Abstract
Background
The first-pass meconium has been suggested as a proxy for the fetal gut microbiota because it is formed in utero. This systematic review and cohort study investigated how pre- and perinatal factors influence the composition of the meconium microbiota.
Methods
We performed the systematic review using Covidence by searching PubMed, Scopus, and Web of Science databases with the search terms “meconium microbiome” and “meconium microbiota”. In the cohort study, we performed 16 S rRNA gene sequencing on 393 meconium samples and analyzed the sequencing data using QIIME2.
Results
Our systematic review identified 69 studies exploring prenatal factors, immediate perinatal factors, and microbial composition in relation to subsequent health of infants but gave only limited comparative evidence regarding factors related to the composition of the meconium microbiota. The cohort study pointed to a low-biomass microbiota consisting of the phyla Firmicutes, Proteobacteria and Actinobacteriota and the genera Staphylococcus, Escherichia-Shigella and Lactobacillus, and indicated that immediate perinatal factors affected the composition of the meconium microbiota more than did prenatal factors.
Conclusions
This finding supports the idea that the meconium microbiota mostly starts developing during delivery.
Impact
It is unclear when the first-pass meconium microbiota develops, and what are the sources of the colonization.
In this systematic review, we found 69 studies exploring prenatal factors, immediate perinatal factors, and microbial composition relative to subsequent health of infants, but there was no consensus on the factors affecting the meconium microbiota development.
In this cohort study, immediate perinatal factors markedly affected the meconium microbiota development while prenatal factors had little effect on it.
As the meconium microbiota composition was influenced by immediate perinatal factors, the present study supports the idea that the initial gut microbiota develops mainly during delivery.
Introduction
The human gut microbiota has become a topic of interest due to its health effects, and the first-pass meconium, i.e., the first stool after birth, formed in utero, is the first easily available sample for investigating the development of this gut microbiota in newborn infants. The meconium microbiota has previously been characterized in several studies,1–8 and interestingly, its composition has been found to be associated with the subsequent health of the children, including infantile colic and overweight.9–11
The microbiota of the first-pass meconium has been suggested as a possible proxy for the fetal gut microbiota12,13 because it is formed in the gut before birth. Alternatively, the initial colonization may start perinatally during delivery.6,14–16 As the timing of the initial colonization of the gut is still unclear, we hypothesized that the influence of prenatal and immediate perinatal factors, including exposure to antibiotics at birth and the mode of delivery, should be compared to elucidate the timing of the early colonization process. Furthermore, as meconium samples are prone to contamination because of their low biomass, there is limited evidence regarding their microbial communities from studies maintaining rigorous control over contamination.17–21
This paper presents a systematic review of previous investigations into the microbiota of the first-pass meconium, followed by a comparison of the effects of prenatal and immediate perinatal factors on the microbiota composition of the first stool in a cohort study of 393 newborn infants using a robust means of controlling for environmental contaminants.
Materials and methods
Systematic review of the literature
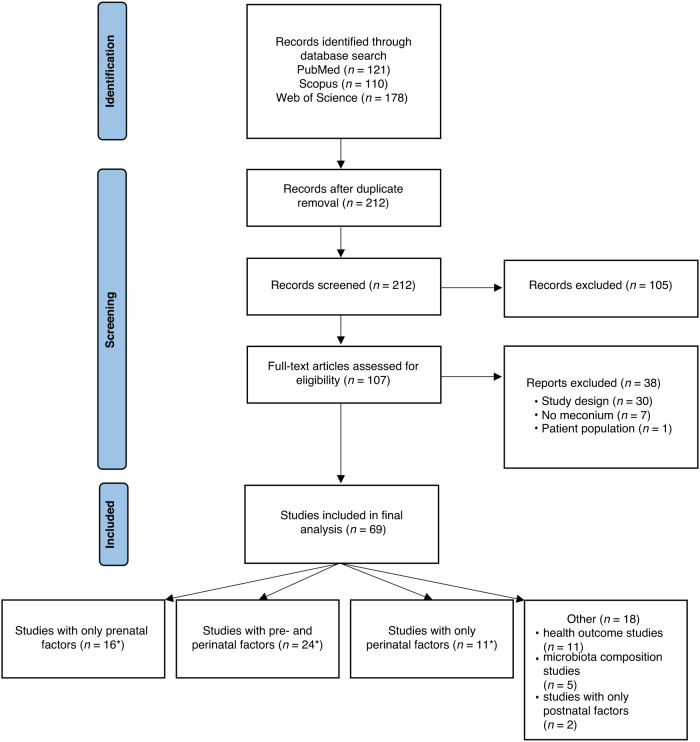
We performed a systematic review of the literature on the meconium microbiota using Covidence, a web-based collaboration software platform that streamlines the production of systematic and other literature reviews (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). We searched PubMed (date: 1.12.2022), Scopus (date: 1.12.2022), and Web of Science (2.12.2022) and used terms “meconium microbiome” and “meconium microbiota” for the literature search. The results were filtered to include only reports written in English and based on original research employing human samples. The search details can be seen in Supplementary Information 1. In the Covidence platform we removed duplicates and performed title and abstract screening, and separately full text screening, during which we also removed articles that did not study meconium or did not include samples of human origin and the results of 16 S sequencing analysis. After evaluating the selected references, we created a summary table with the following information regarding the publications: title, digital object identifier (DOI), number of meconium samples, prenatal factors (maternal characteristics, environmental factors during pregnancy), immediate perinatal factors (exposure to antibiotics during birth, delivery mode), other factors (newborn characteristics and health outcomes), additional methods beyond standard 16 S sequencing such as whole-genome shotgun sequencing and metabolomic analyses, the use of metabolic pathway prediction tools in the analysis, and reported use of negative and positive controls during the laboratory work. Finally, we created a flowchart of the review process using PRISMA.22
Study design and supervision
For the cohort study, we recruited mothers and their newborn infants treated in Oulu University Hospital, Oulu, Finland, between April 27th, 2016, and December 19th, 2018. The hospital serves as the sole primary delivery hospital for a population of 410,000 people, with about 4000 annual births. We invited all mothers delivering via Caesarean section during that period to participate. The pregnant women were recruited by the nurse at their preoperative appointment, and for each participant giving birth via C-section we recruited one mother with a vaginal delivery at the same time. All the participants gave their written informed consent. The Ethics Committee of the Central Finland Hospital District, Finland, found the study plan ethically acceptable (EETTMK:3/2016).
Study population
Altogether 508 mother-neonate pairs were enrolled during the recruitment period: 253 mothers in the vaginal birth group and 255 in the Caesarian section group. For 11 mothers the mode of delivery was changed from vaginal to C-section after recruitment, so that eventually, 242 neonates were born vaginally and 266 via C-section. A subset of these cases has been used in previous reports.6,23 Thus, we had 393 meconium samples with enough fecal material for the present study. In addition, 50 negative control samples of sterile water (HyClone™ HyPure, Thermo Fisher Scientific) were processed with the meconium samples to control for environmental contamination.
Collection of first-pass meconium samples
The first-pass meconium samples were collected as soon as possible after the birth from the diaper to collection tubes using a plastic spoon in the maternity ward and stored at –80 °C until analyses. During analyses, samples were stored at –20 °C.
DNA extraction
DNA was extracted using the DNeasy PowerSoil Pro kit (Qiagen, Hilden, Germany), by suspending 200 mg of sample in 1 ml of PBS and performing bead beating homogenization using Tissuelyzer at 25 Hz for 2 min. Tissuelyzer is recommended to samples which are hard to suspend in the extraction buffer, such as meconium samples, thus, we replaced the kit’s Vortex Adapter instructions with it in the case of meconium samples with more raw material. The samples were then left to incubate on ice for 1 min and homogenization was repeated 1–3 times. After the homogenization, the manufacturer’s instructions were followed. The negative controls and meconium samples with little material were homogenized using the kit’s Vortex Adapter instructions. We performed the extractions on a QIAcube DNA extraction machine (Qiagen). The final elution volume was set at 100 µl, and the quantity and purity of the DNA were measured using a Nanodrop Spectrophotometer (Thermo Scientific).
PCR, sequencing and sequence preprocessing
PCR and sequencing of the V3-V4 hypervariable region of the bacterial 16 S gene took place in the DNA Sequencing and Genomics Laboratory, Institute of Biotechnology, University of Helsinki. PCR was performed using the Phusion HotStart enzyme and a 2-step protocol that started with a PCR using a mix of 341 F (5′ CCTACGGGNGGCWGCAG 3′) and 785 R (5′ GACTACHVGGGTATCTAATCC 3′) primers that included truncated overhangs for the Illumina TruSeq adapter (Supplementary Information 2). For the second PCR dual-index primers selected using Barcosel24 were used to target the overhangs in the first PCR. The ZymoBIOMICS™ Microbial Community DNA Standard was used as a mock community, and the PCR products were pooled, purified and sequenced using a 600 cycle v3 sequencing kit on MiSeq (Illumina, San Diego, CA).
The analysis was conducted with QIIME2 (versions 2021.2 and 2022.8).25 The reads were imported in paired end phred 33 format and demultiplexed. Denoising and chimera filtering were performed with DADA2,26 and the reads were trimmed at base 15 and truncated at base 280 for forward reads and at 220 for reverse reads. Environmental contamination in the 50 negative controls was identified using the R package decontam (version 1.16.0).27 Contamination from the meconium samples was removed by a prevalence-based method where the presence and absence of each sequence is compared between negative controls and true samples. The threshold was set to 0.5, meaning that the sequence is considered a contaminant if it is more prevalent in negative controls than in true samples. Taxa identified as Mitochondria, Eukaryota, Cyanobacteria, and Archaea were omitted from the analyses.6 Overall, 23,481,047 reads were left for further analysis. Alpha rarefaction plots were examined to choose a sampling depth, and the samples were rarefied at 31,069, excluding reads with lower read counts from diversity analyses.
Analysis
To analyze the effect of delivery mode and exposure to intrapartum antibiotics, we grouped the meconium samples into vaginal delivery group without intrapartum antibiotic treatment, vaginal delivery group with intrapartum antibiotic treatment and C-section delivery group. C-section delivery samples were not grouped based on intrapartum antibiotic exposure due to nearly all C-sectionally born infants being exposed to antibiotics. For the within-sample diversity, known as alpha diversity, we calculated Shannon Index and observed features which are unique DNA sequences down to a single nucleotide, and therefore more precise than the operational taxonomic units (OTUs) commonly used previously, to measure differences between the sample types. Statistical significance was confirmed using the Kruskal–Wallis H test with Benjamini–Hochberg correction for p-values to control the false discovery rate (FDR) for multiple comparisons. To calculate between-sample diversity, or beta diversity, we performed Principal Coordinate Analysis using Bray–Curtis Dissimilarity. Statistical significance was confirmed using PERMANOVA with a threshold of p < 0.05. The relative abundances in the taxonomy were calculated at the phylum and genus levels using SILVA database (version 138)28 and statistical significances of these abundances were evaulated using analysis of composition of microbiomes (ANCOM).29 In ANCOM analysis, pairwise tests are performed on the abundance of each feature between sample groups, and an automatic threshold by the framework is set to the number of rejected null hypothesis results known as W. If the value of W exceeds the threshold, the feature is considered differentially abundant between the sample groups. In the case of the most interesting phyla and genera we performed multivariate regression analysis using a linear mixed model, including several prenatal and perinatal factors simultaneously. We used Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) (version 2.5.1)30 to analyze the predicted metabolic pathways by aligning amplicon sequence variants (ASVs) produced during the denoising phase to a reference tree, using HMMER (hmmer.org) for alignment and SEPP31 for placements in the tree, with Integrated Microbial Genomes (IMG) database.32 The resulting tree file was produced with GAPPA.33 The hidden-state prediction of gene families was performed using a script based on the castor R package.34 The pathway abundance predictions were made by regrouping Enzyme Classification (EC) numbers into MetaCyc reactions and predicting the metabolic pathways using MinPath.35 Statistical significances of the metabolic pathway abundances were confirmed with ANCOM. Finally, we used machine learning to create a learning classifier that can predict metadata in our samples and identify the most important genera for the predictions. The random forest nested cross-validation (NCV) learning method was used, and the number of estimators was set at 200. The figures were drawn using ggplot2 (version 3.3.6), grid (version 4.2.1) and GridExtra (version 2.3).
Results
Systematic review of the literature
The search through the databases yielded 409 publications, 197 of which were removed as duplicates. Altogether 212 publications were screened by their titles and abstracts (Fig. 1), after which 105 were removed as irrelevant, leaving 107 publications for full-text review. Among these publications, 38 were not investigating the first-pass meconium or had to be excluded due to not having performed NGS analysis, leaving 69 which explored the following factors influencing the meconium microbiota: prenatal factors (n = 16), immediate perinatal factors, including exposure to antibiotics at birth and delivery mode (n = 11), both pre- and perinatal factors (n = 24) or other factors (n = 18) (Supplementary Information 3). The mean sample size in the publications included was 107 meconium samples with a median of 61 and a range from 8 to 950 (Supplementary Information 3). Of the publications 10 performed a sequencing analysis beyond the standard 16 S of hypervariable regions, including full-length 16 S gene sequencing and whole-genome shotgun sequencing, four used qPCR for quantitative analyses and 13 included a predicted metabolic pathway analysis (Supplementary Information 3).
Fig. 1. Flowchart of the systematic review process (drawn using PRISMA).
*Also includes postnatal factors.
Due to the importance of contamination control, we reviewed the use of technical controls in the included publications. Reporting of using technical controls varied: more than half of the publications (n = 37) did not mention the use of negative or positive controls, and out of the 32 that did report either negative or positive controls (including mock communities, fecal samples, or DNA extractions of singular bacteria), 16 failed to mention the number of negative controls used and four the number of positive controls (Supplementary Information 3). In six studies, a control sample, usually a diaper sample, had already been taken during sample collection to examine possible contaminations (Supplementary Information 3).
Bacterial DNA was found in meconium in all except one included publication. The most commonly studied prenatal factors were gestational age (n = 18) and maternal pregnancy-related health issues (n = 13), while the most common immediate perinatal factor was delivery mode (n = 33) and the most common newborn-related factor was the size of the newborn at birth (n = 11) (Supplementary Information 3). Altogether 24 publications explored the effects of both prenatal and immediate perinatal factors, and 13 of these used bi or multivariate analyses to assess the effects of pre- and perinatal factors on the composition of the meconium microbiota (Supplementary Information 3). The publications were highly heterogeneous regarding what factors were studied and whether their health outcomes were investigated. Overall, the results in all the publications were mixed and inconclusive regarding the effects of pre- and perinatal factors on meconium microbiota. Altogether 17 of 33 studies, analyzing the effect of delivery mode on the meconium microbiota and possible subsequent health, yielded no significant differences. Of the ones that did, vaginally delivered infants’ meconium tended to be enriched with Escherichia-Shigella, Lactobacillus, Bacteroides, and Bifidobacterium, whereas infants’ born via C-section meconium had more Staphylococcus and Corynebacterium. In some studies, C-section delivery and the resulting meconium microbiota was investigated as a risk factor for future health conditions. Generally, maternal pregnancy-related health issues and newborn-related outcomes were found to be associated with an altered meconium microbiota.
Population characteristics in the cohort study
A total of 393 mother-neonate pairs were enrolled in the cohort study, 186 involving children born vaginally and 207 via C-section (Table 1).
Table 1.
Population characteristics of the 393 mother-newborn pairs, including prenatal, immediate perinatal and newborn factors.
| Population characteristics | Cohort size n = 393 |
|---|---|
| Prenatal factors | |
| Maternal age (years) mean (SD) | 30.8 (5.5) |
| Maternal weight at the start of pregnancy (kg) mean (SD)a | 69 (15) |
| Maternal weight at the end of pregnancy (kg) mean (SD)b | 83 (16) |
| Maternal asthma n (%) | 31 (7.6) |
| Maternal allergy n (%) | 122 (31.0) |
| Gestational diabetes n (%)c | 102 (26.0) |
| Smoking during pregnancy n (%) | 38 (9.7) |
| Streptococcus agalactiae-positived | 77 (19.6) |
| Antibiotics during pregnancy n (%) | 98 (24.9) |
| Mean h/week (SD) spent in a foreste | 3.1 (4.2) |
| Number of siblings, mean (SD) | 1.6 (2.4) |
| Fish consumption/week, mean (SD)f | 1.0 (0.7) |
| Meat consumption/week, mean (SD)g | 5.3 (2.5) |
| Immediate perinatal factors | |
| Delivery mode, vaginal delivery n (%) | 186 (47.3) |
| Antibiotics during delivery n (%) | 246 (62.6) |
| Newborn factors | |
| Sex (girl) (%) | 188 (47.8) |
| Gestational age (weeks) mean (SD) | 39.2 (1.6) |
| Birth weight (grams) mean (SD) | 3500 (570) |
| Birth length (cm) mean (SD)h | 50 (2.2) |
| Head circumference (cm) mean (SD)i | 35 (1.7) |
| Apgar 1 min mean (SD) | 8.7 (0.9) |
| Apgar 5 min mean (SD) | 9.1 (0.7) |
| Apgar 15 min mean (SD) | 9.3 (0.6) |
| Postnatal antibiotics n (%)j | 17 (4.3) |
| Meconium sampling time h (SD)k | 9.3 (8.0) |
aWeight at the start of pregnancy not available for 12 mothers.
bWeight at the end of pregnancy not available for 8 mothers.
cDefined by abnormal oral glucose tolerance values.
dStreptococcus agalactiae screening result not reported for 86 mothers.
eWeekly time spent in a forest not available for 30 mothers.
fFish consumption not available for 17 mothers.
gMeat consumption not available for 15 mothers.
hBirth length not available for 8 newborns.
iHead circumference not available for 9 newborns.
j8 children received a combination of benzyl penicillin and tobramycin, 8 received benzyl penicillin alone and 1 received a combination of cefuroxime and tobramycin.
kSampling time not available for 76 meconium samples.
Immediate perinatal factors influencing the microbiota of the meconium
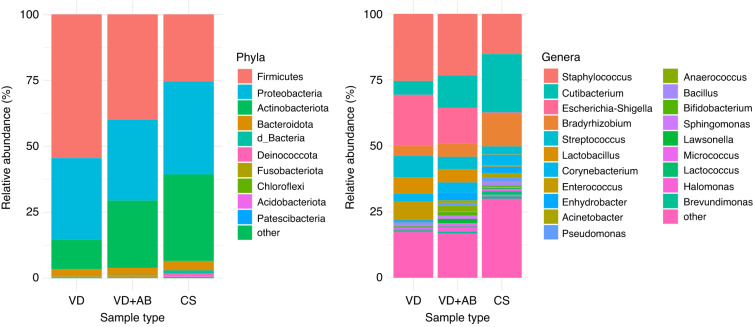
We first analyzed how the mode of delivery and exposure to antibiotics during delivery affected the composition of the meconium microbiota. The 341 samples obtained after preprocessing were divided into three groups: vaginal delivery samples without intrapartum antibiotics (VD, n = 118), vaginal delivery samples with intrapartum antibiotics (VD + AB, n = 39) and C-section delivery samples (CS, n = 184). Comparison of the proportions of the various phyla and genera in the meconium samples showed Firmicutes (VD: 54%, VD + AB: 40%, CS: 26%), Proteobacteria (VD: 31%, VD + AB: 31%, CS: 35%) and Actinobacteriota (VD: 11%, VD + AB: 25%, CS: 33%) to be the most abundant phyla and Staphylococcus the most abundant genus in the vaginal delivery samples (VD: 25%, VD + AB: 23%, CS: 15%) followed by Escherichia-Shigella (VD: 19%, VD + AB: 13%, CS: 0.62%) and Streptococcus (VD: 8.1%, VD + AB: 4.7%, CS: 3%) (Fig. 2 and Supplementary Information 4). Cutibacterium was the most abundant genus in the CS group (VD: 5.2%, VD + AB: 12%, CS: 22%), (Fig. 2 and Supplementary Information 4).
Fig. 2. Bacterial composition of the meconium samples, by delivery mode and the use of intrapartum antibiotics.
VD Vaginal delivery, no intrapartum antibiotics, VD + AB: Vaginal delivery, intrapartum antibiotics, CS: C-section. The 10 most abundant phyla and 20 most abundant genera are listed. The remaining taxa in both plots are assigned to the category “other”.
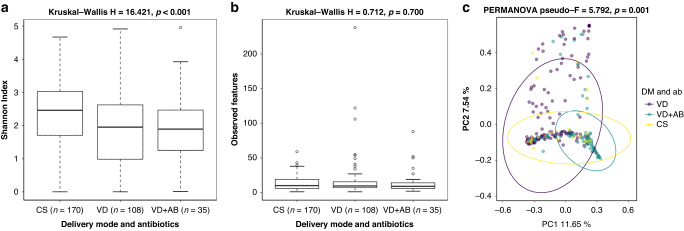
We then analyzed the effect of delivery mode and exposure to intrapartum antibiotics on the alpha and beta diversities of the meconium samples. After rarefying, we had 108 samples from the VD group, 35 from the VD + AB group and 170 from the CS group for the diversity analyses. The Shannon Index showed significant differences between the VD and CS groups (p < 0.001) and between the VD + AB and CS groups (p = 0.016), while the samples from the infants born via C-section were slightly more diverse in their bacterial composition than the vaginal delivery samples in general (Fig. 3a and Supplementary Information 5). The mean number (SD) of observed features in the samples was low in all the groups, however: 17 (27) in VD, 14 (17) in VD + AB, and 13 (9.4) in CS, yielding no significant differences between the groups (Fig. 3b and Supplementary Information 5). In the beta diversity analysis, the meconium samples differed between the VD, VD + AB and CS groups (p = 0.001), and significant differences were found between all the sample groups in the pairwise comparisons of the beta diversity analyses (Fig. 3c and Supplementary Information 5).
Fig. 3. Alpha and beta diversity of meconium samples.
a Shannon Index, b observed features, and c Bray–Curtis Dissimilarity of the meconium samples grouped according to delivery mode and exposure to intrapartum antibiotics. VD Vaginal delivery, no intrapartum antibiotics, VD + AB: Vaginal delivery, intrapartum antibiotics. CS C-section.
ANCOM analysis showed that both delivery mode and exposure to intrapartum antibiotics contributed to the differences in abundance between the samples. Several such differences in phyla and genera were found when comparing the VD and CS groups and in the phyla Actinobacteriota and Firmicutes between the VD and VD + AB groups, while no differentially abundant phyla or genera could be identified between the VD + AB and CS group (Table 2).
Table 2.
Differentially abundant features identified when using ANCOM to compare the sample groups.
| Pairwise comparison | Phyla | W | Genera | W |
|---|---|---|---|---|
| VD vs. VD + AB | Actinobacteriota | 14 | NA | NA |
| VD vs. CS | Actinobacteriota | 20 | Cutibacterium | 430 |
| Firmicutes | 20 | Escherichia-Shigella | 429 | |
| Lactobacillus | 426 | |||
| Enterococcus | 421 | |||
| Bradyrhizobium | 417 | |||
| VD + AB vs. CS | NA | NA | NA | NA |
Differences in abundance were measured at the phylum and genus levels. W: Number of times the zero hypothesis was rejected.
Prenatal factors influencing the microbiota of the meconium
In addition, we performed a univariate analysis of the whole cohort using alpha and beta diversities in the various prenatal factors, including environmental and maternal factors and the newborn size (Supplementary Information 6). The presence of furry pets at home during pregnancy was associated with the number of observed features (p = 0.024) but the alpha and beta diversity analyses showed no significant differences in any other prenatal factors (Supplementary Information 6). We also performed an ANCOM analysis on the same prenatal factors and found small differences in some of them (Supplementary Information 7). The mother’s weight and weight gain during pregnancy affected the abundance of Pelomonas, maternal use of medicines, mostly iron supplements, painkillers, thyroid hormones and blood pressure medicine, and likewise smoking, affected the abundance of Acidovorax, and the consumption of fish and meat during pregnancy affected the abundance of Acidiphilium (Supplementary Information 7). Finally, the newborn’s size at birth influenced the abundance of the genera Rahnella1 and Bryocella (Supplementary Information 7).
Multivariate linear mixed model
We used a multivariate linear mixed model to compare the relative abundances of 10 taxa, the prevalence of which ranged from 32 to 303 zero counts in the samples studied, in the presence of 5 prenatal and 2 immediate perinatal factors (Table 3). When adjusted for these factors in the model, this analysis showed that the phylum Actinobacteriota was significantly more abundant in infants exposed to intrapartum antibiotics than in those not exposed, while the abundance of Firmicutes and Escherichia-Shigella was significantly greater in the vaginal delivery samples and Cutibacterium was in the C-section samples (Supplementary Information 8 and 9). Furthermore, Enterococcus was significantly more abundant in the meconium samples when there were no older siblings in the household during pregnancy (Supplementary Information 8 and 9). The rest of the comparisons yielded no statistically significant results (Supplementary Information 8 and 9).
Table 3.
Pre-and perinatal factors used in the multivariate mixed model and the outcome variables, including 4 phyla, 6 genera and 2 alpha diversity metrics analyzed here.
| Factors studied | Outcome variables | ||
|---|---|---|---|
| Immediate perinatal factors | Prenatal factors | Taxa in the model | Alpha diversity metrics |
| Delivery mode | Maternal age | Actinobacteriota* | Shannon Index |
| Intrapartum antibiotics | Maternal forest exposure | Bacteroidota | Chao1 |
| Maternal weight gain | Firmicutes* | ||
| Presence of furry pets | Proteobacteria | ||
| Presence of older siblings | Cutibacterium* | ||
| Enterococcus# | |||
| Escherichia-Shigella* | |||
| Lactobacillus | |||
| Staphylococcus | |||
| Streptococcus | |||
PICRUSt2 analysis of the meconium microbiota
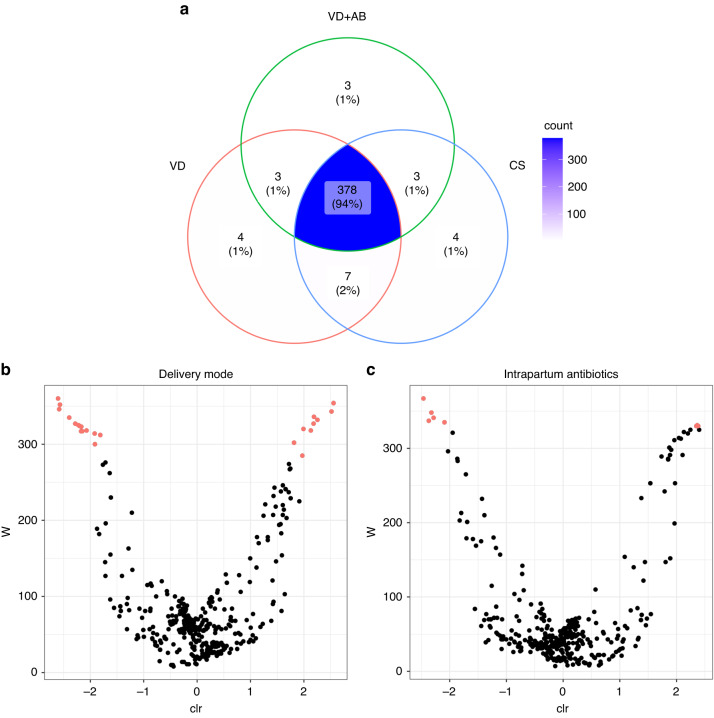
A predicted metabolic pathway analysis performed using the 16 S data for the newborns in our cohort identified a total of 401 predicted pathways, 378 (94%) of which were shared between all three sample groups (Fig. 4a). There were a few pathways that were unique to one group: 4 to VD, 3 to VD + AB and 4 to CS (Fig. 4a). Using ANCOM, we found pathways that differed in abundance depending on both the delivery mode (25 pathways) and exposure to intrapartum antibiotics (8 pathways; see Fig. 4b, c and Supplementary Information 10). The pathways that differed according to the use of intrapartum antibiotics were mainly those involved in fatty acid biosynthesis, the degradation of various metabolites and aerobic respiration, while those that different according to the delivery mode included those responsible for metabolite degradation, biosynthesis of membrane structures, biosynthesis of metabolites and amino acids, aerobic respiration, fatty acid biosynthesis and the urea cycle (Supplementary Information 10).
Fig. 4. PICRUSt2 results of meconium samples based on the mode of delivery and exposure to intrapartum antibiotics.
a A Venn diagram of predicted metabolic pathways shared between the sample groups. b Volcano plot of the abundances of the predicted metabolic pathways that differed according to the delivery mode. c Volcano plot of the predicted metabolic pathway abundances that differed according to intrapartum antibiotic exposure. Pathways that differ in abundance are colored red. The pathway labels were removed from the plots due to overlaps.
Sample classification using machine learning to predict the delivery mode
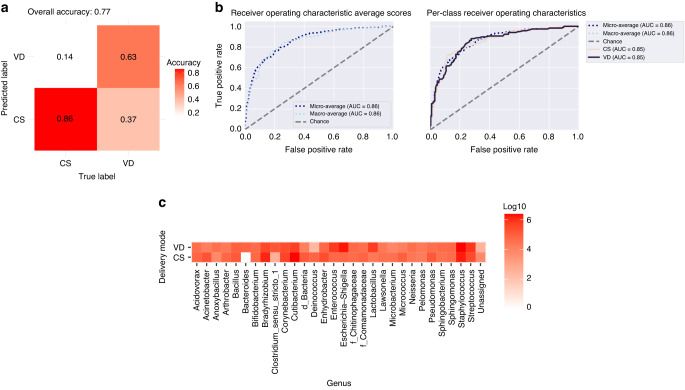
Using the random forest (RF) learning method for classifying our samples, we obtained a model in which the RF classifier managed to predict the delivery mode in 77% of the samples, C-section deliveries being correctly identified more often, at an accuracy rate of 0.86 (86%), while vaginal delivery samples without intrapartum antibiotic treatment were correctly identified with 0.63 (63%) accuracy (Fig. 5a). Receiver Operating Characteristics (ROC) curves for both delivery modes had an area under curve (AUC) of 0.85 (Fig. 5b). The 30 most important genera for distinguishing between the delivery modes are listed in Fig. 5c.
Fig. 5. Random forest classification of the samples according to delivery mode.
a The proportions of the samples that were correctly and incorrectly assigned to the given mode. “True label” (x-axis) means the true delivery mode of the samples and “Predicted label” (y-axis) the delivery mode that the classifier assigned to the samples. b Receiver operating characteristics (ROC) of classifier accuracy, with the area under the curve (AUC) depicted for both the CS and VD samples, together with the micro- and macro-average AUCs. c The 30 most important genera for classifying samples according to delivery mode. The numbers of reads are presented as log10 scores.
Discussion
The systematic review of the literature provided some comparative evidence for the effects of prenatal and immediate perinatal factors on the initial colonization of the gut in neonates, while the cohort study indicated that this early gut colonization was altered at birth according to the mode of delivery and exposure to intrapartum antibiotics. Furthermore, the multivariate model constructed here showed that immediate perinatal factors influenced the microbial composition of the first-pass meconium more than did prenatal factors. This finding supports the idea that the microbiota of the first stool is mainly influenced by the delivery.
The largest differences in the meconium microbiota were found here between infants born via the vaginal route without intrapartum antibiotic treatment and those born via C-section. This finding was further supported by our machine learning analysis, which was able to identify C-section samples accurately. Half of the 33 studies in our systematic review that examined the delivery mode as a contributor to meconium microbiota development2,5,6,13–15,36–60 found that it did indeed have an effect. This implies that if the colonization process starts in utero or during birth, interventions to influence the composition of the infant’s gut microbiota may need to be started early. The importance of an early intervention is emphasized by previous reports that the meconium microbiota has been found to be associated with the subsequent health of the infants, such as neonatal jaundice,46,61,62 NEC,40,60,63,64 allergies and atopy,65,66 disrupted infant growth,10,53,67–71 early-onset neonatal sepsis (EONS)60,72,73 and other conditions.9,60,74–76 One possible intervention to alter the gut microbiota may be the use of probiotics, which have been found to have a positive effect on the newborn gut.60,77
In clinical practice the use of intrapartum antibiotics is often linked with the delivery mode, since intrapartum antibiotics are used to prevent early-onset group B streptococcal (GBS) sepsis in vaginal deliveries usually by administering intravenous penicillin to the 20–30% of pregnant women who are colonized with GBS78–80 while almost all women undergoing C-section are given antibiotics to prevent surgical site infections.80 Thus it is difficult to determine whether the meconium microbiota is shaped by the delivery mode, the use of intrapartum antibiotics, or the combined effect of both. Studies investigating the effect of antenatal37,39,40,49,57,58,64,69,81 and intrapartum13,36,45,72,82 antibiotics on the meconium microbiota have shown variable results. In the present cohort study, intrapartum antibiotics seemed to alter the meconium microbiota development in the infants born vaginally, suggesting that maternal exposure to antibiotics during vaginal birth acts rapidly to modify early gut colonization, probably before the actual birth.
The microbial composition of the first-pass meconium appearing here to be affected mainly by immediate perinatal factors suggests that the meconium microbiota is mostly formed during delivery and not during the fetal period, although we cannot rule out the possibility that the fetus may be exposed to maternal microbiota or microbiota-derived extracellular vesicles23 or metabolites83 allowing prenatal factors to modify these contacts in utero. In addition, we collected extensive data on maternal living environments and lifestyles during pregnancy, such as fish and meat consumption, leisure time spent in forests, the presence of older siblings or furry pets in the home, smoking in the home and maternal weight, and established that these prenatal factors had little effect on the microbial composition of meconium. It is therefore understandable that the prenatal factors considered in our systematic review, including maternal diet,2,37,84,85 residential area,37,44 age,37,44,49,50,53,57,86 ethnicity,3,49 weight,3,38,44,49,53,56,57,75,86 smoking,3,37,44,87 pregnancy-related health issues,36–40,47,49,54,60,71,75,85,86 non-pregnancy-related health issues3,37,38,44,45,49,70,76,88,89 and education,37,44,49 together with environmental pollutants, particularly metals and microplastics58,90 and the presence of furry pets13,45 yielded mixed results. Another commonly studied newborn factor, which we did not analyze in our cohort study, was gestational age,3,4,36–39,44,45,48–50,52,53,57,59,72,91–93 which most reports have found to affect the development of the meconium microbiota. Interestingly, when Yang et al. compared the meconium microbiota of monozygotic twins with that of dizygotic ones, they concluded that the former resembled each other in terms of their gut microbiota more than did the latter,94 implying a possible intrauterine colonization mechanism. These results emphasize the controversial nature of gut microbiota development in newborns as a research topic and point to a need for a carefully planned and controlled study design especially in the case of a low-biomass microbiota.
PICRUSt2 analysis to predict metabolic pathways in our samples yielded significant differences in the abundances of the predicted pathways according to the delivery mode and to a lesser extent the use of intrapartum antibiotics. This suggests that the delivery mode especially affects the major metabolic pathways in meconium microbiota, which may contribute to the function of the microbiota at birth. This contradicts earlier PICRUSt findings, as one study mentions a difference in the pathways of transitional stool, but not in the meconium.42 Furthermore, in an earlier study where shotgun metagenomic sequencing was applied to both meconium and stool collected 1 month after birth, differences in the taxonomic and gene composition were found in later stool samples but not in meconium.95 Finally, studies using whole-genome shotgun sequencing and full length 16 S rRNA gene sequencing did not find significant differences in the meconium samples based on the delivery mode.2,5
The strength of the present study lies in the combination of a systematic review of the literature with a comparison of the impact of prenatal and immediate perinatal factors on the microbiota of the first stool in a large cohort using a multivariate model, together with the use of a large dataset of environmental factors and extensive analyses of their effects on the meconium microbiota. To our knowledge, this is overall the largest cohort study characterizing the meconium microbiota and the environmental factors affecting it to be published to date. We used various methods, including machine learning and predicted metabolic pathway analysis. Since reagent and other laboratory contaminations are common in sequencing studies17 it is crucial in microbiota research to be able to identify such contamination, so that extraneous microbes will not be mistaken for true microbiota findings. We had a large number of negative controls to enable decontamination processes to be introduced during the analysis. This is especially important for low-biomass microbiota research, such as that involving the meconium.
The study had certain limitations, however. We were unable to perform sequencing of the full 16 S gene due to the low biomass of the meconium samples. Although sequencing of the full gene might have improved the accuracy of the bacterial taxonomy to the species level, sequencing of the V3-V4 region of the gene is still common in microbiota studies, even with the possible primer biases.96 Furthermore, we did not include negative controls such as diaper samples to cover possible contamination during sample collection. The genus Cutibacterium is a skin commensal and may be a contamination rather than a true colonizer in the microbiota of the meconium. In an earlier study, we analyzed the diapers of newborns alongside their meconium samples and found that the diapers did not contaminate the meconium microbiota.13 Finally, we were limited in our data analysis in many cases by the small group sizes or by missing data, which may have affected the results.
In conclusion, the systematic review showed that there is not yet a consensus on when the meconium microbiota develops or what factors affect its development. The minor effect of prenatal factors on meconium microbiota development in comparison to the observed effect of immediate perinatal factors, including the mode of delivery and exposure to intrapartum antibiotics, suggests that the initial human gut microbiota is mostly influenced by the delivery. The delivery likely shapes the infant gut microbiota development after birth.
Supplementary Information
Supplementary_information1,2,4,5,7,8,9,10
Acknowledgements
We would like to thank Kaisa Nurminen for the DNA extractions, and the DNA Sequencing and Genomics Laboratory, Institute of Biotechnology, University of Helsinki, for the sequencing of the samples.
Author contributions
T.T. designed the study; M.V.T. designed the DNA extraction, PCR and sequencing; N.P. provided the participant information and metadata; T.P. performed the regression analyses; S.A. and S.M. led the systematic review; J.T. supervised the DNA extraction, analyzed the sequence data, performed the systematic review, and wrote the manuscript with insight from M.V.T., N.P., T.P., S.A., S.M., A.K., J.R., and T.T.
Funding
T.T. would like to thank the Academy of Finland for a Clinical Research grant in 2018–2022 and the Pediatric Research Foundation for a grant in 2019–2022. M.V.T. thanks the Päivikki and Sakari Sohlberg Foundation for grants. J.T. would like to thank The Alma and K. A. Snellman Foundation, Oulu, Finland, for a grant in 2023. Open Access funding provided by University of Oulu including Oulu University Hospital.
Data availability
Raw sequences were submitted to Genbank with the Bioproject number PRJNA905086.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Ethical Committee of Northern Ostrobothnia Hospital District at Oulu University Hospital, Finland, with the decision number EETTMK:3/2016. The participants gave their informed written consent before entering the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41390-023-02783-z.
References
- 1.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu DM, et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017;23:314–326. doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stinson LF, Boyce MC, Payne MS, Keelan JA. The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Front. Microbiol. 2019;10:1124. doi: 10.3389/fmicb.2019.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younge N, et al. Fetal exposure to the maternal microbiota in humans and mice. JCI Insight. 2019;4:1–14. doi: 10.1172/jci.insight.127806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Q, et al. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes. 2020;12:1794266. doi: 10.1080/19490976.2020.1794266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turunen J, et al. Presence of distinctive microbiome in the first-pass meconium of newborn infants. Sci. Rep. 2021;11:19449. doi: 10.1038/s41598-021-98951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallenborn JT, Gunier RB, Pappas DJ, Chevrier J, Eskenazi B. Breastmilk, stool, and meconium: bacterial communities in South Africa. Micro. Ecol. 2022;83:246–251. doi: 10.1007/s00248-021-01758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams N, et al. Investigating the origin of the fetal gut and placenta microbiome in twins. J. Matern. Fetal Neonatal Med. 2022;35:7025–7035. doi: 10.1080/14767058.2021.1936487. [DOI] [PubMed] [Google Scholar]
- 9.Korpela K, et al. Microbiome of the first stool after birth and infantile colic. Pediatr. Res. 2020;88:776–783. doi: 10.1038/s41390-020-0804-y. [DOI] [PubMed] [Google Scholar]
- 10.Korpela, K. et al. Microbiome of the first stool and overweight at age 3 years: a prospective cohort study. Pediatr. Obes.15, e12680 (2020). [DOI] [PubMed]
- 11.Kielenniva K, et al. Microbiota of the first‐pass meconium and subsequent atopic and allergic disorders in children. Clin. Exp. Allergy. 2022;52:684–696. doi: 10.1111/cea.14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romano-Keeler J, Weitkamp J-H. Maternal influences on fetal microbial colonization and immune development. Pediatr. Res. 2015;77:189–195. doi: 10.1038/pr.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapiainen T, et al. Maternal influence on the fetal microbiome in a population-based study of the first-pass meconium. Pediatr. Res. 2018;84:371–379. doi: 10.1038/pr.2018.29. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez-Bello MG, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y-C, et al. Initial meconium microbiome in Chinese neonates delivered naturally or by cesarean section. Sci. Rep. 2018;8:3212–3255. doi: 10.1038/s41598-018-21657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rackaityte E, et al. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 2020;26:599–607. doi: 10.1038/s41591-020-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salter SJ, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Goffau MC, et al. Recognizing the reagent microbiome. Nat. Microbiol. 2018;3:851–853. doi: 10.1038/s41564-018-0202-y. [DOI] [PubMed] [Google Scholar]
- 19.Stinson LF, Keelan JA, Payne MS. Identification and removal of contaminating microbial DNA from PCR reagents: impact on low‐biomass microbiome analyses. Lett. Appl. Microbiol. 2019;68:2–8. doi: 10.1111/lam.13091. [DOI] [PubMed] [Google Scholar]
- 20.Dos Santos, S. J. et al. Early neonatal meconium does not have a demonstrable microbiota determined through use of robust negative controls with cpn 60-based microbiome profiling. Microbiol. Spectr.9, e0006721 (2021). [DOI] [PMC free article] [PubMed]
- 21.Kennedy KM, et al. Fetal meconium does not have a detectable microbiota before birth. Nat. Microbiol. 2021;6:865–873. doi: 10.1038/s41564-021-00904-0. [DOI] [PubMed] [Google Scholar]
- 22.Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020‐compliant flow diagrams, with interactivity for optimised digital transparency and Open. Synth. Campbell Syst. Rev. 2022;18:1–12. doi: 10.1002/cl2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turunen, J. et al. Bacterial extracellular vesicles in the microbiome of first-pass meconium in newborn infants. Pediatr. Res. 1–10 10.1038/s41390-022-02242-1 (2022). [DOI] [PMC free article] [PubMed]
- 24.Somervuo P, et al. BARCOSEL: a tool for selecting an optimal barcode set for high-throughput sequencing. BMC Bioinforma. 2018;19:257. doi: 10.1186/s12859-018-2262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callahan BJ, et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis NM, Proctor DM, Holmes SP, Relman DA, Callahan BJ. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome. 2018;6:226. doi: 10.1186/s40168-018-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandal S, et al. Analysis of composition of microbiomes: a novel method for studying microbial composition. Micro. Ecol. Health Dis. 2015;26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douglas GM, et al. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020;38:685–688. doi: 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirarab, S., Nguyen, N. & Warnow, T. SEPP: SATé-Enabled Phylogenetic Placement. in Biocomputing 2012 247–258 (WORLD SCIENTIFIC, 2011). 10.1142/9789814366496_0024. [DOI] [PubMed]
- 32.Markowitz VM, et al. IMG: the integrated microbial genomes database and comparative analysis system. Nucleic Acids Res. 2012;40:D115–D122. doi: 10.1093/nar/gkr1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czech L, Barbera P, Stamatakis A. Genesis and Gappa: processing, analyzing and visualizing phylogenetic (placement) data. Bioinformatics. 2020;36:3263–3265. doi: 10.1093/bioinformatics/btaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louca S, Doebeli M. Efficient comparative phylogenetics on large trees. Bioinformatics. 2018;34:1053–1055. doi: 10.1093/bioinformatics/btx701. [DOI] [PubMed] [Google Scholar]
- 35.Ye Y, Doak TG. A parsimony approach to biological pathway reconstruction/inference for genomes and metagenomes. PLoS Comput. Biol. 2009;5:e1000465. doi: 10.1371/journal.pcbi.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mshvildadze M, et al. Intestinal microbial ecology in premature infants assessed with non–culture-based techniques. J. Pediatr. 2010;156:20–25. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosalbes MJ, et al. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy. 2013;43:198–211. doi: 10.1111/cea.12063. [DOI] [PubMed] [Google Scholar]
- 38.Hu J, et al. Diversified microbiota of meconium is affected by maternal diabetes status. PLoS One. 2013;8:e78257. doi: 10.1371/journal.pone.0078257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ardissone AN, et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS One. 2014;9:e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heida FH, et al. A necrotizing enterocolitis-associated gut microbiota is present in the meconium: results of a prospective study. Clin. Infect. Dis. 2016;62:863–870. doi: 10.1093/cid/ciw016. [DOI] [PubMed] [Google Scholar]
- 41.Brazier L, et al. Evolution in fecal bacterial/viral composition in infants of two central African countries (Gabon and Republic of the Congo) during their first month of life. PLoS One. 2017;12:e0185569. doi: 10.1371/journal.pone.0185569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller N, et al. Delivery mode and the transition of pioneering gut-microbiota structure, composition and predicted metabolic function. Genes (Basel) 2017;8:364. doi: 10.3390/genes8120364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wampach L, et al. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front. Microbiol. 2017;8:1–21. doi: 10.3389/fmicb.2017.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claassen-Weitz S, et al. HIV-exposure, early life feeding practices and delivery mode impacts on faecal bacterial profiles in a South African birth cohort. Sci. Rep. 2018;8:5078. doi: 10.1038/s41598-018-22244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durack J, et al. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat. Commun. 2018;9:707. doi: 10.1038/s41467-018-03157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong T, et al. Meconium microbiome associates with the development of neonatal jaundice. Clin. Transl. Gastroenterol. 2018;9:e182. doi: 10.1038/s41424-018-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut. 2018;67:1614–1625. doi: 10.1136/gutjnl-2018-315988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escribano E, et al. Influence of a Serratia marcescens outbreak on the gut microbiota establishment process in low-weight preterm neonates. PLoS One. 2019;14:e0216581. doi: 10.1371/journal.pone.0216581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu J, et al. Microbiota of newborn meconium is associated with maternal anxiety experienced during pregnancy. Dev. Psychobiol. 2019;61:640–649. doi: 10.1002/dev.21837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu C-J, et al. Is the delivery mode a critical factor for the microbial communities in the meconium? EBioMedicine. 2019;49:354–363. doi: 10.1016/j.ebiom.2019.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo C, et al. Breastfeeding restored the gut microbiota in caesarean section infants and lowered the infection risk in early life. BMC Pediatr. 2020;20:532. doi: 10.1186/s12887-020-02433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia J, et al. Impact of postnatal antibiotics and parenteral nutrition on the gut microbiota in preterm infants during early life. J. Parenter. Enter. Nutr. 2020;44:639–654. doi: 10.1002/jpen.1695. [DOI] [PubMed] [Google Scholar]
- 53.Lu Q, et al. Alterations of gut microbiota composition in neonates conceived by assisted reproductive technology and its relation to infant growth. Gut Microbes. 2020;12:1794466. doi: 10.1080/19490976.2020.1794466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen T, et al. Gestational diabetes mellitus is associated with the neonatal gut microbiota and metabolome. BMC Med. 2021;19:120. doi: 10.1186/s12916-021-01991-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heida FH, et al. Weight shapes the intestinal microbiome in preterm infants: results of a prospective observational study. BMC Microbiol. 2021;21:219. doi: 10.1186/s12866-021-02279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raspini B, et al. Early life microbiota colonization at six months of age: a transitional time point. Front. Cell Infect. Microbiol. 2021;11:1–14. doi: 10.3389/fcimb.2021.590202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farinella R, et al. Maternal anthropometric variables and clinical factors shape neonatal microbiome. Sci. Rep. 2022;12:2875. doi: 10.1038/s41598-022-06792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naspolini NF, et al. Environmental pollutant exposure associated with altered early-life gut microbiome: results from a birth cohort study. Environ. Res. 2022;205:112545. doi: 10.1016/j.envres.2021.112545. [DOI] [PubMed] [Google Scholar]
- 59.Liu L, et al. Early gut microbiota in very low and extremely low birth weight preterm infants with feeding intolerance: a prospective case-control study. J. Microbiol. 2022;60:1021–1031. doi: 10.1007/s12275-022-2180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westaway JAF, et al. The bacterial gut microbiome of probiotic-treated very-preterm infants: changes from admission to discharge. Pediatr. Res. 2022;92:142–150. doi: 10.1038/s41390-021-01738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding J, et al. Gut microbial alterations in neonatal jaundice pre- and post-treatment. Biosci. Rep. 2021;41:1–13. doi: 10.1042/BSR20210362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, et al. Clinical manifestations of neonatal hyperbilirubinemia are related to alterations in the gut microbiota. Children. 2022;9:764. doi: 10.3390/children9050764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leach ST, et al. Multiple opportunistic pathogens, but not pre-existing inflammation, may be associated with necrotizing enterocolitis. Dig. Dis. Sci. 2015;60:3728–3734. doi: 10.1007/s10620-015-3830-6. [DOI] [PubMed] [Google Scholar]
- 64.Dobbler PT, et al. Low microbial diversity and abnormal microbial succession is associated with necrotizing enterocolitis in preterm infants. Front. Microbiol. 2017;8:1–12. doi: 10.3389/fmicb.2017.02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen X, et al. Dynamic construction of gut microbiota may influence allergic diseases of infants in Southwest China. BMC Microbiol. 2019;19:123. doi: 10.1186/s12866-019-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petersen C, et al. A rich meconium metabolome in human infants is associated with early-life gut microbiota composition and reduced allergic sensitization. Cell Rep. Med. 2021;2:100260. doi: 10.1016/j.xcrm.2021.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, et al. Characteristics of the intestinal microbiota in very low birth weight infants with extrauterine growth restriction. Front. Pediatr. 2019;7:1–9. doi: 10.3389/fped.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terrazzan Nutricionist AC, et al. Meconium microbiome and its relation to neonatal growth and head circumference catch-up in preterm infants. PLoS One. 2020;15:e0238632. doi: 10.1371/journal.pone.0238632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Y, et al. Intrauterine antibiotic exposure affected neonatal gut bacteria and infant growth speed. Environ. Pollut. 2021;289:117901. doi: 10.1016/j.envpol.2021.117901. [DOI] [PubMed] [Google Scholar]
- 70.Grant-Beurmann S, et al. Dynamics of the infant gut microbiota in the first 18 months of life: the impact of maternal HIV infection and breastfeeding. Microbiome. 2022;10:61. doi: 10.1186/s40168-022-01230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu Q, Yang X, Zhang Y, Shan C, Shi Z. Role of the gut microbiota in the increased infant body mass index induced by gestational diabetes mellitus. mSystems. 2022;7:e0046522. doi: 10.1128/msystems.00465-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou, P. et al. Perinatal antibiotic exposure affects the transmission between maternal and neonatal microbiota and is associated with early-onset sepsis. mSphere5, e00984-19 (2020). [DOI] [PMC free article] [PubMed]
- 73.Dornelles LV, et al. Meconium microbiota predicts clinical early-onset neonatal sepsis in preterm neonates. J. Matern. Fetal Neonatal Med. 2022;35:1935–1943. doi: 10.1080/14767058.2020.1774870. [DOI] [PubMed] [Google Scholar]
- 74.Guzzardi MA, et al. Fetal cardiac growth is associated with in utero gut colonization. Nutr. Metab. Cardiovasc. Dis. 2019;29:170–176. doi: 10.1016/j.numecd.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Guzzardi MA, et al. Maternal pre-pregnancy overweight and neonatal gut bacterial colonization are associated with cognitive development and gut microbiota composition in pre-school-age offspring. Brain Behav. Immun. 2022;100:311–320. doi: 10.1016/j.bbi.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Wei Q, et al. Associations of maternal prenatal emotional symptoms with neurodevelopment of children and the neonatal meconium microbiota: a prospective cohort study. Psychoneuroendocrinology. 2022;142:105787. doi: 10.1016/j.psyneuen.2022.105787. [DOI] [PubMed] [Google Scholar]
- 77.Kurath-Koller S, et al. Hospital regimens including probiotics guide the individual development of the gut microbiome of very low birth weight infants in the first two weeks of life. Nutrients. 2020;12:1256. doi: 10.3390/nu12051256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N. Engl. J. Med. 1986;314:1665–1669. doi: 10.1056/NEJM198606263142603. [DOI] [PubMed] [Google Scholar]
- 79.Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013;31:D20–D26. doi: 10.1016/j.vaccine.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 80.Gardemeister S, et al. Cross-sectional study of the proportion of antibiotic use during childbirth in full-term deliveries in Finland. BMC Pregnancy Childbirth. 2023;23:50. doi: 10.1186/s12884-023-05368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong WSW, et al. Prenatal and peripartum exposure to antibiotics and cesarean section delivery are associated with differences in diversity and composition of the infant meconium microbiome. Microorganisms. 2020;8:179. doi: 10.3390/microorganisms8020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bossung, V. et al. Timing of antimicrobial prophylaxis for cesarean section is critical for gut microbiome development in term born infants. Gut Microbes14, 2038855 (2022). [DOI] [PMC free article] [PubMed]
- 83.Pessa-Morikawa T, et al. Maternal microbiota-derived metabolic profile in fetal murine intestine, brain and placenta. BMC Microbiol. 2022;22:46. doi: 10.1186/s12866-022-02457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chu DM, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8:77. doi: 10.1186/s13073-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amandito R, Malik A, Rohsiswatmo R. Metagenomic profiles of the early life microbiome of Indonesian inpatient neonates and their influence on clinical characteristics. Sci. Rep. 2022;12:9413. doi: 10.1038/s41598-022-13496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Su M, et al. Diversified gut microbiota in newborns of mothers with gestational diabetes mellitus. PLoS One. 2018;13:e0205695. doi: 10.1371/journal.pone.0205695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.López-Rabuñal Á, et al. Assessment of tobacco exposure during pregnancy by meconium analysis and maternal interview. J. Anal. Toxicol. 2020;44:797–802. doi: 10.1093/jat/bkaa027. [DOI] [PubMed] [Google Scholar]
- 88.Naudé PJW, et al. Association of maternal prenatal psychological stressors and distress with maternal and early infant faecal bacterial profile. Acta Neuropsychiatr. 2020;32:32–42. doi: 10.1017/neu.2019.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X, et al. Effects of a maternal mindfulness intervention targeting prenatal psychological distress on infants’ meconium microbiota: a randomized controlled trial. Psychoneuroendocrinology. 2022;145:105913. doi: 10.1016/j.psyneuen.2022.105913. [DOI] [PubMed] [Google Scholar]
- 90.Liu, S. et al. The association between microplastics and microbiota in placentas and meconium: the first evidence in humans. Environ. Sci. Technol.10.1021/acs.est.2c04706 (2022). [DOI] [PubMed]
- 91.Hiltunen H, et al. Preterm infant meconium microbiota transplant induces growth failure, inflammatory activation, and metabolic disturbances in germ-free mice. Cell Rep. Med. 2021;2:100447. doi: 10.1016/j.xcrm.2021.100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen W, et al. Postnatal age is strongly correlated with the early development of the gut microbiome in preterm infants. Transl. Pediatr. 2021;10:2313–2324. doi: 10.21037/tp-21-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klopp, J. et al. Meconium microbiome of very preterm infants across Germany. mSphere7, e00808-21 (2022). [DOI] [PMC free article] [PubMed]
- 94.Yang J, et al. Comparison of meconium microbiome in dizygotic and monozygotic twins born by caesarean section (CS) Front. Microbiol. 2020;11:1–12. doi: 10.3389/fmicb.2020.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bittinger K, et al. Bacterial colonization reprograms the neonatal gut metabolome. Nat. Microbiol. 2020;5:838–847. doi: 10.1038/s41564-020-0694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuczynski J, et al. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2011;13:47–58. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_information1,2,4,5,7,8,9,10
Data Availability Statement
Raw sequences were submitted to Genbank with the Bioproject number PRJNA905086.