Abstract
Purpose
This cross-sectional study aimed to assess the association between ultra-processed foods consumption and dietary diversity and micronutrient intake in Australia.
Methods
As part of the Nutrition and Physical Activity Survey (2011–2012), 12,153 participants aged 2 years and above were recruited and interviewed. Dietary intake data were collected by two 24-h dietary recalls using the Automated Multiple-Pass Method. The NOVA classification system was used to group the food items based on the extent and purpose of industrial food processing. The mean micronutrient contents were calculated for the total diet, and for two diet fractions; one made up entirely of ultra-processed foods (NOVA group 4) and the other consisting of all non-ultra-processed foods (aggregation of NOVA food groups 1 to 3). The mean micronutrient content in the ultra-processed and non-ultra-processed food diet fractions were compared. Dietary diversity was measured using the ten Food Group Indicators (FGI) of the Food and Agriculture Organization and was defined as the sum number of FGIs per individual. Multiple linear regression models were used to assess the association between the quintiles of energy contribution of ultra-processed foods, dietary diversity, and micronutrient intake.
Results
A negative association was found between quintiles of energy contribution of ultra-processed foods and dietary diversity (β = − 0.43; p < 0.001). The overall micronutrient content was lower in the diet fraction dominated by ultra-processed foods compared to the non-ultra-processed food diet fraction in the study population. The dietary contents of vitamins A, E, C, B9, B12, zinc, calcium, iron, magnesium, potassium, and phosphorus were reduced significantly with increased consumption of ultra-processed foods, even after adjustment for sociodemographic factors and dietary diversity.
Conclusion
The quintiles of energy contribution of ultra-processed foods were negatively associated with dietary diversity and micronutrient intake in Australia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-023-03245-2.
Keywords: Ultra-processed food, Micronutrient, Dietary diversity, Food consumption, Australia
Introduction
Increasing evidence supports the detrimental impacts of food ultra-processing on human health [1, 2]. Ultra-processed foods (UPFs) are made of processed formulations of low-cost ingredients manufactured with ‘cosmetic’ additives [3, 4]. Nationally representative data have shown that UPFs are contributing to more than half of the dietary energy in some high-income countries [5–7] and about one-third to one-fifth of the energy intake in middle-income nations [8–10]. In Australia [11], France [12], and Japan [13], 30–45% of the daily energy consumption comes from UPFs. Ultra-processed products are becoming dominant in global food systems [14], and Australia alone has experienced a growth rate of 5% in UPFs expenditures between 1989 and 2010 [15].
UPF-rich diets are associated with a wide array of health complications, partly due to low dietary diversification and micronutrient intake. In terms of micronutrients; studies in the US [16], UK [7], Australia [11], Canada [5], Brazil [8], Mexico [17], Chile [18], and Colombia [19] consistently reported that UPF-rich diets are nutritionally unbalanced. In these studies, rises in the share of UPFs were inversely associated with the intake of vitamins A, C, D, E, B12, B6, and β-carotene, thiamine, riboflavin, niacin, folate, zinc, potassium, phosphorus, magnesium, calcium, and iron. In studies in Australia, higher consumption of UPFs was positively associated with non-recommended intake of free sugar, sodium, and saturated fat [11] and negatively related to the overall diet quality [20]. Dietary diversity is known as an important construct of dietary metrics linked to Non-communicable Diseases (NCDs) prevention [21]. In studies among adult men in India [22], primary schoolchildren in Côte d’Ivoire [23], and community-dwelling older people in Thailand [24], low dietary diversity was associated with a higher prevalence of NCDs. In addition to dietary diversity and micronutrient intake, the variety of hyper-palatable foods in UPF-rich diets may promote compulsive eating, which along with the large portion sizes of UPFs, can lead to a excessive energy intake [25]. Also, as a rule, energy-dense UPFs ameliorate the sense of satiety [4], trigger hyperglycemic responses [4], and increase the cardiometabolic and NCDs risk factors [1, 2, 13]. It is estimated that halving UPFs consumption in the UK could reduce cardiovascular disease mortality by 10% by 2030 [26]. Beyond the nutritional facets, industrial food processing can degrade the general characteristics of the original food matrix, which can lead to different health complications [27].
More than half of men and 73% of women aged 2 years and over have a low dietary intake of calcium and 23% of women have different forms of iron deficiency in Australia [28]. Likewise, 7% and 16% of men and women have inadequate intakes of thiamine, respectively, and 9% of women aged 19 years and over have failed to meet their folate requirements from food sources [28]. Given the significance of dietary diversity and micronutrient adequacy in health maintenance across the lifecycle and the connection between inadequate micronutrients intake and higher risk of NCDs, this study aimed to evaluate UPFs consumption in association with dietary diversity and micronutrient intake in Australia. The investigated micronutrients were selected based on data availability and consistent with the literature. Also, UPFs were defined according to the NOVA food classification system. To the best of our knowledge, this is the first study to investigate the NOVA-classified UPFs, dietary diversity, and micronutrient intake in Australia. Australia's unique geographical location relative to other countries can potentially influence the accessibility and availability of specific food items and the food culture, which may limit the generalizability of findings from other countries. Therefore, it is important to study this topic within the Australian context.
Methods
Data source
This cross-sectional study is based on the 2011–2012 National Nutrition and Physical Activity Survey (NNPAS) data, part of the 2011–2013 Australian Health Survey (AHS). The NNPAS data collection was conducted between May 2011 and June 2012 on 9,519 households and included a random sample of Australians selected via stratified, multistage probability cluster sampling. As part of the data collection, 12,153 Australians aged 2 years and above were interviewed [29]. This study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut) reporting guidelines (Supplementary Table 1).
Sociodemographic data were collected for all individuals via face-to-face interviews and included age, sex, educational attainment, income, socioeconomic status, and geographical location [30]. As part of the NNPAS, the dietary intake data were collected through two 24-h dietary recalls administered by trained and experienced interviewers using the Automated Multiple-Pass Method. The Automated Multiple-Pass Method involves five steps to assist the interviewers maximize data collection on the amount, timing, cooking method, and processing level of the consumed food items [31]. The first dietary recall was completed in a face-to-face interview (n = 12,153) and the second recall was done via a telephone interview (n = 7735) conducted eight days or more after the first interview [29]. Dietary information for children aged 2 to 5 years was reported by the child’s parent/guardian (child’s proxy). This method was previously found to be a valid instrument to assess energy intake among children aged 4 to 10 years old [32]. For ages 6–8 years, the child was allowed to assist the proxy and from 9 to 11 years, the child was eligible to be interviewed with the assistance of the proxy, if required [33]. Where permission was granted by a parent/guardian, adolescents aged 12–17 years old were interviewed in person [33], otherwise, questions were answered by the parent/guardian. Energy and micronutrient (vitamins A, C, E, B12, thiamine, riboflavin, niacin, pyridoxine, folate, and zinc, calcium, iron, magnesium, potassium, and phosphorus) intakes were estimated based on the Australian Food Composition Database (AUSNUT 2011–2013). The AUSNUT 2011–2013 Food Composition Database contains information for approximately 5740 foods and beverages and was specifically designed to match the NNPAS dietary intake survey [33, 34]. Given that Australia has certain mandatory food fortification policies, such as flour fortification with folic acid, the nutrition composition of the ingredients and food items takes the micronutrient fortifications into account [35]. We did not analyse information on dietary supplementation.
NOVA classification
Food and beverages recorded in the NNPAS were previously classified according to the NOVA classification system [11] into the following four groups (and subgroups within these groups): Group 1—Unprocessed or minimally processed foods (e.g. rice and other cereals, meat, fish, milk, eggs, fruit, roots and tubers, vegetables, nuts, and seeds); Group 2—Processed culinary ingredients (e.g. table sugar, plant oils, and butter); Group 3—Processed foods (e.g. processed bread and cheese, canned fruit and fish, and salted and smoked meats); Group 4—Ultra-processed foods (UPFs; e.g. confectionaries, savoury snacks, fast food dishes, mass-produced packaged bread, frozen and ready meals, and soft drinks).
Ultra-processed products, which are of interest in this study, are formulations of low-cost ingredients, many of non-culinary use, that result from a sequence of industrial processes [4]. The manufacture of UPFs starts with the extraction of substances existing in intact foods, such as oils, fats, sugars, starches, and protein [3]. Intermediate processes may involve hydrolysis, hydrogenation, and other chemical modifications of the extracted substances [4]. Other steps include the assembling of modified (e.g., hydrogenated oils) and unmodified (e.g., sugar) substances using procedures such as extrusion and pre-frying, the addition of ‘cosmetic’ additives such as flavours, colours, thickeners, or emulsifiers, and sophisticated packaging with the frequent employment of novel synthetic materials [4]. The presence or absence of these ingredients was identified in accordance with the auxiliary AUSNUT data sources (Food details and Food recipe files), which are based on the list of ingredients on the food packages or the company websites [11]. Food items in this study were classified by two evaluators with expertise in the Australian food supply, the AUSNUT 2011–2013 Food Composition Database, and the NOVA classification system. More information regarding the UPFs’ classification system in Australia can be found elsewhere [11].
Dietary diversity
Food items were classified according to the ten Food Group Indicators (FGIs) proposed by the Food and Agriculture Organization of the United Nations (FAO) to measure dietary diversity: (1) grain, white roots and tubers, and plantains (starchy staples); (2) pulses (beans, peas, and lentils); (3) nuts and seeds; (4) dairy; (5) meat, poultry, and fish; (6) eggs; (7) dark green leafy vegetables; (8) vitamin A-rich fruits and vegetables; (9) other vegetables; (10) and other fruits [36]. Ultra-processed products were not included in the FGIs based on the premise that these foods are unhealthy and should not be recommended as part of the diversity indicators. Dietary diversity was established as the number of FGIs (>15 g) consumed by each individual per day. Beverages were considered as part of the FGIs. For instance, FGI 4 includes dairy products such as milk, and groups 7–10 describe different types of fruits, which also includes fruit juices. Ultra-processed and unhealthy beverages such as soda and sugary drinks were not included based on the health-deteriorating notion.
Data analysis
Dietary intake data were adjusted for the Multiple Source Method to account for intra-person variability [37]. The study population excluded women during pregnancy or breastfeeding. The mean contribution (%) of each NOVA food group and subgroup to the total energy intake was calculated. The study population was then stratified into quintiles of the energy contribution of UPFs (first and fifth quintiles representing the lowest and highest consumption of UPFs, respectively). The % energy share of each NOVA food group and subgroup was estimated across those quintiles.
The mean contents of the selected micronutrients were calculated for the total diet (micronutrient density; mg per 1000 kcal) and for two diet fractions made up entirely of ultra-processed (NOVA group 4) versus all non-ultra-processed foods (aggregation of unprocessed or minimally processed foods, processed culinary ingredients and processed foods; NOVA food groups 1 to 3). Independent Samples t test was used to assess the mean differences between the two dietary fractions.
The associations between the energy contribution of UPFs with FGIs by socio-demographic characteristics were studied using adjusted Poisson regression models. The mean micronutrient intake and dietary diversity across the quintiles of the energy contribution of UPFs were studied using linear regression models. The first model was adjusted for participants’ age, sex, educational attainment, income, socioeconomic status, and geographical location, and model 2 was additionally adjusted for dietary diversity. The analyses were based on the first 24-h recall data, which is deemed suitable for the estimation of the group means. Finally, sensitivity analyses were conducted: (i) using the exposure (the percentage of energy explained by UPFs) as a continuous variable (Supplementary Table 2); (ii) using energy-adjusted FGIs (Supplementary Table 3).
Results
Table 1 illustrates the micronutrient density of the total diet and two diet fractions made up of UPFs (NOVA group 4) and non-UPFs (aggregated NOVA groups 1–3). Compared to the UPFs-dominated diet fraction, the fraction made up of non-UPFs had a higher density of all micronutrients (except Vitamins B1, B2, and iron), with differences ranging from 3 times (Vitamin B12) to 1.1 times (Vitamin E) (p for all < 0.001). In particular, vitamins B12 and C were about 3 times higher density in the non-UPFs diet fraction compared to the UPFs fraction (p < 0.001).
Table 1.
Dietary diversity and micronutrient content (standardised for 1000 kcal) of the overall diet and the ultra-processed and non-ultra-processed foods diet fractions; Australian population aged 2 + years (NNPAS 2011–2012; n = 11,862)
| Overall diet | Ultra-processed food diet fraction | Non-ultra-processed food diet fraction | Ratio non-UPFs/UPFs | ||||
|---|---|---|---|---|---|---|---|
| Mean | (SE) | Mean | (SE) | Mean | SE | ||
| Dietary Diversity | |||||||
| Number of Food Group Indicators | 5.55 | 0.02 | – | – | 5.55 | 0.02 | – |
| Vitamins | |||||||
| Vitamin A (RAE) | 393.8 | 7.69 | 204.9 | 2.23 | 559 | 13.5 | 2.7* |
| Vitamin B1 Thiamine (mg) | 0.85 | 0.008 | 1.22 | 0.02 | 0.63 | 0.004 | 0.5* |
| Vitamin B2 Riboflavin (mg) | 0.93 | 0.005 | 1.06 | 0.01 | 0.90 | 0.005 | 0.9* |
| Vitamin B3 Niacin (mg) | 20.2 | 0.07 | 15.8 | 0.12 | 24.2 | 0.11 | 1.5* |
| Vitamin B6 Pyridoxine (mg) | 0.70 | 0.005 | 0.51 | 0.01 | 0.85 | 0.004 | 1.7* |
| Vitamin B9 Folate (mg) | 142.4 | 0.70 | 102.6 | 1.02 | 179.3 | 1.06 | 1.7* |
| Vitamin B12 (μg) | 2.24 | 0.02 | 1.08 | 0.01 | 3.21 | 0.04 | 3.0* |
| Vitamin C (mg) | 49.2 | 0.49 | 44.6 | 1.20 | 55.6 | 0.59 | 1.2* |
| Vitamin E (mg) | 5.09 | 0.30 | 4.84 | 0.04 | 5.45 | 0.04 | 1.1* |
| Minerals | |||||||
| Calcium (mg) | 394.5 | 1.91 | 333.4 | 3.25 | 470.4 | 3.12 | 1.4* |
| Iron (mg) | 5.68 | 0.03 | 6.55 | 0.07 | 5.20 | 0.03 | 0.8* |
| Magnesium (mg) | 160.8 | 0.58 | 130.5 | 0.84 | 190.6 | 0.99 | 1.5* |
| Potassium (mg) | 1403.7 | 4.16 | 1043.3 | 6.02 | 1722.8 | 6.80 | 1.6* |
| Phosphorus (mg) | 714.8 | 1.93 | 590.7 | 3.82 | 835.6 | 2.48 | 1.4* |
| Zinc (mg) | 5.24 | 0.02 | 3.95 | 0.02 | 6.31 | 0.03 | 1.6* |
NNPAS National Nutrition and Physical Activity Survey, SE Standard Error, RAE Retinol Activity Equivalents
*p value for differences with non-ultra-processed foods by using Student’s tests in each micronutrient; p < 0.001 is considered significant
aIncludes NOVA unprocessed or minimally processed foods, processed culinary ingredients and processed foods
Table 2 represents the distribution of sociodemographic variables according to the number of FGIs by the quintiles of the energy contribution of UPFs. The mean percentage of the total energy intake from UPFs was 43.69 ± 0.21. Also, the mean quintiles of energy contribution of UPFs ranged from 20.26% in the first quantile to 68.33% in the fifth quantile. Based on the results, the mean dietary diversity was reduced with increasing energy contribution of the UPFs from quantile 1 to 5 across all sociodemographic groups and subgroups (p < 0.001). Additional models were run also adjusting for the effect of energy intake among different age groups (Supplementary Table 3), and consistent results were found.
Table 2.
Dietary diversity across quintiles of the energy contribution of ultra-processed foods by socio-demographic characteristics; Australian population aged 2 + years (NNPAS 2011–2012; n = 11,862)
| Socio-demographic characteristics | Total sample (%) | Mean (SE) | Mean (SE) FGIs by quintiles of energy contribution of ultra-processed foods¥ | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| All | 100 | 5.55 (0.02) | 6.26 (0.04) | 6.05 (0.05) | 5.86 (0.05) | 5.38 (0.05) | 4.19 (0.05)*** |
| Sex | |||||||
| Male | 50.7 | 5.42 (0.03) | 6.11 (0.06) | 5.94 (0.07) | 5.75 (0.07) | 5.32 (0.07) | 4.12 (0.07)*** |
| Female | 49.3 | 5.68 (0.03) | 6.41 (0.06) | 6.17 (0.06) | 5.98 (0.06) | 5.45 (0.07) | 4.23 (0.08)*** |
| Age group (years) | |||||||
| 2 to 4 | 4.2 | 5.09 (0.09) | 5.90 (0.22) | 5.74 (0.18) | 5.43 (0.19) | 5.07 (0.19) | 4.00 (0.17)*** |
| 5 to 11 | 9.1 | 5.19 (0.07) | 6.79 (0.23) | 5.63 (0.16) | 5.74 (0.16) | 5.62 (0.13) | 4.35 (0.12)*** |
| 12 to 19 | 10.8 | 4.95 (0.08) | 5.68 (0.22) | 5.98 (0.21) | 5.80 (0.16) | 5.16 (0.14) | 4.03 (0.13)*** |
| 20 to 59 | 56.2 | 5.65 (0.03) | 6.29 (0.05) | 6.04 (0.06) | 5.92 (0.07) | 5.40 (0.07) | 4.18 (0.08)*** |
| 60 + | 19.7 | 5.85 (0.04) | 6.29 (0.07) | 6.21 (0.09) | 5.90 (0.08) | 5.43 (0.11) | 4.41 (0.15)*** |
| Years of education | |||||||
| Low (≤ 9) | 12.5 | 5.31 (0.06) | 5.79 (0.12) | 5.82 (0.12) | 5.65 (0.11) | 5.07 (0.15) | 3.87 (0.17)*** |
| Medium (10 to 12 with no graduate degree) | 63.6 | 5.44 (0.03) | 6.21 (0.06) | 5.97 (0.06) | 5.79 (0.06) | 5.37 (0.06) | 4.15 (0.06)*** |
| High (12 with graduate degree) | 23.9 | 5.99 (0.05) | 6.55 (0.07) | 6.35 (0.09) | 6.17 (0.09) | 5.62 (0.10) | 4.60 (0.14)*** |
| SEIFA | |||||||
| Quintile 1—greater disadvantage | 17.9 | 5.18 (0.06) | 5.74 (0.10) | 5.52 (0.13) | 5.82 (0.11) | 5.31 (0.12) | 3.93 (0.12)*** |
| Quintile 2 | 19.8 | 4.35 (0.05) | 6.02 (0.10) | 5.81 (0.11) | 5.62 (0.10) | 5.38 (0.10) | 4.07 (0.11)*** |
| Quintile 3 | 20.8 | 5.54 (0.05) | 6.39 (0.10) | 6.05 (0.10) | 5.88 (0.10) | 5.30 (0.10) | 4.22 (0.11)*** |
| Quintile 4 | 18.8 | 5.72 (0.05) | 6.58 (0.09) | 6.18 (0.09) | 5.87 (0.10) | 5.40 (0.11) | 4.28 (0.15)*** |
| Quintile 5—greater advantage | 22.7 | 5.88 (0.05) | 6.42 (0.07) | 6.47 (0.09) | 6.07 (0.10) | 5.51 (0.11) | 4.54 (0.13)*** |
| Household income | |||||||
| Quintile 1—lower income | 16.4 | 5.25 (0.05) | 5.86 (0.10) | 5.78 (0.11) | 5.63 (0.11) | 5.09 (0.12) | 3.97 (0.13)*** |
| Quintile 2 | 16.0 | 5.41 (0.06) | 6.02 (0.12) | 5.84 (0.11) | 5.83 (0.10) | 5.57 (0.11) | 4.14 (0.13)*** |
| Quintile 3 | 18.5 | 5.54 (0.05) | 6.42 (0.10) | 6.13 (0.10) | 5.73 (0.10) | 5.41 (0.10) | 4.24 (0.12)*** |
| Quintile 4 | 18.7 | 5.60 (0.05) | 6.45 (0.09) | 6.09 (0.11) | 5.95 (0.11) | 5.39 (0.12) | 4.28 (0.12)*** |
| Quintile 5—greater income | 17.0 | 6.02 (0.05) | 6.54 (0.09) | 6.32 (0.12) | 6.27 (0.10) | 5.56 (0.11) | 4.66 (0.15)*** |
| Geographical location | |||||||
| Major cities of Australia | 70.7 | 5.57 (0.03) | 6.28 (0.05) | 6.04 (0.05) | 5.87 (0.06) | 5.33 (0.06) | 4.19 (0.07)*** |
| Inner regional Australia | 19.7 | 5.50 (0.05) | 6.23 (0.11) | 6.09 (0.11) | 5.85 (0.11) | 5.49 (0.10) | 4.20 (0.11)*** |
| Other | 9.6 | 5.47 (0.07) | 6.14 (0.13) | 6.06 (0.12) | 5.81 (0.12) | 5.50 (0.14) | 4.18 (0.15)*** |
NNPAS National Nutrition and Physical Activity Survey, SEIFA Socio-Economic Index for Areas
***p < 0.001 for prevalence ratio estimated using Poisson regression models adjusted by all the sociodemographic characteristics in the table (quintile 1 vs. quintile 5)
¥Percentage of total energy intake from ultra-processed foods. Mean (43.69 ± 0.21); quintiles mean and range: Q1 = 20.26 (0 to 28.72); Q2 = 34.08 (28.73 to 38.79); Q3 = 43.22 (38.80 to 47.76); Q4 = 52.59 (47.76 to 58.12); Q5 = 68.33 (58.12 to 100)
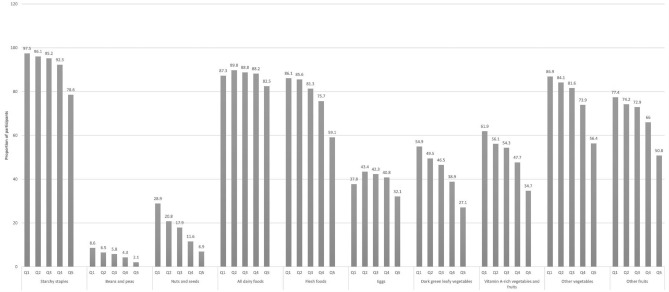
Figure 1 presents the proportion of the study sample who consumed each of the 10 FGIs across quintiles of the energy contribution of UPFs. As illustrated, there had been linear reductions in the proportion of participants that consumed all the ten FGIs across the UPFs-contributed energy quantiles, except in dairy foods and eggs, where the quintiles indicated inverted U-shapes.
Fig. 1.
Proportion of participants who consumed each of the 10 Food Group Indicators (FGIs)† across quintiles of energy contribution of ultra-processed foods¥. Australian population aged 2+ years (NNPAS 2011–2012) (n = 11,862). Notes: NNPAS National Nutrition and Physical Activity Survey. †p value of linear trend < 0.001 across quintiles using regression models adjusted for age, sex, educational attainment, socio-economic status, income and geographical location was observed for all food groups, except dairy (p = 0.09) and eggs (p = 0.01). ¥Percentage of total energy intake from ultra-processed foods. Mean (43.69 ± 0.21); quintiles mean and range: Q1 = 20.26 (0 to 28.72); Q2 = 34.08 (28.73 to 38.79); Q3 = 43.22 (38.80 to 47.76); Q4 = 52.59 (47.76 to 58.12); Q5 = 68.33 (58.12 to 100)
Table 3 represents the distribution of the FGIs and micronutrient intakes across quintiles of the energy contribution of UPFs. After adjustment for potential confounders in model 1, an inverse and statistically significant association was found between dietary diversity and the quintiles of the energy contribution of UPFs (β = − 0.43; p < 0.001). In the adjusted models, the dietary intakes of vitamins A, E, C, B12, niacin, pyridoxine, folate, zinc, calcium, iron, magnesium, potassium, and phosphorus were negatively associated with the consumption of UPFs (p for all < 0.001), while no significant associations at the p = 0.001 level were observed for thiamine and riboflavin. Similar results were found when the exposure was used as a continuous variable in the sensitivity analysis (p < 0.001; Supplementary Table 2).
Table 3.
Dietary diversity and micronutrients intake (standardised for 1000 kcal) across the quintiles of the dietary share of ultra-processed foods. Australian population aged 2 + years (NNPAS 2011–2012; n = 11,862)
| Nutrients | Quintiles of the dietary contribution of ultra-processed foods (% of total dietary energy)¥ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Model 1 | Model 2 | ||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | β | SE | β | SE | |
| Dietary Diversity | ||||||||||||||
| Number of Food Group Indicators | 6.18 | 0.04 | 6.01 | 0.05 | 5.85 | 0.05 | 5.42 | 0.05 | 4.31 | 0.05 | − 0.43 | 0.02*** | – | – |
| Vitamins | ||||||||||||||
| Vitamin A (RAE) | 458.1 | 16.6 | 396.6 | 9.96 | 423.4 | 28.3 | 373.8 | 14.5 | 313.5 | 8.42 | − 30.9 | 4.40*** | − 26.2 | 4.08*** |
| Vitamin B1 Thiamine(mg) | 0.79 | 0.01 | 0.87 | 0.02 | 0.88 | 0.02 | 0.87 | 0.02 | 0.85 | 0.02 | 0.01 | 0.006* | 0.009 | 0.006 |
| Vitamin B2 Riboflavin (mg) | 0.91 | 0.01 | 0.94 | 0.01 | 0.93 | 0.01 | 0.93 | 0.01 | 0.92 | 0.01 | 0.0008 | 0.004 | 0.0002 | 0.004 |
| Vitamin B3 Niacin (mg) | 21.8 | 0.17 | 21.2 | 0.16 | 20.3 | 0.14 | 19.5 | 0.14 | 18.2 | 0.17 | − 0.88 | 0.06 *** | − 0.85 | 0.06*** |
| Vitamin B6 Pyridoxine (mg) | 0.79 | 0.01 | 0.72 | 0.01 | 0.68 | 0.01 | 0.66 | 0.01 | 0.64 | 0.02 | − 0.03 | 0.004 *** | -0.03 | 0.004*** |
| Vitamin B9 Folate (mg) | 160.8 | 1.82 | 148.9 | 1.52 | 143.2 | 1.40 | 136.7 | 1.27 | 122.1 | 1.48 | − 8.93 | 0.52 *** | − 7.72 | 0.56*** |
| Vitamin B12 (μg) | 2.46 | 0.04 | 2.33 | 0.03 | 2.30 | 0.07 | 2.16 | 0.04 | 1.96 | 0.03 | − 0.12 | 0.01 *** | − 0.12 | 0.01*** |
| Vitamin C (mg) | 56.8 | 1.23 | 51.8 | 1.15 | 49 | 0.99 | 46.1 | 0.97 | 41.7 | 1.22 | − 3.59 | 0.39 *** | − 2.96 | 0.41*** |
| Vitamin E (mg) | 5.86 | 0.09 | 5.32 | 0.06 | 5.06 | 0.06 | 4.81 | 0.04 | 4.43 | 0.05 | − 0.33 | 0.02 *** | − 0.24 | 0.02*** |
| Minerals | ||||||||||||||
| Calcium (mg) | 398 | 4.3 | 402.6 | 4.47 | 398 | 4.27 | 393 | 4.2 | 373.9 | 4.46 | − 5.64 | 1.46 *** | − 5.58 | 1.57*** |
| Iron (mg) | 5.87 | 0.07 | 5.92 | 0.06 | 5.73 | 0.06 | 5.67 | 0.06 | 5.17 | 0.07 | − 0.16 | 0.02 *** | − 0.15 | 0.03*** |
| Magnesium (mg) | 183.8 | 1.5 | 170.3 | 1.15 | 159.9 | 1.1 | 152.1 | 1.09 | 139.1 | 1.20 | − 10.7 | 0.44 *** | − 10.1 | 0.46*** |
| Potassium (mg) | 1551.7 | 10.12 | 1465 | 8.69 | 1408 | 8 | 1357 | 8.25 | 1237 | 9.22 | − 73.4 | 3.12 *** | − 64.8 | 3.30*** |
| Phosphorus (mg) | 762.3 | 4.50 | 751 | 4.64 | 717.5 | 3.96 | 697.4 | 3.73 | 643.7 | 4.24 | − 28.9 | 1.46 *** | − 26.2 | 1.54*** |
| Zinc (mg) | 5.84 | 0.07 | 5.55 | 0.05 | 5.28 | 0.04 | 5.05 | 0.04 | 4.51 | 0.04 | − 0.32 | 0.02 *** | − 0.32 | 0.02*** |
NNPAS National Nutrition and Physical Activity Survey, RAE Retinol Activity Equivalents, ¥Percentage of total energy intake from ultra-processed foods. Mean (43.69 ± 0.21); quintiles mean and range: Q1 = 20.26 (0 to 28.72); Q2 = 34.08 (28.73 to 38.79); Q3 = 43.22 (38.80 to 47.76); Q4 = 52.59 (47.76 to 58.12); Q5 = 68.33 (58.12 to 100), Model 1: Adjusted for age, sex, educational attainment, socio-economic status, income and geographical location. Model 2: Adjusted for covariates of Model 1 and the 10 FGI
*p < 0.05, **p < 0.01 and ***p < 0.001 for linear trend across quintiles
Discussion
In this study, dietary diversity and the dietary content of most micronutrients were inversely associated with the energy share of UPFs. Our findings are consistent with other population-based studies in the US [16], Canada [5], Brazil [8], and Mexico [17]. To the best of our knowledge, this is the first study to investigate the consumption of UPFs in association with dietary diversity and micronutrient intake in Australia. In 2019, co-authors of this study used the same data and investigated the dietary intake of macronutrients [11]. The energy contribution of UPFs was positively associated with the dietary intake of total, saturated, and trans fats and negatively linked with dietary fibre intake [11].
As ultra-processed food consumption increases globally, food manufacturers are involving reformulated UPFs in food fortification programs as potential vehicles of micronutrient supplementation regardless of their poor nutritional quality [38]. Although food fortification programs partly address populations’ micronutrient-related malnourishment, fortification of UPFs can maintain or increase their current rates of consumption, thereby resulting in a constant or higher intake of sodium, saturated fats, and sugar and increased risks of NCDs [11]. To tackle the negative consequences of these approaches, it is best to prioritise compliance with the current Australian Dietary Guidelines and the elements of healthy diet, which have a particular emphasis on dietary diversification and consumption of fortified foods that are not ultra-processed [39]. Considering the higher density of micronutrients in unprocessed and minimally processed foods, enhancing dietary diversity is key to achieving the vitamin and mineral intake recommendations at the population level.
In this study, the number of FGIs representing dietary diversity was negatively associated with the energy contribution of UPFs. In addition, the proportion of participants that consumed the ten identified FGIs was higher in quintile 1 of the UPFs energy contribution across all food groups, except for dairy products and eggs. Consistent with the findings of a similar study in Mexico [17], starchy staples were among the highly consumed food group across the UPF quintiles, which decreased from 97.5% in quintile 1 to 78.6% in quintile 5. This can be explained by the fact that corn and rice are still among the primary food sources and essential ingredients of international and Australian cuisines [40].
Compared to minimally processed foods, UPFs may be predominant in the food basket of socioeconomically disadvantaged households in Australia [20, 41]. The significant expense and financial hurdles involved in obtaining nutritious foods pose major barriers to maintaining a healthy diet [41, 42]. This requires exploring the socioeconomic aspects of food processing in the planning and management of public health policies. Recently, Lee and others reported that although recommended healthy diets can cost 20% less than routine diets, they might still be unaffordable for low-income families in Australia [42]. Despite the various interventions and programs in place, the marketing rates of UPFs and the prevalence of obesity and NCDs in Australia have been increasing simultaneously in recent years [11]. This could be a possible sign of a double burden of malnourishment in Australia, which needs to be considered by policymakers when developing dietary guidelines and planning sustainable and equitable food systems.
In this study, the average intake of thiamine, riboflavin, and iron was higher in the UPFs-dominated diet fraction compared with the non-UPFs fraction. Nevertheless, when assessing intakes of thiamine and riboflavin across quintiles of UPF consumption, associations did not remain significant. These results are aligned with similar findings in Brazil [8] and Mexico [17] and can be linked to the high consumption of nutritionally fortified, mass-produced bread and breakfast cereals in the top quintiles of UPFs in Australia [11]. Further investigation is required to evaluate whether these associations remain consistent in recent survey data.
Although extensive food processing is generally criticized, it is important to acknowledge the benefits of processing to societies, as well. This has increased safety and convenience of products, and certain techniques bring benefits such as cooking at normal temperatures, which increases the bioavailability of certain phytonutrients such as lycopene [43]. These are different from the processing techniques applied in UPFs' production, which can alter the food structure and composition and negatively impact the absorption and utilization of nutrients by the body [27].
This study provided evidence to support the detrimental impacts of UPFs on population dietary patterns in Australia. This is consistent with previous studies in different countries and is aligned with the existing dietary guidelines, emphasizing the significance of dietary diversification and prioritization of unprocessed and minimally processed foods over UPFs. Therefore, reducing the share of UPFs in the population's eating patterns can enhance the diet quality in Australia, and help the achievement of micronutrient intake recommendations. Further research in this area is required to validate the findings. Future studies may consider the comparison of population-level micronutrient intake with official dietary guidelines and recommendations in Australia to make firm conclusions.
Our study had several strengths, including the use of the most recent, individual-level dietary data collected from a nationally representative sample of Australian children and adults, which along with the application of valid assessment methods, increases generalizability. Also, in this study, the NOVA food classification system was applied to disaggregated food codes, which enabled the assessment of underestimated food groups and comparisons among different countries. Finally, the assessment of the contribution of foods according to the level of processing to the daily intake of micronutrients provided novel evidence to improve diet quality in Australia. Among the limitations of this study is the collection of dietary data by 24-h recalls, which are subject to errors. A robust method was applied to classify the AUSNUT 2011–2013 food items according to the NOVA system, however, some items may have been misclassified. Also, some items may have been misclassified due to inconsistencies of information indicative of food processing in the datasets.
Conclusion
In this nationally representative study of the Australian population, the UPFs contribution to the energy quantiles was negatively associated with dietary diversity and micronutrient. Therefore, promoting dietary diversity, increasing the consumption of unprocessed and minimally processed foods, and discouraging the consumption of UPFs could improve the overall diet quality in Australia.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
PM and ZH designed the research; PM took care of data management and analyses; PM and ZH interpreted the data; ZH wrote the first draft of the manuscript; PM, ZH, GC, IS, and GS revised each draft for important intellectual content.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. PM received income through an Alfred Deakin Postdoctoral Research Fellowship provided by Deakin University. ZH receives income through the Erasmus Mundus Excellence Scholarship provided by the European Commission.
Declarations
Conflict of interest
The author declares that they have no competing interest.
Ethical standards
This project was declared exempt from ethical review at the Deakin University Human Research Ethics Committee meeting held on 19/07/2021 in accordance with the National Statement on Ethical Conduct in Human Research (2007, updated 2018) section 5.1.22 [44]. This study was a secondary analysis using de-identified data from the ABS Basic Confidentialised Unit Record Files (CURFs), and permission to use the data was obtained. The authors are solely responsible for the opinions, hypotheses and conclusions or recommendations expressed in this publication, and they do not necessarily reflect WHO nor PAHO’s vision.
References
- 1.Lane MM, Davis JA, Beattie S, Gómez-Donoso C, Loughman A, O'Neil A, Jacka F, Berk M, Page R, Marx W. Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies. Obes Rev. 2021;22(3):e13146. doi: 10.1111/obr.13146. [DOI] [PubMed] [Google Scholar]
- 2.Pagliai G, Dinu M, Madarena M, Bonaccio M, Iacoviello L, Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr. 2021;125(3):308–318. doi: 10.1017/S0007114520002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. doi: 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monteiro CA, Cannon G, Levy RB, Moubarac J-C, Louzada MLC, Rauber F, Khandpur N, Cediel G, Neri D, Martinez-Steele E, Baraldi LG, Jaime PC. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–941. doi: 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moubarac J-C, Batal M, Louzada M, Steele EM, Monteiro C. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite. 2017;108:512–520. doi: 10.1016/j.appet.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Baraldi LG, Steele EM, Canella DS, Monteiro CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. 2018;8(3):e020574. doi: 10.1136/bmjopen-2017-020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauber F, Louzada MLdC, Steele EM, Millett C, Monteiro CA, Levy RB. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014) Nutrients. 2018;10(5):587. doi: 10.3390/nu10050587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Costa Louzada ML, Ricardo CZ, Steele EM, Levy RB, Cannon G, Monteiro CA. The share of ultra-processed foods determines the overall nutritional quality of diets in Brazil. Public Health Nutr. 2018;21(1):94–102. doi: 10.1017/S1368980017001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrón-Ponce JA, Sánchez-Pimienta TG, da Costa Louzada ML, Batis C. Energy contribution of NOVA food groups and sociodemographic determinants of ultra-processed food consumption in the Mexican population. Public Health Nutr. 2018;21(1):87–93. doi: 10.1017/S1368980017002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cediel G, Reyes M, da Costa Louzada ML, Steele EM, Monteiro CA, Corvalán C, Uauy R. Ultra-processed foods and added sugars in the Chilean diet (2010) Public Health Nutr. 2018;21(1):125–133. doi: 10.1017/S1368980017001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado PP, Steele EM, Levy RB, Sui Z, Rangan A, Woods J, Gill T, Scrinis G, Monteiro CA. Ultra-processed foods and recommended intake levels of nutrients linked to non-communicable diseases in Australia: evidence from a nationally representative cross-sectional study. BMJ Open. 2019;9(8):e029544. doi: 10.1136/bmjopen-2019-029544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julia C, Martinez L, Allès B, Touvier M, Hercberg S, Méjean C, Kesse-Guyot E. Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Santé study. Public Health Nutr. 2018;21(1):27–37. doi: 10.1017/S1368980017001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koiwai K, Takemi Y, Hayashi F, Ogata H, Matsumoto S, Ozawa K, Machado PP, Monteiro CA. Consumption of ultra-processed foods decreases the quality of the overall diet of middle-aged Japanese adults. Public Health Nutr. 2019;22(16):2999–3008. doi: 10.1017/S1368980019001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker P, Machado P, Santos T, Sievert K, Backholer K, Hadjikakou M, Russell C, Huse O, Bell C, Scrinis G, Worsley A, Friel S, Lawrence M. Ultra-processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obes Rev. 2020;21(12):e13126. doi: 10.1111/obr.13126. [DOI] [PubMed] [Google Scholar]
- 15.Venn D, Banwell C, Dixon J. Australia’s evolving food practices: a risky mix of continuity and change. Public Health Nutr. 2017;20:2549–2558. doi: 10.1017/S136898001600255X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez Steele E, Popkin BM, Swinburn B, Monteiro CA. The share of ultra-processed foods and the overall nutritional quality of diets in the US: evidence from a nationally representative cross-sectional study. Popul Health Metr. 2017;15(1):6. doi: 10.1186/s12963-017-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrón-Ponce JA, Sánchez-Pimienta TG, Rodríguez-Ramírez S, Batis C, Cediel G (2023) Ultra-processed foods consumption reduces dietary diversity and micronutrient intake in the Mexican population. J Hum Nutr Diet 36(1):241–251. 10.1111/jhn.13003 [DOI] [PubMed]
- 18.Cediel G, Reyes M, Corvalán C, Levy RB, Uauy R, Monteiro CA. Ultra-processed foods drive to unhealthy diets: evidence from Chile. Public Health Nutr. 2021;24(7):1698–1707. doi: 10.1017/S1368980019004737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra DC, Costa-Louzada MLd, Moubarac J-C, Bertazzi-Levy R, Khandpur N, Cediel G, Monteiro CA. Association between ultra-processed food consumption and the nutrient profile of the Colombian diet in 2005. Salud pública de méxico. 2019;61:147–154. doi: 10.21149/9038. [DOI] [PubMed] [Google Scholar]
- 20.Marchese L, Livingstone KM, Woods JL, Wingrove K, Machado P. Ultra-processed food consumption, socio-demographics and diet quality in Australian adults. Public Health Nutr. 2022;25(1):94–104. doi: 10.1017/s1368980021003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller V, Webb P, Micha R, Mozaffarian D. Defining diet quality: a synthesis of dietary quality metrics and their validity for the double burden of malnutrition. Lancet Planetary Health. 2020;4(8):e352–e370. doi: 10.1016/S2542-5196(20)30162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolui M, Sarkar S, Ghosh P, Hossain M. Dietary diversity and association with non-communicable diseases (NCDs) among adult men (15–54 years): a cross-sectional study using National Family and Health Survey. India PLOS Global Public Health. 2023;3(4):e0001775. doi: 10.1371/journal.pgph.0001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traoré SG, Kouassi KB, Coulibaly JT, Beckmann J, Gba BC, Lang C, Long KZ, Dao D, Gerber M, Probst-Hensch N, Pühse U, Utzinger J, Bonfoh B. Dietary diversity in primary schoolchildren of south-central Côte d’Ivoire and risk factors for non-communicable diseases. BMC Pediatr. 2022;22(1):651. doi: 10.1186/s12887-022-03684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalermsri C, Ziaei S, Ekström E-C, Muangpaisan W, Aekplakorn W, Satheannopakao W, Moshfiqur Rahman S (2022) Dietary diversity associated with risk of cardiovascular diseases among community-dwelling older people: a national health examination survey from Thailand. Front Nutr 9 [DOI] [PMC free article] [PubMed]
- 25.Monteiro CA, Cannon G, Lawrence M, Costa Louzada Md, Pereira Machado P. Ultra-processed foods, diet quality, and health using the NOVA classification system. Rome: FAO; 2019. p. 49. [Google Scholar]
- 26.Moreira PV, Baraldi LG, Moubarac J-C, Monteiro CA, Newton A, Capewell S, O’Flaherty M. Comparing different policy scenarios to reduce the consumption of ultra-processed foods in UK: impact on cardiovascular disease mortality using a modelling approach. PLoS ONE. 2015;10(2):e0118353. doi: 10.1371/journal.pone.0118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srour B, Kordahi MC, Bonazzi E, Deschasaux-Tanguy M, Touvier M, Chassaing B. Ultra-processed foods andhuman health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol. 2022;7(12):1128–1140. doi: 10.1016/S2468-1253(22)00169-8. [DOI] [PubMed] [Google Scholar]
- 28.Australian Bureau of Statistics (2013) Australian Health Survey: Biomedical Results for Nutrients. National Health Measures Survey (NHMS). Australian Bureau of Statistics, Australia
- 29.Australian Bureau of Statistics (2013) Australian Health Survey: Users' Guide, 2011–13 Canberra2013. http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4363.0.55.001Chapter2002011-13. 2022
- 30.Choi J-H, Yates Z, Veysey M, Heo Y-R, Lucock M. Contemporary issues surrounding folic acid fortification initiatives. Prev Nutr Food Sci. 2014;19(4):247. doi: 10.3746/pnf.2014.19.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Australian Bureau of Statistics (2014) 24-HOUR DIETARY RECALL. Australian Bureau of Statistics. https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/0D6B1FE95EAB8FF3CA257CD2001CA113?opendocument. 2023
- 32.Börnhorst C, Bel-Serrat S, Pigeot I, Huybrechts I, Ottavaere C, Sioen I, De Henauw S, Mouratidou T, Mesana M, Westerterp K. Validity of 24-h recalls in (pre-) school aged children: comparison of proxy-reported energy intakes with measured energy expenditure. Clin Nutr. 2014;33(1):79–84. doi: 10.1016/j.clnu.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Australian Health Survey: Users' Guide - (2013) DATA COLLECTION. Australian Health Survey: Users' Guide, 2011–13. https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4363.0.55.001Chapter2152011-13. Accessed 2023 2013
- 34.Food Standards Australia New Zealand (FSANZ) (2011–2013) Food Composition Database 2014. http://www.foodstandards.gov.au/.
- 35.Food Standards Australia New Zealand (FSANZ) (2016) Vitamins and minerals added to food. https://www.foodstandards.gov.au/consumer/nutrition/vitaminadded/Pages/default.aspx.
- 36.Martin-Prével Y, Allemand P, Wiesmann D, Arimond M, Ballard T, Deitchler M, Dop M-C, Kennedy G, Lee WT, Moursi M (2015) Moving forward on choosing a standard operational indicator of women’s dietary diversity
- 37.Harttig U, Haubrock J, Knüppel S, Boeing H. The MSM program: web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur J Clin Nutr. 2011;65(1):S87–S91. doi: 10.1038/ejcn.2011.92. [DOI] [PubMed] [Google Scholar]
- 38.Kroker-Lobos MF, Mazariegos M, Guamuch M, Ramirez-Zea M. Ultraprocessed products as food fortification alternatives: a critical appraisal from Latin America. Nutrients. 2022;14(7):1413. doi: 10.3390/nu14071413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NHMRC (2013) Eat for Health: Australian Dietary Guidelines; Providing the scientific evidence for healthier Australian diets. Commonwealth of Australia
- 40.Martini D, Godos J, Bonaccio M, Vitaglione P, Grosso G. Ultra-processed foods and nutritional dietary profile: a meta-analysis of nationally representative samples. Nutrients. 2021;13(10):3390. doi: 10.3390/nu13103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee AJ, Kane S, Lewis M, Good E, Pollard CM, Landrigan TJ, Dick M. Healthy diets ASAP—Australian Standardised Affordability and Pricing methods protocol. Nutr J. 2018;17(1):88. doi: 10.1186/s12937-018-0396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee A, Patay D, Herron L-M, Parnell Harrison E, Lewis M. Affordability of current, and healthy, more equitable, sustainable diets by area of socioeconomic disadvantage and remoteness in Queensland: insights into food choice. Int J Equity Health. 2021;20(1):153. doi: 10.1186/s12939-021-01481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matos RA, Adams M, Sabaté J. Review: the consumption of ultra-processed foods and non-communicable diseases in Latin America. Front Nutr. 2021;8:622714. doi: 10.3389/fnut.2021.622714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Health and Medical Research Council, Australian Research Council Universities Australia . National Statement on Ethical Conduct in Human Research 2007 (Updated 2018) Canberra: Commonwealth of Australia; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.