Abstract
Universal varicella vaccination (UVV), as a single dose to children aged 12 to 15 months, was introduced in Korea in 2005. A seroprevalence study is required to upgrade this UVV strategy. The fluorescent antibody to membrane antigen (FAMA) assay is the gold standard for varicella-zoster virus (VZV) immunity testing. However, no standard operating procedure (SOP) has been developed for the FAMA assay, in which either glutaraldehyde or acetone may be used for VZV-infected cell fixation. In this observational study, we aimed to investigate the age-specific seroprevalence in Korean children and adults. Additionally, with glycoprotein enzyme-linked immunosorbent assay (gpELISA) as the reference, we evaluated the performance of the FAMA assay using acetone-fixed cells.
Four hundred sera were analyzed using the FAMA assay (acetone-fixed cells) and gpELISA, comprising 50 subjects from each age category. In the FAMA assay, the seropositivity rate decreased from 82.0% in the 1 to 4-year-old group to 58.0% in the 5 to 9-year-old group (95% confidence interval [CI]: 69.2–90.2 and 44.2–70.6, respectively; P = .009), while that in the gpELISA decreased from 80.0% to 52.0% (95% CI: 67.0–88.8 and 38.5–65.2, respectively; P = .003). In both methods, the seropositivity rates ranged from 95% to 100% in the population aged ≥ 20 years. We observed a significant correlation between the 2 methods, with a correlation coefficient of 0.795 (P < .001). In receiver operating characteristic analysis using the gpELISA results as a reference, the area under the curve for the FAMA assay was very high at 0.995 (95% CI: 0.990–1.000; P < .001). Compared to the gpELISA, the sensitivity, specificity, and kappa value of the FAMA assay were 99.4%, 79.3%, and 0.84 (nearly perfect), respectively. The seropositivity rate of the 5 to 9-year-old group indicated waning immunity over time and supported implementation of a second dose in the UVV program. The results of the FAMA assay were comparable to those of the gpELISA. Although further study is needed to standardize procedures, our results suggest that the FAMA assay using acetone-fixed cells can be used widely and can be included in a universal FAMA assay SOP.
Keywords: fluorescent antibody to membrane antigen, glycoprotein enzyme-linked immunosorbent assay, seroprevalence, standard operating procedure, varicella vaccination
1. Introduction
Varicella caused by varicella-zoster virus (VZV) is a common childhood disease and is generally mild and self-limiting. However, immunocompromised children and adults are at increased risk for severe disease including visceral dissemination to lungs, brain, and liver; secondary bacterial skin infection; and mortality. Varicella is highly contagious, and vaccination is the most effective strategy for its prevention.[1] The World Health Organization recommends implementing a 1- or 2-dose universal varicella vaccination (UVV) program in countries in which varicella is a public health concern. The number of countries introducing UVV programs or using varicella vaccines in the private sector has been increasing.[2]
Korea implemented UVV in 2005; 1 dose of the vaccine is administered to children at 12 to 15 months of age. Clusters of varicella cases are continuously occurring among Korean vaccinated children, and support for provision of a second vaccine dose has emerged among infectious disease specialists.[3] A study of seroprevalence at more than 10 years postvaccination was performed to evaluate the current vaccination strategy.
Seroprevalence studies that identify susceptible populations are often needed before or after UVV implementation, and the demand to assess immunity against VZV has been increasing worldwide. Although a glycoprotein enzyme-linked immunosorbent assay (gpELISA) is widely used to evaluate immunity against VZV, the fluorescent antibody to membrane antigen (FAMA) assay is the gold standard.[4,5] However, no FAMA standard operating procedure (SOP) has been developed, and procedural details differ among laboratories. Health authorities are aware of the need for a FAMA SOP. FAMA assays are currently performed using unfixed VZV-infected cells or VZV-infected cells fixed with acetone or glutaraldehyde. Recently, to increase throughput and efficiency, the use of fixed VZV-infected cells has increased.[6] Several studies have reported seroprevalence against VZV using the glutaraldehyde fixed-cell FAMA assay, and performance of the glutaraldehyde fixation method has been assessed.[7,8] However, studies of the efficacy of the acetone fixed-cell FAMA assay are limited.
The objectives of this study were to investigate age-specific seroprevalence of protection against VZV among Korean children and adults in the post-vaccination era and to evaluate performance of the acetone-fixed-cell FAMA assay using the gpELISA as a reference. The results will provide valuable information for upgrading the current varicella vaccination strategy and for developing a FAMA assay SOP.
2. Materials and methods
2.1. Sera source
Sera stored in the biobanks of Seoul St. Mary Hospital and Gyeongsang National University Hospital were collected between January 2018 to December 2021. These sera were from individuals who had given informed consent for use of their sera in future research. The exclusion criteria were sera with insufficient quantity and sera from individuals with congenital immunodeficiency disease, malignancy, or leukemia. For each serum sample, information on age, sex, and collection date were provided in anonymous form.
We also used residual sera from routine blood tests obtained from pediatric patients hospitalized in Seoul St. Mary Hospital. The sera were centrifuged and stored at −70°C until testing. Written consent for using the residual sera was obtained from parents or legal representatives of the children. Sera with insufficient quantity or from immunocompromised children were excluded. The anonymity of the children was ensured by removing personal identification information such as hospital number and name.
The study design and the sources of sera were approved by the Institutional Review Boards of Seoul St. Mary Hospital (KC21TNSI0686) and Eunpyeong St. Mary Hospital (PC21SNSI0139).
2.2. FAMA assay
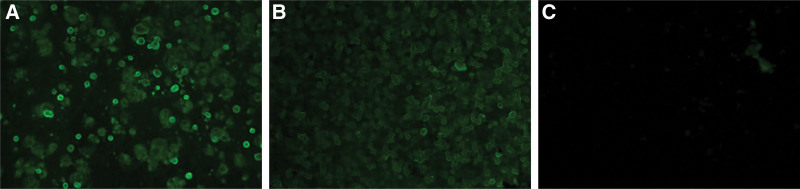
Both FAMA assay and gpELISA were performed on each serum sample at the Vaccine Bio Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea. VZV-infected cells used as antigen in the FAMA assay were provided by Mogam Institute for Biomedical Research (Seoul, Korea), a research institute belonging to GC Pharma, one of the varicella vaccine manufacturers in Korea. MAV/06 strain VZV was cultured in MRC-5 cells and harvested when cytopathic effects were observed in 70% to 80% of cells. VZV-infected cells were fixed with cold acetone for 15 minutes and washed 3 times using phosphate-buffered saline (PBS). Collected sera were serially diluted, mixed with VZV-infected cells, incubated for 30 minutes at 37°C, and washed 3 times using PBS. A secondary antibody, fluorescein isothiocyanate-labeled anti-human immunoglobulin G (IgG) from rabbit (Dako, Hamburg, Germany), was added to mixtures, incubated for 30 minutes at 37°C, and washed 3 times using PBS. Assessment for a fluorescent ring was made independently by 2 investigational technicians. Generally, a 1:4 titer is used as the cutoff in FAMA assay method.[4,5] Thus, a titer of ≥ 1:4 was considered seropositive in this study. For positive reference, WHO standard VZV immunoglobulin was used. For negative reference, sera from gpELISA-negative children with no history of varicella vaccination or infection were used. VZV-infected cells used in the FAMA assay are presented in Figure 1.
Figure 1.
Fluorescence microscopy of VZV-infected cells in FAMA assay. (A) VZV-infected cells fixed with cold acetone for 15 mins used as antigen in the FAMA assay; (B) positive control; (C) negative control. FAMA = fluorescent antibody to membrane antigen, VZV = varicella-zoster virus.
2.3. gpELISA
IgG antibodies against VZV were measured by VaccZyme VZV gpELISA kit (Binding Site, Birmingham, UK) according to the manufacturer instructions. Anti-VZV IgG concentrations < 100 mIU/mL, 100 to 149 mIU/mL, and ≥ 150 mIU/mL were interpreted as negative, equivocal, and positive, respectively. As in previous studies, both equivocal and positive results were considered seropositive.[9,10]
2.4. Statistical analysis
Statistical analysis was performed using MedCalc software (version 19.2.0, MedCalc Software bvba, Ostend, Belgium) and SPSS software (version 19, IBM Co., Armonk, NY, USA). Percentages of subjects demonstrating seropositivity against VZV were calculated with 95% confidence intervals (CIs), and chi-square test was used for comparisons between age groups. In assessment of the acetone-fixed-cell FAMA assay, correlation test values; receiver operating curve (ROC) analysis; and sensitivity, specificity, and kappa value were calculated. Pearson correlation coefficient (r) was calculated to assess correlation between the gpELISA and FAMA assay results. ROC analysis was conducted to evaluate the ability of the FAMA assay to predict gpELISA results, and area under curve (AUC) was calculated with 95% CI. An AUC of 0.5 was considered no discrimination, 0.7 to 0.8 was considered acceptable, 0.8 to 0.9 was considered excellent, and AUC > 0.9 was considered outstanding.[11] Relative to results of gpELISA, diagnostic sensitivity, specificity, and kappa values for the FAMA method were all calculated using 3 different cutoffs, 1:4, 1:8, and 1:16. Kappa value was categorized as poor (< 0.00), slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or nearly perfect (0.81–1.00). For all tests, a P value < .05 was considered significant.
3. Results
3.1. Seroprevalence
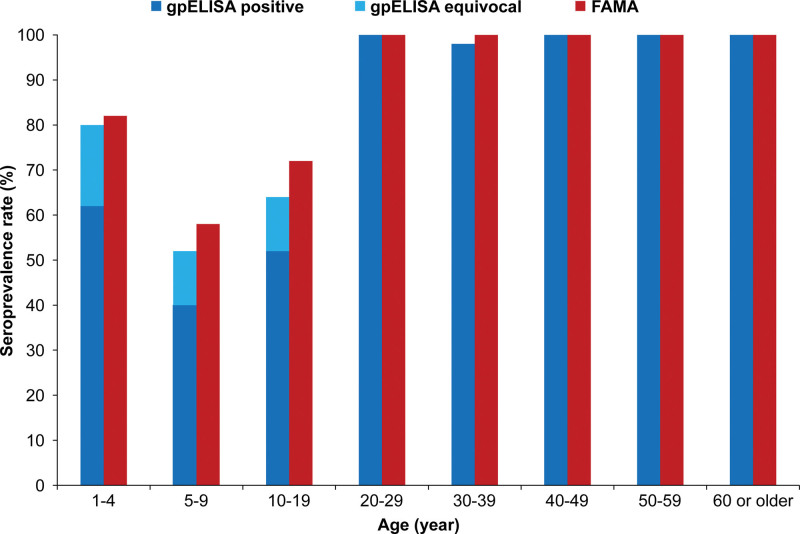
This study included 400 participants, 50 participants in each of 8 age groups (1–4 years; 5–9 years; 10–19 years; 20–29 years; 30–39 year; 40–49 years; 50–59 years; 60 years or older). In the FAMA assay, proportions of seropositive subjects were 82.0% (n = 41/50; 95% CI = 69.2–90.2) in the 1 to 4-year-old group; 58.0% (n = 29/50; 95% CI = 44.2–70.6) in the 5 to 9-year-old group; 72.0% (n = 36/50; 95% CI = 58.3–82.5) in the 10 to 19-year-old group; and 100% (n = 50/50; 95% CI = 92.9–100.0) in the 20 to 29-year-old group, 30 to 39-year-old group, 40 to 49-year-old group, 50 to 59-year-old group, and 60-year or older group. Seropositivity rate sharply decreased from 1 to 4-year to 5 to 9-year groups (P = .009) (Fig. 2).
Figure 2.
Age-specific seroprevalence of VZV measured by acetone-fixed-cell FAMA assay and gpELISA. FAMA = fluorescent antibody to membrane antigen, gpELISA = glycoprotein enzyme-linked immunosorbent assay, VZV = varicella-zoster virus.
When measured by gpELISA, proportions of seropositive subjects were 80.0% (n = 40/50; 95% CI = 67.0–88.8) in the 1 to 4-year-old group, 52.0% (n = 26/50; 95% CI = 38.5–65.2) in the 5 to 9-year-old group, 64.0% (n = 32/50; 95% CI = 50.1–75.9) in the 10 to 19-year-old group, 100% (n = 50/50; 95% CI = 92.9–100.0) in the 20 to 29-year-old group, 98.0% (n = 49/50; 95% CI = 89.5–99.7) in the 30 to 39-year-old group, and 100% (n = 50/50; 95% CI = 92.9–100.0) in the 40 to 49-year-old group, 50 to 59-year-old group and 60-year or older group. Seropositivity rate sharply decreased from the 1 to 4-year-old group to the 5 to 9-year-old group (P = .003) (Fig. 2).
3.2. Comparison of performance of FAMA assay and gpELISA
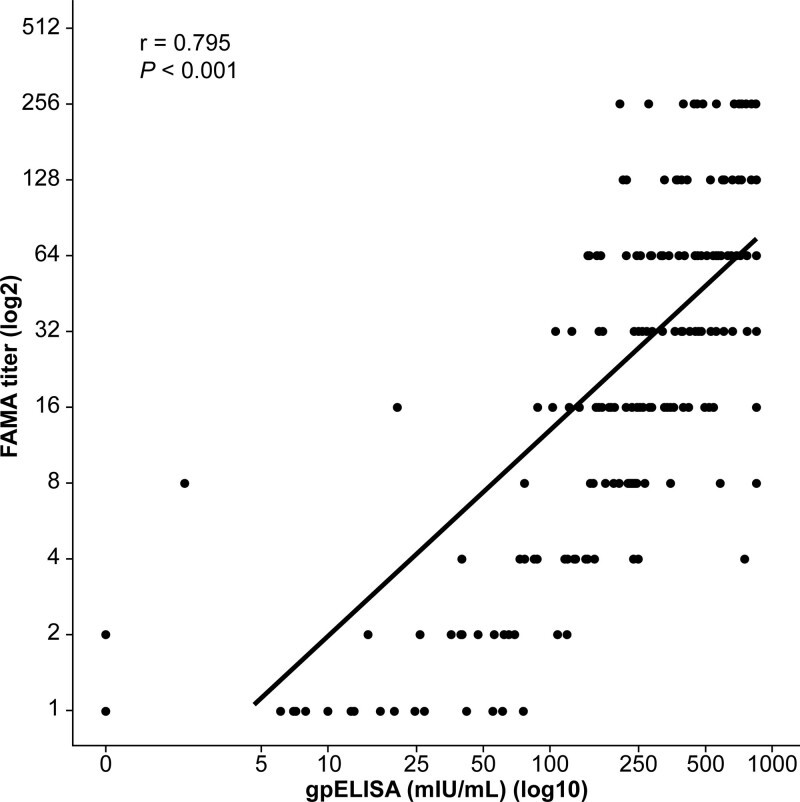
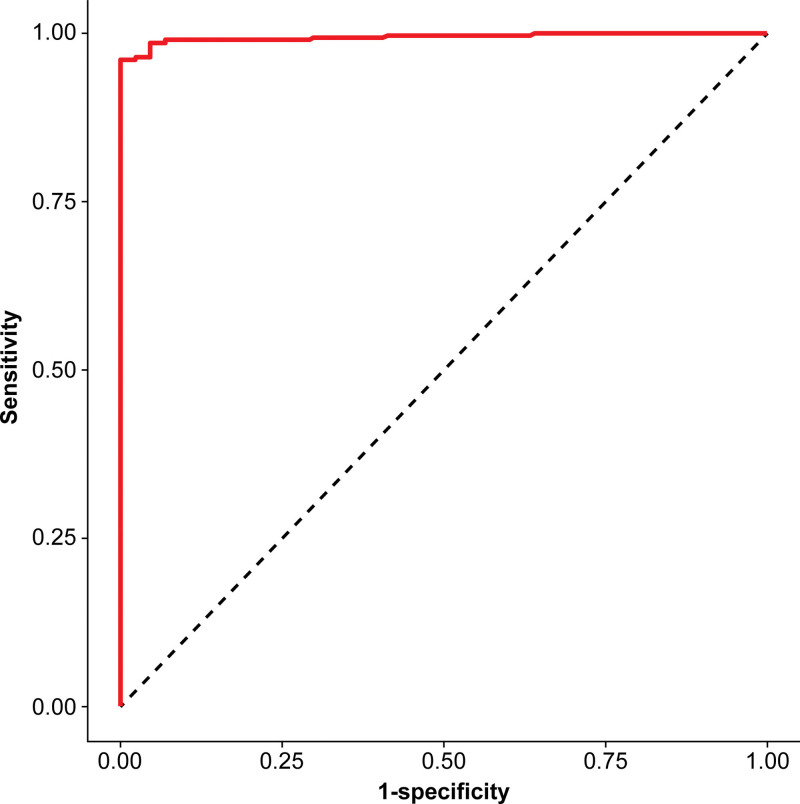
In correlation analysis, significant correlation was observed between gpELISA and FAMA results (coefficient of correlation, 0.795; P < .001) (Fig. 3). In ROC analysis using results of gpELISA as reference, AUC of the FAMA assay was 0.995 (95% CI: 0.990–1.000) (P < .001); this was considered outstanding (Fig. 4). Relative to results of gpELISA, sensitivity, specificity, and kappa value were calculated with different cutoffs: at 1:4, sensitivity was 99.4% and specificity was 79.3%; at 1:8, sensitivity was 95.1%, and specificity was 92.5%; and at cutoff 1:16, sensitivity was 88.2% and specificity 96.2%. The kappa value was 0.848 (nearly perfect) at 1:4, 0.793 (substantial) at 1:8, and 0.638 (substantial) at 1:16. The 1:4 value was the highest shown (Table 1).
Figure 3.
Correlation between results of gpELISA and acetone-fixed-cell FAMA assays. The Pearson r (r) is shown. gpELISA = glycoprotein enzyme-linked immunosorbent assay.
Figure 4.
With gpELISA as reference, performance of the acetone-fixed-cell FAMA assay was evaluated by ROC analysis (AUC = 0.995). AUC = area under the curve, FAMA = fluorescent antibody to membrane antigen, gpELISA = glycoprotein enzyme-linked immunosorbent assay, ROC = receiver operating characteristics.
Table 1.
Comparison between results of gpELISA and FAMA with different cutoffs.
| gpELISA | Sensitivity | Specificity | Kappa value (95% CI) |
||
|---|---|---|---|---|---|
| Positive or equivocal | Negative | ||||
| FAMA 1:4 | |||||
| Positive | 345 | 11 | 99.4% | 79.3% | 0.848 (0.766–0.929) |
| Negative | 2 | 42 | |||
| FAMA 1:8 | |||||
| Positive | 330 | 4 | 95.1% | 92.5% | 0.793 (0.707–0.879) |
| Negative | 17 | 49 | |||
| FAMA 1:16 | |||||
| Positive | 306 | 2 | 88.2% | 96.2% | 0.638 (0.539–0.738) |
| Negative | 41 | 51 | |||
CI = confidence interval, FAMA = fluorescent antibody to membrane antigen, gpELISA = glycoprotein enzyme-linked immunosorbent assay.
4. Discussion
A UVV program was introduced in Korea in 2005. One dose of the vaccine is administered to children 12 to 15 months old. Vaccination coverage has been maintained at higher than 95% since 2008. Based on data from the National Health Insurance Service, varicella cases substantially decreased from approximately 220,000 in 2008 to 80,000 in 2019. However, breakthrough infections are being continuously reported. The highest incidence, nearly 50% of cases, has been observed in children aged 5 to 9 years.[3,12,13] A seroprevalence study is required to assess population immunity and identify susceptible groups.
Our age-specific seroprevalence study had several findings. First, seropositivity rate declined from approximately 80% in the 1 to 4-year-old group to 50% to 60% in the 5 to 9-year-old group. The lower seropositivity rate in the 5 to 9 age group may be the reason for the high incidence of varicella in this age group and indicates that immunity induced by varicella vaccination decreased with time since vaccination. This result is similar to previous publication findings. In a study conducted by Hong et al, vaccine effectiveness decreased over time to 62.6%, 55.4%, and 49.9% after 4, 5 and 6 years, respectively, from the 1-dose varicella vaccination.[3] A seroprevalence study conducted in China also demonstrated an approximate 50% seropositivity rate in children aged 5 to 9 years with a 1-dose varicella vaccination program.[14] Second, seropositivity rate was higher than 90% in all adult, aged 20 and older, groups. This finding is consistent with that of a seroprevalence study conducted within 10 years after UVV initiation in Korea. In studies conducted by Lee et al and Han et al,[15,16] seropositivity rate against VZV was higher than 90% in those aged 20 or older. In our study, in relation to age, we assume that most immunity in subjects aged 20 or older resulted from natural infection; the UVV did not appear to affect the immunity against VZV in the adult group. Third, seropositivity rate in the 10 to 19-year group in our study ranged from 60% to 75%. In a VZV seroprevalence study conducted in 2009 to 2010, Lee et al reported a seropositivity rate higher than 90% in the 10 to 19-year age group[15]; in a VZV seroprevalence study conducted in 2012 to 2013, Han et al reported a seropositivity rate of 80% to 96% in the 10 to 19-year age group. The results of this study suggest that the decrease in 10 to 19-year-old VZV seropositivity indicates that circulation of VZV has been reduced due to UVV. In the varicella vaccination era, exposure to VZV may have been reduced, lowering the probability of gaining natural immunity and increasing the pool of susceptible adolescents and young adults. The unvaccinated and those in whom immunity has waned after vaccination require attention.
The FAMA assay is the most extensively validated VZV antibody detection method and correlates well with identification of those susceptible to varicella. UVV programs and private sector varicella vaccine use have increased, and use of the vaccine will continue to increase with the institution of more UVV programs and the shift from 1-dose to 2-dose programs. VZV antibody detection is required in adults in certain circumstances such as in healthcare professionals and in those who are immunocompromised.[4,5] Since the demand for VZV immunity testing will increase, development of a FAMA assay SOP is needed. The antibody-specific membrane protein of VZV-infected cells, the main detection object of the FAMA test, is well-detected by the acetone fixation method.[6] However, acetone-fixed-cell FAMA assay performance has not been evaluated. In this study, we found a statistical correlation between gpELISA and acetone-fixed-cell FAMA assay. In ROC analysis with gpELISA results as reference, acetone-fixed-cell FAMA assay demonstrated outstanding AUC; with a cutoff of 1:4, the kappa value was nearly perfect (0.848). Kappa values with 1:8 and 1:16 cutoffs were substantial (0.793 and 0.638, respectively). The results of acetone-fixed-cell FAMA assay with a 1:4 cutoff were similar with those of gpELISA.
Despite its valuable findings, limitations of this study need to be addressed. First, only a small sample of sera was collected from only 2 blood banks and 1 hospital. Therefore, the data may not be representative of a national pattern. Second, only sera from healthy individuals were collected in this study; VZV seroprevalence in immunocompromised individuals was not assessed. Third, relative to results of gpELISA, only performance of the FAMA assay using acetone-fixed cells was assessed. Further serologic testing that includes a large sample size from different regions of Korea and from immunocompromised individuals is needed. To standardize the FAMA assay, further studies comparing results among FAMA assay procedures are needed.
To our knowledge, this was the first study to investigate the age-specific seroprevalence of protection against VZV at 10 years after initiation of Korea UVV program. The seropositivity rate in the 5 to 9-year age group was low, 50% to 60% and suggested post-vaccination immunity waning. This supports the implementation of a 2-dose UVV program. These serologic results provide evidence for the need to update Korea vaccination strategy and serve as a reference for other regions. This study also is the first evaluation of the performance of the acetone-fixed-cell FAMA assay using gpELISA as a reference. Based on the results, the acetone-fixed-cell FAMA assay with a cutoff of 1:4 can be used widely in measuring immunity against VZV. Although more data are needed for FAMA assay standardization, this method can be included in any FAMA assay SOP.
Acknowledgments
The serum used for this study was provided by Gyeongsang National University Hospital (a member of the Korea Biobank Network) and Seoul St. Mary Hospital. The authors offer their gratitude for the resources and services provided by biobanks.
Author contributions
Conceptualization: Hye Seon Ji, Ui Yoon Choi, Jin Han Kang.
Data curation: Hye Seon Ji, Kyu Ri Kang, Hyun Mi Kang, Ui Yoon Choi.
Formal analysis: Ui Yoon Choi.
Funding acquisition: Jin Han Kang.
Investigation: Hye Seon Ji, Kyu Ri Kang, Jin Han Kang.
Methodology: Hye Seon Ji, Kyu Ri Kang, Ui Yoon Choi.
Project administration: Jin Han Kang.
Resources: Hye Seon Ji, Kyu Ri Kang, Jin Han Kang.
Software: Hye Seon Ji, Kyu Ri Kang.
Supervision: Ui Yoon Choi, Jin Han Kang.
Validation: Hyun Mi Kang, Soo Young Lee.
Visualization: Ui Yoon Choi.
Writing – original draft: Ui Yoon Choi.
Writing – review & editing: Hye Seon Ji, Kyu Ri Kang, Hyun Mi Kang, Ui Yoon Choi, Soo Young Lee, Jin Han Kang.
Abbreviations:
- AUC
- area under curve
- CI
- confidence interval
- FAMA
- fluorescent antibody to membrane antigen
- gpELISA
- glycoprotein enzyme-linked immunosorbent assay
- IgG
- immunoglobulin G
- PBS
- phosphate-buffered saline
- ROC
- receiver operating curve
- SOP
- standard operating procedure
- UVV
- universal varicella vaccination
- VZV
- varicella-zoster virus
This research was supported by National Institute of Health, funded by the Korea Disease Control and Prevention Agency, Republic of Korea (grant number: 2021ER260201).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Ji HS, Kang KR, Kang HM, Choi UY, Lee SY, Kang JH. Seroprevalence of varicella-zoster virus as measured by fluorescent antibody to membrane antigen assay and glycoprotein enzyme-linked immunosorbent assay more than 10 years after initiation of a universal vaccination program: An observational study. Medicine 2024;103:3(e36931).
Contributor Information
Hye Seon Ji, Email: wlgptjs0315@naver.com.
Kyu Ri Kang, Email: kjhan@catholic.ac.kr.
Hyun Mi Kang, Email: kjhan@catholic.ac.kr.
Soo Young Lee, Email: sylee@catholic.ac.kr.
Jin Han Kang, Email: kjhan@catholic.ac.kr.
References
- [1].Gershon AA. Varicella-zoster virus. In: Cherry J, Harrison GJ, Kaplan SL, et al. (eds). Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier. 2019:1476–84. [Google Scholar]
- [2].World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014-Recommendations. Vaccine. 2016;34:198–9. [DOI] [PubMed] [Google Scholar]
- [3].Hong K, Sohn S, Choe YJ, et al. Waning effectiveness of one-dose universal varicella vaccination in Korea, 2011-2018: a propensity score matched national population cohort. J Korean Med Sci. 2021;36:e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dollard S, Chen MH, Lindstrom S, et al. Diagnostic and immunologic testing for varicella in the era of high-impact varicella vaccination: an evolving problem. J Infect Dis. 2022;226:S450–5. [DOI] [PubMed] [Google Scholar]
- [5].Breuer J, Schmid DS, Gershon AA. Use and limitations of varicella-zoster virus-specific serological testing to evaluate breakthrough disease in vaccinees and to screen for susceptibility to varicella. J Infect Dis. 2008;197:S147–51. [DOI] [PubMed] [Google Scholar]
- [6].Pan D, Wang W, Cheng T. Current methods for the detection of antibodies of varicella-zoster virus: a review. Microorganisms. 2023;11:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wutzler P, Färber I, Wagenpfeil S, et al. Seroprevalence of varicella-zoster virus in the German population. Vaccine. 2001;20:121–4. [DOI] [PubMed] [Google Scholar]
- [8].Sauerbrei A, Farber I, Brandstadt A, et al. Immunofluorescence test for sensitive detection of varicella-zoster virus-specific IgG: an alternative to fluorescent antibody to membrane antigen test. J Virol Methods. 2004;119:25–30. [DOI] [PubMed] [Google Scholar]
- [9].Kim YH, Hwang JY, Shim HM, et al. Evaluation of a commercial glycoprotein enzyme-linked immunosorbent assay for measuring vaccine immunity to varicella. Yonsei Med J. 2014;55:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sauerbrei A, Wutzler P. Serological detection of varicella-zoster virus-specific immunoglobulin G by an enzyme-linked immunosorbent assay using glycoprotein antigen. J Clin Microbiol. 2006;44:3094–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–6. [DOI] [PubMed] [Google Scholar]
- [12].The Korean Pediatric Society. Varicella vaccine. In: Choi EH. (ed). Immunization Guideline: 2021 Report of the Committee in Infectious Diseases. 10th ed. Seoul: The Korean Pediatric Society. 2021:183–99. [Google Scholar]
- [13].Korean Disease Control and prevention Agency. Infectious disease. Main statistics. Available at: https://npt.kdca.go.kr/npt/biz/npp/ist/simple/simplePdStatsMain.do. [Accessed June 6, 2023].
- [14].Suo L, Lu L, Chen M, et al. Antibody induced by one-dose varicella vaccine soon became weak in children: evidence from a cross-sectional seroepidemiological survey in Beijing, PRC. BMC Infect Dis. 2015;15:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee H, Cho HK, Kim KH. Seroepidemiology of varicella-zoster virus in Korea. J Korean Med Sci. 2013;28:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Han SB, Kang KR, Huh DH, et al. Seroepidemiology of varicella-zoster virus in Korean adolescents and adults using fluorescent antibody to membrane antigen test. Epidemiol Infect. 2015;143:1643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]