Abstract
Rationale
The neuropeptide catestatin is an endogenous nicotinic-cholinergic antagonist, which acts as a pleiotropic hormone.
Objective
Catestatin shares several functions with angiogenic factors. We therefore reasoned that catestatin induces growth of new blood vessels.
Methods and Results
Catestatin induced migration, proliferation and anti-apoptosis in endothelial cells and exerted capillary tube formation in-vitro in a matrigel assay and such effects were mediated via G-protein, mitogen-activated protein kinase and Akt. Catestatin -induced endothelial cell functions are further mediated by basic fibroblast growth factor as shown by blockade of effects by a neutralizing fibroblast growth factor antibody. Furthermore, catestatin released basic fibroblast growth factor from endothelial cells and stimulated fibroblast growth factor signaling. Beside its function on endothelial cells, catestatin also exerted effects on endothelial progenitor cells and vascular smooth muscle cells.
In-vivo, catestatin induced angiogenesis in the mouse cornea neovascularization assay and increased blood perfusion and number of capillaries in the hindlimb ischemia model. Beside angiogenesis, catestatin increased density of arterioles/arteries and incorporation of endothelial progenitor cells in the hind-limb ischemia model indicating induction of arteriogenesis and post-natal vasculogenesis.
Conclusion
We conclude that catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism.
Subject code: angiogenesis, peripheral vascular disease, other vascular biology, animal models of human disease
Keywords: angiogenesis, blood vessels, endothelium, endothelial progenitor cells
Introduction
Chromogranin A (CgA), the index member of the chromogranin/secretogranin protein family, is the major soluble protein of catecholamine storage vesicles of sympathetic nerve terminals and the adrenal medulla 1 2. CgA is a pro-protein giving rise to biological active peptides like the dysglycemic hormone pancreastatin 3, the vasodilator vasostatin 4 and catestatin 5 (CST: CgA352-372) which inhibits catecholamine release by acting as a nicotinic cholinergic antagonist resulting in a negative feedback mechanism. Although plasma CgA is high in human essential (hereditary) hypertension, the plasma concentration of catestatin is lower in both established cases and in normotensive subjects with a family history of the disease 6, suggesting a mechanism whereby diminished CST might increase the risk for later development of hypertension. Consistent with the human findings, high blood pressure has been reported in mice after targeted ablation of the Chga gene (Chga-knock-out) and such high blood pressure can be “rescued” either by replacement with catestatin or introduction of the human ortholog in the Chga-knock-out background 7.
Angiogenesis, the growth of new vessels from the pre-existing vasculature, is an important process in many physiological conditions including embryonic development and wound healing. However, defects in the regulation of angiogenesis often result in pathological conditions such as inflammatory diseases, ischemic heart, peripheral vascular diseases, proliferative retinopathy and solid tumors 8. The most potent angiogenic factors are basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF). Besides bFGF and VEGF, a variety of neuropeptides like substance P (SP), secretoneurin (SN) and neuropeptide Y (NPY) exert effects on endothelial cells (ECs) and induce angiogenesis 9-11.
Catestatin shares several features with SP such as vasodilation by CST (Fung MM 2010, in press) and SP 12 and having comparable pI (CST: 12.16 versus SP: 11.14). In addition, CgA was shown to be up-regulated by hypoxia in neuronal cells after transient hypoxia 13, a typical characteristic of angiogenic factors like VEGF. Based on the above similarities of CST with angiogenic peptides we reasoned that CST would exert effects on ECs in-vitro and induce angiogenesis in-vivo. The present communication establishes catestatin as a novel angiogenic cytokine with the potential of inducing therapeutic effects in animal models of ischemia.
Materials and Methods
For further details in materials and methods see supplemental data.
Catestatin peptide and antiserum
Human catestatin and antiserum was described previously 14.
Endothelial progenitor cell (EPC) isolation
EPCs were isolated and cultured and EPC chemotaxis assay was performed as described 15.
Tube formation assay
Tube formation assay was performed as described 10. Some matrigel assays were performed with a mixture of 5000 HUVECs and 3000 DiI LDL-labelled EPCs 16. The association of EPC to capillary structures was quantified using Image J.
Cornea Neovascularisation Assay
Pellets containing 500 ng CST, scrambled CST or VEGF were implanted in C57/BL/6J mice as described 10. On postoperative day 7 mice received an intravenous injection of 500 μg BS1 lectin conjugated to FITC (Vector Laboratories). After euthanasia, enucleated eyes were fixed in 1% paraformaldehyde, corneas dissected and examined by fluorescence microscopy.
Mouse hindlimb ischemia model
C57BL/6 wild-type mice were subjected to unilateral hindlimb surgery. Mice were injected with saline or 20 μg catestatin into thigh and calf muscles after operation and every other day for weeks 1 and 2 and 2 times per week for weeks 3 and 4. Limb necrosis status (see also supplemental data) was determined on days 7, 14 and 28.
Bone marrow transplantation model
Bone marrow transplantation (BMT) was performed as described 15 17. Labelling of functional vessels and of EPCs in BMT-mice was performed 3 weeks after induction of ischemia as described 17. EPCs are expressed as number of double positive cells per high-power field (magnification ×100).
For confocal microscopy a Broadband Confocal Leica TCS SP5 microscope (lenses: HCX PL APO CS 20×0.7 (dry) and HCX PL APO CS 63×1.2 (Water)) was used.
Blood flow measurement
Blood flow measurements were performed using a laser Doppler perfusion image (LDPI) analyzer (Moor Instruments, USA). Blood perfusion is expressed as the LDPI index representing the ratio of left (=operated, ischemic leg) versus right (=not-operated, not-ischemic leg) limb blood flow.
Immunohistochemistry
CD-31 and alpha-smooth muscle actin (SMA) staining were performed as described 18 10. For fluorescent microscopy appropriate secondary antibodies (Alexa 488 for SMA and Alexa 594 for CD31; both from Invitrogen 1:200) were used.
Results
In-vitro Results
1. CST effects on migration, proliferation and apoptosis in ECs in-vitro
CST caused dose dependent induction of chemotaxis in human umbilical vein endothelial cells (HUVEC) with a maximum effect at 1 nmol/L (relative chemotaxis index, CI 1.67±0.06, P<0.01 vs. ctr.; Fig. 1a) comparable to other angiogenic factors like VEGF or SN 10. Blockade of CST by a specific neutralizing antibody completely inhibited CST mediated EC migration indicating specificity of the observed effect (relative CI: CST 1.74±0.06, CST+antibody 1.12±0.06, P<0.01 CST vs. CST+Ab; Fig. 1b). CST-induced chemotaxis was completely abolished by inhibition of G-protein coupled receptors by pertussis toxin (PTX) and mitogen-activated protein kinase (MAPK) by PD indicating CST signaling via G-protein-coupled receptors and MAPK pathway (relative CI: CST 1.74±0.06, CST+PTX 1.0±0.04, CST+PD 1.08±0.05, P<0.01 CST vs. CST+PTX and CST+PD; Fig. 1b). Tyrosine kinase inhibitor Genistein (1 μmol/L) caused only partial inhibition of CST-induced EC migration (relative CI: CST 1.73±0.05, CST+genistein 1.41±0.04; P<0.05 CST vs. CST+genistein).
Figure 1. Effects of catestatin on ECs in-vitro.
1.a. Catestatin induces EC migration. EC chemotaxis is expressed as chemotactic index relative to control cells without treatment. Cells were treated with VEGF (50 ng/ml), secretoneurin (SN, 1 nmol/L) or catestatin with a concentration of 10-7 to 10-15 mol/L (M). Results are means of 3-5 experiments and show that catestatin induces EC chemotaxis with a maximum effect at 10-9 mol/L.* P<0.05; ** P<0.01.
1.b. Catestatin-induced EC migration is blocked by catestatin antibody, pertussis toxin (PTX) and inhibition of MAPK by PD. ECs were treated with 1nmol/L catestatin with or without pre-incubation with catestatin-neutralizing antibody, PTX or PD. Results (n=3) show that catestatin-induced migration is blocked by treatment with respective substances whereas antibody, PTX or PD did not affect basal migration. **P<0.01 CST vs. Ctr; § P<0.05 vs. CST
1.c. Catestatin induces EC proliferation. ECs were treated with 1 nmol/L SN or different concentrations of catestatin, cell number was counted and compared to untreated cells. 1nmol/L catestatin exerted the most potent effect on EC proliferation and this effect was blocked by treatment with a neutralizing catestatin antibody or PD. *P<0.05; **P<0.01 vs. Ctr. § P<0.05 vs. CST; n=3.
1.d. Catestatin induces EC proliferation-BrdU-Assay
Catestatin-induced EC-proliferation was also determined by BrdU assay. VEGF (50 ng/ml) and catestatin (1 nmol/L) significantly increased BrdU incorporation as expressed as relative light units per second. *P<0.05 vs. Ctr; n=3.
1.e. Catestatin inhibits EC apoptosis. ECs were starved and stained for TUNEL and DAPI. (given as % of TUNEL positive cells out of all cells stained by DAPI). Catestatin reduced EC apoptosis (% of TUNEL-positive of DAPI-positive cells) at 1 nmol/L whereas co-incubation with WM (10 nmol/L) inhibited CST-induced protective effect. * P<0.05 vs. Ctr; § P<0.05 vs. CST; n=3. Representative pictures of TUNEL and DAPI stains are shown on the right; arrowheads: examples of double positive cells; arrow in Ctr. DAPI: cell positive for DAPI and negative for TUNEL.
1.f. Catestatin- and VEGF-induced ERK and Akt activation.
ECs were stimulated with catestatin at 1nmol/L and 300 nmol/L, with VEGF (50 ng/ml) (all 10 minutes) with the combination of VEGF and catestatin 300 nmol/L or with 20% FBS and extracts were analyzed for ERK- or Akt-activation by western blotting. Catestatin induced ERK and Akt-phosphorylation at low and even more at high concentrations and did not block VEGF induced stimulation of these pathways. Actin and tubulin stains were used as loading controls.
CST also induced proliferation of HUVEC (as determined by cell number) with a maximum effect at 1nmol/L (relative cell number 1.85±0.11, P<0.01 vs. ctr.) and such effect was blocked by pre-treatment with CST antibody (relative cell number 0.97±0.06, P<0.05 vs. CST) and blockade of MAPK by PD (relative cell number 1.19±0.09, P<0.05 vs. CST) indicating specificity of CST effect and its signaling through MAPK pathway (Fig. 1c). CST-induced EC proliferation was also determined by BrdU staining (Fig. 1d). CST like VEGF significantly increased BrdU incorporation in ECs.
To test the potential effects of CST on EC apoptosis, HUVEC were serum-starved and stained for TUNEL and DAPI. CST caused significant inhibition of apoptosis (% TUNEL positive apoptotic cells of DAPI positive cells: Ctr. 23.0±0.7%, CST 10.4±0.5%; P<0.01 Ctr vs. CST; Fig. 1e). Inhibition of CST-induced anti-apoptotic effect by wortmannin (WM) indicates that PI-3 kinase/Akt pathway mediates this effect (Fig. 1e).
Inhibition of CST-mediated effects by PD and WM indicates involvement of MAPK and PI3-kinase/Akt pathways. CST signaling was evaluated by Western blotting, which revealed activation of extracellular signal-regulated kinase (ERK) and Akt as judged by phosphorylation of ERK and Akt (Fig. 1f). CST was able to stimulate ERK- and Akt-phosphorylation at 1 nmol/L and 300 nmol/L, VEGF was used as positive control. In contrast to the N-terminal CgA peptide vasostatin 19, CST did not inhibit VEGF-induced ERK activation in ECs.
2. CST induction of angiogenesis in-vitro
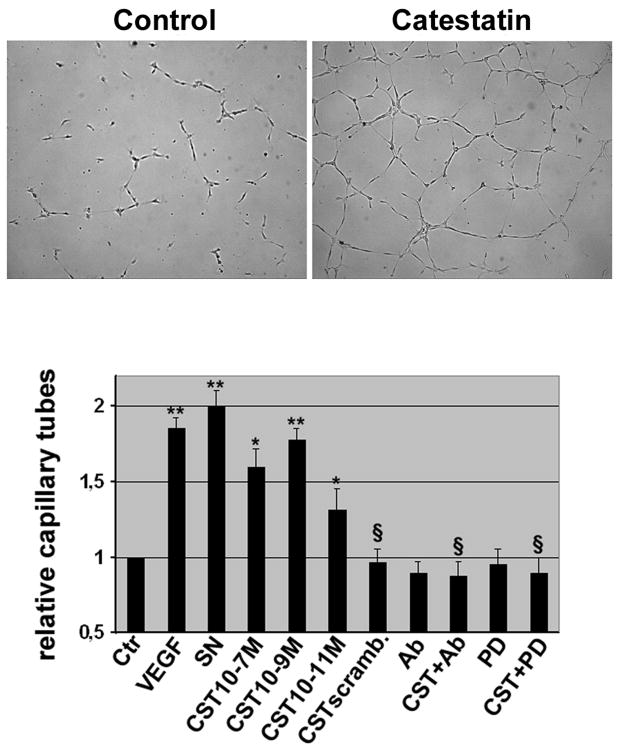
CST induced angiogenesis in-vitro as demonstrated by increments in capillary tube formation in a matrigel assay (Fig. 2) with a maximum effect at 1 nmol/L (relative capillary tube formation 1.77±0.08, P<0.01 vs. ctr.) comparable to VEGF or SN. Conversely, a scrambled peptide exerted no effect. Pretreatment with CST antibody completely abolished CST-induced tube formation (tube formation 0.87±0.1, P<0.01 vs. CST), indicating specificity of observed effects. In addition, CST-induced tube formation was totally inhibited by co-incubation of CST with PD (tube formation 0.89±0.11, P<0.01 vs. CST) indicating CST signaling through MAPK pathway in in-vitro angiogenesis.
Figure 2. Catestatin induces capillary tube formation in-vitro.
ECs were seeded onto matrigel and capillary tubes were counted. VEGF (50 ng/ml) and SN (1 nmol/L) were used as positive control and catestatin induced capillary tube formation with a maximum effect at 1 nmol/L (M). Scrambled catestatin (CST scramb.) was not effective. PD or a neutralizing antibody blocked catestatin-induced tube formation. * P<0.05; ** P<0.01 capillary tubes vs. Ctr; §P<0.01 vs. CST; n=3-5.
In-vivo Results
1. CST effect on cornea neovascularization assay
To further establish the role of CST in angiogenesis we took advantage of the in-vivo cornea neovascularization assay. We found that scrambled CST (Fig. 3 left) did not induce growth of new vessels toward the pellet whereas CST induced growth of arteries out of the limbus artery leading to a capillary network around the pellet. Of note, vessels reaching the limbus vein and presumably representing veins were also observed (Fig. 3, middle). VEGF was used as positive control and showed a distinct pattern of neovascularization with higher density of capillaries at the leading front of vessels (Fig. 3 right).
Figure 3. Catestatin induces angiogenesis in-vivo in the mouse cornea neovascularization assay.
Pellets (P) containing 500 ng of scrambled catestatin, catestatin or VEGF were implanted into the cornea of mice. After 7 days mice received i.v. FITC-BS1-lectin, were sacrificed, corneas dissected and subjected to fluorescent microscopy. Catestatin and VEGF induced growth of arteries (red arrows) originating from the limbus artery (red arrowhead), forming a capillary plexus (white arrow) at the bottom of the pellet and draining the blood via veins (blue arrows) to the limbus vein (blue arrowhead).
2. CST effects on hindlimb ischemia model
Intra-muscular injection of CST into the ischemic limb significantly inhibited tissue necrosis and amputation as shown by reduced necrosis score 14 and 28 days after induction of ischemia and CST treatment (necrosis score day 28: Ctr. 1.8±0.12, CST 1.3±0.1, P<0.05; Fig. 4a). Furthermore, CST improved blood perfusion as judged by laser-Doppler perfusion imaging (LDPI) (Fig. 4b). 3 and 4 wks after induction of ischemia, LDPI ratio increased significantly in CST-treated group as compared to saline-treated group (4 wks: Saline, 0.76±0.04 vs CST, 0.94±0.03, P<0.01; Fig. 4b). Blood vessel density in ischemic muscle immunohistochemistry was determined by CD31 staining (detecting ECs) and by alpha smooth muscle actin (SMA, detecting pericytes/smooth muscle cells). Significantly higher densities of capillaries (CD31 -positive vessels) were noticeable in CST-treated mice 4 weeks after surgery (capillaries/mm2: catestatin 845.5±47.8 and saline 530.4±41.8; P<0.01; Fig. 4c). In addition, we observed increased density of alpha-SMA-positive vessels after CST treatment (alpha-SMA-positive vessels/mm2: catestatin 7.5±0.5 and saline 3.9±0.7; P<0.01; Fig. 4d). These findings of increased density of arterioles/arteries after catestatin treatment are consistent with the induction of arteriogenesis by this peptide.
Figure 4. Catestatin induces therapeutic angiogenesis and arteriogenesis in the mouse hindlimb ischemia model.
4.a. Catestatin reduces necrosis score. After hind-limb ischemia mice (n=10 each group) were treated i.m. with catestatin or saline and evaluated for necrosis/amputation at days 7, 14 and 28. Necrosis score was significantly reduced by catestatin 14 and 28 days after induction of ischemia. Representative pictures are shown.
4.b. Catestatin improves blood perfusion. Limb perfusion was measured by LDPI before and after operation and weekly thereafter for 4 weeks. Results are expressed as LDPI ratio of the operated versus the not operated leg. Catestatin improved perfusion compared to saline 3 and 4 weeks after operation (n=10). Representative pictures are shown.
4.c. Catestatin increases capillary density. Sections from ischemic muscles (after 4 wks) were stained for capillary density by CD31. Values are expressed as CD31+ capillaries/mm2. Catestatin significantly increased capillary density (n=10).
4.d. Catestatin increases density of arteries/arterioles. Sections were also stained for alpha-SMA. Values are expressed as SMA-positive arteries or arterioles/mm2 (n=10). * P<0.05; ** P<0.01 Saline vs. CST for Fig. 4a-4d.
4.e. Immunoflourescence staining. Adjacent sections of CST treated ischemic limb stained for CD-31 and α-SMA. Merging of pictures shows CD-31 positve endothelial cells lining the inner surface of the vessels which are sorrounded by α-SMA-positive smooth muscle cells.
We injected CST in green fluorescence protein (GFP) bone marrow transplanted mice subjected to hindlimb ischemia for 3 weeks to evaluate CST-induced vasculogenesis in-vivo. CST induced incorporation of EPCs as shown by increased number of cells double positive for BS1- lectin (injected i.v. staining host vessels) and for GFP (bone-marrow-derived cells after transplantation). Examination of double positive cells per high powered field (hpf, 100×) revealed the following: Ctr. 9.6±2.1, CST 21.7±2; P<0.001 (Fig. 5a). When we examined peripheral blood of these mice we observed increased numbers of flk-1+/GFP+cells (% of GFP+cells) in CST treated mice (saline: 1.2±0.25, CST: 3.1±0.4, P<0.05). We also used confocal microscopy to improve co-localization of DAPI, GFP and rhodamine lectin positive cells. As shown in Fig. 5b (right bottom picture) cells positive for DAPI, GFP and lectin can be detected (arrowheads).
Figure 5. Catestatin induces postnatal vasculogenesis.
Sections of ischemic muscles (a) were analyzed for bone marrow-derived cells (GFP positive) and merged with lectin positive host vessels (rhodamine positive). Double positive cells were considered as EPCs and counted per high powered field (hpf, 100 ×). Catestatin treatment significantly increased number of incorporated EPCs. * P<0.001; n=7 each group.
Additionally, confocal microscopy was performed (b) and sections were analyzed for DAPI staining (left panel), rhodamine-labeled BS1 lectin (2nd from left) and for GFP+ cells (3rd from left). Merged picture (right panel) shows EPCs positive for DAPI, lectin and GFP (yellow cells, arrowheads).
CST –induced effects are mediated by bFGF
To asses effects of VEGF or bFGF on CST-mediated in-vitro angiogenesis CST was incubated with neutralizing VEGF and bFGF antibodies. Inhibition of VEGF did not influence CST-induced tube formation but bFGF antibody completely inhibited CST-mediated effects (tube formation CST 2.05±0.08, CST+VEGF-Ab 1.98±0.12, CST+bFGF-Ab 1.3±0.06; P<0.05 CST vs. CST+bFGF-Ab; Fig. 6a). Additionally, bFGF antibody inhibited CST-induced MAPK activation in ECs. When we performed a time course of CST-induced MAPK activation in HUVEC a biphasic activation of MAPK was observed with a maximum at 10 and 50 minutes (Fig. 6b). Whereas early activation of MAPK was not influenced by bFGF antibody late activation of MAPK was blocked by bFGF inhibition indicating that CST-mediated late activation of MAPK is mediated by bFGF. As bFGF mRNA was not up-regulated by CST over a broad range of concentrations and time-points (data not shown and supplemental data) we evaluated if CST releases bFGF protein from ECs. We found that ECs stimulated by 1nmol/L CST released bFGF into cell supernatant from 30 min to 6 hours with a maximum at 1 hour (pg/ml: Ctr. 28.6±2.2, CST 53.8±3.4, P<0.01, Fig. 6c). Concentration of bFGF was not changed in heparin wash solutions or cell lysates after CST treatment (data not shown). To evaluate if CST stimulates bFGF signalling we performed studies on FGF-receptor-1 (FGFR1) activation. We observed that CST (after 60 min) like bFGF (after 30 min) stimulated phosphorylation of FGFR1 (Fig. 6d). To investigate the mechanism of bFGF release cells were incubated with substances inhibiting different signalling transduction pathway. We observed that inhibition of MAPK by PD blocked CST-induced bFGF release whereas inhibition of PI3-kinase (by WM), of protein kinase C(by GFX) or of Rho kinase (by Y26732) had no effect (Fig. 6e). We also found that PD and FGF antibody inhibited CST-induced FGFR1 activation indicating that CST transactivates FGF receptor by release of bFGF (Fig. 6f).
Figure 6. Catestatin-induced effects on ECs are mediated by bFGF.
6.a. Catestatin-induced tube formation is blocked by a neutralizing bFGF antibody.
Catestatin-induced tube formation was calculated in the presence or absence of a neutralizing VEGF or bFGF antibody (Ab). Inhibition of bFGF blocked catestatin mediated effect. *P<0.05, **P<0.01 vs. Ctr; § P<0.05, VEGF vs. VEGF+VEGF-Ab, bFGF vs. bFGF+bFGF-Ab or CST vs. CST+bFGF-Ab; n=3.
6.b. Catestatin-induced MAPK activation is blocked by a neutralizing bFGF antibody.
CST induced phosphorylation of ERK in a biphasic manner with a maximum after 10 and 50 minutes. Incubation with a bFGF antibody inhibited late but not early ERK activation induced by CST.
6.c. Catestatin induces bFGF release from ECs
ECs were incubated for different time-points with 1 nmol/L CST and bFGF of supernatants was analyzed by ELISA. CST induces bFGF release with a maximum at 1 hour. **P<0.01, * P<0.05 vs. Ctr; n=3.
6.d. Catestatin induces FGF-receptor-1 activation
HUVEC were treated by bFGF (20 ng/mL) for 30, 60 and 90 minutes and by CST (1nmol/L) for 30, 60, 90 and 180 minutes. FGFR1 activation was studied by immunoprecipitation using FGFR1 antibody and subsequent immunoblotting for phospho-tyrosine and FGFR1. CST, like bFGF induces activation of this receptor.
6e. Catestatin-induced bFGF release is mediated by MAPK
PD blocked CST-induced bFGF release in HUVEC whereas inhibitors of PI3-kinase, protein-kinase C and Rho kinase could not inhibit significant release of bFGF by CST. *P<0.05 vs. Ctr., §p<0.05 vs. CST; n=3.
6.f. Catestatin-induced FGF-receptor-1 activation is blocked by bFGF antibody and inhibition of MAPK.
After 60 min incubation with CST (1 nmol/L) FGFR1 activation was observed by immunoprecipitation. Effect was blocked by PD (10 μmol/L) and bFGF-Ab (1:1000).
6.g. Catestatin induced effects in-vivo are blocked by inhibition of bFGF: necrosis score
After hindlimb ischemia and CST therapy bFGF-Ab impaired necrosis score on day 7, 14 and 28 compared to IgG. P<0.05; n=7.
6.h. Catestatin induced effects in-vivo are blocked by inhibition of bFGF: LDPI
After hindlimb ischemia and CST therapy blood perfusion was significantly reduced in bFGF-Ab treated mice compared to IgG. * P<0.05, **P<0.01 bFGF-Ab vs. IgG; n=7.
6.i. Catestatin-induced vasculogenesis is inhibited by inhibition of bFGF: EPCs in ischemic muscle. EPCs were counted in sections of muscles of GFP bone marrow transplanted mice (GFP+/lectin+ cells). Inhibition of bFGF blocked CST-induced increase of EPCs.* P<0.05 IgG vs. bFGF-Ab; n=7.
6.j. Catestatin-induced vasculogenesis is inhibited by inhibition of bFGF: circulating flk-1+/GFP+ cells. Flk-1+/GFP+ cells 3 weeks after hindlimb ischemia and CST i.m. treatment were significantly inhibited by bFGF-Ab.* P<0.05 IgG vs. bFGF-Ab; n=7.
In-vivo, inhibition of bFGF by a neutralizing antibody inhibited CST-induced effects on angiogenesis as shown by increased necrosis score (Fig. 6g) and impaired blood perfusion in LDPI measurements (d28: CST+IgG 0.75±0.3; CST+bFGF-Ab: 0.46±0.04, n=7, P<0.01, Fig. 6h; Ctr d28: 0.55±0.08, P<0.05 vs. CST+IgG, n=3, data not shown). Additionally, CST-induced vasculogenesis in the GFP bone marrow transplanted mouse was inhibited by blockade of bFGF as demonstrated by reduced GFP+/lectin+ EPCs in ischemic limbs (EPCs/hpf: Ctr: 8.8±0.8, CST+IgG: 15.9±1.2, CST+bFGFAb: 10.8±0.8; P<0.05 CST+IgG vs. CST +bFGF-Ab; Fig. 8i) and reduced circulating flk-1+/GFP+cells (% of GFP+ cells) Ctr: 1.2±0.25, CST+IgG: 3.1±0.4, CST+bFGF Ab: 1.5±0.3; P<0.05 CST+IgG vs. CST+bFGF-Ab; Fig. 8j).
Discussion
Overview
CST was initially described as a potent endogenous nicotinic-cholinergic antagonist inhibiting nicotine-evoked catecholamine secretion from PC12 cells and primary cultures of bovine chromaffin cells. Subsequently, CST was established as a pleiotropic peptide. CST acts as a negative regulator of hypertension 7, inotropy and lusitropy 20. CST induces histamine release in-vivo in rat 21 and in-vitro from mast cells 22. Furthermore, CST induces directed migration of human blood monocytes 14, which qualifies CST to be an inflammatory cytokine 14. Recently, CST has been shown to induce vasodilation in human dorsal hand vein (Fung MM 2010, in press). The present findings establish CST as an angiogenic peptide.
CST induces effects in ECs
Inflammation and hypoxia are usually accompanied or followed by increased generation of blood vessels 8. CgA, the precursor of CST, has been shown to be up-regulated in brain in response to hypoxia 13 as has been reported previously for angiogenic factors like VEGF. Here we found that catestatin exerts several effects on ECs including EC migration and proliferation, and consistent with previous findings in well established angiogenic peptides VEGF and bFGF 23, CST also signals through MAPK pathway to induce these effects. Furthermore, inhibition of CST-induced chemotaxis of EC by pertussis toxin indicates CST signaling through G-proteins. PI-3-kinase/Akt pathway plays a pivotal role in EC survival and we show that CST stimulates Akt phosphorylation, that CST inhibits EC apoptosis and that inhibition of PI3-kinase by wortmannin abrogates CST-mediated effect. These data suggest that CST signals through the PI-3-kinase/Akt pathway to inhibit HUVEC apoptosis.
CST effects on other vascular cells (EPCs, SMCs), vasculogenesis and arteriogenesis
Beside its effects on ECs, CST also induced chemotaxis on other vascular cells like EPCs or SMCs (see supplemental data). CST also exerted incorporation of EPCs into vascular structures in-vitro and inhibition of bFGF blocked this effect indicating that bFGF, a factor reported to attract EPCs 24 mediates CST-induced effects (see below). These findings indicate that this peptide may also induce post-natal vasculogenesis. Consistent with this, we found that CST increases incorporation of EPCs in ischemic hindlimbs in GFP bone marrow transplanted mice, which is a well characterized model to study these cells 16, 17, findings that were also confirmed by confocal microscopy. We also found increased numbers of arteries/arterioles in CST treated ischemic hindlimbs, consistent with induction of arteriogenesis. Upregulation of PDGF-B in ECs might indicate that CST induces maturation of capillaries 25. In contrast, MCP-1 an important regulator of maturation of pre-existing collaterals was not increased by CST 26.
CST effects on angiogenesis in-vivo
Consistent with angiogenic peptides, CST induces angiogenesis in-vivo in the mouse cornea neovascularization assay. This in-vivo finding further strengthened CST's ability to induce angiogenesis in the hindlimb ischemia model. Serial measurements of blood perfusion point out that CST increases perfusion to levels before ligation of the femoral artery yielding a significant better value compared to saline injected animals. These observations establish CST as a novel angiogenic cytokine. In this context it is also interesting that CST precursor CgA was found in motor nerve endplates of skeletal muscles 27. Future studies will have to reveal if CST plays a role in physiological processes like nerve-muscle signal transmission or in the patho-physiology of injured skeletal muscle cells beside its ability to increase blood perfusion to these cells.
CST effects are mediated by bFGF
Indirect angiogenic factors like sonic hedgehog induce their effects by up-regulation of other, direct angiogenic cytokines like VEGF 28. Furthermore, effects of factors like PDGF-BB or prostaglandin-E on vascular cells or angiogenesis have been shown to be mediated by bFGF 29, 30. We therefore tested if CST-mediated effects on ECs depend on VEGF or bFGF and found that inhibition of bFGF by a neutralizing antibody indeed blocked CST-mediated in-vitro angiogenesis and late MAPK activation. Additionally, we found that CST releases bFGF from ECs with a maximum as early as 1 hour and stimulates FGFR1 activation. Release of bFGF was blocked by inhibition of MAPK indicating that this signaling pathway is necessary for CST-induced bFGF release. Furthermore, inhibition of bFGF and MAPK blocked CST-induced FGFR1 activation. This finding as well as the observation that CST-induced FGFR1 activation was delayed compared to bFGF indicates that CST transactivates FGFR1 by release of bFGF. Therefore we propose a model for CST action where bFGF is released from ECs by CST via a MAPK dependent mechanism, stimulates FGFR1, leading to a second, late activation of MAPK after 50 minutes beside a first, direct CST-mediated MAPK activation independent of bFGF after 10 minutes. This prolonged MAPK stimulation seems to be necessary for CST-mediated EC function as shown by blockade of CST-induced capillary tube formation by inhibition of bFGF. A similar effect of bFGF on PDGF-BB-induced vascular SMC function has been reported previously 29. Additionally we also were able to demonstrate that CST-induced effects in-vivo depend on bFGF as shown by increased necrosis, decreased blood perfusion, reduced EPCs in ischemic muscles and reduced flk-1/GFP+ cells in the circulation in mice treated with a neutralizing bFGF-Ab. These findings might indicate that bFGF mediates CST-induced mobilization and homing to ischemic muscle tissue.
It should be pointed out that the N-terminal CgA peptide vasostatin inhibits VEGF-induced angiogensis. In fact, vasostatin blocked VEGF-induced MAPK activation in ECs and inhibited angiogenesis exerted by this factor in-vivo in the matrigel assay 19. These findings indicate selective inhibition of EC function and angiogenesis by the CgA peptides. Future studies will determine why two peptides (vasostatin and CST) derived from CgA exert opposite effects on angiogenesis. It is yet to be determined whether CgA exhibits differential processing to vasostatin and CST in response to different physiological demands. In this context it is also interesting that tumor necrosis factor (TNF)-alpha exhibits dose-dependent, opposing actions on angiogenesis 31.
Of note, SN, a peptide derived from another member of the chromogranin/secretogranin family, secretogranin II, also induces therapeutic angiogenesis 16. Several angiogenic cytokines have been used to treat patients suffering from peripheral arterial or coronary heart disease 32 and CST as well as SN are emerging promising novel candidates in the therapy of these diseases.
Supplementary Material
Novelty and Significance.
What Is Known?
Neuropeptides like substance-P, neuropeptide-Y or secretoneurin induce angiogenesis.
Catestatin (human chromogranin A352-372; bovine chromogranin A344-364) was initially identified as a catecholamine release-inhibitory peptide inhibiting catecholamine secretion in an autocrine/paracrine manner by acting as a nicotinic cholinergic antagonist.
Subsequent studies established catestatin as a pleiotropic peptide which lowers blood pressure. It induces vasodilation is a negative inotrope and has anti-microbial activity. It also stimulates monocyte chemotaxis.
What New Information Does This Article Contribute?
Catestatin induces migration and proliferation of endothelial cells (ECs), and stimulates chemotaxis in vascular smooth muscle cells or endothelial progenitor cells in-vitro. These effects are mediated by the activation of Akt and mitogen-activated protein kinase (MAPK) in ECs.
Catestatin acts as an angiogenic factor in vivo specifically in the cornea neovascularization model and the hindlimb ischemia model. It stimulates the incorporation of endothelial progenitor cells into ischemia hindlimbs.
Both the in-vitro and in-vivo effects of catestatin were blocked by a neutralizing basic fibroblast growth factor (bFGF) antibody, implicating the induction of bFGF-mediated signaling by catestatin via stimulation of bFGF release from ECs and activation of FGF receptor -1.
Summary.
We hypothesized that the neuropeptide catestatin induces the formation of new blood vessels because of its reported effects on vasodilation coupled with hypoxia-induced upregulation of the catestatin-precursor, chromogranin-A.
We found that catestatin induces chemotactic, proliferative and anti-apoptotic effects on ECs in-vitro by activating Akt and MAPK. Catestatin induced angiogenesis in the mouse cornea neovascularization assay. In the hindlimb ischemia model catestatin therapy improved blood flow and reduced necrosis. Immunofluorescent studies revealed increased density of capillaries, arteries and incorporation of endothelial progenitor cells by catestatin implying that the peptide induces angiogenesis, arteriogenesis and postnatal vasculogenesis. The in vitro and in vivo effects of catestatin were inhibited by a neutralizing bFGF antibody indicating that bFGF-mediates the action of catestatin. Of note, catestatin releases bFGF from ECs and stimulates FGF receptor-1 in these cells. These findings indicate that catestatin induces therapeutic angiogenesis in the hindlimb ischemia model. Additional studies are required to evaluate the therapeutic potential of this peptide in other ischemic conditions such as ischemic heart disease.
Acknowledgments
We thank Ursula Stanzl, Eva Huber and Lydia Markut for excellent technical assistance.
Sources of Funding: Dr. Kirchmair was supported by a grant of the Oesterreichische Nationalbank (grant # 10189). Dr. Mahata was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (R01 DA011311 & P01 HL58120).Dr. Theurl was supported by a grant of the Medizinische Forschungsfonds Tirol (grant#202).
Non-standard abbreviations and acronyms
- bFGF
basic fibroblast growth factor
- bFGF-Ab
basic fibroblast growth factor antibody
- CgA
Chromogranin A
- CST
catestatin
- EC
endothelial cells
- EPC
endothelial progenitor cells
- ERK
extracellular signal-regulated kinase
- FGFR1
FGF-receptor-1
- GFP
green fluorescence protein
- HUVEC
human umbilical vein endothelial cells
- LDPI
laser-Doppler perfusion imaging
- MAPK
mitogen-activated protein kinase
- NPY
neuropeptide Y
- PDGF
platelet-derived growth factor
- PTX
pertussis toxin
- SMA
smooth muscle actin
- SMC
smooth muscle cells
- SN
secretoneurin
- SP
substance P
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures: None
References
- 1.O'Connor DT, Frigon RP, Sokoloff RL. Human chromogranin A. Purification and characterization from catecholamine storage vesicles of human pheochromocytoma. Hypertension. 1984;6:2–12. doi: 10.1161/01.hyp.6.1.2. [DOI] [PubMed] [Google Scholar]
- 2.Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324:476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- 4.Aardal S, Helle KB, Elsayed S, Reed RK, Serck-Hanssen G. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J Neuroendocrinol. 1993;5:405–412. doi: 10.1111/j.1365-2826.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 5.Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 9.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. Journal of Clinical Investigation. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchmair R, Gander R, Egger M, Hanley A, Silver M, Ritsch A, Murayama T, Kaneider N, Sturm W, Kearny M, Fischer-Colbrie R, Kircher B, Gaenzer H, Wiedermann CJ, Ropper AH, Losordo DW, Patsch JR, Schratzberger P. The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation. 2004;109:777–783. doi: 10.1161/01.CIR.0000112574.07422.C1. [DOI] [PubMed] [Google Scholar]
- 11.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen WT, Kleinman HK, Grouzmann E, Grant DS. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- 12.Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- 13.Marti E, Ferrer I, Blasi J. Differential regulation of chromogranin A, chromogranin B and secretoneurin protein expression after transient forebrain ischemia in the gerbil. Acta Neuropathol (Berl) 2001;101:159–166. doi: 10.1007/s004010000280. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Beer AG, Theurl M, Schgoer W, Hotter B, Tatarczyk T, Vasiljevic D, Frauscher S, Marksteiner J, Patsch JR, Schratzberger P, Djanani AM, Mahata SK, Kirchmair R. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol. 2008;598:104–111. doi: 10.1016/j.ejphar.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Kirchmair R, Egger M, Walter DH, Eisterer W, Niederwanger A, Woell E, Nagl M, Pedrini M, Murayama T, Frauscher S, Hanley A, Silver M, Brodmann M, Sturm W, Fischer-Colbrie R, Losordo DW, Patsch JR, Schratzberger P. Secretoneurin, an angiogenic neuropeptide, induces postnatal vasculogenesis. Circulation. 2004;110:1121–1127. doi: 10.1161/01.CIR.0000139884.81390.56. [DOI] [PubMed] [Google Scholar]
- 16.Schgoer W, Theurl M, Jeschke J, Beer AG, Albrecht K, Gander R, Rong S, Vasiljevic D, Egger M, Wolf AM, Frauscher S, Koller B, Tancevski I, Patsch JR, Schratzberger P, Piza-Katzer H, Ritsch A, Bahlmann FH, Fischer-Colbrie R, Wolf D, Kirchmair R. Gene therapy with the angiogenic cytokine secretoneurin induces therapeutic angiogenesis by a nitric oxide-dependent mechanism. Circ Res. 2009;105:994–1002. doi: 10.1161/CIRCRESAHA.109.199513. [DOI] [PubMed] [Google Scholar]
- 17.Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, Eaton E, Iwakura A, Tsutsumi Y, Hamada H, Kishimoto S, Thorne T, Kishore R, Losordo DW. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113:2413–2424. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 18.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, Horowitz JR, Symes JF. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996;94:3281–3290. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- 19.Belloni D, Scabini S, Foglieni C, Veschini L, Giazzon A, Colombo B, Fulgenzi A, Helle KB, Ferrero ME, Corti A, Ferrero E. The vasostatin-I fragment of chromogranin A inhibits VEGF-induced endothelial cell proliferation and migration. Faseb J. 2007;21:3052–3062. doi: 10.1096/fj.06-6829com. [DOI] [PubMed] [Google Scholar]
- 20.Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology. 2008;149:4780–4793. doi: 10.1210/en.2008-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy BP, Mahata SK, O'Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/s0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 22.Kruger PG, Mahata SK, Helle KB. Catestatin (CgA344-364) stimulates rat mast cell release of histamine in a manner comparable to mastoparan and other cationic charged neuropeptides. Regul Pept. 2003;114:29–35. doi: 10.1016/s0167-0115(03)00069-7. [DOI] [PubMed] [Google Scholar]
- 23.D'Angelo G, Struman I, Martial J, Weiner RI. Activation of mitogen-activated protein kinases by vascular endothelial growth factor and basic fibroblast growth factor in capillary endothelial cells is inhibited by the antiangiogenic factor 16-kDa N-terminal fragment of prolactin. Proc Natl Acad Sci U S A. 1995;92:6374–6378. doi: 10.1073/pnas.92.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murayama T, Tepper O, Silver M, Ma H, Losordo D, Isner J, Asahara T, Kalka C. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30:967. doi: 10.1016/s0301-472x(02)00867-6. [DOI] [PubMed] [Google Scholar]
- 25.Ramsauer M, D'Amore PA. Getting Tie(2)d up in angiogenesis. J Clin Invest. 2002;110:1615–1617. doi: 10.1172/JCI17326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JY, Leitner B, Lovisetti-Scamihorn P, Winkler H, Dahlstrom A. Proteolytic processing, axonal transport and differential distribution of chromogranins A and B, and secretogranin II (secretoneurin) in rat sciatic nerve and spinal cord. Eur J Neurosci. 1999;11:528–544. doi: 10.1046/j.1460-9568.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 28.Pola R, Ling LE, Silver M, Corbley MJ, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nature Medicine. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 29.Millette E, Rauch BH, Defawe O, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB-induced human smooth muscle cell proliferation depends on basic FGF release and FGFR-1 activation. Circ Res. 2005;96:172–179. doi: 10.1161/01.RES.0000154595.87608.db. [DOI] [PubMed] [Google Scholar]
- 30.Finetti F, Donnini S, Giachetti A, Morbidelli L, Ziche M. Prostaglandin E(2) primes the angiogenic switch via a synergic interaction with the fibroblast growth factor-2 pathway. Circ Res. 2009;105:657–666. doi: 10.1161/CIRCRESAHA.109.203760. [DOI] [PubMed] [Google Scholar]
- 31.Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140:539–544. [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.