Abstract
Background:
Atopic dermatitis (AD) affects approximately 10% of adults worldwide. CM310 is a humanized monoclonal antibody targeting interleukin-4 receptor alpha that blocks interleukin-4 and interleukin-13 signaling. This trial aimed to evaluate the efficacy and safety of CM310 in Chinese adults with moderate-to-severe AD.
Methods:
This multicenter, randomized, double-blind, placebo-controlled, phase 2b trial was conducted in 21 medical institutions in China from February to November 2021. Totally 120 eligible patients were enrolled and randomized (1:1:1) to receive subcutaneous injections of 300 mg CM310, 150 mg CM310, or placebo every 2 weeks for 16 weeks, followed by an 8-week follow-up period. The primary endpoint was the proportion of patients achieving ≥75% improvement in the Eczema Area and Severity Index (EASI-75) score from baseline at week 16. Safety and pharmacodynamics were also studied.
Results:
At week 16, the proportion of EASI-75 responders from baseline was significantly higher in the CM310 groups (70% [28/40] for high-dose and 65% [26/40] for low-dose) than that in the placebo group (20%[8/40]). The differences in EASI-75 response rate were 50% (high vs. placebo, 95% CI 31%–69%) and 45% (low vs. placebo, 95% CI 26%–64%), with both P values <0.0001. CM310 at both doses also significantly improved the EASI score, Investigator's Global Assessment score, daily peak pruritus Numerical Rating Scale, AD-affected body surface area, and Dermatology Life Quality Index compared with placebo. CM310 treatment reduced levels of thymus and activation-regulated chemokine, total immunoglobulin E, lactate dehydrogenase, and blood eosinophils. The incidence of treatment-emergent adverse events (TEAEs) was similar among all three groups, with the most common TEAEs reported being upper respiratory tract infection, atopic dermatitis, hyperlipidemia, and hyperuricemia. No severe adverse events were deemed to be attributed to CM310.
Conclusion:
CM310 at 150 mg and 300 mg every 2 weeks demonstrated significant efficacy and was well-tolerated in adults with moderate-to-severe AD.
Keywords: CM310, Moderate-to-severe atopic dermatitis, Interleukin-4 receptor alpha, Adults
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease that has a global prevalence of up to 20% in children and 10% in adults.[1] Approximately 20% of AD patients experience moderate-to-severe AD.[2] Typical characteristics of moderate-to-severe AD include intense pruritus and chronic or relapsing disease course,[3] which significantly impairs patients' quality of life.[4] Despite topical corticosteroids combined with optimal skin care being the mainstay of AD treatment, many patients with moderate-to-severe AD do not achieve adequate disease control and require systemic immunosuppressants.[5]
As with other atopic diseases, AD is characterized by immune dysregulation, including aberrant activation of type 2 T helper (Th2) cytokines.[6] Th2 immune mediators, including interleukin-4 (IL-4) and IL-13, are known to participate in the pathophysiology of AD by promoting inflammation, fibrosis, impaired epidermal barrier function, and immunoglobulin E (IgE) production.[7–9] In vivo data have identified a potential reduction of chronic pruritus after sensory neuron-specific deletion of IL-4 receptor alpha (IL-4Rα) in mice.[10] Therefore, developing biologics targeting IL-4, IL-13, and IL-4Rα has emerged as a new strategy in the treatment of AD.
Dupilumab, an anti-IL-4Rα antibody, has shown notable therapeutic benefits in multiple phase 3 clinical trials for moderate-to-severe AD.[11–14] These trials have demonstrated significant amelioration in AD clinical manifestations, as assessed by established metrics such as Investigator's Global Assessment (IGA), Eczema Area and Severity Index (EASI), and pruritus Numerical Rating Scale (NRS) score. These findings underscore the favorable efficacy and safety profile of anti-IL-4Rα antibody for individuals with moderate-to-severe AD.
CM310 is a humanized monoclonal antibody that specifically binds to IL-4Rα, preventing its interaction with IL-4 and IL-13. A phase 1b/2a trial (NCT04893941) involving adults with moderate-to-severe AD showed that CM310 was well-tolerated and produced significant improvements in AD signs and symptoms. The epitope of CM310 to IL-4Rα differs from that of dupilumab, as evidenced by the differential cross-species reactivity of CM310 binding to humans, rats, and cynomolgus monkeys, whereas dupilumab binds only to human IL-4Rα. This divergence indicates distinct mechanisms in precluding IL-4Rα signaling and leads to different clinical outcomes. The present study reports findings from a phase 2b trial evaluating the efficacy and safety of CM310 monotherapy in adults with moderate-to-severe AD.
Methods
Ethics approval
This trial was conducted in compliance with the Good Clinical Practice guidelines issued by National Medical Products Administration, and relevant regulations. This study was approved by the Institutional Review Board and Independent Ethics Committee of the Peking University People's Hospital (No. 2020PHA073-001) and the participating study centers. Patients signed a written informed consent form.
Trial design and participants
This multicenter, randomized, double-blind, placebo-controlled, phase 2b trial (ClinicalTrials.gov, NCT04805411) was conducted across 21 study sites in China. The 28-week study period comprised an up to 4-week screening period, followed by 16 weeks of treatment and 8 weeks of follow-up periods. Patients aged 18–70 years, who had been diagnosed with moderate-to-severe AD for at least 12 months (with baseline IGA score of ≥3, EASI score of ≥16, ≥10% body surface area (BSA) of AD involvement, and peak pruritus NRS [PP-NRS] score of ≥4), and who were inadequately controlled with topical corticosteroids, were enrolled in the trial. Detailed inclusion/exclusion criteria are listed in Supplementary Table 1, http://links.lww.com/CM9/B608.
Randomization and blinding
The enrolled patients were randomly allocated to the placebo group or CM310 groups. Patient and drug randomization lists were generated via SAS (version 9.4, SAS Institute Inc., Cary, USA) with a permuted block size of six and then imported into Interactive Web Response System. All patients and trial personnel were blinded to all randomization procedures.
Procedures
Eligible patients were randomized 1:1:1 to receive high-dose CM310 at a dose of 300 mg (loading dose, 600 mg), low-dose CM310 at a dose of 150 mg (loading dose, 300 mg), or matching placebo subcutaneously every 2 weeks for 16 weeks. Throughout the trial, patients were required to apply moisturizer daily. If medically indicated (i.e., to control intolerable AD symptoms), rescue medications with topical corticosteroids, topical calcineurin inhibitors, or systemic glucocorticoids were given at the discretion of the investigator. Patients who were given systemic glucocorticoids were discontinued from the study drug, but continued study evaluations were required.
Outcomes
The primary endpoint was the proportion of EASI-75 responders (EASI scores improved by ≥75% from baseline) at week 16. The secondary efficacy endpoints included the following responder rates at all scheduled time points from baseline to end-of-study (EOS; 24 weeks): achieving IGA 0/1 with ≥2-grade improvement; ≥2-grade improvement in IGA score; EASI-75; EASI-50 and EASI-90 (≥50% and ≥90% improvements from baseline, respectively); and ≥3- or ≥4-point improvements in weekly average of daily PP-NRS score. Additional secondary efficacy outcomes were the percent changes at all scheduled time points in EASI score, weekly average of daily PP-NRS score, and percentage of BSA (%BSA) of AD involvement, as well as the change at all scheduled time points in Dermatology Life Quality Index (DLQI) score.
Safety assessments involved monitoring of treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), physical examination, vital signs, a 12-lead electrocardiogram, and clinical laboratory parameters.
To reflect the pharmacodynamics of CM310 in moderate-to-severe AD, serum biomarkers were evaluated, including thymus and activation-regulated chemokine (TARC), total IgE, lactate dehydrogenase, and blood eosinophils.
Statistical analysis
Sample size calculation is based on the primary endpoint (the proportion of participants achieving EASI-75 at week 16). Assuming that CM310 had similar efficacy results to dupilumab,[15] an enrollment of 30 participants per group would provide the trial with 80% power to detect a 36.6% difference between the CM310 high-dose group and the placebo group (51.3% vs. 14.7%) in the primary endpoint. Statistical tests were two-sided and performed at the 0.05 significance level. Factoring a dropout rate of 25%, 40 participants per group were required with a total of 120 participants.
Both full analysis set and safety set included all randomized participants who received at least one dose of study drug. Full analysis set was used for efficacy analysis by treatment groups as randomized; while safety set was adopted for safety analysis by treatment group as actually received. Pharmacodynamic concentration analysis set included all randomized patients who received any study drug and had at least one corresponding qualified result.
The binary efficacy endpoints (e.g., responders for EASI-50, EASI-75, EASI-90, and IGA 0/1) were analyzed using Wald method and Fisher's exact test. If rescue medication was used or participants had missing value of endpoints for any visit, the patient would be treated as a non-responder from when the rescue was used.
Continuous endpoints (e.g., percent change in EASI score) were analyzed using mixed-effect model for repeated measures, using response variable, group, visit, and group-by-visit interaction as fixed effect and the baseline value as covariates. An unstructured covariance matrix was used to model within-patient errors. Continuous efficacy data were deemed missing once rescue medication was initiated or study withdrawal occurred, and no imputation was made. Least-squares (LS) means estimated by treatment group were provided, along with the difference between these estimates vs. placebo, as well as corresponding standard errors (SEs) and associated 95% confidence intervals (CIs). For biomarker analysis, descriptive statistics were computed, followed by a two-sample t-test to compare each pair of groups.
The following three sensitivity analyses were prespecified to assess the robustness of primary endpoint: (1) the patients would be treated as "non-responders" after rescue medication; and last observation carried forward (LOCF) method was used to impute the missing EASI score at week 16 and further derive the corresponding EASI-75; (2) no handling of efficacy data after rescue medication and non-responder imputation for all missing data; and (3) no handling of efficacy data after rescue medication; and the LOCF method was used to impute the missing EASI score at week 16 and further derive the corresponding EASI-75. The following two sensitivity analyses were prespecified to evaluate the robustness of response for continuous variable EASI (percent change in EASI score from baseline to week 24): (1) data after rescue medication (excluding the onset day) were deemed missing and were analyzed using an analysis of covariance (ANCOVA) model after LOCF imputation; and (2) no handling of efficacy data after rescue medication; all observed values of EASI scores would be included in mixed-effect model for repeated measures (MMRM) for analysis.
We performed post hoc efficacy endpoint analyses on the proportions of responders for EASI-75 and an IGA score of 0/1 with ≥2-point reduction at week 16, which selectively excluded the confounding impact of coronavirus disease 2019 (COVID-19). SAS (SAS Institute Inc., Cary, USA) was used for statistical analysis.
Results
Participants
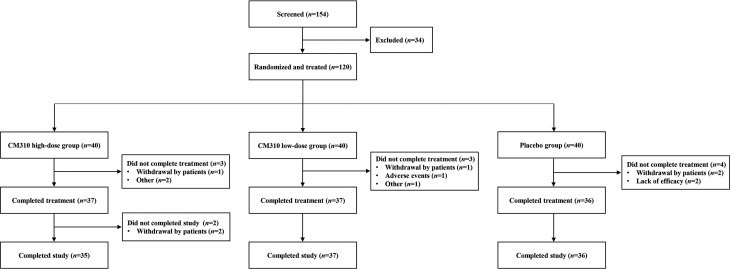
From February to November 2021, 120 patients were randomized to the study treatment (n = 40 per group) [Figure 1]. Altogether 108 (90.0%) patients completed the study, with 12 patients discontinuing the study mainly due to withdrawal of consent.
Figure 1.
Flow diagram of the recruitment of patients with moderate-to-severe atopic dermatitis.
No obvious differences in terms of demographic and baseline characteristics were identified between the treatment groups [Table 1]. All patients were adults with a mean age of 34.5 years, and most were male (79, 65.8%). About half (55, 45.8%) of the patients had moderate AD (IGA score of 3) and the other half (65, 54.2%) had severe AD (IGA score of ≥4).
Table 1.
Demographic and clinical characteristics of patients with moderate-to-severe atopic dermatitis at baseline (full analysis set).
| Characteristics |
CM310 high-dose (n = 40) |
CM310 low-dose
(n = 40) |
Placebo
(n = 40) |
Total
(n = 120) |
|---|---|---|---|---|
| Age (years) | 33.2 ± 13.2 | 36.7 ± 16.4 | 33.7 ± 14.9 | 34.5 ± 14.9 |
| Male | 26 (65) | 29 (73) | 24 (60) | 79 (66) |
| Height (cm) | 169.81 ± 8.90 | 168.73 ± 9.26 | 166.46 ± 8.61 | 168.33 ± 8.96 |
| Weight (kg) | 70.75 ± 14.66 | 68.91 ± 12.73 | 65.85 ± 13.48 | 68.51 ± 13.68 |
| BMI (kg/m2) | 24.40 ± 3.95 | 24.19 ± 4.14 | 23.64 ± 3.71 | 24.08 ± 3.92 |
| Baseline EASI score | 26.25 ± 10.41 | 30.07 ± 13.76 | 26.28 ± 11.14 | 27.53 ± 11.90 |
| Baseline IGA score of 3 | 21 (52) | 14 (35) | 20 (50) | 55 (46) |
| Baseline IGA score of ≥4 | 19 (48) | 26 (65) | 20 (50) | 65 (54) |
| Baseline weekly average of daily PP-NRS score | 7.38 ± 1.62 | 7.48 ± 1.38 | 7.42 ± 1.62 | 7.43 ± 1.53 |
| Baseline %BSA affected by AD | 42.96 ± 22.59 | 47.86 ± 22.00 | 43.31 ± 23.01 | 44.71 ± 22.46 |
| %BSA affected by AD | ||||
| ≥10 to <30 | 13 (32) | 10 (25) | 14 (35) | 37 (31) |
| ≥30 to <50 | 13 (32) | 17 (42) | 13 (32) | 43 (36) |
| ≥50 | 14 (35) | 13 (32) | 13 (32) | 40 (33) |
| Baseline DLQI score | 19.60 ± 5.90 | 17.90 ± 5.70 | 17.50 ± 6.40 | 18.30 ± 6.00 |
| Medical history reported in ≥10% of patients | ||||
| Rhinitis allergic | 14 (35) | 16 (40) | 16 (40) | 46 (38) |
| Hyperlipidemia | 8 (20) | 4 (10) | 2 (5) | 14 (12) |
| Hepatic steatosis | 7 (18) | 5 (12) | 2 (5) | 14 (12) |
| Hyperuricemia | 6 (15) | 4 (10) | 3 (8) | 13 (11) |
| Asthma | 2 (5) | 5 (12) | 6 (15) | 13 (11) |
| Conjunctivitis* | 2 (5) | 4 (10) | 2 (5) | 8 (7) |
| Eczema | 4 (10) | 1 (2) | 2 (5) | 7 (6) |
| Prior systemic treatment | ||||
| Systemic glucocorticoids | 6 (15) | 9 (22) | 5 (12) | 20 (17) |
| Immunosuppressants | 3 (8) | 3 (8) | 4 (10) | 10 (8) |
| JAK inhibitors | 3 (8) | 2 (5) | 0 | 5 (4) |
| Dupilumab | 2 (5) | 3 (8) | 0 | 5 (4) |
Data are presented as n (%) or mean ± standard deviation, unless otherwise indicated. *Conjunctivitis (MedDRA preferred term) included conjunctivitis and conjunctivitis allergic.%BSA: Percentage of body surface area; AD: Atopic dermatitis; BMI: Body mass index; DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; IGA: Investigator's Global Assessment; JAK: Janus kinase inhibitors; MedDRA: Medical Discovery for Regulatory Activities; PP-NRS: Peak pruritus Numerical Rating Scale.
Efficacy
In the full analysis set, the proportions of EASI-75 responders at week 16 were 70% (28/40), 65% (26/40), and 20% (8/40) in high-dose, low-dose, and placebo groups, respectively. The differences in EASI-75 response rate were 50% (high vs. placebo, 95% CI 31%–69%) and 45% (low vs. placebo, 95% CI 26%–64%), with both P values <0.0001. For both EASI-90 and EASI-50 response rates, the CM310 groups demonstrated statistically significant superiority over placebo at week 16 (high vs. placebo, P = 0.0038 and 0.0004, respectively; low vs. placebo, P = 0.0033 and 0.0172, respectively) [Table 2]. The proportions of EASI-50, EASI-75, and EASI-90 responders from baseline at each time point are shown in Supplementary Figure 1A–C, http://links.lww.com/CM9/B608.
Table 2.
Efficacy analysis of patients with moderate-to-severe atopic dermatitis (full analysis set).
| Items |
CM310 high-dose
(n = 40) |
CM310 low-dose
(n = 40) |
Placebo
(n = 40) |
|---|---|---|---|
| Patients with EASI-75 at week 16 | |||
| Number, n (%) | 28 (70) | 26 (65) | 8 (20) |
| Difference (95% CI) vs. placebo, % | 50 (31, 69) | 45 (26, 64) | |
| P value vs. placebo | <0.0001 | <0.0001 | |
| Patients with IGA 0–1 and ≥2-point improvement from baseline at week 16 | |||
| Number, n (%) | 9 (22) | 12 (30) | 5 (12) |
| Difference (95% CI) vs. placebo, % | 10 (–6, 26) | 18 (–0, 35) | |
| P value vs. placebo | 0.3781 | 0.0993 | |
| Patients with ≥2-point improvement in IGA from baseline at week 16 | |||
| Number, n (%) | 19 (48) | 22 (55) | 5 (12) |
| Difference (95% CI) vs. placebo, % | 35 (16, 54) | 42 (24, 61) | |
| P value vs. placebo | 0.0012 | 0.0001 | |
| Patients with EASI-90 at week 16 | |||
| Number, n (%) | 16 (40) | 19 (48) | 4 (10) |
| Difference (95% CI) vs. placebo, % | 30 (12, 48) | 38 (19, 56) | |
| P value vs. placebo | 0.0038 | 0.0004 | |
| Patients with EASI-50 at week 16 | |||
| Number, n (%) | 34 (85) | 32 (80) | 21 (52) |
| Difference (95% CI) vs. placebo, % | 32 (14, 52) | 28 (8, 47) | |
| P value vs. placebo | 0.0033 | 0.0172 | |
| Patients with ≥3-point reduction in weekly average of daily PP-NRS score from baseline at week 16 | |||
| Number, n (%) | 24 (60) | 23 (58) | 10 (25) |
| Difference (95% CI) vs. placebo, % | 35 (15, 55) | 33 (12, 53) | |
| P value vs. placebo | 0.0030 | 0.0060 | |
| Patients with ≥4-point reduction in weekly average of daily PP-NRS score from baseline at week 16 | |||
| Number, n (%) | 18 (45) | 15 (38) | 5 (12) |
| Difference (95% CI) vs. placebo, % | 33 (14, 51) | 25 (7, 43) | |
| P value vs. placebo | 0.0026 | 0.0188 | |
| LS mean percent change in weekly average of daily PP-NRS score from baseline to EOS, mean ± SE | –47.6 ± 5.8 | –33.0 ± 5.8 | –17.1 ± 6.4 |
| Difference (95% CI) vs. placebo, % | –30.5 (–47.6, –13.5) | –15.8 (–33.0, 1.3) | |
| LS mean percent change in EASI score from baseline to EOS, mean ±SE | –81.9 ± 5.2 | –70.5 ± 5.3 | –51.2 ± 5.9 |
| Difference (95% CI) vs. placebo, % | –30.7 (–46.4, –15.0) | –19.2 (–35.0, –3.4) | |
| LS mean percent change in AD-affected %BSA from baseline to EOS, mean ± SE | –74.2 ± 6.7 | –62.6 ± 6.7 | –33.6 ± 7.6 |
| Difference (95% CI) vs. placebo, % | –40.6 (–60.6, –20.6) | –29.0 (–49.0, –9.0) | |
| LS mean change in DLQI score from baseline to EOS, mean ± SE | –10.3 ± 1.1 | –6.5 ± 1.1 | –4.1 ± 1.2 |
| Difference (95% CI) vs. placebo, % | –6.2 (–9.4, –2.9) | –2.4 (–5.6, 0.9) |
%BSA: Percentage of body surface area; AD: Atopic dermatitis; CI: Confidence interval; DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; EOS: End-of-study; IGA: Investigator's Global Assessment; LS: Least-squares; PP-NRS: Peak pruritus Numerical Rating Scale; SE: Standard error.
The proportions of patients achieving an IGA score of 0/1 and a reduction of ≥2 points from baseline at week 16 were numerically higher in high-dose and low-dose groups than that in the placebo group (high vs. placebo, 22% vs. 12%, P = 0.3781; low vs. placebo, 30% vs. 12%, P = 0.0993); significant differences between high-dose and placebo groups were observed from weeks 20 to 24. The proportions of patients achieving ≥2-point reduction in IGA score from baseline at week 16 were statistically significantly higher in the CM310 groups (high vs. placebo, 48% vs. 12%, P = 0.0012; low vs. placebo, 55% vs. 12%, P = 0.0001) [Table 2 and Supplementary Figure 1D,E, http://links.lww.com/CM9/B608]. In addition, EASI-75 (high-dose [73%] and low-dose [71%] vs. placebo [18%]; both P values <0.0001) and IGA score of 0/1 with ≥2-point reduction (35% and 32% vs. 9%, P = 0.023 and 0.033, respectively) in the post hoc efficacy analysis were consistent with the full analysis set (data not shown).
The proportions of patients achieving ≥3-point improvements in the weekly average of daily PP-NRS scores from baseline at week 16 were statistically higher in the CM310 groups than that in the placebo group (high vs. placebo, 60% vs. 25%, P = 0.003; low vs. placebo, 58% vs. 25%, P = 0.006); similar results were observed in the case of the proportions of patients achieving ≥4-point improvements (high vs. placebo, 45% vs. 12%, P = 0.0026; low vs. placebo, 38% vs. 12%, P = 0.0188). The LS mean percent change from baseline in weekly average of daily PP-NRS score favored CM310 vs. placebo starting as early as week 2 (LS mean differences, –1.2% [95% CI –1.8% to –0.5%] for high vs. placebo and –0.8% [95% CI –1.5% to –0.2%] for low vs. placebo), and improved incrementally over the 16-week treatment period. Greater LS mean percent reductions in EASI score and %BSA affected from baseline to EOS and LS mean change in DLQI were found in the CM310 groups vs. placebo [Table 2 and Supplementary Figure 2, http://links.lww.com/CM9/B608].
Across prespecified subgroups based on the demographics and baseline disease characteristics (IGA score, EASI score, and %BSA affected), CM310 generally increased the proportions of patients achieving EASI-75, IGA 0/1 with ≥2-grade improvement, and a reduction of ≥3- and ≥4-point in weekly average of daily PP-NRS score at week 16 [Supplementary Figures 3 and 4, http://links.lww.com/CM9/B608].
During the trial, rescue medication use was less frequent in the CM310 groups than in the placebo group (high-dose, 5%; low-dose, 18%; placebo, 35%). In the sensitivity analyses, the proportion of EASI-75 responders at week 16 remained significantly higher in the CM310 groups than that in the placebo group [Supplementary Table 2, http://links.lww.com/CM9/B608]. Furthermore, both CM310 dose regimens showed greater improvements in the LS mean percent change in EASI score from baseline at week 16 compared to placebo [Supplementary Table 2, http://links.lww.com/CM9/B608].
Safety
The overall rates of TEAEs were similar between the CM310 and placebo groups (62/80 [78%] vs. 30/40 [75%]). Most TEAEs were mild or moderate [Table 3], and no deaths were reported. Four SAEs were reported in 3 (2%) out of 120 patients: hepatitis E in one patient in the low-dose group; infected dermal cyst in one patient and two spinal compression fractures in one patient in the placebo group. Spinal compression fractures led to drug discontinuation, while hepatitis E led to drug and study discontinuation. These four SAEs were considered unrelated to the study drug.
Table 3.
Adverse events of patients with moderate-to-severe atopic dermatitis (safety set).
| Items |
CM310 high-dose
(n = 40) |
CM310 low-dose
(n = 40) |
Placebo
(n = 40) |
|---|---|---|---|
| No. of TEAEs | 64 | 79 | 83 |
| Any TEAE | 30 (75) | 32 (80) | 30 (75) |
| Grade 3 | 0 | 1 (2) | 1 (2) |
| Grade 4/5 | 0 | 0 | 0 |
| No. of study drug-related TEAEs | 10 | 5 | 13 |
| Any study drug-related TEAE | 8 (20) | 5 (12) | 5 (12) |
| Grade 3 | 0 | 0 | 0 |
| Any SAE | 0 | 1 (2) | 2 (5) |
| TEAE leading to treatment discontinuation | 0 | 1 (2) | 1 (2) |
| TEAE leading to study discontinuation | 0 | 1 (2) | 0 |
| Death | 0 | 0 | 0 |
| TEAEs occurring in ≥5% of patients in any treatment group (MedDRA SOC or PT) | |||
| Investigations | 13 (32) | 14 (35) | 15 (38) |
| Blood bilirubin increased | 3 (8) | 2 (5) | 1 (2) |
| White blood cells urine positive | 3 (8) | 2 (5) | 0 |
| Blood uric acid increased | 2 (5) | 2 (5) | 1 (2) |
| Alanine aminotransferase increased | 1 (2) | 2 (5) | 2 (5) |
| Blood creatine phosphokinase increased | 2 (5) | 1 (2) | 2 (5) |
| Urinary occult blood positive | 0 | 2 (5) | 2 (5) |
| Blood triglycerides increased | 1 (2) | 1 (2) | 2 (5) |
| Blood lactate dehydrogenase increased | 1 (2) | 2 (5) | 1 (2) |
| Red blood cells urine positive | 0 | 0 | 3 (8) |
| Aspartate aminotransferase increased | 2 (5) | 0 | 1 (2) |
| White blood cell count increased | 0 | 0 | 2 (5) |
| Blood glucose increased | 0 | 2 (5) | 0 |
| Infections and infestations | 14 (35) | 9 (22) | 13 (32) |
| Upper respiratory tract infection | 8 (20) | 2 (5) | 4 (10) |
| Urinary tract infection | 2 (5) | 0 | 2 (5) |
| Skin infection | 0 | 3 (8) | 0 |
| Nasopharyngitis | 0 | 2 (5) | 1 (2) |
| Folliculitis | 1 (2) | 0 | 2 (5) |
| Skin and subcutaneous tissue disorders | 5 (12) | 6 (15) | 10 (25) |
| Dermatitis atopic | 0 | 3 (8) | 4 (10) |
| Urticaria | 3 (8) | 0 | 0 |
| Erythema | 0 | 0 | 2 (5) |
| Skin erosion | 0 | 0 | 2 (5) |
| Metabolism and nutrition disorders | 5 (12) | 6 (15) | 6 (15) |
| Hyperlipidemia | 4 (10) | 2 (5) | 1 (2) |
| Hyperuricemia | 0 | 5 (12) | 1 (2) |
| Gastrointestinal disorders | 1 (2) | 4 (10) | 3 (8) |
| Dental caries | 0 | 2 (5) | 0 |
| Hepatobiliary disorders | 1 (2) | 0 | 2 (5) |
| Hepatic function abnormal | 0 | 0 | 2 (5) |
| General disorders and administration site conditions | 1 (2) | 2 (5) | 0 |
| Injection site reaction | 0 | 2 (5) | 0 |
Data are presented as n (%), unless otherwise indicated. MedDRA: Medical Discovery for Regulatory Activities; PT: Preferred term; SAE: Serious adverse event; SOC: System organ class; TEAE: Treatment-emergent adverse event. Unless otherwise stated, data are presented as the number and percentage of patients. TEAEs were encoded by MedDRA v24.1.
The most common TEAEs (≥10% in any treatment group) were upper respiratory tract infection, AD, hyperlipidemia, and hyperuricemia [Table 3]. Of these, there was no imbalance in upper respiratory tract infection between the CM310 groups and the placebo group. Patients dosed with placebo reported higher incidence of AD than those treated with CM310 (placebo, 10%; high-dose, 0; low-dose, 8%), whereas hyperlipidemia and hyperuricemia were more common in CM310-treated patients. Conjunctivitis (MedDRA preferred term: conjunctivitis, conjunctivitis allergic, and conjunctivitis bacterial) occurred at a low rate, with one patient receiving 300 mg CM310 and two patients receiving placebo (all moderate) reporting the condition.
No significant abnormalities were observed in physical examination, vital signs, 12-lead electrocardiogram, or laboratory tests among the three groups.
Biomarker
The mean percent change in serum TARC decreased from baseline at week 16 in CM310-treated patients, but increased in placebo-treated patients (high vs. placebo, –63.42% vs. 29.89%, P = 0.0003; low vs. placebo, –49.99% vs. 29.89%, P = 0.0035). However, unlike the high-dose group, where serum TARC remained almost unchanged at week 24 (–61.35%), the mean percent change in serum TARC increased to 2.78% in the low-dose group. The mean percent change in serum total IgE also declined from baseline in CM310-treated patients, but increased in placebo-treated patients (at week 24: high vs. placebo, –54.92% vs. 43.53%, P <0.0001; low vs. placebo, –44.52% vs. 43.53%, P = 0.0001). At week 16, the mean change in lactate dehydrogenase from baseline was higher in the high-dose and low-dose groups than in the placebo group (high vs. placebo, –65.21 U/L vs. –4.57 U/L, P = 0.0001; low vs. placebo, –75.72 U/L vs. –4.57 U/L, P = 0.0005); the mean changes in blood eosinophil count were –0.099 × 109/L, –0.193 × 109/L, and –0.006 × 109/L in the high-dose, low-dose, and placebo groups, respectively (both P values >0.05) [Supplementary Figure 5, http://links.lww.com/CM9/B608].
Discussion
Over a 16-week treatment period, both CM310 regimens (150 mg and 300 mg every 2 weeks) produced marked improvements in AD clinical manifestations, including pruritus, skin lesions, and quality of life. Furthermore, CM310 was well-tolerated with a favorable safety profile.
Clinical trials of dupilumab have shown that dupilumab improves AD symptoms by blocking IL-4Rα.[12,16] Similar to dupilumab, CM310 also specifically binds to the IL-4Rα, blocking the signaling of both IL-4 and IL-13, thereby reducing type 2 inflammatory activity. A phase 3 study of dupilumab in China showed that 57.3% of patients with moderate-to-severe AD achieved EASI-75 response after 16-week treatment, along with an acceptable safety profile.[17] In this trial of CM310, a statistically significant higher proportion of CM310-treated patients achieved EASI-75 compared with placebo starting at week 4 and sustained through week 24. Notably, EASI-75 response rates were maintained in more patients receiving CM310 at 300 mg than 150 mg after drug withdrawal.
The CM310 groups did not exhibit significant superiority in the response rate for achieving an IGA score of 0/1 with ≥2-point reduction at week 16 compared with placebo. However, the response rate of IGA ≥2-point reduction was significantly higher in the CM310 groups than in the placebo group. Excluding the confounding impact of COVID-19, a significantly higher proportion of patients in the CM310 groups achieved an IGA score of 0/1 with ≥2-point reduction than placebo-treated patients.
Pruritus is a debilitating symptom of AD that causes patients to scratch, resulting in further aggravation of AD.[18] In the current trial, 16-week CM310 monotherapy, particularly the high-dose, displayed rapid and substantial amelioration in pruritus, as assessed by PP-NRS. More patients in the CM310 groups vs. the placebo group achieved ≥3- and ≥4-point improvements in the weekly average of daily PP-NRS score from weeks 4 and 6, respectively. Superiority over placebo was also found in the CM310 groups regarding percent change from baseline at week 16 in PP-NRS score. Moreover, in comparison with the CM310 low-dose group, the improvement in pruritus persisted up to week 24 in the CM310 high-dose group, suggesting that the high-dose regimen produced better clinical benefits in improving pruritus symptoms. Achieving an improvement in pruritus may contribute to the improvement of life quality.
CM310 demonstrated favorable tolerability and safety profile. The overall incidence of adverse events was similar across the CM310 and placebo groups, except for higher risks of skin and subcutaneous tissue disorders (including AD, erythema, and skin erosion) in placebo-treated patients. This difference could be attributed to the effect of CM310 in restoring skin-barrier function and decreasing scratching. Patients receiving 300 mg CM310 had a higher incidence of upper respiratory tract infection than those receiving 150 mg, but the incidence of treatment-related upper respiratory tract infection was similar between the two groups (5% vs. 2%). The findings may be impacted by possible external interference and limited sample size. Unlike more common TEAEs (e.g., conjunctivitis, injection site reaction, and nasopharyngitis) reported in dupilumab trials,[19,20] elevated rates of hyperlipidemia and hyperuricemia were observed in CM310-treated patients, presumably due to their daily diet. A higher incidence of hyperuricemia was found in the low-dose group (none was considered to be study drug-related), which might be affected by transient elevations of uric acid. In addition, the incidence of conjunctivitis was low in each cohort. Further studies of CM310 (e.g., ongoing phase 2 extension study and phase 3 study) are necessary to evaluate these AEs.
AD activity and severity have been reported to be associated with Th2-related biomarker levels.[21] In this trial, CM310 led to marked reductions in serum levels of total IgE and lactate dehydrogenase, with IgE decreasing slowly and continuously until week 20 and lactate dehydrogenase showing a rapid initial decrease during the first 4 weeks, followed by a subtle incremental decrease over the reminder of the treatment duration. TARC is a chemotactic factor that selectively attracts Th2 cells.[22] This study showed rapid decrease in serum TARC with CM310 vs. placebo from baseline to week 4, which was maintained through week 16. During safety follow-up period, however, serum TARC level returned to prior treatment in low-dose group and remained unchanged in high-dose group; similar trends were also seen in both CM310 groups regarding DLQI and PP-NRS, suggesting that TARC might be more sensitive to the efficacy of CM310 than other pharmacodynamic biomarkers.
This phase 2b study presents limitations as it focuses solely on Chinese patients with moderate-to-severe AD, and has a small sample size as well as a short treatment duration. Subsequent trials, such as the extension trial (CM310AD100), are underway to assess the long-term efficacy and safety of CM310 in a broader population with moderate-to-severe AD.
In conclusion, both CM310 regimens (150 mg and 300 mg every 2 weeks) significantly improved AD signs and symptoms and quality of life in adults with moderate-to-severe AD, with a favorable safety profile. This trial provides further evidence that IL-4Rα is a key target in the treatment of AD and that CM310 is a promising biologic therapy for adults with moderate-to-severe AD.
Acknowledgments
The authors thank the patients whose participation made this trial possible.
Funding
The development of CM310 has been partially funded by the National 13th Five-Year Plan for Major New Drug Development of China (No. 2017ZX09302010).
Conflicts of interest
This study was sponsored by Keymed Biosciences (Chengdu) Limited. Yingmin Jia, Guoqing Zhao, Jinchun Yan, and Bo Chen are employees of Keymed Biosciences (Chengdu) Limited. All other authors declare no competing interests.Availability of data requests should be addressed by e-mail to the corresponding author.
Supplementary Material
Footnotes
How to cite this article: Zhao Y, Zhang JZ, Yang B, Li JY, Ding YF, Wu LM, Zhang LT, Wang JY, Zhu XH, Zhang FR, Tao XH, Li YM, Zhang CL, Li LF, Lu JY, Diao QC, Lu QJ, Man XY, Li FQ, Xia XJ, Cheng H, Jia YM, Zhao GQ, Yan JC, Chen B. Efficacy and safety of CM310 in moderate-to-severe atopic dermatitis: A multicenter, randomized, doubleblind, placebo-controlled phase 2b trial. Chin Med J 2024;137:200–208. doi: 10.1097/CM9.0000000000002747
References
- 1.Chovatiya R. Atopic dermatitis (Eczema). JAMA 2023;329: 268. doi: 10.1001/jama.2022.21457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck LA Thaçi D Hamilton JD Graham NM Bieber T Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014;371: 130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 3.Liu P Zhao Y Mu ZL Lu QJ Zhang L Yao X, et al. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis. Chin Med J 2016;129: 757–762. doi: 10.4103/0366-6999.178960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rønnstad ATM, Halling-Overgaard AS, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: A systematic review and meta-analysis. J Am Acad Dermatol 2018;79: 448–456.e30. doi: 10.1016/j.jaad.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y Zhang L Ding Y Tao X Ji C Dong X, et al. Efficacy and safety of SHR0302, a highly selective janus kinase 1 inhibitor, in patients with moderate to severe atopic dermatitis: A phase ii randomized clinical trial. Am J Clin Dermatol 2021;22: 877–889. doi: 10.1007/s40257-021-00627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ständer S. Atopic dermatitis. N Engl J Med 2021;384: 1136–1143. doi: 10.1056/NEJMra2023911. [DOI] [PubMed] [Google Scholar]
- 7.Chung KF Dixey P Abubakar-Waziri H Bhavsar P Patel PH Guo S, et al. Characteristics, phenotypes, mechanisms and management of severe asthma. Chin Med J 2022;135: 1141–1155. doi: 10.1097/cm9.0000000000001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai X Utsunomiya R Shiraishi K Mori H Muto J Murakami M, et al. Nuclear IL-33 plays an important role in the suppression of FLG, LOR, keratin 1, and keratin 10 by IL-4 and IL-13 in human keratinocytes. J Invest Dermatol 2021;141: 2646–2655.e6. doi: 10.1016/j.jid.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Gieseck RL, 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 2018;18: 62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 10.Oetjen LK Mack MR Feng J Whelan TM Niu H Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017;171: 217–228.e13. doi: 10.1016/j.cell.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mennini M, Dahdah L, Fiocchi A. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2017;376: 1090. doi: 10.1056/NEJMc1700366. [DOI] [PubMed] [Google Scholar]
- 12.Blauvelt A de Bruin-Weller M Gooderham M Cather JC Weisman J Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017;389: 2287–2303. doi: 10.1016/s0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 13.Silverberg JI Simpson EL Ardeleanu M Thaçi D Barbarot S Bagel J, et al. Dupilumab provides important clinical benefits to patients with atopic dermatitis who do not achieve clear or almost clear skin according to the Investigator's Global Assessment: A pooled analysis of data from two phase III trials. Br J Dermatol 2019;181: 80–87. doi: 10.1111/bjd.17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thaçi D Simpson EL Deleuran M Kataoka Y Chen Z Gadkari A, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: A pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci 2019;94: 266–275. doi: 10.1016/j.jdermsci.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Simpson EL Bieber T Guttman-Yassky E Beck LA Blauvelt A Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375: 2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 16.Paller AS Siegfried EC Thaçi D Wollenberg A Cork MJ Arkwright PD, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol 2020;83: 1282–1293. doi: 10.1016/j.jaad.2020.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y Wu L Lu Q Gao X Zhu X Yao X, et al. The efficacy and safety of dupilumab in Chinese patients with moderate-to-severe atopic dermatitis: A randomized, double-blind, placebo-controlled study. Br J Dermatol 2022;186: 633–641. doi: 10.1111/bjd.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong J, Buddenkotte J, Berger TG, Steinhoff M. Management of itch in atopic dermatitis. Semin Cutan Med Surg 2011;30: 71–86. doi: 10.1016/j.sder.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deleuran M Thaçi D Beck LA de Bruin-Weller M Blauvelt A Forman S, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol 2020;82: 377–388. doi: 10.1016/j.jaad.2019.07.074. [DOI] [PubMed] [Google Scholar]
- 20.Thaçi D Simpson EL Beck LA Bieber T Blauvelt A Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: A randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016;387: 40–52. doi: 10.1016/s0140-6736(15)00388-8. [DOI] [PubMed] [Google Scholar]
- 21.Wollenberg A Beck LA Blauvelt A Simpson EL Chen Z Chen Q, et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: Results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br J Dermatol 2020;182: 1120–1135. doi: 10.1111/bjd.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catherine J, Roufosse F. What does elevated TARC/CCL17 expression tell us about eosinophilic disorders? Semin Immunopathol 2021;43: 439–458. doi: 10.1007/s00281-021-00857-w. [DOI] [PMC free article] [PubMed] [Google Scholar]