Abstract
Mitochondria integrate the key metabolic fluxes in the cell. This role places this organelle at the center of cellular energetics and, hence, mitochondrial dysfunction underlies a growing number of human disorders and age-related degenerative diseases. Here we present novel analytical and technical methods for evaluating mitochondrial metabolism and (dys)function in human muscle in vivo. Three innovations involving advances in optical spectroscopy (OS) and magnetic resonance spectroscopy (MRS) permit quantifying key compounds in energy metabolism to yield mitochondrial oxidation and phosphorylation fluxes. The first of these uses analytical methods applied to optical spectra to measure hemoglobin (Hb) and myoglobin (Mb) oxygenation states and relative contents ([Hb]/[Mb]) to determine mitochondrial respiration (O2 uptake) in vivo. The second uses MRS methods to quantify key high-energy compounds (creatine phosphate, PCr, and adenosine triphosphate, ATP) to determine mitochondrial phosphorylation (ATP flux) in vivo. The third involves a functional test that combines these spectroscopic approaches to determine mitochondrial energy coupling (ATP/O2), phosphorylation capacity (ATPmax) and oxidative capacity (O2max) of muscle. These new developments in optical and MR tools allow us to determine the function and capacity of mitochondria noninvasively in order to identify specific defects in vivo that are associated with disease in human and animal muscle. The clinical implication of this unique diagnostic probe is the insight into the nature and extent of dysfunction in metabolic and degenerative disorders, as well as the ability to follow the impact of interventions designed to reverse these disorders.
Keywords: Cell energy metabolism, noninvasive, clinical spectroscopy, muscle energetics, mitochondrial dysfunction
1. INTRODUCTION
Mitochondrial dysfunction is implicated in a wide range of disorders [1–3], including diabetes [4], neurodegeneration [5] and aging-related dysfunctions [6–8]. Despite these associations between mitochondrial changes and disease, we still know little about the nature and extent of mitochondrial dysfunction in vivo and how these dysfunctions are linked to specific disease pathologies, especially in human tissues. Important progress is reported in this volume concerning characterizing mitochondria defects and dysfunction in isolated tissues [9], [10] and in mouse models [11]. However, there has been a paucity of experimental tools to study mitochondrial function in vivo. Here we describe advances that now make these measurements possible and highlight human muscles as an ideal system to characterize mitochondrial dysfunction and determine the links to disease processes.
New innovations in in vivo spectroscopy from our laboratories permit quantitative measurement of the key mass and energy fluxes (ATP and O2) governed by the mitochondria, as illustrated in Figure 1. We show how to use these fluxes to characterize the mitochondrial coupling efficiency (ATP/O2 or the biochemical term, P/O, which is numerically ATP/O2 divided by 2) [7, 12, 13], as well as the tissue capacities for phosphorylation (ATPmax) and oxidation (O2max). These measurements have been shown to reveal important aspects of mitochondrial dysfunction with disease and age. For example, mitochondrial coupling (ATP/O2) measures the efficiency of oxidative phosphorylation, which declines with age [14, 15], thyroid function [16] and activation of uncoupling protein [17]. Mitochondrial uncoupling has also been shown to be an early stage in the apoptotis process [18], [19] and may play a role in the cell loss that underlies sarcopenia [20]. ATPmax has been used as a measure of mitochondrial phosphorylation capacity in normal and pathological muscle (e.g., aging [21, 22]), but here we show that O2max directly reflects muscle mitochondrial content to provide a noninvasive alternative to analyzing muscle biopsies for oxidative enzyme activity or mitochondrial volume. We also show that these measurements can be made on a wide range of human muscles, which means we can now extend our studies beyond the gold standard for human muscle studies –– the human vastus lateralis. Finally, we show that these methods are sensitive enough to identify the nature of the mitochondrial defect and its consequence on cell function in an individual muscle. The end result of these novel spectroscopic methods is the ability to directly measure mitochondrial function in vivo to diagnose mitochondrial defects and test specific mechanisms responsible for dysfunction with age and disease.
FIGURE 1:
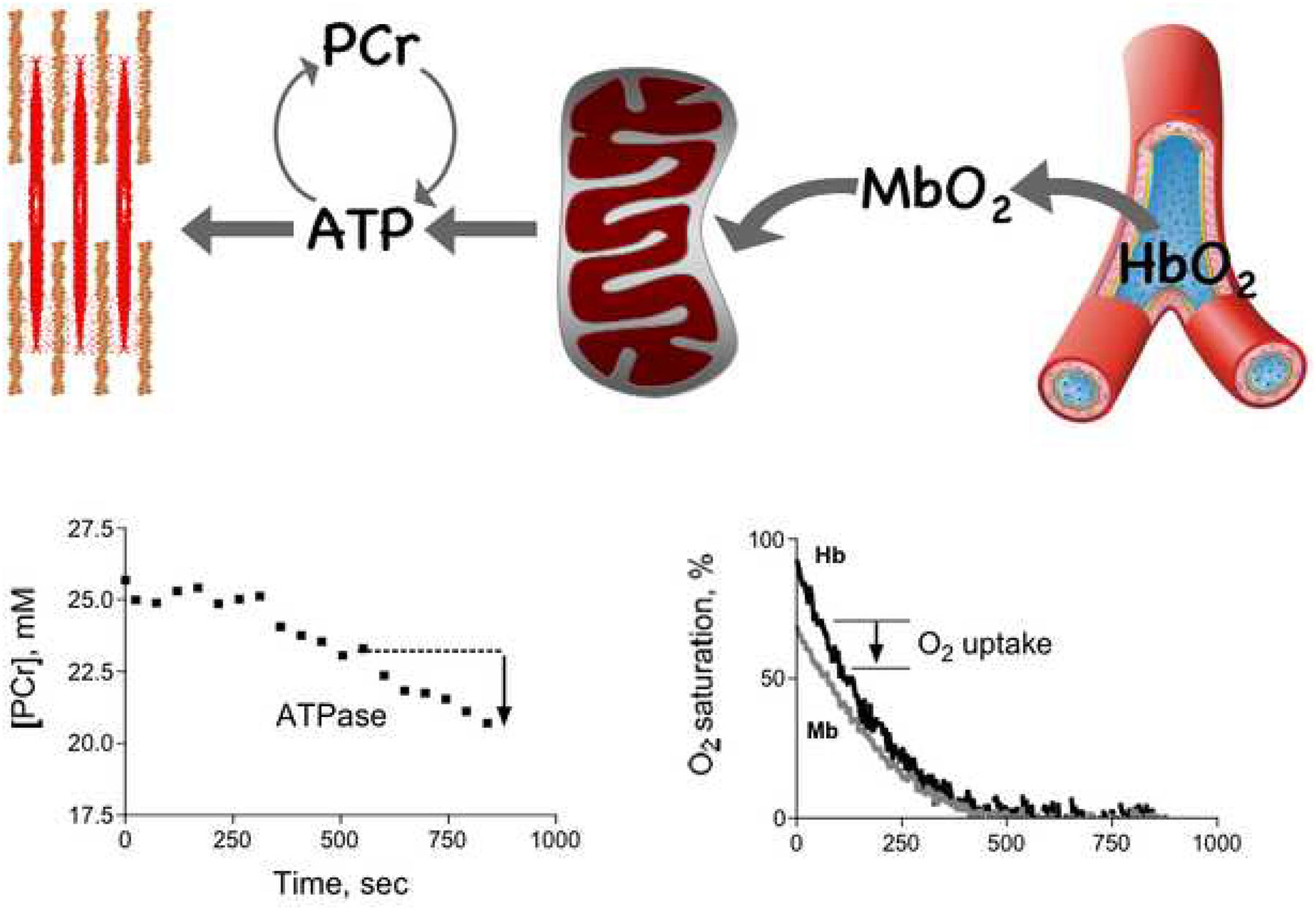
Diagram of the key energy fluxes in the cell and the magnetic resonance spectroscopic (MRS) and optical spectroscopic (OS) methods used to determine fluxes. ATP flux from mitochondria to the cell ATPases is measured by MRS. The figure in the lower left shows the changes in PCr that are the basis for determining the net ATP demand of the cell that must be met by mitochondrial ATP synthesis. Oxygen flux from capillary to mitochondrial oxidation is determineded from OS measurements of oxy- and deoxy- contents of myoglobin (Mb) and hemoglobin (Hb). The figure in the lower right illustrates the changes in Mb- and Hb-O2 saturation that underlie the O2 uptake determination.
2. EXPERIMENTAL PROTOCOL
At the center of our innovations is an in vivo functional test that is used to perturb the cell physiological state. The protocol combines optical (OS) and MR spectroscopy (MRS) to measure the contents and dynamics of key metabolic compounds that together yield mitochondrial energy fluxes that yield P/O, ATPmax, and O2max of single muscles. Figure 2 shows the phases used to measure the chemical contents of the key metabolites (Quantification), and the changes in these compounds (Dynamics and Recovery) to measure flux:
A rest period is used for Quantification of the concentration of the key compounds
The Dynamics phase is used to perturb phosphocreatine [PCr], oxy-myoglobin [Mb-O2], and oxy-hemoglobin [Hb-O2] to determine O2 and ATP fluxes. We achieve this perturbation using ischemia (15 min) induced by a tourniquet to block blood flow and eliminate O2 delivery. Ischemia in excess of 30 min is commonly and safely used in surgery without muscle trauma or damage by avoidance of venous pooling prior to ischemia and careful screening of subjects for susceptibility to blood clots [23]. We have had similar success applying this method in hundreds of bouts over a 15 yr period. In the ATPmax test, an exercise bout is used in lieu of ischemia.
The Recovery phase is initiated by blood flow or exercise ceasation. During this phase, the time constant of this recovery of [PCr] is determined to yield the phosphorylation capacity (ATPmax; see Eq. 3 below). The measurements of ATPmax and ATP/O2 are used to derive the oxidative capacity (O2max) of mitochondria in vivo, as described below (Eq. 4).
FIGURE 2:

, A) Diagram of the 3-phase protocol for measuring mitochondrial function in muscle in vivo. B) Flow diagram of the measurements that define mitochondrial function (ATP/O2) and capacities (ATPmax and O2max). Variables in brackets ([]) are chemical concentrations and delta (Δ) denotes the change in these concentrations during the Dynamics phase; τPCr is the time constant of PCr recovery.
The lower panel in Figure 2 shows a flow diagram of how the measurements made during the mitochondrial function protocol are used to determine P/O2, ATPmax, and O2max of single muscles.
3. OPTICAL SPECTROSCOPY
3.1. Strategy
We determine tissue O2 uptake by quantifying the level of O2 carrying compounds (oxy-myoglobin, [Mb-O2], and oxy-hemoglobin, [Hb-O2], concentrations) at baseline and by following their desaturation during ischemia. Two critical developments that underlie this determination are: 1) separation of the O2 saturations of Mb and Hb in vivo using partial least squares analysis (PLS) [24], [25–28] and 2) measurement of the tissue content of Hb and Mb using a combination of noninvasive OS and MRS methods [29, 30]. Together the relative O2 saturation and total tissue content of these compounds yield a quantitative measure of tissue O2 stores. Ischemia perturbs these O2 stores by eliminating O2 delivery and shifting O2 supply for cell respiration to the Mb and Hb O2 stores. The resulting declines in [Mb-O2] and [Hb-O2] are used to calculate O2 uptake (see eq. 1, below).
3.2. Optical analysis
Our optical apparatus consists of a quartz tungsten halogen source (Acton Research), a spectrometer equipped with a 1024×256 thermoelectrically cooled CCD array detector (Inspectrum, Acton Research), and fiber optic bundles that convey light to and from the sample. The light is equipped with an infrared filter to avoid excessive heating of the tissues during experiments. Two 6.4-mm diameter fiber optic bundles (model G39–368, Edmund Optics, Barrington, NJ) are positioned in contact with the skin overlying the muscle with a center-to-center distance of 1.8 cm for the FDI (first dorsal interosseus muscle) and 2.4 cm for the tibialis anterior muscle (TA, leg muscle). In the near infrared region at 760 nm, these distances between source and detection fiber bundles results in light penetration to approximately 0.6–0.8 cm [31], deep enough to sample the underlying muscle. Care is taken to ensure full contact between the probes and the skin throughout the experiment. We collect spectra from 560 to 805 nm and have typically used a wavelength range of 600–800 nm for our analysis, as described below. Acquisition times range from 300–1000 ms per spectrum.
3.2.1. Separating Mb-O2 and Hb-O2:
The PLS method is an extension of a linear regression approach that extracts information on specific spectral components from complex spectra [25]. The algorithm generates a set of calibration coefficients at each wavelength when calibrated with spectra from solutions with known values of Mb or Hb saturation. In the short time span of an experiment, Mb concentration is fixed in the muscle cell and ischemia ensures that blood volume and Hb concentration do not vary during our measurement of O2 uptake. With these constraints, the PLS algorithm interprets spectral variations with time as changes in Mb or Hb saturation.
The PLS analysis requires a calibration set containing spectra that mimic in vivo spectra. The calibration set contains three concentrations of Intralipid (1, 1.5, and 2%) to take into account the scattering of light in tissue. The concentrations of Mb and Hb are chosen to approximate physiological values based on previous human tissue measurements [32]: 350 μM Mb and 25 μM, 50 μM or 100 μM Hb to account for the expected changes that occur from rest through hyperemia in vivo. Human blood is used to generate Hb solutions for the calibration spectra. Horse Mb (Sigma Chemical Co., M1882) is used in the human calibration set, since pilot experiments established that there are no species related differences in the spectrum based on a comparison of horse and human (cardiac) Mb.
To extract Hb for the calibration set, whole human blood is treated with deionized water to lyse red cells and the resulting solution is passed through a filter to separate soluble Hb from cellular debris. Both Hb and Mb solutions are prepared in 50 mM sodium phosphate buffer (pH 7.4) and are completely reduced by the addition of excess sodium dithionite. Each solution is then passed over a Sephadex G-25 size exclusion column at pH 7.4 to remove the dithionite. Mb and Hb solutions are combined with Intralipid, brought to the appropriate concentration, and equilibrated with 100% O2 before collecting oxygenated spectra. Sodium dithionite is added to the oxygenated solutions before deoxy-Mb and deoxy-Hb spectra are recorded. Fifty spectra of each solution are obtained with the same fiber optic probe configuration as used for collecting in vivo experimental spectra.
In vitro calibration set:
Spectra containing both Mb and Hb absorbances are obtained by mathematically adding spectra from oxy- and deoxy- forms of Mb and Hb in different proportions to create complex spectra ranging from 0 to 100% saturation for both O2 carriers [27]. The known values of Mb and Hb saturation for each calibration spectrum are used to calibrate the PLS. Separate calibration sets are used to determine the calibration coefficients for Mb and Hb saturation. In the Mb saturation calibration set, three scattering levels and three Hb concentrations are represented to mimic chromophore concentrations and scattering coefficients found in muscle. Three Hb concentrations are included to make measurements of Mb saturation less sensitive to changes in total Hb concentration. In the Hb saturation calibration set, all spectra have a single Hb concentration of 50 μM so that the PLS model is sensitive to Hb saturation and not to Hb concentration. Two PLS models (prediction data sets) are formed by providing the algorithm with either a Mb or Hb calibration set and the corresponding values for Mb or Hb saturation. In this way each PLS model is sensitive to a single variable and spectral variations associated with the other chromophores are treated as uncorrelated noise. The optimal number of factors for each chromophore is determined by a PRESS (Prediction Error Sum of Squares) analysis [33]. Typically, 4–5 factors are used.
3.2.2. Quantifying [Mb] and [Hb] in vivo:
Separating Hb from Mb in optical spectra in vivo has been a barrier to measuring cellular and vascular oxygenation and determining O2 uptake in vivo. We quantify the tissue content of these O2 carriers in 3 steps: 1) quantifying total [Mb] using MRS, 2) determining the relative content of Hb and Mb ([Hb]/[Mb]) using a novel optical analytic approach termed wavelength shift analysis [29], and 3) calculating total [Hb] from [Mb] × [Hb]/[Mb].
3.2.2.1. Wavelength Shift Analysis: Quantifying [Hb]/[Mb]:
The relative ratio of the two O2-binding chromophores is determined from tissue that is deoxygenated (15 min of ischemia) using a peak whose position in the spectrum depends on the relative concentration of deoxy-Hb and deoxy-Mb in the tissue. The second derivative of the spectrum is taken to measure the shift of this composite peak, as previously described [29] (second derivative pre-processing is discussed in section 3.2.3.1). The position of this composite peak will shift in wavelength from 757 nm in pure deoxy-Hb toward 761 nm with greater relative content of deoxy-Mb. Thus the wavelength of the composite peak is a measure of the relative deoxy-Hb to deoxy-Mb ratio. The relationship between peak position and proportion of Hb contributing to the optical signal is linear over the entire range of relative Hb contributions (0 – 100%) [29].
3.2.2.2. [Mb] and [Hb] quantification in vivo:
Myoglobin concentration is determined by MRS using the Internal H20 Calibration method in Ref. [30] from the ratio of the maximal deoxy-Mb peak determined at the end of ischemia to the H2O peak area (see Absolute quantification, below). The absolute concentration of Hb is calculated from [Mb] times the ratio [Hb]/[Mb] determined by wavelength shift analysis. This analysis has revealed that Mb represents the dominant O2 binding protein in resting human muscle (up to 80% of the O2 carrying capacity in the FDI and TA muscles) in agreement with Tran et al. [34] who used an MRS approach.
3.2.3. In vivo spectra
3.2.3.1. Spectral preprocessing:
The second derivative with respect to wavelength (hereafter, second derivative spectra) is used to pre-process the in vivo spectra to reduce the impact of baseline shifts and tissue scattering, as well as to improve spectral resolution. Arakaki et al. [27] have shown that spectra with identical Mb saturations and concentrations, but different scattering coefficients, have very similar second derivative spectra. This elimination of baseline shifts may be why accurate PLS estimates result despite differences in pigmentation, small subcutaneous fat layers (<1 cm) and scattering differences between the in vitro calibration sets and the in vivo spectra.
3.2.3.2. Goodness of fit:
Evaluation of the adequacy of the calibration sets in fitting in vivo spectra is accomplished with the Mahalanobis Distance Test (MDT) and Residual Ratio test (RRT). Briefly, the MDT uses a chi-squared distribution to classify data points as members of the calibration set (probability value 1.0 – 0.05) vs. outliers (probability value <0.05) based on a principal component analysis of spectra in the calibration set and those acquired in vivo [27]. For the RRT, the magnitudes of the residuals from each of the data sets can be compared for consistency in spectral variations and to identify species that are present in the in vivo data set but not in the calibration set [24]. The residual ratio is calculated as the ratio of the sum of squares of residuals for an in vivo spectrum to the mean sum of squares of residuals in all of the calibration set spectra. We use a residual ratio <10 as the indication that the PLS model is relevant for an in vivo spectrum [35].
3.2.3.3. Calibration of cellular and vascular oxygenation:
We use a 2-point calibration to establish the end-points (0% and 100% O2 saturation) for the relative Hb and Mb saturations derived from the PLS method. An example of the end-point determinations of Hb saturation are shown in Figure 3 (upper panel). To achieve full Hb-O2 saturation, the subjects breathe 100% O2 for 5 min during which Hb saturation rises to a peak value in human subjects. This peak is used to represent full O2 saturation. Confirmation of Hb-O2 saturation comes from direct measurement of venous partial pressure in resting gastrocnemius muscle in dogs breathing 100% O2, which yielded a PO2 of 285 torr (i.e., ~100% Hb saturation) [36]. The high peak Hb saturation observed during hyperemia in Fig. 3 represents the greatly increased oxy-Hb content in muscle due to higher blood flow (and hemoglobin concentration) as compared to resting muscle. Lower relative changes in saturation with hyperemia are apparent with conventional near-infrared measurements because they combine lower relative change in Mb saturation with that of Hb saturation. Values during hyperemia are not valid for analysis and cannot be used as endpoints for Hb-O2 saturation because of the requirement for a constant [Hb] in the data set, as mentioned above. Complete desaturation of the O2 carriers is achieved by allowing muscle respiration to deplete O2 stores during ischemia. Zero Hb saturation is taken as the asymptote of the desaturation curve after 15 min of blood flow occlusion.
FIGURE 3:

Optical measurements showing for Hb-O2 saturation (upper panel) and Mb-O2 saturation (lower panel) during air breathing (0–3 min), breathing of 100% O2 (4–8 min), ischemia (9–23 min), and the recovery after tourniquet release (>24 min). The horizontal dashed lines show the calibration end-points: 100% O2 saturation at 8 min for the Hb-O2 peak and 27 min for the Mb-O2 peak, and 0% O2 saturation at the end of ischemia at 23 min for both peaks.
Fig. 3 (lower panel) shows that Mb is not 100% saturated in resting muscle in agreement with animal and other human muscle studies [28, 37], [38], [39]. The two-point calibration shown in Fig. 3 yields a resting Mb-O2 saturation of 60–75% in agreement with independent methods [38], but well below the value estimated by an MRS approach (~90%[39]). This difference likely reflects the low signal to noise (S/N) of the deoxy-Mb peak detected by MRS at high oxy-Mb levels [40] and the lack of endpoints in the MRS approach. In contrast, the optical approach has equal S/N at all saturation values and has the fast time resolution (~1 sec) to capture the peak Mb-O2 saturation after release of the tourniquet during hyperemia as a result of high blood flow and O2 delivery. The Mb-O2 saturation during hyperemia is well above the value in resting muscle or that achieved with breathing 100% O2 yielding 60–75% O2 saturation in resting muscle. The end result of these calibrations are %Mb-O2 and %Hb-O2 values that are combined with the muscle content of these O2 carriers to yield the concentration of Mb-O2 and Hb-O2 for the O2 uptake determinations.
3.3. O2 uptake
Figure 1 shows the %Mb-O2 and %Hb-O2 changes reflect the O2 consumption by the mitochondria as the result of blocking O2 delivery by occlusion of blood flow using a tourniquet. The uptake of O2 is determined from the %Mb-O2 and %Hb-O2 changes during the first 2 min of ischemia when Mb-O2 saturation is above the threshold for an O2 limit to mitochondrial respiration (50% Mb-O2 for P50 = 2.4 torr [13, 41], [42]). The O2 uptake rate is calculated as previously described [13, 37]:
| (1) |
where O2 uptake is in mM sec−1, %Mb-O2 and %Hb-O2 are the percentage saturation levels, [Hb] and [Mb] are concentrations in muscle tissue (mM), dissO2 is the amount of O2 dissolved in the intracellular compartment (mM), and Δt is the time period of the determinations (sec). The O2 dissolved in the vascular space is negligible because of the low O2 content of plasma and small plasma volume per muscle volume (<5% of total). Good agreement is evident between our method of measurement of O2 uptake in resting hand muscle (1.4± 0.2 (SD) μM sec −1) and traditional, invasive measurements of O2 uptake determined from blood flow through resting forearm muscles (1.0± 0.44 (SD) μM sec −1; so-called Fick method) [43, 44].
4. MR SPECTROSCOPY
4.1. Strategy
These determinations measure phosphocreatine content ([PCr]) in vivo and use changes in [PCr] to measure ATP flux. The chemical content of PCr is determined by absolute quantification methods using MRS. The rate of change of [PCr] in muscle depleted of O2 (i.e., absence of oxidative ATP supply) measures ATP flux via the creatine kinase reaction (see below).
4.2. Quantification
Here we provide a detailed outline of our methods for quantifying both 31P and 1H metabolites in human muscle. Determination of [Mb], [PCr] and [ATP] involves heteronuclear (1H and 31P) acquisition using a variably tuned coil to detect each of the nuclei with high S/N based on the method of Buchli and Boesiger [30]. Myoglobin concentration is determined using the internal H20 Calibration method in Ref. [30] by taking the ratio of the deoxy-Mb to the H2O peak areas corrected for the gain difference between these two spectral acquisitions as well as the number of acquisitions.
The phosphorus quantification strategy depends on the expected thickness and variability of the subcutaneous lipid within a patient population. For a relatively homogeneous and thin subcutaneous lipid layer (e.g. FDI), we use non-selective acquisition in which the phosphorus signal is quantified via reference to a water scan with a correction for differential MR sensitivity to phosphorus versus proton.
For cases in which the subcutaneous fat layer is not negligible, phosphorus quantification involves selecting a volume of muscle for spectroscopy via a voxel-localized, ISIS acquisition [30]. This acquisition is referenced to a standard curve from a series of phosphorus standards (10 – 50 mM inorganic phosphate, Pi, concentration) that span the expected metabolite concentration ranges to yield the cell concentration of the phosphorus containing compound (PCr, ATP or inorganic phosphate, Pi). These phosphorus standards are prepared to closely match the loading conditions of human tissue for the coil in the study using a total of 75 mM Na+ supplied either from Na2HPO4 or from NaCl.
4.2.1. Spectral analysis:
All spectra related to quantification are analyzed in the time domain using the Advanced Method for Accurate, Robust and Efficient Spectral fitting (AMARES) algorithm in the Java-based Magnetic Resonance User Interface (jMRUI [45]) software, which allows constraint on fits using prior knowledge files [46]. jMRUI software is available from the participants of the EU Network programs (http://www.mrui.uab.es/mrui/).
For the ATPase quantification, the free-induction decays (FID) are line-broadened (using the half width of the resting PCr peak for the broadening value) and Fourier-transformed into spectra. The peak areas in resting muscle are determined by integration of fully relaxed spectra. These areas are used as the reference levels for the changes in PCr and Pi peak areas during the experiments and are determined from the partially saturated spectra as previously described [47]. Protons are assumed to be at a concentration of 110 M in H2O. The final concentrations are corrected for a 10% extracellular water content in muscle [48].
5. ATP Flux
5.1. Resting ATPase:
Figure 1 shows our MRS method of measuring ATP flux from the changes in PCr that accompany anoxia in the muscle via the creatine kinase reaction:
| (2) |
where ADP, H+ and Cr are the adenosine diphosphate, proton and free creatine levels in muscle. Under ischemia, aerobic ATP supply is blocked and the experiment is designed so that glycolytic ATP synthesis is minimal in resting muscle. Since glycolysis provides a parallel source of ATP and, in turn, PCr, the PCr breakdown is a measure of net ATP demand (cell ATP use minus glycolytic ATP supply). We have found in resting muscle that glycolytic ATP supply is small (≤8% on average of the ATP demand [14]). A detailed analysis of the reactions underlying this ATP balance and a careful validation of the chemical energy balance has been made in isolated mouse muscles [49]. A second study validated the MRS approach used to quantify the individual ATP fluxes in vivo [50]. In both validation studies, a chemical energy balance is found between the net ATP turnover and oxidative ATP supply (Fig. 2 and Table IIB in [49]; Fig. 3 in [50]). In addition, the use of anoxia to separate ATP fluxes is validated by the agreement of the net ATP turnover under anoxia to the steady-state O2 uptake (converted to ATP flux using the P/O; [49], Fig. 4 in [50]). These agreements demonstrate the ability to separate and quantitatively measure the sources and sinks of ATP in the muscle cell in vivo. They also demonstrate that anoxia did not change the cell ATP turnover from that found under aerobic conditions. These validations permit the use of net ATP demand as a measure of mitochondrial ATP supply, which in combination with O2 uptake yields mitochondrial P/O in vivo.
FIGURE 4:

Comparison of ATP flux, O2 uptake rates, and P/O in the FDI of adult and elderly subjects from ref [14]. Horizontal solid bars represent the group means and * denotes difference between mean values of the muscles at P<0.05. The horizontal dashed line in the P/O panel defines the theoretical value for well-coupled mitochondria [52, 53, 63]
6. MITOCHONDRIAL FLUXES AND P/O IN VIVO
Here we show that these methods are sensitive enough to identify the nature of the mitochondrial defect and its consequence on cell function in an individual. The power to detect individual differences in ATP and O2 fluxes, as well as P/O in the human FDI is shown in Figure 4. The nearly three-fold range apparent among individuals of each group in the ATP or O2 flux is well above the reproducibility of our measurements (typically ±11% [51]) and therefore reflects individual variation in ATP demand and mitochondrial O2 uptake. The small variance in the ratio of these fluxes (i.e., P/O; CV=7% [14]) means that subjects with a high ATP flux have a corresponding high O2 flux. The P/O in the muscles of the FDI (mean: 2.68 ± 0.05 SE) is close to the values expected for well-coupled mitochondria (P/O = 2.3–2.5 [52] [53], dashed line in Fig. 4). Studies of isolated tissues from adult humans have found similar mitochondrial coupling values [15, 54]. This similarity to the theoretical coupling value suggests that the mitochondria in the FDI muscles are operating at or close to the maximum stoichiometry for oxidative phosphorylation. In contrast, we have found that the tibialis anterior muscle of the human leg is substantially less well-coupled in adults as compared to the FDI [14], which indicates that mitochondrial coupling may not be fixed at the theoretical limit for all tissues.
The ability to detect differences between groups is apparent in the lower average ATP flux and P/O in the FDI of elderly subjects. The drop in ATP flux with age is consistent with the decline in maintenance functions involving ATP with age (i.e. ion transport, protein and DNA synthesis and degradation) [55]. In contrast, no difference is apparent in resting O2 uptake rates with age, indicating that the mitochondria consume the same O2 to generate less ATP in the elderly FDI. This decrease in efficiency and coupling with age is apparent in the decrease in P/O between adult and elderly groups. We have previously shown a decrease in maximum ATP flux per mitochondrion in elderly vastus lateralis of humans [21] and others have reported a decrease in P/O in human fibroblasts with age [15]. Thus, this paired spectroscopic approach is sensitive enough to reveal individual differences in mitochondrial fluxes and coupling and is also capable of detecting changes in flux and capacity with age and disease.
A third benefit of the in vivo approach is the ability to determine the consequence of a mitochondrial defect on cell function. A consequence of the severe uncoupling with age is the inability to maintain ATP levels in the cell [56]. Figure 5 shows this relationship by the lower ATP levels associated with reduced coupling in the FDI, which are found in the elderly subjects. This [ATP] loss may be the first step in the activation of cell death pathways (apoptosis/necrosis) leading to the irreversible muscle fiber loss that is an important aspect of the sarcopenia that is characteristic of the aging process [18, 57]. Thus noninvasive studies of the intact system reveal the connections between mitochondrial and cytosolic properties that are difficult to discern in biopsy tissue yet important to cell survival and longevity.
FIGURE 5:

ATP concentration ([ATP]) as a function of mitochondrial coupling (P/O) in the FDI of adult and elderly subjects from ref [14].
7. MITOCHONDRIAL CAPACITY IN VIVO
The mitochondrial flux measurements are used to quantify the capacities for O2 uptake and ATP production that represent the mitochondrial functional capacity and reflect the mitochondrial content. Measurements of oxidative enzyme activity in tissue biopsies are a common method for characterizing mitochondrial properties [58]. Here we show how to determine mitochondrial capacities noninvasively. The phosphorylation capacity (ATPmax) is measured using the PCr recovery time constant (τPCr) and the PCr level in oxygenated muscle at rest ([PCr]rest)[21]:
| (3) |
Confirmation that ATPmax is a good measure of phosphorylation capacity comes from animal and human studies that have found this rate to vary in direct proportion to the oxidative enzyme activity of healthy muscle [22, 59]. This approach has proved worthwhile in following the improvement of mitochondrial function with a targeted intervention such as reversal of mitochondrial dysfunction with exercise. We have previously reported a substantial increase in the phosphorylation capacity per mitochondrion (ATPmax/mitochondrial volume) with exercise training of the elderly [60], indicating that mitochondrial dysfunction is reversible
The maximal rate of mitochondrial O2 uptake (O2max) is a better predictor of mitochondrial content because of variation in mitochondrial P/O among muscles will result in a range of ATPmax independent of mitochondrial content. The mitochondrial oxidative capacity is derived from ATPmax using the coupling efficiency of mitochondria (P/O):
| (4) |
Figure 6 shows that this in vivo measurement of O2max is directly proportional to the reported mitochondrial content in a range of human muscles. Remarkably, these data points fall along the line that defines maximum oxygen consumption per mitochondrial volume (4 ml O2 (ml mito−1 min−1)) that has been determined in exercising animals (>10 species) by Hoppeler and colleagues [61, 62]. The similar maximum oxygen consumption per mitochondrial volume of the human muscle findings and the value found for a wide range of species provides independent support of the validity of our O2max determination. Thus O2max provides not only a good measure of muscle mitochondrial content but the ratio of O2max to mitochondrial volume provides a metric for identifying mitochondrial oxidative dysfunction and respiratory chain defects in aged and diseased muscle.
FIGURE 6:

Maximum oxygen uptake of muscle measured in vivo vs. mitochondrial content reported for these human muscles. The dashed line represents a constant maximum O2 uptake per mitochondrial volume (4 ml O2 (ml mito−1 min−1)), as reported for animals exercising at the aerobic capacity [61]. Maximum oxygen uptake of muscle is determined using Eq. 4 using data from [14, 21, 64]. Muscle mitochondrial content from [21, 65, 66]. Muscle abbreviations: human first dorsal interosseus (FDI), vastus lateralis (VL) and tibialis anterior (TA).
8. CONCLUSIONS
Here we show innovative spectroscopic tools and measurement protocols that provide a noninvasive biopsy of mitochondrial function and dysfunction in health, degenerative disorders and disease. This noninvasive approach permits study of a wide range of muscles and keeps intact the normal network of metabolic and regulatory connections. New insights from this approach are that mitochondrial coupling efficiency (P/O) is close to theoretical values in some muscles, can differ among human muscles of the same individual, and can decline with age with impact on key signals governing cell fate. The ability to monitor mitochondrial coupling (P/O) and oxidative capacity (O2max), in addition to ATPmax, provide new tools for diagnosing mitochondrial defects and also for assessing the impact of an intervention on both the quality and quantity of mitochondria. The end result is a new window on mitochondria that permits identifying individuals at an early stage of dysfunction to permit targeting interventions to forestall or even reverse the functional and cell loss with age and disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CITATIONS
- 1.Chabi B, Adhihetty PJ, Ljubicic V, and Hood DA (2005) Med Sci Sports Exerc 37, 2102–10. [DOI] [PubMed] [Google Scholar]
- 2.Shigenaga M, Hagen TM, and Ames BN (1994) Proceedings of the National Academy of Sciences, USA 91, 10771–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholls D (2002) Sci Aging Knowledge Environ 2002, pe12. [DOI] [PubMed] [Google Scholar]
- 4.Parish R, and Petersen KF (2005) Curr Diab Rep 5, 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews ZB, Horvath B, Barnstable CJ, Elsworth J, Yang L, Beal MF, Roth RH, Matthews RT, and Horvath TL (2005) J Neurosci 25, 184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, and Shulman GI (2003) Science 300, 1140–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, and Conley KE (2005) J Physiol 569, 467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper ME, Bevilacqua L, Hagopian K, Weindruch R, and Ramsey JJ (2004) Acta Physiol Scand 182, 321–31. [DOI] [PubMed] [Google Scholar]
- 9.Janes M (2008) Methods. [Google Scholar]
- 10.Calvaruso MA, Smeitink J, and Nijtmans L (2008) Methods. [DOI] [PubMed] [Google Scholar]
- 11.Vempati UD, Torraco A, and Moraes CT (2008) Methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcinek DJ (2004) Acta Physiol Scand 182, 343–52. [DOI] [PubMed] [Google Scholar]
- 13.Marcinek DJ, Schenkman KA, Ciesielski WA, and Conley KE (2004) Am J Physiol Cell Physiol 286, C457–63. [DOI] [PubMed] [Google Scholar]
- 14.Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, and Conley KE (2007) Proc Natl Acad Sci U S A 104, 1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greco M, Villani G, Mazzucchelli F, Bresolin N, Papa S, and Attardi G (2003) Faseb J 17, 1706–8. [DOI] [PubMed] [Google Scholar]
- 16.Lebon V, Dufour S, Petersen KF, Ren J, Jucker BM, Slezak LA, Cline GW, Rothman DL, and Shulman GI (2001) J Clin Invest 108, 733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, and Shulman GI (2001) J Biol Chem 276, 20240–4. [DOI] [PubMed] [Google Scholar]
- 18.Dirks AJ, Hofer T, Marzetti E, Pahor M, and Leeuwenburgh C (2006) Ageing Res Rev 5, 179–95. [DOI] [PubMed] [Google Scholar]
- 19.Skulachev VP (2006) Apoptosis 11, 473–85. [DOI] [PubMed] [Google Scholar]
- 20.Bua EA, McKiernan SH, Wanagat J, McKenzie D, and Aiken JM (2002) J Appl Physiol 92, 2617–24. [DOI] [PubMed] [Google Scholar]
- 21.Conley KE, Jubrias SA, and Esselman PE (2000) J Physiol 526.1, 203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCully KK, Fielding RA, Evans WJ, Leigh JS, and Posner JD (1993) J Appl Physiol 75, 813–19. [DOI] [PubMed] [Google Scholar]
- 23.Blaisdell FW (2002) Cardiovasc Surg 10, 620–30. [DOI] [PubMed] [Google Scholar]
- 24.Schenkman KA, Marble DR, Burns DH, and Feigl EO (1999) Appl Spectrosc 53, 332–38. [Google Scholar]
- 25.Arakaki LSL, and Burns DH (1992) Appl Spectrosc 46, 1919–28. [Google Scholar]
- 26.Arakaki LSL (1995), University of Washington.
- 27.Arakaki LSL, Kushmerick MJ, and Burns DH (1996) Applied Spectroscopy 50, 697–707. [DOI] [PubMed] [Google Scholar]
- 28.Arakaki LS, Burns DH, and Kushmerick MJ (2007) Appl Spectrosc 61, 978–85. [DOI] [PubMed] [Google Scholar]
- 29.Marcinek DJ, Amara CE, Matz K, Conley KE, and Schenkman KA (2007) Appl Spectrosc 61, 665–9. [DOI] [PubMed] [Google Scholar]
- 30.Buchli R, and Boesiger P (1993) Magn Reson Med 30, 552–8. [DOI] [PubMed] [Google Scholar]
- 31.Cui W, Kumar C, and Chance B (1991) Proc SPIE 1431, 180–91. [Google Scholar]
- 32.Sylven C, Jansson E, and Böök K (1984) Cardiovasc Res 18, 443–46. [DOI] [PubMed] [Google Scholar]
- 33.Haaland DM, and Thomas EV (1988) Anal Chem 60, 1193–202. [Google Scholar]
- 34.Tran TK, Sailasuta N, Kreutzer U, Hurd R, Chung Y, Mole P, Kuno S, and Jue T (1999) Am J Physiol 276, R1682–90. [DOI] [PubMed] [Google Scholar]
- 35.Miller CE (2000) J. Chemom 14, 513–28. [Google Scholar]
- 36.Grassi B, Gladden LB, Stary CM, Wagner PD, and Hogan MC (1998) J Appl Physiol 85, 1404–12. [DOI] [PubMed] [Google Scholar]
- 37.Marcinek DJ, Ciesielski WA, Conley KE, and Schenkman KA (2003) Am J Physiol Heart Circ Physiol 285, H1900–8. [DOI] [PubMed] [Google Scholar]
- 38.Voter WA, and Gayeski TEJ (1995) Am J Physiol 269, H1328–H41. [DOI] [PubMed] [Google Scholar]
- 39.Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, and Carlier PG (2006) J Physiol 571, 415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson RS, Newcomer SC, and Noyszewski EA (2001) J Appl Physiol 91, 2679–85. [DOI] [PubMed] [Google Scholar]
- 41.Schenkman KA, Marble DR, Burns DH, and Feigl EO (1997) J Appl Physiol 82, 86–92. [DOI] [PubMed] [Google Scholar]
- 42.Haseler LJ, Hogan MC, and Richardson RS (1999) J Appl Physiol 86, 2013–8. [DOI] [PubMed] [Google Scholar]
- 43.Zurlo F, Larson K, Bogardus C, and Ravussin E (1990) J Clin Invest 86, 1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Beekvelt MC, Colier WN, Wevers RA, and Van Engelen BG (2001) J Appl Physiol 90, 511–9. [DOI] [PubMed] [Google Scholar]
- 45.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, and Graveron-Demilly D (2001) Magma 12, 141–52. [DOI] [PubMed] [Google Scholar]
- 46.Vanhamme L, van den Boogaart A, and Van Huffel S (1997) J Magn Reson 129, 35–43. [DOI] [PubMed] [Google Scholar]
- 47.Blei ML, Conley KE, and Kushmerick MJ (1993) J Physiol (Lond) 465, 203–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjøgaard G, and Saltin B (1982) Am J Physiol 243, R271–R80. [DOI] [PubMed] [Google Scholar]
- 49.Crow MT, and Kushmerick MJ (1982) J Gen Physiol 79, 147–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kemper WF, Lindstedt SL, Hartzler LK, Hicks JW, and Conley KE (2001) Proc Natl Acad Sci U S A 98, 723–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blei ML, Conley KE, Odderson IB, Esselman PC, and Kushmerick MJ (1993) Proc Natl Acad Sci U S A 90, 7396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholls D, and Ferguson S (2002) Bioenergetics 3, Academic Press, London. [Google Scholar]
- 53.Brand MD (2005) Biochem Soc Trans 33, 897–904. [DOI] [PubMed] [Google Scholar]
- 54.Kuznetsov AV, Kunz WS, Saks V, Usson Y, Mazat JP, Letellier T, Gellerich FN, and Margreiter R (2003) Anal Biochem 319, 296–303. [DOI] [PubMed] [Google Scholar]
- 55.Yarasheski KE (2003) J Gerontol A Biol Sci Med Sci 58, M918–22. [DOI] [PubMed] [Google Scholar]
- 56.Nicholls DG (2004) Aging Cell 3, 35–40. [DOI] [PubMed] [Google Scholar]
- 57.Izyumov DS, Avetisyan AV, Pletjushkina OY, Sakharov DV, Wirtz KW, Chernyak BV, and Skulachev VP (2004) Biochim Biophys Acta 1658, 141–7. [DOI] [PubMed] [Google Scholar]
- 58.Reichmann H, Hoppeler H, Mathieu CO, von Bergen F, and Pette D (1985) Pflugers Arch 404, 1–9. [DOI] [PubMed] [Google Scholar]
- 59.Paganini AT, Foley JM, and Meyer RA (1997) Am J Physiol. 272, C501–C10. [DOI] [PubMed] [Google Scholar]
- 60.Jubrias SA, Esselman PC, Price LB, Cress ME, and Conley KE (2001) J Appl Physiol 90, 1663–70. [DOI] [PubMed] [Google Scholar]
- 61.Hoppeler H (1990) Respir Physiol 80, 137–45. [DOI] [PubMed] [Google Scholar]
- 62.Hoppeler H, and Lindstedt SL (1985) J. Exp. Biol 115, 355–64. [DOI] [PubMed] [Google Scholar]
- 63.Lehninger AL, Nelson DL, and Cox MM (1993) Principles of Biochemistry, Worth Publishing, New York, NY. [Google Scholar]
- 64.Jubrias SA, Crowther GJ, Shankland EG, Gronka RK, and Conley KE (2003) J Physiol 553, 589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoppeler H (1986) Int J Sports Med 7, 187–204. [DOI] [PubMed] [Google Scholar]
- 66.Jerusalem F, Engel A, and Peterson H (1975) Neurology 25, 127–34. [DOI] [PubMed] [Google Scholar]


