Abstract
Objective:
Medical adhesives are used to secure wound care dressings and other critical devices to the skin. While high peel-strength adhesives provide more secure skin attachment, they are difficult to remove from the skin and are correlated with medical adhesive-related skin injuries (MARSI), including skin tears, and an increased risk of infection. Lower-adhesion medical tapes may be applied to avoid MARSI, leading to dressing or device dislodgement and further medical complications.
Method:
This paper reports on the clinical testing of a new, high-adhesion medical tape, ThermoTape (University of Washington, US), designed for low skin trauma upon release. ThermoTape was benchmarked with Tegaderm (3M, US) and Kind Removal Tape (KRT) (3M, US). All three tapes were applied to both the left and right forearm of healthy volunteers and were removed 24 hours later—the right arm without applying heat and the left arm by applying a heat pack for 30 seconds before removal. Tape wear, self-reported pain (0–10 scale) and skin redness 15 minutes after removal were recorded.
Results:
This was a 53-subject comparative, single-blind clinical trial. There were clinically and statistically significant results supporting reduced pain during removal of ThermoTape with warming, with an average 58% decrease in pain, paired with a statistically significant 45% reduction in skin redness (p<0.01 for both values). In contrast, there were statistically insignificant differences in pain and redness for removal of Tegaderm and KRT with warming. ThermoTape after warming, in comparison with Tegaderm without warming, produced a reduced pain score of >1 on the -10 Wong–Baker/Face pain scale, which was statistically significant (p<0.01).
Conclusion:
These results provide compelling evidence that warming ThermoTape prior to removal can reduce pain and injury when compared with standard medical tapes. This could allow for stronger attachment of wound care dressings and critical medical devices while reducing cases of MARSI.
Declaration of interest:
The authors declare no conflict of interest; the University of Washington will manage the patent filings and any future royalty sharing.
Keywords: acrylic-based adhesives, MARSI, medical adhesive, medical device, medical tape, skin injury, temperature-responsive adhesion, wound, wound care, wound dressing, wound healing
Medical adhesive tapes are an integral part of healthcare delivery due to their versatility in securing wound dressings or critical medical devices to the skin, such as intravenous (IV) lines and nasogastric tubes. Medical tapes consist of pressure-sensitive adhesive (PSA) applied to a backing that functions as a carrier for the adhesive, providing various structural and protective properties.1 The selection of a specific backing and PSA determines the characteristics of the tape, such as the adhesion level, water resistance and wear time.1–3 For example, silicone-based adhesives, such as Kind Removal Tape (KRT) (3M, US), generally have lower adhesion; and acrylic adhesives, such as Durapore (3M, US) and Tegaderm (3M, US), generally have higher adhesion.3,4 Long-term skin contact or repeated removal of high-adhesion tapes from the same location on the body can lead to painful and time-consuming removal processes which are correlated to medical adhesive-related skin injuries (MARSI).2 Furthermore, younger patients may recall and react to painful medical procedures well into their adulthood. The effects of negative pain experiences can result in anticipation of subsequent painful experiences and possible avoidance of medical care as an adult.5 Implementing solutions such as ThermoTape (University of Washington, US) provides a low-resource solution to improving the experience, and subsequent memories, around such painful experiences.5
MARSI is defined as ‘erythema and/or other manifestations of cutaneous abnormality that persists 30 minutes or more after the removal of the adhesive’.2 These injuries often occur during tape removal and include skin tears, blisters and stripping of the skin;4 they cause pain and discomfort and can increase the risk of infections, increase wound size and delay wound healing.4 All of these morbidities can increase medical costs, negatively impact patient safety and lead to lower patient satisfaction.6 MARSI is prevalent but is known to be underreported.4 Prevalence rates vary between studies; one study reported it to be as high as 13% in the general population,7 with 98.6% of nurses considering skin tears ‘common’ or ‘very common’.8 A cross-sectional, multicentre epidemiological study involving 697 adults aged 18–89 years showed a MARSI prevalence rate of 19.7%, noting that the risk increased for patients >50 years old.9 While MARSI can occur at any age, it is more common in the young and in older people, due to the fragility of their skin, with MARSI having an incidence rate of over 50% in paediatric care intensive care units.10 In addition to a lower quality of care, the additional cost of MARSI includes follow-up doctors’ visits in 10% of cases, additional medical supplies, and an average of 24 minutes of additional treatment time per patient.11
To avoid MARSI, low-adhesion tapes are often used as an alternative to higher-adhesion medical tapes.3 However, these low-adhesion attachment tapes are associated with a higher risk of device dislodgement, which is classified as a medical error.2,12 As a result, many nurses avoid low-adhesion medical tapes and are left with high-adhesion medical tapes that carry the risk of MARSI during removal.3 Even when nurses select high-adhesion tapes, these can still lead to dressing or device dislodgement.3 It has been reported that frequent and daily dislodgements occur, with 68% of respondents to a survey reporting ‘often’, ‘daily’ or ‘multiple times daily’ occurrence.13 For IV lines, the mean rate of dislodgement is 17.5%, with all of these cases risking medical complications that would be classified as a medical error.14 It was also found that dislodgement was responsible for 50% of all cases where an IV had to be replaced.14 The average cost for IV lines ranges between $28–35, although the actual cost is dependent on the type of IV used, geographical location (device costs appear to depend on location of purchaser) and the other medical technologies used to support it.14 When failure occurs, another IV is required, possibly leading to an extended hospital stay, an increase in treatment cost and infection.
IV placement is a painful experience, with no certainty that one attempt will achieve an adequate placement. The most common source of pain in children in hospitals is procedural pain.15 Increased IV placement events will negatively impact the patient’s experience. A catheter-related bloodstream infection (CR-BSI) results in an additional 7–20 days in a care facility, with additional costs of up to $56,000 per patient.14 The total annual cost of CR-BSI is $2.3 billion per year in US intensive care cases.14 Wound care dressing dislodgements lead to an exposed wound, which can lead to infection and extended healing times. Many of these dislodgements occur with commercialised high-adhesion medical tapes, while ThermoTape is a higher-adhesion medical tape. The current design of medical tapes may be at their maximum adhesion levels as they already result in a high incidence of MARSI. From our >100 stakeholder interviews we learned that nurses caring for children would prefer to have significantly higher skin adhesion to keep critical devices, such as IV lines and nasogastric tubes, secure and maintain wound care dressings to prevent infection, reinjury and other complications, but that their current high-adhesion tapes still lead to regular dislodgement.16 Nurses indicated that they must choose either high-adhesion tapes, which risks MARSI, or lower-adhesion tapes and increase the risk of dislodgement. From current reviews of the literature and our >100 interviews during a National Science Foundation’s Innovation Corps (NSF I-Corps) project, there is an unmet need for higher-adhesion medical tape that can be removed without the risk of MARSI.
ThermoTape is a high-adhesion tape that can transition to lower-adhesion by applying a heat pack at the time of removal. ThermoTape incorporates a high-adhesion PSA which addresses the unmet needs noted above. A recent ThermoTape publication on prototype development and pilot clinical testing demonstrated in vitro functionality, and the pilot clinical tests indicated that a larger blinded clinical study would be informative to clinicians.17 Using a temperature-controlled peel force instrument, the study found a 67% reduction in ThermoTape strength when removed from a low-density polyethylene substrate having been warmed to 45°C from 35°C, and a 66% reduction in pain was observed in human pilot clinical testing.14 The follow-on study (to a series of smaller clinical studies) presented in this paper is a clinical trial to compare ThermoTape performance against industry standard high- and low-adhesion medical tapes.
Method
Polymer synthesis
All polymers used in this study were prepared using the same copolymerisation procedure described in detail in a previous publication.17
Atomic force microscopy (AFM)
Surface morphology of the PSA dried coatings was analysed by tapping mode phase contrast AFM. Images were taken with an Oxford Instruments Asylum Research Jupiter XR AFM (Asylum Research, US) using BudgetSensors Tap300Al-G tips. The cantilever was tuned to a free amplitude of 300mV and operated at a set point optimised to obtain the highest possible average phase, ensuring the images were obtained in attractive mode within a range of phase between 100–180 degrees. AFM was performed on a sample from each batch of ThermoTape to ensure that each batch would perform as expected in the trial.
Peel testing
Peel testing was conducted using a test apparatus constructed based on Test Method F of ASTM D 3330/D 3330 M.18 Adhesive tape was applied to a temperature-controlled low-density polyethylene (LDPE) substrate after it reached a target temperature, and was left for one minute before initiating the test. The tape was secured to the substrate using a ChemInstruments RD-1000 rolldown machine with a 4.5-pound roller (ChemInstruments, US). A standard peel rate of 100mm/minute was used at a peel angle of 180°. Tape samples were tested at numerous substrate temperatures. The peel force data were analysed with MATLAB Version R2022a (The Math Works Inc., US). Peel testing was performed on a 1×3cm sample from each batch of ThermoTape to ensure that each batch would perform as expected in the trial.
Prototype tape fabrication
The temperature-sensitive polymer (TSP), synthesised by copolymerisation of 73% weight/weight (w/w) C14-alkyl acrylate and 27% w/w C18-alkyl acrylate, was dissolved in the solvent-based LOCTITE DURO-TAK AH 115 PSA (Henkel Corporation, Germany) by stirring for two hours at room temperature while 4.5μm-thick polyethylene terephthalate (PET) substrate sheets were prepared. Each sheet was fixed by its corners and side-centres to a sheet of paper which maintained PET rigidity during fabrication and handling. The substrate-liner sets were sanitised with a quick spray of general use quality electronics duster and then with a five-minute cycle from a model 256 ultraviolet-ozone (UVO) cleaner (Jetlight, US). They were then pneumatically fixed to the stage of a slot die sheet coater (FOM Technologies, FOM alphaSC, Denmark) inside a controlled-environment room that was set to 21°C and 40% humidity. The coating programme was set to dispense 600μl/min and travel the bed at 6cm/minute. A 30ml syringe was filled with the PSA-TSP mixture and loaded into a syringe pump. The slot die was primed with the mixture, positioned at one end of the PET sheet, and lowered to allow clearance for a 0.203mm feeler gauge between the meniscus guide plate and the substrate. The mixture was dispensed to allow meniscus formation with a length of 0.1–3.3cm and the coating programme was initiated. When the film deposition was complete the die head was raised, the film-PET-paper composite was removed from the pneumatic stage and put into a nitrogen cure oven (VWR International, US) at 120°C for 10 minutes. This process was repeated until the mixture was exhausted. Each cured composite was covered with a sheet of Slick Paper silicone release liner (Oil Slick, US) and rolled once with a 4.5kg roller to ensure uniform application. The uncoated and nonconforming areas were trimmed with a commercial grade paper cutter and a set of final cuts provided our desired 2.54×5.08±0.24cm geometry.
Tape preparation
Prior to application, ThermoTape and Tegaderm Transparent Dressing Film (10.1cm×10m, 3M, US) were prepared in 2.5×5.1cm samples. ThermoTape was prepared with a liner system that allowed for a similar application style as Tegaderm, where the adhesive is not touched during application. A 2.5×5.1cm section of KRT (2.5cm×5m, 3M, US) was peeled from the roll and cut at the time of tape application.
Study design and setting
This was a single-blind clinical trial; therefore, the subjects were unaware of the tape identities, or that warming would decrease adhesion in one of them. To minimise the risk to the subjects, the inclusion and exclusion criteria were selected to reduce the risk of skin tears during the trial. The inclusion criteria specified subjects aged 18–25 years old to reduce the risk of skin tears. The exclusion criteria included a history of eczema, MARSI or allergies to medical adhesives.
Flyers advertising the study were posted around the University of Washington Seattle campus with a link to a survey. The survey collected respondents’ sex and email addresses and verified that they were aged 18–25 years old and did not meet any of the exclusion criteria. If they were eligible for the study, they could then use a scheduler to select a time slot for tape application and removal. When the subjects arrived at their appointment, they were given a written consent form to read and sign before tape application. Tape application and removal took place in a temperature-controlled building.
Before tape application, both forearms were cleaned using a 70% isopropyl alcohol Prep Pad (Clever Health, US), with one wipe used per arm. A surgical skin marker (Viscot Medical, US) was used to draw lines to indicate the areas of tape application. The first area of tape application was 3.8cm distal to the elbow, with the second and third areas 5cm and 10cm distal to the first area, respectively. Following the pre-tape application preparations, ThermoTape, KRT and Tegaderm were applied to each forearm in a location specified by a spreadsheet. This spreadsheet was output by a Python program, which provided random placement locations for the three tapes on individual forearms for all 53 subjects. A single researcher applied and removed all tapes to and from the subjects to eliminate variability. The tapes were applied with the 5.1cm length wrapping around the forearm, as shown in Fig 1. After each application, a finger was used to rub the tape on the forearm to ensure that the tape was fully adhered. Once all tapes were applied, subjects were requested to not participate in strenuous physical activity and to not get the tapes wet for the duration of the 24-hour study. The limitation on strenuous physical activity was implemented to reduce sweat. The team wanted to limit moisture buildup under the tape and focus on demonstrating the outcome of pain reduction with applied heat. This moisture buildup could have reduced the peel force upon removal or led to increased tape wear. We wanted to compare ThermoTape performance against an industry standard that is used in hospitals. Future trials will allow a wide range of physical activity. Subjects were given an activity log to record any physical activity. The log included details on the activity, duration and the state of the tape after the activity.
Fig 1.
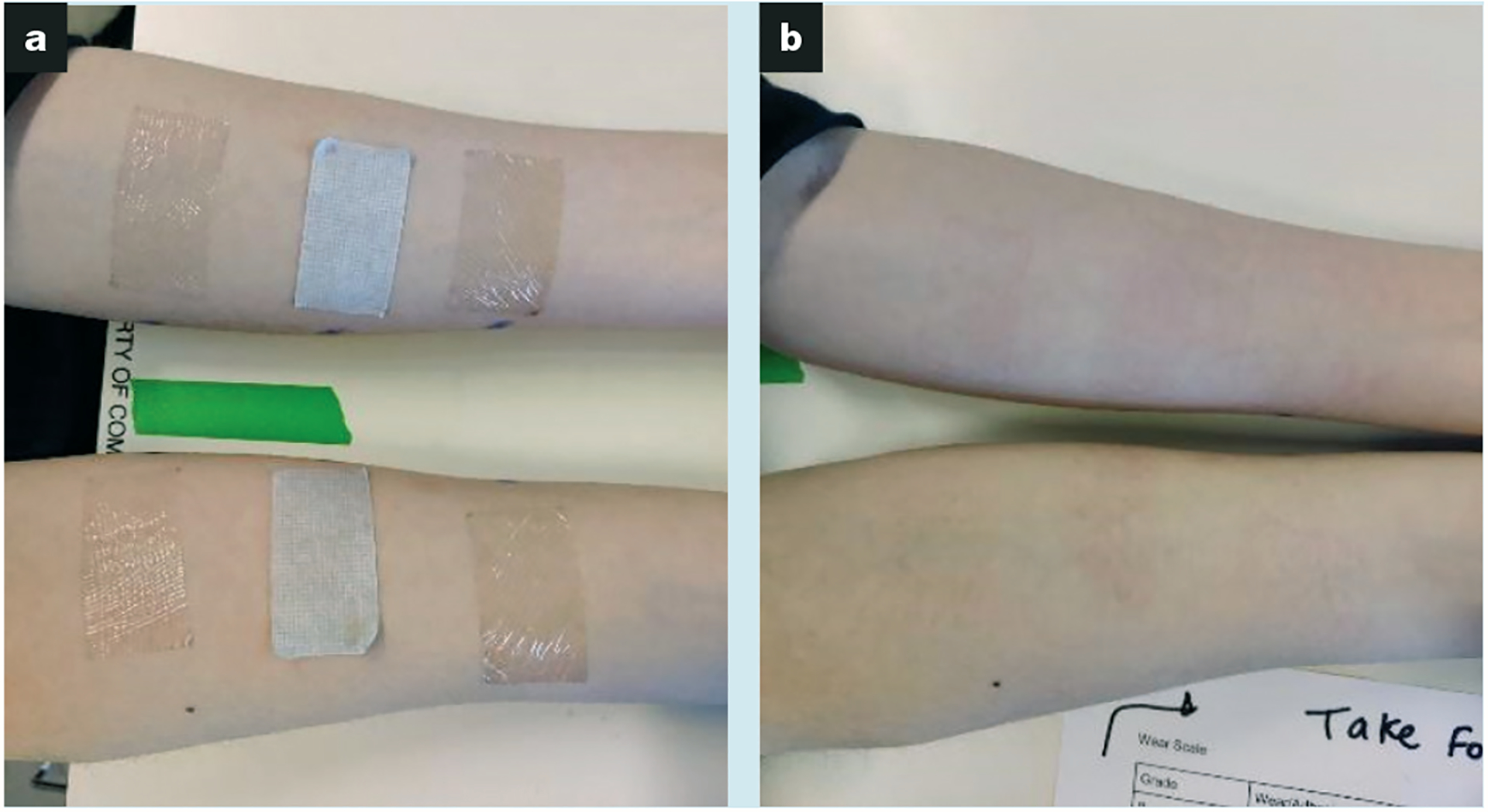
Subject forearms. At 24 hours after application and immediately before removal of ThermoTape, Kind Removal Tape and Tegaderm which were randomly placed on the left and right forearms (a); and at 15 minutes after removal to document the locations of any skin redness (b)
When subjects returned to have the tapes removed, the team inspected the activity log and ensured that subjects had not got the tape wet or participated in strenuous physical activity. Each piece of tape was analysed for wear before removal using a 0–7 scale, as shown in Table 1. A visual pain scale of 0–10 was placed in front of them, and the removal process began. After each piece was removed, the subject would be asked to report their pain on a 0–10 scale, as shown in Fig 2. Tapes were removed from the right arm first without the use of a heat pack. Removal was initiated at an already lifted corner, or if all corners were adhered, a corner was lifted, and the tape was peeled at 180° at a constant rate. The peel rate depended on the strength of the adhesion. If adhesion was high, the tape was peeled more slowly to avoid skin tears. If adhesion was low, the peel rate was faster. If a hair was encountered, the tape was removed following the root of the hair to the tip.
Table 1.
The wear scale used in this trial
| 0 | Test strip off |
| 1 | Test strip almost off (hanging) |
| 2 | 3/4 of test strip off |
| 3 | 1/2 of test strip off |
| 4 | 1/4 of test strip off |
| 5 | 3–4 edges lifted |
| 6 | 1–2 edges lifted |
| 7 | All corners adhered firmly |
Fig 2.
Wong-Baker FACES Pain Rating Scale
Tape on the subject’s left arm was removed following the same procedure as above, but a Dynarex Instant Hot Pack (Dynarex Corporation, US) was applied to each tape prior to removal. Prior to heat pack activation, the corner of each tape sample was lifted so that removal could begin immediately after heat pack application. The heat pack was then activated and kneaded for one minute before application and applied to the tape for 30 seconds before removal. Each piece was removed immediately after warming. The heat pack was kneaded for 20 seconds between each tape removal and then applied to the next tape, and the process was repeated.
Each subject was asked to wait for 15 minutes following tape removal to examine for skin redness caused by removal. As demonstrated by Krejsa et al.,20 there is little change in redness between tape removal and 30 minutes post-removal, so a shorter time was used. After 15 minutes, the redness from the removal site was visually inspected in comparison with surrounding skin, and recorded on a 0–4 scale, as shown in Table 2. The reduction in redness was found by calculating the percentage change in the recorded redness for tape removed with and without heat. Subjects were emailed a $50 Tango Card after completing the study.
Table 2.
The redness scale used in this trial
| 0 | None: no evidence of erythema other than natural skin tone |
| 1 | Slight: barely perceptible increase in light pink colouration; localised or diffuse |
| 2 | Mild: perceptible increase in light pink coloration; localised or diffuse |
| 3 | Moderate: diffuse pink colouration; localised or diffuse areas of reddened skin |
| 4 | Severe: intense redness, diffuse or localised |
In the case where a skin tear occurred, Vinyl gloves and Brava Adhesive Remover Wipes (Coloplast, US) were available to aid in tape removal. Neosporin (Johnson & Johnson Consumer Inc., US), and First Aid Sheer Adhesive Pads (7.6×10.2cm, Rite Aid, US) were available to treat the tear.
Statistical analysis
Statistical analysis was performed in Excel (Microsoft Corp., US), with two-tailed paired t-tests and binomial distribution functions used to show statistical significance. Results were considered significant when p≤0.05.
Ethical considerations
This study was approved by the University of Washington Institutional Review Board (IRB) committee B. This trial has been registered with ClinicalTrials.gov (NCT05449600).19 All subjects provided written informed consent before tape application. The inclusion and exclusion criteria lowered the risk of skin tears, and the researchers were prepared with Brava, Neosporin and bandages in case tape removal was not possible without inflicting skin tears or high levels of pain. Subjects were informed to indicate if pain was too high, in which case Brava would be used to assist with removal.
Results
The primary outcomes from this trial include quantifiable data on tape wear, pain during removal, and skin redness after removal. Tape was applied to 54 subjects, and 53 returned for tape removal; one subject did not return for removal. Of the 53 that completed the trial, the results from seven subjects were removed for the following reasons: two subjects went swimming; one had slight irritation around several tapes when she returned for removal and admitted that she had a history of mild eczema but did not report it on the survey. For one subject, the heat pack was too hot on her skin, and so a paper barrier was applied. To be consistent with all other subjects, this subject was removed. An additional three subjects had some tapes that had completely fallen off. For the analysis, these subjects were removed as there was no associated pain or redness values. This left a total of 47 subjects with complete data for comparative analysis.
As shown in Fig 3a, the percentage change in pain before and after heating was 58% for ThermoTape, −4.8% for Tegaderm and 8.3% for KRT. A paired t-test for ThermoTape with and without heat yielded a p-value of 3.74×10−13, 0.43 for Tegaderm, and 0.66 for KRT. A sign test showed the difference in pain before and after heating. An increase indicated that pain increased with heat, ‘same’ indicated there was no change in pain with heat, and a decrease indicated that pain decreased with heat. Fig 4 shows that, for ThermoTape, most subjects experienced a decrease in pain with heat, with just one subject having an increase. Out of 47 subjects, 40 reported a decrease in pain with heat, of whom 36/40 reported a decrease of ≥50%. Of the remaining 6/40 who reported no change in pain, four had pain values of 0 with and without heat, and so there was no possibility of a decrease. With Tegaderm and KRT, most subjects did not have a change in pain with heat, with a similar number showing either an increase or a decrease. A binomial distribution function was used on the number of increased and decreased values, with the ‘same’ values omitted. This yielded a p-value of 1.91×10−11 for ThermoTape, 0.66 for Tegaderm and 0.39 for KRT. Fig 3b shows the average difference in pain by subtracting the pain score with heat from the pain score without heat. The average difference in pain for ThermoTape was 1.57, with a paired t-test p-value of 6.11×10−13. Tegaderm and KRT had average differences in pain close to zero, with paired t-test p-values of 0.58 and 0.66, respectively. Comparing the clinically relevant conditions of Tegaderm with no heat and ThermoTape with heat, the average pain difference was 1.05, with ThermoTape having a lower average pain. The paired t-test p-value of these two datasets was 3.6×10−8. ThermoTape without heat and Tegaderm without heat had an average pain difference of 0.53, with ThermoTape having a higher average pain score.
Fig 3.
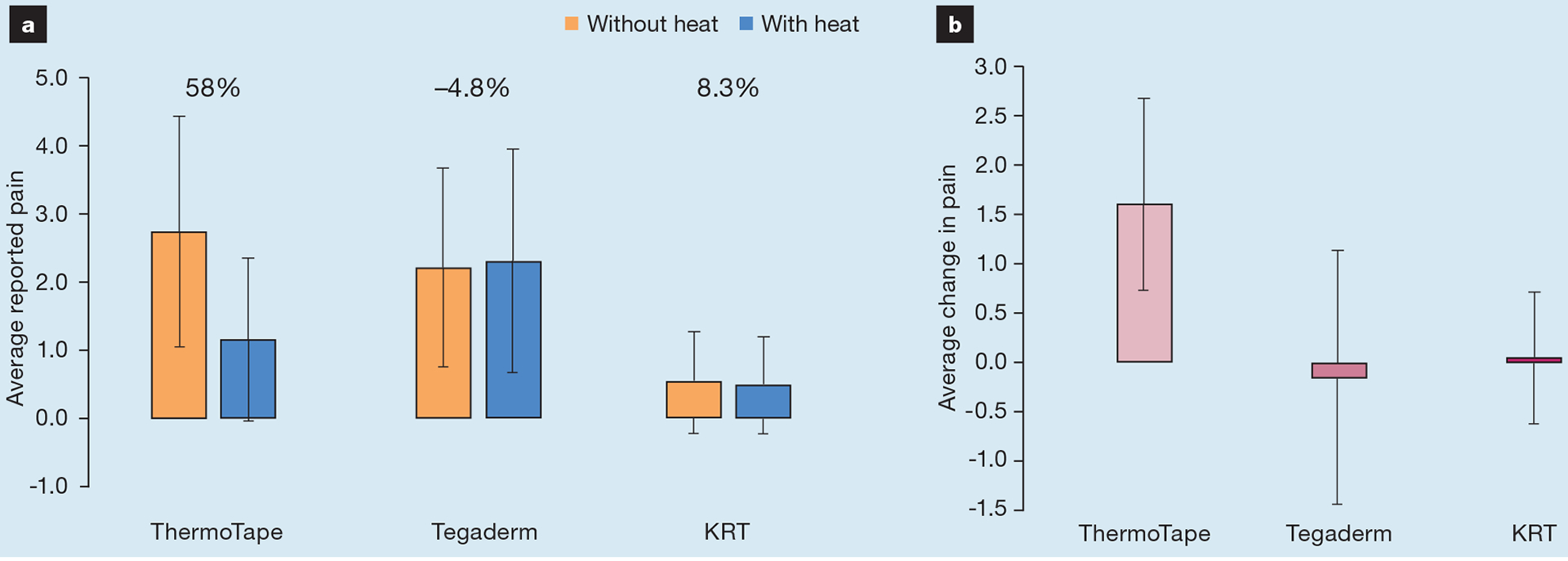
The average reported pain with and without heat. The percentage change in pain before and after heating (a) and the associated average change in pain when heat was applied (b). KRT—Kind Removal Tape
Fig 4.
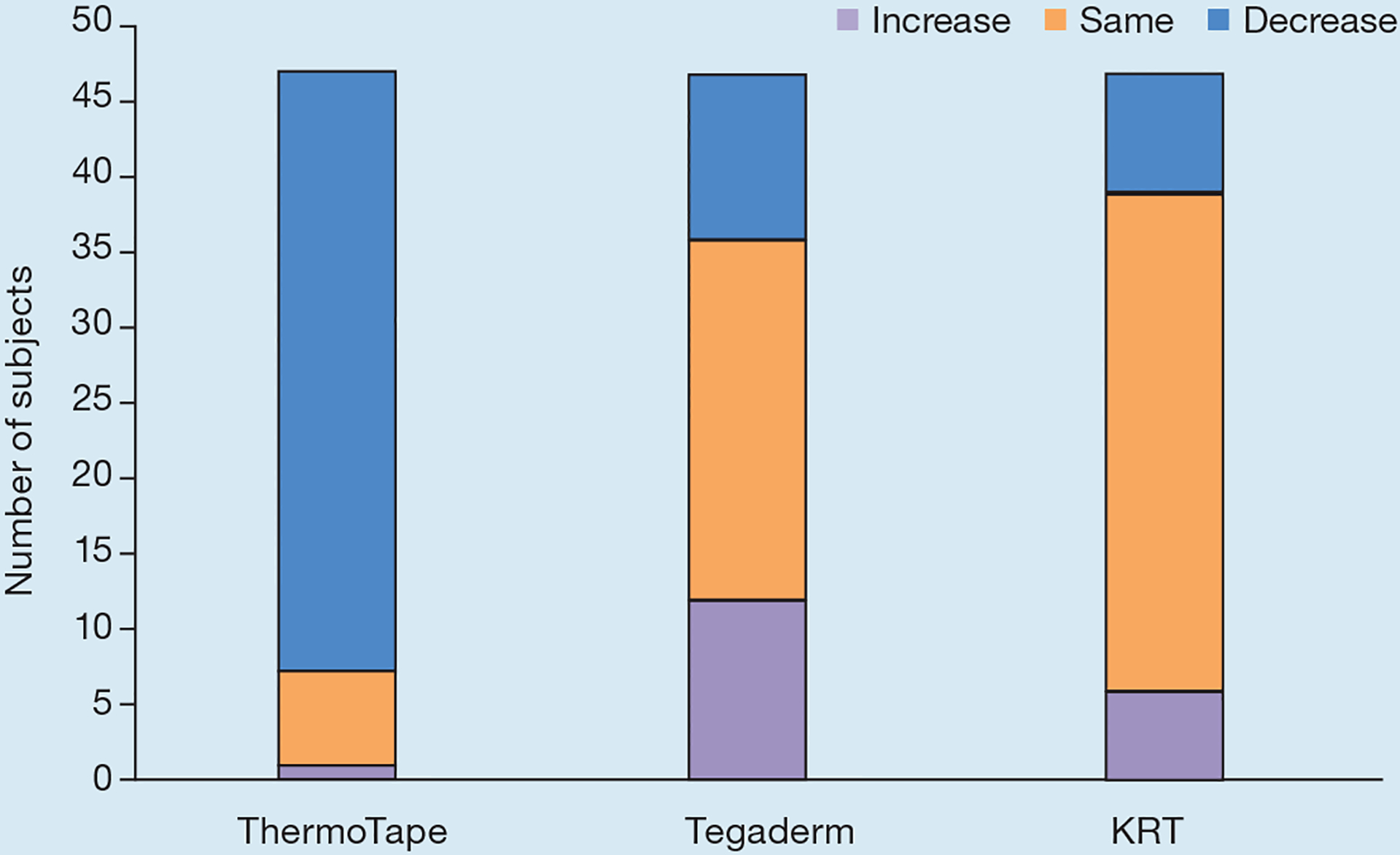
Sign test for the difference in pain before and after heat. An increase indicates that pain increased with heat, ‘same’ indicates there was no change in pain with heat, and a decrease indicates that pain decreased with heat. KRT—Kind Removal Tape
Wear values for both arms were combined, as shown in Fig 5. ThermoTape exhibited slightly more wear than Tegaderm, with a paired t-test p-value of 0.054. ThermoTape and Tegaderm exhibited less wear than KRT, with paired t-test p-values of 5.35×10−11 and 1.02×10−18, respectively.
Fig 5.
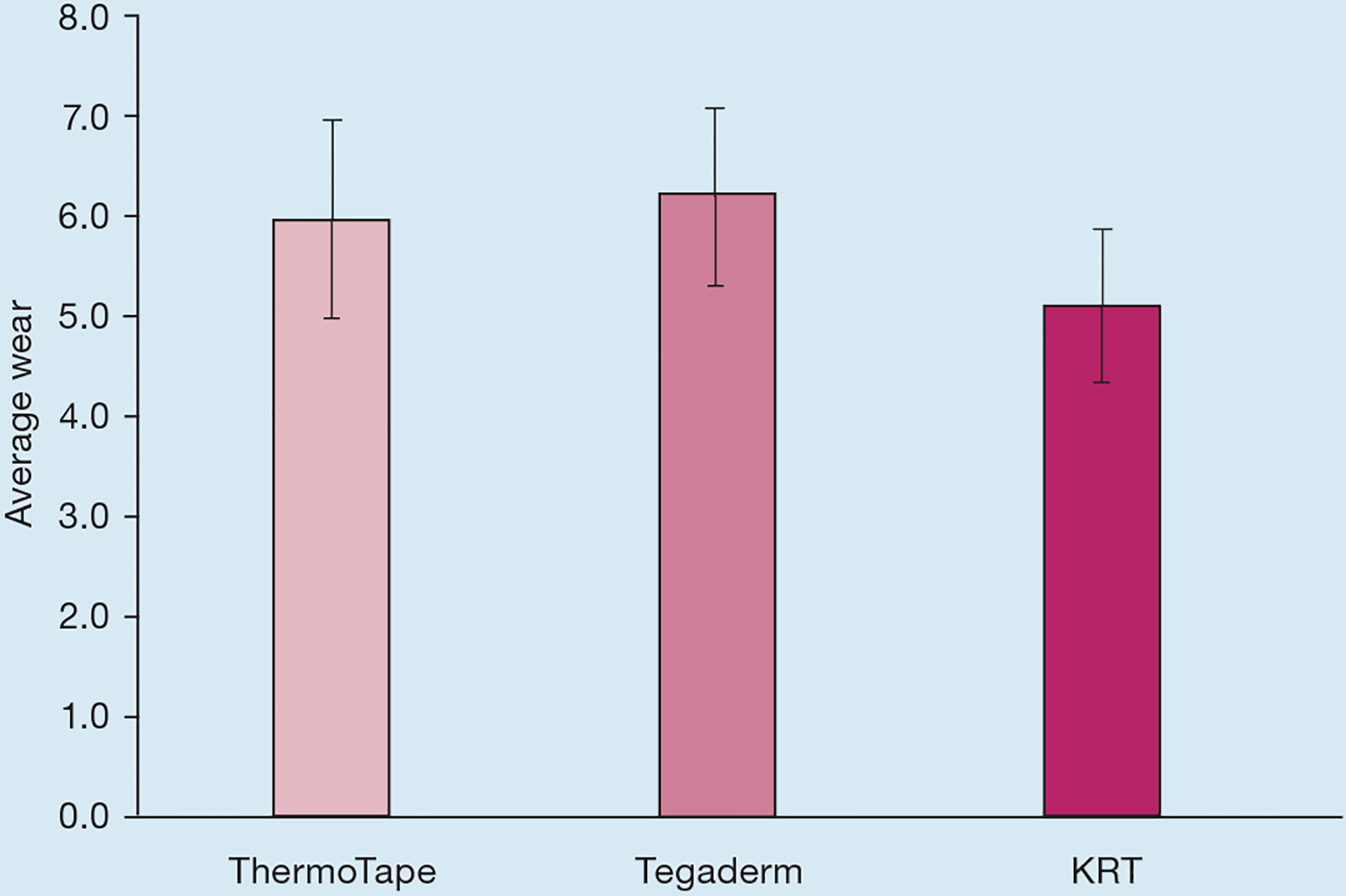
Average wear values 24 hours after application for ThermoTape, Tegaderm and Kind Removal Tape (KRT). The left and right forearms were combined, and so this data consists of 94 samples of each tape
As shown in Fig 6, ThermoTape had a 45% reduction in redness when heat was applied before removal, with an associated paired t-test p-value of 1.8×10−4. Tegaderm had a 23% increase in redness when heat was applied prior to removal, with an associated p-value of 0.07. KRT had a 4.5% increase in redness when heat was applied before removal, with an associated p-value of 0.8. As shown in the sign test (Fig 7), 18 subjects experienced a decrease in redness with heat, while two experienced an increase. Of the 27 subjects who experienced no change in redness, 15 were zeros with and without heat. Most subjects did not experience a change in redness for Tegaderm and KRT with and without heat. A binomial distribution function was used on the number of increased and decreased values, with the ‘same’ value left out. This yielded a p-value of 4.02×10−4 for ThermoTape, 0.18 for Tegaderm and 0.55 for KRT.
Fig 6.
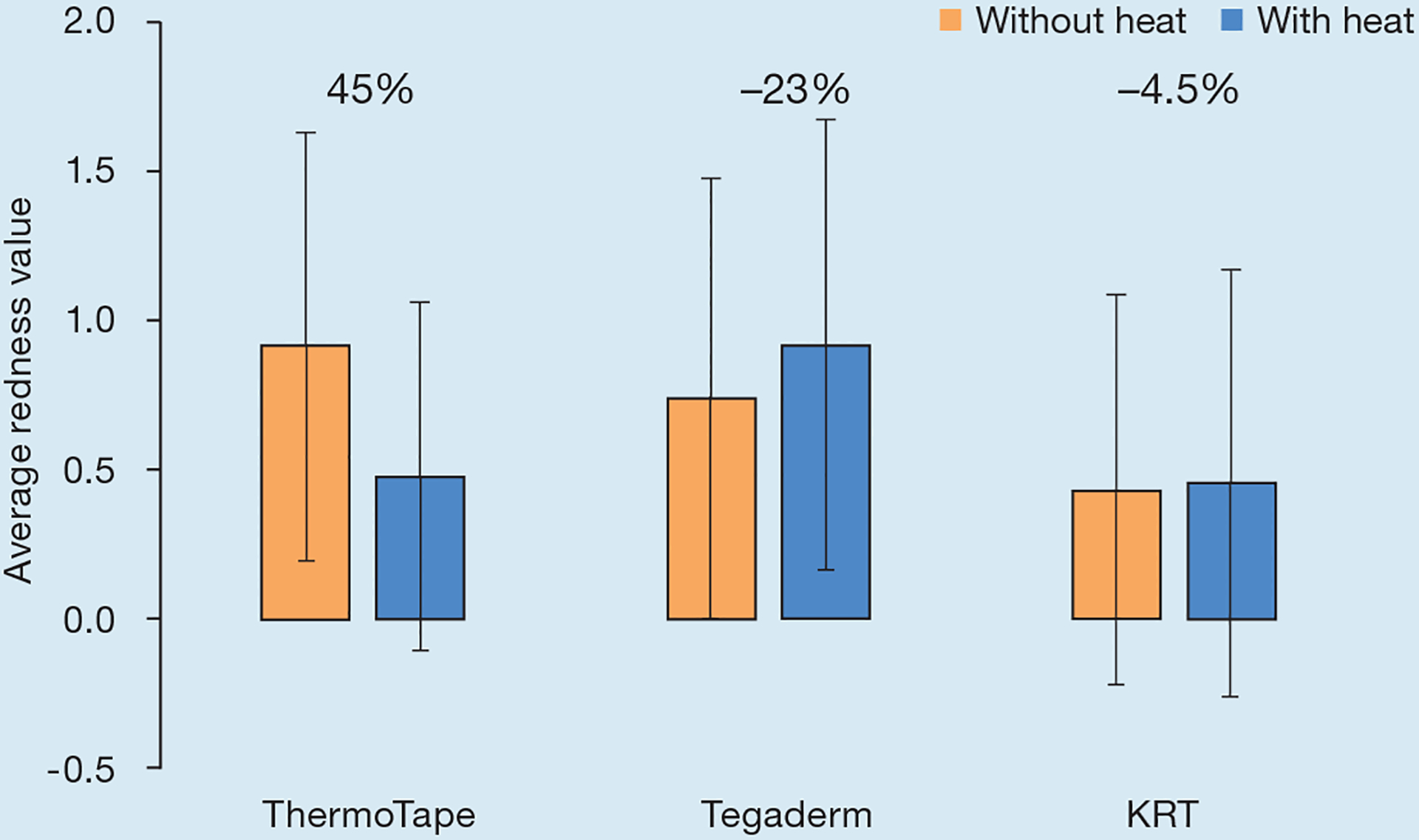
The average redness from tape removal with and without heat, with the percentage change in redness with and without heat reported on the chart. KRT—Kind Removal Tape
Fig 7.
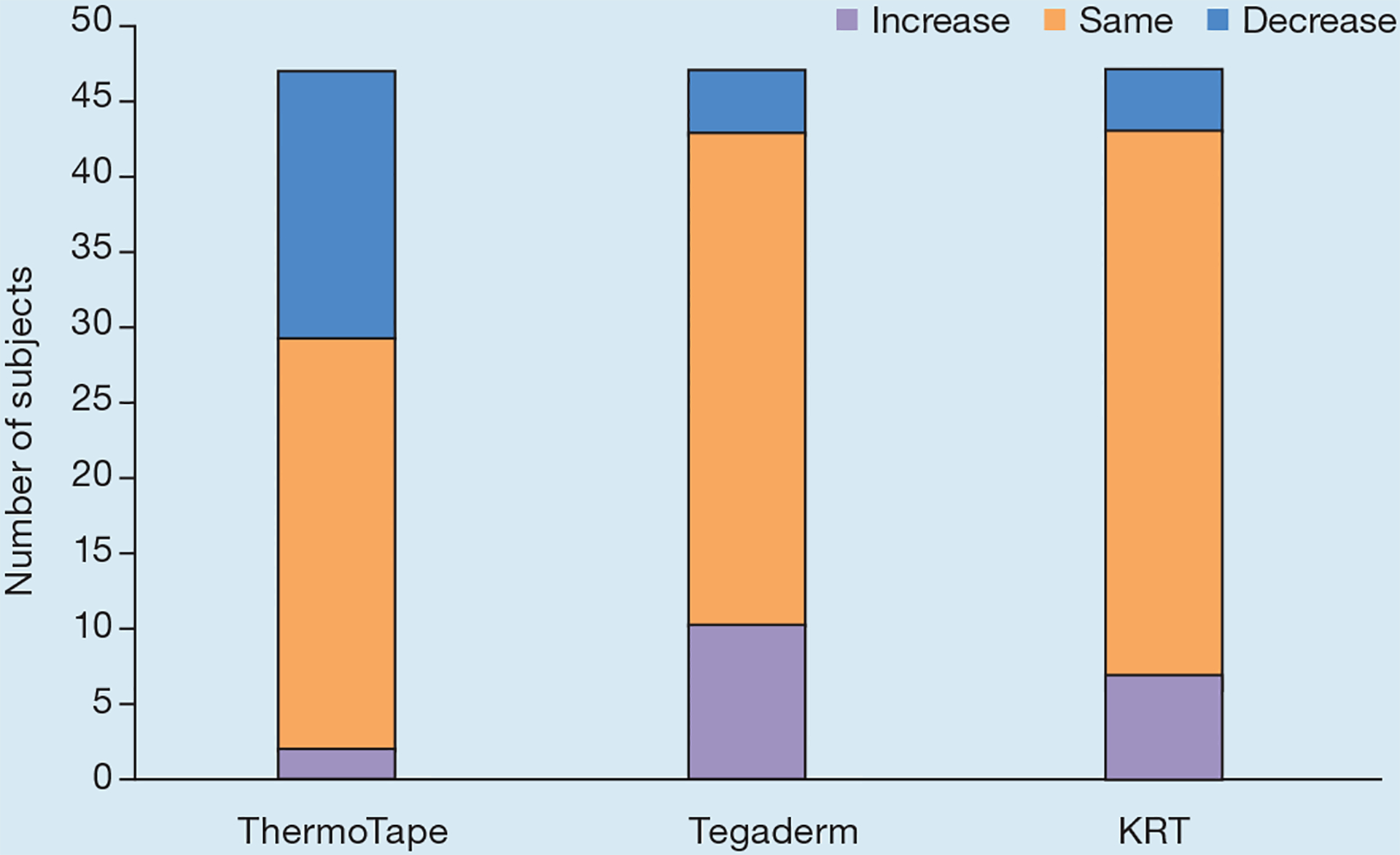
Sign test for the difference in redness before and after heat for ThermoTape, Tegaderm and Kind Removal Tape (KRT). An increase indicates that redness increased with heat, ‘same’ indicates there was no change in redness with heat, and a decrease indicates that redness decreased with heat
Our lean process controls, analysis feedback loops, and growing familiarity with the process allowed an increase in quality that was visible from the meniscus area reduction of the fabricated sheets and consistent AFM images, as reported in our previous publication.14 AFM testing of all 26 sheets from four batches yielded an average circle equivalent (CE) diameter of 48.72±13.04nm. AFM phase images from three sheets are shown in Fig 8. Peel testing from each batch verified that all batches used in the trial exhibited at least a 67% reduction in peel strength from 35°C to 45°C. The average peel strength reduction of the batches used in the trial was 72±4.8%.
Fig 8.
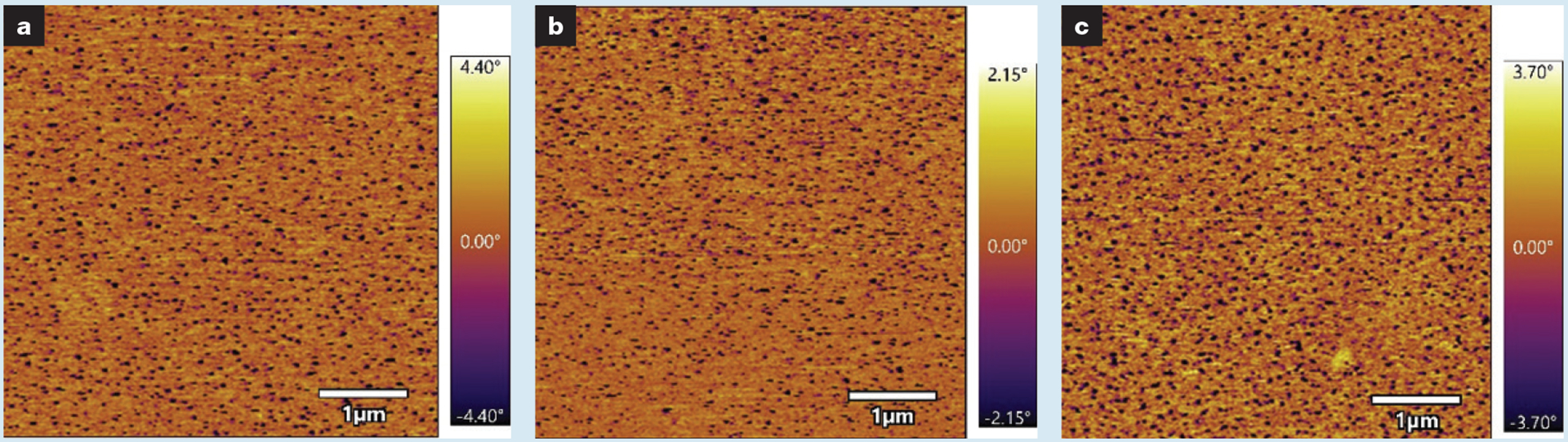
Atomic force microscopy phase images of ThermoTape samples used in the trial. Each of these images is from a separate sheet of ThermoTape showing similar nanodomain morphology and the level of process control. Areas of low phase (dark) correspond to temperature-sensitive polymer nanodomains
Discussion
In developing the first clinical trial for ThermoTape, previous studies were referenced in the development of the testing protocol. For the selection criteria, young adults with healthy skin were chosen as the test population to reduce the risk of skin tears. Other adhesive studies had a similar age range requirement paired with the exclusion criteria of eczema or adhesive allergies.21,22 Other trials generated randomised locations for medical adhesive placement with a computer program.20,23,24 Subjects were asked to avoid getting the adhesives wet to best test the adhesion of the tapes on skin, which was seen in several other studies.22–24 Tapes had been applied on the volar forearms with locations marked off for consistency in another study.18 Tapes had been rubbed following application to the skin to ensure that they would adhere consistently in other studies.21,23 Tape removal was performed similarly in another trial, where a corner was lifted, and then the tape was removed at a 180° angle.23 Tegaderm and KRT were selected as product benchmarks for the study, as both tapes had been used in previous studies and represented higher and lower-adhesion tapes, respectively.25,26 Many other studies focused on tape wear, pain self-reported from removal, and erythema (redness)—properties that were assessed in this trial following the measurement procedures from these previous studies.21–24,27 In some previous studies, a visual analogue scale (VAS) was used to measure pain; however, it was found that there is a 0.94 correlation factor between VAS and other pain scales, allowing the pain scale to be a preferential selection.22,23 The Wong–Baker/Faces pain scale is preferable for cross-cultural interactions when compared to VAS, and therefore Wong–Baker was selected.28 The wear and redness tables, as well as the Wong–Baker images, are from a clinical study by Krejsa et al.20 With a protocol based on previous medical adhesive studies, this is the first clinical trial of the initial prototype of ThermoTape, and the first temperature-sensitive, high-adhesion tape that has undergone a single-blind clinical trial to the authors’ knowledge.
The inclusion and exclusion criteria were chosen so that subjects had a minimal risk of skin tears during the trial. Given these precautions, no skin tears occurred during the trial. The trial provided statistically significant results supporting pain reduction of ThermoTape with warming. The minimum clinically significant difference in acute pain was defined in a previous study, with a threshold of 12 out of 100 for the VAS for the general population, and 10 out of 100 for children aged 8–15 years.29,30 Modifying this for our similar 0–10 scale, given the 0.94 correlation factor between the two scales, this would be around a 1.2 difference for the general population or 1.0 for children for clinical significance. Additionally, since the change in pain of 1.57 was above 1.2 on the 0–10 pain scale, this result is deemed clinically significant. This means that the patient can feel the difference between ThermoTape with and without heat. This is paired with statistically insignificant results supporting pain reduction for Tegaderm and KRT with warming. As a single-blind study, the subject was unaware of the tape identities and that warming would decrease adhesion in one of them. Additionally, tape location was randomised on each arm—so ThermoTape and Tegaderm may have been in the same forearm location for some subjects. As shown in Fig 1a, ThermoTape and Tegaderm were indistinguishable from each other on the skin, further reducing bias. This is further supported by the statistically significant ThermoTape sign test results, where 40/47 subjects reported a decrease in pain when heat was used to remove ThermoTape, with only one subject reporting an increase in pain. Coupled with the statistically insignificant Tegaderm and KRT test results, we can conclude that ThermoTape demonstrated a clinically noticeable decrease in pain when heat was applied before removal, and KRT and Tegaderm did not.
When focusing on the clinically relevant conditions, there is a statistically significant difference between ThermoTape in the way it was designed to be used (heated upon removal) and Tegaderm in the way it is used clinically (no heating upon removal). The pain difference between the two is 1.05. This is over the clinically significant threshold of 1.0 for children, and therefore children would be expected to feel the difference clinically. This does not exceed the clinically significant threshold of 1.2 for the general population, but it is close, so many patients will feel this difference clinically. ThermoTape had a higher pain value when removed without heat than Tegaderm with or without heat and so it likely had higher skin adhesion than Tegaderm over the 24-hour period.
ThermoTape had statistically significant results for reducing redness from tape removal when heat was applied, reducing redness by 45%. This indicates that ThermoTape with heat can not only reduce pain, but potentially reduce MARSI. Tegaderm exhibited a 23% increase in redness, and KRT a 4% increase in redness when heat was used. While these were not statistically significant results, they indicate that redness possibly increased with heat on these tapes. As they are not temperature-sensitive, the redness could result from warming of the skin with the heat pack instead of irritation or erythema. Despite this possible baseline increase in skin redness with heating, ThermoTape still exhibited a significant reduction in redness with heat. ThermoTape with heat had an average redness of 0.48, while KRT without heat had an average redness of 0.43 on the 0–4 scale. The associated paired t-test p-value was 0.69, so there is no statistically significant difference in redness from removal between the way ThermoTape should be used (with heat), and the way KRT should be used (without heat). This indicates that ThermoTape has a comparatively lower trauma release compared with KRT, which is a silicone tape specifically designed for low-trauma release. However, redness from KRT removal was not expected, as silicone adhesives are typically associated with the absence of skin redness.27 While 0.43 is low on the redness scale, the elevated temperatures in Seattle during the trial could have trapped sweat under the tape, which could have contributed to the observed redness.
As expected, ThermoTape and Tegaderm exhibited less wear than KRT, as KRT is a low-adhesion silicone tape. ThermoTape and Tegaderm did not have a statistically significant difference in wear. This was unexpected, as ThermoTape was made on a 4.5μm PET film backing, a stiff clear polymer which lacks breathability when compared with the more conformable Tegaderm polyurethane (PU) backing, which has a high moisture vapour transmission rate (MVTR). Sweat, reported on the activity logs of 13 subjects, was under their tape samples upon removal, or they had sweaty arms on tape application. Given that Seattle was amid a heatwave at the time of the study, a high MVTR was important to prevent moisture accumulation under the tape. The study was completed during the month of July 2022 with outdoor temperatures ranging from 21–32°C over the three weeks of the study. There were three cases in which tape samples fell off subjects, and they all occurred in the last week of the study, where high temperatures ranged from 32–34°C. Most of the samples that fell off were ThermoTape or Tegaderm, with KRT often staying on, likely due to the superior MVTR conferred by a woven backing. Several subjects had moisture accumulation under ThermoTape when it was removed. Despite the limitation of PET, ThermoTape did not have a statistically significant difference in wear when compared with Tegaderm. Considering the higher average pain of ThermoTape without heat compared with Tegaderm, and the similar average wear values of ThermoTape and Tegaderm, ThermoTape likely has slightly higher skin adhesion than Tegaderm. This stronger skin adhesion is desirable, as it enables stronger attachment of wound care dressings or critical medical devices to the skin, which could decrease accidental dislodgement. Dislodgement of a wound care dressing would expose the wound and could lead to infection, and dislodgement of a medical device, such as a peripheral IV line, could lead to severe medical complications. Having higher adhesion without the associated risk of MARSI would add value to the healthcare system.
A trial benchmark, KRT, had low pain and redness values in the trial but experienced the most wear among the three tapes. This was expected, as KRT is a low-adhesion silicone-based tape. In a comparative study with KRT and standard higher-adhesion tapes involving 200 nurses, 75% were dissatisfied with KRT due to unreliable adherence.27 A 2019 study showed there was little evidence that silicone tapes were gentler on skin than microporous tapes, with many studies funded by 3M, having small sample sizes and short wear duration.31 These low-adhesion tapes, such as KRT, are associated with a higher risk of device dislodgement, which was reflected in the high wear values during the trial.2,12 Given the risk of device dislodgement, nurses often choose higher-adhesion tapes, leading to a higher risk of MARSI, reserving KRT for only the highest-risk patients.
The other trial benchmark, Tegaderm, is a transparent medical dressing, which is available in individually-wrapped films. This is a higher-adhesion tape commonly used to secure wound care dressings and IV lines to the skin. For this study, we used Tegaderm cut into strips, instead of applying the entire dressing, so that all three tapes would be the same size, and Tegaderm and ThermoTape would have a similar appearance. Tegaderm has been used on all age groups and has proved to be more effective than other medical tapes at securing wound care dressings and IV lines.32 As noted in the European Wound Management Association (EWMA) position document, Pain at wound dressing changes, removing dressings should be done in a way that avoids unnecessary wound manipulation and prevents damages to the healing structures in and around the wound.33 Tegaderm without heat had higher redness and pain than ThermoTape with heat, and performed similarly to ThermoTape in wear. This indicates that ThermoTape may perform better than Tegaderm at maintaining wound integrity during dressing changes.
Lumina, a company in Sweden, has attempted to address the need for a higher-adhesion tape with low-trauma release. Lumina uses an optical wand to reduce tape adhesion before removal. The near-ultraviolet, light-actuated debonding method uses a photo-initiator that causes a chemical reaction which decreases the cohesiveness of the adhesive.34 This approach does change the tape adhesion and has been shown to reduce pain upon removal and increase patient satisfaction.35,36 Cohesive failure can leave adhesive residue on the removal surface, which needs to be removed by a solvent.37 Additionally, the optical wand must be determined safe for clinical use by a regulatory body, and it must always be available when tape is being removed. On top of increasing costs, nurses interviewed for NSF I-Corps said they did not want a separate device to remove medical tape, and instead preferred heat packs, as these are familiar and are readily available around the hospital.
Ultrathin 4.5μm PET was used for the ThermoTape backing. However, the industry standard is thicker, nonwoven PU, which is used for Tegaderm. Our prototype tape was made on PET for this clinical trial as it is simpler to do so as the PSA can be directly coated and dried on the PET backing. Ultrathin PET is a suitable short-term substitute for PU ThermoTape clinical testing. Future plans include making ThermoTape with a PU backing that is preferred for covering wound care dressings or peripheral IV lines for long-term wear. Because our PSA can be coated and dried on a liner and easily transferred to PU using a commercial laminator, future ThermoTape prototypes will be fabricated in a pilot roll-to-roll manufacturing facility.
Limitations
A limitation is that although the participants were considered blinded, KRT is visibly distinctive from ThermoTape and Tegaderm, so participants could be familiar with the product.
Special consideration was given to lean manufacturing methods. Each process was reviewed for opportunities to reduce time and budget consumption. Documentation, collaboration between researchers, continual dimensional analysis, cycle time analysis, advanced preparation of substrate materials, and overlap of independent processes were implemented towards the goal of an efficient and precisely repeatable fabrication. This process was rewarded with consistent AFM and peel testing results.
AFM and peel force results indicate improvements in ThermoTape from a previous publication.17 The average reduction in peel force from 35°C to 45°C is higher, which is coupled with a slight size increase in the average phase-separated nanometre-sized domains with a tighter standard deviation (SD). This is paired with generally small SD values, when comparing laboratory-scale ThermoTape prototypes with 3M products. ThermoTape had similar average pain SD values when compared with Tegaderm, suggesting that the ThermoTape batches used in this trial were consistently fabricated.
Conclusion
ThermoTape exhibited unique performance in this first clinical trial, and superior performance when compared to the benchmarks of Tegaderm and KRT, after short-term wear. ThermoTape demonstrated a 58% reduction in pain with heat, which was both clinically and statistically significant. This was coupled with a 45% reduction in redness for ThermoTape, indicating a possible reduction in MARSI in addition to a reduction in pain. ThermoTape without heat had higher pain values and similar wear to Tegaderm, indicating that ThermoTape potentially had higher skin adhesion than Tegaderm. This would enable stronger attachment of wound care dressings and medical devices to the skin. Comparing the clinically relevant conditions of Tegaderm with no heat and ThermoTape with heat, the average pain difference was 1.05, with ThermoTape having a lower average pain score. These results provide compelling evidence that warming ThermoTape prior to removal can reduce pain and injury when compared with Tegaderm, while also allowing for stronger attachment of wound care dressings and critical medical devices. Future clinical trials are planned using Seattle area hospital systems to test ThermoTape performance on patient populations.
Reflective questions.
What medical complications could arise from wound care dressing or device dislodgements?
What are some of the challenges and barriers to avoiding medical adhesive-related skin injuries and device dislodgement in a clinical setting with the existing solutions?
Heat packs were used as the heat source in this trial, but would they be the best option in a clinical setting? If not, what would be the best option and why?
Acknowledgements
The team would like to thank the Washington Clean Energy Testbeds for the use of their PSA coating and diagnostic equipment; Kevin C Cain, Senior Biostatistician at the Institute of Translational Health Sciences (ITHS), for his biostatistics mentorship; and Michael R Krejsa, Principal Applications Engineer at Henkel Corporation, for his technical guidance. Funding for the clinical trial testing and analysis was provided by ITHS New Interdisciplinary Academic Collaboration award (UL1 TR002319, www.iths.org). In preparation for this study, the manufacturing improvements and pilot clinical studies were supported by WE-REACH (WR-20-038, NIH U01 HL152401 and Washington Research Foundation). Clinical need analysis was provided by NSF I-Corps grant #2149741. This project was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1 TR002318. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.United States Food and Drug Administration Department of Health and Human Services. Code of Federal Regulations Title 21, Section 880.5240: Medical adhesive tape and adhesive bandage. https://tinyurl.com/mw9ewsa4 (accessed 31 August 2023)
- 2.McNichol L, Lund C, Rosen T, Gray M. Medical adhesives and patient safety: state of the science: consensus statements for the assessment, prevention, and treatment of adhesive-related skin injuries. J Wound Ostomy Continence Nurs 2013; 40(4):365–380. 10.1097/WON.0b013e3182995516 [DOI] [PubMed] [Google Scholar]
- 3.Taroc A-M. Staying out of sticky situations: how to choose the right tape for your patient. Wound Care Advis 2015; 4:21–26 [Google Scholar]
- 4.Fumarola S, Allaway R, Callaghan R et al. Overlooked and underestimated: medical adhesive-related skin injuries. J Wound Care 2020; 29(Sup3c):S1–S24. 10.12968/jowc.2020.29.Sup3c.S1 [DOI] [PubMed] [Google Scholar]
- 5.Noel M, McMurtry CM, Chambers CT, McGrath PJ, Children’s memory for painful procedures: the relationship of pain intensity, anxiety, and adult behaviors to subsequent recall. J Pediatr Psychol 2010; 35(6): 626–636, 10.1093/jpepsy/jsp096 [DOI] [PubMed] [Google Scholar]
- 6.Gao C, Yu C, Lin X et al. Incidence of and risk factors for medical adhesive-related skin injuries among patients: a cross-sectional study. J Wound Ostomy Continence Nurs 2020; 47(6):576–581. 10.1097/WON.0000000000000714 [DOI] [PubMed] [Google Scholar]
- 7.Farris MK, Petty M, Hamilton J et al. Medical adhesive-related skin injury prevalence among adult acute care patients: a single-center observational study. J Wound Ostomy Continence Nurs 2015; 42(6):589–598. 10.1097/WON.0000000000000179 [DOI] [PubMed] [Google Scholar]
- 8.McNichol L, Bianchi J. Medical adhesive-related skin injuries (MARSI) made easy. Wounds UK 2016;12(4):1–4 [Google Scholar]
- 9.Zhao H, He Y, Wei Q, Ying Y. Medical adhesive–related skin injury prevalence at the peripherally inserted central catheter insertion site: a cross-sectional, multiple-center study. J Wound Ostomy Continence Nurs 2018; 45(1):22–25. 10.1097/WON.0000000000000394 [DOI] [PubMed] [Google Scholar]
- 10.Kim MJ, Jang JM, Kim HK et al. Medical adhesives-related skin injury in a pediatric intensive care unit: a single-center observational study. J Wound Ostomy Continence Nurs 2019; 46(6):491–496. 10.1097/WON.0000000000000592 [DOI] [PubMed] [Google Scholar]
- 11.Maene B Hidden costs of medical tape-induced skin injuries. Wounds UK 2013; 9(1):46–50 [Google Scholar]
- 12.Tanios MA, Epstein SK, Livelo J, Teres D. Can we identify patients at high risk for unplanned extubation? A large-scale multidisciplinary survey. Respir Care 2010; 55(5):561–568 [PubMed] [Google Scholar]
- 13.Moureau N. Impact and safety associated with accidental dislodgement of vascular access devices: a survey of professions, settings, and devices. J Assoc Vasc Access 2018; 23(4):203–215. 10.1016/j.java.2018.07.002 [DOI] [Google Scholar]
- 14.Helm R, Klausner J, Klemperer J et al. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs 2015; 38(3):189–203. 10.1097/NAN.0000000000000100 [DOI] [PubMed] [Google Scholar]
- 15.Dastgheyb S, Fishlock K, Daskalakis C et al. Evaluating comfort measures for commonly performed painful procedures in pediatric patients. J Pain Res 2018; 11:1383–1390. 10.2147/JPR.S156136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Science Foundation. Award Abstract #2149741. https://tinyurl.com/vd9tbvyx (accessed 30 August 2023)
- 17.Swanson S, Bashmail R, Fellin C et al. Prototype development of a temperature-sensitive high-adhesion medical tape to reduce medical-adhesive-related skin injury and improve quality of care. Int J Mol Sci 2022; 23(13):7164. 10.3390/ijms23137164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ASTM International. Standard N. D3330/D330M. Standard test method for peel adhesion of pressure-sensitive tape. 2010. http://tinyurl.com/3c7vjecn (accessed 4 September 2023) [Google Scholar]
- 19.ClinicalTrials.gov. ThermoTape high-adhesion medical tape comparison study. http://tinyurl.com/fz32tnwt (accessed 4 September 2023)
- 20.Krejsa M, Luciano A, Butterworth D, Layser K. Clinical methods for evaluation of wear and pain in medical pressure sensitive adhesives. 2021. http://tinyurl.com/2fssfuta (accessed 4 September 2023)
- 21.Drzymalski DM, Ward K, Hernandez JM et al. The effect of Tegaderm versus EyeGard on eyelid erythema during general anesthesia: a randomized-controlled trial. Can J Anaesth 2020; 67(5):560–567. 10.1007/s12630-020-01588-6 [DOI] [PubMed] [Google Scholar]
- 22.Woo KY, Coutts PM, Price P et al. A randomized crossover investigation of pain at dressing change comparing 2 foam dressings. Adv Skin Wound Care 2009; 22(7):304–310. 10.1097/01.ASW.0000305483.60616.26 [DOI] [PubMed] [Google Scholar]
- 23.Grove GL, Zerweck CR, Houser TP et al. A randomized and controlled comparison of gentleness of 2 medical adhesive tapes in healthy human subjects. J Wound Ostomy Continence Nurs 2013; 40(1):51–59. 10.1097/WON.0b013e318276f2a4 [DOI] [PubMed] [Google Scholar]
- 24.Lin YS, Ting PS, Hsu KC. Comparison of silicone sheets and paper tape for the management of postoperative scars: a randomized comparative study. Adv Skin Wound Care 2020; 33(6):1–6. 10.1097/01.ASW.0000661932.67974.7d [DOI] [PubMed] [Google Scholar]
- 25.Grove GL, Zerweck CR, Ekholm BP et al. Randomized comparison of a silicone tape and a paper tape for gentleness in healthy children. J Wound Ostomy Continence Nurs 2014; 41(1):40–48. 10.1097/01.WON.0000436669.79024.b0 [DOI] [PubMed] [Google Scholar]
- 26.Zeng LA, Lie SA, Chong SY. Comparison of medical adhesive tapes in patients at risk of facial skin trauma under anesthesia. Anesthesiol Res Pract 2016; 2016:4878246. 10.1155/2016/4878246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manriquez S, Loperfido B, Smith G. Evaluation of a new silicone adhesive tape among clinicians caring for patients with fragile or at-risk skin. Adv Skin Wound Care 2014; 27(4):163–170. 10.1097/01.ASW.0000444646.43044.df [DOI] [PubMed] [Google Scholar]
- 28.Pathak A, Sharma S, Jensen MP. The utility and validity of pain intensity rating scales for use in developing countries. Pain Rep 2018; 3(5):e672. 10.1097/PR9.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J 2001; 18(3):205–207. 10.1136/emj.18.3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med 2001; 37(1):28–31. 10.1067/mem.2001.111517 [DOI] [PubMed] [Google Scholar]
- 31.Santos A, Terra A, Nogueira J et al. Silicone tape versus micropore tape to prevent medical adhesive-related skin injuries: systematic review and meta-analysis. J Bras Econ Saúde 2019; 11(3):271–282. 10.21115/JBES.v11.n3.p271-82 [DOI] [Google Scholar]
- 32.Kellam B, Fraze DE, Kanarek KS. Central line dressing material and neonatal skin integrity. Nutr Clin Pract 1988; 3(2):65–68. 10.1177/011542658800300265 [DOI] [PubMed] [Google Scholar]
- 33.European Wound Management Association. Position document. Pain at wound dressing changes. 2002. http://tinyurl.com/yedpdt36 (accessed 4 September 2023) [Google Scholar]
- 34.Tunius M Switchable adhesives, patent number AU2011234266B2. 2011. https://tinyurl.com/ye296ttm (accessed 14 September 2023)
- 35.Schmitz M, Rogmans S, Kasparek S, Mustafi N. Pilot-study Lumina switchable post-op dressing & postsurgical wounds: a non-interventional, non-placebo-controlled, national pilot study. J Surg 2020; 8(5):153–157. 10.11648/j.js.20200805.12 [DOI] [Google Scholar]
- 36.Schmitz M Pilot-study switchable film dressing and NPWT: a non-interventional, non-placebo-controlled, national pilot study. Wound Medicine 2019; 26(1):100153. 10.1016/j.wndm.2019.100153 [DOI] [Google Scholar]
- 37.Wokovich A, Prodduturi S, Doub W et al. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur J Pharm Biopharm 2006; 64(1):1–8. 10.1016/j.ejpb.2006.03.009 [DOI] [PubMed] [Google Scholar]


