Abstract
Heart diseases cause over 17.9 million total deaths globally, making them the leading source of mortality. The aim of this review is to describe the characteristic mechanical, chemical and cellular properties of human cardiac tissue and how these properties can be mimicked in 3D bioprinted tissues. Furthermore, the authors review how current healthy cardiac models are being 3D bioprinted using extrusion-, laser- and inkjet-based printers. The review then discusses the pathologies of cardiac diseases and how bioprinting could be used to fabricate models to study these diseases and potentially find new drug targets for such diseases. Finally, the challenges and future directions of cardiac disease modeling using 3D bioprinting techniques are explored.
Keywords: bioprinting, cardiac tissues, cardiomyocytes, disease modelling, review, stem cells
Graphical abstract:
Tweetable abstract:
In this article, the authors discuss how #3dbioprinting can be used to model healthy and diseased cardiac tissue.
Executive summary
Background
Heart diseases, specifically cardiovascular disease, are the leading cause of global mortality.
The most common models used to study cardiac diseases include rodent models and 2D cell culture.
Properties of cardiac tissue & their translation to bioprinting applications
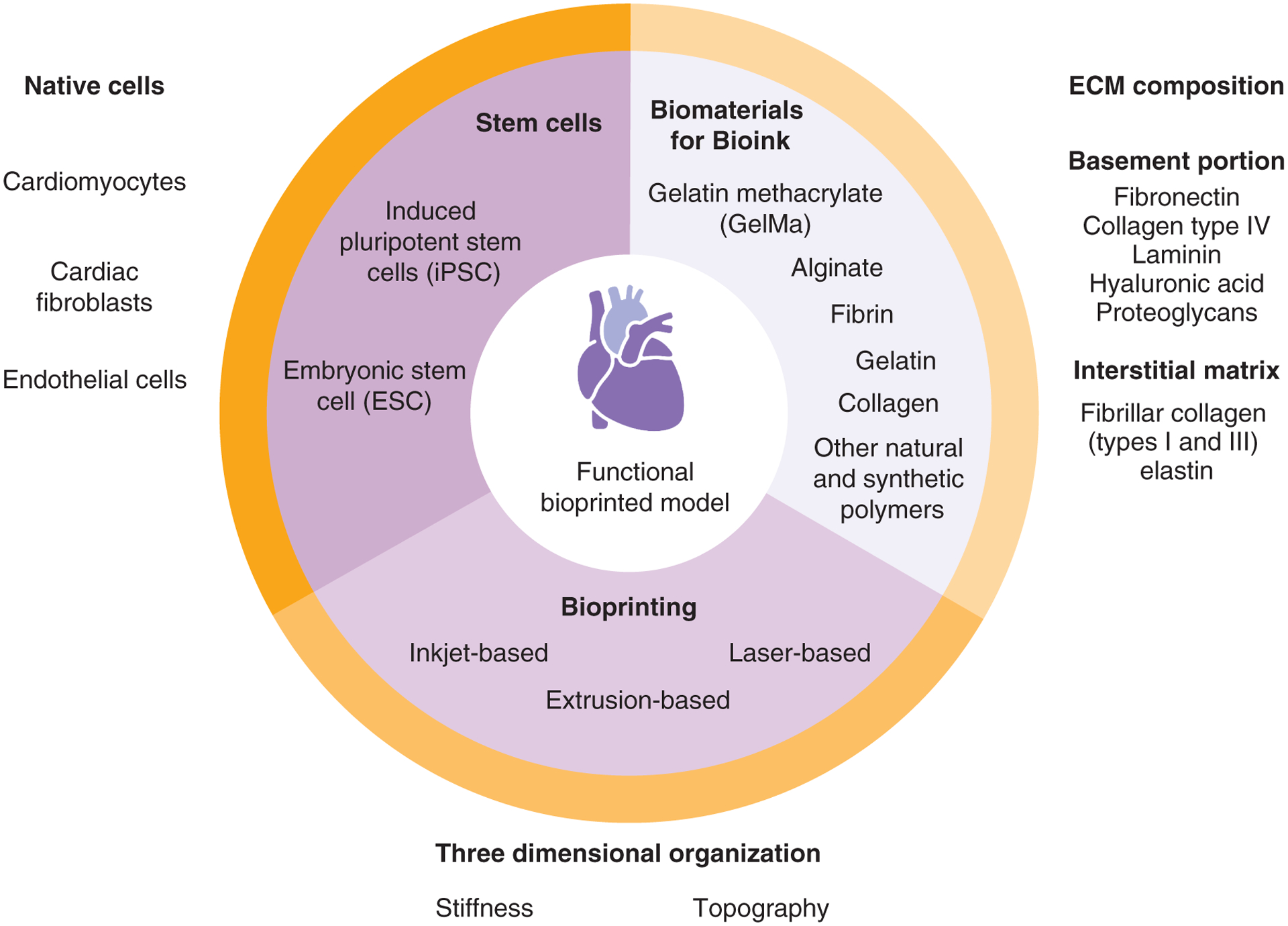
Biomaterials that can be used to mimic cardiac tissue include alginate, fibrinogen, collagen, gelatin-methacrylate, gelatin and other natural and synthetic polymers.
How healthy cardiac tissues are 3D bioprinted
Healthy cardiac models are being bioprinted using extrusion-, laser- and inkjet-based techniques.
Cardiac diseases & modeling techniques
Cardiac diseases can be grouped into four groups: coronary and vascular diseases, arrhythmias, structural heart diseases and acute conditions such as myocardial infarction and stroke.
3D modeling techniques include 3D printing/bioprinting and organoid models.
Current bioprinted models are mainly being used for regenerative medicine or as a preprocedural tool.
Potential challenges when 3D bioprinting models of cardiac diseases
Developing tissues with complex vascular networks will be challenging, and the biomaterials must not interfere with cardiac action potentials.
Large quantities of cells are required to print physiologically relevant models, and a variety of cells must be used, which poses its own challenges.
Future perspective
3D models will potentially serve as a tool for personalized modeling; however, this comes with challenges such as standardization, the safety and efficacy of the tissue and cost.
Bioreactors and machine learning could serve as tools to enhance the reproducibility and scalability of tissues.
WHO has classified cardiovascular disease as the leading cause of global mortality [1]. It was estimated that in 2019, 17.9 million people died from heart disease, representing 32% of all deaths globally [1]. In Canada alone, about 14 adults die every hour due to a diagnosed heart disease, making it the second leading cause of death in Canada [2], whereas in the USA, heart disease is the leading cause of death for both men and women [3]. It is also estimated that heart disease costs the USA $220 billion (USD) each year, which includes the cost of healthcare services and medication [3]. Heart disease can be classified by the function or structure of the heart it affects, including the arteries, blood vessels, ventricles, atria and muscles. First, coronary artery and vascular disease are the most common type of cardiovascular disease, and they occur when the coronary arteries and other blood vessels are blocked or narrowed, respectively [1]. Second, heart rhythm disorders (arrhythmias) cause the heart to beat too fast, too slow or irregularly and this causes sudden disruptions in blood flow [1]. Third, structural heart diseases are characterized as structural defects in different parts of the heart, including the valves, walls and muscles [1]. These diseases include cardiomyopathy, congenital heart disease and heart valve disease. Finally, heart conditions such as heart attack, stroke and heart failure are also a leading cause of death, but they are typically acute events that are caused by other heart conditions [1].
There are a variety of cardiac models that have been used to study new drugs and the pathophysiology of cardiac diseases. In vivo studies using animals such as mice, rats, rabbits, canines, sheep and pigs are the most widely used [4]. Out of all these animals, rodents (mice and rats) are the most popular because they are easier to handle, they are lower in cost and, since they have a short gestation period (~21 days), genetically modified models can be created in a short period of time [4]. Although studies using rodents provide valuable information about human cardiac diseases, some cardiovascular parameters are starkly different from humans [4,5]. For example, a rodent’s heart rate is between 310 and 840 b.p.m., whereas humans have an average heart rate of 72 b.p.m. [4]. A rodent’s heart also has different excitation and contraction properties when compared with a human heart, and the body weight of rodents (0.02–0.063 kg) is also very different from the average human weight (50–86 kg) [4]. These are all significant differences that can limit the translation of findings from rodent studies to humans. Larger animals such as canines, pigs and sheep would more closely resemble the human heart; however, the cost of acquiring these animals is significantly higher, making them less desirable [4]. The zebrafish blastema model has emerged as a useful cardiac model to study heart regeneration and human cardiovascular diseases [6,7]. Zebrafish are transparent and can easily be genetically manipulated, which makes them easy to work with for phenotypic assays [6]. However, the zebrafish heart has only two chambers with a single atrium and ventricle, so it does not directly mimic the human heart [6]. Researchers and pharmaceutical companies have also utilized 2D monolayer in vitro models to study cardiac diseases and drug efficacy in preclinical trials [5]. However, these models lack the complex microenvironment, physiological characteristics and functions of cardiac tissue. Thus, 3D-tissue models, including organs-on-chips, 3D-scaffolds and 3D-bioprinted models have grown in popularity, since they address the limitations of 2D models [5,8–11].
3D bioprinting has become one of the most advanced techniques to mimic the microenvironment of cardiac tissue [12]. This method can generate physiologically relevant models that can be used as an in vitro system to evaluate biological responses. This multidisciplinary technique is low-cost and efficient, and it allows researchers to generate highly defined geometries using biomaterials while maintaining cellular viability and functionality [13]. In general, the process of bioprinting consists of the simultaneous deposition of cells and biomaterials in a layer-by-layer fashion, forming a construct that can morphologically and structurally mimic native tissue architectures [14]. A variety of techniques are being used for cardiac tissue engineering, which include inkjet, extrusion and laser-based bioprinting [15,16].
One of the obstacles in bioprinting is finding a balance between printability and biocompatibility. The mechanical properties of the biomaterials must be compatible with the printing technique to achieve the desired resolution and it must mimic the native tissue to allow for the required dynamic cell behaviors [17]. For this reason, the fabrication of tissues requires suitable bioinks, a solution developed specifically to support cells and allow for proper printability. The distinct and complex biochemical composition of each tissue requires unique components to provide the necessary cues to maintain cell phenotype, viability, function and maturation [18]. However, for cardiac tissue, studies suggest that bioinks must also be electrically conductive to generate a functional model [19]. Most bioinks used in cardiac tissue engineering are naturally derived from humans or other animals. However, there are synthetic bioinks that some researchers have used to create cardiac models. For example, polyvinyl alcohol has been used as a sacrificial bioink, and a polyester urethane urea cardiac patch, with stem cells, was developed and implanted in a mouse model [20,21]. This review focuses on the most commonly used bioink components, including collagen and fibrin, because these are components that are naturally found in a human heart.
Current reviews focus on the challenges faced when bioprinting cardiac tissues in general, but there is a lack of literature that describes the potential challenges when bioprinting cardiac disease models. Thus, this review aims to discuss the current literature on how cardiac tissues have been bioprinted and the specific challenges faced when trying to 3D bioprint models of cardiac diseases, including arrhythmias, vascular disease and structural disease. First, the authors describe the important mechanical, chemical and cellular properties of cardiac tissue and how these properties can be mimicked in 3D bioprinted tissues. Next, they review how current healthy cardiac models are being 3D bioprinted. They also introduce the pathologies of cardiac diseases and how bioprinting could be used to create models to study these diseases and their potential challenges. Finally, future directions of cardiac disease modeling using 3D bioprinting techniques are discussed. This review aims to provide a concise perspective on bioprinting cardiac disease models, with the hope that it will help others understand potential challenges, so that better solutions can be developed. If patient-specific cardiac disease models can be engineered, it will reduce the need for animal models, which do not directly mimic the human heart, and thus will potentially increase the success of future therapies.
Properties of cardiac tissue & their translation to bioprinting applications
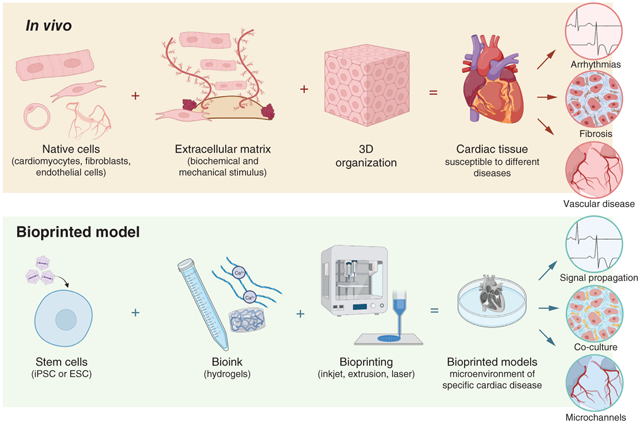
Figure 1 shows the different components of the heart and how bioprinting can be used to replicate these characteristics. This section discusses the properties of cardiac tissues in depth.
Figure 1. How the native properties of cardiac tissue, including the native cells and embryonic stem cell composition, can be translated to a functional 3D bioprinted model.
ECM: Extracellular matrix.
Cells found in cardiac tissue
To pump blood, individual cardiomyocytes synchronously contract and relax to generate rhythmic contraction–relaxation cycles. Atrial and ventricular cardiomyocytes form the muscular walls of the cardiac atrial and ventricular chambers, respectively. For blood to flow through the chambers, these cells exhibit different action potential (AP) properties [22]. Pacemaker cells, also known as nodal cardiomyocytes, are responsible for generating and dictating the heart’s rhythm, while Purkinje fibers are responsible for the orientation of the electrical stimulus throughout the heart [22]. Cardiac fibroblasts are one of the most abundant cells in the myocardium [23]. These cells surround cardiomyocytes and bridge tissue layers, contributing to the biochemical, mechanical and electrical properties of the heart [24]. Due to the proliferative potential of fibroblasts and their ability to synthesize extracellular matrix (ECM) proteins, the density of these cells in cardiac tissue must be kept at equilibrium; otherwise, a fibrous environment can emerge [24]. Finally, endothelial cells also play an important role in heart function. Forming the inner layer of blood and lymphatic vessels, endothelial cells can control vasomotor tone, blood flow, vascular permeability, leukocyte trafficking and angiogenesis [25]. Due to these functions, these cells are in constant communication with cardiomyocytes and fibroblasts, promoting angiogenic signaling, inflammation and ECM deposition [26].
Both 2D and 3D models utilize primary cells, cell lines or stem cells [5]. Primary cells are directly obtained from human tissue or that of other animal species and are not genetically or virally transformed, which allows them to maintain the cellular behaviors found in vivo [5]. However, primary cells have a short life span and limited proliferation capacity, and they require invasive surgical techniques [5]. For these reasons, cell lines are the most utilized cells in in vitro models, since they have unlimited proliferation capacity and are standardized [5]. However, their cellular behavior can easily change depending on the passage number and culture conditions [5]. Due to the limitations of these cells, stem cells have grown in popularity for modeling cardiac tissues due to their ability to differentiate to relatively pure (50–90%) cardiomyocytes (CMs) [5]. Their proliferation capacity and maturation can be adjusted depending on a variety of factors [5]. However, stem cells derived from embryos (ESCs) present various ethical issues and are difficult to obtain. Therefore, human-induced pluripotent stem cells (HiPSCs) are more commonly used because they are directly generated from somatic cells using the Yamanaka factors [27].
The ECM of cardiac tissue
In cardiac tissue, the ECM has structural functions by providing support and strength for the cells’ contractile movement [28]. The ECM also has nonstructural functions by accommodating multiple proteins with growth factors and cell receptor-binding properties [28]. Although the ECM has a wide range of roles in the maintenance of cardiac tissue, its hallmark is the ability to support a reliable behavior during events of dynamic mechanical load, such as pulsatile blood pressure and flow [28]. This relation is known as mechanobiology, and its effects on homeostasis are directly related to the proteins that compose the cardiac ECM [29]. In general, the ECM of cardiac tissue can be viewed as a basement membrane and interstitial matrix [30]. The basement portion of the ECM contains specialized molecules, such as fibrin, collagen type IV, laminin, hyaluronic acid and proteoglycans, which promote cellular functionality through interactions with surface receptors [31]. Fibrillar collagen (types I and III) and elastin make up the interstitial matrix of cardiac tissue, and these components are responsible for the structural and mechanical integrity of the tissue [32].
Cardiac tissue has several mechanical characteristics, all of which play important roles. Stiffness and topography show a significant impact on the behavior of cardiac cells [33]. Stiffness can be defined as a material’s resistance to deformation by an applied force, and it can be measured by Young’s modulus. Studies in rat myocardium have found that stiffness can significantly increase in diseased environments, such as infarcts and fibrosis [34]. Topography can be defined as the structural characteristics of the ECM at the surface level. Cardiac tissue has specific topography characterized by the cells’ parallel alignment, known as a Young’s modulus, which provides structural stability and tensile strength [35].
Biomaterials needed to mimic cardiac tissue
In designing bioink, it is vital that there is an understanding of the chemical, physical and mechanical properties, so that cardiac cells can be properly supported. Among the biomaterials used to mimic cardiac tissue and provide proper printability, some options seem to recur throughout studies, such as gelatin methacrylate, alginate, fibronectin and gelatin [12–14,17,21,36–39].
Gelatin methacrylate
Gelatin methacrylate is an engineered, gelatin-based material that has become popular in tissue engineering due to its versatility, biocompatibility and ability to photo cure [40]. When associated with high-resolution techniques, such as laser-based bioprinters, gelatin methacrylate was shown to be able to generate scaffolds with complex microarchitecture and able to reproduce stimulus of native cardiac ECM topography [41].
Alginate
Alginate is a natural polysaccharide derived from the cell walls of algae. Due to its unique properties and renewable origin, this material is becoming one of the most popular components of bioinks [42]. However, cells do not adhere well to this material; therefore, it has limited capacity for the maintenance of cardiac cells [43]. In addition, the use of alginate in cardiac implants suggests that the material is poorly conductive and can impede the propagation of cardiac AP [44]. These drawbacks can be solved when this material is associated in a bioink with other components. It was demonstrated that extruded constructs of alginate and platelet-rich plasma exhibit suitable mechanical properties and the viability of cardiac cocultures [21]. Also, in laser-based bioprinter applications, alginate has shown promising results when associated with carbon nanotubes, providing proper electrical and mechanical properties [19].
Fibrin
Fibrin is a fibrillar protein of extreme importance in the blood clotting cascade. Fibrin is a great biomaterial because it replicates the ECM and stimulates cell adhesion, proliferation and differentiation [45]. Moreover, fibrin is physiologically biodegradable through a mechanism that allows ECM replacement and integration when new tissue is formed [46]. This fibrillar protein exhibits structural integrity, having high tensile strength and adhesion strength, enabling cells to adhere to it, along with its biodegradability in soft tissues [46]. In 3D-bioprinted cardiac tissue, fibrin has been shown to support and help orientate iPSC-derived CMs [13]. Using a droplet-based bioprinter, fibrin has also been shown to help CMs proliferate and beat synchronously [14].
Gelatin
Gelatin is a natural material derived from collagen that is known for its large number of applications due its ability to solubilize in warm water and form physical hydrogels at low temperatures. For bioprinting, especially extrusion-based, this feature has been useful because bioink viscosity can be changed with temperature [47]. During the printing process, the gelatin physically gelatinizes and temporarily stabilizes the hydro gel scaffolds, reducing shear stress, and during incubation, this material melts [48]. Also, gelatin has enzymatic cleavage sites, which cells can degrade, so this material can be used as a temporary scaffold until the cells secrete their own ECMs [48]. However, due to the low mechanical stability of gelatin, it is usually modified with other components to be compatible with tissue-engineering applications [49]. In cardiac bioprinting, combinations of gelatin with fibrin and fibrinogen have shown promising results [36,38]. Additionally, gelatin can act as a sacrificial material, which makes it useful when printing complex architectures [37].
Collagen
Collagen is an abundant protein in the cardiac ECM and is known to promote cellular adhesion and mechanical strength, and it enables structural organization [50]. Although these reasons popularize collagen over cardiac tissue engineering, its use in bioprinting faces difficulties due its poor self-supporting property and low viscosity [19]. Unmodified collagen gelation is typically achieved through self-assembly driven by temperature, which is difficult to control [17]. For this reason, collagen is often modified or associated with other materials. To demonstrate the potential of this material when this drawback is counteracted, a modified pH change to drive collagen self-assembly was used to replicate a functional extruded construct with patient-specific anatomical structures [17]. Also, a newly developed form of type I collagen, named Viscoll, has shown promising viscoelastic properties in extrusion bioprinting, making it a potential alternative for cardiac tissue models [51].
Other materials
Other natural and synthetic polymers have shown interesting properties for cardiac tissue modeling. The combination of gelatin and hyaluronic acid seems to improve cardiac maturation and longevity in animal engraftments [52], suggesting this long-chain disaccharide could be useful for long-term models. Other interesting materials for cardiac tissue engineering include silk fibroin hydrogels; although these can form nanocrystals, when crosslinked with other materials, this natural polymer improved the mechanical and biological properties of bioprinted constructs [53]. As a result of its hydrophobic composition and polarity, silk fibroin offers a promising alternative to improve the properties of printed models in terms of strength, resistance and longevity.
Thermoplastics have been used, though not widely, for bioprinting cardiac tissue. Synthetic biodegradable polymers such as polylactide, polyglycolic acid and their copolymer, polylactide-glycolic acid, are attractive materials due to their strong mechanical properties, processing flexibility and low immunological responses [54]. Additionally, in one study, polylactide-glycolic acid associated with carbon nanofibers could align CMs while improving electrical and mechanical properties [55]. Also, surface treatments can help improve cell adhesion and proliferation [56]. As a result, these polymeric materials hold great potential for improving specific properties and developing more accurate models of cardiac tissue.
How healthy cardiac tissues are 3D bioprinted
This section discusses the methods for 3D bioprinting cardiac tissues. Figure 2 provides a visual comparison of these techniques for easy reference.
Figure 2. Comparison of inkjet-, extrusion- and laser-based bioprinters for cardiac model development.
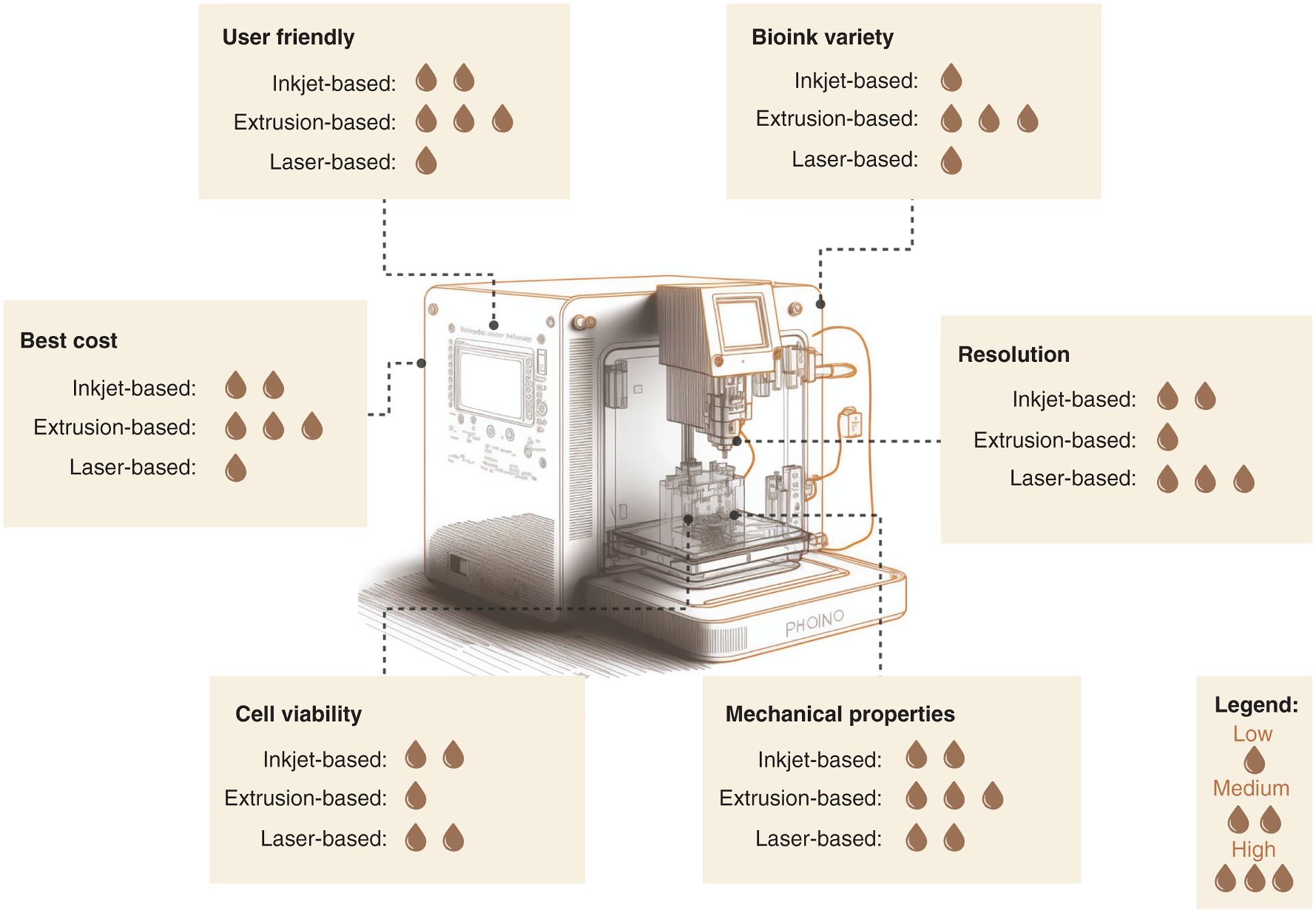
The highest cell viability was found in inkjet- and laser-based bioinks, whereas extrusion-based had the lowest. For mechanical properties, the extrusion-based provided more options. For resolution, the best was laser-based, followed by inkjet- and finally extrusion-based. For bioink variety, the best was extrusion-based bioprinters. The most and least user-friendly were extrusion- and laser-based bioprinters, respectively. Finally, the most affordable was extrusion-based, followed by inkjet and laser bioprinters.
Inkjet-based bioprinting
The origins of 3D bioprinting can be traced back to the initial stage of cellular bioprinted structures using inkjet printing technology, which is an approach first introduced by Thomas Boland et al. [57–59]. Inkjet-based bioprinting (IBB) is a method used frequently for biological applications. This technique deposits a defined volume of a cell-encompassing bioink onto a supporting material through distinctive energy sources (thermal and piezoelectric) and a droplet-based mechanism [16,58,60]. Effective deposition is contingent on the bioink’s possession of certain physical properties, including viscosity, density and surface tension [61]. The necessity for certain bioink properties produces limitations, as encountered by the bioink’s low-viscosity requisite, leading to constructs with deficient mechanical properties [15,57]. Even so, IBB can generate high-resolution 3D structures swiftly and at a low cost, as well as exhibit cellular viabilities of 80% [15,58,60,61].
The use of IBB in the fabrication of functional cardiac tissue was demonstrated by Xu et al. [62]. In this study, the authors printed layered 3D cardiac constructs with a particular “half heart” design composed of alginate/gelatin gels encapsulating CMs and crosslinked with CaCl2. Having undergone electrical simulations, the 3D cardiac pseudostructures exhibited functional excitation–contraction pairing, with visible rhythmic contraction of CMs within the 3D-printed structures, along with recurrent beating of the structures as a whole [62]. These results demonstrate IBB’s capability in producing functional cardiac constructs.
Extrusion-based bioprinting
Extrusion-based bioprinting (EBB) is the most popular 3D bioprinting technique when generating cardiac structures [15,63,64]. EBB utilizes a computer-controlled system to eject bioink strands through a nozzle and onto a surface, creating a layer-by-layer 3D structure [16,65]. Typically, the biomaterial is inserted into a metallic or plastic syringe and extruded by route of a pneumatic, piston-driven or screw-driven force [66]. The pneumatic mechanism utilizes air pressure to achieve extrusion, as opposed to the mechanical technique (i.e., piston- or screw-driven), which uses vertical and rotational forces [61]. EBB is characterized by its efficiency in depositing biomaterials with high cell densities (108–109 cells/ml), similar to physiological cell densities [15]. Using EBB’s multinozzle features and rapid print speed, intricate structures can be created using a variety of biomaterials and cell types [16]. There are, however, certain limitations of EBB, including low resolution (200 μm; contrasting laser- or inkjet- based processes), low cellular viability due to shear stress and highly viscous bioinks, causing harm to cellular function/morphology [15,66].
In a study conducted by Wang et al., the authors implemented EBB in the fabrication of functional cardiac structures using a three-axis stage system with multiple extruding modules containing pneumatic pressure control [65]. Cardiomyocytes isolated from infant rat hearts were encapsulated in a fibrin-based bioink, composing the hydro gel. The hydro gel, along with a sacrificial hydro gel and a sustaining polymeric frame, were extruded, producing cardiac structures exhibiting coordinated contraction while in culture, suggesting that the cells were mature. In a different study, Zhang et al. created vascularized cardiac tissue through EBB [67]. Endothelial cells (embedded in microfibrous hydro gel scaffolds via bioprinting) were granted the ability to migrate by using a composite bioink, generating a confluent endothelium layer, with the assembly of the endothelial cells echoing the architecture of blood vessels [67].
Laser-based bioprinting
According to Agarwal et al., laser-assisted bioprinting (LAB), or laser-induced forward transfer, employs a high-intensity laser, which impels the bioink droplets within a noncontact mode [16]. There are three primary components within LAB, including a pulsed laser beam; a target plate (the ribbon), which is covered by the bioink; and a receiving substrate. By means of a transparent ribbon, the laser beam passes through it and reaches the substrate, expelling a cell-loaded bioink onto the substrate. The substrate is typically covered with hydrogels, minimizing the impact of preceding situated droplets. Programmable features of LAB include laser frequency, intensity and motion control. With LAB being a nozzle-free process, nozzle clogging is avoided. LAB allows for high cell densities (~108 cells/ml) owning high resolution (10–100 μm) and permits an ample range in biomaterial viscosities (1–2000 mPa/s) [16]. The LAB technique, however, is also accompanied by certain limitations, including its limited capability of expelling various cell types. LAB can also be expensive, is a sluggish process, and is commonly characterized by small structures with limited clinical applications [16].
In a study conducted by Gaebel et al., the authors created a cardiac patch and seeded human umbilical vein endothelial cells and human mesenchymal stem cells on a polyester urethane urea cardiac patch [20]. Cultivation of the cardiac patches was performed in vitro or transplanted in vivo onto the portion of the heart being infarcted. The results conveyed modified growth characteristics of cocultured human umbilical vein endothelial cells and human mesenchymal stem cells, making it possible to achieve an increased vessel formation. Prominent functional improvement of infarcted hearts after transplantation of a laser-induced forward transfer tissue-engineered cardiac patch was also noted [20].
Cardiac diseases & modeling techniques
Coronary artery & vascular diseases
Coronary artery disease (CAD) is caused by the blockage or narrowing of coronary arteries, which is commonly due to the buildup of plaques (atherosclerosis) [1]. This causes a mismatch between myocardial oxygen supply and demand. Other common vascular diseases include cerebrovascular disease and peripheral arterial disease, which affect the blood vessels supplying the brain and arms/legs, respectively [1]. The treatment for these diseases includes revascularization and various pharmacological therapies using antiplatelet agents and statins [68]. Revascularization is where blood flow is restored to the heart by performing an angioplasty or stenting procedure [69]. 3D bioprinting has gained popularity in revascularization procedures, specifically with stenting. For example, Lu et al. 3D printed a bioresorbable stent with the goal of treating cerebrovascular disorders [11]. They developed a novel stent that enabled antistenosis and disappeared after vessel endothelization [11]. Endothelial cells were also seeded in the stents and good proliferation capabilities were observed [11]. 3D bioprinting has been used for regenerative purposes with the idea of creating patient-specific tissue or stents that can be implanted. However, there are no 3D-bioprinted models to study diseases or new drug targets. These studies are usually performed in animal models such as rodents or porcine. 3D printing, on the other hand (no cells or biomaterials), has been used extensively to create models of complex coronary anomalies, which have been used to simulate potential vascular surgical procedures [70–72]. Aside from 3D-printed models, organoid models have also been used as a potential tool for studying cardiovascular diseases. For example, a study by Liang et al. developed vascularized cardiac organoids by differentiating hiPSCs via the Wnt signaling pathway to cardiomyocytes and endothelial-like cells [73]. Their chambered model exhibited more mature membrane potentials and it proved to be a better model for studying cardiotoxicity [73].
Arrhythmias
Cardiac arrhythmias can be classified into two groups, bradyarrhythmia and tachyarrhythmia, both of which are caused by abnormalities in electrical impulses of the myocardium. Bradyarrhythmias are slow heart rate arrhythmias (<60 beats/min) and there are two main types: sinus bradycardia, which originates from the sinus node, and atrioventricular blocks, which are characterized by an interruption or delay of the electrical signals between the atria and ventricles [5]. On the contrary, tachyarrhythmias are fast heart rate arrhythmias (>100 beats/min), which can originate in the sinoatrial node, atrial myocardium or atrioventricular node or below the atrioventricular node [74]. Common subtypes of this disease include atrial fibrillation, atrial tachycardia, ventricular tachycardia and atrial flutters [74]. Rhythmic disorders have mainly been studied in 2D models and animal models [74]. However, a recent study developed human 3D microtissues generated by embedding HiPSC-derived CMs in a microwell system [75]. After disrupting gap junctions using cyclodextrin, the authors found that the microtissues started to exhibit rhythmic disorders [75]. Although this study did not use 3D bioprinting techniques, it has been suggested that 3D bioprinting may be useful when developing antiarrhythmia drugs because models can be engineered using cells derived from patients with a genetic predisposition to arrhythmias [76].
Structural heart diseases
Heart diseases can vary and may present themselves due to other health conditions or they may be present at birth. The latter are known as congenital heart disease, and it affects about one in four children [77]. There are varying levels of severity from mild to critical; for example, atrial septal defect can be considered a mild congenital heart disease because it is caused by a small hole in the wall dividing the right and left atria [78]. On the other hand, coarctation of the aorta is considered a critical congenital heart disease because a portion of the aorta is abnormally narrow, which prevents oxygen-rich blood from being sent to the rest of the body [78]. Cardiomyopathies, another type of structural heart disease, affects the heart muscle by causing either the walls of the heart to thicken (hypertrophic) or the chambers of the heart to become too large (dilated) [79]. These diseases can be acquired from other pre-existing conditions such as arrhythmias, long-term blood pressure or other health issues, but they can also be inherited [79]. There are several components to managing cardiomyopathies, including pharmacological approaches and lifestyle changes. However, severe cases require heart transplantation, ablation procedures or surgically implanted devices. 3D bioprinting could serve as an alternative approach for some of these severe cases. For example, Park et al. developed a stem cell-laden 3D-bioprinted cardiac patch, which was used for ischemic cardiomyopathy caused by myocardial infarction (heart attack) [80]. After a heart attack was induced in a mouse model, the patch reduced scar tissue formation and improved cardiac function [80]. Another study developed a 3D cardiac coculture model containing CMs, fibroblasts and microvalvular endothelial cells to study the affects of microgravity [81]. The authors also suggest that the model can be used to study cellular crosstalk in cardiac atrophy to better understand the disease pathology [81]. 3D heart organoid models have also been developed by Lewis-Israeli et al. to study congenital heart defects [82]. This group differentiated hiPSCs using a three-step Wnt signaling modulation procedure to develop a heart model. Their multicellular models developed chambers and complex vasculature, which were used to re-create a metabolic disorder that is associated with congenital heart defects [82].
Potential challenges when 3D bioprinting models of cardiac diseases
Figure 3 shows how different types of heart disease can be modeled using bioprinting. This section details the challenges of modeling these different types of diseases.
Figure 3.
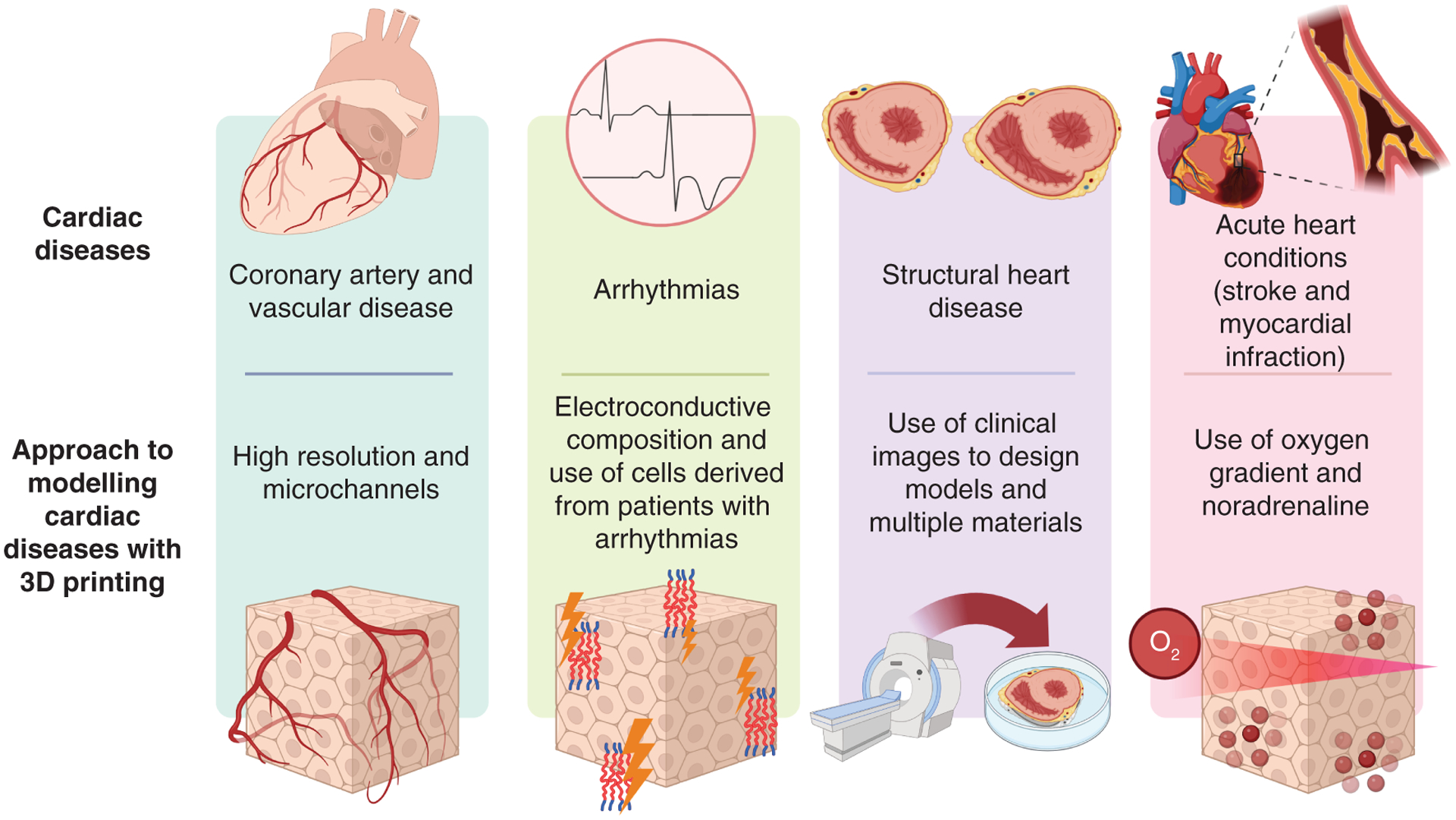
How cardiac diseases (vascular, arrhythmia, structural and acute conditions) can be modeled using 3D bioprinting.
CAD & vascular disease
Vascularization is the most important factor when trying to mimic vascular-based diseases such as CAD. The most common strategy when developing vascularized tissue is using iPSCs from patients and differentiating them to CMs and endothelial cells to generate a coculture model [13,37,38]. For the bioink, hydrogels containing a patient’s ECM have been shown to generate parenchymal cardiac tissue and blood vessels [37,38]. Natural bioinks such as alginate, collagen and fibrinogen have also demonstrated the ability to support multicellular tissues [13,17,21]. However, due to the complexity of human vascular networks, one of the major challenges when engineering vascularized tissue is the poor resolution of the bioprinters. To overcome this, the use of freeform reversible embedding of suspended hydrogels (FRESH) or sacrificial materials has been useful [17,21]. Using the FRESH technique and modified collagen to crosslink through pH, a 20 μm filament resolution was generated and shown to provide rapid cellular infiltration and microvascularization [17]. Also, studies using polyvinyl alcohol as sacrificial material have shown improvement over the flexibility of printed construct, as well as the ability to generate microfluidic channels for endothelial vascularization [21]. Another challenge to overcome is how to arrange the cardiac cells in a way that is physiologically relevant [35]. Hydro gel frames are a new strategy used to orient cells in a desired 3D shape [13,38]. Although a lot of progress has been made in producing vascular tissues, this challenge continues to be one of the main limitations when generating diseased cardiac models, specifically for vascular disease [39].
Arrhythmias
The rhythm of the heart is controlled by cardiac APs, which cause coordinated contractions of CMs, followed by relaxation. These cardiac APs are a result of cell-membrane channels opening and closing, releasing ions and causing AP signals from one cell to another [83]. Some arrhythmias are caused by changes in ionic currents through the Na+ or Ca2+ ion channels [83]. For this reason, when engineering an arrhythmia model using bioprinting techniques, it is important that the biomaterials and printing technique used do not interfere with ionic currents. As mentioned previously, alginate is a biomaterial that has great printability characteristics, but it can interfere with the propagation of APs and has poor conductive properties [44]. Therefore, in arrhythmic models, it may be best to exclude alginate as a biomaterial and incorporate collagen or gelatin. On the other hand, if alginate is necessary, other electroconductive materials can be added to the bioink, so that signals can be transmitted cell to cell. A fine balance would also need to be found where enough alginate is added, so that the biomaterial is printable, and the concentration of the conductive material is adequate to allow for AP signals to propagate. For example, Roshanbinfar et al. incorporated PEDOT:PSS (an electroconductive polymer) into a collagen–alginate hydro gel, making a fibrous microstructure that was similar to native cardiac ECM [84]. It was found that the primary CMs had improved maturation and beating properties [84]. For the cell source, cells derived from patients with a family history of arrhythmias may be the most ideal for disease and drug studies [76]. To extend, hiPSCs could be reprogrammed from the somatic cells of a patient and differentiated to CMs, so that a 3D microphysiological model could be developed, allowing disease progression to be studied and new antiarrhythmic drugs to be found [76].
Structural heart diseases
To model structural heart diseases such as cardiomyopathies and other neonatal diseases, it is more important that a human-size physiological model be created rather than a microphysiological model. This is because structural irregularities typically occur between different chambers of the heart, so all these components should be mimicked in the model. In this case, FRESH bioprinting may serve as a powerful tool because larger and more complex models can be created [85]. Additionally, FRESH bioprinting has higher resolution, allowing vascular architectures to be constructed [85]. Patient-specific models can also be constructed by using high-quality clinical images of the heart, segmenting it into layers and bioprinting each layer using either laser-based bioprinters or extrusion-based bioprinters [86]. This would allow researchers to study uncommon structural diseases that are patient-specific, so that preprocedural planning, device sizing and disease studies could be conducted [86]. Apart from the bioprinting technique, modeling of these diseases would require a multitude of cell lines, including CMs, fibroblasts, endothelial cells, pacemaker cells and others, so that the full heart structure could be accurately produced. Also, various biomaterials would also need to be incorporated, so that each chamber of the heart and myocardium could be mimicked. This is a large feat that will most likely require multiple biomaterials and bioprinting techniques.
Acute heart conditions (stroke & myocardial infarction)
Although strokes and heart attacks occur due to other, pre-existing heart conditions, the end stage of these acute events result in the damaging of the myocardium, which can cause an increase in arrhythmic disorders and reduce cardiac functionality [87]. During these events, the release of noradrenaline by the adrenergic nervous system increases in an attempt to restore cardiac functionality by augmenting its contractility, which can further damage the tissue [87]. For this reason, a potential strategy to study these acute conditions is to explore the cross-communication between the adrenergic nervous system and damaged myocardium [88]. To date, no bioprinted models have been developed to study myocardial infarction. Instead, 3D studies using spheroids have successfully mimicked the desired environment [89]. To achieve this, a coculture model containing cardiac cells was exposed to a gradient of oxygen concentrations; then noradrenaline was added to the spheroids to induce an apoptotic response and generate “infarction gradients” that were able to mimic zones of infarcted cardiac tissue. With this model, it was possible to observe a reduction of calcium-handling protein expression, CM death and fibrosis, allowing the assessment of responses to clinically relevant drugs [89]. In this way, 3D bioprinting strategies could be incorporated into this approach to include mechanical loading and other conditions that better replicate the functional characteristics of these conditions. Although multiple cell lines can be used when 3D bioprinting, the inclusion of immune cells is still challenging due to the complexities of the in vivo pathways, and the effects of reperfusion injury are difficult to replicate (Table 1) [17,21].
Table 1.
Outlining the different model designs, materials, cell types, printing methods and functionalities of all 3D bioprinting cardiac models discussed in this review.
| Study | Model design | Materials | Cell type | Printing method | Functionality | Ref. |
|---|---|---|---|---|---|---|
| Xu et al. | Half heart with a 1 cm inner diameter and two connected ventricles | Alginate/gelatin crosslinked with calcium chloride | Mammalian cardiomyocytes | Inkjet-based bioprinting | Microscopic and macroscopic contractile function in vitro | [62] |
| Wang et al. | Constructs either string form or patch form; entire construct was 1.8 × 1.6 cm2 and 0.6 mm thick | Fibrin, gelatin, aprotinin, glycerol, and hyaluronic acid; a sacrificial hydro gel of gelatin, glycerol and hyaluronic acid used to support the cell-laden hydrogel while printing | Cardiomyocytes from infant rat hearts | Extrusion-based bioprinting | Spontaneous synchronous contraction; cardiac tissues were formed with electromechanically coupled cardiac cells | [65] |
| Zhang et al. | 3D microfibrous scaffolds with anisotropic arrangements (crosshatch pattern) | Alginate and gelatin methacryloyl | Human umbilical endothelial cells and neonatal rat cardiomyocytes | Extrusion-based bioprinting | Aligned endothelialized myocardium with spontaneous and synchronous contraction capabilities | [67] |
| Gaebel et al. | Human umbilical vein endothelial cells printed in two layers in an orthogonal grid pattern with a 90 μm grid-line distance, followed by mesenchymal cells in two layers | Polyester urethane urea | Human umbilical endothelial cells and human mesenchymal stem cells | Laser-based bioprinting | Observed vascular tube formation; functional improvement of infarcted hearts after transplantation in rats | [20] |
| Lu et al. | Symmetrical stent structure with wires forming a uniform diamond shape | Poly (p-dioxanone)and Stabaxol −1 | Endothelial cells | Extrusion-based bioprinting | Good proliferation when endothelial cells were seeded into the bioresorbable stents | [11] |
| Park et al. | Patches with a thickness of 3 mm and diameter of 8 mm | Heart-derived extracellular matrix hydro gel | Bone marrow-derived mesenchymal stem cells | Extrusion-based bioprinting | High cell survival rate and significant improvements in cardiac function and vessel formation | [80] |
| Alonzo et al. | Annular ringlike scaffolds | Gelatin and alginate | Human cardiomyocytes, fibroblasts and microvascular endothelial cells | Extrusion-based bioprinting | Heterocellular cardiac cell interactions, paracrine signaling and cardiac contractions | [81] |
Future perspective
In terms of 3D bioprinting cardiac tissues, we speculate that cardiac models will become more intricate with vascular networks and the inclusion of immune cells, which will allow researchers to better mimic diseases such as CAD, arrhythmias and acute heart conditions. This will require researchers to merge various bioprinting techniques simultaneously to accurately reproduce the complexities of human cardiac tissue.
Additionally, physicians have been moving toward personalized medicine to better identify patient-specific treatment, and 3D-printed models using patient-derived iPSCs can serve as a tool for these personalized models. Also, autologous hiPSC cardiac models could serve as transplantable tissues with potentially lower immune rejections, since the patients’ own cells would be used. As an example, recent clinical trials are transplanting patient-derived MSCs for ear restoration, which paves the way for implanting cardiac tissues. Despite this progress, there are several challenges associated with 3D bioprinting in clinical trials. One of the main challenges is ensuring the safety and efficacy of the printed tissue. Due to the relative youth of 3D printing technology, there is not much standardization in the field, which makes it difficult to ensure that printed tissue meets safety and efficacy requirements. Furthermore, it is difficult to predict potential complications or side effects associated with 3D-printed tissue due to a lack of understanding of its long-term effects. Moreover, the technology is expensive and complex, and the 3D bioprinting process is highly specialized and technical, requiring significant investment in equipment, materials and expertise.
With respect to model development, implementing this technology poses a challenge due to the intrinsic difficulty of growing cells, as well as incubating and maintaining printed constructs, processes that require extensive human involvement and limit the development of human-size models. Consequently, improvements in bioreactor use will be necessary to overcome these limitations and enhance reproducibility and scalability. In addition, to achieve functional cardiac models, bioprinting is an essential process that permits the incorporation of necessary elements in fabricating desired architectures and specific functions. Thus, optimization of the printing parameters is crucial when creating viable tissues. In order to overcome these difficulties, machine learning is a promising approach. Supervised and unsupervised algorithms have proven robust computational capacity, allowing an association among process, material and performance with regard to the bioprinting process, thus demonstrating the possibility of utilizing machine learning in enhancing manufacturability and the quality of attained structures [90]. With the complexity of cardiac tissue, it is vital to optimize the bioprinting process and identify standard printing parameters that will achieve high throughput and consistency of developed models.
Conclusion
3D bioprinting serves as a powerful tool for generating models of cardiac tissues especially when combined with stem cells. This process enables the study of cardiovascular diseases with several advantages for modeling such diseases in comparison with 2D cell culture and animal models. Future work will address challenges with this process, including scaling up cell culture production and translating this work for clinical applications.
Financial & competing interests disclosure
SM Willerth is the founder and CEO of Axolotl Biosciences, a biotechnology company focused on 3D printing. This work was supported by an NSERC Discovery Grant, the Canadian Research Chairs program, and the Canadian Institutes of Health Research Project Grant Program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Cardiovascular Diseases (CVDs). WHO, Geneva, Switzerland: (2021). www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [Google Scholar]
- 2.Heart Diseases in Canada. Public Health Agency of Canada, Ottawa, Canada: (2021). www.canada.ca/en/public-health/services/publications/diseases-conditions/heart-disease-canada.html [Google Scholar]
- 3.Heart Disease Facts. Centers for Disease Control and Prevention, MD, USA: (2022). www.cdc.gov/heartdisease/facts.htm [Google Scholar]
- 4.Milani-Nejad N, Janssen PML. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharm. Ther 141(3), 235–249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article provides interesting insights into the advantages and disadvantages of small and large animal models, which is important for this review, since it provides insights into the need for other types of models such as bioprinted models.
- 5.Savoji H, Mohammadi MH, Rafatian N et al. Cardiovascular disease models: a game changing paradigm in drug discovery and screening. Biomaterials 198, 3–26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asnani A, Peterson RT. The zebrafish as a tool to identify novel therapies for human cardiovascular disease. Dis. Model. Mech 7(7), 763–767 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 29(11), 611–620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan AJ, Brougham CM, Garciarena CD, Kerrigan SW, O’Brien FJ. Towards 3D in vitro models for the study of cardiovascular tissues and disease. Drug Discov. Today 21(9), 1437–1445 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov 14(4), 248–260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rioux Y, Fradette J, Maciel Y, Bégin-Drolet A, Ruel J. Biofabrication of sodium alginate Hydro gel scaffolds for heart valve tissue engineering. Int. J. Mol. Sci 23(15), 8567 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Hu X, Yuan T et al. 3D-printed poly (p-dioxanone) stent for endovascular application: in vitro evaluations. Polymers 14(9), 1755 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bejleri D, Streeter BW, Nachlas ALY et al. A bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv. Healthc. Mater 7(23), 1800672 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiullari F, Costantini M, Milan M et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep 8(1), 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chikae S, Kubota A, Nakamura H et al. Three-dimensional bioprinting human cardiac tissue chips of using a painting needle method. Biotechnol. Bioeng 116(11), 3136–3142 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Khanna A, Ayan B, Undieh AA, Yang YP, Huang NF. Advances in three-dimensional bioprinted stem cell-based tissue engineering for cardiovascular regeneration. J. Mol. Cell. Cardiol 169, 13–27 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal T, Fortunato GM, Hann SY et al. Recent advances in bioprinting technologies for engineering cardiac tissue. Mater. Sci. Eng. C 124, 112057 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A, Hudson AR, Shiwarski DJ et al. 3D bioprinting of collagen to rebuild components of the human heart. Science (New York, NY) 365(6452), 482–487 (2019). [DOI] [PubMed] [Google Scholar]; • With the extrusion method combined with freeform reversible embedding of suspended hydrogels, high-resolution collagen constructs were bioprinted successfully with functional results.
- 18.Yu C, Ma X, Zhu W et al. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 194, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izadifar M, Chapman D, Babyn P, Chen X, Kelly ME. UV-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng. Part C Methods 24(2), 74–88 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Gaebel R, Ma N, Liu J et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 32(35), 9218–9230 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Zou Q, Grottkau BE, He Z et al. Biofabrication of valentine-shaped heart with a composite hydro gel and sacrificial material. Mater. Sci. Eng. C 108, 110205 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Ng SY, Wong CK, Tsang SY. Differential gene expressions in atrial and ventricular myocytes: insights into the road of applying embryonic stem cell-derived cardiomyocytes for future therapies. Am. J. Physiol. Cell Physiol 299(6), C1234–C1249 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol 293(3), H1883–H1891 (2007). [DOI] [PubMed] [Google Scholar]
- 24.MacKenna D Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc. Res 46(2), 257–263 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Talman V, Kivelä R. Cardiomyocyte–endothelial cell interactions in cardiac remodeling and regeneration. Front. Cardiovasc. Med 5(101), 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation 122(9), 928–937 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4), 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Fomovsky GM, Thomopoulos S, Holmes JW. Contribution of extracellular matrix to the mechanical properties of the heart. J. Mol. Cell. Cardiol 48(3), 490–496 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin IL, Hool L, Choi YS. A review of in vitro platforms for understanding cardiomyocyte mechanobiology. Front. Bioeng. Biotechnol 7(133), 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva AC, Pereira C, Fonseca ACRG, Pinto-Do-Ó P, Nascimento DS. Bearing my heart: the role of extracellular matrix on cardiac development, homeostasis, and injury response. Front. Cell Dev. Biol 8(621644), 8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebleu VS, MacDonald B, Kalluri R. Structure and function of basement membranes. Exp. Biol. Med. (Maywood, NJ) 232(9), 1121–1129 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Lindsey ML, Jung M, Hall ME, Deleon-Pennell KY. Proteomic analysis of the cardiac extracellular matrix: clinical research applications. Expert Rev. Proteom 15(2), 105–112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan W, Bjorkman KK, Choi ES, Panepento AL, Anseth KS, Leinwand LA. Cardiac myocytes respond differentially and synergistically to matrix stiffness and topography. doi: 10.1101/682930 (2019) (Epub ahead of print). [DOI]
- 34.Bhana B, Iyer RK, Chen WLK et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng 105(6), 1148–1160 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Ariyasinghe NR, Lyra-Leite DM, McCain ML. Engineering cardiac microphysiological systems to model pathological extracellular matrix remodeling. Am. J. Physiol. Heart Circ. Physiol 315(4), H771–H789 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anil Kumar S, Alonzo M, Allen SC et al. A visible light-cross-linkable, fibrin–gelatin-based bioprinted construct with human cardiomyocytes and fibroblasts. ACS Biomater. Sci. Eng 5(9), 4551–4563 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A bioink made of fibrin and gelatin able to crosslink with visible light was developed to increase the cell viability of bioprinted cardiac cells.
- 37.Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci 6(11), 1900344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Functional vascularized constructs were bioprinted; this feature improved cell maintenance and allowed thicker constructs to be generated.
- 38.Tsukamoto Y, Akagi T, Akashi M. Vascularized cardiac tissue construction with orientation by layer-by-layer method and 3D printer. Sci. Rep 10(1), 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • A new approach based on using layer-by-layer overlapping allows orientation and vascularization of cardiac constructions.
- 39.Koti P, Muselimyan N, Mirdamadi E, Asfour H, Sarvazyan NA. Use of GelMA for 3D printing of cardiac myocytes and fibroblasts. J. 3D Print. Med 3(1), 11–22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klotz BJ, Gawlitta D, Rosenberg AJWP, Malda J, Melchels FPW. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotech. 34(5), 394–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Miller K, Ma X et al. Direct 3D bioprinting of cardiac micro-tissues mimicking native myocardium. Biomaterials 256, 120204 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawar SN, Edgar KJ. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials 33(11), 3279–3305 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Pati F, Jang J, Ha D-H et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Comm. 5(1), 1–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izadifar M, Babyn P, Kelly ME, Chapman D, Chen X. Bioprinting pattern-dependent electrical/mechanical behavior of cardiac alginate implants: characterization and ex vivo phase-contrast microtomography assessment. Tissue Eng. C Method 23(9), 548–564 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Laurens N, Koolwijk P, De Maat MPM. Fibrin structure and wound healing. J. Thromb. Haemost 4(5), 932–939 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Benwood C, Chrenek J, Kirsch RL et al. Natural biomaterials and their use as bioinks for printing tissues. Bioengineering 8(2), 27 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tajima S, Tobita M, Mizuno H. Current status of bone regeneration using adipose-derived stem cells. Histol. Histopathol 33(7), 619–627 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Ji S, Guvendiren M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front. Bioeng. Biotechnol 5, 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Huang S, Liu Y et al. Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci. Rep 8(1), 8020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araña M, Gavira JJ, Peña E et al. Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials 35(1), 143–151 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Osidak EO, Karalkin PA, Osidak MS et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med 30(3), 1–12 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Gaetani R, Feyen DAM, Verhage V et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 61, 339–348 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Vettori L, Sharma P, Rnjak-Kovacina J, Gentile C. 3D bioprinting of cardiovascular tissues for in vivo and in vitro applications using hybrid hydrogels containing silk fibroin: state of the art and challenges. Curr. Tissue Microenviron. Rep 1(4), 261–276 (2020). [Google Scholar]
- 54.Guo B, Ma PX. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Sci. China Chem 57(4), 490–500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asiri AM, Marwani HM, Khan SB, Webster TJ. Understanding greater cardiomyocyte functions on aligned compared to random carbon nanofibers in PLGA. Int. J. Nanomedicine 10, 89–96 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang X, Zauscher S, Klitzman B et al. Peptide interfacial biomaterials improve endothelial cell adhesion and spreading on synthetic polyglycolic acid materials. Ann. Biomed. Eng 38(6), 1965–1976 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Boland T, Mironov V, Gutowska A, Roth EA, Markwald RR. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat. Rec. A Discov. Mol. Cell. Evol. Biol 272A(2), 497–502 (2003). [DOI] [PubMed] [Google Scholar]
- 58.Fang Y, Guo Y, Liu T et al. Advances in 3D bioprinting. Chinese Journal of Mechanical Engineering: Additive Manufacturing Frontiers 1(1), 100011 (2022). [Google Scholar]
- 59.Puluca N, Lee S, Doppler S et al. Bioprinting approaches to engineering vascularized 3D cardiac tissues. Curr. Cardiol. Rep 21(9), 90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams D, Thayer P, Martinez H, Gatenholm E, Khademhosseini A. A perspective on the physical, mechanical and biological specifications of bioinks and the development of functional tissues in 3D bioprinting. Bioprinting 9, 19–36 (2018). [Google Scholar]
- 61.Jain P, Kathuria H, Dubey N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials 287, 121639 (2022). [DOI] [PubMed] [Google Scholar]
- 62.Xu T, Baicu C, Aho M, Zile M, Boland T. Fabrication and characterization of bio-engineered cardiac pseudo tissues. Biofabrication 1(3), 035001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalhori D, Zakeri N, Zafar-Jafarzadeh M, Moroni L, Solati-Hashjin M. Cardiovascular 3D bioprinting: a review on cardiac tissue development. Bioprinting 28, e00221 (2022). [Google Scholar]
- 64.Bonatti AF, Vozzi G, Kai Chua C, De Maria C. A deep learning approach for error detection and quantification in extrusion-based bioprinting. Mater. Today: Proc 70, 131–135 (2022). [Google Scholar]
- 65.Wang Z, Lee SJ, Cheng H-J, Yoo JJ, Atala A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 70, 48–56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pati F, Jang J, Lee JW, Cho D-W. Extrusion bioprinting. In: Essentials of 3D Biofabrication and Translatio. Atala A, Yoo JJ (Eds). Academic Press, MA, USA, 123–152 (2015). [Google Scholar]
- 67.Zhang YS, Arneri A, Bersini S et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 110, 45–59 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fihn SD, Gardin JM, Abrams J et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 126(25), 3097–3137 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Lawton JS, Tamis-Holland JE, Bangalore S et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol 79(2), e21–e129 (2022). [DOI] [PubMed] [Google Scholar]
- 70.Misra A, Walters HL, Kobayashi D. Utilisation of a three-dimensional printed model for the management of coronary-pulmonary artery fistula from left main coronary artery. Cardiol. Young 29(3), 431–434 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Oliveira-Santos M, Oliveira Santos E, Marinho AV et al. Patient-specific 3D printing simulation to guide complex coronary intervention. Rev. Port. Cardiol 37(6), 541.e541–541.e544 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Velasco Forte MN, Byrne N, Valverde Perez I et al. 3D printed models in patients with coronary artery fistulae: anatomical assessment and interventional planning. EuroIntervention 13(9), e1080–e1083 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Liang P-Y, Chang Y, Jin G, Lian X, Bao X. Wnt signaling directs human pluripotent stem cells into vascularized cardiac organoids with chamber-like structures. Front. Bioeng. Biotechnol 10, 10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haghjoo M Tachyarrhythmias. In: Practical Cardiology (2nd Edition). Haghjoo M, Maleki M, Alizadehasl A (Eds). Elsevier, 257–277 (2022). [Google Scholar]
- 75.Williams K, Liang T, Massé S et al. A 3-D human model of complex cardiac arrhythmias. Acta Biomater. 132, 149–161 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Birla RK, Williams SK. 3D bioprinting and its potential impact on cardiac failure treatment: an industry perspective. APL Bioeng. 4(1), 010903 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics 131(5), e1502–1508 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jenkins KJ, Correa A, Feinstein JA et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge. Circulation 115(23), 2995–3014 (2007). [DOI] [PubMed] [Google Scholar]
- 79.Narula J, Maron BJ, Narula N, Arbustini E. Classification of Cardiomyopathies. In: Hurst’s The Heart (14th Edition). Narula J, Fuster V, Harrington RA, Eapen ZJ (Eds). McGraw-Hill Education, NY, USA, 1–16 (2017). [Google Scholar]
- 80.Park BW, Jung SH, Das S et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci. Adv 6(13), eaay6994 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alonzo M, El Khoury R, Nagiah N, Thakur V, Chattopadhyay M, Joddar B. 3D biofabrication of a cardiac tissue construct for sustained longevity and function. ACS Appl. Mater. Interfaces 14(19), 21800–21813 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis-Israeli YR, Wasserman AH, Gabalski MA et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Comm 12(1), 5142 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borchard U, Hafner D. Ion channels and arrhythmias. Z. Kardiol 89(Suppl. 3), S6–S12 (2000). [PubMed] [Google Scholar]
- 84.Roshanbinfar K, Vogt L, Greber B et al. Electroconductive biohybrid hydro gel for enhanced maturation and beating properties of engineered cardiac tissues. Adv. Funct. Mater 28(42), 1803951 (2018). [Google Scholar]
- 85.Mirdamadi E, Tashman JW, Shiwarski DJ, Palchesko RN, Feinberg AW. FRESH 3D bioprinting a full-size model of the human heart. ACS Biomater. Sci. Eng 6(11), 6453–6459 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Vukicevic M, Filippini S, Little SH. Patient-specific modeling for structural heart intervention: role of 3D printing today and tomorrow (CME). Methodist Debakey Cardiovasc. J 16(2), 130–137 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mills RJ, Hudson JE. Bioengineering adult human heart tissue: how close are we? APL Bioeng. 3(1), 010901 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mills R, Hudson J. An in vitro model of myocardial infarction. Nat. Biomed. Eng 4(4), 366–367 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Richards DJ, Li Y, Kerr CM et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng 4(4), 446–462 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun J, Yao K, Huang K, Huang D. Machine learning applications in scaffold based bioprinting. Mater. Today: Proc 70, 17–23 (2022). [Google Scholar]


