Abstract
Diabetic kidney disease (DKD) is a common chronic microvascular complication of diabetes mellitus. Although studies have indicated the therapeutic potential of mesenchymal stem cells (MSCs) for DKD, the underlying molecular mechanisms remain unclear. Herein, we explored the renoprotective effect of placenta-derived MSCs (P-MSCs) and the potential mechanism of SIRT1/FOXO1 pathway-mediated autophagy in DKD. The urine microalbumin/creatinine ratio was determined using ELISA, and renal pathological changes were detected by special staining techniques. Immunofluorescence was used for detecting the renal tissue expression of podocin and nephrin; immunohistochemistry for the renal expression of autophagy-related proteins (LC3, Beclin-1, SIRT1, and FOXO1); and western blotting and PCR for the expression of podocyte autophagy- and pathway-related indicators. We found that P-MSCs ameliorated renal tubular injury and glomerular mesangial matrix deposition and alleviated podocyte damage in DKD rats. PMSCs enhanced autophagy levels and increased SIRT1 and FOXO1 expression in DKD rat renal tissue, whereas the autophagy inhibitor 3-methyladenine significantly attenuated the renoprotective effect of P-MSCs. P-MSCs improved HG-induced Mouse podocyte clone5(MPC5)injury, increased podocyte autophagy, and upregulated SIRT1 and FOXO1 expression. Moreover, downregulation of SIRT1 expression blocked the P-MSC-mediated enhancement of podocyte autophagy and improvement of podocyte injury. Thus, P-MSCs can significantly improve renal damage and reduce podocyte injury in DKD rats by modulating the SIRT1/FOXO1 pathway and enhancing podocyte autophagy.
Keywords: Placenta-derived mesenchymal stem cells, autophagy, diabetic kidney disease, SIRT1/FOXO1 pathway, podocyte injury
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disease common worldwide. In 2015, an estimated 415 million people were living with diabetes globally, which was almost double the number in 2000, and the number is expected to increase to 642 million by 2040 [1]. Diabetic kidney disease (DKD) is a serious microvascular complication of diabetes mellitus, affecting 35–40% of patients with diabetes [2], and is one of the main causes of end-stage renal disease (ESRD). Its pathological features include capillary basement membrane thickening and diffuse and nodular glomerulosclerosis. In the early stages of DKD, glomerular lesions are characterized by hyperfiltration and increased albumin excretion. Albuminuria is a major characteristic of DKD and is caused by failure of the glomerular filtration barrier (GFB), which is composed of glomerular endothelial cells, glomerular basement membrane (GBM), and podocytes. Diabetic nephropathy is closely related to podocyte injury, one of the earliest glomerular injuries that contributes to the occurrence and development of DKD, in the early stage of diabetes [3, 4]. As the disease progresses, proteinuria gradually increases, the estimated glomerular filtration rate gradually decreases, and renal fibrosis leads to renal failure [5]. Glomerular hyperfiltration is associated with abnormal hemodynamics, glucose metabolism, and genetic susceptibility. However, the specific molecular mechanisms underlying DKD are not fully understood.
Autophagy is a cellular process that degrades damaged organelles and malformed or nonfunctional proteins [6, 7]. Under physiological conditions, autophagy occurs at the basal level to maintain homeostasis [8]. However, it induces nutrient regeneration and cell death under various stress conditions [9, 10]. Autophagy is essential for maintaining podocyte homeostasis. Impaired autophagy in podocytes leads to GFB dysfunction, macroalbuminuria, and severe glomerulosclerosis [11]. The occurrence and development of DKD are related to many pathological factors, including the downregulation of autophagy, podocyte apoptosis and shedding, and epithelial-mesenchymal transformation (EMT) [12–14]. Some studies have shown that autophagy is inhibited in the kidney tissues of rats in a nephritis model of diabetes mellitus. Therefore, local renal autophagy is a potential therapeutic target for DKD [15, 16]. The phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway is a well-known upstream signaling pathway of autophagy [17]. SIRT1 is a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase that directly affects autophagy. It is also an important regulator of the transcription factor forkhead box O1 (FOXO1). Its deacetylates FOXO1 to upregulate its expression, which in turn induces autophagy to reduce the renal damage caused by oxygen free radicals [18, 19]. Thus, SIRT1/FOXO1 signaling pathway is associated with autophagy.
Mesenchymal Stem Cells (MSCs) have strong self-renewal ability, differentiation potential, anabolic activity, immunomodulatory effects, and can secrete paracrine anti-inflammatory and cytoprotective factors [20–22], making them widely applicable in the field of regenerative medicine. Therefore, MSCs have promising prospects in the treatment of DKD. Yue Y et al. [23] revealed that human umbilical cord-derived MSCs (HUCDMSCs) therapy effectively preserved the residual renal function, reduced proteinuria and remarkably reduced the inflammation, oxidative stress, and mitochondrial damage in DKD rats. The potential renoprotective effects of MSC-derived exosomes are based on their upregulation of autophagy and inhibition of the mTOR pathway, as well as their anti-fibrotic effects in diabetic nephropathy models [24]. JUNG et al. [25] found that placental chorion plate-derived MSCs (CP-MSCs) significantly increased the level of autophagy in the liver of rats injured by carbon tetrachloride (CCl4). In addition, co-culture of CCl4-treated primary rat hepatocytes with CP-MSCs reduced necrotic cells and increased autophagy signals and regenerative factors. SIRT1 regulates the deacetylation of FOXO1 and plays a crucial role in regulating cellular autophagy. Studies have shown that MSCs enhance autophagy and resist apoptosis [26]. In a study in which bone marrow-derived MSCs (BM-MSCs) were co-cultured with INS-1 cells exposed to high glucose (HG) medium, BM-MSCs enhanced the expression of Beclin-1 and LC3-II, increased the formation of autophagosomes and autolysosomes, and decreased cell apoptosis [27]. In conclusion, the therapeutic effects of MSCs are associated with alterations in autophagy levels in tissues and host cells. Placenta-derived MSCs (P-MSCs) are easier to obtain than MSCs from other sources and do not require invasive procedures. Therefore, they have become an ideal source of stem cells for regenerative medicine. However, to our knowledge, there are few relevant studies on the efficacy and safety of P-MSCs in the treatment of diabetic nephropathy.
In this study, we established a streptozotocin (STZ)-induced diabetes rat model and an HG-induced podocyte injury model to determine whether P-MSCs play a role in renal protection by regulating autophagy, and to explore the potential mechanism of SIRT1/FOXO1 pathway-mediated autophagy in P-MSC treatment of renal injury in the two models.
2. Methods
2.1. Experimental animals
Male Sprague–Dawley (SD) rats (210 ± 10 g, 6 weeks old) were purchased from Hunan Laike Jingda Experimental Animal Co., Ltd. (Changsha, China). They were fed at 26 °C, 12 h light/dark cycle and they were free to eat and drink. All experiments were conducted in accordance with the relevant regulations for animal experimentation and ethics. Our study protocol has been approved by the Research Committee of the First Affiliated Hospital of Nanchang University (Approval number: 2020-04).
The rats were randomly divided into four groups: NC, DKD, P-MSCs, and P-MSC + 3-MA group, with 10 rats per group. The NC group was fed normally and injected with the same dose of normal saline, whereas the other groups were injected intraperitoneally with STZ (60 mg/kg). After 72 h, BG was detected in the tail vein for three consecutive days using a BG meter, and the random BG level was higher than 16.7 mmol/L three consecutive times, indicating that the T1DM model was successfully established. After eight weeks of continuous feeding, T1DM rats were maintained in a state of chronic hyperglycemia for a long time, resulting in diabetic chronic kidney injury. After eight weeks, the random BG level was higher than 16.7 mmol/L for three consecutive times, and they were included in the DKD model group. In the P-MSC and P-MSC + 3-MA groups, P-MSCs (1 × 106) were injected via the tail vein once a week, three times. The P-MSC + 3-MA group was intraperitoneally injected with 3-MA (15 mg/Kg/d) for eight weeks.
The body weights of the rats were recorded once a week, BG was measured once every 2 weeks, and urine protein was collected once in 24 h. After the end of the treatment (at the end of the 8th week), urine samples were collected to detect urinary microalbumin (mALB) and urinary creatinine (Ucr). Rats in each group were anesthetized by intraperitoneal injection of 10% chloral hydrate, and blood and kidney tissues were collected. Owing to excessive fluctuations in short-term BG levels and glucose intolerance, seven rats died by the end of 8 weeks of treatment, including two rats in the DKD group, one rat in the P-MSC group, and four rats in the P-MSC + 3-MA group.
2.2. Detection of renal function
BG, TG, LDL-C, and BUN levels were measured using an automatic biochemical analyzer at the First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China. Rat urinary microalbumin was detected using a rat microalbuminuria (MAU/ALB) ELISA Kit (Cusabio) according to the manufacturer’s instructions. Rat urinary creatinine was detected using a creatinine assay kit (R&D Systems China Co., Ltd.), and the UACR was calculated and expressed in mg/g.
2.3. Histopathological and immunohistochemical analysis
Kidney tissues were fixed with 4% paraformaldehyde and embedded in paraffin to prepare 4 μm tissue sections for HE and PAS staining. Based on the swelling of renal tubular epithelial cells, intertubular hemorrhage, vacuolar degeneration, and necrotic tubules, histopathological changes were evaluated according to the degree of 0–3 tubular damage. The criteria were as follows: 0, no damage; 1, <25%; 2, 25–50%; 3, >50%.
Immunohistochemical staining was performed using anti-SIRT1 (Abcam, Cambridge, MA, USA), anti-FoxO1 (Cell Signaling Technology, Danvers, MA, USA), anti-Beclin1 (Proteintech, Wuhan, China), and anti-LC3 (Proteintech) primary antibodies. Light microscopy and image acquisition were performed, and ImageJ software was used for semi-quantitative analysis of specific protein expression.
2.4. Immunofluorescence staining
The frozen kidney tissue of rats was cut into 5 μm thick sections for indirect immunofluorescence staining of podocyte markers podocin and nephrin. Rabbit polyclonal antibodies against podocin (1:200 dilution, Proteintech) and nephrin (1:200 dilution, Abcam) were used as primary antibodies. Cy3-labeled goat anti-rabbit immunoglobulin (Servicebio) was used as a secondary antibody.
Cells cultured on glass slides were washed twice with cold phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 20 min. After washing thrice with phosphate buffered saline (PBS), the cells were treated with 0.1% Triton X-100 for 5 min, blocked with 3% fetal bovine serum (FBS) in PBS for 30 min at room temperature, incubated with the specific primary antibody, podocin (1:200 dilution, Proteintech), and then stained with Cy3-labeled secondary antibody. Semi-quantitative analysis of specific protein expression was performed using fluorescence microscopy, and the mean gray value, representing the mean fluorescence intensity, was analyzed using ImageJ.
2.5. Cell culture and treatment
Mouse podocyte clone5(MPC5) was purchased from Guangzhou Gineo Biological Technology Co., Ltd. (Guangzhou, Guangdong, China). Human P-MSCs were provided by Jiangxi Han’s United Stem Cell Technology Co. Ltd. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Solarbio Science & Technology Co., Ltd., Beijing, China) containing 10% FBS. Cells were treated with different concentrations of HG(50mM), and MPC5 cells and P-MSCs were co-cultured at a ratio of 10:1.
2.6. Cell transfection
Mouse podocyte cells were transfected with siRNAs targeting SIRT1 (Santa Cruz Biotechnology, Dallas, TX, USA), or control (negative control) with Lipofectamine 2000 (Thermo Fisher Scientific, Shanghai, China). After 24 h of transfection, the cells were treated with P-MSCs and HG.
2.7. Western blotting
Total protein was extracted from MPC5 cells using RIPA lysis buffer (Applygen, Beijing, China), and protein concentration was determined using a protein detection kit (TransGen Biotech, Beijing, China). The primary antibodies used in this study were podocin (1:500; Proteintech); Beclin-1 (1:1000; Proteintech), LC3 (1:500; Proteintech), FOXO1 (1:1000; Cell Signaling Technology), and SIRT1 (1:1000; Abcam). ECL (Tiangen Biochemical Technology, Beijing, China) was used to detect immunoreactive bands after incubation with the corresponding horseradish peroxidase-conjugated secondary antibodies.
2.8. RT-PCR analysis
Total RNA from MPC5 cells was prepared using the TRIzol RNA isolation system, according to the manufacturer’s instructions (Applygen, Beijing, China), and the concentration of the extracted total RNA was detected using NanoDrop2000. The first strand of cDNA was synthesized with 2 μg of RNA and 20 μL of reaction buffer at 42 °C using the RT/RI Enzyme Mix (Tiangen Biochemical Technology) and random primers. The amplification reaction was performed on a real-time fluorescent quantitative PCR instrument (Thermo Fisher Scientific, Shanghai, China) using 1 μL cDNA as a template and a standard PCR kit (QIAgen, Germany). The sequences of primer pairs were as follows: SIRT1 (forward) 5′-AGAACCACCAAAGCGGAAAA-3′ and (reverse) 5′-AATCCCACAGGAGACAGAAACC-3′; β-actin (forward) 5′-AACAGTCCGCCTAGAAGCAC-3′ and (reverse) 5′-CGTTGACATCCGTAAAGACC-3′.
2.9. Statistical analysis
All statistical results were expressed as mean ± standard deviation (Mean ± SD). The experimental data were sorted and analyzed using SPSS25.0 software. One-way analysis of variance and least significant difference tests were used to compare differences between groups. A p-value <0.05 represents a statistically significant difference.
3. Results
3.1. P-MSCs reduced blood glucose and lipid, improved renal function and pathological injury in DKD rats
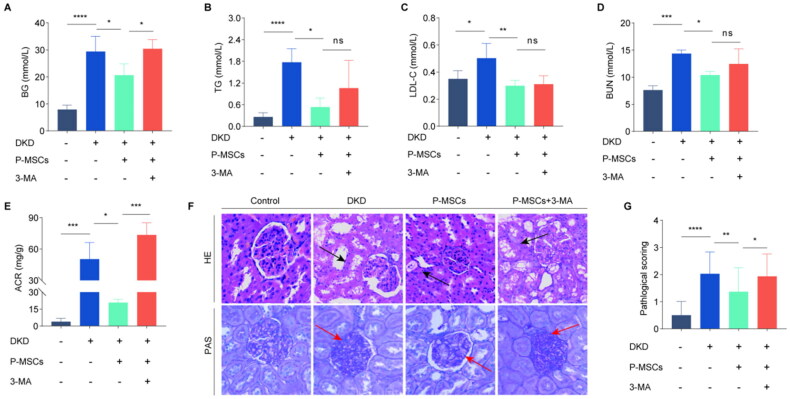
After 8 weeks of P-MSCs and 3-methyladenine (3-MA) treatment, blood glucose (BG), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and blood urea nitrogen (BUN) levels and urinary albumin-creatinine ratio (UACR) were significantly lower in the treatment group than in the DKD group (p < 0.01) (Figure 1(A,E)). 3-MA inhibited the effects of P-MSCs on BG and UACR levels in DKD rats. Compared with the P-MSCs group, the LDL-C, TG, and BUN levels in the P-MSCs + 3-MA group increased, but the differences were not statistically significant (p > 0.05). Hematoxylin and eosin (H&E) staining was used to evaluate the degree of renal tubule injury in rat kidney tissue. The results showed that renal tubular epithelial cells in DKD rats were swollen, vacuolated, and necrotic, and that P-MSCs improved the pathological changes in the renal tubules (Figure 1(F,G)). P-MSCs significantly reduced renal tubular injury (p < 0.01)), whereas 3-MA inhibited the protective effects of P-MSCs on renal tubular injury (p < 0.05). Proliferation of the mesangial matrix was evaluated using periodic acid–Schiff (PAS) staining. The results showed a significant increase in the number of glomerular mesangial cells, deposition of mesangial matrix, and thickening of basement membrane in DKD rats. P-MSCs significantly improved mesangial matrix proliferation, whereas 3-MA inhibited the effect of P-MSCs on the pathological structure (Figure 1F). These observations clearly indicate that P-MSCs can improve glucose and lipid metabolism and renal function and reduce renal injury in DKD rats.
Figure 1.
Effects of placenta-derived mesenchymal stem cells (P-MSCs) on blood glucose (BG), lipid, renal function, and renal pathological structure in diabetic rats. A: Effect of P-MSCs on BG in diabetic kidney disease (DKD) rats; B: Effect of P-MSCs on triglyceride (TG) in DKD rats; C: Effect of P-MSCs on low-density lipoprotein cholesterol (LDL-C) in DKD rats; D: Effect of P-MSCs on blood urea nitrogen (BUN) in DKD rats; E: Effect of P-MSCs on urinary albumin-to-creatinine ratio (UACR) in DKD rats; F: Effect of P-MSCs on renal tubule and glomerulus in DKD rats; G: Pathological score of renal tubular injury(there are 6 rats in each group, and 5 pathological pictures are randomly selected for each group, so there are 30 pictures in each group to analyze the pathological score.)(nsP >0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3.2. P-MSCs alleviated podocyte damage in DKD rats
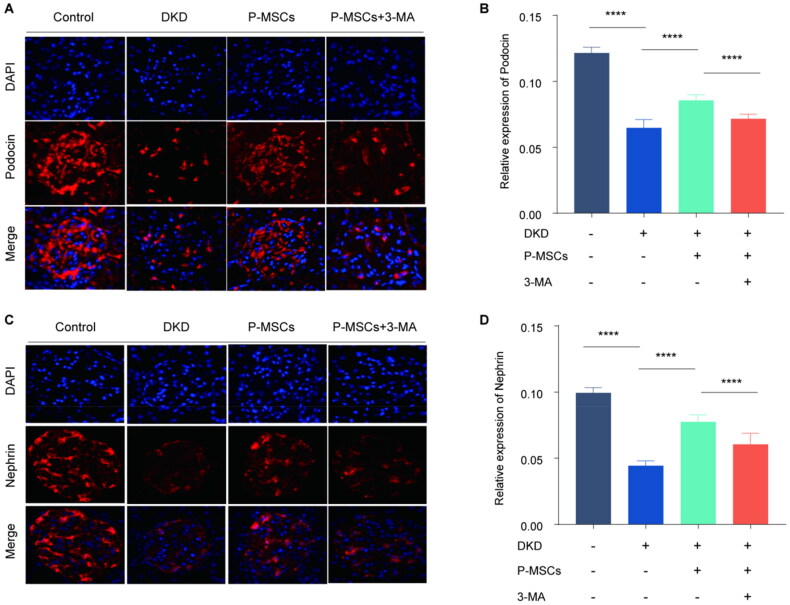
Podocin and nephrin expression levels in the DKD group were significantly lower than those in the normal control (NC) group but were significantly higher than those in the P-MSC group. Furthermore, the expression of podocyte fissure membrane protein in the P-MSCs +3-MA group was significantly lower than that in the P-MSC group (Figure 2). These data suggest that P-MSCs can improve renal podocyte injury, whereas 3-MA inhibits the protective effect of P-MSCs on renal podocyte injury in DKD rats.
Figure 2.
Effects of placenta-derived mesenchymal stem cells (P-MSCs) and 3-methyladenine (3-MA) on glomerular podocyte fissure membrane proteins podocin and nephrin in diabetic kidney disease (DKD) rats. A-B: Immunofluorescence was used to detect the expression of podocin in rat glomerulus. C-D: Effects of P-MSCs and 3-MA on nephrin in DKD rats. (****p < 0.0001).
3.3. P-MSCs promoted autophagy likely by activating the SITR1/FOXO1 pathway in DKD rats
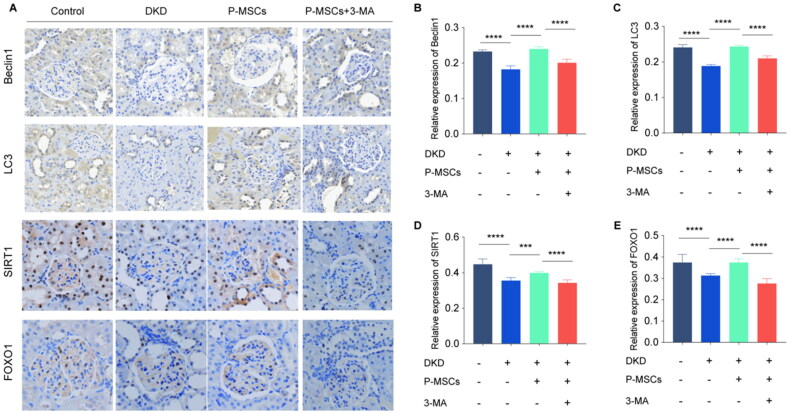
Immunohistochemical staining was used to evaluate the expression of the autophagy-related proteins Beclin1 and LC3 in the renal tissue of the rats in each group. The results showed that the expression of Beclin1 and LC3 in the renal tissue of DKD rats was significantly decreased and that P-MSCs promoted autophagy, whereas 3-MA inhibited the autophagy-promoting effect of P-MSCs (Figure 3(A,B,C)). Moreover, the levels of SITR1 and FOXO1 were reduced in the renal tissues of DKD rats. P-MSCs could significantly upregulate the expression levels of SITR1 and FOXO1; however, 3-MA inhibited the P-MSC-mediated upregulation of SITR1 and FOXO1 (Figure 3(A,D,E)). These observations indicate that the protective effect of P-MSCs in the DKD rats may be related to SIRT1/FOXO1-mediated autophagy.
Figure 3.
Effects of placenta-derived mesenchymal stem cells (P-MSCs) and 3-methyladenine (3-MA) on autophagy-related proteins, SITR1 and FOXO1 in renal tissue of diabetic kidney disease (DKD) rats. A: Expression of autophagy-related proteins Beclin1, LC3, SITR1, and FOXO1 was evaluated by immunohistochemical staining in renal tissue of rats in each group; B: Effects of P-MSCs and 3-MA on Beclin1 in renal tissue of DKD rats; C: Effects of P-MSCs and 3-MA on LC3 in renal tissue of DKD rats D: Effects of P-MSCs and 3-MA on SITR1 in renal tissue of DKD rats; E: Effects of P-MSCs and 3-MA on FOXO1 in renal tissue of DKD rats. (***p < 0.001,****p < 0.0001).
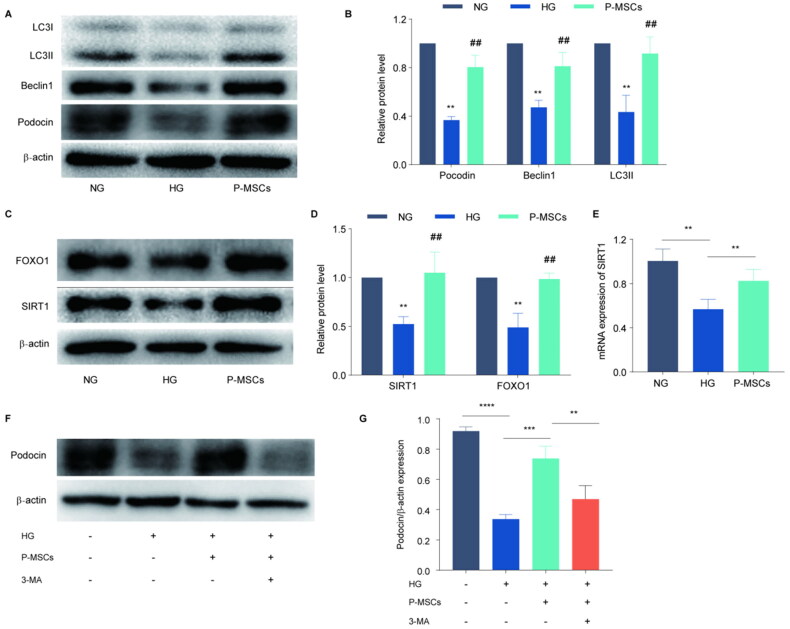
3.4. P-MSCs improved podocyte injury and autophagy and promoted SIRT1 and FOXO1 expression in vitro
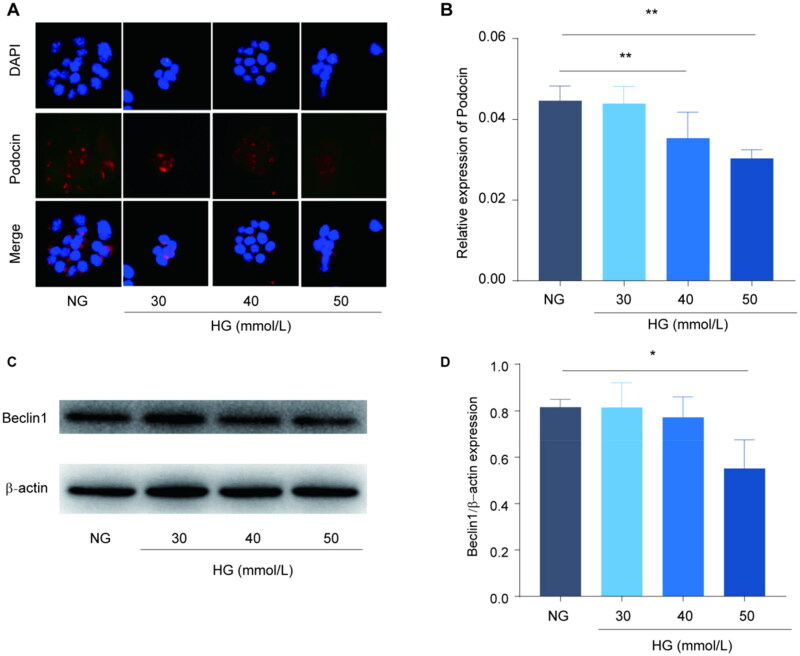
We next investigated the therapeutic effects of P-MSCs on podocyte and found that high glucose (HG) inhibited autophagy and induced podocyte damage in a concentration-dependent manner (p < 0.05) (Figure 4). For this, we established a podocyte injury model by treating podocytes with 50 mmol/L HG. Our results showed that podocin expression in the HG group was significantly lower than that in the normal glucose (NG) group (p < 0.01); However, podocin expression in the HG + P-MSC group was significantly higher than that in the HG group (Figure 5(A, B)). These results suggested that P-MSCs inhibited HG-induced MPC5 injury. The expression levels of LC3-II and Beclin1 were detected using western blotting. The results showed an increase in the expression levels of LC3-II and Beclin1 in the P-MSC-treated group, indicating an increase in MPC5 autophagy. Western blot analysis showed that LC3-II and Beclin1 in the HG group were significantly decreased compared with the NG group (p < 0.01). An increase in the protein expression levels of LC3-II and Beclin1 in the P-MSC group, compared with the HG group (p < 0.01) (Figure 5(A,B)), suggested that HG levels can inhibit MPC5 autophagy and that P-MSCs can promote MPC5 autophagy in HG culture. Protein expression levels of SIRT1 and FOXO1 were also detected by western blotting, which showed that, compared with the NG group, HG inhibited SIRT1 and FOXO1 protein expression, while P-MSCs significantly up-regulated SIRT1 and FOXO1 protein expression (p < 0.01) (Figure 5(C D)). At the same time, PCR results showed that compared with the NG group, HG inhibited SIRT1 mRNA expression, but P-MSCs significantly weakened the inhibitory effect of HG on SIRT1 mRNA expression (Figure 5E). Compared with the P-MSC group, podocin expression in the P-MSC + 3-MA group was significantly decreased (p < 0.01) (Figure 5(F, G)). These results suggested that P-MSCs protect podocytes by activating autophagy and upregulating SIRT1 and FOXO1 expression.
Figure 4.
Effects of high glucose on podocyte- and autophagy-related proteins Beclin1 of podocytes. A-B: Immunofluorescence was used to detect the expression of podocin expression in podocytes with different concentrations of high glucose; C-D: Western blot was used to detect the effects of different concentrations of high glucose on podocyte autophagy. (*p < 0.05, **p < 0.01).
Figure 5.
Effects of placenta-derived mesenchymal stem cells (P-MSCs) and 3-methyladenine (3-MA) on autophagy-related proteins and SIRT1, FOXO1 in high glucose-induced MPC5 cells A-B: Effects of P-MSCs on podocin and autophagy-related proteins (Beclin1 and LC3) in high glucose-induced MPC5; C-D: Western blot was used to detect the expression of SIRT1 and FOXO1 in high glucose-induced MPC5 cells treated with P-MSCs; E: PCR was used to evaluate SIRT1 mRNA expression in high glucose-induced MPC5 cells by P-MSCs; F-G: Effects of P-MSCs and 3-MA on podocin in high glucose-induced MPC5 cells. (**p < 0.01, ***p < 0.001, ****p < 0.000, ## p < 0.001).
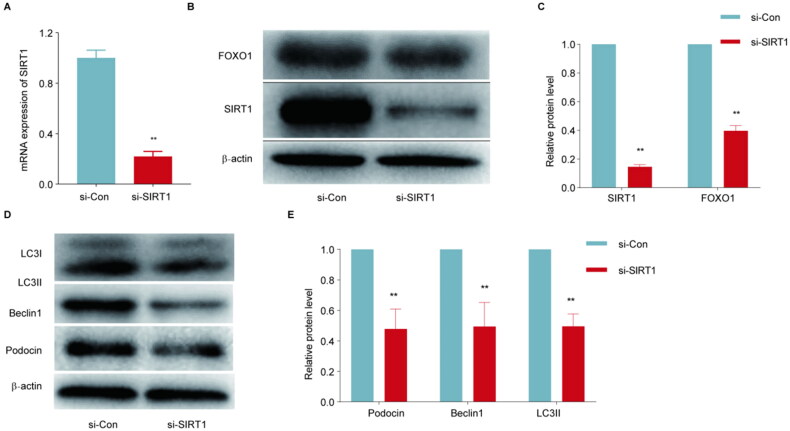
3.5. P-MSCs inhibited HG‐induced MPC5 injury via activating SIRT1/FOXO1‐mediated autophagy
To determine whether P-MSCs inhibited MPC5 damage through SIRT1/FOXO1 signaling pathway, SIRT1 expression levels in MPC5 cells treated with HG or P-MSCs were downregulated using siRNA, and then the culture was continued in medium containing 50 mM HG. Western blotting and PCR verified that the expression of SIRT1 protein and mRNA was significantly downregulated after treatment with siRNA SIRT1(Figure 6(A B)).
Figure 6.
Effects of SIRT1 siRNA on SIRT1/FOXO1 and autophagy-related proteins in MPC5 cells treated with high glucose and placenta-derived mesenchymal stem cells (P-MSCs). A: SIRT1 siRNA inhibited P-MSCs from promoting SIRT1 mRNA expression in high glucose-induced MPC5 cells; B-C: Effects of SIRT1 siRNA on SIRT1 and FOXO1 expression in MPC5 cells treated with high glucose and P-MSCs; D-E: Effects of SIRT1 siRNA on podocin and autophagy-related proteins in MPC5 cells treated with high glucose and P-MSCs. (**p < 0.01).
As shown in Figure 6(D, E), the downregulation of SIRT1 expression in MPC5 cells significantly reduced podocin expression (p < 0.01). These results suggest that P-MSCs protect MPC5 cells from HG injury via the SIRT1/FOXO1 signaling pathway.
To explore the role of the SIRT1/FOXO1 signaling pathway in autophagy, siRNA transfection was used to downregulate the expression level of SIRT1 in MPC5 cells treated with HG and P-MSCs. The expression levels of FOXO1, LC3, and Beclin1 in each group of cells were analyzed by western blotting. The results showed that SIRT1 expression was downregulated in MPC5 cells and the expression levels of FOXO1, LC3-II, and Beclin1 were significantly decreased (p < 0.01) (Figure 6(B,C,D,E)). These results suggest that P-MSCs promote HG-induced MPC5 autophagy via the SIRT1 the FOXO1 signaling pathway.
4. Discussion
With the improvement of people’s living standards and changes in lifestyle, the number of patients with diabetes is increasing at an alarming rate over the past 30 years, with DKD becoming the most frequent cause of ESRD in both developed and developing countries [28, 29]. In the multifactorial pathogenesis of DKD, the oxidative stress caused by long-term hyperglycemia, activation of growth factors such as transforming growth factor-β (TGF-β), glomerular hypertension, production of advanced glycation end-products, and podocyte injury are believed to play important roles in kidney injury [30, 31]. DKD is characterized by persistent elevated proteinuria, GBM thickening, and extracellular matrix accumulation, leading to inhibition of autophagic flux, increased podocyte injury, and progressive renal dysfunction [32]. At present, the main therapeutic drugs for DKD include: renin-angiotensin-aldosterone system (RAS) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors and Finerenone, which is a selective nonsteroidal mineralocorticoid receptor antagonist (MRA) [33,34].
Patients with DKD and ESRD impose a heavy economic burden on their families and society, making early detection and treatment particularly important.
MSCs are mesoderm-derived adult stem cells with strong self-renewal ability and differentiation potential and are widely used in regenerative medicine. They are mainly used as P-MSCs, BM-MSCs, adipose-derived MSCs (AD-MSCs), and umbilical cord MSCs (UC-MSCs). Numerous studies have validated the role of MSCs in many diseases, including osteoarthritis, pulmonary fibrosis, systemic lupus erythematosus, and diabetic nephropathy [35–40]. We learned that MSCs can decreasing blood glucose through islet function recovery, islet cell proliferation, and insulin sensitivity improvement [41]. In a clinical study, Packham et al. [42] found that a single intravenous injection of MPCs showed a tendency to stabilize or improve the estimated and measured glomerular filtration rates at 12 weeks with a good safety profile. Animal studies have shown that MSCs can reduce the severity of DKD in rats by improving proteinuria, serum creatinine/urea ratio, glomerular hypertrophy, mesangial expansion and sclerosis, foot process loss, renal tubular injury, and interstitial fibrosis [39, 43–45]. The therapeutic effect of MSCs is mainly related to their paracrine mechanism, anti-inflammatory activity, secretion of cytoprotective factors, reduction of oxidative stress, and inhibition of the mTOR pathway [5, 24, 44]. Through these mechanisms, MSCs can improve the intrarenal environment and slow down or reverse key pathogenic pathways, including glomerular barrier dysfunction, proinflammatory cell infiltration, tubular epithelial cell stress, and progressive interstitial fibrosis.
The results of this study showed that the BG, TG, LDL-C, BUN, and UACR levels in DKD rats significantly decreased after 8 weeks of P-MSC treatment, suggesting that P-MSCs can improve glucose and lipid metabolism and renal function in DKD rats. Moreover, we found that P-MSCs alleviated tubular injury and GBM thickening, and the expression levels of the glomerular podocyte markers podocin and nephrin in the renal tissue of DKD rats significantly increased after P-MSC treatment, suggesting that P-MSCs can significantly reduce glomerular podocyte injury in the renal tissue of DKD rats.
Autophagy is a conserved and tightly regulated process that maintains cellular homeostasis. Autophagy-related protein 6 (ATG6 or Beclin-1) is the most important regulator of autophagy, and its upregulation usually represents increased autophagic activity [46]. Autophagy plays key roles in protecting cells under both normal and diseased states, including immunity, inflammation, adaptation to stress, development and aging, metabolic and neurodegenerative diseases, and cancer [8]. Many studies have shown that impaired autophagy is associated with the pathogenesis of diabetic nephropathy. Barbosa et al. [47] reported that autophagy was decreased in the renal tubular cells of STZ-induced rats, and insulin treatment or insulin replacement with islet transplantation promoted autophagy. Vallon et al. [48] found the expression levels of p62/SQSTM1, a substrate of the autophagy-lysosomal degradation pathway, were elevated in diabetic mice, indicating impaired autophagy function. Podocytes, or glomerular visceral epithelial cells, are terminally differentiated cells that line the glomerular sacs that enclose capillaries [49]. Podocyte injury often results in proteinuria, and the extent of podocyte injury and loss plays a decisive role in the speed of DKD progression [50]. Because podocytes do not replicate, intracellular degradation systems are essential for maintaining their homeostasis. Autophagy is primarily responsible for the degradation of proteins and organelles, and hence for maintaining homeostasis, in podocytes. Its dysfunction is considered an important inducer of podocyte injury [51, 52]. It has been reported that autophagy is activated in adriamycin-induced nephropathy to protect podocytes from injury [53]. In Podo-ATG7 podocyte-specific knockout mice, doxorubicin treatment results in podocyte injury, glomerulopathy, and proteinuria [54]. Our animal experiments also showed that autophagy is downregulated in kidney tissue, and podocytes are severely damaged in DKD rats. Furthermore, the results of our vitro experiment showed that HG levels could lead to the downregulation of autophagy and damage to podocytes.
SIRT1 is a highly conserved NAD+-dependent deacetylase that is activated in response to energy limitation and acts as a cellular sensor to detect energy availability and regulate metabolism in various tissues [55]. It forms molecular complexes with essential components of the autophagy machinery such as ATG5, ATG7, and LC3, and directly deacetylates these components in an NAD-dependent manner [56]. The most frequently deacetylated transcription factors are the members of the FOXO family [57], which participate in the regulation of cell proliferation, apoptosis, metabolism, DNA damage repair, and other processes in various organs or tissues such as the heart, skeletal muscle, liver, and brain tissue by activating cell autophagy. The transcriptional activation ability of the FOXO family members is specifically regulated by their acetylation/deacetylation and phosphorylation/dephosphorylation processes [58]. Among them, FOXO1 plays a key role in cellular stress adaptation and directly regulates the transcription of autophagy-related genes. In addition, acetylated FOXO1 can interact with ATG7, leading to enhanced expression of BNIP3 [59,60]. Studies have reported that metformin and resveratrol may reduce kidney injury in diabetic rats by inducing the SIRT1/FOXO1 autophagy signal axis [61, 62] and that the SIRT1/FOXO1 pathway can regulate autophagy in endothelial cells, myocardium, and kidney tissues [63–65]. Therefore, the SIRT1/FOXO1 pathway is an important regulator of autophagy.
The expression levels of SIRT1 and FOXO1 are decreased in the kidney tissue of diabetic animal models, and the upregulation of the SIRT1/FOXO1 pathway reduces BG levels, improves oxidative stress, and enhances autophagy, thereby reducing renal damage. In contrast, 3-MA, a PI3K inhibitor, causes kidney injury. In this study, we treated DKD rats with P-MSCs in combination with the autophagy inhibitor 3-MA. The results showed that P-MSCs significantly upregulated the expression of SIRT1, FOXO1, LC3II, and Beclin1 in the renal tissues of rats. These results suggested that P-MSCs upregulated SIRT1/FOXO1 expression and enhanced autophagy. However, the intervention of the autophagy inhibitor weakened the autophagy-enhancing effect of P-MSCs, leading to renal pathological disorder and increased UACR in DKD rats. These results suggest that the inhibition of autophagy can block the protective effect of P-MSCs on the kidneys of DKD rats, and its mechanism is related to the SIRT1/FOXO1 pathway. In vitro, we confirmed that different HG concentrations can reduce the expression of LC3II and Beclin1. With an increase in the concentration gradient, the damage to MPC5 autophagy was more serious, and P-MSCs could reverse the inhibition of MPC5 autophagy.
We observed that HG affected the expression of SIRT1, FOXO1, and autophagy in MPC5 cells, and that the upregulation of FOXO1 expression and autophagy was significantly reduced when SIRT1 was silenced by siRNA. These results suggest that the effect of P-MSCs on the upregulation of HG-induced MPC5 autophagy is mediated by SIRT1/FOXO1 pathway.
This study has limitations. First, after T1DM induction, there may be a bias that only rats resistant to hyperglycemic injury will survive; therefore, results such as blood glucose and ACR might be underestimated. Second, The study concludes that P-MSCs can improve blood glucose and blood lipid levels, but the specific mechanism of its action is not explored in depth, and further research in this direction is needed. Third, this study did not explore whether P-MSCs or exosomes play an important role in the improvement of diabetic kidney injury. The role of P-MSCs-secreted exosomes in diabetic kidney disease needs to be further explored.
5. Conclusion
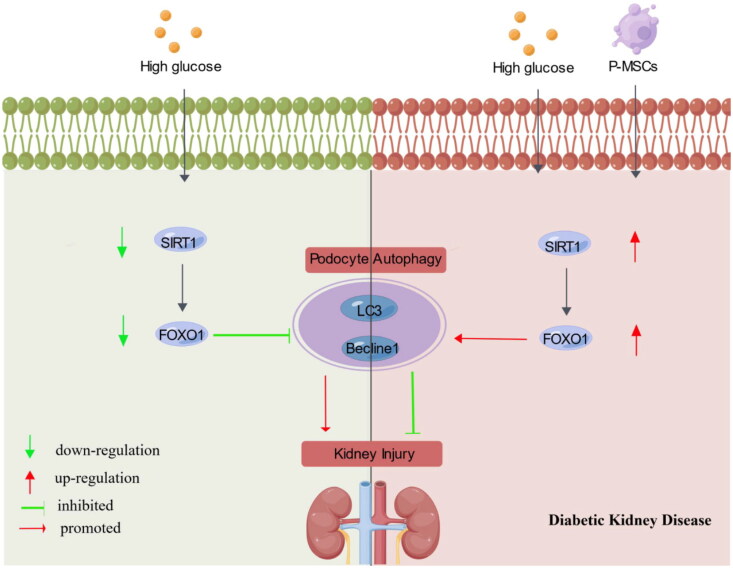
This study indicates that P-MSCs alleviate diabetic kidney injury by regulating the SIRT1/FOXO1 pathway and upregulating autophagy in kidney tissues and podocytes (Figure 7).
Figure 7.
Schematic diagram of the effects of P-MSCs on podocyte in DKD via enhancing SIRT1/FOXO1 pathway-mediated autophagy(visual abstract).
Acknowledgements
The authors would like to thank the Stem Cell Engineering Research Center of Jiangxi Province for providing human placenta-derived mesenchymal stem cells.
Funding Statement
This study was supported by grants from the Jiangxi Provincial Key R&D Plan (no. 20201BBG71006), the National Natural Science Funds of China (no. 8216030486), the Natural Science Foundation of Jiangxi Province (no. 20224BAB216009), and the Science and Technology Plan of Jiangxi Health Planning Committee (no. SKJP220217702).
Ethical approval and consent to participate
Our study protocol has been approved by the Research Committee of the First Affiliated Hospital of Nanchang University (Approval number: 2020-04). All procedures in this study were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. There are no human subjects in this article and informed consent is not applicable.
Authors’ contributions
Design: Jixiong Xu
Conduct/data collection: Jiao Wang and Honghong Liu.
Analysis: Jiao Wang, Honghong Liu, and Guanru Yue.
Writing manuscript: Jiao Wang and Honghong Liu.
Consent for publication
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Ogurtsova K, Da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:1–12. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Koye DN, Magliano DJ, Nelson RG, et al. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25(2):121–132. doi: 10.1053/j.ackd.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reidy K, Kang HM, Hostetter T, et al. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124(6):2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye H, Bai X, Gao H, et al. Urinary podocalyxin positive-element occurs in the early stage of diabetic nephropathy and is correlated with a clinical diagnosis of diabetic nephropathy. J Diabetes Complications. 2014;28(1):96–100. doi: 10.1016/j.jdiacomp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Griffin TP, Martin WP, Islam N, et al. The promise of mesenchymal stem cell therapy for diabetic kidney disease. Curr Diab Rep. 2016;16(5):42. doi: 10.1007/s11892-016-0734-6. [DOI] [PubMed] [Google Scholar]
- 6.Nakatogawa H, Suzuki K, Kamada Y, et al. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10(7):458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 7.He C, Klionsky DJ.. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43(1):67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi AM, Ryter SW, Levine B.. Autophagy in human health and disease. N Engl J Med. 2013;368(7):651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Ng S, Wang J, et al. Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways. Autophagy. 2015;11(4):629–642. doi: 10.1080/15548627.2015.1023981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravanan P, Srikumar IF, Talwar P.. Autophagy: the spotlight for cellular stress responses. Life Sci. 2017;188:53–67. doi: 10.1016/j.lfs.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Kume S, Koya D.. Autophagy: a novel therapeutic target for diabetic nephropathy. Diabetes Metab J. 2015;39(6):451–460. doi: 10.4093/dmj.2015.39.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai H, Liu Q, Liu B.. Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res. 2017;2017:2615286. doi: 10.1155/2017/2615286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pontrelli P, Oranger A, Barozzino M, et al. Deregulation of autophagy under hyperglycemic conditions is dependent on increased lysine 63 ubiquitination: a candidate mechanism in the progression of diabetic nephropathy. J Mol Med. 2018;96(7):645–659. doi: 10.1007/s00109-018-1656-3. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Iwano M, Suzuki D, et al. Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54(4):653–664. doi: 10.1053/j.ajkd.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Liu WJ, Gan Y, Huang WF, et al. Lysosome restoration to activate podocyte autophagy: a new therapeutic strategy for diabetic kidney disease. Cell Death Dis. 2019;10(11):806. doi: 10.1038/s41419-019-2002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Sun W, Cheng Y, et al. Role of sirtuin-1 in diabetic nephropathy. J Mol Med . 2019;97(3):291–309. doi: 10.1007/s00109-019-01743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, et al. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26(12):2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Qiao L, Shao J.. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281(52):39915. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 19.Ji L, Wang Q, Huang F, et al. FOXO1 overexpression attenuates tubulointerstitial fibrosis and apoptosis in diabetic kidneys by ameliorating oxidative injury via TXNIP-TRX. Oxid Med Cell Longev. 2019;2019:3286928–3286914. doi: 10.1155/2019/3286928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Hu G, Su J, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20(5):510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 22.Caplan AI, Dennis JE.. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 23.Yue Y, Yeh JN, Chiang JY, et al. Intrarenal arterial administration of human umbilical cord-derived mesenchymal stem cells effectively preserved the residual renal function of diabetic kidney disease in rat. Stem Cell Res Ther. 2022;13(1):186. doi: 10.1186/s13287-022-02857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebrahim N, Ahmed IA, Hussien NI, et al. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells. 2018;7(12):226. doi: 10.3390/cells7120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung J, Choi JH, Lee Y, et al. Human placenta-derived mesenchymal stem cells promote hepatic regeneration in CCl4 -injured rat liver model via increased autophagic mechanism. Stem Cells. 2013;31(8):1584–1596. doi: 10.1002/stem.1396. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Zhou J, Zhang D, et al. Bone marrow-derived mesenchymal stem cells enhance autophagy via PI3K/AKT signalling to reduce the severity of ischaemia/reperfusion-induced lung injury. J Cell Mol Med. 2015;19(10):2341–2351. doi: 10.1111/jcmm.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao K, Hao H, Liu J, et al. Bone marrow-derived mesenchymal stem cells ameliorate chronic high glucose-induced β-cell injury through modulation of autophagy. Cell Death Dis. 2015;6(9):e1885–e1885. doi: 10.1038/cddis.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. Jama. 2016;316(6):602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low SK, Sum CF, Yeoh LY, et al. Prevalence of chronic kidney disease in adults with type 2 diabetes mellitus. Ann Acad Med Singap. 2015;44(5):164–171. doi: 10.47102/annals-acadmedsg.V44N5p164. [DOI] [PubMed] [Google Scholar]
- 30.Xiao L, Wang M, Yang S, et al. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy. Biomed Res Int. 2013;2013:987064–987067. doi: 10.1155/2013/987064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu Q, Li Y, Jin J, et al. Curcumin alleviates diabetic nephropathy via inhibiting podocyte mesenchymal transdifferentiation and inducing autophagy in rats and MPC5 cells. Pharm Biol. 2019;57(1):778–786. doi: 10.1080/13880209.2019.1688843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Li R, Shi W, et al. NFAT2 inhibitor ameliorates diabetic nephropathy and podocyte injury in db/db mice. Br J Pharmacol. 2013;170(2):426–439. doi: 10.1111/bph.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamazaki T, Mimura I, Tanaka T, et al. Treatment of diabetic kidney disease: current and future. Diabetes Metab J. 2021;45(1):11–26. doi: 10.4093/dmj.2020.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frampton JE. Finerenone: first approval. Drugs. 2021;81(15):1787–1794. doi: 10.1007/s40265-021-01599-7. [DOI] [PubMed] [Google Scholar]
- 35.Lee WS, Kim HJ, Kim KI, et al. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, Placebo-Controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504–511. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S, Cui G, Peng C, et al. Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res Ther. 2018;9(1):110. doi: 10.1186/s13287-018-0846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D, Huang S, Yuan X, et al. The regulation of the treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol. 2017;14(5):423–431. doi: 10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Rong P, Ma X, et al. Paracrine effect of mesenchymal stem cell as a novel therapeutic strategy for diabetic nephropathy. Life Sci. 2018;215:113–118. doi: 10.1016/j.lfs.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Li K, Liu X, et al. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cells Dev. 2013;22(23):3074–3086. doi: 10.1089/scd.2013.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang E, Han B, Zhang Q, et al. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res Ther. 2020;11(1):336. doi: 10.1186/s13287-020-01852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Shan SK, Guo B, et al. The multi-therapeutic role of MSCs in diabetic nephropathy. Front Endocrinol. 2021;12:671566. doi: 10.3389/fendo.2021.671566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Packham DK, Fraser IR, Kerr PG, et al. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine. 2016;12:263–269. doi: 10.1016/j.ebiom.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JH, Park J, Hwang SH, et al. Delayed treatment with human umbilical cord blood-derived stem cells attenuates diabetic renal injury[J]. Transplant Proc. 2012;44(4):1123–1126. doi: 10.1016/j.transproceed.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 44.Lv S, Cheng J, Sun A, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting oxidative stress. Diabetes Res Clin Pract. 2014;104(1):143–154. doi: 10.1016/j.diabres.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Ezquer F, Giraud-Billoud M, Carpio D, et al. Proregenerative microenvironment triggered by donor mesenchymal stem cells preserves renal function and structure in mice with severe diabetes mellitus. Biomed Res Int. 2015;2015:164703–164723. doi: 10.1155/2015/164703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine B, Kroemer G.. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbosa Júnior AdA, Zhou H, Hültenschmidt D, et al. Inhibition of cellular autophagy in proximal tubular cells of the kidney in streptozotocin-diabetic and uninephrectomized rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;61(6):359–366. doi: 10.1007/BF02890439. [DOI] [PubMed] [Google Scholar]
- 48.Song P, Huang W, Onishi A, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;317(1):F207–F217. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wickelgren I. First components found for new kidney filter. Science. 1999;286(5438):225–226. doi: 10.1126/science.286.5438.225. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Zheng S, Ma C, et al. Research progress on exosomes in podocyte injury associated with diabetic kidney disease. Front Endocrinol. 2023;14:1129884. doi: 10.3389/fendo.2023.1129884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li G, Li CX, Xia M, et al. Enhanced epithelial-to-mesenchymal transition associated with lysosome dysfunction in podocytes: role of p62/sequestosome 1 as a signaling hub. Cell Physiol Biochem. 2015;35(5):1773–1786. doi: 10.1159/000373989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takagi-Akiba M, Asanuma K, Tanida I, et al. Doxorubicin-induced glomerulosclerosis with proteinuria in GFP-GABARAP transgenic mice. Am J Physiol Renal Physiol. 2012;302(3):F380–9. doi: 10.1152/ajprenal.00502.2010. [DOI] [PubMed] [Google Scholar]
- 54.Yi M, Zhang L, Liu Y, et al. Autophagy is activated to protect against podocyte injury in adriamycin-induced nephropathy. Am J Physiol Renal Physiol. 2017;313(1):F74–f84. doi: 10.1152/ajprenal.00114.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nogueiras R, Habegger KM, Chaudhary N, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105(9):3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daitoku H, Sakamaki J, Fukamizu A.. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim Biophys Acta. 2011;1813(11):1954–1960. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Ferdous A, Battiprolu PK, Ni YG, et al. FoxO, autophagy, and cardiac remodeling. J Cardiovasc Transl Res. 2010;3(4):355–364. doi: 10.1007/s12265-010-9200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kroemer G, Mariño G, Levine B.. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sengupta A, Molkentin JD, Yutzey KE.. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284(41):28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Liu LQ, Xu LL, et al. Metformin alleviates renal injury in diabetic rats by inducing Sirt1/FoxO1 autophagic signal axis. Clin Exp Pharmacol Physiol. 2020;47(4):599–608. doi: 10.1111/1440-1681.13226. [DOI] [PubMed] [Google Scholar]
- 62.Wu L, Zhang Y, Ma X, et al. The effect of resveratrol on FoxO1 expression in kidneys of diabetic nephropathy rats. Mol Biol Rep. 2012;39(9):9085–9093. doi: 10.1007/s11033-012-1780-z. [DOI] [PubMed] [Google Scholar]
- 63.Wang B, Yang Q, Sun YY, et al. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice[J]. J Cell Mol Med. 2014;18(8):1599–1611. doi: 10.1111/jcmm.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Q, Hu Y, Jiang M, et al. Effect of autophagy regulated by Sirt1/FoxO1 pathway on the release of factors promoting thrombosis from vascular endothelial cells[J]. Int J Mol Sci. 2019;20(17):4132. doi: 10.3390/ijms20174132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren H, Shao Y, Wu C, et al. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell Endocrinol. 2020;500:110628. doi: 10.1016/j.mce.2019.110628. [DOI] [PubMed] [Google Scholar]