Abstract
The prognostic value of preoperative white blood cell to hemoglobin ratio (WHR) and fibrinogen to albumin ratio (FAR) in colorectal cancer (CRC) is unknown. The purpose of this study was to analyze the correlation between preoperative WHR and FAR and the prognosis of CRC patients. The retrospective study analyzed the medical records of 207 patients with colorectal cancer who were admitted to Linyi People’s Hospital between June 1, 2017 and June 1, 2021. The receiver operator curve was used to determine the cutoff value of 4.604 for WHR and 0.086 for FAR, and the patients were divided into high and low groups for comparative analysis of clinical data. Cox proportional hazards regression models were used to assess independent risk factors for disease-free survival (DFS) and overall survival (OS) in univariate and multifactorial analyses. Kaplan–Meier methods were used for survival analysis and logrank tests were used to assess survival differences. Multifactorial Cox analysis showed that tumor pathological stage (HR = 6.224, 95% CI:3.063–12.647, P < .001), and WHR (HR = 3.681, 95% CI:1.768–7.401, P < .001) were the independent risk factors for DFS in CRC patients. Tumor pathological stage (HR = 4.080, 95% CI:1.992–8.360, P < .001), and WHR (HR = 3.397, 95% CI:1.662–6.940, P = .001) were independent risk factors for OS. High levels of WHR and high levels of FAR were associated with lower DFS (P < .001) and OS (P < .001).CRC patients with both higher WHR and FAR had significantly lower DFS (P < .001) and OS (P < .001). DFS and OS may be shorter in CRC patients with high WHR and high FAR, perhaps associated with poor prognosis in CRC patients, and WHR and FAR may be potential CRC prognostic markers.
Keywords: colorectal cancer, fibrinogen to albumin ratio, prognosis, survival, white blood cell to hemoglobin ratio
1. Introduction
Today, colorectal cancer (CRC) is the third most common malignancy worldwide and the second leading cause of cancer-related death.[1] In recent years, CRC morbidity and mortality have decreased in some developed countries in Europe and the United States; in contrast, CRC morbidity and mortality continue to increase in China.[2] However, colorectal cancer progresses slowly, from colorectal adenoma to colorectal cancer in the whole process of about 10–15 years,[3] so early screening of colorectal cancer becomes especially important. It has been demonstrated that patients with CRC diagnosed by screening or surveillance have a significantly better prognosis compared to symptomatic patients.[4] Endoscopy is the gold standard for monitoring the progression of colorectal cancer. However, because it is an invasive test, some patients may not tolerate it and it is costly, increasing the financial burden of patients. Therefore, we need intuitive, convenient, and economical indicators to predict the survival prognosis of CRC patients for patients to choose more active and effective treatment.
A growing number of studies have shown that inflammation plays a key role in tumorigenesis and metastasis through multiple mechanisms.[5,6] Inflammatory markers can assess the prognosis of tumors after surgery and are prognostic factors for tumors.[7] Many inflammatory mediator-based neogenetic markers, such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR), can be used to evaluate the prognosis of cancer patients and have attracted widespread interest because they are intuitive, readily available, and inexpensive markers.[8,9] Recently it has been shown that preoperative white blood cells to hemoglobin ratio (WHR) are related to poor prognosis in patients with hepatocellular carcinoma after hepatectomy.[10] Meanwhile, preoperative fibrinogen to albumin ratio (FAR) has been shown to be associated with poor prognosis in a variety of cancers.[11–14] However, the effects of preoperative white blood cell to hemoglobin ratio (WHR) and fibrinogen to albumin ratio (FAR) on CRC patients have not been fully elucidated. Therefore, the aim of this study was to investigate the effect of preoperative WHR and FAR on the prognosis of CRC patients.
2. Methods
This retrospective study analyzed medical records of patients diagnosed with colorectal cancer and who underwent radical resection of colorectal cancer at Linyi People’s Hospital between June 1, 2017 and June 1, 2021. The inclusion criteria were as follows: 1. Colorectal cancer was confirmed by colonoscopic pathology. 2. Radical resection of colorectal cancer. 3. Age > 18 years. 4. Preoperative blood tests were performed 1 week before surgery. 5. No previous antitumor therapy 6. Diseases that do not affect blood indicators such as blood system diseases, autoimmune diseases. The exclusion criteria were: 1. Prior or concurrent other tumor diseases 2. Preoperative neoadjuvant radiotherapy and chemotherapy 3. Tumor complicated with infection 4. Preoperative hormone therapy 5. Severe dysfunction of liver and kidney function. All patients underwent laparoscopic R0 resection. In rectal cancer, after resection of the tumor, the anastomosis is located low and close to the anus, as well as in colorectal cancer, the intestinal dilatation and edema are obvious, and their own general condition is very poor, we usually perform protective loop ileostomy. All patients were clinically staged according to the Union for International Cancer Control Colorectal Cancer 2017 8th edition TNM method. All patients with stage IV disease may undergo surgery if the tumor is resectable. In addition neoadjuvant chemotherapy and radiotherapy are required after surgery. This study was approved by the Medical Ethics Committee, Linyi People Hospital with the waiver of informed written consent in view of the retrospective nature of the study.
2.1. Follow-up of patients
After surgery, patients were followed up every 3 months for 2 years, every 6 months for 2 to 5 years, and annually after 5 years. Typically, the postoperative complications that we have observed include anastomotic fistula and intestinal obstruction. Anastomotic fistulas are treated according to grades A, B, and C. Grade A fistulas can be recovered with dietary control; grade B fistulas require interventions that include parenteral nutritional support, anti-inflammation, and adequate local drainage; and grade C fistulas require surgical intervention. Intestinal obstruction in most patients can be improved by dietary modifications such as fasting and eating smaller meals. A small percentage require surgical intervention. Postoperative adjuvant therapy such as chemotherapy and radiotherapy according to the pathological stage. If the tumor recurs, it needs to be evaluated whether it can be palliated by radiotherapy as well as chemotherapy and whether it can be removed by surgery. Adjuvant treatment regimens and relapse management were uniform throughout the study period. DFS (disease-free survival) is defined as the time from surgery to local recurrence or distant metastasis. OS (overall survival) is the time from first diagnosis to death from any cause.
2.2. Definition
The white blood cell to hemoglobin ratio (WHR) and fibrinogen to albumin ratio (FAR) were calculated respectively according to the following equations: (white blood cell count [number/mm3])/(10*hemoglobin level [g/L]),[15] plasma fibrinogen level (g/L)/plasma albumin level (g/L).
2.3. Statistical analysis
SPSS 26.0 statistical software was used for data analysis. The receiver operator curve was used to calculate the Youden index to determine the optimum cutoff values of WHR and FAR for survival analysis. Pearson χ2 test was used to verify the relationship between WHR, FAR, and baseline clinical characteristics of CRC patients. DFS and OS were calculated according to Kaplan–Meier survival analysis and compared using log-rank test. Cox proportional hazards regression model was used to evaluate the independent risk factors for DFS and OS. P < .05 was considered statistically significant.
3. Results
3.1. Baseline clinical characteristics of patients
As shown in Table 1, a total of 207 CRC patients were included in this study. The median follow-up time was 45 (25–62) months. Among them, 112 cases were male and 95 cases were female. Age > 65 years accounted for 52.7%. BMI > 24 accounted for 37.7%. Among them, colon cancer was 42.0% and rectal cancer was 58.0%. Tumor pathology TNM stage I–IV accounted for 15.5%, 36.2%, 41.5%, and 6.8%, respectively. CEA > 5 was 38.6%.CA19-9 > 30 was 15.9%. There were 82 cases (39.6%) with WHR ≤ 4.604 and 125 cases (60.4%) with WHR > 4.604. FAR ≤ 0.086 was 119 cases (57.5%) and FAR > 0.086 was 88 cases (42.5%).
Table 1.
Clinicopathological variables of patients with CRC.
| Variables | n | % |
|---|---|---|
| Gender | ||
| Male | 112 | 54.1 |
| Female | 95 | 45.9 |
| Age | ||
| ≤65 | 98 | 47.3 |
| >65 | 109 | 52.7 |
| BMI | ||
| ≤24 | 129 | 62.3 |
| >24 | 78 | 37.7 |
| Tumor site | ||
| Right colon | 52 | 25.1 |
| Transvers colon | 18 | 8.7 |
| Left colon | 17 | 8.2 |
| Rectum | 120 | 58.0 |
| Protective loop ileostomy | ||
| Yes | 101 | 48.8 |
| No | 106 | 51.2 |
| Pathological stage | ||
| I | 32 | 15.5 |
| II | 75 | 36.2 |
| III | 86 | 41.5 |
| IV | 14 | 6.8 |
| CEA | ||
| ≤5 | 127 | 61.4 |
| >5 | 80 | 38.6 |
| CA19-9 | ||
| ≤30 | 174 | 84.1 |
| >30 | 33 | 15.9 |
| WHR | ||
| ≤4.604 | 82 | 39.6 |
| >4.604 | 125 | 60.4 |
| FAR | ||
| ≤0.086 | 119 | 57.5 |
| >0.086 | 88 | 42.5 |
3.2. Association between WHR, FAR, and baseline clinical characteristics of CRC patients
As shown in Table 2, there were no significant differences between the low and high WHR groups in terms of gender (P = .059), age (P = .535), BMI (P = .578), tumor location (P = .199), pathological stage (P = .457), CEA (P = .622), and CA19-9 (P = .719). For FAR, only gender (P = .032) differences were statistically significant.
Table 2.
Relationship between WHR, FAR, and baseline clinical characteristics of CRC patients.
| Variables | WHR ≤ 4.604 | WHR > 4.604 |
P value |
FAR ≤ 0.086 | FAR > 0.086 |
P value |
|---|---|---|---|---|---|---|
| n = 82 (%) | n = 125 (%) | n = 119 (%) | n = 88 (%) | |||
| Gender | .059 | .032 | ||||
| Male | 51 (62.2) | 61 (48.8) | 72 (60.5) | 40 (45.5) | ||
| Female | 31 (37.8) | 64 (51.2) | 47 (39.5) | 48 (54.5) | ||
| Age | .535 | .852 | ||||
| ≤65 | 41 (50.0) | 57 (45.6) | 57 (47.9) | 41 (46.6) | ||
| >65 | 41 (50.0) | 68 (54.4) | 62 (52.1) | 47 (53.4) | ||
| BMI | .578 | .807 | ||||
| ≤24 | 53 (64.6) | 76 (60.8) | 75 (63.0) | 54 (61.4) | ||
| >24 | 29 (35.4) | 49 (39.2) | 44 (37.0) | 34 (38.6) | ||
| Tumor site | .263 | .412 | ||||
| Right colon + transvers colon | 24 (29.3) | 46 (36.8) | 43 (36.1) | 27 (30.7) | ||
| Left colon + rectum | 58 (70.7) | 79 (63.2) | 76 (63.9) | 61 (69.3) | ||
| Protective loop ileostomy | .650 | .192 | ||||
| Yes | 42 (51.2) | 60 (48.0) | 54 (45.4) | 48 (54.5) | ||
| No | 40 (48.8) | 65 (52.0) | 65 (54.6) | 40 (45.5) | ||
| Pathological stage | .457 | .671 | ||||
| I + II | 45 (54.9) | 62 (49.6) | 60 (50.4) | 47 (53.4) | ||
| III + IV | 37 (45.1) | 63 (50.4) | 59 (49.6) | 41 (46.6) | ||
| CEA | .622 | .771 | ||||
| ≤5 | 52 (63.4) | 75 (60.0) | 72 (60.5) | 55 (62.5) | ||
| >5 | 30 (36.6) | 50 (40.0) | 47 (39.5) | 33 (37.5) | ||
| CA19-9 | .719 | .436 | ||||
| ≤30 | 68 (82.9) | 106 (84.8) | 98 (82.4) | 76 (86.4) | ||
| >30 | 14 (17.1) | 19 (15.2) | 21 (17.6) | 12 (13.6) |
3.3. WHR and FAR cutoff value
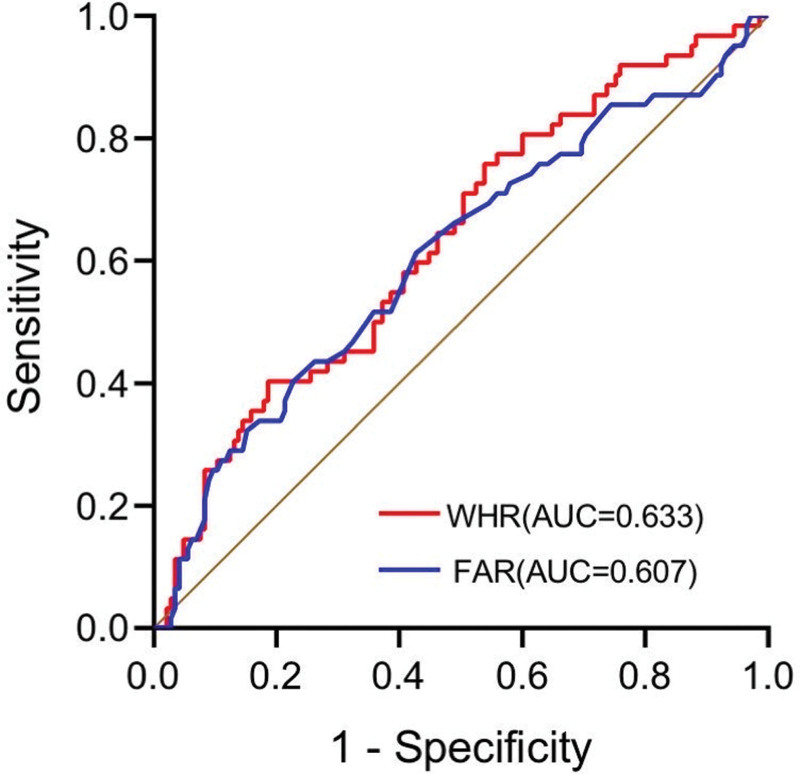
The receiver operator curves of WHR and FAR are shown in Figure 1. According to the Youden index, the optimal cutoff value of WHR was 4.604, and the optimal cutoff value of FAR was 0.086. It was divided into 2 groups, high and low, according to the optimal cutoff value.
Figure 1.
ROC curves of WHR, FAR. FAR = fibrinogen to albumin ratio, ROC = receiver operator curve, WHR=white blood cell to hemoglobin ratio.
3.4. Kaplan–Meier survival analysis
Kaplan–Meier survival curves of WHR and FAR on DFS and OS of CRC patients are shown in Figure 2. The 5-year DFS rate was 13.6% and 5-year OS rate was 4.1% in the high-WHR group, and the 5-year DFS rate was 79.3% and 5-year OS rate was 75.0% in the low-WHR group, and the DFS (P < .001, Fig. 2A) and OS (P < .001, Fig. 2B) were significantly lower in the high-WHR group than in the low-WHR group. The 5-year DFS rate was 36.3% and 5-year OS rate was 0.0% in the high-FAR group, and the 5-year DFS rate was 70.1% and 5-year OS rate was 49.0% in the low FAR group. Similarly, the 5-year DFS rate (P < .001, Fig. 2C) and 5-year OS rate (P < .001, Fig. 2D) were significantly lower in the high-FAR group than in the low FAR group. This implies that high WHR, FAR may be connected with poor prognosis in CRC patients. Our objective was to exploit the role of preoperative WHR combined with FAR in the prognosis of patients with CRC. Therefore, we divided the patients into 4 groups: 1. WHR ≤ 4.604 and FAR ≤ 0.086 (n = 76); 2. WHR ≤ 4.604 and FAR > 0.086 (n = 6); 3. WHR > 4.604 and FAR ≤ 0.086 (n = 43); 4. WHR > 4.604 and FAR > 0.086 (n = 82). The final results showed that patients with both higher WHR and FAR had shorter DFS (P < .001, Fig. 3A) and OS (P < .001, Fig. 3B) than patients in other subgroups.
Figure 2.
Kaplan–Meier analysis curves of WHR and FAR on DFS and OS in colorectal cancer patients.(A) Kaplan–Meier analysis curve of WHR on DFS in colorectal cancer patients. (B) Kaplan–Meier analysis curve of WHR on OS in colorectal cancer patients. (C) Kaplan–Meier analysis curve of FAR on DFS in colorectal cancer patients. (D) Kaplan–Meier analysis curve of FAR on OS in colorectal cancer patients. DFS = disease-free survival, FAR = fibrinogen to albumin ratio, OS = overall survival, ROC = receiver operator curve, WHR=white blood cell to hemoglobin ratio.
Figure 3.
Kaplan–Meier analysis curves for DFS (A) and OS (B) after WHR combined with FAR. DFS = disease-free survival, FAR = fibrinogen to albumin ratio, OS = overall survival, WHR=white blood cell to hemoglobin ratio.
3.5. Univariate and multivariate analyses of DFS and OS
Analysis of the univariate Cox proportional risk regression model showed that tumor pathological stage (HR = 6.266, 95% CI: 3.090–12.706, P < .001), WHR (HR = 4.361, 95% CI: 2.337–8.138, P < .001), and FAR (HR = 2.532, 95% CI: 1.478–4.336, P = .001) were risk factors for DFS. In addition, gender, age, BMI, tumor location, protective loop ileostomy, CEA, CA19-9, intestinal obstruction, diabetes, hypertension were, NLR, LMR, and PLR were not significantly associated with DFS (Table 3). Meanwhile, tumor pathological stage (HR = 4.976, 95% CI: 2.450–10.109, P < .001), WHR (HR = 5.503, 95% CI: 2.997–10.106, P < .001), and FAR (HR = 3.832, 95% CI: 2.193–6.696, P < .001) were associated with OS and were risk factors for OS, while male (HR = 0.597, 95% CI: 0.359–0.993, P = .047), LMR (HR = 2.185, 95% CI: 1.285–3.717, P = .004) were protective factors in OS (Table 3).
Table 3.
Univariate survival analysis associated with DFS and OS.
| Variables | DFS | OS | ||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 0.666 (0.404–1.097) | .11 | 0.597 (0.359–0.993) | .047 |
| Age | ||||
| ≤65 | 1 | 1 | ||
| >65 | 1.043 (0.633–1.719) | 0.868 | 0.966 (0.586–1.594) | 0.893 |
| BMI | ||||
| ≤24 | 1 | 1 | ||
| >24 | 1.106 (0.666–1.838) | 0.696 | 1.054 (0.634–1.752) | 0.839 |
| Tumor site | ||||
| Right colon + transvers colon | 1 | 1 | ||
| Left colon + rectum | 0.717 (0.434–1.185) | 0.194 | 0.804 (0.486–1.331) | 0.397 |
| Protective loop ileostomy | ||||
| No | 1 | 1 | ||
| Yes | 0.779 (0.467–1.298) | 0.338 | 0.786 (0.471–1.311) | 0.357 |
| Pathological stage | ||||
| I + II | 1 | 1 | ||
| III + IV | 6.266 (3.090–12.706) | <0.001 | 4.976 (2.450–10.109) | <0.001 |
| CEA | ||||
| ≤5 | 1 | 1 | ||
| >5 | 1.575 (0.957–2.592) | 0.074 | 1.368 (0.827–2.262) | 0.222 |
| CA19-9 | ||||
| ≤30 | 1 | 1 | ||
| >30 | 1.575 (0.868–2.857) | 0.135 | 1.445 (0.796–2.622) | 0.226 |
| Anastomotic fistula | ||||
| No | 1 | 1 | ||
| Yes | 1.182 (0.710–1.967) | 0.52 | 1.434 (0.822–2.503) | 0.204 |
| Intestinal obstruction | ||||
| No | 1 | |||
| Yes | 1.393 (0.845–2.297) | 0.193 | 1.511 (0.913–2.502) | 0.108 |
| Diabetes | ||||
| No | 1 | 1 | ||
| Yes | 1.106 (0.666–1.838) | 0.696 | 1.054 (0.634–1.752) | 0.839 |
| Hypertension | ||||
| No | 1 | 1 | ||
| Yes | 1.106 (0.666–1.838) | 0.696 | 1.054 (0.634–1.752) | 0.839 |
| NLR | ||||
| ≤3.91 | 1 | 1 | ||
| >3.91 | 1.336 (0.804–2.220) | 0.264 | 1.441 (0.866–2.396) | 0.16 |
| LMR | ||||
| >3.89 | 1 | 1 | ||
| ≤3.89 | 1.495 (0.894–2.500) | 0.125 | 2.185 (1.285–3.717) | 0.004 |
| PLR | ||||
| ≤141.07 | 1 | 1 | ||
| >141.07 | 1.444 (0.846–2.466) | 0.178 | 1.163 (0.677–1.966) | 0.585 |
| WHR | ||||
| ≤4.604 | 1 | 1 | ||
| >4.604 | 4.361 (2.337–8.138) | <0.001 | 5.503 (2.997–10.106) | <0.001 |
| FAR | ||||
| ≤0.086 | 1 | 1 | ||
| >0.086 | 2.532 (1.478–4.336) | 0.001 | 3.832 (2.193–6.696) | <0.001 |
The results of multifactorial Cox analysis showed that tumor pathological stage (HR = 6.224, 95% CI: 3.063–12.647, P < .001), and WHR (HR = 3.681, 95% CI: 1.768–7.401, P < .001) were the independent risk factors for DFS in CRC patients. Tumor pathological stage (HR = 4.231, 95% CI: 2.062–8.680, P < .001) and WHR (HR = 3.261, 95% CI: 1.552–6.665, P = .002) were independent risk factors for OS, however, LMR (HR = 0.584, 95%CI: 0.346–0.987, P = .044) was an independent protective factor (Table 4).
Table 4.
Multivariate survival analysis associated with DFS and OS.
| Variables | DFS | OS | ||
|---|---|---|---|---|
| HR(95%CI) | P value | HR(95%CI) | P value | |
| Gender | ||||
| Female | – | 1 | ||
| Male | – | 0.708 (0.421–1.191) | .193 | |
| Pathological stage | ||||
| I + II | 1 | 1 | ||
| III + IV | 6.224 (3.063–12.647) | <.001 | 4.231 (2.062–8.680) | <.001 |
| LMR | ||||
| ≤3.89 | – | 1 | ||
| >3.89 | – | 0.584 (0.346–0.987) | .044 | |
| WHR | ||||
| ≤4.604 | 1 | 1 | ||
| >4.604 | 3.681 (1.768–7.401) | <.001 | 3.216 (1.552–6.665) | .002 |
| FAR | ||||
| ≤0.086 | 1 | 1 | ||
| >0.086 | 1.265 (0.647–2.357) | .464 | 1.644 (0.836–3.233) | .149 |
4. Discussion
Statistically, infection and chronic inflammation account for approximately ¼ of all cancer cases.[16] Inflammation predisposes to cancer and promotes tumorigenesis at all stages. Cancer cells as well as surrounding stromal and inflammatory cells engage in carefully coordinated reciprocal interactions to form the inflammatory tumor microenvironment (TME).[17] The idea that cancer may be the cause or result of chronic inflammation has raised concerns about the link between inflammation and cancer.[18] Previous studies have suggested that tumor-associated chronic inflammation and cytokines may be involved in cancer development and progression, promoting malignant cell transformation and carcinogenesis.[19] Moreover, increasing evidence suggests that inflammatory markers, such as NLR, PLR, and LMR, may be of great significance in predicting the survival prognosis of cancer patients.
The TME contains a number of subspecies of leukocytes such as neutrophils, lymphocytes, monocytes, etc.[20] Leukocytes are an important component of the immune system and their elevation signals an impaired immune system. Leukocytes, the main inflammatory component of TME, can promote tumor growth and progression through different mechanisms.[21] One study found that higher white blood cells were positively associated with cancer mortality.[22] Ahmed Abu-Zaid et al[23] verified by meta-analysis that preoperative leukocytosis was associated with poor survival outcome in patients with endometrial cancer. Anemia is one of the most common complications in cancer patients.[24] Anemia causes the formation of a hypoxic environment in the body, which promotes the secretion of angiogenic factors, the formation of neovascularization, and accelerates the proliferation and metastasis of tumor cells.[25] Preoperative anemia has been reported to be significantly linked to poor patient prognosis in several types of malignancies.[26–30] Xuan-Zhang Huang et al[31] conducted a meta-analysis on the prognostic value of preoperative anemia in gastric cancer, which involved 154,36 patients with gastric cancer, and found that preoperative anemia predicted poor prognosis in gastric cancer by calculating the risk ratio (HR) and 95% confidence interval (CI). Based on the above studies, we speculate that white blood cell to hemoglobin ratio (WHR) may have a close association with colorectal cancer progression as well as outcome and may be an inflammatory indicator for predicting the prognosis of colorectal cancer patients. In this retrospective research, we found that patients with high WHR had shorter DFS and OS, indicating that high WHR was associated with a worse prognosis. This was also supported by the results of our multifactorial analysis, in which WHR was an independent risk factor for DFS and OS (Table 4).
Fibrinogen is rarely mentioned as a biomarker of inflammation.[32] Fibrinogen is synthesized in the liver and secreted into the circulation, and its levels rise in response to tissue damage, infection, or inflammation.[33,34] In addition, it promotes tumor cell invasion and migration and also stimulates angiogenesis.[12,35] Many of the previous studies have shown that preoperative fibrinogen is associated with a poorer prognosis in many cancers, such as colon, gastric, bladder, and gallbladder cancers.[36–39] Hypoalbuminemia is not uncommon in inflammatory diseases.[40] Tumors can degrade proteins such as albumin mediated by the ubiquitin-proteasome pathway. Furthermore, pretreatment albumin levels have prognostic significance for cancer.[41] Cheng-Chou Lai et al[42] found that low preoperative serum albumin was a risk factor for poor outcome in colon cancer. Takaaki Fujii et al retrospectively analyzed 157 breast cancer patients and showed that preoperative low albumin was associated with poor prognosis in breast cancer patients.[43] FAR is the ratio of fibrinogen to albumin and has been shown to have a poor association with the prognosis of several malignancies.[44–46] Our results show that the higher the FAR, the shorter the DFS and OS of CRC patients. However, according to our multivariate analysis, FAR may not be an independent risk factor affecting the prognosis of patients (P > .05, Table 4).
As mentioned above, a number of studies have found WHR or FAR to have predictive value in the prognosis of tumors. However, there are few studies on colorectal cancer when the 2 are combined. Thus, we combined the 2 to observe their effects on the prognosis of colorectal cancer patients. Ultimately, our data showed that patients in the WHR > 4.604 and FAR > 0.086 groups had significantly shorter DFS and OS than the other 3 groups, suggesting that WHR combined with FAR has great predictive value for CRC.
The shortcoming is that there are some aspects of this study that need to be refined. Firstly, the study was a single-center retrospective, non-randomized study with a not very large sample size and possible confounding factors. For example, tumor staging did not correlate with WHR as well as FAR, which was unexpected and may be related to our small sample size. Second, since it was a retrospective study, we only used the results of blood tests of patients 1 week before surgery and did not dynamically evaluate the FAR of patients. Third, the study needs to be further validated by prospective cohort studies.
5. Conclusion
In conclusion, WHR may be an independent risk factor affecting patients’ prognosis, while FAR as another independent risk factor needs further study. In addition, WHR combined with FAR might be associated with poor clinical prognosis in colorectal cancer.
Author contributions
Conceptualization: Haifeng Zhang, Chunlei Lu.
Data curation: Weijia Wang.
Methodology: Mingxiao Guo, Xiaoming Zhang.
Software: Kang Li, Jing Yan.
Supervision: Zhaoyong Zhang.
Writing – original draft: Kang Li, Jing Yan.
Writing – review & editing: Haifeng Zhang.
Abbreviations:
- CI
- confidence interval
- CRC
- colorectal cancer
- DFS
- disease-free survival
- FAR
- fibrinogen to albumin ratio
- HR
- risk ratio
- LMR
- lymphocyte to monocyte ratio
- NLR
- neutrophil to lymphocyte ratio
- OS
- overall survival
- PLR
- platelet to lymphocyte ratio
- TME
- tumor microenvironment
- UICC
- Union for International Cancer Control
- WHR
- white blood cell to hemoglobin ratio
This study was supported by the National Natural Science Foundation of China (Grant no. 81500688) and Shandong Provincial Natural Science Foundation, China (ZR2021MH362, ZR2015HL033).
This study was approved by the Medical Ethics Committee, Linyi People Hospital with the waiver of informed written consent in view of the retrospective nature of the study.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Li K, Yan J, Zhang H, Lu C, Wang W, Guo M, Zhang X, Zhang Z. Prognostic value of preoperative white blood cell to hemoglobin ratio and fibrinogen to albumin ratio in patients with colorectal cancer. Medicine 2024;103:3(e37031).
Contributor Information
Kang Li, Email: 3264277939@qq.com.
Jing Yan, Email: 2430077188@qq.com.
Chunlei Lu, Email: 13953988559@163.com.
Weijia Wang, Email: weijia2003sun@163.com.
Mingxiao Guo, Email: gmx1211@163.com.
Xiaoming Zhang, Email: zzywky@126.com.
Zhaoyong Zhang, Email: zzywky@126.com.
References
- [1].Weng J, Li S, Zhu Z, et al. Exploring immunotherapy in colorectal cancer. J Hematol Oncol 2022;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li N, Lu B, Luo C, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: a comparison among China, Europe, and northern America. Cancer Lett. 2021;522:255–68. [DOI] [PubMed] [Google Scholar]
- [3].Wu Z, Li Y, Zhang Y, et al. Colorectal cancer screening methods and molecular markers for early detection. Technol Cancer Res Treat. 2020;19:1533033820980426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Leijssen LGJ, Dinaux AM, Kunitake H, et al. Detrimental impact of symptom-detected colorectal cancer. Surg Endosc. 2020;34:569–79. [DOI] [PubMed] [Google Scholar]
- [5].Hibino S, Kawazoe T, Kasahara H, et al. Inflammation-induced tumorigenesis and metastasis. Int J Mol Sci . 2021;22:5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21:653–67. [DOI] [PubMed] [Google Scholar]
- [7].Xiao Z, Wang X, Chen X, et al. Prognostic role of preoperative inflammatory markers in postoperative patients with colorectal cancer. Front Oncol. 2023;13:1064343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lin ZQ, Ma C, Cao W-Z, et al. Prognostic significance of NLR, PLR, LMR and tumor infiltrating T lymphocytes in patients undergoing surgical resection for hilar cholangiocarcinoma. Front Oncol. 2022;12:908907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mandaliya H, Jones M, Oldmeadow C, et al. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res 2019;8:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shen X, Wang W, Niu X. Neutrophil lymphocyte ratio to albumin ratio and white blood cell to hemoglobin ratio as prognostic markers for hepatocellular carcinoma patients who underwent curative hepatectomy. Int J Gen Med 2021;14:5029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fang L, Yan F-H, Liu C, et al. Systemic inflammatory biomarkers, especially fibrinogen to albumin ratio, predict prognosis in patients with pancreatic cancer. Cancer Res Treat 2021;53:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li R, Song S, He X, et al. Relationship between fibrinogen to albumin ratio and prognosis of gastrointestinal stromal tumors: a Retrospective Cohort Study. Cancer Manag Res 2020;12:8643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu WY, Zhang H-H, Xiong J-P, et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol. 2018;24:3281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mai RY, Bai T, Luo X-L, et al. Preoperative fibrinogen-to-albumin ratio predicts the prognosis of patients with hepatocellular carcinoma subjected to hepatectomy. BMC Gastroenterol. 2022;22:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng HL, Lu J, Xie J-W, et al. Exploring the value of new preoperative inflammation prognostic score: white blood cell to hemoglobin for gastric adenocarcinoma patients. BMC Cancer 2019;19:1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–80. [DOI] [PubMed] [Google Scholar]
- [17].Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [19].Landskron G, De la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol 2020;119:199–245. [DOI] [PubMed] [Google Scholar]
- [21].Wu L, Saxena S, Singh RK. Neutrophils in the tumor microenvironment. Adv Exp Med Biol. 2020;1224:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shankar A, Wang JJ, Rochtchina E, et al. Association between circulating white blood cell count and cancer mortality: a Population-Based Cohort Study. Arch Intern Med. 2006;166:188–94. [DOI] [PubMed] [Google Scholar]
- [23].Abu-Zaid A, Alomar O, Baradwan S, et al. Preoperative leukocytosis correlates with unfavorable pathological and survival outcomes in endometrial carcinoma: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;264:88–96. [DOI] [PubMed] [Google Scholar]
- [24].Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology (Huntingt). 2006;70:34–48. [DOI] [PubMed] [Google Scholar]
- [25].Wicks EE, Semenza GL. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest. 2022;132:e159839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Njølstad TS, Engerud H, Werner HMJ, et al. Preoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas. Gynecol Oncol. 2013;131:410–5. [DOI] [PubMed] [Google Scholar]
- [27].Taylor M, Abah U, Hayes T, et al. Preoperative anemia is associated with worse long-term survival after lung cancer resection: a Multicenter Cohort Study of 5,029 Patients. J Cardiothorac Vasc Anesth. 2022;36:1373–9. [DOI] [PubMed] [Google Scholar]
- [28].Ferran-Carpintero A, Domínguez-García A, Muñoz-Rodríguez J, et al. Impact of anemia on the survival of patients undergoing radical cystectomy for bladder cancer. Actas Urol Esp (Engl Ed) 2020;44:489–96. [DOI] [PubMed] [Google Scholar]
- [29].Deng Y, Weng M, Zhang J. Preoperative anemia and long-term survival in patients undergoing colorectal cancer surgery: a retrospective cohort study. World J Surg Oncol. 2023;21:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang Y, Chen YY, Chen DT, et al. Impact of preoperative anemia on relapse and survival in breast cancer patients. BMC Cancer 2014;14:844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang XZ, Yang Y-C, Chen Y, et al. Preoperative anemia or low hemoglobin predicts poor prognosis in gastric cancer patients: a meta-analysis. Dis Markers. 2019;2019:7606128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Maurel M, Castagné R, Berger E, et al. Patterning of educational attainment across inflammatory markers: findings from a multi-cohort study. Brain Behav Immun. 2020;90:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–904. [DOI] [PubMed] [Google Scholar]
- [34].Tennent GA, Brennan SO, Stangou AJ, et al. Human plasma fibrinogen is synthesized in the liver. Blood. 2007;109:1971–4. [DOI] [PubMed] [Google Scholar]
- [35].Shu YJ, Weng H, Bao R-F, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer 2014;14:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Papila Kundaktepe B, Papila C. The clinical significance of preoperative plasma fibrinogen levels and platelet counts in resectable colon cancer. World J Surg Oncol. 2021;19:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang Y, Liu N, Liu C, et al. High fibrinogen and platelets correlate with poor survival in gastric cancer patients. Ann Clin Lab Sci. 2020;50:457–62. [PubMed] [Google Scholar]
- [38].Li X, Shu K, Zhou J, et al. Preoperative plasma fibrinogen and d-dimer as prognostic biomarkers for non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2020;18:11–19.e1. [DOI] [PubMed] [Google Scholar]
- [39].Jiang C, Li Y, Li Y, et al. Fibrinogen promotes gallbladder cancer cell metastasis and extravasation by inducing ICAM1 expression. Med Oncol. 2022;40:10. [DOI] [PubMed] [Google Scholar]
- [40].Moshage HJ, Janssen JA, Franssen JH, et al. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. 1987;79:1635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lai CC, You J-F, Yeh C-Y, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis. 2011;26:473–81. [DOI] [PubMed] [Google Scholar]
- [43].Fujii T, Tokuda S, Nakazawa Y, et al. Implications of low serum albumin as a prognostic factor of long-term outcomes in patients with breast cancer. In Vivo 2020;34:2033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu H, Zhang W, Ye P, et al. Preoperative circulating fibrinogen to albumin ratio in predicting 5-year prognosis of oral cancer radical surgery. Neoplasma. 2022;69:1246–52. [DOI] [PubMed] [Google Scholar]
- [45].Chen W, Shan B, Zhou S, et al. Fibrinogen/albumin ratio as a promising predictor of platinum response and survival in ovarian clear cell carcinoma. BMC Cancer 2022;22:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Barone B, Napolitano L, Reccia P, et al. Preoperative fibrinogen-to-albumin ratio as potential predictor of bladder cancer: a Monocentric Retrospective Study. Medicina (Kaunas) 2022;58:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]