Abstract
Bovine alveolar macrophages (BAM) were examined for the expression of β-defensins and to determine whether their expression could be upregulated by bacterial lipopolysaccharide (LPS), as observed with β-defensins expressed in bovine tracheal epithelial cells. Four β-defensins were expressed constitutively in BAM, with bovine neutrophil β-defensin (BNBD)-4 and BNBD-5 being the most predominant. This is the first evidence of β-defensin gene expression in a mature myeloid cell. LPS had no effect on β-defensin expression in BAM, even though tumor necrosis factor alpha (TNF-α) production was induced. Nonbacterial inflammatory particles had little effect on β-defensin gene expression or TNF-α production in BAM. We hypothesize that constitutively expressed β-defensins of alveolar macrophages may have a role in lung host defense.
β-Defensins are cysteine-rich antimicrobial peptides found in neutrophils and epithelial cells in mammalian and avian species (22). Their broad-range antibiotic activity is proposed to contribute to host defense by eliminating or preventing the colonization of pathogenic organisms at a variety of anatomic sites (7). The inducible expression of β-defensins in tracheal epithelial cells (TEC) by lipopolysaccharide (LPS) and tumor necrosis factor alpha (TNF-α) (6, 25) has suggested a role for these peptides in host defense of the airway (5–7). Impairment of β-defensin activity in the respiratory tract recently has been implicated in the pathophysiology of cystic fibrosis (15, 30). Induction of a β-defensin gene in human skin cells by bacteria has also been observed (16).
In the cow, β-defensins are encoded by a large gene family expressed in a wide variety of tissues (7a, 14). Thirteen β-defensins have been isolated from bovine neutrophils (28), although gene expression is restricted to mature myelopoietic cells (28a). Other sites of β-defensin gene expression include the pseudostratified columnar epithelial cells of the trachea (5), squamous epithelial cells of the tongue (27), and simple columnar epithelial cells of the distal small intestine and colon (30a). To date, β-defensin gene expression has not been reported in macrophages, although defensins have been shown to exhibit antimicrobial activity against intracellular pathogens of macrophages (24).
Air-borne environmental inflammatory agents such as residual oil fly ash (ROFA), SiO2, and asbestos have been shown to stimulate the production of reactive oxygen species by alveolar macrophages (AM) in vitro (18). In vivo studies indicate that these inflammatory agents cause an increase in susceptibility to pulmonary infections in mice (17). Furthermore, they induce the release of proinflammatory cytokines in both AM and TEC. Asbestos and silica have also been shown to activate NF-κB in TEC (9, 19). These results suggest that these agents stimulate a host defense response. We hypothesized that β-defensin genes may be expressed in AM and that induction of these genes in AM is part of a cellular host defense response. We therefore examined the expression of β-defensin genes in bovine alveolar macrophages (BAM) in response to the inflammatory agents LPS, SiO2, and ROFA and two forms of asbestos, crocidolite and chrysotile.
Lungs were obtained from freshly killed cows, placed on ice, and used within 2 h of slaughter. BAM were isolated by lavage of excised cow lungs with pyrogen-free Hank’s balanced salt solution without Ca2+ and Mg2+. Cells were washed with medium (RPMI) and determined to consist of >90% viable macrophages. Cells were cultured in RPMI medium containing 2 mM l-glutamine, 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). A total of 2 × 106 cells were adhered to 10-cm-diameter dishes for 2 h, washed twice with RPMI, and cultured for 2 to 7 days at 37°C with 5% CO2 and 95% humidity prior to treatment. After 2 days, no neutrophils were observed by differential staining, consistent with published data (21). SiO2 (Sigma), TiO2 (Sigma), and asbestos (National Institute of Environmental Health Sciences) (29) were baked at 190°C for 4 h to render them LPS free as determined by Limulus assay (sensitivity = 0.125 endotoxin units/ml; Associates of Cape Cod). ROFA, a combustion emission source ambient air particulate, contained negligible (2.5 pg/mg) LPS (8). BAM were either unstimulated or induced for 18 h with 1 μg of LPS per ml or with ROFA, SiO2, chrysotile, and crocidolite, at concentrations of 10 to 100 μg/ml. LPS, SiO2, and asbestos are known to stimulate cytokine release from rat AM at these concentrations (11, 20, 29). TiO2 was used at similar concentrations as an inert, noninflammatory control for the effects of the particles (10). Cell lysates (RNeasy; Qiagen) and supernatants were then collected and stored at −70°C for analysis of β-defensin gene expression and TNF-α. An aliquot of supernatant was stored at 4°C for lactate dehydrogenase (LDH) analysis.
To allow us to identify β-defensin mRNAs in BAM, we used 3′ rapid amplification of cDNA ends (RACE) (13). Bovine β-defensin mRNAs characteristically have unusually high sequence identity in their 5′ untranslated region and coding region, allowing the design of an oligonucleotide primer with a sequence common to the β-defensins. Total mRNA was isolated from cultured BAM, and reverse transcription (RT) was carried out by SuperScript II reverse transcriptase (Life Technologies, Bethesda, Md.) with oligodeoxythymidylic acid as a primer. A portion of the reaction product was amplified by 3′ RACE with a gene-specific primer from the conserved region, BBD-1S (5′ GCCAGCATGAGGCTCCAT 3′), and the oligodeoxythymidylic acid adapter primer supplied by the manufacturer (Life Technologies). The amplified product was purified and ligated into pBluescript SK II+ (Promega, Madison, Wis.) by standard techniques. Several clones from each experiment were subjected to DNA sequence analysis (Sequenase). RT-PCR was also carried out with this cDNA template, with BBD-1S and a downstream primer from a region highly conserved among β-defensins, BBD-2A (5′ AACAGGTGCCAATCTGT 3′). Figure 1A shows the DNA sequences obtained from the cloned PCR products along with their predicted amino acid sequences. Sequence analysis of 20 clones from RT-PCR indicated that the majority of the amplified products encode two known bovine neutrophil β-defensins, BNBD-4 and BNBD-5; a single clone was also obtained for each of two sequences whose derived amino acid sequences indicated that they were β-defensins. One was an isoform of bovine tracheal antimicrobial peptide (7), with a single amino acid substitution in the mature peptide region. This peptide sequence has been purified from bovine trachea (3a), suggesting that it is a natural isoform rather than an artifact of PCR. A search of GenBank databases indicated that the second sequence encoded another bovine epithelial β-defensin, enteric β-defensin (30a). Figure 1B shows the amino acid sequence of these precursor molecules aligned with the consensus sequence for mature β-defensins.
FIG. 1.
β-Defensins produced in BAM. (A) DNA sequence obtained by 3′ RACE and RT-PCR of total BAM mRNA. Primer sequences are underlined. (B) Predicted amino acid sequences of mature peptides from the DNA sequence shown in panel A. These sequences are those for BNBD-4 and -5, enteric β-defensin (EBD), and the tracheal antimicrobial peptide isoform (TAP*). Also shown is the consensus sequence for β-defensins.
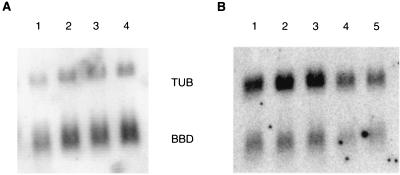
We hypothesized that the expression of these β-defensin genes in BAM might be regulated by bacterial components such as LPS and by nonmicrobial, LPS-free, environmental inflammatory particles. Northern blot analysis was performed on total mRNA from these cells by standard techniques and visualized by phosphorimage analysis. Representative blots are shown in Fig. 2, indicating challenge with LPS, SiO2, and TiO2 (Fig. 2A) as well as a dose-response challenge with chrysotile and a single concentration of ROFA (Fig. 2B). SiO2 and crocidolite were also tested at concentrations of 10 to 100 μg/ml, with similar results. A time course of challenge with crocidolite from 2 to 48 h and LPS from 2 to 18 h showed no changes in β-defensin mRNA levels (data not shown). Quantitation of the data from at least three experiments for each concentration was normalized to control levels (data not shown). The results indicate that LPS and the inflammatory particles have no significant effects on β-defensin mRNA levels in BAM.
FIG. 2.
Expression of β-defensins in cultured BAM. Total mRNA was fractionated onto a 1% agarose gel, transferred to a nylon membrane, and hybridized with an antisense oligonucleotide probe for β-defensins (BBD) as well as one for α-tubulin (TUB). Visualization of results is by phosphorimage analysis. (A) Lane 1, no stimulation; lane 2, LPS, 1 μg of P. aeruginosa LPS per ml; lane 3, 10 μg of silica per ml; lane 4, 10 μg of titanium dioxide per ml. (B) Lane 1, no stimulation; lane 2, 25 μg of chrysotile asbestos per ml; lane 3, 50 μg of chrysotile asbestos per ml; lane 4, 100 μg of chrysotile asbestos per ml; lane 5, 50 μg of ROFA per ml.
BAM activation was measured by assessing TNF in culture supernatants with the WEHI 13VAR cell line, an actinomycin D-sensitive, stable subclone of WEHI 164 clone 13 (sensitivity, ≤8 pg/ml) (1, 12). Units of TNF-α were determined by comparing the dilution of sample giving 50% cytotoxicity with that of recombinant human TNF (Genzyme). Cellular damage was assessed by measuring LDH released into the supernatants. A Cobas Fara II clinical chemistry analyzer was used to perform a colorimetric assay for LDH (Sigma). No cell damage was initiated with the concentrations of asbestos, SiO2, TiO2, or ROFA used in our study; LDH concentrations in the supernatants of treated cells were not different from those of controls and ranged from 4 to 50 U/liter. In our study, 1 μg of Pseudomonas aeruginosa LPS per ml induced 9.3 ng of TNF-α per ml in BAM cell supernatants, but no TNF-α was detected in any of the particulate-stimulated cell supernatants. While asbestos and SiO2 have been shown to stimulate rat alveolar macrophages (11, 29) and RAW 264.7 cells (4) to transcribe and secrete TNF, other studies have shown that rat, human, and bovine macrophages have not been as responsive to TNF-α induction by mineral dusts (3, 23, 26). Therefore, the lack of TNF-α induction by these particles in our study is consistent with results from other studies. Our results suggest that while LPS was able to stimulate BAM to secrete levels of TNF-α similar to those reported in other studies (2, 31), BAM are insensitive to the induction of TNF-α by these particles.
In summary, we have shown that BAM highly express several members of the β-defensin gene family. This is the first example of β-defensin mRNA in a mature myeloid cell. While β-defensin peptides are extraordinarily abundant in mature neutrophils, their gene expression is limited to the promyelocyte stage (28a). Unlike β-defensin genes in TEC, however, β-defensin genes expressed in BAM are not upregulated by LPS. Inflammatory nonmicrobial, environmental particles such as ROFA, SiO2, and asbestos had no significant effect on β-defensin gene expression in BAM and did not induce the secretion of TNF-α, a proinflammatory cytokine, in our study. Previous studies suggest that antimicrobial peptides in general and β-defensins in particular play a crucial role in epithelium-based host defense of the large airway. Our data indicate that β-defensin genes are constitutively active at high levels in AM, suggesting that these cells utilize antimicrobial peptides in protection of the lung. In future studies we will examine the role these peptides play in host defense under pathogenic conditions.
Acknowledgments
We thank Judy Richards for performing the LDH analysis and Kevin Dreher for supplying the ROFA (Pulmonary Toxicology Branch, Experimental Toxicology Division, NHEERL, U.S. EPA); Petia Simeonova and Dori Gormelec, NIEHS, for supplying the asbestos used in this study; and Max Cohen for supplying the bovine tissues. We also appreciate the comments of M. J. Selgrade and C. L. Bevins.
This research was a collaboration supported by the U.S. EPA (L. K. Ryan) and grants to G. Diamond from the USDA (project no. 9504034) and the NIH (R29HL53400). M. Bhat was supported by the NIH (HL5578901).
REFERENCES
- 1.Abukhabar K S, Armstrong J A, Ho M. Type-I interferons (IFN-α and β) suppress cytotoxin (tumor necrosis factor-α and lymphotoxin) production by mitogen-stimulated human peripheral blood mononuclear cells. J Leukocyte Biol. 1992;52:165–172. doi: 10.1002/jlb.52.2.165. [DOI] [PubMed] [Google Scholar]
- 2.Adams J L, Czuprynski C J. Bacterial lipopolysaccharide induces release of tumor necrosis factor-α from bovine peripheral blood monocytes and alveolar macrophages in vitro. J Leukocyte Biol. 1990;48:549–556. doi: 10.1002/jlb.48.6.549. [DOI] [PubMed] [Google Scholar]
- 3.Becker S, Soukup J M, Gilmour M I, Devlin R B. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996;141:637–648. doi: 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- 3a.Bevins, C. L. Personal communication.
- 4.Claudio E, Segade F, Wrobel K, Ramos S, Lazo P S. Activation of murine macrophages by silica particles in vitro is a process independent of silica-induced cell death. Am J Respir Cell Mol Biol. 1995;13:547–554. doi: 10.1165/ajrcmb.13.5.7576690. [DOI] [PubMed] [Google Scholar]
- 5.Diamond G, Jones D E, Bevins C L. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc Natl Acad Sci USA. 1993;90:4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond G, Russell J P, Bevins C L. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Tracheal antimicrobial peptide, a novel cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Diamond, G., and C. L. Bevins. Unpublished data.
- 8.Dreher K L, Jaskot R H, Lehmann J R, Richards J H, McGee J K. Soluble transition metals mediate residual oil fly ash induced acute lung injury. J Toxicol Environ Health. 1997;50:285–305. [PubMed] [Google Scholar]
- 9.Driscoll K E, Howard B W, Hassenbein D G, Janssen Y W, Mossman B T. Oxidative stress and silica-induced increases in nuclear NF-κB binding activity in alveolar epithelial cells. Am J Respir Crit Care Med. 1995;151:A712. [Google Scholar]
- 10.Driscoll K E, Maurer J K. Cytokine and growth factor release by alveolar macrophages: potential biomarkers of pulmonary toxicity. Toxicol Pathol. 1991;19:398–405. doi: 10.1177/0192623391019004-108. [DOI] [PubMed] [Google Scholar]
- 11.Dubois C M, Bissonnette E, Rola-Pleszczynski M. Asbestos fibers and silica particles stimulate rat alveolar macrophages to release tumor necrosis factor. Am Rev Respir Dis. 1989;139:1257–1264. doi: 10.1164/ajrccm/139.5.1257. [DOI] [PubMed] [Google Scholar]
- 12.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 Clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 13.Frohman M A, Dush M K, Martin G R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher D S, Jr, Ryan A M, Diamond G, Bevins C L, Womack J E. Somatic cell mapping of beta-defensin genes to cattle syntenic group U25 and fluorescence in situ localization to chromosome 27. Mamm Genome. 1995;6:554–556. doi: 10.1007/BF00356177. [DOI] [PubMed] [Google Scholar]
- 15.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 16.Harder J, Bartels J, Christophers E, Schroder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 17.Hatch G E, Boykin E, Graham J A, Lewtas J, Pott F, Loud K, Mumford J L. Inhalable particles and pulmonary host defense: in vivo and in vitro effects of ambient air and combustion particles. Environ Res. 1985;36:67–80. doi: 10.1016/0013-9351(85)90008-8. [DOI] [PubMed] [Google Scholar]
- 18.Hatch G E, Gardner D E, Menzel D B. Stimulation of oxidant production in alveolar macrophages by pollutant and latex particles. Environ Res. 1980;23:121–136. doi: 10.1016/0013-9351(80)90099-7. [DOI] [PubMed] [Google Scholar]
- 19.Janssen Y M, Barchowsky A, Treadwell M, Driscoll K E, Mossman B T. Asbestos induces nuclear factor kappa B (NF-κB) DNA-binding activity and NF-κB-dependent gene expression in tracheal epithelial cells. Proc Natl Acad Sci USA. 1995;92:8458–8462. doi: 10.1073/pnas.92.18.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaire I, Ouellet S. Distinctive profile of alveolar macrophage-derived cytokine release induced by fibrogenic and nonfibrogenic mineral dusts. J Toxicol Environ Health. 1996;47:465–478. doi: 10.1080/009841096161618. [DOI] [PubMed] [Google Scholar]
- 21.Lopez A F, Williamson D J, Gamble J R, Begley C G, Harlan J M, Kelbanoff S J, Waltersdorph A, Wong G, Clark S C, Vadas M A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin E, Ganz T, Lehrer R I. Defensins and other endogenous peptide antibiotics of vertebrates. J Leukocyte Biol. 1995;58:128–136. doi: 10.1002/jlb.58.2.128. [DOI] [PubMed] [Google Scholar]
- 23.Mosbach M, Weiner-Schmuck M, Seidel A. Influence of coexposure of ozone with quartz, latex, albumin, and LPS on TNF-a and chemotactic factor release by bovine alveolar macrophages in vitro. Inhalation Toxicol. 1996;8:625–638. [Google Scholar]
- 24.Ogata K B, Linzer B A, Zuberi R I, Ganz T, Lehrer R I, Catanzaro A. Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium-Mycobacterium intracellulare. Infect Immun. 1992;60:4720–4725. doi: 10.1128/iai.60.11.4720-4725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell J P, Diamond G, Tarver A P, Scanlin T F, Bevins C L. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect Immun. 1996;64:1565–1568. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savici D, He B, Geist L J, Monick M M, Hunninghake G W. Silica increases tumor necrosis factor (TNF) production, in part, by upregulating the TNF promoter. Exp Lung Res. 1994;20:613–625. doi: 10.3109/01902149409031740. [DOI] [PubMed] [Google Scholar]
- 27.Schonwetter B S, Stolzenberg E D, Zasloff M A. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 28.Selsted M E, Tang Y-Q, Morris W L, McGuire P A, Novotny M J, Smith W, Henschen A H, Cullor J S. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem. 1993;268:6641–6648. [PubMed] [Google Scholar]
- 28a.Selsted, M. E. Personal communication.
- 29.Simeonova P P, Luster M I. Iron and reactive oxygen species in the asbestos-induced tumor necrosis factor-α response from alveolar macrophages. Am J Respir Cell Mol Biol. 1995;12:676–683. doi: 10.1165/ajrcmb.12.6.7539275. [DOI] [PubMed] [Google Scholar]
- 30.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 30a.Tarver, A. P., D. P. Clark, G. Diamond, J. P. Russell, H. Erdjument-Bromage, P. Tempst, K. S. Cohen, D. E. Jones, R. W. Sweeney, M. Wines, S. Hwang, and C. L. Bevins. Enteric β-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 31.Yoo H S, Maheswaran S K, Lin G, Townsend E L, Ames T R. Induction of inflammatory cytokines in bovine alveolar macrophages following stimulation with Pasteurella haemolytica lipopolysaccharide. Infect Immun. 1995;63:381–388. doi: 10.1128/iai.63.2.381-388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]