Abstract
Background
It has been thought that intramuscular ADP and phosphocreatine (PCr) concentrations are important regulators of mitochondorial respiration. There is a threshold work rate or metabolic rate for cellular acidosis, and the decrease in muscle PCr is accelerated with drop in pH during incremental exercise. We tested the hypothesis that increase in muscle oxygen consumption ( o2mus) is accelerated with rapid decrease in PCr (concomitant increase in ADP) in muscles with drop in pH occurs during incremental plantar flexion exercise.
o2mus) is accelerated with rapid decrease in PCr (concomitant increase in ADP) in muscles with drop in pH occurs during incremental plantar flexion exercise.
Methods
Five male subjects performed a repetitive intermittent isometric plantar flexion exercise (6-s contraction/4-s relaxation). Exercise intensity was raised every 1 min by 10% maximal voluntary contraction (MVC), starting at 10% MVC until exhaustion. The measurement site was at the medial head of the gastrocnemius muscle. Changes in muscle PCr, inorganic phosphate (Pi), ADP, and pH were measured by 31P-magnetic resonance spectroscopy.  o2mus was determined from the rate of decrease in oxygenated hemoglobin and/or myoglobin using near-infrared continuous wave spectroscopy under transient arterial occlusion. Electromyogram (EMG) was also recorded. Pulmonary oxygen uptake (
o2mus was determined from the rate of decrease in oxygenated hemoglobin and/or myoglobin using near-infrared continuous wave spectroscopy under transient arterial occlusion. Electromyogram (EMG) was also recorded. Pulmonary oxygen uptake ( o2pul ) was measured by the breath-by-breath gas analysis.
o2pul ) was measured by the breath-by-breath gas analysis.
Results
EMG amplitude increased as exercise intensity progressed. In contrast, muscle PCr, ADP,  o2mus, and
o2mus, and  o2pul did not change appreciably below 40% MVC, whereas above 40% MVC muscle PCr decreased, and ADP,
o2pul did not change appreciably below 40% MVC, whereas above 40% MVC muscle PCr decreased, and ADP,  o2mus, and
o2mus, and  o2pul increased as exercise intensity progressed, and above 70% MVC, changes in muscle PCr, ADP,
o2pul increased as exercise intensity progressed, and above 70% MVC, changes in muscle PCr, ADP,  o2mus, and
o2mus, and  o2pul accelerated with the decrease in muscle pH (~6.78). The kinetics of muscle PCr, ADP,
o2pul accelerated with the decrease in muscle pH (~6.78). The kinetics of muscle PCr, ADP,  o2mus, and
o2mus, and  o2pul were similar, and there was a close correlation between each pair of parameters (r = 0.969~0.983, p < 0.001).
o2pul were similar, and there was a close correlation between each pair of parameters (r = 0.969~0.983, p < 0.001).
Conclusion
With decrease in pH muscle oxidative metabolism accelerated and changes in intramuscular PCr and ADP accelerated during incremental intermittent isometric plantar flexion exercise. These results suggest that rapid changes in muscle PCr and/or ADP with mild acidosis stimulate accelerative muscle oxidative metabolism.
Background
Skeletal muscle respiratory control is a cardinal issue in the field of muscle energetics. Early work on isolated mitochondria identified ADP as an important stimulator of mitochondrial respiration [1]. Thereafter, it has been verified that ADP is a control signal of muscle oxidative phosphorylation in many studies [2-7]. During steady state phase of muscle contraction, muscle O2 consumption ( o2mus) linearly correlates with intramuscular phosphocreatine (PCr) concentration at varying intensities under relatively stable muscle pH conditions [8-10]. It has also been demonstrated that muscle PCr and pulmonary oxygen uptake (
o2mus) linearly correlates with intramuscular phosphocreatine (PCr) concentration at varying intensities under relatively stable muscle pH conditions [8-10]. It has also been demonstrated that muscle PCr and pulmonary oxygen uptake ( o2pul) show similar kinetics during the transition from rest to steady state exercise in humans in a non-steady state condition [11-13]. In addition, Rossiter et al. [14] demonstrated that muscle PCr and slowly developing supplementary component (slow component) of
o2pul) show similar kinetics during the transition from rest to steady state exercise in humans in a non-steady state condition [11-13]. In addition, Rossiter et al. [14] demonstrated that muscle PCr and slowly developing supplementary component (slow component) of  o2pul show similar response during a high intensity constant load exercise with decreased pH condition. Therefore, it has been thought that intramuscular ADP and PCr concentrations are important regulators of skeletal muscle oxidative metabolism [1-14].
o2pul show similar response during a high intensity constant load exercise with decreased pH condition. Therefore, it has been thought that intramuscular ADP and PCr concentrations are important regulators of skeletal muscle oxidative metabolism [1-14].
Although  o2pul has been used as an indicator of muscle oxidative metabolism [11-14], it does not specifically indicate oxygen consumption each of the exercising muscle group(s). Near-infrared continuous wave spectroscopy (NIRcws) has unique capability for non-invasively evaluating of O2 kinetics in an objective portion of tissue with high-time resolution. NIRcws was first applied to the study of exercising skeletal muscle in humans in 1991 [15]. Since then, many more groups have applied this technique [16-20].
o2pul has been used as an indicator of muscle oxidative metabolism [11-14], it does not specifically indicate oxygen consumption each of the exercising muscle group(s). Near-infrared continuous wave spectroscopy (NIRcws) has unique capability for non-invasively evaluating of O2 kinetics in an objective portion of tissue with high-time resolution. NIRcws was first applied to the study of exercising skeletal muscle in humans in 1991 [15]. Since then, many more groups have applied this technique [16-20].  o2mus can be determined using NIRcws with transient arterial occlusion [8], and its validity was confirmed [21]. The rate of decrease in oxygenated hemoglobin and/or myoglobin (HbO2/MbO2) under conditions in which interruption of the O2 supply to the muscle (arterial occlusion) reflects
o2mus can be determined using NIRcws with transient arterial occlusion [8], and its validity was confirmed [21]. The rate of decrease in oxygenated hemoglobin and/or myoglobin (HbO2/MbO2) under conditions in which interruption of the O2 supply to the muscle (arterial occlusion) reflects  o2mus [8,21,22]. Therefore, this NIRcws technique enables us to determine
o2mus [8,21,22]. Therefore, this NIRcws technique enables us to determine  o2mus during exercise where metabolic condition changes diversely.
o2mus during exercise where metabolic condition changes diversely.
It has been reported that there is a threshold work rate or metabolic rate for cellular acidosis (pHT) and that, above pHT, the decrease in muscle PCr is accelerated during incremental exercise [23-25]. If muscle oxidative metabolism is closely related with muscle PCr even under acidotic condition, it would be predicted that acceleration in increase in  o2mus coincided with decrease in pH. However, there is no evidence for the effect of decrease in pH on muscle oxidative metabolism during incremental exercise.
o2mus coincided with decrease in pH. However, there is no evidence for the effect of decrease in pH on muscle oxidative metabolism during incremental exercise.
The aim of this study was to measure  o2mus, ADP, and PCr during incremental exercise where muscle pH changed from stable to decreasing condition. We hypothesized that the increase in
o2mus, ADP, and PCr during incremental exercise where muscle pH changed from stable to decreasing condition. We hypothesized that the increase in  o2mus, increase in ADP and decrease in PCr occurred similar kinetics throughout incremental exercise. When exercise intensity increased above pHT, there is a possibility that the accelerative decrease in PCr stimulates accelerative increase in muscle oxidative metabolism during incremental exercise. To test the second hypothesis that with decrease in pH accelerative decrease in PCr could be responsible for the increase in
o2mus, increase in ADP and decrease in PCr occurred similar kinetics throughout incremental exercise. When exercise intensity increased above pHT, there is a possibility that the accelerative decrease in PCr stimulates accelerative increase in muscle oxidative metabolism during incremental exercise. To test the second hypothesis that with decrease in pH accelerative decrease in PCr could be responsible for the increase in  o2mus, we identified the inflexion point of pH, PCr, ADP, cytosolic free energy of ATP hydrolysis (ΔGATP),
o2mus, we identified the inflexion point of pH, PCr, ADP, cytosolic free energy of ATP hydrolysis (ΔGATP),  o2mus, and
o2mus, and  o2pul during incremental exercise. We predicted that when exercise intensity increased above the level which decrease in pH occurred, PCr, ADP, ΔGATP,
o2pul during incremental exercise. We predicted that when exercise intensity increased above the level which decrease in pH occurred, PCr, ADP, ΔGATP,  o2mus, and
o2mus, and  o2pul would show greater change than that obtained during stable pH condition during incremental exercise.
o2pul would show greater change than that obtained during stable pH condition during incremental exercise.
Methods
Subjects
Five male volunteers, aged between 22 and 34 years, participated in this study. All subjects were healthy, non-smokers, and free of known diseases. All subjects were fully informed of the risks involved in this study, and we obtained written informed consent from each. This study was approved by the Institutional Committee for the protection of human subjects.
Experimental design
Each subject sat on a platform in an upright sitting position with his right leg positioned horizontally. The subjects performed the same exercise procedure five times on different occasions: once (day 1) with the 31-phosphorus-magnetic resonance spectroscopy (31P-MRS) measurement, twice (day 2, 3) with the respiratory gas analysis, once (day 4) with the NIRcws measurement for determination of  o2mus, and once (day 5) with the EMG record. With the exception of the 31P-MRS measurement, the other four measurements were performed outside the MRS magnet. During these four measurements the subjects inserted a leg into a cylindrical plastic pipe of the same diameter and length as the bore of the MRS magnet. For each measurement, whether in the magnet or the plastic pipe, the leg was held in a fixed position by a cradle.
o2mus, and once (day 5) with the EMG record. With the exception of the 31P-MRS measurement, the other four measurements were performed outside the MRS magnet. During these four measurements the subjects inserted a leg into a cylindrical plastic pipe of the same diameter and length as the bore of the MRS magnet. For each measurement, whether in the magnet or the plastic pipe, the leg was held in a fixed position by a cradle.
Exercise Protocols
On occasions of the experiment, maximal voluntary contractions (MVC) was measured prior to the principal experiment, and each subject's exercise load was set based on the MVC of each. The MVC of isometric plantar flexion was measured by pushing against a foot pedal with connected force transducer. MVC was measured three times with sufficient rest (> 3 min) between each performance. The maximum value was used as the MVC. After sufficient rest in an upright sitting position, the subjects performed repetitive intermittent isometric plantar flexion exercise with the right leg in the same position. One duty cycle of contraction and relaxation consisted of a 6-s contraction and a 4-s relaxation. With the use of a visual feedback meter, the subjects were directed to perform using the prescribed force. Additionally, the experimental director continuously verified force. Exercise intensity was increased incrementally every 60 s by 10% MVC, starting from 10% MVC to an intensity at which the subject could no longer maintain the required force. A backrest was placed behind the subject during exercise. To fix position of the subject and to limit involvement of muscles other than calf muscle, the contact area of the backrest and subject's body was limited as small as possible. The height of backrest was 21 cm, and area of contact against subject's body was limited to lower back only. The subjects were instructed not to exert muscles other than the calf muscle to the best of their ability during the exercise, and they were fully familiarized with the exercise prior to the experiment.
31P-MRS
31P-MRS signals were obtained by an NMR spectrometer (Otsuka Electronics Co. Ltd.) with a 2.0-T superconducting 26-cm bore magnet. A double tuned (1H and 31P), 3.0-cm diameter radio frequency surface coil tuned to 34.58 MHz with 60-μs pulse width was used for the phosphorus signal. Pulse repetition time was 2 s. Five pulses were averaged to obtain a free induction decay (FID). Therefore, a spectrum was obtained every 10 s. Twelve spectra were averaged during the pre-exercise resting period, and three spectra were averaged during exercise. The surface coil was placed on the medial head of the gastrocnemius muscle (m.MG), and the coil and leg were held in a fixed position in the magnet by a cradle. All 31P-MRS spectra were fitted to a Lorentzian line shape using the least-squares method. The relative area and frequency of the individual peaks were determined (Otsuka Electronics software) to calculate the areas of PCr, inorganic phosphate (Pi), and β-ATP peaks. The PCr and Pi intensities were normalized using the sum of PCr and Pi to avoid influence from possible changes in the sensitivity of 31P-MRS signals. Saturation correction was performed using saturation factors of PCr, Pi, and β-ATP peaks, which were calculated by comparing the data from the 2-s and fully relaxed spectra. The saturation factors of PCr, Pi, and β-ATP peaks in this study were 1.330, 1.081, and 1.184, respectively. The intracellular pH was calculated from the median chemical shift between the Pi and PCr peaks [26]. Changes in muscle PCr are expressed as a percentage of the pre-exercise resting value.
To convert peak areas to concentrations, the β-ATP peak was assumed to represent total ATP and was set at 8.2 mM [27-29]. [PCr] and [Pi] could then be estimated as the product of the areas to ATP (as PCr to β-ATP and Pi to β-ATP) and 8.2 mM. Total creatine (TCr) was assumed to be equal to the sum of PCr and Pi ([TCr] = [PCr] + [Pi]), and TCr was assumed to be constant throughout the experiment [10]. ADP was calculated with the assumption that equilibrium of the Cr kinase (CK) reaction [23,30,31]:
[ADP] = {0.74 [ATP]([TCr] - [PCr])} / {(1.66 × 109)(10^-pHobs) [PCr]} (1)
The constant 0.74 is the estimated monovalent ion activity coefficient [31] that corrects for the fact that pHobs is an activity, subscript obs indicates observed factors, and 1.66 × 109 is the equilibrium constant for CK. Free magnesium was assumed to be 1 mM and unchanging throughout the experiment [32]. Cytosolic free energy of ATP hydrolysis (ΔGATP) was also calculated [23,30,31]:
ΔGATP = ΔGO + RT ln ([ADP] [Pi] / [ATP]) + RT ln [10^-(pHobs-7)] (2)
ΔGO is Gibb's free energy, R is gas constant, and T is absolute temperature. ΔGO is taken to be -32 kJ/mol at pH7.0[31], RT at 37°C is 2.58.
NIR spectroscopy
NIR signals were obtained by NIRcws (HEO-200, OMRON Co. Ltd.). The NIRcws probe contained a light source and an optical detector with a distance of 3.0 cm between the light source and detector to provide sensory input for the unit. A pair of two-wavelength light emitting diodes, with wavelengths of 760 and 840 nm, was used as the light source. A silicon photodiode was used as the photodetector. The NIRcws probe was placed on the m.MG, and the probe and leg were held in a fixed position by a cradle in a plastic pipe that mimicked the bore of the MRS magnet. Changes in HbO2 and/or MbO2, deoxygenated Hb and/or Mb, and total hemoglobin and/or myoglobin (THb/TMb) were calculated by the least squares method using data from the changes in the absorbance of these different wavelengths of light. The sampling time of the data was 0.1 s.
 o2mus was measured using NIRcws with the transient arterial occlusion technique described previously in detail [8,21,22].
o2mus was measured using NIRcws with the transient arterial occlusion technique described previously in detail [8,21,22].  o2mus was determined by the rate of decrease in HbO2/MbO2 during arterial occlusion. Since the changes in HbO2/MbO2 measured by NIRcws show a dynamic balance between O2 supply and O2 consumption, the rate of decrease in HbO2/MbO2 during arterial occlusion reflects the
o2mus was determined by the rate of decrease in HbO2/MbO2 during arterial occlusion. Since the changes in HbO2/MbO2 measured by NIRcws show a dynamic balance between O2 supply and O2 consumption, the rate of decrease in HbO2/MbO2 during arterial occlusion reflects the  o2mus [8,21,22]. Arterial occlusion was performed for 1 min during rest, and for 6 s once every 30 s during isometric contraction. Timing for arterial occlusion during exercise took place at the third and the sixth contraction of each intensity i.e. at 20–26 s and 50–56 s of each minute. The
o2mus [8,21,22]. Arterial occlusion was performed for 1 min during rest, and for 6 s once every 30 s during isometric contraction. Timing for arterial occlusion during exercise took place at the third and the sixth contraction of each intensity i.e. at 20–26 s and 50–56 s of each minute. The  o2mus was expressed as a value relative to that obtained at rest (fold of resting).
o2mus was expressed as a value relative to that obtained at rest (fold of resting).
Respiratory gas analysis
 o2pul was measured during the pre-exercise resting period and throughout the exercise period by the breath-by-breath gas analysis method using an Aeromonitor AE-280 (Minato Medical Science Co. Ltd.) [33]. This system consists of a microcomputer, a hot-wire flow-sensor, and oxygen and carbon dioxide analyzers (zirconium element-based oxygen analyzer and infra-red carbondioxide analyzer). Prior to the experiments, the flow-sensor and gas analyzers were calibrated with a known volume of room air at several mean flow rates and gas mixtures of known concentration, respectively. To improve the signal-to-noise ratio of
o2pul was measured during the pre-exercise resting period and throughout the exercise period by the breath-by-breath gas analysis method using an Aeromonitor AE-280 (Minato Medical Science Co. Ltd.) [33]. This system consists of a microcomputer, a hot-wire flow-sensor, and oxygen and carbon dioxide analyzers (zirconium element-based oxygen analyzer and infra-red carbondioxide analyzer). Prior to the experiments, the flow-sensor and gas analyzers were calibrated with a known volume of room air at several mean flow rates and gas mixtures of known concentration, respectively. To improve the signal-to-noise ratio of  o2pul, each subject performed the exercise session for
o2pul, each subject performed the exercise session for  o2pul measurement twice on different days, and the dual measurement data were subsequently averaged.
o2pul measurement twice on different days, and the dual measurement data were subsequently averaged.
Surface electromyograms
Surface electromyography (EMGs) were obtained from the m.MG, lateral head of the gastrocnemius muscle (m.LG), and soleus muscle (m.SOL) using a bipolar, silver-silver chloride electrode (10 mm diameter sample area) with a fixed inter-electrode spacing of 30 mm (Nihon Koden Co., Japan) during incremental plantar flexion exercise. The EMG signal was sampled at a rate of 2000 Hz using available software (BIOPAC Systems, Inc., USA) and stored on computer disk for later analysis. The root mean square of the EMG signal (rmsEMG) was calculated. Prior to the principal experiment the subjects performed MVC, and the rmsEMG was normalized as 100% at MVC.
Data analysis
Analysis of each parameter was performed every 30 s as the procedure is shown in figure 1. Except for  o2mus, all data were averaged over 30 s. The data for
o2mus, all data were averaged over 30 s. The data for  o2mus were obtained at the third (20–26 s) and sixth (50–56 s) contractions of each intensity. The reason
o2mus were obtained at the third (20–26 s) and sixth (50–56 s) contractions of each intensity. The reason  o2mus was measured only once during three contraction phases was to avoid the limitations to exercise performance caused by interrupting the blood flow. The value of the third contraction was used to represent the first 30 s of each minute, and the value of the sixth contraction was used to represent the last 30 s of each minute. All averaged data were shown from pre-exercise rest to the first 30 s at 80% MVC exercise at which every subject was able to perform. The logarithms of the individual metabolic parameters (pH, PCr, ADP, ΔGATP,
o2mus was measured only once during three contraction phases was to avoid the limitations to exercise performance caused by interrupting the blood flow. The value of the third contraction was used to represent the first 30 s of each minute, and the value of the sixth contraction was used to represent the last 30 s of each minute. All averaged data were shown from pre-exercise rest to the first 30 s at 80% MVC exercise at which every subject was able to perform. The logarithms of the individual metabolic parameters (pH, PCr, ADP, ΔGATP,  o2mus
o2mus  o2pul) were plotted against exercise intensity in order to determine a break point of metabolic change based on the method of determining lactate threshold [34]. These plots were best fit by a piecewise linear regression model with a breakpoint.
o2pul) were plotted against exercise intensity in order to determine a break point of metabolic change based on the method of determining lactate threshold [34]. These plots were best fit by a piecewise linear regression model with a breakpoint.
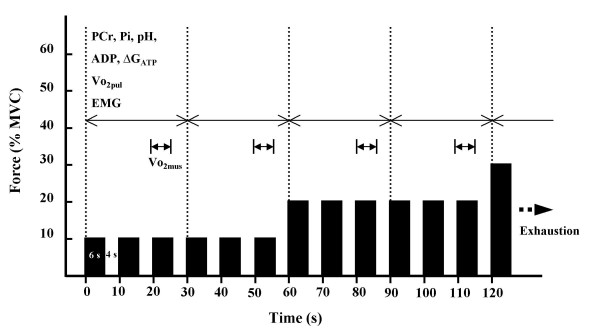
Figure 1.
Procedure for data analysis. Each parameter was analyzed every 30 s. Muscle phosphocreatine (PCr), inorganic phosphate (Pi), pH, estimated ADP and free energy of ATP hydrolysis (ΔGATP), pulmonary oxygen uptake ( o2pul), and electromyogram (EMG) were averaged over 30 s. The data for muscle oxygen consumption (
o2pul), and electromyogram (EMG) were averaged over 30 s. The data for muscle oxygen consumption ( o2mus) were obtained during the third (20–26 s) and sixth (50–56 s) contractions at each intensity. The
o2mus) were obtained during the third (20–26 s) and sixth (50–56 s) contractions at each intensity. The  o2mus value of the third contraction was used to represent the first 30 s of each minute, whereas the
o2mus value of the third contraction was used to represent the first 30 s of each minute, whereas the  o2mus value of the sixth contraction was used to represent the last 30 s of each minute.
o2mus value of the sixth contraction was used to represent the last 30 s of each minute.  Division of data analysis (30s).
Division of data analysis (30s). 
 o2mus measurement (6 s; once per three contraction phases).
o2mus measurement (6 s; once per three contraction phases).
Confirmation of reproducibility
Since the subjects performed the same exercise procedure five times, we were able to obtain five sets of performance data. The maximal exercise intensity the subjects were able to perform during the exercise protocol was 80–90% MVC (450–510 s). The maximal intensity at which each subject was able to perform was the same throughout five exercise sessions. The coefficient of variation for exercise duration was 0.91%. Regarding time course change and peak value,  o2pul did not differ significantly during the two measurements. There was a significant correlation between each time measurement for individual pulmonary
o2pul did not differ significantly during the two measurements. There was a significant correlation between each time measurement for individual pulmonary  o2(r = 0.981~0.993, p < 0.001).
o2(r = 0.981~0.993, p < 0.001).
Statistical analyses
Data are expressed as means ± SD. The data were compared to determine significant changes in the values of each parameter every 30 s compared with the values obtained during the first 30 s of exercise (the first 30 s at 10% MVC), and the 30 s of exercise immediately before. One-way analysis of variance (ANOVA) for repeated measures was used to determine the significance of time course changes in each parameter, and Fisher's PLSD post hoc comparisons were used to determine the significance of differences of each parameter every 30 s. A linear regression analysis was used to examine the relationship between each parameter. P < 0.05 was defined as statistically significant.
Results
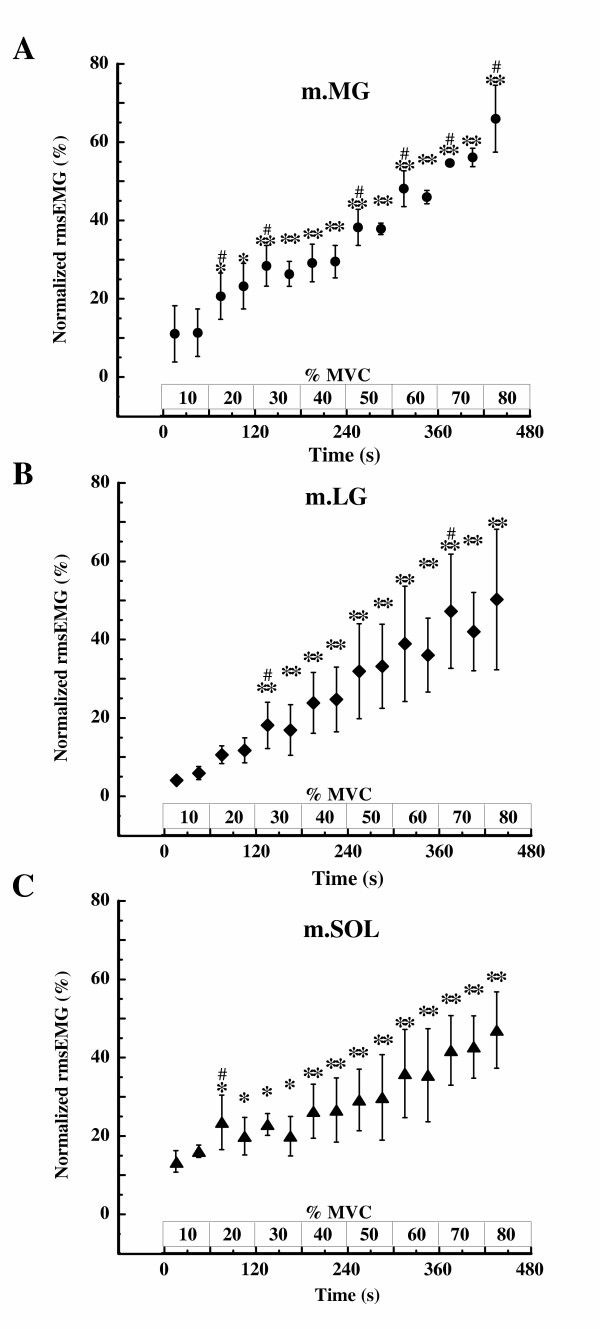
Fig. 2 shows the time course changes in normalized rmsEMG of m.MG, m.LG, and m.SOL. The rmsEMG in those muscles increased similarly with increasing exercise intensity. The rmsEMG of m.MG for each of the first 30 s at 20%, 30%, 50%, 60%, 70%, and 80% MVC differed significantly from that during the 30 s of exercise immediately before (i.e., prior intensity) (p < 0.05). Throughout the exercise, the change in rmsEMG of m.MG was largest in the three muscle groups.
Figure 2.
Changes in root mean square of EMG (rmsEMG) during incremental intermittent isometric plantar flexion exercise. Changes in rmsEMG at (A) the medial head of the gastrocnemius muscle (m.MG), (B) the lateral head of gastrocnemius muscle (m.LG), and (C) the soleus muscle (m.SOL) during incremental intermittent isometric plantar flexion exercise. Data are represented as relative values obtained during maximal voluntary contraction (MVC) as 100%. Values shown are means ± SD of 5 subjects. * p < 0.05, ** p < 0.01 vs. the value during the first 30 s at 10% MVC (first 30 s of exercise). #p < 0.05 vs. the value obtained during the 30 s of exercise immediately before.
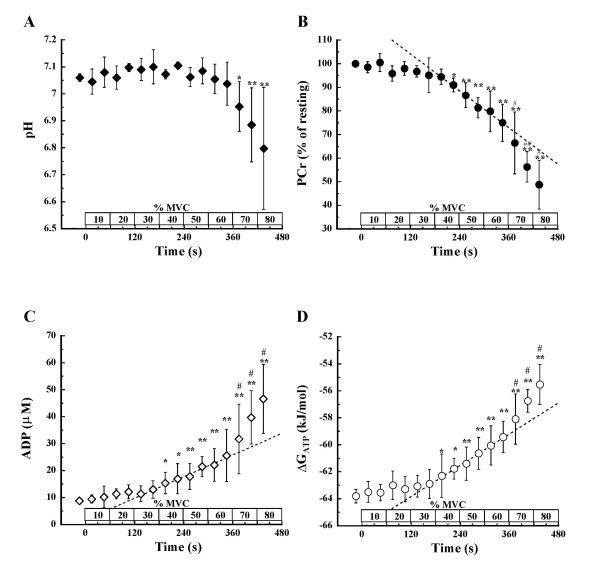
Fig. 3A shows the time course of changes in intramuscular pH. We found that pH was relatively constant, from resting values (7.06 ± 0.01) until 60% MVC (7.04 ± 0.08), but it decreased significantly (p < 0.05) at 70% MVC and with exercise progression, being 6.78 ± 0.22 at the end of exercise.
Figure 3.
Changes in muscle pH, PCr, ADP, and ΔGATP during incremental intermittent isometric plantar flexion exercise. Changes in (A) pH, (B) PCr, (C) ADP, and (D) ΔGATP during incremental intermittent isometric plantar flexion exercise. A dotted line in each panel B, C, and D represents a linear regression line which is drawn to obtain the highest correlation coefficient above 40% MVC, at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. Values shown are means ± SD of 5 subjects. * p < 0.05, **p < 0.01 vs. the value during the first 30 s at 10% MVC (first 30 s of exercise). #p < 0.05 vs. the value obtained during the 30 s of exercise immediately before.
Fig. 3B shows the time course changes in intramuscular PCr. We found that there were significant differences after the last 30 s at 40% MVC when compared with the value obtained during the first 30 s at 10% MVC (p < 0.05), and that PCr decreased with progression of exercise. Above 70% MVC, the values were significantly different when compared with those obtained during the 30 s of exercise immediately before. A linear regression line was drawn to obtain the highest correlation coefficient above the last 30 s of 40% MVC, at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. The PCr deviated downward from the regression line above 70% MVC.
Fig. 3C shows the time course changes in estimated ADP. We found that ADP slightly increased from rest (8.8 ± 0.9 μM) until the last 30 s of 30% MVC (13.0 ± 3.2 μM), whereas above 40% MVC, these values differed significantly from those obtained during the first 30 s at 10% MVC (p < 0.05). Thereafter, ADP increased with progression of exercise, being significantly different above 70% MVC compared with the value obtained during the 30 s of exercise immediately before. At the end of exercise, ADP was 46.6 ± 12.8 μM. A linear regression line was drawn to obtain the highest correlation coefficient above the first 30 s of 40% MVC, at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. The ADP deviated upward from the regression line above 70% MVC.
Fig. 3D shows the time course changes in estimated ΔGATP. We found that ΔGATP changed only slightly from rest (-63.8 ± 0.5 kJ/mol) until the last 30 s of 30% MVC (-62.9 ± 1.1 kJ/mol), whereas above 40% MVC, these values differed significantly from those obtained during the first 30 s at 10% MVC (p < 0.05). Thereafter, ΔGATPincreased with progression of exercise, being significantly different above 70% MVC compared with the value obtained during the 30 s of exercise immediately before. At the end of exercise, ΔGATP was -55.1 ± 1.9 kJ/mol. A linear regression line was drawn to obtain the highest correlation coefficient above the first 30 s of 40% MVC, at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. The ΔGATP deviated upward from the regression line above 70% MVC.
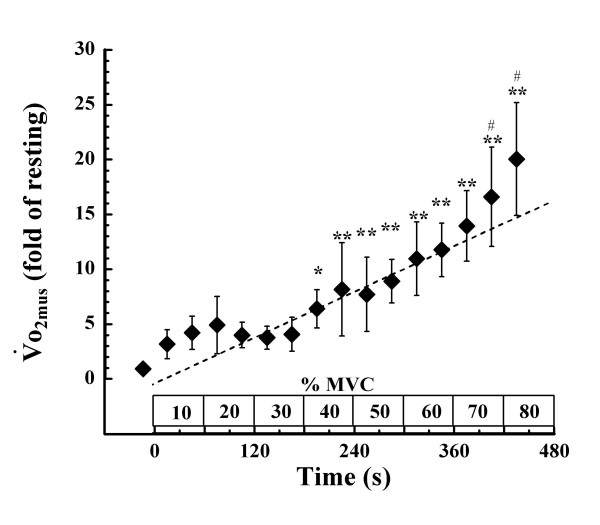
Fig. 4 shows the time course changes in  o2mus.
o2mus.  o2mus also showed slight changes during exercise below 40% MVC. Above 40% MVC, however, there were significant differences when compared with the value obtained during the first 30 s at 10% MVC.
o2mus also showed slight changes during exercise below 40% MVC. Above 40% MVC, however, there were significant differences when compared with the value obtained during the first 30 s at 10% MVC.  o2mus subsequently increased with progression of exercise, and the values obtained during the last 30 s at 70% MVC and the first 30 s at 80% MVC differed significantly from the value during the 30 s of exercise immediately before. The peak value of
o2mus subsequently increased with progression of exercise, and the values obtained during the last 30 s at 70% MVC and the first 30 s at 80% MVC differed significantly from the value during the 30 s of exercise immediately before. The peak value of  o2mus was 21.3 ± 5.2 fold higher than its resting value. A linear regression line was drawn to obtain the highest correlation coefficient above the first 30 s of 40% MVC, at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. The
o2mus was 21.3 ± 5.2 fold higher than its resting value. A linear regression line was drawn to obtain the highest correlation coefficient above the first 30 s of 40% MVC, at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. The  o2mus deviated upward from the regression line above 70% MVC.
o2mus deviated upward from the regression line above 70% MVC.
Figure 4.
Changes in  o2mus during incremental intermittent isometric plantar flexion exercise. A dotted line represents a linear regression line which is drawn to obtain the highest correlation coefficient above the first 30 s of 40% MVC at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. The
o2mus during incremental intermittent isometric plantar flexion exercise. A dotted line represents a linear regression line which is drawn to obtain the highest correlation coefficient above the first 30 s of 40% MVC at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. The  o2mus is expressed as a value relative to that obtained at rest (fold of resting). Values shown are means ± SD of 5 subjects. *p < 0.05, **p < 0.01 vs. the value during the first 30 s at 10% MVC (first 30 s of exercise) # p < 0.05 vs. the value obtained during the 30 s of exercise immediately before.
o2mus is expressed as a value relative to that obtained at rest (fold of resting). Values shown are means ± SD of 5 subjects. *p < 0.05, **p < 0.01 vs. the value during the first 30 s at 10% MVC (first 30 s of exercise) # p < 0.05 vs. the value obtained during the 30 s of exercise immediately before.
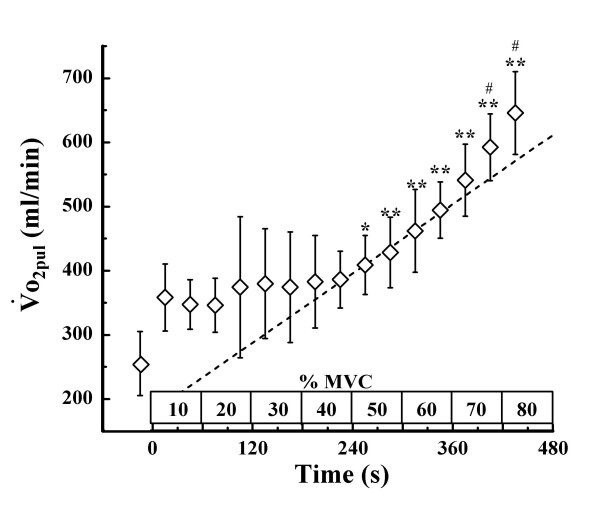
Fig. 5 shows the time course changes in  o2pul. We found that
o2pul. We found that  o2pul changed only slightly, and there was little difference relative to exercise intensity up to 40% MVC. When the exercise intensity was raised above 50% MVC, the value of
o2pul changed only slightly, and there was little difference relative to exercise intensity up to 40% MVC. When the exercise intensity was raised above 50% MVC, the value of  o2pul differed significantly from that obtained during the first 30 s at 10% MVC. Thereafter,
o2pul differed significantly from that obtained during the first 30 s at 10% MVC. Thereafter,  o2pul increased with progression of exercise, and the values obtained during the last 30 s at 70% MVC and the first 30 s at 80% MVC were significantly different from the value obtained during the 30 s of exercise immediately before. The peak value of of
o2pul increased with progression of exercise, and the values obtained during the last 30 s at 70% MVC and the first 30 s at 80% MVC were significantly different from the value obtained during the 30 s of exercise immediately before. The peak value of of  o2pul was 684.8 ± 64.8 ml/min, which different from the resting value of
o2pul was 684.8 ± 64.8 ml/min, which different from the resting value of  o2pul (Δ
o2pul (Δ o2pul) by 364.8 ± 74.3 ml/min. A linear regression line was drawn to obtain the highest correlation coefficient above the first 30 s of 50% MVC, at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. The
o2pul) by 364.8 ± 74.3 ml/min. A linear regression line was drawn to obtain the highest correlation coefficient above the first 30 s of 50% MVC, at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC. The  o2pul deviated upward from the regression line above 70% MVC.
o2pul deviated upward from the regression line above 70% MVC.
Figure 5.
Changes in  o2mus during incremental intermittent isometric plantar flexion exercise. A dotted line represents a linear regression line which is drawn to obtain the highest correlation coefficient above the first 30 s of 50% MVC at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC Values shown are means ± SD of 5 subjects. * p < 0.05, **p < 0.01 vs. the value during the first 30 s at 10%MVC(first 30 s of exercise), # p < 0.05 vs. the value obtained during the 30 s of exercise immediately before.
o2mus during incremental intermittent isometric plantar flexion exercise. A dotted line represents a linear regression line which is drawn to obtain the highest correlation coefficient above the first 30 s of 50% MVC at which significant difference was observed when compared with the value obtained during the first 30 s at 10% MVC Values shown are means ± SD of 5 subjects. * p < 0.05, **p < 0.01 vs. the value during the first 30 s at 10%MVC(first 30 s of exercise), # p < 0.05 vs. the value obtained during the 30 s of exercise immediately before.
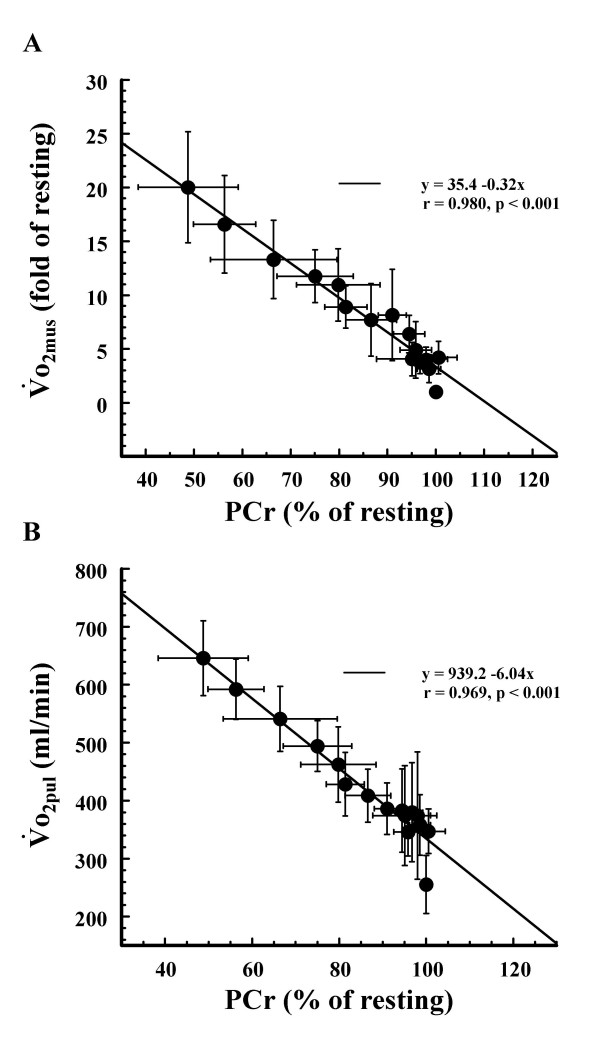
When we examind the relationship between the averaged muscle PCr and the averaged  o2mus, we observed a significant inverse correlation between the two (r = 0.980, p < 0.001) (Fig 6A). There was also a significant inverse correlation between averaged muscle PCr and averaged
o2mus, we observed a significant inverse correlation between the two (r = 0.980, p < 0.001) (Fig 6A). There was also a significant inverse correlation between averaged muscle PCr and averaged  o2pul (r = 0.969, p < 0.001) (Fig 6B).
o2pul (r = 0.969, p < 0.001) (Fig 6B).
Figure 6.
Relationships between muscle PCr and  o2mus, muscle PCr and
o2mus, muscle PCr and  o2pul. Relationships between (A) averaged muscle PCr and
o2pul. Relationships between (A) averaged muscle PCr and  o2mus and (B) averaged muscle PCr and
o2mus and (B) averaged muscle PCr and  o2pul. Values shown are means ± SD of 5 subjects.
o2pul. Values shown are means ± SD of 5 subjects.
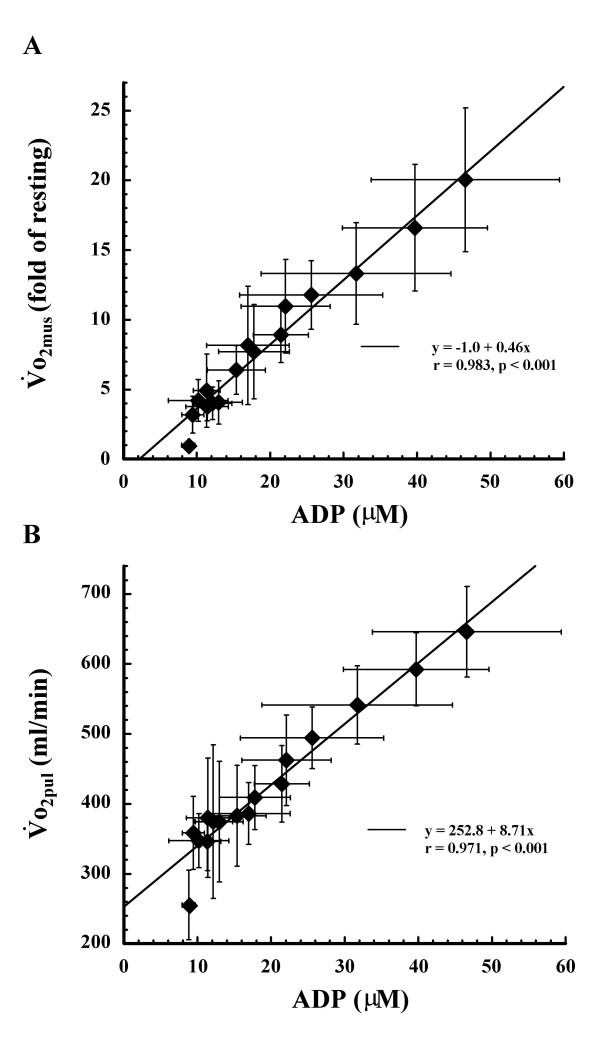
We also determined the relationship between the averaged ADP and the averaged  o2mus (Fig. 7A) and between the averaged ADP and the averaged
o2mus (Fig. 7A) and between the averaged ADP and the averaged  o2pul (Fig. 7B). There was a significant correlation betwene ADP and
o2pul (Fig. 7B). There was a significant correlation betwene ADP and  o2mus (r = 0.983, p < 0.001) and between ADP and
o2mus (r = 0.983, p < 0.001) and between ADP and  o2pul (r = 0.971, p < 0.001). Individual correlation between ADP and
o2pul (r = 0.971, p < 0.001). Individual correlation between ADP and  o2mus(r = 0.916~0.963, p < 0.001) and between ADP and
o2mus(r = 0.916~0.963, p < 0.001) and between ADP and  o2pul (r = 0.902~0.974, p < 0.001) were seen in all subjects (Figures not shown). Additionally, there was a significant positive correlation between averaged
o2pul (r = 0.902~0.974, p < 0.001) were seen in all subjects (Figures not shown). Additionally, there was a significant positive correlation between averaged  o2mus and averaged
o2mus and averaged  o2pul (r = 0.975, p < 0.001) (Figure not shown).
o2pul (r = 0.975, p < 0.001) (Figure not shown).
Figure 7.
Relationships between muscle ADP and  o2mus, muscle ADP and
o2mus, muscle ADP and  o2pul. Relationships between (A) averaged muscle ADP and
o2pul. Relationships between (A) averaged muscle ADP and  o2mus and (B) averaged muscle ADP and
o2mus and (B) averaged muscle ADP and  o2pul. Values shown are means ± SD of 5 subjects.
o2pul. Values shown are means ± SD of 5 subjects.
The logarithms of individual metabolic parameters (pH, PCr, ADP, ΔGATP,  o2mus,
o2mus,  o2pul) were best fit by the piecewise regression model with an inflexion point ranging from 60 to 70% MVC, and individual break points for all metabolic parameters were the same intensity in all subject. There were significant intra-individual correlations between each pair of metabolic parameters (r = 0.971~0.988, p < 0.001). 0.001)
o2pul) were best fit by the piecewise regression model with an inflexion point ranging from 60 to 70% MVC, and individual break points for all metabolic parameters were the same intensity in all subject. There were significant intra-individual correlations between each pair of metabolic parameters (r = 0.971~0.988, p < 0.001). 0.001)
Discussion
The main finding of this study was that increase in  o2mus accelerated coincidentally with drop in muscle pH over 70% MVC during incremental intermittent isometric contraction. Changes in muscle PCr ADP, ΔGATP, and
o2mus accelerated coincidentally with drop in muscle pH over 70% MVC during incremental intermittent isometric contraction. Changes in muscle PCr ADP, ΔGATP, and  o2pul also accelerated simultaneously with drop in pH. In addition, the kinetics of each metabolic parameter was similar, and there were significant correlations between each pair of parameters (r = 0.969~0.983, p < 0.001).
o2pul also accelerated simultaneously with drop in pH. In addition, the kinetics of each metabolic parameter was similar, and there were significant correlations between each pair of parameters (r = 0.969~0.983, p < 0.001).
It has been thought that intramuscular ADP and PCr concentration are important regulators of mitochondrial respiration [1-14,35,36]. However, there is no evidence that examined relationship between muscle oxidative metabolism and muscle PCr or ADP during incremental exercise where muscle pH changed from stable to decreasing condition. According to the PCr shuttle hypothesis [37] and other biochemical hypotheses [38], control of respiration is exerted linearly at the mitochondria by the declining PCr and concomitant rise in cytosolic Cr. These hypotheses [37,38] are based on observations of linear changes in muscle respiration relative to increasing contraction intensity under relatively stable pH conditions. The greater rate of breakdown of PCr under acidotic conditions [23-25], if still tightly coupled to oxidative phosphorylation, would predict that  o2mus increases nonlinearly with increasing contraction intensity. In this study, the increase in
o2mus increases nonlinearly with increasing contraction intensity. In this study, the increase in  o2mus is actually accelerated with rapid decrease in PCr during a conspicuous drop in pH, to ~ 6.78. Additionally, the accelerated increase in
o2mus is actually accelerated with rapid decrease in PCr during a conspicuous drop in pH, to ~ 6.78. Additionally, the accelerated increase in  o2mus coincided with abrupt increase in ADP. Consequently, our results indicated that muscle oxidative metabolism is closely related with muscle PCr and ADP even under mild acidotic conditions. Therefore, it is suggested that rapid changes in muscle PCr and/or ADP, coincided with drop in pH, are factor(s) that accelerate muscle oxidative metabolism during incremental intermittent isometric contraction.
o2mus coincided with abrupt increase in ADP. Consequently, our results indicated that muscle oxidative metabolism is closely related with muscle PCr and ADP even under mild acidotic conditions. Therefore, it is suggested that rapid changes in muscle PCr and/or ADP, coincided with drop in pH, are factor(s) that accelerate muscle oxidative metabolism during incremental intermittent isometric contraction.
The accelerated changes in PCr, ADP,  o2mus,
o2mus,  o2pul, and the calculated ΔGATP above 70% MVC coincided with the decrease in pH, indicating that metabolic demand changes nonlinearly with increasing exercise intensity (Fig. 3, Fig. 4, Fig. 5). In contrast, others have shown that, although above pHT muscle PCr rapidly decreases, ΔGATP increases linearly with increasing intensity throughout dynamic plantar flexion exercise [23].
o2pul, and the calculated ΔGATP above 70% MVC coincided with the decrease in pH, indicating that metabolic demand changes nonlinearly with increasing exercise intensity (Fig. 3, Fig. 4, Fig. 5). In contrast, others have shown that, although above pHT muscle PCr rapidly decreases, ΔGATP increases linearly with increasing intensity throughout dynamic plantar flexion exercise [23].  o2pul rises linearly with increasing work rate during bicycle exercise [39,40], and ΔGATP shows a linear increase with increasing work rate during dynamic plantar flexion exercise [23] consistent with pulmonary
o2pul rises linearly with increasing work rate during bicycle exercise [39,40], and ΔGATP shows a linear increase with increasing work rate during dynamic plantar flexion exercise [23] consistent with pulmonary  o2 [39,40]. One possible explanation for the different results between the earlier studies [23,39,40] and ours is the difference of load setting. Previous dynamic exercise studies were incremented by prescribed work rate, until either the frequency of contraction/s or the full range of motion could no longer be sustained [23] or until maximal
o2 [39,40]. One possible explanation for the different results between the earlier studies [23,39,40] and ours is the difference of load setting. Previous dynamic exercise studies were incremented by prescribed work rate, until either the frequency of contraction/s or the full range of motion could no longer be sustained [23] or until maximal  o2pul was attained by increments of 15–30W/min ramp loaded bicycle exercise [39,40]. In contrast, we loaded using % MVC and reached 80–90% MVC at the end of exercise. Although % MVC was not expressed in those previous studies, it is possible that the peak intensity attained in our study was higher in those previously reported [23,39,40]. It is therefore conceivable that a larger amount of type II fibers were recruited in our study during exercise above the intensity where drop of muscle pH occurred. Since the energy cost of type II fibers is larger than that of type I fibers [41,42], an increase in type II fiber recruitment may produce greater changes in muscle PCr, ADP,
o2pul was attained by increments of 15–30W/min ramp loaded bicycle exercise [39,40]. In contrast, we loaded using % MVC and reached 80–90% MVC at the end of exercise. Although % MVC was not expressed in those previous studies, it is possible that the peak intensity attained in our study was higher in those previously reported [23,39,40]. It is therefore conceivable that a larger amount of type II fibers were recruited in our study during exercise above the intensity where drop of muscle pH occurred. Since the energy cost of type II fibers is larger than that of type I fibers [41,42], an increase in type II fiber recruitment may produce greater changes in muscle PCr, ADP,  o2mus,
o2mus,  o2pul and ΔGATP above the intensity during which a decrease in pH occurs.
o2pul and ΔGATP above the intensity during which a decrease in pH occurs.
Another explanation for the different results between the earlier study [23] and ours (i.e. linear vs. nonlinear increase in ΔGATP) may be the difference in types of muscle contraction (i.e. concentric vs. intermittent isometric contraction). A nonlinear relationship between heat production, an indicator of ATP turnover rate, and force production during voluntary isometric contractions has been reported, although EMG activity continued to increase linearly with force production [43]. In addition, it is impossible to determine mechanical work for this type of static contraction. Therefore, voluntary isometric contraction does not necessarily show linear relationships between energy demand and exercise intensity or muscle electrical activity.
One might criticize that despite the increasing exercise intensity in the initial phases, up to 30–40% MVC, there were only small changes in energy metabolism. At the onset of exercise,  o2pul showed a steep increase, which remained stable until 40% MVC.
o2pul showed a steep increase, which remained stable until 40% MVC.  o2pul and heart rate often exceed their steady state levels at the onset of exercise (phase I) during very low work rates [44]. This abrupt increase in
o2pul and heart rate often exceed their steady state levels at the onset of exercise (phase I) during very low work rates [44]. This abrupt increase in  o2pul is due to the rapid elevation of cardiac output that drives mixed venous blood through the lungs [44]. It is possible, therefore, that phase I
o2pul is due to the rapid elevation of cardiac output that drives mixed venous blood through the lungs [44]. It is possible, therefore, that phase I  o2pul exceeded the oxygen demand from the initial phase of exercise in this study. PCr, ADP, ΔGATP,
o2pul exceeded the oxygen demand from the initial phase of exercise in this study. PCr, ADP, ΔGATP,  o2mus, and
o2mus, and  o2pul also changed only slightly during exercise below 30–40% MVC. We found however, that rmsEMG of m.MG, the same site as 31P-MRS and NIRcws measurements, increased with increasing exercise intensity, and that rmsEMG of m.LG and m.SOL changed similarly with m.MG (Fig. 2). These results indicate that, although energy consumption changed slightly below 30–40% MVC, muscle electrical activity changed significantly with increased exercise intensity. It has been demonstrated that heat production increased only moderately with increasing contraction intensity during isometric contraction at low intensities, though EMG increased relative to contraction intensity [43]. Therefore, it appears that little metabolic change during exercise at low intensities is a characteristic of isometric contraction.
o2pul also changed only slightly during exercise below 30–40% MVC. We found however, that rmsEMG of m.MG, the same site as 31P-MRS and NIRcws measurements, increased with increasing exercise intensity, and that rmsEMG of m.LG and m.SOL changed similarly with m.MG (Fig. 2). These results indicate that, although energy consumption changed slightly below 30–40% MVC, muscle electrical activity changed significantly with increased exercise intensity. It has been demonstrated that heat production increased only moderately with increasing contraction intensity during isometric contraction at low intensities, though EMG increased relative to contraction intensity [43]. Therefore, it appears that little metabolic change during exercise at low intensities is a characteristic of isometric contraction.
One limitation of our study is that the bore diameter of the 31P-MRS magnet used in this study was small (26cm), and it only permitted us to perform intermittent isometric plantar flexion. Isometric contraction is sensitive to occlude blood flow [45]. Therefore, one concern is that limited blood flow affected the results of this study. However, as far as we observe EMGs, the subjects fully relaxed between contractions even at highest intensities. In addition, metabolic parameters (pH, PCr, ΔGATP) of this study reached at the end of exercise were approximately same levels as reported data which performed incremental dynamic plantar flexion exercise [23]. The result obtained in this study could be comparable to the previous study that used dynamic exercise [23].
Although EMGs of plantar flexor muscles increased with increasing exercise intensity it is impossible to entirely eliminate the possibility that increases in  o2pul could include
o2pul could include  o2mus from other muscles besides the plantar flexors. These should include muscles that maintain posture during exercise especially at high intensity. However, we observed a linear relationship between calf
o2mus from other muscles besides the plantar flexors. These should include muscles that maintain posture during exercise especially at high intensity. However, we observed a linear relationship between calf  o2mus and
o2mus and  o2pul (r = 0.975, p < 0.001). Moreover, the
o2pul (r = 0.975, p < 0.001). Moreover, the  o2pul kinetics was similar to muscle ADP and PCr which are thought to be important regulators of muscle oxidative metabolism. Therefore, we believe that the increase in
o2pul kinetics was similar to muscle ADP and PCr which are thought to be important regulators of muscle oxidative metabolism. Therefore, we believe that the increase in  o2pul primarily derives from the increase in
o2pul primarily derives from the increase in  o2mus in the active calf muscle.
o2mus in the active calf muscle.
Conclusion
 o2mus changed similarly with PCr and ADP throughout incremental intermittent isometric plantar flexion exercise. The increase in
o2mus changed similarly with PCr and ADP throughout incremental intermittent isometric plantar flexion exercise. The increase in  o2mus accelerated under mild acidosis during exercise at high intensity. The point of acceleration coincided with rapid changes in muscle PCr and ADP. The results of this study suggest that rapid decrease in PCr (concomitant accelerative increase in ADP) under mild acidotic condition stimulates accelerative muscle oxidative metabolism during incremental intermittent isometric exercise at high intensity.
o2mus accelerated under mild acidosis during exercise at high intensity. The point of acceleration coincided with rapid changes in muscle PCr and ADP. The results of this study suggest that rapid decrease in PCr (concomitant accelerative increase in ADP) under mild acidotic condition stimulates accelerative muscle oxidative metabolism during incremental intermittent isometric exercise at high intensity.
Authors' contributions
Toshiyuki Homma conceived the experimental design, carried out the experiment, and drafted the manuscript. Takafumi Hamaoka, M.D., Ph.D, participated in the design and coordination of the study, and directed throughout the study. Takayuki Sako, Ph.D., Motohide Murakami, M.D., Ph.D., Kazuki Esaki and Ryotaro Kime, Ph.D, participated in the study design and carried out the experiment. Toshihito Katsumura, MD., Ph.D, participated in the design and coordination of the study. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The authors thank Mr. Eric Sell, Miss Kathryn Kempf, and Mr. Toshio Kimura for their help in writing the English manuscript. We also thank the entire staff of the Department of Preventive Medicine and Public Health, Tokyo Medical University, for their helpful advice and technical assistance.
Contributor Information
Toshiyuki Homma, Email: homma.toshiyuki@jiss.naash.go.jp.
Takafumi Hamaoka, Email: kyp02504@nifty.com.
Takayuki Sako, Email: sako@fc.jwu.ac.jp.
Motohide Murakami, Email: qyw13211@nifty.com.
Kazuki Esaki, Email: esaki@taiiku.tsukuba.ac.jp.
Ryotaro Kime, Email: kime@tokyo-med.ac.jp.
Toshihito Katsumura, Email: kats@tokyo-med.ac.jp.
References
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. L Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990;258:C377–389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- Chance B, Leigh JS, Jr, Kent J, McCully K, Nioka S, Clark BJ, Maris JM, Graham T. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci USA. 1986;83:9458–9462. doi: 10.1073/pnas.83.24.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp GJ, Manners DN, Clark JF, Bastin ME, Radda GK. Theoretical modelling of some spatial and temporal aspects of the mitochondrion/creatine kinase/myofibril system in muscle. Mol Cell Biochem. 1998;184:249–289. doi: 10.1023/A:1006848726795. [DOI] [PubMed] [Google Scholar]
- Conley KE, Kemper WF, Crowther GJ. Limits to sustainable muscle performance: interaction between glycolysis and oxidative phosphorylation. J Exp Biol. 2001;204:3189–3194. doi: 10.1242/jeb.204.18.3189. [DOI] [PubMed] [Google Scholar]
- McCully KK, Natelson BH, Iotti S, Sisto S, Leigh JS., Jr Reduced oxidative muscle metabolism in chronic fatigue syndrome. Muscle Nerve. 1996;19:621–625. doi: 10.1002/(SICI)1097-4598(199605)19:5<621::AID-MUS10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Sahlin K. Physical exercise and mitochondrial function in human skeletal muscle. Exerc Sport Sci Rev. 2002;30:129–137. doi: 10.1097/00003677-200207000-00007. [DOI] [PubMed] [Google Scholar]
- Hamaoka T, Iwane H, Shimomitsu T, Katsumura T, Murase N, Nishio S, Osada T, Kurosawa Y, Chance B. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol. 1996;81:1410–1417. doi: 10.1152/jappl.1996.81.3.1410. [DOI] [PubMed] [Google Scholar]
- Mahler M. First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between QO2 and phosphorylcreatine level. Implications for the control of respiration. J Gen Physiol. 1985;86:135–165. doi: 10.1085/jgp.86.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 1988;254:C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT. Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. J Appl Physiol. 1996;81:1331–1338. doi: 10.1152/jappl.1996.81.3.1331. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol. 1999;518:921–932. doi: 10.1111/j.1469-7793.1999.0921p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipp BJ, Rossiter HB, Ward SA, Avery D, Doyle VL, Howe FA, Griffiths JR. Simultaneous determination of muscle 31P and O2 uptake kinetics during whole body NMR spectroscopy. J Appl Physiol. 1999;86:742–747. doi: 10.1152/jappl.1999.86.2.742. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Howe FA, Kowalchuk JM, Griffiths JR, Whipp BJ. Dynamics of intramuscular 31P-MRS P(i) peak splitting and the slow components of PCr and O2 uptake during exercise. J Appl Physiol. 2002;93:2059–2069. doi: 10.1152/japplphysiol.00446.2002. [DOI] [PubMed] [Google Scholar]
- McCully KK, Kakihira H, Vandenborne K, Kent-Braun J. Noninvasive measurements of activity-induced changes in muscle metabolism. J Biomech. 1991;24 Suppl 1:153–161. doi: 10.1016/0021-9290(91)90385-Z. [DOI] [PubMed] [Google Scholar]
- Bhambhani YN. Muscle oxygenation trends during dynamic exercise measured by near infrared spectroscopy. Can J Appl Physiol. 2004;29:504–523. doi: 10.1139/h04-033. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bulow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (MRS) in health and disease. Scand J Med Sci Sports. 2001;11:213–222. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- McCully KK, Hamaoka T. Near-infrared spectroscopy: what can it tell us about oxygen saturation in skeletal muscle? Exerc Sport Sci Rev. 2000;28:123–127. [PubMed] [Google Scholar]
- Quaresima V, Lepanto R, Ferrari M. The use of near infrared spectroscopy in sports medicine. J Sports Med Phys Fitness. 2003;43:1–13. [PubMed] [Google Scholar]
- Sako T, Hamaoka T, Higuchi H, Kurosawa Y, Katsumura T. Validity of NIR spectroscopy for quantitatively measuring muscle oxidative metabolic rate in exercise. J Appl Physiol. 2001;90:338–344. doi: 10.1152/jappl.2001.90.1.338. [DOI] [PubMed] [Google Scholar]
- Murakami M, Katsumura T, Hamaoka T, Osada T, Sako T, Higuchi H, Esaki K, Kime R, Shimomitsu T. Effects of epinephrine and lactate on the increase in oxygen consumption of nonexercising skeletal muscle after aerobic exercise. J Biomed Opt. 2000;5:406–410. doi: 10.1117/1.1289143. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Buchthal SD, Zanconato S, Cooper DM. Changes in potential controllers of human skeletal muscle respiration during incremental calf exercise. J Appl Physiol. 1994;77:2169–2176. doi: 10.1152/jappl.1994.77.5.2169. [DOI] [PubMed] [Google Scholar]
- Marsh GD, Paterson DH, Thompson RT, Driedger AA. Coincident thresholds in intracellular phosphorylation potential and pH during progressive exercise. J Appl Physiol. 1991;71:1076–1081. doi: 10.1152/jappl.1991.71.3.1076. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983;1:77–94. [PubMed] [Google Scholar]
- Kushmerick MJ, Meyer RA. Chemical changes in rat leg muscle by phosphorus nuclear magnetic resonance. Am J Physiol. 1985;248:C542–C549. doi: 10.1152/ajpcell.1985.248.5.C542. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest Methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Henriksson J, Katz A, Sahlin K. Redox state changes in human skeletal muscle after isometric contraction. J Physiol. 1986;380:441–451. doi: 10.1113/jphysiol.1986.sp016296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Katz A, Henriksson J. Redox state and lactate accumulation in human skeletal muscle during dynamic exercise. Biochem J. 1987;245:551–556. doi: 10.1042/bj2450551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstow TJ, Buchthal S, Zanconato S, Cooper DM. Muscle energetics and pulmonary oxygen uptake kinetics during moderate exercise. J Appl Physiol. 1994;77:1742–1749. doi: 10.1152/jappl.1994.77.4.1742. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Meyer RA, Brown TR. Regulation of oxygen consumption in fast- and slow-twitch muscle. Am J Physiol. 1992;263:C598–C606. doi: 10.1152/ajpcell.1992.263.3.C598. [DOI] [PubMed] [Google Scholar]
- Lawson JW, Veech RL. Effects of pH and free Mg2+on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- Koike A, Yajima T, Adachi H, Shimizu N, Kano H, Sugimoto K, Niwa A, Marumo F, Hiroe M. Evaluation of exercise capacity using submaximal exercise at a constant work rate in patients with cardiovascular disease. Circulation. 1995;91:1719–1724. doi: 10.1161/01.cir.91.6.1719. [DOI] [PubMed] [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ. Improved detection of lactate threshold during exercise using a log-log transformation. J Appl Physiol. 1985;59:1936–1940. doi: 10.1152/jappl.1985.59.6.1936. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol. 2001;537:291–303. doi: 10.1111/j.1469-7793.2001.0291k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol. 2002;541:991–1002. doi: 10.1113/jphysiol.2001.012910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the "phosphocreatine shuttle". Am J Physiol. 1984;246:C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
-
Connett RJ, Honig CR. Regulation of
 O2 in red muscle: do current biochemical hypotheses fit in vivo data? Am J Physiol. 1989;256:R898–R906. doi: 10.1152/ajpregu.1989.256.4.R898. [DOI] [PubMed] [Google Scholar]
O2 in red muscle: do current biochemical hypotheses fit in vivo data? Am J Physiol. 1989;256:R898–R906. doi: 10.1152/ajpregu.1989.256.4.R898. [DOI] [PubMed] [Google Scholar] - Hansen JE, Sue DY Oren A, Wasserman K. Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. Am J Cardiol. 1987;59:669–674. doi: 10.1016/0002-9149(87)91190-8. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Davis JA, Torres F, Wasserman K. A test to determine parameters of aerobic function during exercise. J Appl Physiol. 1981;50:217–221. doi: 10.1152/jappl.1981.50.1.217. [DOI] [PubMed] [Google Scholar]
- Barclay CJ, Constable JK, Gibbs CL. Energetics of fast- and slow-twitch muscles of the mouse. J Physiol. 1993;472:61–80. doi: 10.1113/jphysiol.1993.sp019937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka MN, Petrofsky JS, Phillips CA. Energy cost of submaximal isometric concentrations in cat fast and slow twitch muscles. Pflugers Arch. 1981;390:164–168. doi: 10.1007/BF00590201. [DOI] [PubMed] [Google Scholar]
- Saugen E, Vøllestad NK. Nonlinear relationship between heat production and force during voluntary contractions in humans. J Appl Physiol. 1995;79:2043–2049. doi: 10.1152/jappl.1995.79.6.2043. [DOI] [PubMed] [Google Scholar]
- Sietsema KE, Daly JA, Wasserman K. Early dynamics of O2 uptake and heart rate as affected by exercise work rate. J Appl Physiol. 1989;67:2535–2541. doi: 10.1152/jappl.1989.67.6.2535. [DOI] [PubMed] [Google Scholar]
- Wesche J. The time course and magnitude of blood flow changes in the human quadriceps muscles following isometric contraction. J Physiol. 1986;377:445–462. doi: 10.1113/jphysiol.1986.sp016197. [DOI] [PMC free article] [PubMed] [Google Scholar]