Abstract
Extracellular vesicles (EVs) are membrane-bound nanoparticles released by cells and are an important means of intercellular communication in physiological and pathological states. We provide an overview of recent advances in the understanding of EV biogenesis, cargo selection, recipient cell effects, and key considerations in isolation and characterization techniques. Studies on the physiological role of EVs have relied on cell-based model systems due to technical limitations of studying endogenous nanoparticles in vivo. Several recent studies have elucidated the mechanistic role of EVs in liver diseases, including nonalcoholic fatty liver disease, viral hepatitis, cholestatic liver disease, alcohol-associated liver disease, acute liver injury, and liver cancers. Employing disease models and human samples, the biogenesis of lipotoxic EVs downstream of endoplasmic reticulum stress and microvesicles via intracellular activation stress signaling are discussed in detail. The diverse cargoes of EVs including proteins, lipids, and nucleic acids can be enriched in a disease-specific manner. By carrying diverse cargo, EVs can directly confer pathogenic potential, for example, recruitment and activation of monocyte-derived macrophages in NASH and tumorigenicity and chemoresistance in hepatocellular carcinoma. We discuss the pathogenic role of EVs cargoes and the signaling pathways activated by EVs in recipient cells. We review the literature that EVs can serve as biomarkers in hepatobiliary diseases. Further, we describe novel approaches to engineer EVs to deliver regulatory signals to specific cell types, and thus use them as therapeutic shuttles in liver diseases. Lastly, we identify key lacunae and future directions in this promising field of discovery and development.
Introduction
Intercellular communication is a fundamental facet of the organized existence of multicellular organisms. Since the earliest description of externalized membranous vesicles with ectoenzymes (214), the identification of prostate-derived vesicles which promoted sperm motility (198), and as a mechanism for the removal of iron and transferrin receptors in reticulocytes (66, 168), extracellular vesicles (EVs) have emerged as important mediators in intercellular communication. Along with the recognition of their important role in communication, there has been an increase in our understanding of how EVs are formed, released, targeted to, and communicated with recipient cells. Knowledge of the biophysical properties of EVs and their unique cargoes has led to a plethora of bulk and targeted isolation methods, development of biomarkers, and the exploration of natural or engineered EVs as acellular nanotherapeutics. This article encompasses the basic biology of EVs, as is understood from various models, and the relevance of EVs to liver health and liver diseases with data from models and human correlations.
Classification and Formation
EVs are membrane-enclosed nanoscale particles that have heterogenous size, cell of origin, content, and function. Structurally, the EV membrane is composed of a phospholipid bilayer that is in the same orientation as the cell of origin with extracellularly exposed lipids and transmembrane proteins on the outside. The EV lumen encloses cargoes that are derived from the cytoplasm of the cell of origin. EVs released from live cells can be further classified into two broad categories, microvesicles (ectosomes, or microparticles) and exosomes, based on their mechanism of formation and release (Figure 1) (209). Another category of nonmembranous nanoparticles, termed exomere, has recently been identified. Exomeres are ≤50 nm in size and can be isolated by ultracentrifugation or asymmetric flow field-flow fractionation (240, 241). Although exomeres can transfer functional cargo, their role in pathophysiology has not been widely studied yet. Microvesicles are formed by outward budding of the plasma membrane and have a wide size range from 50 nm to 1 μm in diameter. Exosomes are approximately 100 nm in diameter and are formed by invagination and inward budding of the membrane of the multivesicular body to give rise to intraluminal vesicles (ILVs) which are released as exosomes (91, 145, 217). Due to overlapping proteo-lipidomic markers and size, and the lack of gold standard markers for exosomes and microvesicles, a simpler classification system is commonly employed. In this classification, EVs are grouped according to size into small EVs (sEVs) which are less than 200 nm, or medium/large (m/l EVs) ≥200 nm in diameter. sEVs are likely comprised of exosomes and microvesicles, whereas large EVs are mostly microvesicles, based on the size constraints on exosomes as they are formed within multivesicular bodies (MVBs). While the physiological functions of EVs are poorly understood and likely diverse, multiple studies have elucidated that EVs play a pivotal role in intercellular communication in health and disease (196).
Figure 1.
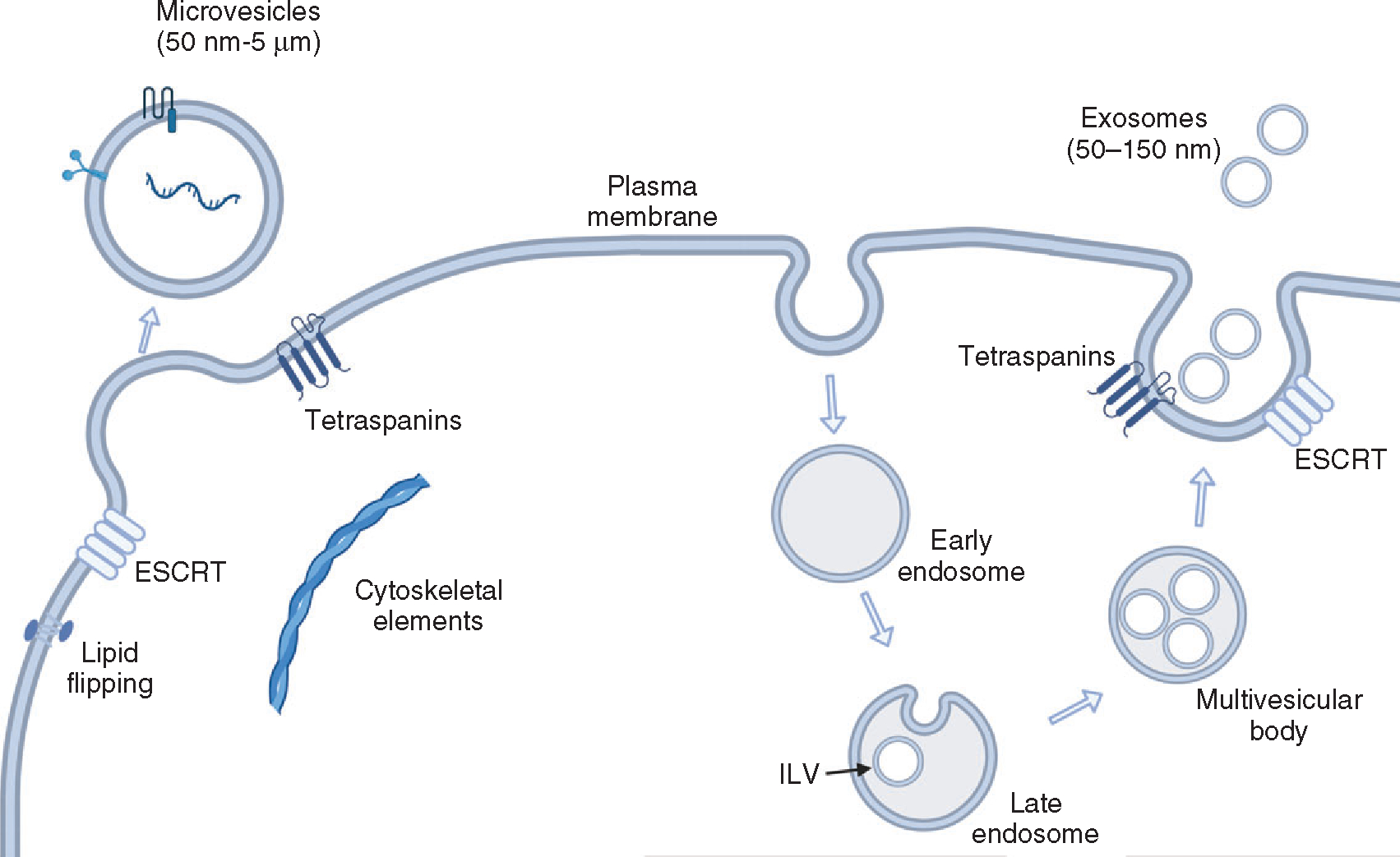
Biogenesis of extracellular vesicles. Microvesicles are formed directly from plasma membrane budding by sequestering cytosolic cargo. Exosomes arise via endosomal maturation into multivesicular bodies (MVB). MVBs contain intraluminal vesicles generated by invagination of the limiting membrane and eventually released at the plasma membrane as exosomes.
Biogenesis and release pathways
Exosomes start out as ILVs contained in intracellular MVBs, also called as multivesicular endosomes, generated by a double invagination of the phospholipid bilayered plasma membrane. The first invagination forms a cup-shaped structure termed the early-sorting endosome that contains surface proteins as well as extracellular components. These mature into late-sorting endosomes, and inward invagination of their membrane leads to the formation of ILVs, thus maturing into MVBs (217). MVBs have three well-defined trafficking fates within a cell (19). They may fuse with lysosomes leading to their degradation, may recycle as endosomes, or a subpopulation of MVBs may fuse with the plasma membrane—via subunits of the endosomal sorting complex required for transport (ESCRT)—and their component ILVs are secreted as exosomes (136). The ESCRT family of proteins drives cargo sorting into and scission of the limiting membrane via formation of successive complexes at the surface of MVBs (77). Specifically, the ESCRT complex consisting of hepatocyte growth factor-regulated tyrosine kinase substrate (HRS or HGS), and ESCRT-I mediate clustering of ubiquitinated cargoes onto the limiting membrane of MVBs, and subsequently, ESCRT-III is recruited via ESCRT-II to initiate membrane budding and fission. Biogenesis of exosomes can also occur independent of ESCRT, for instance, by the generation of ceramide, from sphingomyelin via a neutral type II sphingomyelinase, that in turn generates membrane subdomains that impart a spontaneous membrane curvature (57). Alternatively, MVBs may be targeted to lysosomes by retrograde transport on microtubules via the Rab-GTPase RAB7, (181) or instead toward exosome secretion depending on the ubiquitination status of RAB7 (194). Other RABs such as RAB27 A and B also mediate exosome docking at the plasma membrane and subsequent secretion via actin cytoskeleton reorganization (166).
On the other hand, microvesicles are formed directly at the plasma membrane. Relatively less is understood about this process, which starts with plasma membrane vesicle budding, followed by a scission step leading to release. There is overlap with mechanisms involved in the biogenesis of exosomes, for example, ESCRT proteins, or the biogenesis of ceramide (152, 161). Broadly, a physical restructuring of the plasma membrane and underlying actin cytoskeleton occurs whereby phosphatidylserine (PS) is exposed from the inner to the outer leaflet, resulting in budding as microvesicles. This is mediated by changes in membrane lipid, protein, and Ca2+ content by enzymatic machineries that include aminophospholipid translocases (flippases and floppases), scramblases, and calpain (173). Another described mechanism is the case of arrestin-domain-containing protein 1 (ARRDC1)-mediated microvesicle formation, which are uniformly approximately 50 nm in size, and released by the interaction of ARRDC1 with ESCRT proteins by recruitment of tumor susceptibility gene 101 (TSG101) (161). Further, elements of the cytoskeleton such as Rho family of small GTPases and of the Rho-associated protein kinase (ROCK) can regulate actin dynamics, and microvesicle biogenesis, for example, in tumor cells (116). Another example of microvesicle release mechanisms includes the interaction between adenosine diphosphate-ribosylation factor 6, or ARF6, and extracellular signal-regulated kinase (ERK), leading to phosphorylation of myosin light chain kinase (70). Lastly, apoptotic bodies refer to microvesicles released by blebbing of the plasma membrane of cells undergoing apoptosis (76), and oncosomes are atypically large vesicles released by cancer cells (155).
Lipo-proteomic microdomains play a role in the nucleation of EVs. These are clustered on the limiting membrane of the MVBs for exosomes or on the plasma membrane for microvesicles and are integral for the recruitment of specific cytoplasmic cargo. For instance, incorporation of ILVs into the MVB lumen occurs when charged multivesicular body protein 4, or CHMP4B, interacts with apoptosis-linked gene-2 interacting protein X (ALIX), an ESCRT-III binding protein (10). The dissociation of this complex and recycling of ESCRT components is mediated by the vacuolar protein sorting 4 (VPS4) complex, and subsequent endosomal budding can occur by ESCRT-dependent or ESCRT-independent mechanisms. In the case of proteolipid cargoes, trafficking into and secretion as exosomes occurs via the interaction of ceramide generated through the salvage pathway, and tetraspanins such as cluster of differentiation 63 (CD63) and CD81 (42, 213). MVBs travel within a cell via the microtubule network and dock on the luminal side of the plasma membrane with the help of MVB-docking proteins. Several proteins including the Rab family described previously, soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex, or SNARE (82), Syntenin-1, TSG101 are implicated in the process of EV interaction with the plasma membrane prior to their secretion. The separation of EVs into exosomes and microvesicles is based entirely on which membrane compartment EVs originate from. It is not surprising, given the integration of many membranous compartments and trafficking pathways in the cell, that there is overlap in the molecular mediators of exosome and microvesicle formation and release. Manipulation of key mediators by loss- or gain-of-function experiments often impacts the release of all EVs. In subsequent sections, we will employ the inclusive term EV and specify exosome or microvesicle where these distinctions are clearer.
Cargo selection
The nature of EV cargo is determined by the cell of origin and the physiological and pathological state of the donor cell. EVs can contain various cytosolic or membranous constituents, such as nucleic acids, lipids, metabolites, or proteins, and EV cargo may be a component of the EV biogenesis machinery. The process of cargo trafficking into future EVs can occur when endosomes interact with the trans-Golgi network or endoplasmic reticulum (ER). For example, given its wellknown role in targeting proteins for vesicular trafficking, ubiquitination of protein cargo can mediate the process of enrichment in endosomes (18). Similarly, palmitoylation and farnesylation can recruit proteins to lipid rafts with subsequent inclusion in EVs (158). Sphingolipids, such as ceramide, play a role in the formation of ILVs, and for instance, ceramide-enriched EVs are released by lipotoxic hepatocytes—which refers to the cellular hallmark in nonalcoholic steatohepatitis (NASH) characterized by accumulation of toxic lipids leading to organelle dysfunction and cell injury (88). Mechanistically, ER stress in lipotoxic hepatocytes activates inositol-requiring enzyme-1α, or IRE1A, and downstream splicing of X-box binding protein 1, or XBP1 messenger RNA (mRNA) generating a transcription factor, which in turn promotes transcription of the serine palmitoyltransferase genes, resulting in ceramide biosynthesis and release of EVs, both in mouse models and human NASH (34). Specifically, a ceramide transport protein, StAR-related lipid transfer domain 11 (STARD11), transports ceramide synthesized de novo at the ER to the Golgi by nonvesicular transport for sorting into EVs (49). In the case of nucleic acids, proteins such as sumoylated heterogeneous nuclear ribonucleoproteins A2/B1, or HNRPA2B1, and Y-box binding protein 1, or YB-1, can bind to targeting sequences and sort micro RNAs (miRs) into endosomes (192, 215). Normally, the RNA-induced silencing complex (RISC) machinery is capable of slicing mRNA via Argonaute2 (AGO2) and its accumulation in cytoplasmic processing bodies, or P-bodies (150). However, AGO2 can also interact with MVBs in structures called GW-bodies and result in the secretion of AGO2-miR complexes as exosomes (151). This process is inhibited by Kirsten rat sarcoma virus proto-oncogene (KRAS)-dependent activation of mitogen-activated protein kinase (MAPK)/ERK signaling and thus is a novel effect of mutant KRAS in colorectal cancer (149). Much of the characterization of EVs is based on isolated nanoparticles, thus a discussion of isolation methods and their caveats is germane to this article.
EV Isolation Methods
The ability to isolate EVs from biological samples, such as body fluids and cell culture media, is essential for investigating EV properties and functions. EV isolation is also critical for the development of EV-based therapeutic, diagnostic, and prognostic applications. Biological samples are complex and contain nonvesicular macromolecular structures with similar biophysical properties to EVs, such as lipoprotein particles and protein aggregates, which can be co-isolated along with EVs (14). A variety of techniques have been developed to purify EVs from biologic samples based on EV biophysical properties or antigenic features (Table 1; Figure 2). Each isolation method has its inherent advantages and drawbacks, and potential biases toward certain EV subpopulations. Isolation methods differ from each other, especially in terms of usability (e.g., ease-of-use, workload, scalability), EV purity (e.g., presence of non-EV contaminants), and EV yield (230). The choice of isolation method can also impact the results of downstream applications, such as RNA or proteomic profiling of EVs (216, 218). Given the fact that the choice of purification technique affects the nature of isolated EVs, a detailed reporting of the isolation protocol and experimental parameters has been recommended in the 2018 guidelines of the International Society for Extracellular Vesicles (209). There is also a great necessity to standardize the isolation protocols to improve study-to-study comparability of results and increase reproducibility across different laboratories and applications (228). In addition, as the field moves toward clinical applications of EVs, isolation of clinical-grade EVs will require robust and reproducible methods that can be scaled up. The frequently used techniques for EV purification are briefly described below. Informed by the intended downstream use of isolated EVs, a combination of two or more methods is also commonly used.
Table 1.
Advantages and Drawbacks of Extracellular Vesicle Isolation Methods
| Isolation method | Advantages | Drawbacks |
|---|---|---|
|
| ||
| Differential ultracentrifugation | • Common method | • Low scalability |
| • Medium to high yield | • Labor-intensive | |
| • Time-consuming | ||
| • Possible aggregation or rupture of EVs | ||
| • Possible contamination with lipoproteins | ||
| Density gradient ultracentrifugation | • Potential to identify EV subpopulations | • Low scalability |
| • High purity | • Labor-intensive | |
| • Low amount of non-EV contaminants | • Time-consuming | |
| Size-exclusion chromatography | • Fast and straightforward protocol | • Often needs another method to concentrate isolated EVs |
| • Preserved integrity of isolated EVs | ||
| • Highly scalable | ||
| Ultrafiltration | • Straightforward protocol | • Less common method |
| Tangential flow filtration | • Good EV yield | • Often needs another method to concentrate isolated EVs |
| • No EV aggregation | ||
| • Batch-to-batch consistency | • Favorable for functional EV studies | |
| • Suitable for large starting volumes | ||
| Immunoaffinity-based capture | • High purity | • Lower yield |
| • Possibility of isolating subpopulations expressing a certain marker | • May not isolate all EVs | |
| • Higher cost | ||
| Precipitation | • Fast and straightforward protocol | • High amount of non-EV contaminants |
| • Polymer remnants in the isolated EV sample | ||
Figure 2.
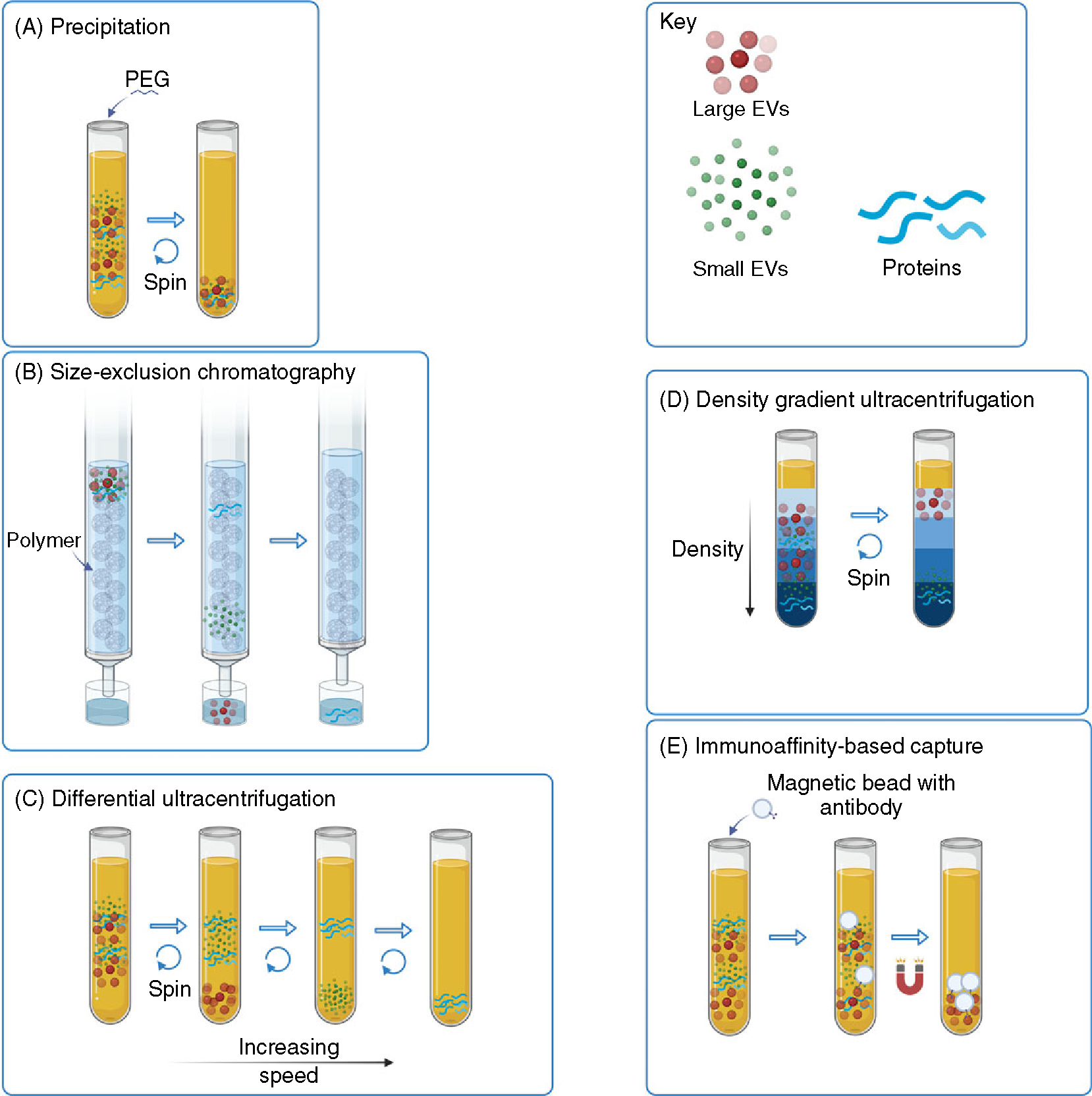
Methods of extracellular vesicle isolation. Various methods of EV isolation are suited for different scenarios based on required yield, purity, and specificity for an EV subtype. Often, separation from non-EV lipoproteins in biological fluids that have similar size and density is also required. Some examples depicted are (A) precipitation, for example, with polyethylene glycol (B) size exclusion chromatography (C) differential centrifugation (dUC) (D) density gradient ultracentrifugation, and (E) immunoaffinity-based capture.
Differential ultracentrifugation
Differential ultracentrifugation is the most widely applied method to isolate EVs, as indicated by the results of a worldwide survey (53). The original isolation protocol was introduced by Thery et al. (207); however, various modifications of this protocol are currently being used in the field. In general, the procedure involves several consequential centrifugation steps, starting with lower speeds to remove cells, cellular debris, and large vesicles, followed by high-speed centrifugation at 100,000 to 120,000 g to pellet sEVs (228). Compared to other common methods, differential ultracentrifugation allows for the isolation of EVs with relatively moderate purity and medium to high yield (216, 230). The shortcoming, however, is that differential ultracentrifugation lacks scalability and is labor-intensive and time-consuming relative to the volumes that can be processed. Recent reports also highlight the possibility that the high g-force during ultracentrifugation may affect EV integrity and functional properties due to vesicle rupture or aggregation (59, 131). Differential ultracentrifugation is often combined with other EV isolation methods, especially where larger volumes of purified EVs must be concentrated.
Density gradient ultracentrifugation
Density gradient ultracentrifugation is used primarily to further purify crude EV samples. The EV sample is subjected to ultracentrifugation using a discontinuous sucrose-based density gradient, iodixanol (OptiPrep™) gradient, or sucrose cushion as a variation of the sucrose gradient. In density gradient ultracentrifugation, EVs separate within the gradient solution based on their floatation speed and equilibrium density. The gradient isolation method has the potential to identify EV subpopulations with different biophysical properties. EVs isolated using density gradient ultracentrifugation have high purity, low amount of non-EV contaminants, and relatively moderate yield (83, 137, 216, 230). This approach is, however, more labor-intensive and time-consuming compared to differential ultracentrifugation.
Size-exclusion chromatography
EV isolation procedures based on size-exclusion chromatography are increasingly becoming popular in recent years (132). This method separates EVs based on their hydrodynamic radius as the sample is transported through a column filled with a stationary phase made of a porous polymer or silica-based material. Larger particles have less access to the pore volume compared to smaller molecules and, therefore, elute from the column earlier than smaller particles and soluble proteins. Thus, isolated EV samples have a relatively low amount of non-EV contaminants (137, 199). Also, size-exclusion chromatography may better preserve the integrity of isolated EVs, compared to differential ultracentrifugation, as there is minimal mechanical stress. Size-exclusion chromatography has gained increasing recognition because it can be compatible with good manufacturing practice and is highly scalable (230). This EV isolation approach is also relatively fast, user-friendly, and economical compared to ultracentrifugation.
Ultrafiltration
Ultrafiltration is another procedure by which EVs can be purified based on their size. Although, ultrafiltration is relatively less common as a primary EV isolation method (53). The pore size of the ultrafiltration membrane ranges between 10 and 100 kDa (219). Biological samples can be subjected to either centrifuge-driven or pressure-driven ultrafiltration, although centrifuge-based protocols may be preferred (137). Ultrafiltration was suggested to recover more EVs compared to differential ultracentrifugation (137). Conveniently, ultrafiltration can also be used to concentrate already isolated EVs to smaller volumes of samples.
Tangential flow filtration
Tangential flow filtration is a relatively recently introduced method for EV isolation. This method uses a similar principle to ultrafiltration, where EVs are separated based on their size using a porous membrane. However, in tangential flow filtration, the sample is flowing along the porous, low protein-binding membrane instead of toward the membrane as in the ultrafiltration procedure. Tangential flow filtration seems to be superior to differential ultracentrifugation in terms of EV yield, EV aggregation, and batch-to-batch consistency (17). Tangential flow filtration is an attractive approach because it also concentrates large volumes of starting samples, albeit it usually needs to be followed by an additional EV isolation method, such as ultracentrifugation.
Immunoaffinity-based capture
Relatively specific isolation of EVs can be achieved by immunoaffinity purification. The main principle of this approach is binding between an EV surface protein and a specific antibody (Figure 3). The antibodies can be immobilized on a variety of media, including magnetic beads, chromatography matrices, and microfluidic devices. Capture antibodies against established EV markers, such as tetraspanins—CD63, CD81, and CD9, are commonly used (197). Given the heterogeneity of EVs and the diversity in their protein content, a combination of multiple capture antibodies is desirable to improve the yield of isolated EVs. On the other hand, using a single antibody can facilitate the purification of a certain EV subpopulation expressing the specific marker of interest, as has been done for hepatocyte-derived EVs (162, 187, 206). Because immunocapture techniques result in high EV purity (230), this method is suitable for isolating EVs from complex samples, such as plasma. The EV yield may be lower compared to other techniques, given that not all EVs may express the marker or combination of markers used for immunocapture (228). Because of the higher cost, immunoaffinity-based capture is more practical for samples with a smaller starting volume.
Figure 3.
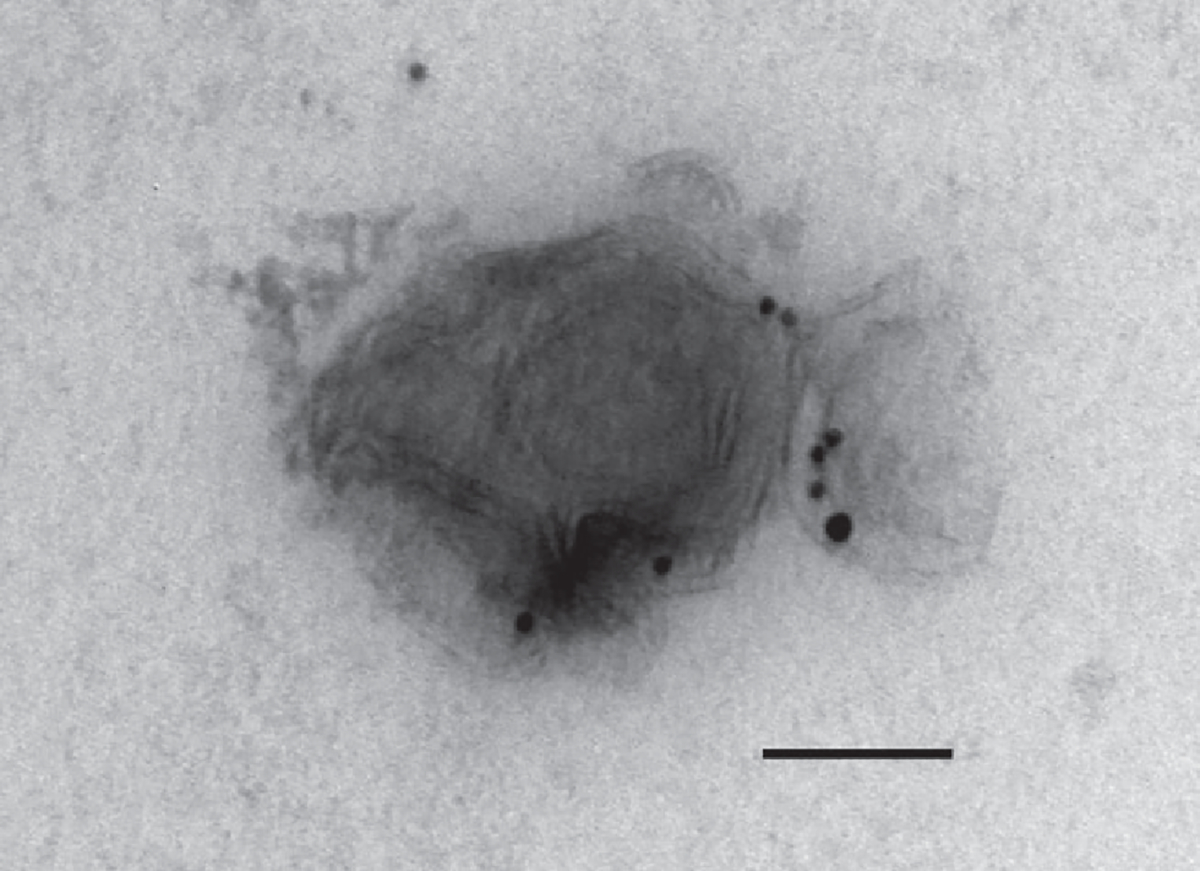
Small EVs from hepatocyte cell line. Cell culture supernatant was fixed and processed for immunogold-based detection of cytochrome P450 2E1 (Cyp2E1, smaller particle size) and asialoglycoprotein 2 (Asgr2, larger particle size) to demonstrate the detection of hepatocyte-derived EVs by this technique. Magnification 150k ×, scale bar 100 nm.
Precipitation
EVs can also be isolated based on their solubility within a solution. Volume-excluding polymers, such as polyethylene glycol (PEG), have been used to precipitate EVs from diverse biofluids. The PEG-based reagent is first incubated with the sample, such as cell culture media or body fluids. By tying up water molecules, the PEG forces less-soluble components, including EVs, out of the solution. The precipitate containing EVs can then be isolated using low-speed centrifugation or filtration. Several proprietary reagents using PEG-based EV enrichment are commercially available. Although the precipitation method is convenient and easy to use, isolated EV samples have typically low purity and high amounts of contaminating proteins (137, 199, 216). Possible issues may also arise from PEG polymer remnants in the isolated EV sample.
Kinetics of Endogenous EVs
Accurate information about the half-life, biodistribution, and clearance of endogenous EVs is essential to understanding their biological roles and is mandatory in employing EVs as therapeutic agents. Due to technical reasons related to the ease of labeling exogenously administered EVs in comparison to the limitations of intra-vital visualization of nanoparticles, most of the published studies describe data collected from systemically administered EVs by intravenous, intraperitoneal, subcutaneous, or oral routes in mouse models rather than endogenous EVs (108, 146). Model organisms like the zebrafish embryo, discussed below, are emerging tools to trace EVs in vivo (78, 79, 221).
Methods that monitor EV biodistribution in vivo are based on molecular imaging and include bioluminescent imaging (BLI), fluorescence imaging (FLI), radionuclide labeling for nuclear imaging, and ultrasmall superparamagnetic iron oxide nanoparticles labeling for magnetic resonance imaging (MRI) (50). Here we discuss studies employing BLI and FLI to assess the half-life of EVs. In BLI, light generated from a luciferase enzyme-substrate is used as a readout of EVs derived from the cells transfected with the vector containing an imaging reporter gene Gaussia luciferase (GLuc). While FLI is achieved by either direct labeling of EV membranes with fluorescent dyes like PHK26 and DiI reagents or indirect labeling of EVs with a fluorescent protein tag attached to an EV protein. A recent study by Matsumoto et al. estimated the half-life of mouse plasma sEVs labeled with PKH26 and GLuc-lactadherin (GLuc-LA) reporter protein at 7 min based on pharmacokinetic analysis (146). GLuc-LA binds EVs through LA, which has high affinity for PS, a component of the EV membrane. Hence, the transfer of GLuc-LA to lipoprotein particles in the circulation was reduced and the accuracy of EV labeling was increased. In vivo imaging demonstrated that GLuc-LA-labeled mouse plasma EVs home mainly to the liver and are eliminated by hepatic macrophages. Macrophage depletion by clodronate liposome treatment in mice delayed EV clearance from the circulation. Likewise, Lai et al. employed a combination of GLuc and metabolic biotinylation to create human embryonic kidney (HEK) 293T cell-derived EVs with this engineered reporter (EV-GLucB) for multimodal imaging by bioluminescence and fluorescence-mediated tomography (108). This methodology showed predominant localization of intravenously administered EVs in the spleen, followed by the liver. Consistent with the study by Matsumoto et al., Lai et al. showed that GLucB-labeled EVs have a half-life of less than 30 min in vivo in most tissues. Monitoring of blood and urine EVs relative to organ accumulation of EVs demonstrated that EVs initially underwent a rapid distribution phase followed by a longer elimination phase via hepatic and renal routes within 6 h. In addition, the study showed that subcutaneous xenograft tumors had increased avidity for EV uptake, within 1 h of systemic administration. This study suggested that blood flow and metabolism may govern EV uptake as the xenografts are metabolically active and have enhanced angiogenesis. Bala et al. examined the biodistribution of EV-associated-miR-155 using a miR-155 knockout (KO) mouse model. The authors demonstrated that intravenous administration of synthetic miR-155- loaded EVs into miR-155-KO mice resulted in rapid distribution of these EVs mostly to the liver, followed by adipose tissue, muscle, and the kidneys. MiR-155 was found both in isolated hepatocytes and liver mononuclear cells of recipient KO mice, indicating cellular uptake of circulating EVs by more than one cell type within the liver. This study showed rapid biodistribution and uptake of EVs in vivo, with maximal plasma levels at 5 min after injection, a 50% decrease in circulating levels after 30 min, and undetectable levels after 4 h (11). Likewise, primary hepatocyte-derived EVs radiolabeled with zirconium-89 and injected intravenously into C57BL/6J mice were immediately and completely taken up by the liver when visualized by positron emission tomography (34). Moreover, in C57Bl/6 mice tumor necrosis factor (TNF)/d-galactosamine-induced hepatic injury significantly increased the homing of parenterally administered fluorescently labeled murine mesenchymal stem cell (MSC)-derived EVs, supporting the use of systemic administration of MSC-EV to target the injured liver (62). Although the liver is the main organ of uptake of exogenously administered EVs, EV biodistribution varies based on the dose, the route of injection, the cell of origin, and the presence of tumor tissues. In addition, EVs can be genetically engineered to preferentially home to a specific tissue or organ by targeting an abundant receptor (227). Taken together these data suggest that the circulatory half-life of exogenously administered EVs is short, and likely does not exceed 30 min. In addition, exogenously administered EVs home preferentially to the liver, especially in liver injury, where they are taken up predominantly by hepatocytes and hepatic macrophages. However, the half-life of endogenous EVs is largely unknown partly limited by the experimental tools available to visualize and track endogenous EVs. EVs can affect target cells’ responses in many ways, these are discussed in detail in the next section.
Target Cell Responses
EVs secreted by one cell (donor cell or originating cell) have the capacity to trigger phenotypic changes in a recipient cell and, therefore, are considered essential mediators of cell-to-cell communication during both normal physiologic processes and pathological conditions. This intercellular cross-talk via EVs can occur in a paracrine manner within the tissue microenvironment or in an endocrine fashion after EVs travel via circulation to distant sites. To communicate the information, EVs first recognize the recipient (target) cell, followed by signal transduction, which induces cellular response. Target cell recognition and interaction are likely governed by EV properties, such as vesicle size and surface composition. For example, EVs may recognize recipient cells by processes involving a ligand-receptor interaction, which is conferred by the specific proteins on the target cell surface and the EV surface (29). Thus, these particular EV surface proteins can be viewed as EV homing signals. EV homing signals are best exemplified by integrins expressed on tumor-derived EVs and their role in the formation of a premetastatic niche. Tumor EVs bearing integrin αVβ5 are destined to liver resident macrophages Kupffer cells (KCs), representing liver tropism, while tumor EVs with integrin α6β1 and α6β4 are preferentially targeted to lung fibroblasts and epithelial cells, mediating lung tropism (75). The interaction between EVs and target cells can also be exploited to specifically direct engineered EVs to desired target cells. To this end, EVs can be manipulated to express a surface ligand that will help those vesicles be recognized by specific target cells expressing a cognate receptor. For instance, nanoparticles with asialoglycoprotein receptor ligands can be quite specifically targeted to hepatocytes and hepatocellular carcinoma cells, which are the major cell types expressing asialoglycoprotein receptors (176). Conversely, EVs can also carry proteins that can protect from cellular uptake. For example, EVs bearing CD47, an integrin-associated protein that inhibits phagocytosis, were less taken up by monocytes and macrophages, resulting in an increased half-life of these EVs in the circulation (92). Thus, EVs can have features mediating both positive and negative selection mechanisms in regard to interactions with target cells.
EV-induced signal transduction in target cells likely occurs at the level of the cell surface (without EV internalization) or following EV uptake (i.e., internalization of EVs by recipient cells) with EV cargo delivery. The relative contribution of these two distinct mechanisms to target cell signal transduction and responses is currently unclear. At the cell surface level, EV-associated ligands can stimulate target cell receptors to induce signaling similarly to soluble or cell membrane-anchored ligands. This has been well documented by EVs bearing major histocompatibility complex (MHC)-peptide complexes activating T cell receptors on antigen-specific T lymphocytes (37, 185, 186, 212). In liver pathology, several known ligand-receptor interactions have been described between EVs and target cells. For example, lipotoxic hepatocyte-derived EVs carrying C-X-C motif chemokine ligand 10 (CXCL10) and TNF-related apoptosis-inducing ligand (TRAIL) interact with C-X-C motif chemokine receptor 3, or CXCR3 and TRAIL receptor-expressing macrophages, respectively (72, 80). Other EV cargoes involved in liver disease pathogenesis are discussed in the following sections in the context of disease relevance. To test whether signal transduction occurs at the cell surface and does not require EV uptake can be determined experimentally using a variety of inhibitors of EV uptake, reviewed by Mulcahy et al. in detail (159).
EVs also incite target cell responses after EV content is transferred inside the recipient cell upon EV uptake. EV uptake by recipient cells can be mediated by a variety of mechanisms: clathrin-mediated endocytosis, caveolin-dependent endocytosis, macropinocytosis, phagocytosis, lipid raft-mediated uptake, and surface membrane fusion (159). Among these, clathrin-mediated endocytosis and caveolin-dependent endocytosis appear to be the primary pathways for EV internalization by most cell types. Certain cell types likely prefer one mechanism of EV uptake over another. Dendritic cells and macrophages, for instance, seem to internalize EVs primarily by phagocytosis (45, 142). EV size may also influence the preferred mechanism of cellular uptake. Smaller vesicles are more likely to be taken up by endocytosis, while large EVs or aggregates of sEVs are more suitable substrates for phagocytosis (29).
Compelling evidence has shown that EV cargo, including protein, lipids, nucleic acid, metabolites, etc., induces downstream effects within the recipient cells following EV internalization (93). The fate of EVs after cellular uptake can vary but often involves targeting to endosomes, lysosomes for degradation, or recycling and extracellular release. Questions remain about the exact mechanisms by which EV content is delivered to the cytosol upon EV endocytosis by the recipient cell, assuming that step is required for intracellular signaling. First reports demonstrating EV cargo delivery into recipient cells focused on the functional transfer of RNA species (178, 215). More recently, the cytosolic release of EV cargo was demonstrated in a quantitative manner, indicating that about 30% of up-taken EVs reach the cytosol (13). This study also suggested that EV content release requires endosomal acidification. Several studies have also demonstrated EV membrane fusion to the recipient cell plasma membrane using fluorescent quenching of lipid probes (157, 169). In addition, fusion may also occur between EVs and the limiting membrane of endocytic compartments (1, 13). In these circumstances, EV-encapsulated content would be directly delivered into the cytosol. Despite the limited knowledge regarding how EV content induces intercellular signaling following EV uptake, a broad spectrum of cellular responses and phenotypic changes in target cells has been described. The effects of EVs on target cells as it pertains to liver physiology and pathophysiology are discussed in respective sections below.
Physiological Roles of EVs
The isolation, labeling, and characterization of EVs have created an opportunity to advance our understanding of the physiological roles of EVs. Our understanding of the role of EVs in health has predominantly relied on in vitro observations with extrapolations implying similar functional relevance in vivo and observations in model systems (196, 221). In the zebrafish embryo, yolk syncytial layer-derived exosomes were found to play a cell proliferative role in the growth of the caudal vein plexus, which was attenuated in height when exosome release was inhibited. Similarly, emerging literature supports a role for EVs in numerous cellular functions including protein homeostasis, cellular proliferation, tissue maintenance via intercellular communication, regulation of immune response, inter-organ communication, and modulation of the organism’s metabolism. Like soluble factors, EVs may exert autocrine, paracrine, or long-range (endocrine function). The identification of numerous bioactive proteins, lipids, and nucleic acid species in EVs has further supported a physiological role for EVs. Notably, EVs are enriched in accessory molecules such as MHC I and II, and may contain peptide-MHC-I, and -II complexes along with other co-stimulatory molecules. In keeping with the detection of these cargoes, EVs from dendritic cells can mediate activation in B cells, T cells, and macrophages (12, 208, 222). These observations suggest a role for EVs in immune homeostasis. EV surface proteins contain eat-me signals, such as PS, and do not eat-me signals such as CD47 and CD24 (95, 182). It remains to be determined whether changes in the expression of eat-me versus do not eat-me signals occur as EVs shift from a physiological role to a pathological role. For example, an increase in do not eat-me signals may increase the half-life of EVs and allow communication with cells at a higher concentration and at distant sites, a phenomenon already observed in cancer-derived EVs.
At a cellular level, heat shock protein (HSP)-40 or HSP70-laden EVs maintain non-cell-autonomous protein homeostasis and preserve organismal proteostasis, by improving the protein-folding environment. These EVs can potentially compensate for the imbalanced state of the HSP among different cells (203). This physiological function of EVs might have implications in liver disorders associated with misfolded proteins and ER overload or stress like alpha 1 anti-trypsin deficiency. The unique microanatomy of the liver, including the organization of hepatocytes into plates around sinusoids which are lined by a fenestrated endothelium, and secretion of bile into canaliculi and eventually cholangiocyte-lined bile ducts also offers several compartments in which EVs may play distinct physiological roles. Hepatocyte-derived EVs can communicate with hepatic stellate cells (HSCs), KCs, and liver sinusoidal endothelial cells (LSECs). These signaling pathways have been identified in various disease models, which are discussed below in detail; however, their role in physiological intercellular communication in the liver is an area ripe for future investigation. Indeed, biliary EVs are sensed by primary cilia on cholangiocytes and decrease cholangiocyte proliferation (144).
Pathophysiological Roles of EVs
EVs are recognized to mediate near-range and long-range signaling in many diseases including acute and chronic liver diseases and malignancies. Early studies examined the contribution of EVs to immune responses by serving as a vehicle for antigen presentation by MHC-I and II complexes. Raposo et al. demonstrated that EVs derived from B-lymphocytes presented antigens bound to the MHC class II. These EV-associated complexes induced an MHC class II-restricted T-cell response, supporting the role of EVs in antigen presentation in vivo (177). Subsequent studies have demonstrated that peptide-MHC complexes on EVs can stimulate cytotoxic or helper T cells directly or indirectly via the antigen-presenting cells (APCs). Peptide-MHC complexes of EVs can be directly presented to T cells without the need for peptide-MHC complex reprocessing through cross-dressing. EVs may have an immunostimulatory role by transferring antigens that activate APCs or an immunosuppressive role on APCs that contributes to the induction of regulatory T cells (180). Known EV-induced immune responses in liver diseases are discussed in subsequent sections; however, this remains an area with many unanswered questions. EVs have been implicated in processes of injury, repair, regeneration, fibrosis as well as carcinogenesis in several liver diseases. We discuss these specific roles in select diseases next.
Nonalcoholic steatohepatitis
NASH is characterized by cross-talk between several different cell types. Pathogenically, hepatocyte lipotoxicity and immune cell-mediated inflammation, both hallmarks of NASH, have suggested an important contribution of intercellular communication in mediating liver injury and inflammation. Consequently, the release of EVs carrying different cargoes with functional relevance in disease pathobiology has been described in human NASH as well as in vitro and in vivo models (Table 2; Figure 4). Initial observations demonstrated an increase in circulating EVs in human plasma from NASH patients (88) and in mouse NASH models (72, 120). Further examination of the relevance of EVs in NASH led to the identification of several bioactive EVs cargoes and mechanisms of release of lipotoxic hepatocyte-derived EVs. For example, EVs carrying CXCL10 released from lipotoxic hepatocytes via mixed lineage kinase 3 (MLK3) mediated mechanism acted as a macrophage chemoattractant (80). In another study, lipotoxic hepatocyte-derived EVs were capable of inducing migration and tube formation in an endothelial cell line in vitro and angiogenesis in mice through vanin-1, a surface cargo protein (174). Further, circulating levels of these EVs enriched in miR-122, a liver-specific miR was increased in a murine diet-induced NASH model, and correlated with disease stage. Similarly, plasma in mice with diet-induced NASH, and patients with NASH contained elevated hepatocyte-derived exosomes carrying mitochondrial DNA that activated Toll-like receptor (TLR) 9 signaling on myeloid cells and a downstream proinflammatory response (52). In a study from our group, lipotoxic ER stress in hepatocytes directly enriched EVs with ceramide which, via conversion to sphingosine 1 phosphate (S1P), was a chemoattractant to proinflammatory macrophages that expressed S1P receptors (88). Indeed, tail-vein injection of these hepatocyte-derived EVs caused accumulation of pro-inflammatory macrophages in the liver, which was ameliorated by genetic or pharmacologic disruption of unfolded protein response sensor IRE1α (34).
Table 2.
Potential of Circulating Extracellular Vesicles as Biomarkers in Human NAFLD
| Comparison groups | Association | Cargo | References |
|---|---|---|---|
|
| |||
| NASH with F3 fibrosis versus cirrhotic NASH and healthy control | Hepatocyte-derived EVs, isolated from serum, correlated with clinical characteristics and disease severity | Specific proteomic signatures | (175) |
| NASH versus HCV and healthy control | Microparticles from invariant natural killer T cells and macrophages/monocytes, correlated with severity of NASH | Not assessed | (101) |
| NASH with F3–4 versus early NAFLD | Leukocytes or endothelial cell EVs inversely correlated with fibrosis | Not assessed | (226) |
| NASH with early fibrosis versus steatosis | EVs in serum are increased in NASH | Integrin β1 | (60) |
| NASH with early fibrosis versus steatosis and obese controls | EVs in plasma | C16:0 ceramide, and its metabolite sphingosine 1 phosphate | (88) |
| Obese with elevated ALT versus obese with normal ALT versus lean subjects with normal ALT | Hepatocyte-derived EVs in plasma correlated with ALT | mitochondrial DNA | (52) |
| NASH with advanced versus early fibrosis | Circulating hepatocyte-derived exosomes in serum increased in advanced fibrosis | miR 122 and miR 192 | (115) |
| NASH versus NAFLD and healthy control | Hepatocyte-derived exosomes are increased in NASH | miR-192-5p | (134) |
| NASH versus chronic hepatitis C, chronic hepatitis B, and healthy controls | The miR expression profile in exosome rich fraction from serum correlated with etiology | miR panel (miR- 225-5p, -1275, -368, -762, 320c, -451, -1974, -630, -1207-5p, -720, -1246, and -486-5p) | (160) |
Figure 4.
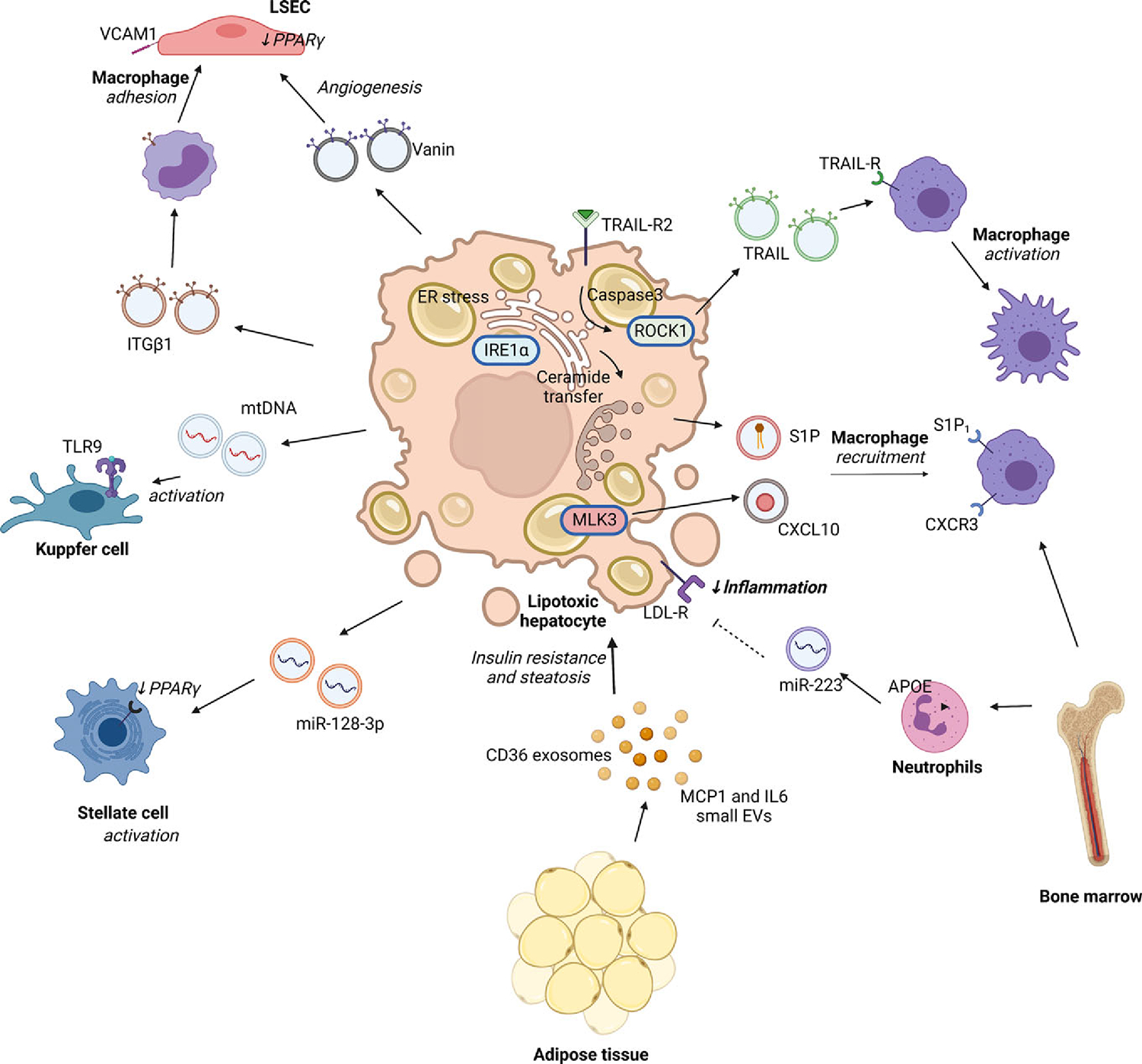
Role of extracellular vesicles in nonalcoholic steatohepatitis. EVs mediate multi-organ cross-talk—between the liver, adipose tissue, and bone marrow; as well as multi-cellular cross-talk—between hepatocytes, non-parenchymal cells in the liver, and immune cells. Lipotoxic hepatocytes activate intracellular stress responses including endoplasmic reticulum (ER) stress-mediated activation of inositol requiring enzyme 1 α (IRE1α), activation of serine threonine Rho- kinase 1 (ROCK1) and caspase 3, and mixed lineage kinase 3 (MLK3) which increase the formation and release of EVs. These EVs are heterogeneous and carry diverse cargo such as proteins including Vanin, TRAIL, CXCL10, nucleic acids including microRNAs (mir-128–3p, miR-223) and mitochondrial DNA (mtDNA), and lipids such as sphingosine 1 phosphate (S1P) which serve ligands for a multitude receptor-mediated or epigenetic regulatory signaling pathways in recipient cells. Though factors targeting subsets of EVs to specific recipient cells remain unknown, studies demonstrate that specific cell types respond to certain cargoes. For example, lipotoxic ER stress in hepatocytes can increase release of EVs containing ceramide-derived S1P which is a chemoattractant to proinflammatory circulating macrophages expressing S1P receptors. Lipotoxic EVs also contain CXC motif chemokine ligand 10 (CXCL10), TNFα-related apoptosis-inducing ligand (TRAIL), mtDNA which engages CXC motif chemokine receptor 3 (CXCR3), TRAIL-receptor (TRAIL-R), and Toll-like receptor 9 (TLR9), respectively. In contrast, hepatocyte uptake via the low-density lipoprotein receptor (LDL-R), of miR-223 enriched EVs from neutrophils may have an anti-inflammatory effect in NASH. Vanin-enriched hepatocyte-derived EVs and miR-128–3p enriched EVs increase hepatic stellate cell activation. Integrin beta 1 (ITGB1) in hepatocyte-derived EVs is internalized by circulating monocytes to increase their adhesion to liver sinusoidal endothelial cells (LSEC). Adipose tissue-derived EVs containing monocyte chemotactic protein 1 (MCP1), interleukin 6 (IL6), and cluster of differentiation 36 (CD36) influence hepatocyte insulin resistance and lipid metabolism. Cells and tissues are denoted in bold, and the biological effect is italicized.
EVs are also implicated in cross-talk with HSCs and mediating fibrosis progression in NASH. Lipotoxic hepatocytes-derived EVs possess a miR expression pattern that significantly amplified profibrotic pathways in HSCs (115). Specifically, miR-128–3p as EV cargo suppressed peroxisome proliferator-activated receptor (PPAR)-γ expression in HSCs. Conversely, EVs can also affect hepatocytes in NASH. EVs isolated from visceral adipose tissue in obese compared to lean patients caused an increase in expression of profibrotic genes such as tissue inhibitor of matrix metalloproteinase-1, or TIMP-1 and integrin ανβ5 and decreased matrix metalloproteinase-7 and plasminogen activator inhibitor-1 in a hepatocyte cell line (99). Moreover, EVs derived from lipotoxic hepatocytes are enriched with the adhesion molecule integrin β1 and enhance the adhesion of proinflammatory monocytes to their cognate ligand on LSEC, vascular cell adhesion molecule 1, or VCAM1, and their homing to the liver in NASH (60). A recent study has demonstrated in NASH that bacteria-derived and host-derived fecal EVs increase intestinal permeability and activate proinflammatory and profibrotic signaling in HSCs (46). In contrast, uptake of neutrophil-derived miR-223-enriched EVs via low-density lipoprotein receptor on hepatocytes inhibited hepatic inflammatory and fibrogenic gene expression in NASH (70). Given their diverse roles in pathobiology, and correlation with progression in disease activity, EVs also have tremendous potential as a clinical biomarker in NASH which is reviewed in a subsequent section.
NASH is a hepatic manifestation of obesity-associated metabolic syndrome, which is also characterized by expansion and sterile inflammation of adipose tissue depots. Indeed, a growing body of literature has identified a role for EVs in interorgan cross-talk in obesity-associated metabolic syndrome (2). The adipokine role of adipose tissue circulating EV miRs was examined in a loss-of-function paradigm using mice with an adipose-tissue-specific knockout of the microRNA-processing enzyme Dicer (ADicerKO). In this study, adipose tissue-derived EVs miRNA reduced the fibroblast growth factor (FGF) 21 expression in the liver and the circulation and was associated with improved glucose tolerance. This function was reconstituted with wild-type brown adipose tissue transplant in ADicerKO mice (210). Gonadal white adipose tissue-derived exosomes enriched in miR-222 worsened hepatic insulin resistance by suppressing insulin receptor substrate 1 expression (118). Similarly, human adipose tissue explant-derived EVs influenced insulin sensitivity in hepatocytes in vitro (106). Indeed, proteomic analysis of exosomes from primary human preadipocytes identified 884 proteins or “exoadipokines”, several of which were significantly associated with liver diseases and metabolic syndrome by pathway analysis (67). Furthermore, EV release may serve as a mechanism to offload the cell from unneeded ceramide and may have a protective role in insulin resistance and nonalcoholic fatty liver disease (NAFLD). Obata et al. reported that adiponectin, an adipocyte-derived circulating factor with known pleiotropic protective properties, accumulates in the vascular endothelium through binding with T-cadherin, a glycosylphosphatidylinositol, or GPI-anchored cadherin. This binding interaction of the adiponectin-T cadherin system enhanced EV biogenesis and secretion by endothelial cells, leading to reduction of ceramide in the aorta of mice (98, 164). Adipose tissue from high-fat diet-fed mice induced TSG101 facilitated CD36 sorting into exosomes that were then endocytosed by hepatocytes to induce lipid accumulation and inflammation (231). Separately, adipose tissue macrophages in obese mice secreted miRNA-containing exosomes that robustly impaired hepatocyte insulin action and in vivo insulin sensitivity (236). Conversely, lipotoxic hepatocyte-derived EVs contain miRNAs that enhanced adipocyte lipid accumulation in mice fed a high-fat diet and correlated with BMI in NAFLD patients (247).
Viral hepatitis
Viral hepatitis affects 397 million individuals worldwide, with both hepatitis B virus (HBV) and HCV infection cases combined (104). Long-term infection may lead to cirrhosis and liver cancer. Viruses hijack the EV biogenesis machinery to increase infectivity and transmissibility. Moreover, EVs released from infected cells may modulate the host immune system against the virus (129). EVs from HCV-transfected or HBV-infected hepatocytes contain viral RNA (39, 105). HBV-RNA-enriched EVs induce the expression of natural killer group 2D ligand, or NKG2D on macrophages, which in turn stimulates interferon γ (IFN-γ) from natural killer (NK) cells (105). Kakizaki et al. showed that macrophages treated with EVs from HBV-infected hepatocytes can also upregulate programmed death-ligand 1 (PD-L1) expression, which is known to bind to programmed cell death protein 1 (PD-1) on T cells and suppress T cell activation (90). The same group described an immunosuppressive function of EVs from HBV-infected hepatocytes in vivo (89). In regard to HCV, HCV-RNA-enriched EVs can activate plasmacytoid dendritic cells which produce type I IFN (39). EVs derived from infected hepatocytes can also contain viral DNA, which is taken up by uninfected hepatocytes leading to detectable HBV DNA levels in hepatocytes (234). Moreover, EVs containing both HBV RNA and DNA from chronic hepatitis B patients were taken up by NK cells isolated from healthy donors leading to decreased NK cell cytotoxicity, TNFα, and IFNγ expression (234). Similarly, monocytes treated with EVs from HCV-infected hepatocytes increased their galectin-9 expression (68), where galectin-9 in turn, promoted the expansion of regulatory T cells and apoptosis of HCV-specific T cells (153). Syntenin-1, an intracellular adaptor protein, has a role in exosome biogenesis and HCV envelope glycoprotein E2 enrichment in EVs. These E2-coated EVs rendered HCV infectivity less susceptible to antibody neutralization in hepatoma cells and primary human hepatocytes (36). Nonenveloped viruses, such as hepatitis A virus (HAV), can also hijack the exosome biogenesis machinery to form a quasi-enveloped virus. In the case of HAV, this happens through interaction of the HAV structural protein pX with one of the exosome biogenesis proteins, ALIX, leading to the secretion of virions and foreign proteins through exosome-like vesicles (84). In line with this study, the delivery of exosome cargo containing viral RNA of HAV is mediated by the PS receptor HAV cellular receptor 1 (HAVCR1) and the cholesterol transporter Niemann-Pick disease, type C1 (NPC1), indicating that viral infection, via this exosome mimicry mechanism, does not require an envelope glycoprotein (31).
MiRNAs present a particular interest in the context of viral hepatitis. MiR-21 and miR-29a are enriched in EVs from plasmid HBV-transfected hepatocytes, and these EVs downregulate the proinflammatory interleukin (IL)-12 expression (105). Furthermore, EVs from patients with chronic hepatitis B transferred miR-25–3p to a hepatocyte cancer cell line enhancing their proliferation (167), suggesting that EVs from infected host cells may promote the progression of viral hepatitis toward liver cancer. Furthermore, miR-19a was enriched in EVs from HCV-infected hepatocytes and these EVs activate signal transducer and activator of transcription 3, or STAT3-mediated tumor growth factor β (TGFβ) signaling in HSCs leading to matrix deposition (38).
Altogether, these studies suggest that hepatitis viruses utilize the EV biogenesis machinery to promote their transmissibility and escape from the host immune system. Hence, EVs promote viral persistence, unproductive immune responses, and an inflammatory microenvironment in the liver which promote the progression to end-stage liver disease (129).
Cholestatic liver disease
Cholestatic liver diseases include primary biliary cholangitis and primary sclerosis cholangitis (PSC), which are characterized by cholestasis, inflammation, and fibrosis. In cholangiopathies, sustained proliferation of cholangiocytes occurs as a response to injury, commonly called the ductular reaction. Cholangiocyte proliferation can be regulated by EVs, in physiological and pathological conditions (104). In this regard, sEVs derived from bile interact with cholangiocyte primary cilia to decrease ERK1/2 phosphorylation, increase miR-15a expression and inhibit cholangiocyte proliferation (144). In line with this observation, EVs from liver stem cells enriched with miR let-7 are shown to reduce ductular reaction mediators and biliary fibrosis through the inhibition of nuclear factor kappa B, or NF-κB and IL-13 signaling pathways (148). On the other hand, serum levels of EV-associated long noncoding RNA (lncRNA) H19 correlate with the severity of human PSC (126). LncRNA H19 can be transferred into hepatocytes via cholangiocyte-derived EVs where they promote cholestatic injury by suppressing small heterodimer partner expression (126). EV-associated H19 also induces cholangiocyte proliferation by upregulating high-mobility group AT-hook 2, or HMGA2 levels (229), HSC activation, and matrix deposition (133). Other studies have shown that H19-enriched EVs promote macrophage activation, differentiation, and chemotaxis through C-C motif chemokine ligand 2 (CCL-2)/C-C motif chemokine receptor 2 (CCR-2) signaling pathways (127) and S100 calcium-binding protein A11 (S100A11) (94). Recently, it has been shown that polarized cholangiocytes release distinct sEV populations from their apical versus their basolateral domains. In an ESCRT-dependent manner, apical EVs differ from basolateral EVs by their composition as well as their number released in the extracellular milieu. A perturbation of the ESCRT machinery may participate in unbalanced EV release and disease progression (35). Further studies are needed to decipher the role of EVs in cholangiopathies.
Fibrosis
Activation of HSCs is crucial for the initiation and progression of fibrogenesis (103). Activated HSCs have been shown to release pro-fibrotic EVs which can induce other HSC activation in a paracrine manner. HSCs stimulated with platelet-derived growth factor (PDGF) release fibrogenic EVs enriched with PDGF receptor α (PDGFRα) (102). Mechanistically, PDGF binding to PDGFRα promotes tyrosine 720 phosphorylation which recruits Src homology region 2-containing protein tyrosine phosphatase-2 (SHP2). Subsequently, SHP2 inhibits PDGFRα degradation and promotes its enrichment in EVs (102). In patients, PDGFRα is enriched in circulating EVs in case of cirrhosis as compared to healthy individuals (102). Moreover, the release of the fibrogenic EVs is increased through activation of mammalian target of rapamycin (mTOR) signaling and inhibition of multivesicular body autophagic degradation (51). Subsequently, the fibrogenic EVs downstream mTOR and SHP2 induce HSC migration in vitro and liver fibrosis in vivo (51, 102). Another group reported that EVs released from activated HSCs contain less Twist family basic helix-loop-helix transcription factor 1 (Twist1) and miR-214 than nonactivated HSCs. Acting in a paracrine manner, these EVs promote the expression of cellular communication network factor 2, or CCN2 in recipient HSCs and their activation (24). It would be interesting to validate the role of TWIST1 inhibitor, harmine (237), on the release of pro-fibrotic EVs and ultimately liver fibrosis.
While the above studies demonstrate the role of EVs derived from activated HSCs, other studies focus on the role of EVs from other cell types on HSC behavior during liver fibrosis. In this regard, lipotoxic EVs derived from stressed hepatocytes carry miR-128–3p and can be taken up by HSCs (174). In turn, miR-128–3p downregulates PPAR-γ leading to HSC activation and liver fibrosis. In addition, in mouse models of liver injury, increased levels of EV-associated miR-128–3p correlate with fibrosis (174). EVs from injured hepatocytes are also enriched with miR-192 and induce fibrogenic signaling in HSCs (115). Besides hepatocytes, endothelial cells also release EVs enriched with sphingosine kinase 1 which promotes HSC migration. Indeed, these EVs are endocytosed in a dynamin-dependent mechanism and induce protein kinase B (AKT) phosphorylation in recipient HSCs and subsequent cell migration (223).
Acute liver injury
Acute liver failure (ALF) is induced by a severe and unresolved insult to the liver. The most known cause of ALF is acetaminophen (APAP)-mediated acute hepatotoxicity. EVs from mice treated with APAP promote hepatocyte apoptosis and increased expression of TNFα/IL-1β in recipient mice (28). The release of EVs from CD133+ hematopoietic stem cells is increased in mice treated with APAP and this is mediated by CD39. Moreover, circulating EVs from patients with liver injury contain high levels of CD39 (184). CD39 is an ectonucleotidase that limits adenosine triphosphate (ATP) induced inflammation by depleting extracellular ATP in APAP-induced liver injury (74). Nevertheless, it remains unknown whether CD39 containing hematopoietic stem cell-derived EVs are involved in limiting inflammation in ALF. In vitro, EVs from umbilical cord MSCs attenuated APAP-induced hepatocyte cell death (130). Hence, EVs from hematopoietic stem cells and MSCs might share similar protective and regenerative properties in ALF. However, more studies are needed to decipher their role in animal models of ALF.
Hepatectomy and ischemia-reperfusion
In case of hepatocellular carcinoma (HCC) or liver failure, the surgical removal of a portion of the liver, partial hepatectomy, or removal of the entire liver to facilitate an orthotopic liver transplantation, is the main therapeutic strategy and it is accompanied by ischemia-reperfusion. After partial hepatectomy, hepatocytes proliferate to restore the liver mass. Partial hepatectomy in rats is associated with an increase in circulating EVs with the enrichment of inflammation-related miR-150 and miR-155 and regeneration-related miR-21 and miR-33 (22). Thymus cell antigen 1+ cell-derived EVs promote the proliferation of hepatocyte progenitors via IL17B receptor signaling. In a rat model of partial hepatectomy, these EVs increased hepatocyte progenitor cluster number and size (81). Hepatocyte proliferation is also promoted by platelets and their interaction with HSCs and LSECs, independently of EVs (154). In a mouse model of hepatectomy, LSECs uptake large EVs from serum mononuclear cells which transfer miR-142–3p leading to abrogated TNFα levels and endothelial cell apoptosis (107). EVs from hepatocytes promote recipient hepatocyte proliferation in vitro, as well as in vivo in mouse models of partial hepatectomy and ischemia/reperfusion. Indeed, EVs from hepatocytes contain neutral ceramidase and sphingosine kinase 2 which promotes the synthesis of S1P in recipient hepatocytes (163). Interestingly, hepatocytes and KCs which undergo mechanical stress due to hepatectomy release lysosomal compartment-derived vesicles which are enriched with ATP to induce the proliferation of hepatocytes and liver regeneration (58). Therapeutically, human umbilical cord MSC-derived EVs promote liver regeneration following hepatectomy in rats by transporting miR-124 and inhibiting Forkhead box protein G1, or FOXG1 (195).
Ischemia/reperfusion is inherent during surgeries such as liver transplantation or hepatectomy. Ischemia/reperfusion promotes interferon regulatory factor 1, or IRF-1-mediated EV release and the transcription of RAB27A, a small GTPase important for the release of MVB content as exosomes (233). These EVs are enriched in oxidized phospholipids which lead to neutrophil activation and hepatic injury (233). On a therapeutic note, in a preclinical mouse model of ischemia/reperfusion EVs derived from human liver stem cells and mesenchymal stromal cells are beneficial by reducing hepatic injury, inflammation, and promote the proliferation of hepatocytes (4, 20).
Primary tumors of the hepatobiliary system
Protumorigenic and antitumorigenic effects have been described for cancer-derived EVs depending on (i) the context of their interaction with the surrounding cells in the tumor micro- and macroenvironment, (ii) specific stage of cancer, and (iii) immune status for both HCC and cholangiocarcinoma (CCA) (Figure 5) (232). The miR cargo of HCC cell line (Hep3B and PLC/PRF/5)-derived EVs demonstrated that while most of the miRs were conserved with donor cells, there was selective enrichment of a few miRs in EVs (100). EVs could transfer miRs from donor HCC cells to recipient HCC cells leading to an increase in their proliferation and colony-forming ability (100, 224). Interestingly, the vacuolar protein sorting 4 homolog A (VPS4A) protein, which is downregulated in human HCC, modulated the secretion of oncogenic and tumor-suppressive miRs into exosomes suggesting that downregulation of VPS4A may promote tumor growth via a reduction in tumor-suppressive miRs or an increase in oncogenic miRs in EVs (224). HCC EVs can also functionally transfer long intergenic noncoding RNA, regulator of reprogramming, or linc-RoR, leading to increased survival of recipient HCC cells (202). EVs from other cells in the tumor microenvironment, have also been implicated in HCC growth (233). Cancer-associated fibroblasts (CAFs) play a critical role in tumor progression, and depletion of miR-320a in CAFs and CAF- derived EVs was associated with an increase in tumor progression (233).
Figure 5.
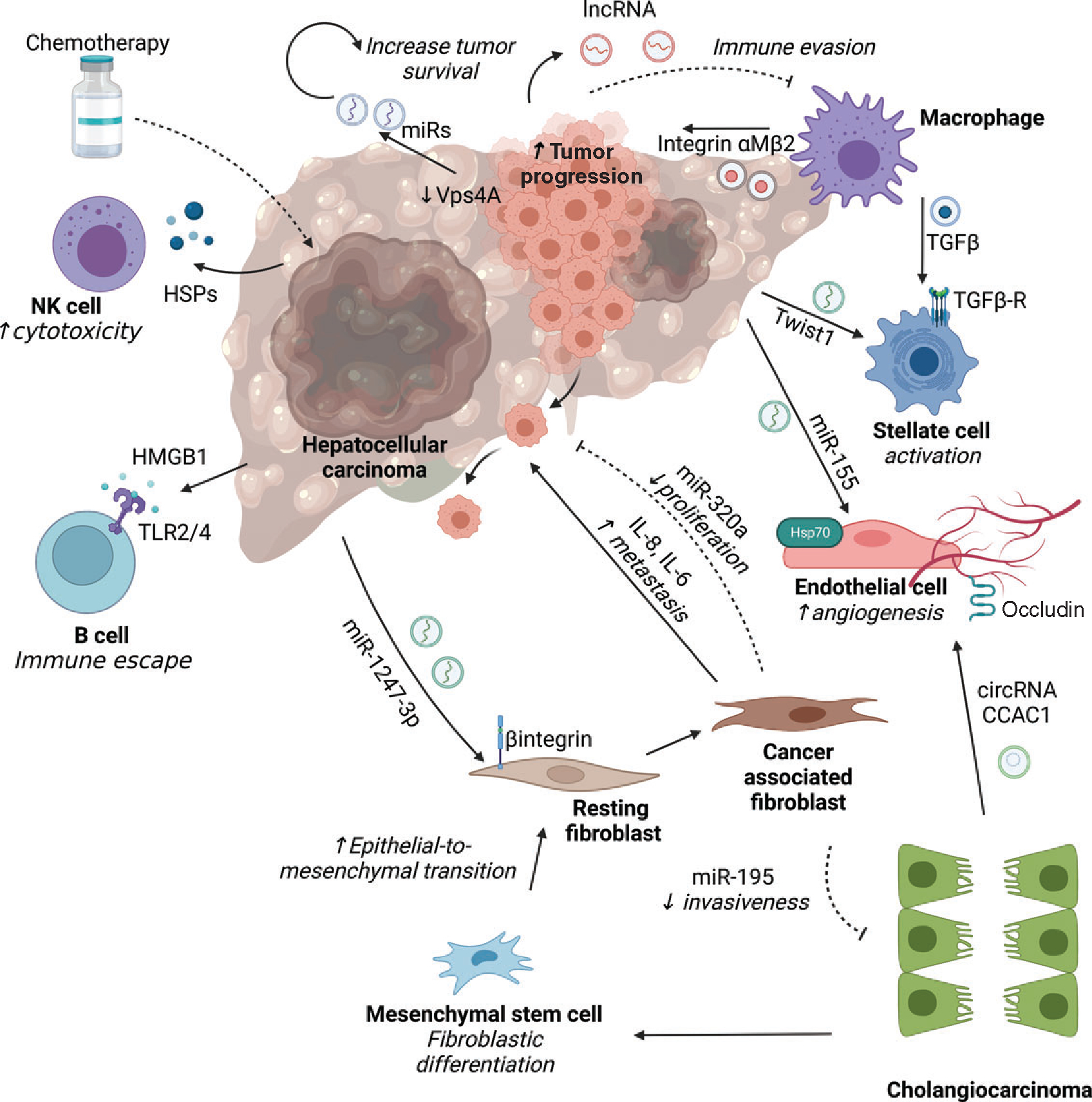
Role of extracellular vesicles in primary tumors of the liver. In hepatocellular carcinoma (HCC) as well as cholangiocarcinoma (CCA), EVs are implicated in cross-talk between cancer cells and other cell types inhabiting the tumor microenvironment. In HCC, tumor cells mediate a feed-forward loop with tumor cells by downregulating expression of the vacuolar protein sorting 4 homolog A (VPS4A) that promotes secretion of EVs with bioactive cargo such as microRNA (miRs) with oncogenic potential or long noncoding RNA (lincRNA-regulator of reprogramming) which confer chemoresistance. HCC-derived EVs mediate diverse processes such as angiogenesis via miR-155 containing EVs that activate heat shock proteins 70 on endothelial cells, epithelial-mesenchymal transition via miR-1247–3p that activate β-integrin on fibroblasts, and stellate cell activation via Twist1. Conversely, cancer-associated fibroblast (CAF) derived EVs carrying interleukins (IL6/8) promote HCC metastatic potential via tranglutaminase2 signaling, while miR-320a reduces tumor progression by suppressing PBX homeobox 3 (PBX3) signaling. Crosstalk with immune cells occurs via tumor-derived exosomes containing high mobility group box 1 (HMGB1) that mediate immune escape by binding to toll-like receptors 2/4 on B cells, and heat shock proteins that activate cytotoxic natural killer (NK) cells after exposure to chemotherapy. Tumor-associated macrophage-derived exosomes that contain integrinαMβ2 and TGFβ boost the migratory potential of HCC by activating matrix metalloproteinase-9 signaling and stellate cell activation respectively. In cholangiocarcinoma, EVs containing circular RNAs (circ-CCAC1) disrupt endothelial barrier integrity by downregulating intercellular junction proteins such as occludin and promote angiogenesis. CCA-EVs promote tumor stroma formation by inducing fibroblast differentiation of mesenchymal stem cells, and conversely CAF-derived EVs carrying miR-195 inhibit CCA growth. Cell types are denoted in bold, and the biologic effect is italicized.
In addition to effects on tumor cell proliferations, HCC EVs may promote angiogenesis via miR-155 and potentially via HSP70 and lysyl oxidase-live 4 (123, 147, 238). EVs have been demonstrated as critical mediators of metastases in a paradigm where tumor-derived EVs educate the metastatic niche, thus promoting metastases formation (32). In the context of HCC, EVs derived from immune cells stimulated by tumor cell culture supernatants, increased cell migration and metastases via the transfer of integrin α(M)β2 from immune cells to tumor cells (141). EVs enriched in miR-1247–3p from highly metastatic HCC converted fibroblasts to CAFs promoting tumor growth in the lung metastatic niche (44). EV cargoes have been implicated in chemoresistance, for instance, exosomal miR-32–5p from HCC cell lines activated the phosphoinositide 3-kinase, or PI3K/Akt pathway and promoted angiogenesis and epithelial-mesenchymal transition (48). Tumor derived-EVs also modulate immunosurveillance of tumors—for example, EVs containing the damage-associated molecular pattern, high mobility group box protein 1 (HMGB1), activated regulatory B cells, and contributed to B cell-mediated immune escape in HCC (235). CCA EVs may also mediate immune escape in vivo based on observations that CCA cell line-derived EVs reduced the cytotoxicity of cytokine-induced killer cells (23). In contrast, EVs released from HepG2 cells that were resistant to anti-HCC drugs stimulated greater HSP-specific NK cell cytotoxicity (140).
Cholangiocarcinoma growth is characterized by a highly desmoplastic stroma with poor vascular supply (193). This stroma is enriched in alpha-smooth muscle actin-positive CAFs and tumor-associated macrophages (179). EVs also play an important role in tumor cell-microenvironment interactions. Katsumi et al. showed that CCA cell line-derived EVs contain the damage-associated molecular pattern (DAMP), S100A11, which leads to upregulation of proinflammatory and profibrogenic pathways in macrophages via the receptor for advanced glycation end products, or RAGE (94). CCA cell line-derived EVs have been demonstrated to exert effects on many recipient cell types, including other CCA cells, MSCs, and immune cells leading to protumorigenic or antitumorigenic responses. Tumor cell-derived EVs in cholangiocarcinoma promoted the differentiation of MSCs into fibroblasts leading to the generation of a tumor-permissive stroma (63). In another study, the protein cargo of CCA-derived EVs was distinct from nontransformed normal cholangiocytes (41). These CCA-derived EVs were internalized and induced migration and invasion of recipient cholangiocytes with associated upregulation of β-catenin and inhibition of E-cadherin expression.
Diagnostic Potential of EVs
The potential for EVs to be used as biomarkers is exciting for multiple reasons—they are (i) stable and abundant in various body fluids, (ii) obtained with relative ease in a minimally invasive fashion, (iii) sampled repeatedly across multiple time points, and (iv) assayed for precise biological origin and cargo composition to inform on the pathophysiological stimuli that led to EV release. We discuss specific examples in select diseases next.
Nonalcoholic steatohepatitis and alcoholic hepatitis
In a 2012 study, levels of circulating EVs were compared in patients with chronic hepatitis C (CHC) and NAFLD. Patients with NAFLD and NASH, but not CHC had greater number of EVs from invariant NKT cells and monocytes, and these were correlated positively with ALT as well as the NAFLD activity score (NAS) (101). Using flow cytometry, circulating EVs were defined to be hepatocyte-derived based on HepPar1 or asialoglycoprotein receptor (ASGPR) expression, correlated with liver chemistry abnormalities, and were reduced after bariatric surgery. In a recent study, using a novel nanoplasmon enhanced scattering, or nPES assay in patients with NAFL, levls of EVs and hepatocyte-derived EVs in plasma correlated with grade of steatosis and inflammation (162). Further, given the significance of lipid species as hepatotoxic stimuli in NASH (6), as well as in EV biogenesis, sphingolipid cargo composition in hepatocyte EVs correlated with EV abundance, NAS score, and decreased after NAFLD resolution in bariatric surgery-induced weight loss.
Similarly, in alcoholic hepatitis (AH), which represents the acute and severe form of alcohol-associated liver disease (ALD), there is an unmet need for a safe, noninvasive, and reliable modality for diagnosis and prognostication. Currently, these are dependent on clinical criteria and the use of scores that utilize blood-based biochemical tests, such as the model for end-stage liver disease (MELD) or Maddrey’s discriminant function, albeit without specificity for AH pathobiology. In a study that included 36 healthy controls, 29 patients with other etiologies of severe end-stage liver disease, and 131 patients with varying degrees of ALD (heavy drinkers, AH, decompensated alcohol-associated cirrhosis), EVs were isolated from plasma and their sphingolipid cargo analyzed by tandem mass spectroscopy. EV abundance was greatest in AH patients compared to all other subgroups and correlated with their MELD score. EV sphingolipid composition correlated independently with the severity of AH and improved the performance of the current standard-of-care (MELD score) in predicting 90-day mortality (187).
Primary hepatobiliary cancer
EV concentration and EV cargoes differ during early and late stages of cancer progression and during recurrence and metastasis hence holding the potential to profile EVs using new omics technologies (111). Diagnostic potential of HCC-derived EVs has been elegantly demonstrated in a recent manuscript which employed a multi-model method based on marker (ASGPR1, Epithelial cell adhesion molecule or EPCAM, and CD147) antibody labeling of EVs, followed by covalent chemistry capture and release on a nanowire substrate followed by quantitative polymerase chain reaction (PCR) for the detection of HCC-associated gene signature based on 10 mRNAs (200). By this approach, the authors reported area under the receiver operating characteristic curve (AUC) for distinguishing cancer from noncancer of 0.87 (95% confidence interval (CI), 0.80–0.94), HCC versus other cancer AUC of 0.95 (95% CI, 0.90–1.00), and early-stage HCC versus cirrhosis an AUC of 0.93 (95% CI, 0.86–1.00). The EV-based biomarker outperformed the currently employed serum alpha-fetoprotein level. Taking a different technical approach employing covalent chemistry-mediated HCC EV capture and immuno-PCR three population of EVs were identified to develop a score for the early detection of HCC (201). Urinary EVs also have the potential to serve as a biomarker for HCC and may be amenable to point-of-care in-home testing. Glycoproteins LG3BP, PIGR, and KNG1 were upregulated in HCC-derived urinary EVs, in comparison to normal controls (117). These very exciting and potentially transformative studies will need independent verification in population-based cohorts and comparison with other blood-based assays such as circulating tumor cells and circulating extracellular RNAs.
In terms of tumor biology, a separate study demonstrated that CD90+ liver cancer cells secreted EVs which were able to promote angiogenesis by enhancing the release of vascular endothelial growth factor, or VEGF, and increasing its receptors on human umbilical vein endothelial cells (30). It was found that motile HCC secrete EVs which are highly enriched in MET-2 and MET-9 protein, which facilitates their metastatic activity (69). Others have demonstrated that EV-long noncoding RNAs may serve as biomarkers for HCC (97) and Shi et al. demonstrated a correlation between serum exosomal miR-638 and HCC, which showed decreased survival rates in patients having a low concentration of exosomal miR-638 in their serum (191). HCC viability and spread can be analyzed by measuring the level of serum miR-9–3p which in turn is inversely related to the expression of FGF-5, which plays a pivotal role in cell proliferation (205). In another study a new population of protumor TIM-1+ regulatory B (Breg) cells, phenotypically being CD5highCD24−CD27−/+CD38+/high, was discovered. Exosomes from HCC were enriched in HMGB1 which facilitated the conversion of B-cells to TIM-1+ Breg cells through TLR 2/4 and mitogen-activated protein kinase pathways. An increase number of TIM-1+ Breg cells are seen infiltrating the tumor and lead to immunosuppression via increased levels of IL-10 and impairing CD8+ T cells (117, 235).
Cholangiocarcinoma presents with two major problems, firstly it is a very aggressive tumor and secondly, late presentation due to lack of specific symptoms. Therefore, there is a dire need for better diagnostics and therapeutics. Biliary EVs hold a unique diagnostic potential for CCA as EVs derived from biliary epithelial cells or CCA cells are likely to be enriched in bile, in comparison to the diverse cellular sources of circulating EVs. miR-195 is an inhibitor of tumor growth and was shown to be decreased in CCA and bordering stromal cells, further, Ling li and colleagues demonstrated inhibition of tumor growth in a CCA rat model by injecting miR-195 enriched EVs (122). In a prospective cohort study, EV concentrations were significantly higher in, and capable of discriminating between malignant versus nonmalignant CBD strictures with high accuracy (188). Likely reflecting changes in microRNAs and lncRNAs in donor CCA tumor cells, a panel of microRNAs in biliary EVs yielded a sensitivity of 67% and a sensitivity of 96% for the diagnosis of CCA (121) and lncRNAs were correspondingly enriched in biliary EVs (54). Circulating EVs in patients with CCA demonstrate a proteomic signature which could also serve as a diagnostic tool (7). A follow-up study from the same group applied machine learning approaches to demonstrate utility of EV proteomics for the early and etiology-specific diagnosis of CCA (113). MiR-200–3p was also found to be enriched in circulating EVs in patients with CCA and had a higher AUC than carbohydrate antigen 19–9 (CA19–9) for the diagnosis of CCA (189). For the spread of tumor, there is a need for cross-talk between cancer and surrounding cells and EVs are responsible for this interaction which further leads to activation of MAPK pathway activation (9). The qualitative analysis of EVs is also of great importance as concentration of some proteins like AMPN, VNN1, and PIGR are markedly increased in CCA versus healthy controls and it carries a better diagnostic value as compared to CA19–9 during early stages (7). Some of the EV-miRs can have oncogenic characteristics and are elevated in CCA, two of them miR-30d-5p and miR-92a-3p were shown to be markedly increased in bile of patients suffering from CCA (64). Besides providing an early diagnosis for CCA, EVs can provide us differential diagnosis regarding conditions with overlapping features especially when it comes to early lesions of CCA versus PSC. For instance, long noncoding RNA MALAT1 gene expression is augmented in serum EVs of CCA patients as compared to PSC (112). Another prominent study demonstrated the interaction between CCA and MSCs and how EVs released from CCA can augment the expression of myofibroblast markers like alpha-smooth muscle actin and fibroblast activation protein and phenotypically change MSCs and promote stromal growth. CCA EVs can further increase the expression of IL-6 from the MSCs which increases the spread of CCA by STAT3 pathway (63). Tumor-derived EVs were found to be elevated in circulation in patients with HCC and CCA and declined after curative resection (87), suggesting that EVs could be used to monitor for tumor recurrence.
Therapeutic Potential
EVs are being studied as a potential therapeutic tool due to their diverse signaling cargoes in homeostasis and disease which can be manipulated for a desired effect, building on their advantages of stability and immunological privilege, and improved safety profile in comparison with cellular therapies (91, 227). Therapeutic EVs can be categorized based on the cellular source of EVs, additionally whether donor cells are autologous or heterologous, whether they serve as a delivery vehicle for specific cargo, or whether the EVs themselves are the therapeutic agent, and whether EVs are modified to be delivered to specific recipient cells. Cellular therapies, in particular stem cell therapies, are inherently associated with a tumorigenic risk (114). On the other hand, EVs are not self-replicating, therefore, bereft of the tumorigenic risk associated with proliferating stem cells. EVs are a natural product, thus, devoid of potentially harmful synthetic materials. The natural homing of systemically administered EVs to the liver, where they are rapidly cleared, though a disadvantage to EV therapeutics for nonliver indications, makes it relatively easier to target liver diseases with lower doses of EVs than required for delivery to other organs (34, 227). Within the liver, systemically administered EVs can be detected in hepatocytes, mononuclear cells, endothelial cells, and HSCs (11, 25). These studies suggest that within the liver, differential cellular uptake of EVs may depend on cell surface upregulation of receptors, such as complement receptors, integrin receptors, or sphingosine 1-phosphate receptors (80, 128, 239). Therefore, it is predictable that several studies have demonstrated that EVs are safe and well tolerated. Furthermore, efficacy of EV therapy has been demonstrated in both mouse models and human disease.
EVs can be actively loaded with cargoes after isolation or by exploiting a donor cell’s intrinsic machinery to sort endogenous cargo into EVs. Diverse cargo such as pharmacological drug products, immune modulators, small interfering RNA (siRNA), or antisense oligonucleotides can be incorporated into EVs by biochemical and biophysical methods such as coincubation (170), electroporation, sonication (65), pH-gradient, freeze-thaw, or EV transfection (138). Alternatively, overexpression of specific therapeutic cargoes in producer cells can result in enriched EVs (165, 225, 244). EVs can also be loaded with specific cargoes with passive incubation, albeit with much lower efficiency (110). There are several examples demonstrating that EVs can also be engineered to enhance specific target cell delivery or cargo. For instance, by transfecting cells to produce EVs enriched with surface ligands (a targeting peptide such as an integrin) (3, 211) receptor-mediated cell-type targeting could be achieved. Additionally, specific markers such as CD47 on EVs confer signal-regulatory protein alpha-mediated protection from phagocytosis. Examples of engineered EVs studied in the treatment of HCC include EV-based transfer of the sodium iodine transporter from donor cells to recipient HCC cells enhancing 125I uptake and conferring radioiodine-sensitivity (238), delivery of stellate cell EVs containing tumor-suppressive miR-335–5p (123), or bone marrow-MSC EVs containing siRNA against glucose regulatory protein 78, or GRP78 (119). These approaches studied in cancer led to an ongoing phase I clinical trial in patients with KRASG12D mutation-associated pancreatic cancer (ClinicalTrials.gov identifier: NCT03608631). Some EVs such as those produced by macrophages are also capable of crossing the blood-brain barrier utilizing intrinsic mechanisms such as integrins (lymphocyte function-associated antigen 1, LFA-1; intercellular adhesion molecule 1, ICAM-1) or the carbohydrate-binding C-type lectin receptors. Alternatively, EVs can be modified to express cholesterol-conjugated RNA aptamers on their membranes (172), thereby achieving cell-specific delivery of siRNA and thus impair tumor growth in different cancer models. Lastly, RNA-binding proteins such as synaptotagmin-binding cytoplasmic RNA-interacting protein, or SYNCRIP in hepatocyte exosomal miR sorting machinery (183) may be targeted to modulate packaging of specific RNAs into EVs.
The therapeutic efficacy of EVs derived from differentiated cells, and from MSCs has been demonstrated in many models of liver diseases. EVs from diverse terminally differentiated cells have been tested in preclinical models of liver diseases. In hepatic ischemic-reperfusion injury (IRI), intravenous administration of hepatocyte-derived EVs induced a concentration-dependent increase in hepatocyte proliferation due to transfer of neutral ceramidase and sphingosine kinase 2 (163). The transfer of this cargo led to an increase in the synthesis of sphingosine 1-phosphate which stimulated hepatocyte proliferation. Orally-administered plant-derived EVs, in particular, ginger-derived shogaol-enriched EVs have protected against alcohol-induced liver injury by activation of TLR 4 and nuclear factor erythroid 2-related factor 2 (Nrf2) mediated antioxidant responses (248). EVs derived from stem cells isolated from cryopreserved adult human hepatocytes enhanced regeneration in a rat model with 70% partial hepatectomy (71). In this study, mRNA which encodes argonaute 2 (AGO2) was transferred to the regenerating livers by EVs. In liver fibrosis mouse models the administration of plasma-derived EV from healthy mice was effective in reducing hepatocyte apoptosis, inflammatory cytokines, transaminases, and fibrosis (25). Several microRNAs, miR-34c, miR-151–3p, miR-483–5p, miR-532–5p, and miR-687, were identified as the mediators of the antifibrotic effects of healthy plasma EVs. This group also demonstrated that normal hepatocyte-cell line-derived EVs were also effective in ameliorating liver injury, inflammation, and fibrosis in a carbon tetrachloride (CCl4) mouse model of fibrosis (125). Along the same lines, purified exosome product (PEP) derived from human plasma has been employed in different disease models, such as tendon repair and ischemic wound healing, where PEP promoted neovascularization, matrix remodeling, and tissue growth (190). However, the role of PEP in enhancing recovery after acute or chronic liver injury, without promoting an exuberant profibrogenic response, remains to be experimentally proven.
Mesenchymal stem cell-derived EVs
Liver regenerative and replacement therapies are a promising alternative or bridge to liver transplantation in patients with end-stage chronic liver disease or ALF. Such therapies have been developed with extracorporeal liver support devices, cellular transplantation approaches, and now with the recognition that EVs retain the therapeutic potential of MSC, it follows that MSC EVs are being examined for their potential in treating acute and chronic liver diseases. MSCs are multipotent mesoderm-derived stem cells which can be isolated from several sources such as bone marrow, umbilical cord, and adipose tissue. MSCs have been demonstrated to have repair, regenerative, proangiogenic, and immunomodulatory properties, via paracrine signaling, in preclinical models of injury and autoimmunity, leading to several clinical trials examining their efficacy in human diseases. MSC EVs offer improved safety over the risks associated with cellular therapies while retaining several advantages, including immunomodulation and tissue repair properties. The immunomodulatory properties of MSC EVs have been demonstrated in experimental models that activate many different immune cell types. MSC EVs can attenuate CpG-induced B cell activation and proliferation (16) and phytohemagglutinin-induced proliferation of peripheral blood mononuclear cells including T cells and B Cells (56). Tolerogenic molecules on MSC EVs, such as PD-L1, galectin-1, and TGF-β, inhibit autoreactive lymphocyte proliferation in a model of experimental autoimmune encephalomyelitis (156). Similarly, angiogenic, antiinflammatory, and reparative properties of MSC EVs have been demonstrated in models of myocardial ischemia (8, 109, 246), acute kidney injury (15), traumatic brain injury (96, 243), and spinal cord injury (139). In keeping with observations in other organ systems and disease states, the therapeutic benefit of MSC EVs has been demonstrated in models of acute and chronic liver diseases.
IRI is a prototypical model for acute liver injury with relevance to liver transplantation, liver resection, and hypoperfusion-induced shock liver. Other acute models of liver injury include partial hepatectomy and toxins such as carbon tetrachloride and d-galactosamine/lipopolysaccharide. In these models of acute liver injury, the administration of MSC EVs of various cellular origins has ameliorated injury (Table 3) (4, 40, 62, 71, 171). These studies also indicate a concentration response to the protective effects of MSC EVs, and also that species mismatch does not appear to be a barrier to MSC EV transplantation, as human MSC EVs were effective in reducing fibrosis and liver tumors in mice administered chronic carbon tetrachloride chronically (85). Human adipose MSC EVs exerted an antifibrotic effect on HSCs in vivo and in vitro by decreasing the proliferation and increasing apoptosis of activated HSCs via PI3K/AKT/mTOR inhibition (245). Proteins, nucleic acid species, and lipids have been implicated in MSC EVs for the observed salutary effects (Table 3), though given the pleiotropic consequences of MSC EV administration, multiple signaling molecules are likely involved. Herein lies one of the limitations of MSC EVs that there is an incomplete understanding of MSC Evs’ mechanism of action.
Table 3.
Studies Investigating Mesenchymal Stem Cell-derived Extracellular Vesicles in Liver Disease
| Model of liver disease | Source of MSC EVs | Bioactive cargo | References |
|---|---|---|---|
|
| |||
| TNF-α/Act-D in mice | Mouse bone marrow | Y-RNA-1 | (62) |
| D-Gal-N/LPS in mice | Human menstrual blood | Multiple cytokines | (27) |
| D-Gal-N/LPS in mice | TNFα-treated human umbilical cord | miR-299-3p | (242) |
| D-Gal-N/LPS in mice | Adipose tissue | H19 | (86) |
| D-Gal-N/LPS in mice | Adipose tissue | miR-17 | (135) |
| Ischemia reperfusion injury (IRI) in mice | Human bone marrow | (4) | |
| IRI in mice | Mouse bone marrow | (61) | |
| ConA in mice | Mouse bone marrow | (204) | |
| CCl4 in mice | Human placenta | (21) | |
| 2/3 partial hepatectomy + IRI in rats | Rat bone marrow | (33) | |
| CCl4 in rats | Rat bone marrow | (33) | |
| Chronic CCl4 in mice | Human umbilical cord blood | (124) | |
| Mdr2−/− mice | Human bone marrow | (5) | |
| Liver antigens S100 | Mouse bone marrow | miR-223 | (26) |
There are additional manufacturing and regulatory considerations relevant to EV therapeutics. EVs are natural cell-derived which gives rise to issues with scalability and variable potency from batch to batch. Two-dimensional (2D) monolayer culture is not amenable to continuous large-scale production of EVs from viable cells. There have been advances in EV biomanufacturing with a shift from 2D monolayer culture to hollow fiber bioreactors (55), which offer several advantages including scalability, viability, decreased manual handling, real-time monitoring, and prolonged production of EVs. In recent years, several EV therapeutics have received approval for investigational new drug, or IND applications from the Food and Drug Administration (FDA). These advances have led to several currently recruiting clinical trials assessing a therapeutic response to EV products from diverse sources for acute myocardial infarction, chronic middle ear infection, and venous trophic lesions among others. On the other hand, the FDA has also issued public safety alerts regarding unregulated and unapproved use of EV therapeutics. Suffice it to say that EVs have immense therapeutic potential, however, will require careful FDA-compliant preclinical studies and clinical trials before their widespread clinical use.
Conclusion
We have discussed several aspects of EV pathobiology above, and in the following section, we focus on the key challenges and gaps in our knowledge and emerging experimental tools to separate the different EV subpopulations based on their compositions, and biogenesis. To date, there is no isolation technique that can differentiate exosomes from microvesicles, since small microvesicles can overlap with the size of exosome (73). Another potential challenge is the selective packaging of EV cargo and whether a predominant cargo in EV is unique or redundant among the different sEV subpopulations (exosomes versus microvesicles). Accumulating studies support the notion that signaling EV cargo is selectively released from the cell of origin into EVs based on the environmental condition or insult (34, 60, 80, 88). In addition, studies over the last few years allowed imaging of MVB fusion with the plasma membrane in real time using a CD63-pHluorin construct facilitating the examination of exosome release at the single-cell resolution (220). However, our understanding of the journey of the EVs across the extracellular space and the circulation, the recognition of EVs by their target cells, and the EV cargo uptake by the destined cell is evolving (143). Nonetheless, tracing circulating EVs to their cell of origin and confirmation of the biological function of EV in vivo is another hot area of research (249). In a recent study, Zomer et al. used the Cre-LoxP system to identify tumor cells that take up EVs in vivo. EVs released by malignant tumors were enriched with mRNAs that promote migration and metastasis. Authors employed intravital imaging to show that the less malignant tumor cells that take up EVs display enhanced migratory behavior and metastatic capacity (249). This study indicates the feasibility of tracing EVs to their cell origin and their ability to elicit a functional signal in the recipient cells in vivo. In addition, model organisms, like the zebrafish embryo, are emerging tools to examine EV biogenesis and trace EVs in vivo (78, 221). Furthermore, the dynamic changes of the source of circulating EVs over the course of disease progression and their cell/tissue of origin were recently described in mouse model of dietary NASH (120). Nanoscale flow cytometry was employed and fluorescently labeled antibodies for cell-specific markers ASGPR1 and cytochrome P450 family 2 subfamily E member 1, or CYP2E1 as markers of hepatocyte-derived EVs (Figure 3); galectin 3 as a marker of macrophage-derived EVs; common epitope on lymphocyte antigen 6 complex, locus G/C1, or Ly6G and Ly6C as a marker of neutrophil-derived EVs; and CD61 as a marker of platelet-derived EVs. In accordance with liver inflammation, macrophage- and neutrophil-derived EVs were significantly elevated at 24 weeks of the feeding study, while hepatocyte-derived EVs increased as early as 10 weeks of feeding and remained elevated afterward. However, although hepatocyte-derived EVs were elevated, they were less abundant than platelet-derived, macrophage-derived, and neutrophil-derived EVs in NASH, and hepatocyte-derived EVs represented a small fraction of the total circulating EVs, no more than 0.02%. Theoretically, the concentration of hepatocyte-derived EVs is much higher within the hepatic microenvironment and may further increase in the microenvironment and decrease in the circulation in advanced liver disease when LSECs lose their fenestrae and develop a basement membrane a process known as capillarization (79). Hence, LSEC capillarization may increase the concentration gradient of EVs in the liver and enhance their role as a catalyst of the inflammatory process in chronic liver diseases.
Liao et al. examined the role of concentration gradient of EVs using a microfluidic gradient generator of EVs derived from hepatocytes under lipotoxic treatment and defined the role of EVs concentration gradient in macrophage chemotaxis (128). Given their high concentration at their site of origin, it is biologically plausible to assume that EVs are more equipped to execute a short-range versus long-range intercellular communication. Nonetheless, emerging data suggest that EVs are destined to interact with a specific cell population based on a combination of receptor-ligand interaction between the EVs and the target cells regardless of the distance of their journey (60, 145).
Furthermore, EV docking and uptake by the target cells are crucial, but largely unclear processes. In addition, how uptaken EV cargo evades degradation in the target cells, and how cargo signals are transmitted to a particular intracellular target (e.g., mitochondria or nucleus) although key aspects in exploiting EV in therapeutic drug delivery, are still an enigma (47).
Finally, EVs associated with a disease may serve as a biomarker in some instances without concrete evidence of a pathogenic role in the disease progression. Circulating EVs have been promoted as liquid biopsy, a noninvasive tool for early detection, monitoring for progression, and response to therapy in various liver diseases. The potential role of EVs as a biomarker is supported by the following EV properties. First, EVs protect their protein and nucleic acid cargo from degradation due to membrane coating; second, EVs facilitate the identification of low abundance diagnostic nucleic acid, lipids, or protein and third, EVs reflect the metabolic status of the cell of origin (43). However, many challenges in using EVs as biomarkers are encountered mainly due to lack of reproducibility of data obtained by different research group and the different protocols employed in sample processing (Table 1).
On the bright side, the analysis of the individual EV is evolving, and our knowledge gap is diminishing with the invention and application of new disease models and state-of-the-art technology including reporter mouse models and zebrafish embryos, immune affinity-based methods, and nanoscale flow cytometry.
Table 4.
List of Abbreviations
| Abbreviation | Expansion |
|---|---|
|
| |
| AGO2 | Argonaute2 |
| AH | Alcoholic hepatitis |
| AKT | Protein kinase B |
| ALD | Alcohol-associated liver disease |
| ALF | Acute liver failure |
| ALIX | Apoptosis-linked gene-2 interacting protein X |
| APAP | Acetaminophen |
| APC | Antigen-presenting cell |
| ARRDC1 | Arrestin-domain-containing protein 1 |
| ASGPR | Asialoglycoprotein receptor |
| ATP | Adenosine triphosphate |
| AUC | Area under the receiver operating characteristic curve |
| CA 19-9 | Carbohydrate antigen 19-9 |
| CAF | Cancer-associated fibroblast |
| CCA | Cholangiocarcinoma |
| CD | Cluster of differentiation |
| CHC | Chronic hepatitis C |
| DAMP | Damage-associated molecular pattern |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| ESCRT | Endosomal sorting complex required for transport |
| EV | Extracellular vesicle |
| FDA | Food and Drug Administration |
| FGF | Fibroblast growth factor |
| GLuc | Gaussia luciferase |
| HAV | Hepatitis A virus |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HMGB1 | High mobility group box protein 1 |
| HSC | Hepatic stellate cell |
| IFN | Interferon |
| IL | Interleukin |
| ILV | Intraluminal vesicle |
| IRI | Ischemia-reperfusion injury |
| KC | Kupffer cell |
| KO | Knockout |
| KRAS | Kirsten rat sarcoma virus proto-oncogene |
| LSEC | Liver sinusoidal endothelial cells |
| MAPK | Mitogen-activated protein kinase |
| MELD | Model for End-Stage Liver Disease |
| MHC | Major histocompatibility complex |
| miR | microRNA |
| mRNA | messenger RNA |
| MSC | Mesenchymal stem cell |
| mTOR | Mammalian target of rapamycin |
| MVB | Multivesicular body |
| NAFLD | Nonalcoholic fatty liver disease |
| NAS | NAFLD activity score |
| NASH | Nonalcoholic steatohepatitis |
| NK | Natural killer |
| PCR | Polymerase chain reaction |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PDGF | Platelet-derived growth factor |
| PEG | Polyethylene glycol |
| PPAR | Peroxisome proliferator-activated receptor |
| PS | Phosphatidylserine |
| PSC | Primary sclerosis cholangitis |
| ROCK | Rho-associated protein kinase |
| S1P | Sphingosine 1 phosphate |
| sEV | Small extracellular vesicle |
| SHP2 | Src homology region 2-containing protein tyrosine phosphatase-2 |
| siRNA | Small interfering RNA |
| STAT | Signal transducer and activator of transcription |
| TGF | Tumor growth factor |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TSG101 | Tumor susceptibility gene 101 |
| VPS4A | Vacuolar protein sorting 4 homolog A |
Didactic Synopsis.
Major teaching points
The nomenclature of extracellular vesicles and their classification into exosomes and microvesicles is based on the molecular machineries involved in their biogenesis.
EV cargo includes proteins, lipids, and nucleic acids which allows them to communicate various biological signals between diverse cell types in health and disease.
There are several techniques to isolate and characterize EVs, with differing yield and purity.
EVs are implicated in the pathobiology of many liver diseases, and thus have powerful diagnostic potential as a biomarker of disease severity.
Given their stability in biological fluids, and their ability to target specific cell types, EVs can also be engineered to serve therapeutic roles in liver diseases.
Acknowledgements
Petra Hirsova received grant support from the AASLD Foundation (Pinnacle Research Award), Mayo Clinic Center for Biomedical Discovery, and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under the Award Numbers P30DK084567 and R01DK130884. Harmeet Malhi received grant support from the NIDDK under award R01DK111378 and from the NIAAA under award AA21788. Samar H. Ibrahim received grant support by R01DK122948 from the NIDDK. Enis Kostallari is supported by the AASLD Foundation (Pinnacle Research Award), NIDDK (P30DK084567), Gilead and Regenerative Medicine Minnesota (P008848105).
References
- 1.Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, van der Goot FG. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep 5: 986–996, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbar N, Azzimato V, Choudhury RP, Aouadi M. Extracellular vesicles in metabolic disease. Diabetologia 62: 2179–2187, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29: 341–345, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Anger F, Camara M, Ellinger E, Germer CT, Schlegel N, Otto C, Klein I. Human mesenchymal stromal cell-derived extracellular vesicles improve liver regeneration after ischemia reperfusion injury in mice. Stem Cells Dev 28: 1451–1462, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Angioni R, Cali B, Vigneswara V, Crescenzi M, Merino A, Sanchez-Rodriguez R, Liboni C, Hoogduijn MJ, Newsome PN, Muraca M, Russo FP, Viola A. Administration of human MSC-derived extracellular vesicles for the treatment of primary sclerosing cholangitis: Preclinical data in MDR2 knockout mice. Int J Mol Sci 21: 8874, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apostolopoulou M, Gordillo R, Koliaki C, Gancheva S, Jelenik T, De Filippo E, Herder C, Markgraf D, Jankowiak F, Esposito I, Schlensak M, Scherer PE, Roden M. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care 41: 1235–1243, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Arbelaiz A, Azkargorta M, Krawczyk M, Santos-Laso A, Lapitz A, Perugorria MJ, Erice O, Gonzalez E, Jimenez-Aguero R, Lacasta A, Ibarra C, Sanchez-Campos A, Jimeno JP, Lammert F, Milkiewicz P, Marzioni M, Macias RIR, Marin JJG, Patel T, Gores GJ, Martinez I, Elortza F, Falcon-Perez JM, Bujanda L, Banales JM. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 66: 1125–1143, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10: 301–312, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Arunsan P, Chaidee A, Cochran CJ, Mann VH, Tanno T, Kumkhaek C, Smout MJ, Karinshak SE, Rodpai R, Sotillo J, Loukas A, Laha T, Brindley PJ, Ittiprasert W. Liver fluke granulin promotes extracellular vesicle-mediated crosstalk and cellular microenvironment conducive to cholangiocarcinoma. Neoplasia 22: 203–216, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14: 677–685, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Bala S, Csak T, Momen-Heravi F, Lippai D, Kodys K, Catalano D, Satishchandran A, Ambros V, Szabo G. Biodistribution and function of extracellular miRNA-155 in mice. Sci Rep 5: 10721, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedford P, Garner K, Knight SC. MHC class II molecules transferred between allogeneic dendritic cells stimulate primary mixed leukocyte reactions. Int Immunol 11: 1739–1744, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Thery C, Lavieu G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun 12: 1864, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan K, Martin K, FitzGerald SP, O’Sullivan J, Wu Y, Blanco A, Richardson C, Mc Gee MM. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep 10: 1039, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7: e33115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant 22: 369–379, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S, Bergese P, Wolfram J. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells 7: 273, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis 35: 398–403, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Buschow SI, Nolte-’t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJ, Raposo G, Wubbolts R, Wauben MH, Stoorvogel W. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 10: 1528–1542, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Calleri A, Roggio D, Navarro-Tableros V, De Stefano N, Pasquino C, David E, Frigatti G, Rigo F, Antico F, Caropreso P, Patrono D, Bruno S, Romagnoli R. Protective effects of human liver stem cell-derived extracellular vesicles in a mouse model of hepatic ischemia-reperfusion injury. Stem Cell Rev Rep 17: 459–470, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao H, Yue Z, Gao H, Chen C, Cui K, Zhang K, Cheng Y, Shao G, Kong D, Li Z, Ding D, Wang Y. In vivo real-time imaging of extracellular vesicles in liver regeneration via aggregation-induced emission luminogens. ACS Nano 13: 3522–3533, 2019. [DOI] [PubMed] [Google Scholar]
- 22.Castoldi M, Kordes C, Sawitza I, Haussinger D. Isolation and characterization of vesicular and non-vesicular microRNAs circulating in sera of partially hepatectomized rats. Sci Rep 6: 31869, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JH, Xiang JY, Ding GP, Cao LP. Cholangiocarcinoma-derived exosomes inhibit the antitumor activity of cytokine-induced killer cells by down-regulating the secretion of tumor necrosis factor-alpha and perforin. J Zhejiang Univ Sci B 17: 537–544, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Chen R, Kemper S, Charrier A, Brigstock DR. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: Role of exosomes in horizontal transfer of Twist1. Am J Physiol Gastrointest Liver Physiol 309: G491–G499, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Chen R, Kemper S, Cong M, You H, Brigstock DR. Therapeutic effects of serum extracellular vesicles in liver fibrosis. J Extracell Vesicles 7: 1461505, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Lu FB, Chen DZ, Wu JL, Hu ED, Xu LM, Zheng MH, Li H, Huang Y, Jin XY, Gong YW, Lin Z, Wang XD, Chen YP. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol 93: 38–46, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood- derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther 8: 9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho YE, Seo W, Kim DK, Moon PG, Kim SH, Lee BH, Song BJ, Baek MC. Exogenous exosomes from mice with acetaminophen-induced liver injury promote toxicity in the recipient hepatocytes and mice. Sci Rep 8: 16070, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M, De Leo G, Alessandro R. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer 14: 155, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costafreda MI, Abbasi A, Lu H, Kaplan G. Exosome mimicry by a HAVCR1-NPC1 pathway of endosomal fusion mediates hepatitis A virus infection. Nat Microbiol 5: 1096–1106, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17: 816–826, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damania A, Jaiman D, Teotia AK, Kumar A. Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury. Stem Cell Res Ther 9: 31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dasgupta D, Nakao Y, Mauer AS, Thompson JM, Sehrawat TS, Liao CY, Krishnan A, Lucien F, Guo Q, Liu M, Xue F, Fukushima M, Katsumi T, Bansal A, Pandey MK, Maiers JL, DeGrado T, Ibrahim SH, Revzin A, Pavelko KD, Barry MA, Kaufman RJ, Malhi H. IRE1A stimulates hepatocyte-derived extracellular vesicles that promote inflammation in mice with steatohepatitis. Gastroenterology 159: 1487–1503 e1417, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies BA, Morton LO, Jefferson JR, Rozeveld CN, Doskey LC, LaRusso NF, Katzmann DJ. Polarized human cholangiocytes release distinct populations of apical and basolateral small extracellular vesicles. Mol Biol Cell 31: 2463–2474, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng L, Jiang W, Wang X, Merz A, Hiet MS, Chen Y, Pan X, Jiu Y, Yang Y, Yu B, He Y, Tu Z, Niu J, Bartenschlager R, Long G. Syntenin regulates hepatitis C virus sensitivity to neutralizing antibody by promoting E2 secretion through exosomes. J Hepatol 71: 52–61, 2019. [DOI] [PubMed] [Google Scholar]
- 37.Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol 165: 1259–1265, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Devhare PB, Sasaki R, Shrivastava S, Di Bisceglie AM, Ray R, Ray RB. Exosome- mediated intercellular communication between hepatitis C virus-infected hepatocytes and hepatic stellate cells. J Virol 91: e02225–16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreux M, Garaigorta U, Boyd B, Decembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 12: 558–570, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du Y, Li D, Han C, Wu H, Xu L, Zhang M, Zhang J, Chen X. Exosomes from human- induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect liver against hepatic ischemia/reperfusion injury via activating sphingosine kinase and sphingosine- 1-phosphate signaling pathway. Cell Physiol Biochem 43: 611–625, 2017. [DOI] [PubMed] [Google Scholar]
- 41.Dutta S, Reamtong O, Panvongsa W, Kitdumrongthum S, Janpipatkul K, Sangvanich P, Piyachaturawat P, Chairoungdua A. Proteomics profiling of cholangiocarcinoma exosomes: A potential role of oncogenic protein transferring in cancer progression. Biochim Biophys Acta 1852: 1989–1999, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Edgar JR, Eden ER, Futter CE. Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic 15: 197–211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eguchi A, Kostallari E, Feldstein AE, Shah VH. Extracellular vesicles, the liquid biopsy of the future. J Hepatol 70: 1292–1294, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, Cao D, Tang L, Tang S, Wu M, Yang W, Wang H. Tumor-derived exosomal miR-1247–3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun 9: 191, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic 11: 675–687, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Fizanne L, Villard A, Benabbou N, Recoquillon S, Soleti R, Delage E, Wertheimer M, Vidal- Gómez X, Oullier T, Chaffron S, Martínez MC, Neunlist M, Boursier J, Andriantsitohaina R. Faeces-derived extracellular vesicles participate in the onset of barrier dysfunction leading to liver diseases. J Extracell Vesicles 12: e12303, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.French KC, Antonyak MA, Cerione RA. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin Cell Dev Biol 67: 48–55, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, Yang Y, Wang S, Wang J, Nan K, Yao Y, Tian T. Exosomal microRNA-32–5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res 37: 52, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Fukushima M, Dasgupta D, Mauer AS, Kakazu E, Nakao K, Malhi H. StAR-related lipid transfer domain 11 (STARD11)-mediated ceramide transport mediates extracellular vesicle biogenesis. J Biol Chem 293: 15277–15289, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gangadaran P, Hong CM, Ahn BC. Current perspectives on in vivo noninvasive tracking of extracellular vesicles with molecular imaging. Biomed Res Int 2017: 9158319, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, Cooper SA, Cao S, Shah VH, Kostallari E. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol 73: 1144–1154, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest 126: 859–864, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J Extracell Vesicles 5: 32945, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ge X, Wang Y, Nie J, Li Q, Tang L, Deng X, Wang F, Xu B, Wu X, Zhang X, You Q, Miao L. The diagnostic/prognostic potential and molecular functions of long non-coding RNAs in the exosomes derived from the bile of human cholangiocarcinoma. Oncotarget 8: 69995–70005, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gobin J, Muradia G, Mehic J, Westwood C, Couvrette L, Stalker A, Bigelow S, Luebbert CC, Bissonnette FS, Johnston MJW, Sauve S, Tam RY, Wang L, Rosu-Myles M, Lavoie JR. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immunomodulatory antigenic signature of the producer cell. Stem Cell Res Ther 12: 127, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomzikova MO, Kletukhina SK, Kurbangaleeva SV, Neustroeva OA, Vasileva OS, Garanina EE, Khaiboullina SF, Rizvanov AA. Mesenchymal stem cell derived biocompatible membrane vesicles demonstrate immunomodulatory activity inhibiting activation and proliferation of human mononuclear cells. Pharmaceutics 12: 577, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goni FM, Alonso A. Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta 1788: 169–177, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Gonzales E, Julien B, Serriere-Lanneau V, Nicou A, Doignon I, Lagoudakis L, Garcin I, Azoulay D, Duclos-Vallee JC, Castaing D, Samuel D, Hernandez-Garcia A, Awad SS, Combettes L, Thevananther S, Tordjmann T. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol 52: 54–62, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guan S, Yu H, Yan G, Gao M, Sun W, Zhang X. Characterization of urinary exosomes purified with size exclusion chromatography and ultracentrifugation. J Proteome Res 19: 2217–2225, 2020. [DOI] [PubMed] [Google Scholar]
- 60.Guo Q, Furuta K, Lucien F, Gutierrez Sanchez LH, Hirsova P, Krishnan A, Kabashima A, Pavelko KD, Madden B, Alhuwaish H, Gao Y, Revzin A, Ibrahim SH. Integrin beta1-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol 71: 1193–1205, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haga H, Yan IK, Borrelli DA, Matsuda A, Parasramka M, Shukla N, Lee DD, Patel T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transpl 23: 791–803, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haga H, Yan IK, Takahashi K, Matsuda A, Patel T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem Cells Transl Med 6: 1262–1272, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles 4: 24900, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han HS, Kim MJ, Han JH, Yun J, Kim HK, Yang Y, Kim KB, Park SM. Bile-derived circulating extracellular miR-30d-5p and miR-92a-3p as potential biomarkers for cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 19: 41–50, 2020. [DOI] [PubMed] [Google Scholar]
- 65.Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL, Kabanov AV, Batrakova EV. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J NeuroImmune Pharmacol 15: 487–500, 2020. [DOI] [PubMed] [Google Scholar]
- 66.Harding C, Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun 113: 650–658, 1983. [DOI] [PubMed] [Google Scholar]
- 67.Hartwig S, De Filippo E, Goddeke S, Knebel B, Kotzka J, Al-Hasani H, Roden M, Lehr S, Sell H. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim Biophys Acta, Proteins Proteomics 1867: 140172, 2019. [DOI] [PubMed] [Google Scholar]
- 68.Harwood NM, Golden-Mason L, Cheng L, Rosen HR, Mengshol JA. HCV-infected cells and differentiation increase monocyte immunoregulatory galectin-9 production. J Leukoc Biol 99: 495–503, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, Ngai SM, Chan TF, Wong N. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis 36: 1008–1018, 2015. [DOI] [PubMed] [Google Scholar]
- 70.He Y, Rodrigues RM, Wang X, Seo W, Ma J, Hwang S, Fu Y, Trojnar E, Matyas C, Zhao S, Ren R, Feng D, Pacher P, Kunos G, Gao B. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J Clin Invest 131: e141513, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med 14: 1605–1618, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology 150: 956–967, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, Gores GJ, Malhi H. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology 64: 2219–2233, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoque R, Sohail MA, Salhanick S, Malik AF, Ghani A, Robson SC, Mehal WZ. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol 302: G1171–G1179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 104: 2761–2766, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol 20: 4–11, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hyenne V, Ghoroghi S, Collot M, Bons J, Follain G, Harlepp S, Mary B, Bauer J, Mercier L, Busnelli I, Lefebvre O, Fekonja N, Garcia-Leon MJ, Machado P, Delalande F, Lopez AA, Silva SG, Verweij FJ, van Niel G, Djouad F, Peinado H, Carapito C, Klymchenko AS, Goetz JG. Studying the fate of tumor extracellular vesicles at high spatiotemporal resolution using the zebrafish embryo. Dev Cell 48: 554–572, 2019. [DOI] [PubMed] [Google Scholar]
- 79.Ibrahim SH. Sinusoidal endotheliopathy in nonalcoholic steatohepatitis: Therapeutic implications. Am J Physiol Gastrointest Liver Physiol 321: G67–G74, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, Goodfellow VS, Malhi H, Gores GJ. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 63: 731–744, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ichinohe N, Ishii M, Tanimizu N, Kon J, Yoshioka Y, Ochiya T, Mizuguchi T, Hirata K, Mitaka T. Transplantation of Thy1+ cells accelerates liver regeneration by enhancing the growth of small hepatocyte-like progenitor cells via IL17RB signaling. Stem Cells 35: 920–931, 2017. [DOI] [PubMed] [Google Scholar]
- 82.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol 7: 631–643, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of exosome composition. Cells 177: 428–445 e418, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang W, Ma P, Deng L, Liu Z, Wang X, Liu X, Long G. Hepatitis A virus structural protein pX interacts with ALIX and promotes the secretion of virions and foreign proteins through exosome-like vesicles. J Extracell Vesicles 9: 1716513, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang W, Tan Y, Cai M, Zhao T, Mao F, Zhang X, Xu W, Yan Z, Qian H, Yan Y. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int 2018: 6079642, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin Y, Wang J, Li H, Gao S, Shi R, Yang D, Wang X, Wang X, Zhu L, Wang X, Chen C, Ning K, Gao Z, Xu J, Fu Q. Extracellular vesicles secreted by human adipose-derived stem cells (hASCs) improve survival rate of rats with acute liver failure by releasing lncRNA H19. EBioMedicine 34: 231–242, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Julich-Haertel H, Urban SK, Krawczyk M, Willms A, Jankowski K, Patkowski W, Kruk B, Krasnodebski M, Ligocka J, Schwab R, Richardsen I, Schaaf S, Klein A, Gehlert S, Sanger H, Casper M, Banales JM, Schuppan D, Milkiewicz P, Lammert F, Krawczyk M, Lukacs-Kornek V, Kornek M. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol 67: 282–292, 2017. [DOI] [PubMed] [Google Scholar]
- 88.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res 57: 233–245, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kakizaki M, Yamamoto Y, Otsuka M, Kitamura K, Ito M, Kawai HD, Muramatsu M, Kagawa T, Kotani A. Extracellular vesicles secreted by HBV-infected cells modulate HBV persistence in hydrodynamic HBV transfection mouse model. J Biol Chem 295: 12449–12460, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kakizaki M, Yamamoto Y, Yabuta S, Kurosaki N, Kagawa T, Kotani A. The immunological function of extracellular vesicles in hepatitis B virus-infected hepatocytes. PLoS One 13: e0205886, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 367: eaau6977, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546: 498–503, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, Contag CH. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A 112: E1433–E1442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katsumi T, Guicciardi ME, Azad A, Bronk SF, Krishnan A, Gores GJ. Activated cholangiocytes release macrophage-polarizing extracellular vesicles bearing the DAMP S100A11. Am J Phys Cell Phys 317: C788–C799, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaur S, Singh SP, Elkahloun AG, Wu W, Abu-Asab MS, Roberts DD. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol 37: 49–59, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A 113: 170–175, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim SS, Baek GO, Ahn HR, Sung S, Seo CW, Cho HJ, Nam SW, Cheong JY, Eun JW. Serum small extracellular vesicle-derived LINC00853 as a novel diagnostic marker for early hepatocellular carcinoma. Mol Oncol 14: 2646–2659, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest 129: 4041–4049, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koeck ES, Iordanskaia T, Sevilla S, Ferrante SC, Hubal MJ, Freishtat RJ, Nadler EP. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: A novel paradigm for obesity-related liver disease. J Surg Res 192: 268–275, 2014. [DOI] [PubMed] [Google Scholar]
- 100.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: A mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology 54: 1237–1248, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, Schuppan D. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology 143: 448–458, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kostallari E, Hirsova P, Prasnicka A, Verma VK, Yaqoob U, Wongjarupong N, Roberts LR, Shah VH. Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha- enriched extracellular vesicles promote liver fibrosis in mice through SHP2. Hepatology 68: 333–348, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kostallari E, Shah VH. Pericytes in the liver. Adv Exp Med Biol 1122: 153–167, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kostallari E, Valainathan S, Biquard L, Shah VH, Rautou PE. Role of extracellular vesicles in liver diseases and their therapeutic potential. Adv Drug Deliv Rev 175: 113816, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kouwaki T, Fukushima Y, Daito T, Sanada T, Yamamoto N, Mifsud EJ, Leong CR, Tsukiyama-Kohara K, Kohara M, Matsumoto M, Seya T, Oshiumi H. Extracellular vesicles including exosomes regulate innate immune responses to hepatitis B virus infection. Front Immunol 7: 335, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kranendonk ME, Visseren FL, van Herwaarden JA, Nolte-’t Hoen EN, de Jager W, Wauben MH, Kalkhoven E. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring) 22: 2216–2223, 2014. [DOI] [PubMed] [Google Scholar]
- 107.Kuhn S, Splith K, Ballschuh C, Feldbrugge L, Krenzien F, Atanasov G, Benzing C, Hau HM, Engelmann C, Berg T, Schulte Am Esch J, Pratschke J, Robson SC, Schmelzle M. Mononuclear-cell-derived microparticles attenuate endothelial inflammation by transfer of miR-142–3p in a CD39 dependent manner. Purinergic Signal 14: 423–432, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8: 483–494, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4: 214–222, 2010. [DOI] [PubMed] [Google Scholar]
- 110.Lamichhane TN, Jay SM. Production of extracellular vesicles loaded with therapeutic cargo. Methods Mol Biol 1831: 37–47, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lapitz A, Arbelaiz A, Olaizola P, Aranburu A, Bujanda L, Perugorria MJ, Banales JM. Extracellular vesicles in hepatobiliary malignancies. Front Immunol 9: 2270, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lapitz A, Arbelaiz A, O’Rourke CJ, Lavin JL, Casta A, Ibarra C, Jimeno JP, Santos-Laso A, Izquierdo-Sanchez L, Krawczyk M, Perugorria MJ, Jimenez-Aguero R, Sanchez-Campos A, Riano I, Gonzalez E, Lammert F, Marzioni M, Macias RIR, Marin JJG, Karlsen TH, Bujanda L, Falcon- Perez JM, Andersen JB, Aransay AM, Rodrigues PM, Banales JM. Patients with cholangiocarcinoma present specific RNA profiles in serum and urine extracellular vesicles mirroring the tumor expression: Novel liquid biopsy biomarkers for disease diagnosis. Cells 9: 721, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lapitz A, Azkargorta M, Milkiewicz P, Olaizola P, Zhuravleva E, Grimsrud MM, Schramm C, Arbelaiz A, O’Rourke CJ, La Casta A, Milkiewicz M, Pastor T, Vesterhus M, Jimenez-Agüero R, Dill MT, Lamarca A, Valle JW, Macias RIR, Izquierdo-Sanchez L, Castaño YP, Caballero-Camino FJ, Riaño I, Krawczyk M, Ibarra C, Bustamante J, Nova-Camacho LM, Falcon-Perez JM, Elortza F, Perugorria MJ, Andersen JB, Bujanda L, Karlsen TH, Folseraas T, Rodrigues PM, Banales JM. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis and prognostication of cholangiocarcinoma. J Hepatol S0168-8278: 00159–9, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 19: 998–1004, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee YS, Kim SY, Ko E, Lee JH, Yi HS, Yoo YJ, Je J, Suh SJ, Jung YK, Kim JH, Seo YS, Yim HJ, Jeong WI, Yeon JE, Um SH, Byun KS. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep 7: 3710, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li B, Antonyak MA, Zhang J, Cerione RA. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31: 4740–4749, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li D, Jia S, Wang S, Hu L. Glycoproteomic analysis of urinary extracellular vesicles for biomarkers of hepatocellular carcinoma. Molecules 28: 1293, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li D, Song H, Shuo L, Wang L, Xie P, Li W, Liu J, Tong Y, Zhang CY, Jiang X, Li J, Zhang Y. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging (Albany NY) 12: 22719–22743, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J Nanobiotechnol 16: 103, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li J, Liu H, Mauer AS, Lucien F, Raiter A, Bandla H, Mounajjed T, Yin Z, Glaser KJ, Yin M, Malhi H. Characterization of cellular sources and circulating levels of extracellular vesicles in a dietary murine model of nonalcoholic steatohepatitis. Hepatol Commun 3: 1235–1249, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li L, Masica D, Ishida M, Tomuleasa C, Umegaki S, Kalloo AN, Georgiades C, Singh VK, Khashab M, Amateau S, Li Z, Okolo P, Lennon AM, Saxena P, Geschwind JF, Schlachter T, Hong K, Pawlik TM, Canto M, Law J, Sharaiha R, Weiss CR, Thuluvath P, Goggins M, Shin EJ, Peng H, Kumbhari V, Hutfless S, Zhou L, Mezey E, Meltzer SJ, Karchin R, Selaru FM. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 60: 896–907, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li L, Piontek K, Ishida M, Fausther M, Dranoff JA, Fu R, Mezey E, Gould SJ, Fordjour FK, Meltzer SJ, Sirica AE, Selaru FM. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology 65: 501–514, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li R, Wang Y, Zhang X, Feng M, Ma J, Li J, Yang X, Fang F, Xia Q, Zhang Z, Shang M, Jiang S. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer 18: 18, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 22: 845–854, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li X, Chen R, Kemper S, Brigstock DR. Extracellular vesicles from hepatocytes are therapeutic for toxin-mediated fibrosis and gene expression in the liver. Front Cell Dev Biol 7: 368, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li X, Liu R, Huang Z, Gurley EC, Wang X, Wang J, He H, Yang H, Lai G, Zhang L, Bajaj JS, White M, Pandak WM, Hylemon PB, Zhou H. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans. Hepatology 68: 599–615, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li X, Liu R, Wang Y, Zhu W, Zhao D, Wang X, Yang H, Gurley EC, Chen W, Hylemon PB, Zhou H. Cholangiocyte-derived exosomal lncRNA H19 promotes macrophage activation and hepatic inflammation under cholestatic conditions. Cells 9: 190, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liao CY, Song MJ, Gao Y, Mauer AS, Revzin A, Malhi H. Hepatocyte-derived lipotoxic extracellular vesicle sphingosine 1-phosphate induces macrophage chemotaxis. Front Immunol 9: 2980, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lim HK, Jeffrey GP, Ramm GA, Soekmadji C. Pathogenesis of viral hepatitis-induced chronic liver disease: Role of extracellular vesicles. Front Cell Infect Microbiol 10: 587628, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin MJ, Li S, Yang LJ, Ye DY, Xu LQ, Zhang X, Sun PN, Wei CJ. Plasma membrane vesicles of human umbilical cord mesenchymal stem cells ameliorate acetaminophen-induced damage in HepG2 cells: A novel stem cell therapy. Stem Cell Res Ther 11: 225, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles 4: 29509, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu DSK, Upton FM, Rees E, Limb C, Jiao LR, Krell J, Frampton AE. Size-exclusion chromatography as a technique for the investigation of novel extracellular vesicles in cancer. Cancers (Basel) 12: 3156, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu R, Li X, Zhu W, Wang Y, Zhao D, Wang X, Gurley EC, Liang G, Chen W, Lai G, Pandak WM, Robert Lippman H, Bajaj JS, Hylemon PB, Zhou H. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes hepatic stellate cell activation and cholestatic liver fibrosis. Hepatology 70: 1317–1335, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu XL, Pan Q, Cao HX, Xin FZ, Zhao ZH, Yang RX, Zeng J, Zhou H, Fan JG. Lipotoxic hepatocyte-derived exosomal microRNA 192–5p activates macrophages through rictor/Akt/forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology 72: 454–469, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu Y, Lou G, Li A, Zhang T, Qi J, Ye D, Zheng M, Chen Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 36: 140–150, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: For good or for bad. Curr Opin Cell Biol 35: 69–77, 2015. [DOI] [PubMed] [Google Scholar]
- 137.Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Moller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles 4: 27031, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, Liu Y. Exosomes derived from miR-122- modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol 8: 122, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu Y, Zhou Y, Zhang R, Wen L, Wu K, Li Y, Yao Y, Duan R, Jia Y. Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front Neurosci 13: 209, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 287: 15874–15885, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ma J, Cai W, Zhang Y, Huang C, Zhang H, Liu J, Tang K, Xu P, Katirai F, Zhang J, He W, Ye D, Shen GX, Huang B. Innate immune cell-derived microparticles facilitate hepatocarcinoma metastasis by transferring integrin αMβ2 to tumor cells. J Immunol 191: 3453–3461, 2013. [DOI] [PubMed] [Google Scholar]
- 142.Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G, Cerf-Bensussan N, Heyman M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 132: 1866–1876, 2007. [DOI] [PubMed] [Google Scholar]
- 143.Margolis L, Sadovsky Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol 17: e3000363, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, Radtke B, Splinter PL, LaRusso NF. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol 299: G990–G999, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21: 9–17, 2019. [DOI] [PubMed] [Google Scholar]
- 146.Matsumoto A, Takahashi Y, Chang HY, Wu YW, Yamamoto A, Ishihama Y, Takakura Y. Blood concentrations of small extracellular vesicles are determined by a balance between abundant secretion and rapid clearance. J Extracell Vesicles 9: 1696517, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Matsuura Y, Wada H, Eguchi H, Gotoh K, Kobayashi S, Kinoshita M, Kubo M, Hayashi K, Iwagami Y, Yamada D, Asaoka T, Noda T, Kawamoto K, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M. Exosomal miR-155 derived from hepatocellular carcinoma cells under hypoxia promotes angiogenesis in endothelial cells. Dig Dis Sci 64: 792–802, 2019. [DOI] [PubMed] [Google Scholar]
- 148.McDaniel K, Wu N, Zhou T, Huang L, Sato K, Venter J, Ceci L, Chen D, Ramos-Lorenzo S, Invernizzi P, Bernuzzi F, Wu C, Francis H, Glaser S, Alpini G, Meng F. Amelioration of ductular reaction by stem cell derived extracellular vesicles in MDR2 knockout mice via lethal-7 microRNA. Hepatology 69: 2562–2578, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, Patton JG, Weaver AM. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep 15: 978–987, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meister G Argonaute proteins: Functional insights and emerging roles. Nat Rev Genet 14: 447–459, 2013. [DOI] [PubMed] [Google Scholar]
- 151.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 26: 707–721, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Menck K, Sonmezer C, Worst TS, Schulz M, Dihazi GH, Streit F, Erdmann G, Kling S, Boutros M, Binder C, Gross JC. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles 6: 1378056, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M, Dobrinskikh E, Busson P, Polyak SJ, Hirashima M, Rosen HR. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One 5: e9504, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meyer J, Balaphas A, Fontana P, Morel P, Robson SC, Sadoul K, Gonelle-Gispert C, Buhler L. Platelet interactions with liver sinusoidal endothelial cells and hepatic stellate cells lead to hepatocyte proliferation. Cells 9: 1243, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Minciacchi VR, You S, Spinelli C, Morley S, Zandian M, Aspuria PJ, Cavallini L, Ciardiello C, Reis Sobreiro M, Morello M, Kharmate G, Jang SC, Kim DK, Hosseini-Beheshti E, Tomlinson Guns E, Gleave M, Gho YS, Mathivanan S, Yang W, Freeman MR, Di Vizio D. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 6: 11327–11341, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett 147: 47–54, 2012. [DOI] [PubMed] [Google Scholar]
- 157.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119: 756–766, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moreno-Gonzalo O, Fernandez-Delgado I, Sanchez-Madrid F. Post-translational add- ons mark the path in exosomal protein sorting. Cell Mol Life Sci 75: 1–19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3: 24641, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Murakami Y, Toyoda H, Tanahashi T, Tanaka J, Kumada T, Yoshioka Y, Kosaka N, Ochiya T, Taguchi YH. Comprehensive miRNA expression analysis in peripheral blood can diagnose liver disease. PLoS One 7: e48366, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain- containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A 109: 4146–4151, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nakao Y, Amrollahi P, Parthasarathy G, Mauer AS, Sehrawat TS, Vanderboom P, Nair KS, Nakao K, Allen AM, Hu TY, Malhi H. Circulating extracellular vesicles are a biomarker for NAFLD resolution and response to weight loss surgery. Nanomedicine 36: 102430, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol 64: 60–68, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Obata Y, Kita S, Koyama Y, Fukuda S, Takeda H, Takahashi M, Fujishima Y, Nagao H, Masuda S, Tanaka Y, Nakamura Y, Nishizawa H, Funahashi T, Ranscht B, Izumi Y, Bamba T, Fukusaki E, Hanayama R, Shimada S, Maeda N, Shimomura I. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 3: e99680, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21: 185–191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12: 19–30; sup pp. 11–13, 2010. [DOI] [PubMed] [Google Scholar]
- 167.Ouyang Y, Tang Y, Fu L, Peng S, Wu W, Tan D, Fu X. Exosomes secreted by chronic hepatitis B patients with PNALT and liver inflammation grade >/= A2 promoted the progression of liver cancer by transferring miR-25–3p to inhibit the co-expression of TCF21 and HHIP. Cell Prolif 53: e12833, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cells 33: 967–978, 1983. [DOI] [PubMed] [Google Scholar]
- 169.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 284: 34211–34222, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pascucci L, Cocce V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Vigano L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J Control Release 192: 262–270, 2014. [DOI] [PubMed] [Google Scholar]
- 171.Peng Z, Bu J, McLuckey SA. The generation of dehydroalanine residues in protonated polypeptides: Ion/ion reactions for introducing selective cleavages. J Am Soc Mass Spectrom 28: 1765–1774, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pi F, Binzel DW, Lee TJ, Li Z, Sun M, Rychahou P, Li H, Haque F, Wang S, Croce CM, Guo B, Evers BM, Guo P. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat Nanotechnol 13: 82–89, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Piccin A, Murphy WG, Smith OP. Circulating microparticles: Pathophysiology and clinical implications. Blood Rev 21: 157–171, 2007. [DOI] [PubMed] [Google Scholar]
- 174.Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L, Pinatel EM, Alisi A, Nobili V, Feldstein AE. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cell Mol Gastroenterol Hepatol 1: 646–663 e644, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Povero D, Yamashita H, Ren W, Subramanian MG, Myers RP, Eguchi A, Simonetto DA, Goodman ZD, Harrison SA, Sanyal AJ, Bosch J, Feldstein AE. Characterization and proteome of circulating extracellular vesicles as potential biomarkers for NASH. Hepatol Commun 4: 1263–1278, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pranatharthiharan S, Patel MD, Malshe VC, Pujari V, Gorakshakar A, Madkaikar M, Ghosh K, Devarajan PV. Asialoglycoprotein receptor targeted delivery of doxorubicin nanoparticles for hepatocellular carcinoma. Drug Deliv 24: 20–29, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183: 1161–1172, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20: 847–856, 2006. [DOI] [PubMed] [Google Scholar]
- 179.Rimassa L, Personeni N, Aghemo A, Lleo A. The immune milieu of cholangiocarcinoma: From molecular pathogenesis to precision medicine. J Autoimmun 100: 17–26, 2019. [DOI] [PubMed] [Google Scholar]
- 180.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14: 195–208, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol 185: 1209–1225, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G, Altevogt P. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol 107: 563–571, 2007. [DOI] [PubMed] [Google Scholar]
- 183.Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, Battistelli C, Alonzi T, Weisz A, Tripodi M. The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell Rep 17: 799–808, 2016. [DOI] [PubMed] [Google Scholar]
- 184.Schmelzle M, Splith K, Andersen LW, Kornek M, Schuppan D, Jones-Bamman C, Nowak M, Toxavidis V, Salhanick SD, Han L, Esch JS, Jonas S, Donnino MW, Robson SC. Increased plasma levels of microparticles expressing CD39 and CD133 in acute liver injury. Transplantation 95: 63–69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol 179: 1489–1496, 2007. [DOI] [PubMed] [Google Scholar]
- 186.Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Thery C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 106: 216–223, 2005. [DOI] [PubMed] [Google Scholar]
- 187.Sehrawat TS, Arab JP, Liu M, Amrollahi P, Wan M, Fan J, Nakao Y, Pose E, Navarro- Corcuera A, Dasgupta D, Liao CY, He L, Mauer AS, Avitabile E, Ventura-Cots M, Bataller RA, Sanyal AJ, Chalasani NP, Heimbach JK, Watt KD, Gores GJ, Gines P, Kamath PS, Simonetto DA, Hu TY, Shah VH, Malhi H. Circulating extracellular vesicles carrying sphingolipid cargo for the diagnosis and dynamic risk profiling of alcoholic hepatitis. Hepatology 73: 571–585, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Severino V, Dumonceau JM, Delhaye M, Moll S, Annessi-Ramseyer I, Robin X, Frossard JL, Farina A. Extracellular vesicles in bile as markers of malignant biliary stenoses. Gastroenterology 153: 495–504 e498, 2017. [DOI] [PubMed] [Google Scholar]
- 189.Shen L, Chen G, Xia Q, Shao S, Fang H. Exosomal miR-200 family as serum biomarkers for early detection and prognostic prediction of cholangiocarcinoma. Int J Clin Exp Pathol 12: 3870–3876, 2019. [PMC free article] [PubMed] [Google Scholar]
- 190.Shi G, Wang Y, Wang Z, Thoreson AR, Jacobson DS, Amadio PC, Behfar A, Moran SL, Zhao C. A novel engineered purified exosome product patch for tendon healing: An explant in an ex vivo model. J Orthop Res 39 (8): 1825–1837, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shi M, Jiang Y, Yang L, Yan S, Wang YG, Lu XJ. Decreased levels of serum exosomal miR-638 predict poor prognosis in hepatocellular carcinoma. J Cell Biochem 119 (6): 4711–4716, 2018. [DOI] [PubMed] [Google Scholar]
- 192.Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. elife 5: e19276, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: Clinical implications and therapeutic targeting. Hepatology 59: 2397–2402, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Song P, Trajkovic K, Tsunemi T, Krainc D. Parkin modulates endosomal organization and function of the endo-lysosomal pathway. J Neurosci 36: 2425–2437, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Song XJ, Zhang L, Li Q, Li Y, Ding FH, Li X. hUCB-MSC derived exosomal miR-124 promotes rat liver regeneration after partial hepatectomy via downregulating Foxg1. Life Sci 265: 118821, 2021. [DOI] [PubMed] [Google Scholar]
- 196.Stahl PD, Raposo G. Extracellular vesicles: Exosomes and microvesicles, integrators of homeostasis. Physiology (Bethesda) 34: 169–177, 2019. [DOI] [PubMed] [Google Scholar]
- 197.Stam J, Bartel S, Bischoff R, Wolters JC. Isolation of extracellular vesicles with combined enrichment methods. J Chromatogr B Anal Technol Biomed Life Sci 1169: 122604, 2021. [DOI] [PubMed] [Google Scholar]
- 198.Stegmayr B, Ronquist G. Promotive effect on human sperm progressive motility by proteasomes. Urol Res 10: 253–257, 1982. [DOI] [PubMed] [Google Scholar]
- 199.Stranska R, Gysbrechts L, Wouters J, Vermeersch P, Bloch K, Dierickx D, Andrei G, Snoeck R. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J Transl Med 16: 1, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sun N, Lee YT, Zhang RY, Kao R, Teng PC, Yang Y, Yang P, Wang JJ, Smalley M, Chen PJ, Kim M, Chou SJ, Bao L, Wang J, Zhang X, Qi D, Palomique J, Nissen N, Han SB, Sadeghi S, Finn RS, Saab S, Busuttil RW, Markovic D, Elashoff D, Yu HH, Li H, Heaney AP, Posadas E, You S, Yang JD, Pei R, Agopian VG, Tseng HR, Zhu Y. Purification of HCC-specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat Commun 11: 4489, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sun N, Zhang C, Lee YT, Tran BV, Wang J, Kim H, Lee J, Zhang RY, Wang JJ, Hu J, Zhang Z, Alsudaney MS, Hou KC, Tang H, Zhang TX, Liang IY, Zhou Z, Chen M, Yeh AH, Li W, Zhou XJ, Chang HR, Han SB, Sadeghi S, Finn RS, Saab S, Busuttil RW, Noureddin M, Ayoub WS, Kuo A, Sundaram V, Al-Ghaieb B, Palomique J, Kosari K, Kim IK, Todo T, Nissen NN, Tomasi ML, You S, Posadas EM, Wu JX, Wadehra M, Sim MS, Li Y, Wang HL, French SW, Lu SC, Wu L, Pei R, Liang L, Yang JD, Agopian VG, Tseng HR, Zhu Y. HCC EV ECG score: An extracellular vesicle-based protein assay for detection of early-stage hepatocellular carcinoma. Hepatology 77: 774–788, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci 127: 1585–1594, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, Wada K, Nagai Y. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc Natl Acad Sci U S A 112: E2497–E2506, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tamura R, Uemoto S, Tabata Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm Regen 36: 26, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tang J, Li Y, Liu K, Zhu Q, Yang WH, Xiong LK, Guo DL. Exosomal miR-9–3p suppresses HBGF-5 expression and is a functional biomarker in hepatocellular carcinoma. Minerva Med 109: 15–23, 2018. [DOI] [PubMed] [Google Scholar]
- 206.Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics 12: 587–598, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 3: 3–22. Chapter 3: Unit 3. 22, 2006. [DOI] [PubMed] [Google Scholar]
- 208.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3: 1156–1162, 2002. [DOI] [PubMed] [Google Scholar]
- 209.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542: 450–455, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35: 2383–2390, 2014. [DOI] [PubMed] [Google Scholar]
- 212.Tkach M, Kowal J, Zucchetti AE, Enserink L, Jouve M, Lankar D, Saitakis M, Martin-Jaular L, Thery C. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J 36: 3012–3028, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247, 2008. [DOI] [PubMed] [Google Scholar]
- 214.Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ectoenzyme in the form of micro-vesicles. Biochim Biophys Acta 645: 63–70, 1981. [DOI] [PubMed] [Google Scholar]
- 215.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. [DOI] [PubMed] [Google Scholar]
- 216.Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles 3: 24858, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213–228, 2018. [DOI] [PubMed] [Google Scholar]
- 218.Veerman RE, Teeuwen L, Czarnewski P, Gucluler Akpinar G, Sandberg A, Cao X, Pernemalm M, Orre LM, Gabrielsson S, Eldh M. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J Extracell Vesicles 10: e12128, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vergauwen G, Dhondt B, Van Deun J, De Smedt E, Berx G, Timmerman E, Gevaert K, Miinalainen I, Cocquyt V, Braems G, Van den Broecke R, Denys H, De Wever O, Hendrix A. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci Rep 7: 2704, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Verweij FJ, Bebelman MP, Jimenez CR, Garcia-Vallejo JJ, Janssen H, Neefjes J, Knol JC, de Goeij-de Haas R, Piersma SR, Baglio SR, Verhage M, Middeldorp JM, Zomer A, van Rheenen J, Coppolino MG, Hurbain I, Raposo G, Smit MJ, Toonen RFG, van Niel G, and Pegtel DM. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J Cell Biol 217: 1129–1142, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Verweij FJ, Revenu C, Arras G, Dingli F, Loew D, Pegtel DM, Follain G, Allio G, Goetz JG, Zimmermann P, Herbomel P, Del Bene F, Raposo G, van Niel G. Live tracking of inter-organ communication by endogenous exosomes in vivo. Dev Cell 48: 573–589, 2019. [DOI] [PubMed] [Google Scholar]
- 222.Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol 14: 713–722, 2002. [DOI] [PubMed] [Google Scholar]
- 223.Wang R, Ding Q, Yaqoob U, de Assuncao TM, Verma VK, Hirsova P, Cao S, Mukhopadhyay D, Huebert RC, Shah VH. Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1-phosphate-dependent migration. J Biol Chem 290: 30684–30696, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin HM, Zhou R, Shang CZ, Cao J, He H, Han QF, Liu PQ, Zhou G, Min J. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology 61: 1284–1294, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wei Z, Zhang X, Yong T, Bie N, Zhan G, Li X, Liang Q, Li J, Yu J, Huang G, Yan Y, Zhang Z, Zhang B, Gan L, Huang B, Yang X. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun 12: 440, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Welsh JA, Scorletti E, Clough GF, Englyst NA, Byrne CD. Leukocyte extracellular vesicle concentration is inversely associated with liver fibrosis severity in NAFLD. J Leukoc Biol 104: 631–639, 2018. [DOI] [PubMed] [Google Scholar]
- 227.Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4: 26316, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte’t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2, 2013. DOI: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xiao Y, Liu R, Li X, Gurley EC, Hylemon PB, Lu Y, Zhou H, Cai W. Long noncoding RNA H19 contributes to cholangiocyte proliferation and cholestatic liver fibrosis in biliary atresia. Hepatology 70: 1658–1673, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: Toward clinical application. J Clin Invest 126: 1152–1162, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yan C, Tian X, Li J, Liu D, Ye D, Xie Z, Han Y, Zou MH. A high-fat diet attenuates AMPK alpha1 in adipocytes to induce exosome shedding and nonalcoholic fatty liver development in vivo. Diabetes 70: 577–588, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol 2011: 842849, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yang MQ, Du Q, Goswami J, Varley PR, Chen B, Wang RH, Morelli AE, Stolz DB, Billiar TR, Li J, Geller DA. Interferon regulatory factor 1-Rab27a regulated extracellular vesicles promote liver ischemia/reperfusion injury. Hepatology 67: 1056–1070, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol 14: 465–475, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, Zhang T, Cao Y, Pan H, Zhang L, Wang G, Deng Y, Yang Y, Chen G. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1(+) regulatory B cell expansion. J Immunother Cancer 6: 145, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cells 171: 372–384 e312, 2017. [DOI] [PubMed] [Google Scholar]
- 237.Yochum ZA, Cades J, Mazzacurati L, Neumann NM, Khetarpal SK, Chatterjee S, Wang H, Attar MA, Huang EH, Chatley SN, Nugent K, Somasundaram A, Engh JA, Ewald AJ, Cho YJ, Rudin CM, Tran PT, Burns TF. A first-in-class TWIST1 inhibitor with activity in oncogene-driven lung cancer. Mol Cancer Res 15: 1764–1776, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yukawa H, Suzuki K, Aoki K, Arimoto T, Yasui T, Kaji N, Ishikawa T, Ochiya T, Baba Y. Imaging of angiogenesis of human umbilical vein endothelial cells by uptake of exosomes secreted from hepatocellular carcinoma cells. Sci Rep 8: 6765, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang G, Huang X, Xiu H, Sun Y, Chen J, Cheng G, Song Z, Peng Y, Shen Y, Wang J, Cai Z. Extracellular vesicles: Natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J Extracell Vesicles 10: e12030, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S, Hoshino A, Matei I, Kenific CM, Nakajima M, Mutvei AP, Sansone P, Buehring W, Wang H, Jimenez JP, Cohen-Gould L, Paknejad N, Brendel M, Manova- Todorova K, Magalhaes A, Ferreira JA, Osorio H, Silva AM, Massey A, Cubillos-Ruiz JR, Galletti G, Giannakakou P, Cuervo AM, Blenis J, Schwartz R, Brady MS, Peinado H, Bromberg J, Matsui H, Reis CA, Lyden D. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20: 332–343, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang Q, Higginbotham JN, Jeppesen DK, Yang YP, Li W, McKinley ET, Graves-Deal R, Ping J, Britain CM, Dorsett KA, Hartman CL, Ford DA, Allen RM, Vickers KC, Liu Q, Franklin JL, Bellis SL, Coffey RJ. Transfer of functional cargo in exomeres. Cell Rep 27: 940–954 e946, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang S, Jiang L, Hu H, Wang H, Wang X, Jiang J, Ma Y, Yang J, Hou Y, Xie D, Zhang Q. Pretreatment of exosomes derived from hUCMSCs with TNF-alpha ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci 246: 117401, 2020. [DOI] [PubMed] [Google Scholar]
- 243.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg 122: 856–867, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 39: 133–144, 2010. [DOI] [PubMed] [Google Scholar]
- 245.Zhang Z, Shang J, Yang Q, Dai Z, Liang Y, Lai C, Feng T, Zhong D, Zou H, Sun L, Su Y, Yan S, Chen J, Yao Y, Shi Y, Huang X. Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J Nanobiotechnol 21: 29, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182- regulated macrophage polarization. Cardiovasc Res 115: 1205–1216, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhao Y, Zhao MF, Jiang S, Wu J, Liu J, Yuan XW, Shen D, Zhang JZ, Zhou N, He J, Fang L, Sun XT, Xue B, Li CJ. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat Commun 11: 719, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D, Feng W, McClain CJ, Zhang HG. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles 4: 28713, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SIJ, Wurdinger T, Pegtel DM, van Rheenen J. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cells 161: 1046–1057, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


