased resistance to 5FU was observed upon overexpression of any A9-containing tRNAs, which are also not substrates for Trm10 modification in S. cerevisiae (Fig. 1C). Even though overexpressed tRNATrp remains unmodified in the trm10Δ background, we hypothesize that increased abundance of this tRNA is sufficient to rescue growth in the presence of 5FU, leading us to further examine whether the trm10Δ hypersensitivity to 5FU is caused by decreased tRNATrp levels that are insufficient for the cell.
FIGURE 1.
Overexpression of Trm10 substrate tRNATrp rescues the trm10Δ growth defect in 5FU. Strains were grown overnight in SD-Leu media, serially fourfold diluted from a starting OD600 of 1, plated on SD-Leu plates with indicated concentrations of 5FU, and incubated for 4 d at 30°C. (A) G9-containing tRNAs overexpressed in the trm10Δ strain. Whether the tRNA is modified in vivo or only by purified Trm10 in vitro is indicated on the left of each figure. The modification status of tRNAArg(CCU) has not been determined in S. cerevisiae. S. cerevisiae tRNALys(CUU) contains m2G9 and can be modified by Trm10 upon overexpression of the enzyme in vivo, but has not been tested further as a Trm10 substrate in vitro. (B) G9-containing tRNAs overexpressed in TRM10 cells. (C) A9-containing tRNAs overexpressed in trm10Δ cells.
Levels of mature tRNATrp are significantly depleted in trm10Δ strains
To determine whether the inability of the trm10Δ strain to grow in the presence of 5FU is correlated with the abundance of tRNATrp, we performed northern analysis using a probe targeting mature tRNA via its anticodon stem and D-loop sequences (Supplemental Fig. S1A), which lack other modifications that would predictably block primer binding. Interestingly, we observed significantly lower levels of mature tRNATrp in the trm10Δ strain compared to the isogenic TRM10 control, even in the absence of 5FU (Fig. 2A, compare lane 7 with lane 3, quantified in Fig. 2B). A representative blot of the same RNA species is shown at lower exposure using an alternative chemiluminescent detection strategy in Supplemental Figure S1B, which reveals the same trends. Therefore, even though there is not a detectable growth phenotype of the trm10Δ strain in the absence of 5FU, levels of mature tRNATrp are already significantly decreased compared to the wild-type strain. Consistent with the 5FU hypersensitivity of the trm10Δ strain, the added stress of growth in 5FU further depleted levels of mature tRNATrp in the trm10Δ strain to extremely low levels (<5% of the amount in the wild-type strain) (Fig. 2B). Presumably, the abundance of tRNATrp in trm10Δ cells grown in 5FU is not sufficient to sustain translation and therefore explains the loss in viability. In agreement with the rescued growth upon tRNATrp overexpression, mature tRNATrp levels increased in each of the tRNATrp overexpressing strains. Although the abundance of tRNATrp does not fully recover to wild-type levels in the trm10Δ background, the levels measured in the trm10Δ strain with tRNATrp overexpressed +5FU are similar to those observed in the trm10Δ strain −5FU where there is no growth phenotype (Fig. 2B). The inability of tRNATrp to reach wild-type levels in these strains is likely due to the fact that the tRNA remains unmodified, and therefore is still subject to quality control pathways that act to remove the incorrectly modified tRNA from the cells.
FIGURE 2.
Levels of tRNATrp decrease upon deletion of trm10 and are almost entirely depleted upon growth of this strain in 5FU. (A) Northern analysis of RNA derived from the indicated strains grown with and without 5FU. Mature tRNATrp, mature tRNAGly(GCC), and 5S rRNA levels were all detected using 5′ radiolabeled probes (Supplemental Table S2). A representative northern blot using chemiluminescent detection is shown in Supplemental Figure S1B that displays the same RNAs at a lower exposure for comparison. (B) Quantification of relative tRNA levels from strains shown in A. Relative tRNA levels were calculated by comparing the observed intensity for each tRNA to the normalized abundance observed in the TRM10 strain (lane 3) set to 1. Triplicate data were plotted with each data point shown; error bars denote standard deviation. Two sample t-tests assuming equal variances were performed, and one-tailed P-values were shown with red asterisks under black bars for comparison of tRNATrp levels or a black asterisk or n.s. (no significant difference) over white bars for comparison of tRNAGly(GCC) levels. Specific P-values from other t-test comparisons are shown in Supplemental Table S1.
As a control, we performed northern blots targeting another Trm10 substrate, tRNAGly(GCC) whose overexpression in the trm10Δ strain does not rescue 5FU growth hypersensitivity (Fig. 1A). As expected, quantification of the northern data did not reveal any dramatic changes in the abundance of tRNAGly(GCC) in any of the tested strains (Fig. 2B). We reproducibly observed a small, but statistically significant (P ≤ 0.05), decrease in abundance of tRNAGly in trm10Δ cells when compared to TRM10 cells grown under the same conditions (Fig. 2B, white bar comparing TRM10 vs. trm10Δ in the absence of 5FU; Supplemental Table S1 listing P-values for other comparisons). The addition of 5FU to the media has, if anything, a slightly positive effect on the abundance of tRNAGly(GCC), although statistical analysis indicates that none of the apparent changes rise to the level of significance observed with tRNATrp. These results indicate that tRNATrp alone remains more greatly affected by loss of the m1G9 modification, consistent with its unique ability to restore growth in 5FU (Fig. 2B; Supplemental Table S1). Unsurprisingly, the overexpression of tRNATrp does not detectably affect the abundance of tRNAGly under any tested condition. Overall, the observed pattern of mature tRNA levels suggests that the presence of the m1G9 modification is selectively important for maintaining sufficient levels of tRNATrp under 5FU growth conditions.
Levels of pre-tRNATrp accumulate in a Trm10- and 5FU-dependent manner
Hybridization of the tRNATrp probe that targets sequences in the mature tRNA also revealed the presence of higher migrating precursor tRNATrp (pre-tRNATrp) species in several strains (Fig. 2A). We note that the tRNAGly(GCC) probe hybridizes to a similar region of the mature tRNA, yet did not similarly indicate the presence of pre-tRNAGly in any of the strains (Fig. 2A). However, because the presence of the pre-tRNATrp intron sequence would disrupt base-pairing with the mature tRNATrp targeting probe, we chose not to use this probe to directly quantify the impact on pre-tRNATrp processing in Figure 2. Instead, to further assess potential tRNA processing defects associated with these conditions, we probed the same RNAs with oligonucleotide probes designed to target specific pre-tRNATrp species (Chatterjee et al. 2022). Two probes targeted two of the six tRNATrp 5′-leader sequences (named GAT and GTT based on the last 3 nt of the distinct 5′-leader sequences for each tRNATrp gene), and one probe targeted intron-containing pre-tRNATrp (Fig. 3; Supplemental Tables S2 and S3). Notably, all precursor probes included some exon sequence to facilitate sufficient hybridization to the target RNA; therefore, mature tRNA is also detected in each of these assays to varying extents (as described in more detail below).
FIGURE 3.
tRNATrp precursors accumulate in trm10Δ strains and in TRM10 strains grown in 5FU. (A) Diagram indicating the three pre-tRNATrp species detected by northern probes. Orange and yellow lines indicate the hybridization of 5′ leader probes (GAT and GTT, indicating the last 3 nt of the targeted leader sequence, respectively), while green lines indicate the hybridization of the intron probe (sequences listed in Supplemental Table S2). Images are not to scale, but bent lines indicate probe sequences that lose hybridization to intron-containing and mature tRNAs. (B) Northern analysis of RNA derived from the indicated strains using 5'-biotinylated probes and visualized with chemiluminescence. The bar to the left of each set of results indicates the probe that was utilized (colored as in A). (C) Quantification of relative total pre-RNA levels (for all pre-tRNA species combined) from strains shown in B. Relative tRNA levels were calculated by comparing the observed intensity for each RNA to the normalized abundance observed in the trm10Δ strain plus tRNATrp overexpression (lane 5), to allow for quantifying the full range of increased and decreased pre-tRNA levels. Duplicate data were plotted, with each end of the error bars showing each data point.
tRNATrp is unique in S. cerevisiae in that it is the only tRNA that is processed by the removal of the 3′ trailer sequence prior to the removal of the 5′ leader sequence (O'Connor and Peebles 1991; Kufel and Tollervey 2003). Therefore, the three species that are observed in northerns with pre-tRNA probes are (i) the initial transcript that contains a 5′ leader, 3′ trailer, and an intron, (ii) the 3′ processed (5′ leader- and intron-containing) pre-tRNATrp, and finally, (iii) the end-processed, but still intron-containing pre-tRNATrp that will be exported to the cytoplasm, where tRNA splicing takes place on the surface of the mitochondria (Fig. 3A). The absence of a band for mature tRNATrp in TRM10 wild-type cells observed with either of the two 5′-leader-targeting probes is explained by the fact that these probes both overlap the G9 nucleotide (Fig. 3B). The m1G9 modification in TRM10 cells blocks base-pairing, and in the trm10Δ strains that lack m1G9 modification, mature tRNATrp is readily detected with both 5′-leader hybridizing probes (Fig. 3B, compare the mature tRNA band in lane 3 vs. lane 7 for both leader probes). Consistent with this result, the intron probe readily detected mature tRNATrp from both TRM10 and trm10Δ cells, with levels of mature tRNATrp that mirror those measured with the mature tRNA-targeting probe used in Figure 2 that also does not overlap G9 (Supplemental Fig. S2D).
In wild-type (TRM10) cells, pre-tRNATrp species do not accumulate in the absence of 5FU, even upon tRNATrp overexpression, suggesting that accumulation of pre-tRNATrp cannot be simply attributed to overwhelming the tRNA processing machinery with excess tRNATrp when m1G9 is present (Fig. 3B, lanes 1 and 3). However, the addition of 5FU to TRM10 cells causes significant pre-tRNATrp accumulation even though the m1G9 modification is present, suggesting a processing defect for tRNATrp that is likely caused by the incorporation of 5FU into the tRNA, separate from the effects of Trm10. Interestingly, however, deletion of trm10 alone also causes a significant accumulation of precursor tRNATrp levels, which is observed even in the absence of 5FU (Fig. 3B, compare lanes 3 and 7). Similar patterns of total pre-tRNATrp accumulation with distinct Trm10- and 5FU-dependence were observed upon quantification of results with all three pre-tRNA-directed oligos (Fig. 3C).
Overall, in wild-type cells, pre-tRNATrp species accumulate to similar levels in the presence of 5FU regardless of tRNATrp overexpression (Fig. 3B,C, lanes 1 and 2 vs. lanes 3 and 4). However, the results in the context of trm10 deletion are very different, where overexpression of tRNATrp leads to a large 5FU-dependent increase in pre-tRNATrp (Fig. 3B, lane 5 vs. 6), but pre-tRNATrp accumulation remains unchanged by the addition of 5FU to the trm10Δ strain alone (Fig. 3B, lane 7 vs. 8). These data indicate that the loss of m1G9 causes an accumulation of tRNATrp precursors that is separate from (and additive with) the effects of 5FU.
To test whether the 5FU or trm10Δ effects could be attributed to a specific processing defect, the northern intensities of each of the three individual pre-tRNA species were quantified separately (Supplemental Fig. S2A–C). All three pre-tRNA-targeting probes showed the same pattern of pre-tRNA abundance changes on both 5′-leader containing pre-tRNA species (the initial transcript and the 3′-processed [5′-extended] pre-tRNA), reflecting the fact that both of these pre-tRNA species can completely hybridize to all three DNA probes (Fig. 3A). Neither deletion of trm10 nor addition of 5FU significantly perturbs the 3′-end processing step for pre-tRNATrp, since we did not observe higher relative levels of initial transcript (Supplemental Fig. S2A) at the expense of 3′-processed species (Supplemental Fig. S2B) when comparing levels in any of the same strains. However, the amounts of end-matured intron-containing pre-tRNA reveal some intriguing differences (Supplemental Fig. S2C). For comparing the abundance of intron-containing species, we relied on quantification using the probe targeting intron-containing pre-tRNATrp, since end-matured intron-containing pre-tRNATrp completely hybridizes to this intron probe (green), but not the two leader probes (orange and yellow) (Fig. 3A). Interestingly, both 5′-leader containing species (Supplemental Fig. S2A,B) accumulate to substantially higher levels than intron-containing pre-tRNA (Supplemental Fig. S2C) in TRM10 strains +5FU (Supplemental Table S4). The 5FU-dependent increase in 5′-extended pre-tRNA is approximately 18- to 37-fold in these strains, whereas the intron-containing pre-tRNA only increases approximately sevenfold in TRM10 strains +5FU (Supplemental Table S4). Thus, 5FU appears to preferentially impair 5′-leader removal from pre-tRNATrp, leading to relatively more accumulation of 5′-extended precursors as a fraction of the total pre-tRNA species in these cells. Separately, comparison of individual pre-tRNA species between TRM10 and trm10Δ strains does not indicate significant accumulation of any individual pre-tRNA species in the absence of Trm10 (Supplemental Fig. S1; Supplemental Table S4), suggesting that the negative effect on pre-tRNATrp processing due to deletion of trm10 cannot be as strongly attributed to a specific defect in a particular step of the pre-tRNA processing pathway.
Deletion of met22 rescues growth hypersensitivity of trm10Δ strains to 5FU
To identify the pathway by which tRNATrp levels are reduced upon loss of m1G9 modification, we created double deletion strains of trm10Δ and genes coding for enzymes associated with several known tRNA quality control pathways in S. cerevisiae. The rationale for these experiments is that inactivation of a tRNA quality control pathway that degrades hypomodified tRNATrp would stabilize the tRNA and rescue the 5FU growth defect, as has been observed for other targets of tRNA surveillance. We used homologous recombination to replace each gene. The trm10Δ strain available from the yeast genome deletion collection has a kanamycin-resistant (kanMX) cassette replacing the TRM10 coding sequence, enabling us to introduce alleles of met22, xrn1, and rat1 that had been constructed with other drug-resistant cassettes (see Table 1 for full strain genotypes) (Chernyakov et al. 2008). However, we also constructed a trm10Δ::natMX strain and demonstrated that it exhibits the same 5FU hypersensitivity of the trm10Δ::kanMX deletion strain (Fig. 4A), to enable the use of kanMX deletion alleles to target other quality control genes that were already available from the yeast deletion collection.
TABLE 1.
Saccharomyces cerevisiae strains used in this study
FIGURE 4.
Deletion of met22 in trm10Δ strain rescues growth sensitivity to 5FU. Strains were grown overnight in SC or SD-Leu media (if containing a tRNA overexpression plasmid), serially fourfold diluted from a starting OD600 of 1, plated on plates of corresponding media with indicated concentrations of 5FU, and incubated for 4 d at 30°C. (A) Double and triple mutants with trm10Δ and other possible genes that have been implicated in 5′–3′ tRNA decay were tested for ability to revert 5FU hypersensitivity; control TRM10 strains are shown with the same mutations, as indicated. Note that the trm10Δ 5FU hypersensitive phenotype is observed with both kanR and natR gene replacements (see control strains at top of panel). (B) Double mutants of trm10Δ with genes associated with 3′–5′ tRNA decay, and control TRM10 strains with the same mutations, as indicated. (C) Overexpression of tRNATrp or tRNAHis from plasmids was performed in the indicated TRM10 or trm10Δ strain backgrounds. The results with the vector control strain in TRM10 and trm10Δ cells are shown in the top panel.
Met22 is an enzyme of methionine biosynthesis that, when deleted, causes accumulation of its substrate, adenosine 3′,5′ bis-phosphate (pAp). This metabolite is a potent inhibitor of the 5′–3′ exonucleases Xrn1 and Rat1, which have both been shown to degrade aberrant tRNA in the context of the RTD quality control pathway (Murguia et al. 1996; Dichtl et al. 1997). Targets of RTD are stabilized in the presence of met22Δ because accumulated pAp inhibits these 5′–3′ exonucleases (Alexandrov et al. 2006; Chernyakov et al. 2008). Double deletion of trm10 and met22 rescued growth in the presence of 5FU (Fig. 4A). We considered whether the observed growth rescue by met22Δ is consistent with RTD-mediated degradation of mature tRNATrp that lacks m1G9, which is then exacerbated in the presence of 5FU. To test this, we created double mutant strains with trm10Δ and either or both of the RTD-associated 5′–3′ exonucleases, XRN1 and RAT1. XRN1 is a nonessential gene in S. cerevisiae, allowing us to create trm10Δ xrn1Δ double deletion strains, but RAT1 is essential, so we introduced a rat1-107 allele used previously to demonstrate the role of this enzyme in the degradation of specific hypomodified tRNA by RTD (Chernyakov et al. 2008). Although the rat1-107 mutation is not a null allele, it has been consistently able to restore tRNA levels of all RTD substrates tested to date (Chernyakov et al. 2008; Tasak and Phizicky 2022), and its lack of temperature sensitivity helps to avoid confounding effects of temperature in the growth assays.
Interestingly, unlike for other RTD quality control mechanisms discovered to date, neither RTD-associated mutation alone was able to reverse the trm10Δ phenotype, as both trm10Δ xrn1Δ and trm10Δ rat1-107 strains exhibited the same hypersensitivity to 5FU as the single trm10Δ mutant (Fig. 4A). To test possible redundancy of Xrn1/Rat1 in tRNATrp surveillance, we created the triple trm10Δ xrn1Δ rat1-107 strain, but again, observed no restoration of 5FU-resistant growth. This led us to reason that the effects of met22Δ are occurring through another, yet unidentified, Met22-dependent mechanism for tRNA surveillance and removal that does not require either Xrn1 or Rat1 5′–3′ exonuclease activity. Another S. cerevisiae 5′–3′ exonuclease (Dxo1) has not been associated with met22Δ or observed to act on tRNA. Nonetheless, because of its mechanistic similarity to the RTD exonucleases, we also constructed the trm10Δ dxo1Δ strain (Chang et al. 2012; Yun et al. 2018). Again, we observed no change in 5FU hypersensitivity, ruling out Dxo1 as the Met22-dependent enzyme that is acting on tRNATrp (Fig. 4A). We note that Met22-dependent degradation of intron-containing pre-tRNA by an as of yet unidentified nuclease has been identified in S. cerevisiae, but since that pathway senses pre-tRNA, a distinct pathway must participate in mature tRNATrp decay described here (Tasak and Phizicky 2022). The TRAMP complex also degrades hypomodified tRNAiMet in S. cerevisiae, but neither deletion of trf4 (an essential component of the TRAMP complex) nor the rrp6 subunit of the nuclear exosome that participates in degradation of the tRNA after polyadenylation by the TRAMP complex was able to rescue the 5FU growth defect of the trm10Δ strain, arguing against a role for the TRAMP complex in tRNATrp quality control (Fig. 4B; Kadaba et al. 2004; Callahan and Butler 2010). The lack of even partial growth rescue in either the trm10Δ trf4Δ or trm10Δ rrp6Δ strains also argues against a mechanism involving both RTD and TRAMP, as was recently observed with tRNAiMet (Tasak and Phizicky 2022).
To rule out the possibility that there is a new cause for 5FU hypersensitivity in the context of the double and triple deletion strains tested in Figure 4, we overexpressed tRNATrp and a control tRNAHis (which is not a Trm10 substrate and does not affect 5FU sensitivity) in each double and triple deletion strain (Fig. 4C). In all cases, transformation of the plasmid directing overexpression of tRNATrp, but not tRNAHis, was able to restore wild-type levels of growth in the presence of 5FU in the double and triple mutant strains, as is observed for trm10Δ alone. These results indicate that insufficiency of tRNATrp remains the cause of 5FU hypersensitivity that persists in each strain, and not that another tRNA becomes limiting in the absence of the relevant tRNA quality control enzymes. Therefore, we conclude that mature tRNATrp lacking the m1G9 modification is recognized in a novel manner by the cell for degradation. Growth hypersensitivity of trm10Δ to 5FU is rescued by deletion of met22, yet not by inactivation of any of the known exonuclease-dependent pathways that have been associated with Met22 to date.
Growth rescue in the met22Δ trm10Δ strain correlates with restored abundance of mature tRNATrp
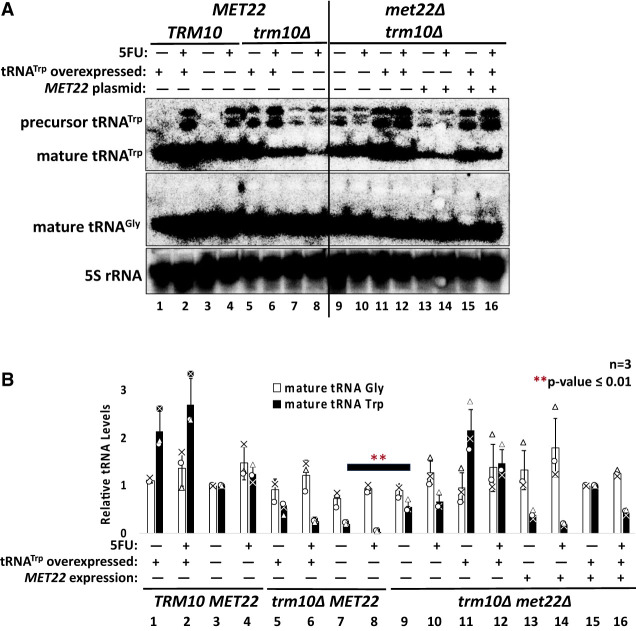
Although decreased abundance of tRNATrp appears to remain the barrier to viability in the met22Δ trm10Δ strains in the presence of 5FU (Fig. 4C), the lack of an effect of other tRNA quality control mutants raised questions about whether there is another previously undescribed Met22-associated mechanism that does not involve inhibiting tRNA decay due to pAp accumulation. Thus, we quantified mature tRNATrp and tRNAGly levels in RNA isolated from the trm10Δ met22Δ strain under each of the previously tested growth conditions, with and without MET22 expressed from a plasmid in the strain (Fig. 5; Supplemental Fig. S1C).
FIGURE 5.
Mature tRNATrp levels are rescued upon met22 deletion in the trm10Δ strain. (A) Northern analysis of RNA derived from the indicated strains probed for mature tRNATrp, mature tRNAGly, and 5S rRNA, which were all detected using 5′ radiolabeled probes (Supplemental Table S2). Strains shown in lanes 1–8 are wild-type for MET22; whereas strains shown in lanes 9–16 are met22Δ strains, with lanes 13–16 also containing a CEN URA3 MET22 plasmid for complementation of met22Δ. A representative northern blot visualized at lower exposure is shown in Supplemental Figure S1C for comparison. (B) Quantification of relative mature tRNA levels from strains shown in A. Normalized RNA levels (to 5S rRNA) were calculated for each strain, and for lanes 1–8, lane 3 was set to 1 for determining relative tRNA levels, and for lanes 9–16, lane 15 was set to 1 for determining relative tRNA levels, in order to better visualize the positive and negative changes associated with each set of strains on the same axis. Triplicate data were plotted, with individual data points for tRNAGly(GCC) and tRNATrp shown. Two-tailed t-tests assuming equal variance were performed, and one-tailed P-value is represented by asterisks comparing tRNATrp levels in the trm10Δ and the trm10Δmet22Δ strain. P-values for comparisons not shown here are listed in Supplemental Table S1.
In trm10Δ met22Δ strains, levels of mature tRNATrp increased as expected based on the ability of met22Δ to rescue growth in 5FU (Fig. 5B, compare mature tRNATrp levels in MET22 trm10Δ strains in lanes 9 and 10 vs. levels in met22Δ trm10Δ strains in lanes 7 and 8). This remains consistent with the known mechanism for Met22 that involves inhibition of 5′–3′ exonucleases that degrade mature hypomodified tRNA, despite the fact that neither of its known RTD-associated enzymes appears to be involved in quality control of tRNATrp. Although expression of MET22 from a CEN plasmid did not restore the 5FU hypersensitive growth phenotype to the trm10Δ met22Δ strain (Supplemental Fig. S3), the abundance of tRNATrp was detectably decreased by expression of MET22 in the complemented strain (Fig. 5, for example, lanes 13 vs. 9 and 14 vs. 10). Plasmid-borne Met22 thus partially complements the molecular phenotype (decay of tRNATrp) but appears not to be expressed to sufficient levels to fully counteract the stabilizing effect of met22Δ on tRNATrp abundance, and thus to enable reversion to 5FU sensitive growth of the trm10Δ strain.
Despite the ability of met22Δ to rescue growth and increase mature tRNATrp levels in the trm10Δ background, pre-tRNATrp species are still detected in the trm10Δ met22Δ cells (Fig. 5A, lanes 9–12). We quantified pre-tRNATrp accumulation in the trm10Δ met22Δ cells by directly probing for pre-tRNA species using the same pre-tRNA oligonucleotide probes used in Figure 3 (Supplemental Fig. S4). These data confirmed that although the expression of MET22 in the trm10Δ met22Δ background resulted in significant destabilization of the mature tRNATrp (Supplemental Fig. S4A, compare lanes 1 vs. 5, 2 vs. 6, 3 vs. 7, or 4 vs. 8), the relative abundance of pre-tRNA species was not significantly affected in the same strains (Supplemental Fig. S4). The much more substantial impact of met22Δ on mature tRNATrp levels rather than on pre-tRNA accumulation suggests that 5FU hypersensitivity of the trm10Δ strain is mostly driven by degradation of the mature tRNA rather than by the defect in pre-tRNA processing.
DISCUSSION
Here, we used genetic approaches to demonstrate that the previously observed 5FU hypersensitive phenotype associated with deletion of the tRNA m1G9 methyltransferase Trm10 in S. cerevisiae is uniquely due to a destabilizing effect on tRNATrp, but not on other Trm10 substrate tRNAs (Figs. 1 and 2). Moreover, the negative effect on tRNATrp is also detected in the absence of m1G9 modification alone and is further exacerbated when trm10Δ cells are grown in the presence of 5FU (Figs. 1 and 2). We also discovered that growth in the presence of 5FU causes a processing defect in tRNATrp that results in accumulation of partially processed tRNATrp species (Fig. 3). Separately, trm10Δ strains also accumulate tRNATrp precursors even in the absence of 5FU (Fig. 3). These data suggest that pre-tRNATrp processing steps are particularly sensitive to perturbations in the physical attributes of this tRNA. We showed that deletion of met22 rescued the 5FU hypersensitive growth phenotype of the trm10Δ strain due to increased levels of mature tRNATrp in the met22Δ trm10Δ strain that are presumably sufficient to maintain translation even with the hypomodified tRNA (Figs. 4 and 5). However, the inactivation of other genes in known tRNA decay pathways in S. cerevisiae did not similarly rescue the trm10Δ 5FU growth defect (Fig. 4), indicating that Met22 acts through some other yet unknown tRNA decay pathway to remove inappropriately modified tRNATrp from the cells. The fact that Trm10 modifies 13 different cytosolic tRNAs in S. cerevisiae, but that the biological importance of the m1G9 modification seems to only be significant for one of its substrates (tRNATrp) provides yet another example of a tRNA modification that does not impact all the tRNAs that contain it equally (Phizicky and Alfonzo 2010; Howell and Jackman 2019). The evolutionary origins and molecular basis for the activity of Trm10 on multiple S. cerevisiae tRNAs, despite its biological importance for only one tRNA, remains to be fully understood.
To date, decay of mature tRNAs due to RTD has been demonstrated for three S. cerevisiae tRNAs, tRNASer(CGA) and tRNASer(UGA), and tRNAVal(AAC) (Alexandrov et al. 2006; Chernyakov et al. 2008; Whipple et al. 2011; Dewe et al. 2012). Hypomodification due to genetic disruption of at least five other tRNA modification enzymes, including Trm8 (m7G46), Trm1 (m2,2G26), Trm4 (m5C), Tan1 (ac4C12), or Trm44 (Um44) in different combinations has been implicated in these tRNA quality control events, and in each case impacts only one or two tRNAs from among all possible substrates for each enzyme. Interestingly, even though the abundance of some of these RTD substrates is detectably impacted upon mutation of the modification enzyme(s) alone, as we also observed for tRNATrp (Fig. 2), growth defects are not observed until the addition of another stressor to the cells, such as growth at high temperature (Dewe et al. 2012). The example we provide here is the first demonstration that growth in the presence of 5FU can similarly destabilize a tRNA that is already impacted by the loss of a modification from one of its substrates. The fact that not all substrates of each modifying enzyme are similarly affected by the loss of modifications underscores the challenges associated with understanding the function of individual tRNA modifications and their enzymes. These results also help to rationalize the long-standing conundrum surrounding the general lack of observed growth defects associated with genetic mutants in many of these highly conserved tRNA modification enzymes in S. cerevisiae, including Trm10, despite the demonstrated impact of Trm10 deficiency on human health.
The toxic effects of 5FU that lead to its widespread use as an antitumor drug have been difficult to attribute to the impact on a specific nucleic acid, since 5FU is salvaged and becomes part of the total nucleotide pool. 5FU can become both an inhibitor of thymidylate synthase in the form of 5FdUMP and can be incorporated into biological RNA molecules in the form of 5FUTP. Examples of negative effects of 5FU on pre-mRNA splicing and translation have been revealed, but the demonstration that a large number of tRNA modification enzyme genes were the most sensitive to 5FU in the genome-wide deletion collection suggested that impacts of 5FU on tRNA function may be even more biologically significant (Gustavsson and Ronne 2008; Hoskins and Butler 2008; Bash-Imam et al. 2017; Ge et al. 2017). Here, we provide the first molecular explanation for the effect of 5FU on tRNA function. Moreover, the observation that these effects (at least for the trm10Δ strain) occur predominantly on a single tRNA (Trp) despite the incorporation of 5FU into all tRNA species, is intriguing. One possible rationale for the negative impact of 5FU on RNA is due to the fact that the presence of 5FU inhibits the formation of pseudouridine (Ψ) modification, which is generally thought to have a stabilizing impact on RNA and is one of the most abundant modifications of all RNAs, but is especially abundant in tRNA (Davis and Poulter 1991; Davis 1995; Hoskins and Butler 2008). The role of pseudouridylation in trm10Δ 5FU hypersensitivity has also been indicated by a previous study that identified a strong genetic interaction between the TRM10 and PUS3 genes (Han et al. 2015). Intriguingly, and consistent with the results described here, even though multiple tRNAs in the cell contain both Pus3- (Ψ38 and/or Ψ39) and Trm10-catalyzed modifications, only overexpression of tRNATrp (naturally containing Ψ39, not Ψ38) partially rescued growth of the trm10Δ pus3Δ strain (Han et al. 2015). Together these results implicate loss of pseudouridylation due to the incorporation of 5FU into tRNATrp as a major mechanism that contributes to the observed 5FU hypersensitive phenotype of the trm10Δ strain (Bowles and Jackman 2023). The specific sensitivity of tRNATrp may be related to the below-average predicted stability of its anticodon stem where Ψ39 modification occurs. However, we note that another Trm10/Pus3 substrate tRNAArg(UCU) is also among the tRNAs with a below-average stability anticodon stem and is not similarly affected by 5FU (Han et al. 2015). Additionally, tRNATrp contains Ψ at six different positions, which is the largest number of Ψ found in any of the 12 elongator tRNAs modified by Trm10. It is thus possible that 5FU incorporation at other positions may also contribute to the observed instability of tRNATrp. It will be interesting to continue to characterize the structural impact of 5FU incorporation into tRNATrp and other tRNAs as the basis for the observed hypersensitive phenotypes.
The observation that met22Δ stabilizes tRNATrp levels implied that a similar mechanism to that observed for other Met22-dependent tRNA quality control pathways, in which accumulation of the Met22 substrate pAp inhibits exonucleases such as Rat1 and Xrn1, could have been acting on tRNATrp. However, the identity of the nuclease(s) that act to degrade hypomodified tRNATrp remains unknown since none of the currently known mutants of pathways that are known to participate in tRNA decay (RAT1, XRN1, TRF4, or RRP6) rescue the trm10Δ growth defect (Fig. 4). For other tRNAs degraded by RTD, mutation of either xrn1 or rat1 is sufficient to restore levels of the affected tRNA. However, the lack of 5FU growth rescue in the triple trm10Δ xrn1Δ rat1-107 strain (Fig. 4) confirms that quality control of tRNATrp is not just a new variant of RTD in which the 5′–3′ exonucleases are acting redundantly to degrade tRNATrp. Therefore, there must be some other Met22-responsive change in tRNA decay that explains the growth rescue in the trm10Δ met22Δ strain, which could include the possibility of Met22-sensitive nucleases that have not yet been identified.
Met22 has also been implicated in the surveillance of pre-tRNA containing mutations that affect the structure of the intron–exon junction of any of several pre-tRNAs through Met22-dependent pre-tRNA decay (MPD), leading to accumulation of end matured, intron-containing pre-tRNA (Payea et al. 2015). Although deletion of trm10 or growth in 5FU also causes accumulation of pre-tRNAs (Fig. 3), no change in accumulation of pre-tRNAs was observed in a met22Δ-dependent manner that would suggest met22Δ is similarly acting to stabilize pre-tRNATrp. Thus, MPD is ruled out as a possible mechanism for the quality control pathway for mature tRNATrp associated with trm10Δ. Interestingly, some crossover between RTD and the nuclear TRAMP pathway was recently observed, with initiator tRNA lacking the essential m1A58 modification sensed for degradation by both pathways (Tasak and Phizicky 2022). In this scenario, both met22Δ and trf4Δ were required to rescue the trm6-504 mutation, which has decreased m1A58 levels. This was the first instance associating 5′–3′ RTD and 3′–5′ nuclear surveillance exonucleases in targeting the same hypomodified tRNA. Our study only found a growth rescue of the trm10Δ strain upon growth in 5FU with pairwise deletion of met22 and did not detect any similar synergy between its associated pathways (Fig. 4).
Two distinct mechanisms impact pre-tRNATrp processing, with an accumulation of pre-tRNATrp observed either only in the presence of 5FU (even in TRM10 strains) or in trm10Δ strains (even in the absence of 5FU). Interestingly, in TRM10 cells grown with 5FU, although all three pre-tRNATrp precursors accumulate to some degree, accumulation of the 5′-leader containing tRNA is greater than that of end-processed intron-containing tRNA (Fig. 3; Supplemental Fig. S2; Supplemental Table S4). This result suggests that there is a more substantial impact of 5FU on the 5′-end processing of pre-tRNATrp than on the removal of the intron. tRNATrp genes in S. cerevisiae are the only set of pre-tRNA in which all of the six different 5′-leader sequences contain a U at the −1 position (Supplemental Table S3; Chan and Lowe 2009). When cells are grown under 5FU conditions, it is likely that 5FU is incorporated into these pre-tRNA transcripts resulting in the presence of a 5FU at the −1 position in at least some tRNAs. Based on the unique processing of tRNATrp in yeast, which unlike all other pre-tRNAs already has a slower 5′-leader removal than 3′-trailer removal, it seems possible that the incorporation of 5FU at the position immediately next to Ribonuclease P-mediated 5′-end cleavage has a disproportionately negative impact on the efficiency of 5′-end leader removal causing some additional accumulation of 5′-extended precursors. Understanding the molecular basis for the negative impact of the loss of m1G9 on pre-tRNATrp processing is more complicated since there is no known role for m1G9 in either 5′-end processing or intron splicing. It is also possible that retrograde transfer of partially processed pre-tRNATrp species also contributes to the quality control of tRNATrp by removing aberrant hypomodified pre-tRNA from the cytoplasm, as has been observed already for several other intron-containing pre-tRNA (Kramer and Hopper 2013). Another possibility for pre-tRNATrp accumulation aside from processing defects in the 5′-end maturation step could be that the decreased levels of mature tRNATrp cause upregulation of tRNATrp transcription to compensate for the loss of the mature transcript. However, we note that overexpression of tRNATrp in the TRM10 wild-type strain does not cause detectable pre-tRNA accumulation, suggesting that the defects that primarily drive pre-tRNA accumulation are in the processing step(s) themselves by a mechanism that remains to be determined.
MATERIALS AND METHODS
tRNA overexpression plasmids
A set of 2µ LEU2 plasmids containing each of 38 different tRNA genes with their endogenous upstream and downstream genomic sequences was utilized as described in Han et al. (2015). This collection of plasmids provided by the Phizicky lab did not contain the gene for one Trm10 substrate elongator tRNA, and therefore, a plasmid was constructed for the expression of this tRNAIle(AAU) species. This overexpression vector was created by amplifying the sequence of tRNAIle(AAU) from S. cerevisiae genomic DNA (YNCI0005W) with 28 and 36 bp of endogenous upstream and downstream sequences, respectively, using iProof PCR. BglII and XhoI sites were introduced with the primers to enable ligation into the same AVA0577 2µ LEU2 tRNA overexpression vector used for the rest of the tRNA overexpression collection.
Creation of yeast strains
Strains used in this study are listed in Table 1. Deletion strains generated for this study were created by amplifying the gene of interest from existing single deletion strains obtained either from the yeast genome deletion collection or generously provided by Eric Phizicky (Giaever et al. 2002). Polymerase chain reactions (PCRs) were performed using iProof polymerase (Bio-Rad) and primers designed to anneal between 150 and 400 bp upstream and downstream from each target gene. PCR products were purified using the NucleoSpin Gel and PCR Clean-up Kit (Machery-Nagel).
Genomic DNA for PCR amplification of target deletion genes was isolated from indicated strains as follows. Colonies of each strain were inoculated into 5 mL media for overnight growth at 30°C. Cells were pelleted (5000g for 17 min at room temperature), resuspended in DNA prep buffer containing 100 mM NaCl, 10 mM Tris-HCl pH 8.0, 2% Triton X-100, and 1% SDS, and then an equal volume of PCA (phenol:chloroform:isoamyl alcohol, 25:24:1) was added to the resuspended cells, followed by glass bead lysis. After vortexing at high speed for 3 min, another equal volume of TE pH 8.0 was added to the vortexed cells so buffer:PCA:1× TE is at a 1:1:1 ratio. After separation by centrifugation, the aqueous layer was transferred to a new tube, ethanol precipitation was performed, and nucleic acids were resuspended in 1× TE pH 8.0; 10 mg/mL RNase A (Thermo Scientific) was added to degrade RNA in the sample. Ethanol precipitation was again performed, and the pellet was resuspended in 1× TE pH 8.0 and quantified by Nanodrop prior to transformation.
Linear transformations were performed using the lithium acetate (LiOAc) method. To generate competent cells, 25 mL cultures of the target strain were grown to an OD600 of 1.5–2, at which point 14 mL of cells were pelleted (4000 rpm for 15 min at room temperature) and washed two times with 0.1 M LiOAc. Cells were resuspended in 140 μL 0.1 M LiOAc and 32 μL salmon sperm DNA (10 mg/mL Invitrogen). Purified PCR product (250–500 ng) was added to 75 μL competent cells and incubated at 30°C for 15 min, followed by the addition of 450 μL 60% PEG 3350 1 M LiOAc and further incubation at 30°C for 30 min. After the addition of DMSO, cells were heat shocked at 42°C for 15 min, pelleted (4000g for 3 min), resuspended in 600 μL YPD, and allowed to recover by incubation at 30°C for 5 h with shaking (200 rpm). Cells were again pelleted and resuspended in 125 μL ddH2O, and half the volume was plated on YPD plates under selective conditions for each drug resistance cassette (100 µg/mL nourseothricin sulfate [Nat], 300 µg/mL hygromycin B [Hyg], 500 µg/mL G148 [Kan], or 75 μg/mL phleomycin [Ble]). Transformants were obtained after 2–3 d of growth at 30°C, and all strains were confirmed by replica plating to test for the expected drug and/or nutrient resistance, followed by PCR amplification and sequencing of the relevant gene deletion region from genomic DNA.
The trm10Δ strain available from the yeast deletion collection contains a kanR marker in place of the TRM10 gene. A second trm10Δ strain was created using the natR marker to simplify the creation of double deletion strains using other available kanR gene replacement strains from the deletion collection. The natR gene sequence was amplified from the pAG25 vector (graciously donated by Anita Hopper's lab) using primers designed to the common promoter/terminator regions and iProof polymerase and transformed into the trm10::kanR strain as described above, with selection on YPD + Nat (100 µg/mL) plates for swapping of the drug markers to create a trm10::natR strain.
Strains containing either a 2µ LEU2 tRNA overexpression plasmid, or CEN URA3 MET22 vector (graciously donated by Eric Phizicky's lab), were transformed into respective strains using the one-step transformation, as described previously (Chen et al. 1992), and plated onto SD-Leu, SD-Ura, or SD-Leu-Ura plates, as appropriate for each marker for selection of transformants.
Drop tests for growth in 5FU conditions
Single colonies of each strain were inoculated in 5 mL of either SD-Leu (for strains containing LEU2 tRNA overexpression plasmids) or SC media and grown overnight at 30°C. Each culture was diluted to an OD600 of 1 in the same media and used as the starting point for fourfold serial dilutions of each strain. Plates containing the indicated amount of 5FU (Sigma-Aldrich) were spotted with 2 μL of each dilution sample, and after spots were allowed to dry at room temperature, plates were incubated at 30°C. Images were taken after 3–4 d growth. Supplemental Figure S2 strains were grown in liquid SD-Leu-Ura media to maintain the CEN URA3 plasmid but were plated onto SD-Leu plates for growth comparison to other Ura-strains.
RNA isolation
To isolate RNA for northern analysis, cultures were grown in either SD-Leu media (if containing a LEU2 plasmid) or SD-Leu-Ura media (if also containing a CEN URA3 plasmid) in the absence of 5FU to late log phase. These cultures were used to inoculate 1 L cultures at a starting OD600 of 0.01 for growth at 30°C in the presence or absence of 1 µg/mL 5FU. After 12–14 h of growth, cultures that exhibit 5FU hypersensitivity (such as the trm10Δ strain) exhibit a clear growth defect compared to wild-type cells, and cells were harvested at this point (Supplemental Table S5). Cells were pelleted and resuspended in ddH2O at 300 OD600 units/mL. Low molecular weight RNA was isolated using the yeast hot-phenol method. Pelleted cells (300 OD600) were resuspended in 4 mL RNA extraction buffer (0.1 M NaOAc pH 5.2, 20 mM EDTA pH 8.0, 1% SDS). Phenol (saturated with 100 mM Tris-Cl pH 7.5) was added at a 1:1 ratio with resuspended cells and vortexed every 2.5 min for 30 sec for a total of 20 min with incubation at 55°C in between vortexing. Cell debris was pelleted (5000 rpm for 6.5 min at 4°C), the aqueous layer was transferred to a new tube, and an equal volume of PCA was added, shaken to mix, and centrifuged (5000g, 20 min, 4°C). The PCA-extracted aqueous layer was transferred to a new tube and this step was repeated, followed by ethanol precipitation to pellet the total RNA. Pellets were resuspended in 900 μL 1× TE pH 8.0 and 100 μL 3 M NaOAc pH 5.2 for a second ethanol precipitation. The final purified RNA pellets were resuspended in 500 μL ddH2O, and the total RNA was quantified by Nanodrop and stored at −80°C.
Northern blotting
Purified RNA (2–10 μg) was resolved by electrophoresis on a 10% polyacrylamide, 8 M urea gel after addition of 10× northern dye (50% glycerol, 0.3% bromophenol blue, 0.3% ethidium bromide). RNA was transferred to a Hybond-N+ membrane (Amersham) using voltage transfer. RNA was crosslinked to the membrane using the Optimal Crosslink setting on a SpectroLinker XL-1000 UV crosslinker (Spectronics Corporation). Membranes were visualized using either 5′-end radiolabeled or 5′-end biotin probes (Supplemental Table S2).
For northerns with radiolabeled probes, membranes were prehybridized for 3 h while rotating at 50°C in 25 mL prewarmed prehybridization buffer containing 5× SSC (3 M NaCl and 0.3 M NaCitrate), 50% formamide, 5× Denhardt's solution, 1% SDS, and 100 μg/mL salmon sperm DNA (denatured at 95°C for 5 min and chilled on ice before addition). Denhardt's solution contained 2% w/v BSA fraction V, 2% w/v Ficoll 400, and 2% w/v polyvinylpyrrolidone. Prehybridization buffer was removed and replaced with prewarmed hybridization buffer (5× SSC buffer, 50% formamide, 5× Denhardt's solution, 1% SDS, 5% dextran sulfate) including ∼10 pmol of 5′-end labeled probe for incubation while rotating overnight at 50°C. Oligos were 5′-end radiolabeled at a final concentration of 4 μM with T4 PNK (NEB) and γ-32P-ATP after incubation for 1 h at 37°C followed by enzyme inactivation at 72°C for 10 min. A BioGel P6 column (Bio-Rad) removed excess labeled ATP. Each membrane was washed four times for 15 min each with low stringency wash buffer (2× SSC buffer and 0.05% SDS) at room temperature. Membranes were exposed for 2 h and imaged on a Typhoon imager (Cytiva). To reprobe the same membrane for a different RNA species, the membrane was stripped with pre-boiled stripping buffer (1% SDS in ddH2O) at 85°C for 15 min while rotating, followed by storage in 1× TBE.
For northerns performed with 5′-biotin probes (protocol courtesy of KM McKenney, RP Connacher, and AC Goldstroham, pers. comm.), membranes were washed in 2× SSC buffer for 15 min while rocking, and then prehybridized while rotating in prewarmed ULTRAhyb Ultrasensitive Hybridization Buffer (Thermo Scientific) at 42°C for 1 h. Of the 5′-biotin DNA oligo, 5 nM was added to the prewarmed ULTRAhyb buffer and incubated while rotating overnight at 42°C. The membrane was washed two times with low stringency wash buffer (2× SSC, 0.1% SDS), and two times with high stringency wash buffer (0.1× SSC, 0.1% SDS) while rotating at 42°C for 15 min each. Biotin detection was performed using the Chemiluminescent Nucleic Acid Detection Module (Thermo Scientific 89880) and imaged with the Omega Lum G (GelCompany) with 10 sec to 4 min exposure, depending on the intensity of the bound probe. Reprobing was performed after stripping using the same conditions described above, although the prehybridization step could be omitted for reprobing with biotin-labeled oligonucleotides. For both radiolabeled and biotin probes, the hybridization buffer was reused up to four times, with the addition of new biotinylated oligonucleotides for each northern experiment.
The intensity of hybridized RNA on each membrane was quantified using ImageQuant (Cytiva Life Sciences). Mature and precursor tRNA levels were normalized to 5S levels on each membrane (tRNA/5S). One strain on each membrane was then set to a value of 1 (as indicated in figure legends) and the normalized intensity observed for all other strains normalized to the intensity of the same RNA in the standard strain, as indicated in each figure legend. Two-sample assuming equal variance t-tests were performed on triplicate measurements for each sample in order to determine statistical significance, as indicated in Figures 2 and 5, and in Supplemental Table S1.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We would like to thank Eric Phizicky for providing yeast strains and tRNA overexpression plasmids used in this work and for valuable conversations during manuscript preparation. We also thank Anita Hopper and Regina Nostromo for yeast strains and for advice on genetic protocols and northern analysis. We would also like to acknowledge our valuable collaboration and discussions with Sarah Strassler and Graeme Conn as this work was carried out. We thank Abi Hubacher for performing preliminary experiments and Dmitri and Elena Kudryashov for assistance with chemiluminescent imaging experiments. This research was supported by NIH R01GM130135 (to J.E.J.) and the OSU Center for RNA Biology Graduate Fellowship (to I.E.B.).
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.079861.123.
MEET THE FIRST AUTHOR
Isobel Bowles.

Meet the First Author(s) is an editorial feature within RNA, in which the first author(s) of research-based papers in each issue have the opportunity to introduce themselves and their work to readers of RNA and the RNA research community. Isobel Bowles is the first author of this paper, “A tRNA-specific function for tRNA methyltransferase Trm10 is associated with a new tRNA quality control mechanism in Saccharomyces cerevisiae.” Isobel attained her B.S. in ACS chemistry from Butler University in 2018 and is currently a PhD candidate in The Ohio State University Biochemistry Graduate Program in Jane Jackman's lab. In the laboratory, she studies the tRNA-modifying enzyme Trm10, with the goal to better characterize both its biological relevance in S. cerevisiae and to define tRNA structural elements that promote Trm10 substrate specificity.
What are the major results described in your paper and how do they impact this branch of the field?
These results provide the first evidence of the biological significance of Trm10 m1G9 modification in S. cerevisiae and strengthen the trend of tRNA-modifying enzymes that act on multiple tRNAs in the cell but are only essential for a subset of those substrates, in this case, tRNATrp. This work also complicates the current understanding of tRNA quality control in the field, as hypomodified tRNATrp is sensed for degradation by MET22 but none of the exonucleases known to degrade hypomodified tRNA, revealing further questions as to how a S. cerevisiae cell targets hypomodified tRNA for degradation. Additionally, the results reveal interesting effects of the antitumor drug 5FU on noncoding RNA, with greater implications for defining its mechanism of action.
What led you to study RNA or this aspect of RNA science?
Upon looking for graduate schools and rotating in labs, the work of the Jackman lab interested me as I could use my background in enzymology from my undergraduate research to apply to the interesting field of RNA biology. Both the broad roles of RNA in the cell and the ever-increasing number of diseases related to lack of RNA modification piqued my interest, and I became enamored with my project. And, to be perfectly candid, Jane had assembled a vivacious and interesting team that I aspired to be a part of, as enjoying my work environment makes the drive to perform science all the greater.
During the course of these experiments, were there any surprising results or particular difficulties that altered your thinking and subsequent focus?
The discovery that the presence of 5FU in growth media caused wild-type cells to accumulate pre-tRNATrp was wholly surprising, and although not entirely parsed out here, it was exciting to reveal further questions the laboratory will pursue in future.
What are some of the landmark moments that provoked your interest in science or your development as a scientist?
From a young age, I have been an inquisitive person who found order in a chaotic world through better understanding why things work the way they do. This trait, combined with love of applied learning, drives my curiosity to continue problem solving through research. Growing up, I was lucky to have educators in K-12 who made learning science fun and relevant, including middle school “Girls in Science” events that broadened my understanding of scientific concepts. The numerous mentors I've had throughout my career have been pivotal in my development as a scientist, including my undergraduate research mentors Geoffrey Hoops and Jeremy Johnson, who took me on as a second year and provided me the opportunity to learn foundational research skills, giving me confidence to pursue a PhD, and my graduate dissertation mentor Jane Jackman who has been pivotal in helping me become an independent scientist. Further, the opportunity to meet and interact with both leaders in my broader scientific field and those at my stage at conferences, including the RNA Society, always furthers my interest in science, which works best when it is both encouraging and collaborative.
If you were able to give one piece of advice to your younger self, what would that be?
Don't be so hard on yourself, and it is okay to not know all the answers; otherwise, there would be no reason to perform research.
Are there specific individuals or groups who have influenced your philosophy or approach to science?
I greatly admire the work of Eric Phizicky and Anita Hopper, who along with their lab members have been instrumental to the field of yeast genetics. Their parsed-out methodologies contributed to and influenced much of the work demonstrated in this paper. More broadly, I am driven to make science more approachable to all and I appreciate the scientists who put in the extra work to do so.
What are your subsequent near- or long-term career plans?
As I finish up my dissertation work, I love working in the lab and performing research, and hope to continue those passions into a postdoc or work in industry. I would ideally use my RNA biology background to perform work contributing to research in disease cures and/or prevention. I have also always had a passion for social justice and could see myself pursuing work in science policy at some point in the long term.
REFERENCES
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. 2006. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21: 87–96. 10.1016/j.molcel.2005.10.036 [DOI] [PubMed] [Google Scholar]
- Bash-Imam Z, Thérizols G, Vincent A, Lafôrets F, Polay Espinoza M, Pion N, Macari F, Pannequin J, David A, Saurin JC, et al. 2017. Translational reprogramming of colorectal cancer cells induced by 5-fluorouracil through a miRNA-dependent mechanism. Oncotarget 8: 46219–46233. 10.18632/oncotarget.17597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt EK, Martinez NM, Gilbert WV. 2020. Regulation and function of RNA pseudouridylation in human cells. Annu Rev Genet 54: 309–336. 10.1146/annurev-genet-112618-043830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles IE, Jackman JE. 2023. Diversity in biological function and mechanism of the tRNA methyltransferase Trm10. Acc Chem Res 56: 3595–3603. 10.1021/acs.accounts.3c00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan KP, Butler JS. 2010. TRAMP complex enhances RNA degradation by the nuclear exosome component Rrp6. J Biol Chem 285: 3540–3547. 10.1074/jbc.M109.058396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PP, Lowe TM. 2009. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res 37: D93–D97. 10.1093/nar/gkn787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Jiao X, Chiba K, Oh C, Martin CE, Kiledjian M, Tong L. 2012. Dxo1 is a new type of eukaryotic enzyme with both decapping and 5′-3′ exoribonuclease activity. Nat Struct Mol Biol 19: 1011–1017. 10.1038/nsmb.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee K, Marshall WA, Hopper AK. 2022. Three tRNA nuclear exporters in S. cerevisiae: parallel pathways, preferences, and precision. Nucleic Acids Res 50: 10140–10152. 10.1093/nar/gkac754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DC, Yang BC, Kuo TT. 1992. One-step transformation of yeast in stationary phase. Curr Genet 21: 83–84. 10.1007/BF00318659 [DOI] [PubMed] [Google Scholar]
- Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM. 2008. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev 22: 1369–1380. 10.1101/gad.1654308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino C, Toivonen S, Diaz Villamil E, Atta M, Ravanat JL, Demine S, Schiavo AA, Pachera N, Deglasse JP, Jonas JC, et al. 2018. Pancreatic β-cell tRNA hypomethylation and fragmentation link TRMT10A deficiency with diabetes. Nucleic Acids Res 46: 10302–10318. 10.1093/nar/gky839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR. 1995. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res 23: 5020–5026. 10.1093/nar/23.24.5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Poulter CD. 1991. 1H-15N NMR studies of Escherichia coli tRNAPhe from hisT mutants: a structural role for pseudouridine. Biochemistry 30: 4223–4231. 10.1021/bi00231a017 [DOI] [PubMed] [Google Scholar]
- Dewe JM, Whipple JM, Chernyakov I, Jaramillo LN, Phizicky EM. 2012. The yeast rapid tRNA decay pathway competes with elongation factor 1A for substrate tRNAs and acts on tRNAs lacking one or more of several modifications. RNA 18: 1886–1896. 10.1261/rna.033654.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichtl B, Stevens A, Tollervey D. 1997. Lithium toxicity in yeast is due to the inhibition of RNA processing enzymes. EMBO J 16: 7184–7195. 10.1093/emboj/16.23.7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Karijolich J, Zhai Y, Zheng J, Yu YT. 2017. 5-Fluorouracil treatment alters the efficiency of translational recoding. Genes (Basel) 8: 295. 10.3390/genes8110295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Véronneau S, Dow S, Lucau-Danila A, Anderson K, André B, et al. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. 10.1038/nature00935 [DOI] [PubMed] [Google Scholar]
- Gillis D, Krishnamohan A, Yaacov B, Shaag A, Jackman JE, Elpeleg O. 2014. TRMT10A dysfunction is associated with abnormalities in glucose homeostasis, short stature and microcephaly. J Med Genet 51: 581–586. 10.1136/jmedgenet-2014-102282 [DOI] [PubMed] [Google Scholar]
- Gustavsson M, Ronne H. 2008. Evidence that tRNA modifying enzymes are important in vivo targets for 5-fluorouracil in yeast. RNA 14: 666–674. 10.1261/rna.966208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Kon Y, Phizicky EM. 2015. Functional importance of Ψ38 and Ψ39 in distinct tRNAs, amplified for tRNAGln(UUG) by unexpected temperature sensitivity of the s2U modification in yeast. RNA 21: 188–201. 10.1261/rna.048173.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann J, Frank P, Löffler E, Bennett KL, Gerner C, Rossmanith W. 2008. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell 135: 462–474. 10.1016/j.cell.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Hopper AK, Huang HY. 2015. Quality control pathways for nucleus-encoded eukaryotic tRNA biosynthesis and subcellular trafficking. Mol Cell Biol 35: 2052–2058. 10.1128/MCB.00131-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins J, Butler JS. 2008. RNA-based 5-fluorouracil toxicity requires the pseudouridylation activity of Cbf5p. Genetics 179: 323–330. 10.1534/genetics.107.082727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell NW, Jackman JE. 2019. Impact of chemical modification on tRNA function. In eLS. John Wiley and Sons, Ltd., Chichester, UK. 10.1002/9780470015902.a0028527 [DOI]
- Howell NW, Jora M, Jepson BF, Limbach PA, Jackman JE. 2019. Distinct substrate specificities of the human tRNA methyltransferases TRMT10A and TRMT10B. RNA 25: 1366–1376. 10.1261/rna.072090.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igoillo-Esteve M, Genin A, Lambert N, Désir J, Pirson I, Abdulkarim B, Simonis N, Drielsma A, Marselli L, Marchetti P, et al. 2013. tRNA methyltransferase homolog gene TRMT10A mutation in young onset diabetes and primary microcephaly in humans. PLoS Genet 9: e1003888. 10.1371/journal.pgen.1003888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman JE, Alfonzo JD. 2013. Transfer RNA modifications: nature's combinatorial chemistry playground. Wiley Interdiscip Rev RNA 4: 35–48. 10.1002/wrna.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman JE, Montange RK, Malik HS, Phizicky EM. 2003. Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. RNA 9: 574–585. 10.1261/rna.5070303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J. 2009. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res 37: D159–D162. 10.1093/nar/gkn772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J. 2004. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev 18: 1227–1240. 10.1101/gad.1183804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempenaers M, Roovers M, Oudjama Y, Tkaczuk KL, Bujnicki JM, Droogmans L. 2010. New archaeal methyltransferases forming 1-methyladenosine or 1-methyladenosine and 1-methylguanosine at position 9 of tRNA. Nucleic Acids Res 38: 6533–6543. 10.1093/nar/gkq451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelawala L, Grayhack EJ, Phizicky EM. 2008. Identification of yeast tRNA Um44 2'-O-methyltransferase (Trm44) and demonstration of a Trm44 role in sustaining levels of specific tRNASer species. RNA 14: 158–169. 10.1261/rna.811008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EB, Hopper AK. 2013. Retrograde transfer RNA nuclear import provides a new level of tRNA quality control in Saccharomyces cerevisiae. Proc Natl Acad Sci 110: 21042–21047. 10.1073/pnas.1316579110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufel J, Tollervey D. 2003. 3′-processing of yeast tRNATrp precedes 5′-processing. RNA 9: 202–208. 10.1261/rna.2145103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Zhou X, Chen X, Huang K, Wu W, Fu J, Li Y, Polychronakos C, Dong GP. 2020. tRNA methyltransferase 10 homologue A (TRMT10A) mutation in a Chinese patient with diabetes, insulin resistance, intellectual deficiency and microcephaly. BMJ Open Diabetes Res Care 8: e001601. 10.1136/bmjdrc-2020-001601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al. 2013. MODOMICS: a database of RNA modification pathways—2013 update. Nucleic Acids Res 41: D262–D267. 10.1093/nar/gks1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murguia JR, Bellés JM, Serrano R. 1996. The yeast HAL2 nucleotidase is an in vivo target of salt toxicity. J Biol Chem 271: 29029–29033. 10.1074/jbc.271.46.29029 [DOI] [PubMed] [Google Scholar]
- Narayanan M, Ramsey K, Grebe T, Schrauwen I, Szelinger S, Huentelman M, Craig D, Narayanan V, Group CRR. 2015. Case report: compound heterozygous nonsense mutations in TRMT10A are associated with microcephaly, delayed development, and periventricular white matter hyperintensities. F1000Res 4: 912. 10.12688/f1000research.7106.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JP, Peebles CL. 1991. In vivo pre-tRNA processing in Saccharomyces cerevisiae. Mol Cell Biol 11: 425–439. 10.1128/MCB.11.1.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payea MJ, Guy MP, Phizicky EM. 2015. Methodology for the high-throughput identification and characterization of tRNA variants that are substrates for a tRNA decay pathway. Methods Enzymol 560: 1–17. 10.1016/bs.mie.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payea MJ, Hauke AC, De Zoysa T, Phizicky EM. 2020. Mutations in the anticodon stem of tRNA cause accumulation and Met22-dependent decay of pre-tRNA in yeast. RNA 26: 29–43. 10.1261/rna.073155.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky EM, Alfonzo JD. 2010. Do all modifications benefit all tRNAs? FEBS Lett 584: 265–271. 10.1016/j.febslet.2009.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky EM, Hopper AK. 2023. The life and times of a tRNA. RNA 29: 898–957. 10.1261/rna.079620.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siklar Z, Kontbay T, Colclough K, Patel KA, Berberoglu M. 2023. Expanding the phenotype of TRMT10A mutations: case report and a review of the existing cases. J Clin Res Pediatr Endocrinol 15: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E, Vivante A, Barel O, Levy-Shraga Y. 2022. TRMT10A mutation in a child with diabetes, short stature, microcephaly and hypoplastic kidneys. J Clin Res Pediatr Endocrinol 14: 227–232. 10.4274/jcrpe.galenos.2020.2020.0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassler SE, Bowles IE, Dey D, Jackman JE, Conn GL. 2022. Tied up in knots: untangling substrate recognition by the SPOUT methyltransferases. J Biol Chem 298: 102393. 10.1016/j.jbc.2022.102393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. 2021. The expanding world of tRNA modifications and their disease relevance. Nat Rev Mol Cell Biol 22: 375–392. 10.1038/s41580-021-00342-0 [DOI] [PubMed] [Google Scholar]
- Swinehart WE, Henderson JC, Jackman JE. 2013. Unexpected expansion of tRNA substrate recognition by the yeast m1G9 methyltransferase Trm10. RNA 19: 1137–1146. 10.1261/rna.039651.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasak M, Phizicky EM. 2022. Initiator tRNA lacking 1-methyladenosine is targeted by the rapid tRNA decay pathway in evolutionarily distant yeast species. PLoS Genet 18: e1010215. 10.1371/journal.pgen.1010215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres AG, Batlle E, Ribas de Pouplana L. 2014. Role of tRNA modifications in human diseases. Trends Mol Med 20: 306–314. 10.1016/j.molmed.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Vilardo E, Nachbagauer C, Buzet A, Taschner A, Holzmann J, Rossmanith W. 2012. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase—extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res 40: 11583–11593. 10.1093/nar/gks910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardo E, Amman F, Toth U, Kotter A, Helm M, Rossmanith W. 2020. Functional characterization of the human tRNA methyltransferases TRMT10A and TRMT10B. Nucleic Acids Res 48: 6157–6169. 10.1093/nar/gkaa353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple JM, Lane EA, Chernyakov I, D'Silva S, Phizicky EM. 2011. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev 25: 1173–1184. 10.1101/gad.2050711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew TW, McCreight L, Colclough K, Ellard S, Pearson ER. 2016. tRNA methyltransferase homologue gene TRMT10A mutation in young adult-onset diabetes with intellectual disability, microcephaly and epilepsy. Diabet Med 33: e21–e25. 10.1111/dme.13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JS, Yoon JH, Choi YJ, Son YJ, Kim S, Tong L, Chang JH. 2018. Molecular mechanism for the inhibition of DXO by adenosine 3′,5′-bisphosphate. Biochem Biophys Res Commun 504: 89–95. 10.1016/j.bbrc.2018.08.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung A, Kori M, Burundukov E, Ben-Yosef T, Tatoor Y, Granot E. 2015. Homozygous deletion of TRMT10A as part of a contiguous gene deletion in a syndrome of failure to thrive, delayed puberty, intellectual disability and diabetes mellitus. Am J Med Genet A 167: 3167–3173. 10.1002/ajmg.a.37341 [DOI] [PubMed] [Google Scholar]