Abstract
Objective:
To determine the relationship of COVID-19 symptoms to COVID-19 antibody positivity among unvaccinated pregnant women in low-and middle-income countries (LMIC).
Design:
COVID-19 infection status measured by antibody positivity at delivery was compared with the symptoms of COVID-19 in the current pregnancy in a prospective, observational cohort study in seven LMICs
Setting:
The study was conducted among women in the Global Network for Women’s and Children’s Health’s Maternal and Newborn Health Registry (MNHR), a prospective, population-based study in Kenya, Zambia, the Democratic Republic of the Congo (DRC), Bangladesh, Pakistan, India (Belagavi and Nagpur sites), and Guatemala.
Population:
Pregnant women enrolled in the ongoing pregnancy registry at study sites.
Methods:
Data on COVID-19 symptoms during the current pregnancy were collected by trained staff between October 2020 and June 2022. COVID-19 antibody testing was performed on samples collected at delivery. The relationship between COVID-19 antibody positivity and symptoms were assessed using generalized linear models with a binomial distribution adjusting for site and symptoms.
Main Outcome Measures:
COVID-19 antibody status and symptoms of COVID-19 among pregnant women.
Results:
Among 19,218 non-vaccinated pregnant women who were evaluated, 14.1% of antibody-positive women had one or more symptoms compared to 13.4% in antibody-negative women. Overall, 85.3% of antibody-positive women reported no COVID-19 symptoms during the present pregnancy. Reported fever was significantly associated with antibody status [RR 1.10 (1.03, 11.18) (p=0.008)]. A multiple variable model adjusting for site and all 8 symptoms during pregnancy showed similar results [RR 1.13 (1.04, 1.23) (p=0.012). Other symptoms were not significantly related to antibody positivity.
Conclusions:
In a population-based cohort in LMICs, unvaccinated pregnant women who were antibody positive had slightly more symptoms during their pregnancy and a small but significantly greater increase in fever. However, for prevalence studies, evaluating COVID-19 related symptoms does not appear to be useful in differentiating pregnant women who have had a COVID-19 infection.
Keywords: COVID-19 antibody, COVID-19 symptoms, pregnant women, Low-and Middle-Income Countries
Introduction
SARS-CoV-2 or COVID-19 infection has posed a significant burden to health care systems worldwide. More than 6 million people are believed to have died from this infection. Pregnancy and its outcomes have also been adversely affected by this infection [1, 2]. There are many unknowns with respect to pregnant women and COVID-19 infection during the current pandemic. [3] One issue that needs attention is whether putative COVID-19 symptoms are useful in determining population rates of COVID-19 infections.
The Centre for Disease Control (CDC) has issued guidance regarding the potential symptoms related to COVID-19. Subsequently, the World Health Organization (WHO) also issued guidance on using symptoms to identify a COVID-19 infection. [4] However, varied presentation of symptoms of COVID-19 were observed during pregnancy in most of the published literature. These reports emphasized that most pregnant women infected with COVID-19 were relatively asymptomatic and were less likely to manifest COVID-19 related symptoms than non-pregnant women. [5,6] A WHO systematic review of 11 observational studies suggested that of women in the reproductive age who tested positive for COVID-19, a greater proportion of pregnant women were asymptomatic; symptoms when present were also mild in nature. [7] Nevertheless, in a study of nearly 400,000 women of reproductive age with COVID-19, the CDC found that pregnant women had a greater risk for mortality and morbidity than non-pregnant women of reproductive age [8]. An issue with many of these studies is that they were not population-based and focused on symptomatic women with the disease documented by the presence of antigens at the time of illness.
COVID-19 infection in pregnant women without classical presentation presents an added challenge regarding service provision, prevention and management. Additionally, there is a need to correlate the symptoms with the disease assessed by presence of COVID-19 antibodies. This paper presents the results pertaining to COVID-19 symptoms among pregnant women from our ongoing Maternal Newborn Health Registry (MNHR) population across seven low-and-middle income countries (LMICs) and if these symptoms were related to the infection status measured by the presence of antibodies in the maternal serum.
Methods
This study was conducted by the Global Network for Women’s and Children’s Health Research (Global Network), a multi-country research network funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). [9] The COVID-19 study among pregnant women was undertaken within the infrastructure of the ongoing Global Network’s Maternal and Neonatal Health Registry (MNHR). The MNHR is a prospective, population-based observational study that includes all pregnant women, their newborns and their outcomes in defined geographic communities (clusters). [10,11] Each cluster includes approximately 300 – 500 births annually, with 8–10 clusters currently available at each of the Global Network sites in western Kenya, Zambia (Kafue and Chongwe), the Democratic Republic of the Congo (DRC) (North and South Ubangi Province), Pakistan (Thatta in Sindh Province), India (Belagavi and Nagpur), Guatemala (Chimaltenango) and Bangladesh (District Tangail). The MNHR has been ongoing in all sites since 2008, except for the DRC, which joined in 2014, and the Bangladesh site, which joined in 2018.
Trained study health care staff (registry administrators [RAs]) identified pregnant women in their respective clusters. Following informed consent, these women were enrolled into the MNHR. [10,11] The RAs obtained basic health and demographic information at enrolment, and recorded the date of the last menstrual period (LMP) and/or early ultrasound report to assess gestational age (GA). Maternal height, weight and haemoglobin were also documented. The next visit was carried out following delivery of the women to collect information on pregnancy outcomes as well as health care received during delivery. The maternal and newborn health status was collected at 42 days post-delivery. The outcomes were based on the review of medical records and birth attendant and family interviews. During the active phase of the pandemic, the study staff were often collected the information by telephone or by home visits with necessary precautions.
The pregnant women enrolled in the MNHR were re-consented to participate in the COVID-19 study as this involved collection of additional information and samples for antibody assessments. The timing of the COVID-19 study initiation varied by site (beginning in October 2020 for Pakistan; in November 2020, for Bangladesh, Guatemala, Nagpur, and India; in December 2020, in Kenya and Belagavi India; and in February 2021 in Zambia) and the data collection continued until the end of June 2022 across all the study sites. Since we did not have the resources to perform antibody testing on all women delivering, we asked each site to collect a blood sample on a maximum of 170 women per month. Some sites collected fewer samples. At the time of the blood sample collection, a questionnaire was administered asking about potential COVID-19 related symptoms before the pregnancy, during the pregnancy, and at the time of the sample collection. (For this study we present responses related to the current pregnancy). The maternal serum sample was generally collected at delivery, however a 2-week window for antibody collection was allowed by protocol. The antibody test results for the samples collected in the Global Network sites were analysed and linked to data in the MNHR. The assay was performed by laboratory staff at each of the sites as per the manufacturer’s protocol with quality oversite by Research Triangle Institute (RTI), USA staff. [12]
Symptoms of COVID-19 infection included fever, cough, shortness of breath or breathing difficulty, chills, muscle pain, headache, sore throat, and loss of taste or smell, based on the WHO criteria. [13] Mothers who received one or more doses of COVID-19 vaccine were documented. This analysis was undertaken among women had never received any type of COVID-19 vaccine.
Statistical Analysis
Pregnant women who delivered at ≥ 20 weeks of gestation, had not received a COVID-19 vaccination and provided a serum sample at delivery with a COVID-19 antibody test completed were included in this analysis. Maternal demographic characteristics, antibody positivity and COVID-19 symptoms during pregnancy are presented with frequencies and percentages. To assess the relationship between COVID-19 symptoms among unvaccinated pregnant women and antibody positivity, relative risks and 95% confidence intervals were obtained from generalized linear models with terms for site and symptom with a separate log binomial model for each COVID-19 symptom. Additionally, relative risks and 95% confidence intervals were obtained from a multivariable generalized linear model adjusting for site and all eight COVID-19 symptoms reported during pregnancy. All analyses were conducted in SAS v. 9.4 (SAS Institute, Cary, NC, USA).
Ethical considerations
This study was reviewed and approved by ethics review committees of all participating sites: INCAP, Guatemala; University of Zambia, Zambia; Moi University, Kenya; Aga Khan University, Pakistan; KLE Academy of Higher Education and Research’s Jawaharlal Nehru Medical College, Belagavi, India; Lata Medical Research Foundation, Nagpur, India; Kinshasa School of Public Health, Democratic Republic of the Congo; and the International Centre for Diarrhoeal Disease Research, Bangladesh. The Institutional Review Boards at each US partner university and the Data Coordinating Centre (RTI International) also approved the protocol. All women provided informed consent for participation in the study, the data and sample collection.
Results
Across all the GN sites, from October 2020 to June 2022, 54,400 pregnant women were screened and 50,424 women had pregnancy outcomes ≥ 20 weeks of gestation. A serum sample was obtained from 22,888 women at delivery and antibody results were available for 22,241 participants. Figure 1 summarizes the study enrolment. 19,218 of these women were unvaccinated and these women comprised the analytical population for this study.
Figure 1:
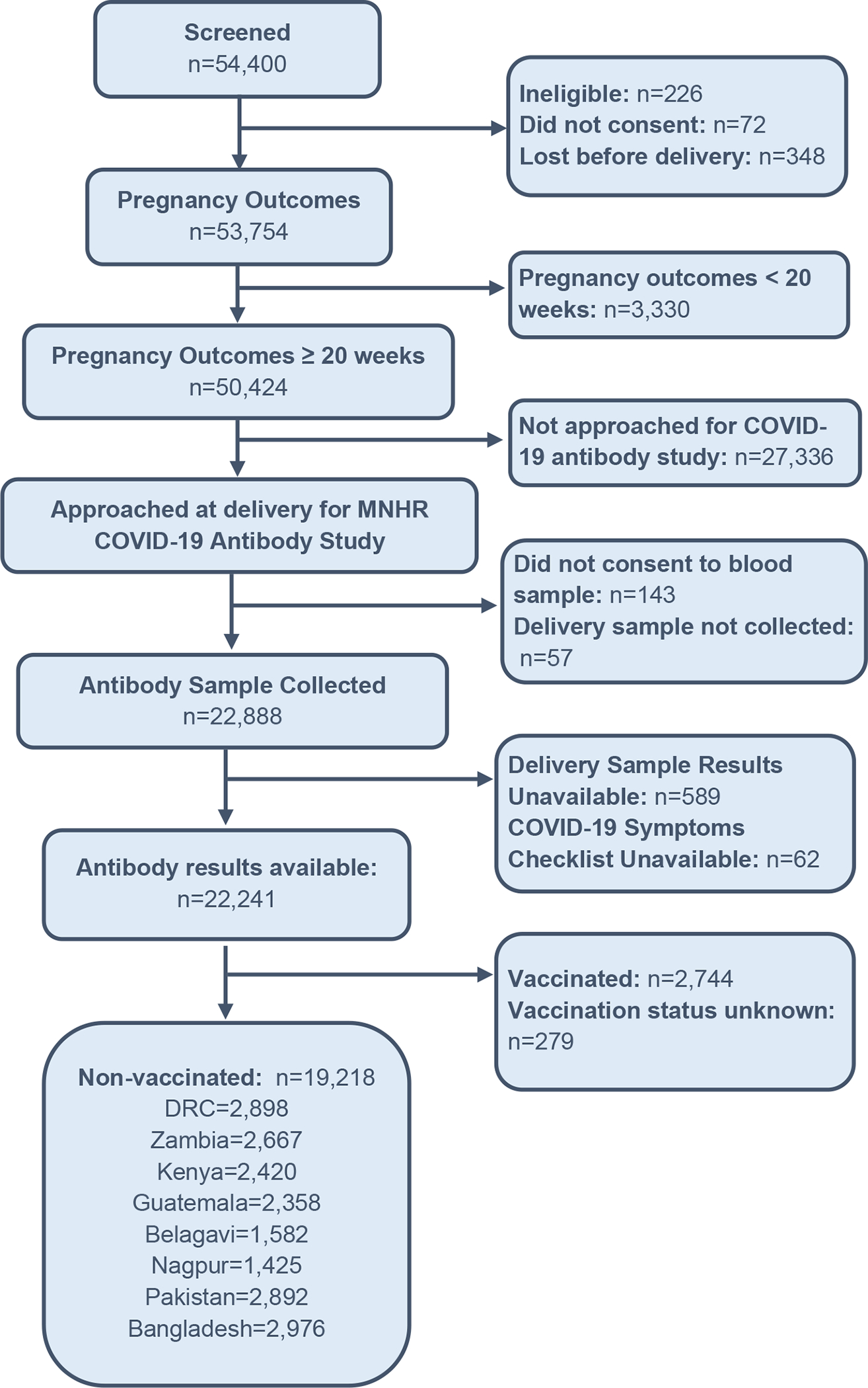
Enrolment in the Global Network COVID-19 Antibody Study
Table 1 presents the maternal characteristics of all participants with antibody assessments with known vaccination status in column 1. In column 2 are those with antibody assessments who were not vaccinated. The later 19,218 women are the study population. Over all, the maternal characteristics were similar in both groups.
Table 1:
Comparison of the Maternal Characteristics of all Women with COVID-19 Results Available with the Characteristics of Non-Vaccinated Women who had COVID-19 Results Available
| Variable | Women with COVID-19 antibody results available n = 22,241 (%) |
Non-vaccinated women with COVID-19 antibody results available n =19,218 (%) |
|---|---|---|
| Education | 22,236 | 19,213 |
| No schooling | 4,388 (19.7) | 4,094 (21.3) |
| 1–6 years | 4,300 (19.3) | 3,834 (20.0) |
| 7–12 years | 11,893 (53.5) | 10,035 (52.2) |
| ≥ 13 years | 1,655 (7.4) | 1,250 (6.5) |
| Age (years) | 22,239 | 19,216 |
| < 20 | 3,520 (15.8) | 3,222 (16.8) |
| 20–35 | 17,446 (78.4) | 14,835 (77.2) |
| > 35 | 1,273 (5.7) | 1,159 (6.0) |
| Parity | 22,240 | 19,217 |
| 0 | 7,260 (32.6) | 6,094 (31.7) |
| 1–2 | 9,779 (44.0) | 8,346 (43.4) |
| > 2 | 5,201 (23.4) | 4,777 (24.9) |
| Delivery location | 22,241 | 19,218 |
| Facility | 18,839 (84.7) | 16,113 (83.8) |
| Home/other | 3,402 (15.3) | 3,105 (16.2) |
| ANC visits | 22,222 | 19,202 |
| 0 | 1,368 (6.2) | 1,327 (6.9) |
| 1 | 1,375 (6.2) | 1,311 (6.8) |
| 2 | 2,308 (10.4) | 2,180 (11.4) |
| 3 | 3,862 (17.4) | 3,563 (18.6) |
| ≥ 4 | 13,309 (59.9) | 10,821 (56.4) |
Table 2 shows the percent of non-vaccinated women overall and by site by antibody status. Among the non-vaccinated women, overall, 34.3% were antibody positive. The positivity rates ranged from 23.0% in Guatemala to 56.8% in Nagpur India.
Table 2.
COVID-19 Antibody Status at Delivery in Non-Vaccinated Women by Study Site
| Variable | Overall n (%) |
DRC n (%) |
Zambia n (%) |
Kenya n (%) |
Guatemala n (%) |
India (Belagavi) n (%) |
India (Nagpur) n (%) |
Pakistan n (%) |
Bangladesh n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Antibody Results in Non- Vaccinated Women, n (%) | 19,218 | 2,898 | 2,667 | 2,420 | 2,358 | 1,582 | 1,425 | 2,892 | 2,976 |
| Positive | 6,584 (34.3) | 1,090 (37.6) | 900 (33.7) | 752 (31.1) | 543 (23.0) | 655 (41.4) | 810 (56.8) | 776 (26.8) | 1,058 (35.6) |
| Negative | 12,146 (63.2) | 1,699 (58.6) | 1,744 (65.4) | 1,550 (64.0) | 1,762 (74.7) | 889 (56.2) | 569 (39.9) | 2,070 (71.6) | 1,863 (62.6) |
| Indeterminate | 488 (2.5) | 109 (3.8) | 23 (0.9) | 118 (4.9) | 53 (2.2) | 38 (2.4) | 46 (3.2) | 46 (1.6) | 55 (1.8) |
Table 3 shows the number and percent of women overall and by site by antibody status with any COVID-19 symptoms and the number and percent with 1 to 3 COVID-19 symptoms. Overall, 14.1% of antibody-positive women reported having one or more symptoms compared to 13.4% of symptoms in antibody-negative women. The reported range in the percent of women reporting any symptoms was large, ranging from about 2% in Pakistan to about 32% in Bangladesh. However, the difference in symptoms between antibody-positive and antibody-negative women within the sites was generally small, and only in Belagavi did antibody positive women have substantially more symptoms compared to antibody-negative women (24.3% vs 13.4%). Overall, 85.3% of antibody-positive women reported no COVID-19 symptoms during their pregnancy compared to 86.6% of antibody-negative women. Conversely, 14.7% of antibody-positive women vs 13.4% of antibody-negative women reported one or more symptoms. Overall, the antibody-positive women reported slightly greater numbers of 1 symptom (8.4% vs 7.3%), similar numbers of 2 symptoms (4.0% for each) and slightly more ≥3 symptoms (2.4 vs 2.1%). The mean number of symptoms present also varied among the sites, but generally there were only small differences within the sites among those women who were antibody-positive and antibody-negative.
Table 3.
Symptoms Potentially Related to Maternal COVID-19 infection During Pregnancy Among Non-Vaccinated Women by COVID-19 Antibody Status at Delivery and by Site. (n and %)
| Variables | Overall | DRC | Zambia | Kenya | Guatemala | India (Belagavi) | India (Nagpur) | Pakistan | Bangladesh | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | |
| Delivery ≥ 20 weeks, n (%) | 6,584 (35.2) | 12,146 (64.8) | 1,090 (39.1) | 1,699 (60.9) | 900 (34.0) | 1,744 (66.0) | 752 (32.7) | 1,550 (67.3) | 543 (23.6) | 1,762 (76.4) | 655 (42.4) | 889 (57.6) | 810 (58.7) | 569 (41.3) | 776 (27.3) | 2,070 (72.7) | 1,058 (36.2) | 1,863 (63.8) |
| ≥ 1 symptom during pregnancy, n (%) | 969 (14.7) | 1,623 (13.4) | 201 (18.4) | 340 (20.0) | 76 (8.4) | 190 (10.9) | 76 (10.1) | 186 (12.0) | 51 (9.4) | 136 (7.7) | 159 (24.3) | 119 (13.4) | 23 (2.8) | 22 (3.9) | 15 (1.9) | 60 (2.9) | 368 (34.8) | 570 (30.6) |
| Number of symptoms during pregnancy, n (%) | 6,584 | 12,146 | 1,090 | 1,699 | 900 | 1,744 | 752 | 1,550 | 543 | 1,762 | 655 | 889 | 810 | 569 | 776 | 2,070 | 1,058 | 1,863 |
| Asymptomatic | 5,615 (85.3) | 10,523 (86.6) | 889 (81.6) | 1,359 (80.0) | 824 (91.6) | 1,554 (89.1) | 676 (89.9) | 1,364 (88.0) | 492 (90.6) | 1,626 (92.3) | 496 (75.7) | 770 (86.6) | 787 (97.2) | 547 (96.1) | 761 (98.1) | 2,010 (97.1) | 690 (65.2) | 1,293 (69.4) |
| 1 Symptom | 550 (8.4) | 884 (7.3) | 91 (8.3) | 141 (8.3) | 44 (4.9) | 122 (7.0) | 31 (4.1) | 62 (4.0) | 32 (5.9) | 94 (5.3) | 115 (17.6) | 95 (10.7) | 16 (2.0) | 17 (3.0) | 7 (0.9) | 34 (1.6) | 214 (20.2) | 319 (17.1) |
| 2 Symptoms | 261 (4.0) | 480 (4.0) | 71 (6.5) | 123 (7.2) | 21 (2.3) | 39 (2.2) | 31 (4.1) | 82 (5.3) | 11 (2.0) | 30 (1.7) | 21 (3.2) | 15 (1.7) | 6 (0.7) | 4 (0.7) | 5 (0.6) | 15 (0.7) | 95 (9.0) | 172 (9.2) |
| 3+ Symptoms | 158 (2.4) | 259 (2.1) | 39 (3.6) | 76 (4.5) | 11 (1.2) | 29 (1.7) | 14 (1.9) | 42 (2.7) | 8 (1.5) | 12 (0.7) | 23 (3.5) | 9 (1.0) | 1 (0.1) | 1 (0.2) | 3 (0.4) | 11 (0.5) | 59 (5.6) | 79 (4.2) |
We next looked at the individual symptoms among antibody-positive and antibody-negative women overall and by site. (Table 4) Overall, fever was the most common symptom both in women with and without antibodies (7.8% vs 6.5%). Additionally, cough (5.1% vs 4.8%) and gastrointestinal symptoms such as vomiting and/or diarrhoea (5.4% vs 4.9%) were observed in slightly higher proportions among women with antibodies. Conversely, new onset loss of taste or smell was more common among women without antibodies. There were large differences in the reported rates of some of the symptoms among the sites, but there was little difference within the sites between women with and without antibodies.
Table 4:
Maternal Symptoms Potentially Related to Maternal COVID-19 Infection During Pregnancy Among Non-Vaccinated Women by COVID-19 Antibody Status at Delivery and by Site
| Symptoms during Pregnancy | Overall | DRC | Zambia | Kenya | Guatemala | India (Belagavi) | India (Nagpur) | Pakistan | Bangladesh | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibody Results | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − |
| Fever, n (%) | 515 (7.8) | 794 (6.5) | 124 (11.4) | 213 (12.5) | 29 (3.2) | 61 (3.5) | 37 (4.9) | 110 (7.1) | 29 (5.4) | 48 (2.7) | 41 (6.3) | 24 (2.7) | 12 (1.5) | 8 (1.4) | 10 (1.3) | 24 (1.2) | 233 (22.0) | 306 (16.4) |
| Cough, n (%) | 337 (5.1) | 585 (4.8) | 53 (4.9) | 82 (4.8) | 40 (4.4) | 89 (5.1) | 15 (2.0) | 45 (2.9) | 26 (4.8) | 68 (3.9) | 36 (5.5) | 29 (3.3) | 11 (1.4) | 13 (2.3) | 6 (0.8) | 26 (1.3) | 150 (14.2) | 233 (12.5) |
| Shortness of breath, n (%) | 29 (0.4) | 38 (0.3) | 3 (0.3) | 3 (0.2) | 6 (0.7) | 9 (0.5) | 6 (0.8) | 2 (0.1) | 4 (0.7) | 7 (0.4) | 3 (0.5) | 3 (0.3) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.1) | 6 (0.6) | 12 (0.6) |
| Chills, n (%) | 136 (2.1) | 253 (2.1) | 72 (6.6) | 122 (7.2) | 13 (1.4) | 24 (1.4) | 31 (4.1) | 77 (5.0) | 6 (1.1) | 8 (0.5) | 13 (2.0) | 8 (0.9) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 11 (0.5) | 1 (0.1) | 2 (0.1) |
| Muscle pain, n (%) | 135 (2.1) | 247 (2.0) | 41 (3.8) | 74 (4.4) | 7 (0.8) | 22 (1.3) | 15 (2.0) | 50 (3.2) | 5 (0.9) | 6 (0.3) | 18 (2.8) | 6 (0.7) | 0 (0.0) | 1 (0.2) | 7 (0.9) | 21 (1.0) | 42 (4.0) | 67 (3.6) |
| New loss of taste or smell, n (%) | 75 (1.1) | 140 (1.2) | 10 (0.9) | 29 (1.7) | 10 (1.1) | 18 (1.0) | 5 (0.7) | 17 (1.1) | 4 (0.7) | 8 (0.5) | 9 (1.4) | 2 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.0) | 37 (3.5) | 64 (3.4) |
| Vomiting or diarrhea, n (%) | 356 (5.4) | 592 (4.9) | 63 (5.8) | 125 (7.4) | 24 (2.7) | 69 (4.0) | 29 (3.9) | 66 (4.3) | 1 (0.2) | 10 (0.6) | 124 (19.0) | 84 (9.5) | 6 (0.7) | 5 (0.9) | 3 (0.4) | 17 (0.8) | 106 (10.0) | 216 (11.6) |
| Sore throat, n (%) | 52 (0.8) | 97 (0.8) | 1 (0.1) | 4 (0.2) | 2 (0.2) | 7 (0.4) | 0 (0.0) | 4 (0.3) | 11 (2.0) | 42 (2.4) | 7 (1.1) | 2 (0.2) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 30 (2.8) | 38 (2.0) |
The relationship between antibody positivity and COVID-19 symptoms were assessed using generalized linear models with a binomial distribution adjusting for site and symptom (Table 5). Reported fever was significantly associated with antibody status with a relative risk (RR) of 1.10 (1.03, 11.18) (p=0.008). A multiple variable model adjusting for site and all eight symptoms during pregnancy showed similar results. In this analysis, women with antibody positivity had a RR of fever of 1.13 (1.04, 1.23), (p=0.012). Other symptoms were not significantly related to antibody positivity.
Table 5.
COVID-19 Antibody Positivity and Symptoms During Pregnancy Among Non-Vaccinated Women
| Putative Symptoms of COVID-19 during Pregnancy | Delivery Sample Results | Single Symptom Models 1 | Multiple Symptoms Model 2 | |||
|---|---|---|---|---|---|---|
| Positive | Negative | RR (95% CI) | p-Value | RR (95% CI) | p-Value | |
| Fever, n (%) | 515 (7.8) | 794 (6.5) | 1.10 (1.03, 1.18) | 0.008 | 1.13 (1.04, 1.23) | 0.004 |
| Cough, n (%) | 337 (5.1) | 585 (4.8) | 1.03 (0.94, 1.12) | 0.534 | 0.98 (0.89, 1.08) | 0.677 |
| Shortness of breath, n (%) | 29 (0.4) | 38 (0.3) | 1.27 (0.97, 1.67) | 0.082 | 1.25 (0.96, 1.64) | 0.103 |
| Chills, n (%) | 136 (2.1) | 253 (2.1) | 0.97 (0.84, 1.11) | 0.628 | 0.91 (0.78, 1.05) | 0.207 |
| Muscle pain, n (%) | 135 (2.1) | 247 (2.0) | 0.99 (0.86, 1.14) | 0.904 | 0.95 (0.82, 1.10) | 0.468 |
| New loss of taste or smell, n (%) | 75 (1.1) | 140 (1.2) | 0.97 (0.81, 1.17) | 0.775 | 0.96 (0.79, 1.16) | 0.669 |
| Vomiting or diarrhea, n (%) | 356 (5.4) | 592 (4.9) | 1.01 (0.93, 1.10) | 0.748 | 1.00 (0.91, 1.09) | 0.960 |
| Sore throat, n (%) | 52 (0.8) | 97 (0.8) | 1.12 (0.90, 1.39) | 0.327 | 1.08 (0.86, 1.35) | 0.507 |
Relative risks and 95% confidence intervals are obtained from generalized linear models with a Binomial distribution and a log link adjusting for site and one symptom.
Relative risks and 95% confidence intervals are obtained from a generalized linear model with a Binomial distribution and a log link adjusting for site and all eight symptoms during pregnancy.
Discussion
During the COVID-19 pandemic, many health care systems across the world used symptoms suggested by various professional organizations to define infection with COVID-19. [14] Clinical assessment of symptoms was used to determine the presence of COVID-19 disease in many resource limited settings. [15] To determine the usefulness of symptoms in determining COVID-19 infections, we evaluated the relationship between putative COVID-19 symptoms and COVID-19 antibody status among non-vaccinated pregnant women at delivery in eight sites in seven LMICs.
Main findings
Across the Global Network sites over a period of 21 months (between October 2020 through June 2022), 22,241 pregnant women at ≥ 20 weeks of gestation who had provided a serum sample at delivery were evaluated. For this study, we specifically focused on the 19,218 women who were not vaccinated for COVID-19. Among these women, 6,584 (34.3%) were positive for COVID-19 antibodies. We compared the potential symptoms of COVID-19 reported during the pregnancy and the results of antibody positivity. Overall, 14.7% of antibody-positive women had one or more symptoms compared to 13.4% of women who were antibody-negative. While there were large differences in the rates of reported symptoms between the sites, within the sites there were only small differences in overall symptom rates between those with antibodies and those without antibodies. Similarly, while the individual sites had large differences in the rates of specific symptoms, within the sites there were only small differences in the rates of specific symptoms when antibody positive and antibody negative women were compared. To better understand these relationships, we created a generalized linear model with a binomial distribution and a log link adjusting for site which showed that among the symptoms, only fever was significantly associated with antibody status and this remained true when the model was further adjusted for all eight symptoms during pregnancy along with site.
Interpretation
Most pregnant women infected with COVID-19 have either mild illness or are asymptomatic (and therefore might not get medical care or testing). Thus, antibody estimation allowed us to better understand the infection rate in the population. [16, 17] In a population of non-vaccinated women, we assumed that the presence of COVID-19 antibodies were a marker of prior COVID-19 infection. Among these non-vaccinated women, we determined if COVID-19 symptoms were related to COVID-19 antibody status. In this analysis, only fever was significantly related to antibody positivity, and that relationship was weak. In addition, our analysis clearly demonstrated that the majority of the unvaccinated pregnant women with COVID-19 antibodies were asymptomatic. Equally important was the finding that the symptoms reported were generally similar in the women with and without COVID-19 antibodies.
A large multi-site WHO study conducted across many states in India compared the pregnant women with COVID-19 disease with a matched control. Though this study used antigen rather than antibody to define disease and was hospital based, the results are in many ways comparable to our study. Most cases were asymptomatic and those who were symptomatic mostly had mild disease. Fever, like in our study, was the most common symptom. [18] There are many reports of COVID-19 related symptoms in women with active disease often defined by an acute illness, hospital admission and the presence of COVID-19 antigens. Importantly, these reports were generally not population-based but mostly included women with known disease. [19–24] The types and rates of symptoms in this type of study are clearly different than those found in our population-based study.
Strengths and Limitations
The presence of the ongoing, prospective, population-based MNH registry with a large sample size representing three regions of south Asia, sub-Saharan Africa and Central America, across seven LMIC countries with a standardized data collection method poses a unique strength to the study. [16] The results of antibody tests were not available at the time of symptom assessment, thus ruling out interviewer bias. Limitations include that we undertook analysis of presence of antibodies at delivery, and antibody estimation was not aligned with the actual presence of symptoms. One of the weaknesses of this study was that we did not know when the women who were antibody positive became antibody positive or were sure if the women became antibody positive during the pregnancy. We do however know on a population level that most of the antibody positivity increase in our sites occurred in the last 9 months of this study.
Conclusions
Symptoms in non-vaccinated pregnant women who were COVID-19 antibody positive were compared to those who were antibody negative. Antibody positive women had slightly more symptoms during their pregnancy and a small but significantly greater increase in fever. However, the differences were small. The relatively small differences in symptoms seen between antibody positive vs. antibody negative women make it unlikely that symptomology will be useful to discriminate between antibody positive and negative women in population-based epidemiologic studies. Therefore, it does not appear that one can use COVID-19 related symptoms to differentiate pregnant women who have a COVID-19 infection. The presence of one or more of these symptoms might lead a clinician to suspect a COVID-19, especially during the pandemic, but it appears that using symptoms to diagnose a COVID-19 infection might lead to an inaccurate diagnosis.
Acknowledgements
We thank the women who participated in this study. We appreciate the study staff in the field i.e., registry administrators, laboratory technicians and community health workers and the Global Network Sites Investigators for their valuable contribution.
Funding information
This study was funded by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). [Grant/Award Number: U24HD092094]. Staff from the funder had input into the study design and reviewed the data in this report. However, the views presented in the paper do not necessarily represent those of the NICHD.
Funding:
Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), USA. Grant/Award Number: U24HD092094
Footnotes
Conflict of Interests
None declared. Completed disclosure of interests’ forms are available on request.
References
- 1.World Health Organization. Rolling updates on coronavirus disease (COVID-19). Geneva: WHO; 2020. Accessed at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen Last accessed on November 25, 2022. [Google Scholar]
- 2.Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, Critchley HOD. Pregnancy and COVID-19. Physiol Rev. 2021. Jan 1;101(1):303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ntounis T, Prokopakis I, Koutras A, Fasoulakis Z, Pittokopitou S, Valsamaki A, et al. Pregnancy and COVID-19. J Clin Med. 2022. Nov 9;11(22):6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centre for Disease Prevention and Control; Symptoms of COVID-19. Accessed at https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html Last accessed on February 4, 2023.
- 5.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vousden N, Bunch K, Morris E, Simpson N, Gale C, O’Brien P, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: A national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS One. 2021. May 5;16(5):e0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ (Clinical research ed). 2020;370:m3320–m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020. Nov 6;69(44):1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koso-Thomas M, McClure EM, Global Network for Women’s and Children’s Heatlh Research Investigators. The Global Network for Women’s and Children’s Health Research: a model of capacity-building research. Semin Fetal Neonatal Med. 2015;20(5):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goudar SS, Carlo WA, McClure EM, Pasha O, Patel A, Esamai F, et al. The Maternal and Newborn Health Registry Study of the Global Network for Women’s and Children’s Health Research. Int J Gynaecol Obstet. 2012;118(3):190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClure EM, Garces AL, Hibberd PL, Moore JL, Goudar SS, Saleem S, et al. The Global Network Maternal Newborn Health Registry: a multi-country, community-based registry of pregnancy outcomes. Reprod Health. 2020;17(Suppl 2):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Saleem S, Billah SKM, Kim J, Moore JL, Ghanchi NK, et al. COVID-19 antibody positivity over time and pregnancy outcomes in seven low-and-middle-income countries: A prospective, observational study of the Global Network for Women’s and Children’s Health Research. BJOG. 2022; 00: 1–11. DOI: 10.1111/1471-0528.17366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. COVID-19. Accessed at: https://www.who.int/health-topics/coronavirus#tab=tab_3 Last accessed on February 4, 2023
- 14.Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (Lond). 2020. Mar;20(2):124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alimohamadi Y, Sepandi M, Taghdir M, Hosamirudsari H. Determine the most common clinical symptoms in COVID-19 patients: a systematic review and meta-analysis. J Prev Med Hyg. 2020. Oct 6;61(3):E304–E312. doi: 10.15167/2421-4248/jpmh2020.61.3.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases, Centre for Disease Prevention and Control. Accessed at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/geographic-seroprevalence-surveys.html (Last accessed on Feb 2, 2023).
- 17.Khalil A, Hill R, Ladhani S, Pattisson K, O’Brien P. SARS-CoV-2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet Gynecol 0: 2020. doi: 10.1016/j.ajog.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Bhatla N, Sharma KA, Agarwal R, Verma A, Perumal V, et al. SCOPE: Surveillance of COVID-19 in pregnancy- results of a multicentric ambispective case-control study on clinical presentation and maternal outcomes in India between April to November 2020. PLoS One. 2023; 6; 18(3): e0272381. doi: 10.1371/journal.pone.0272381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020; 382: 2163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Toro F, Gjoka M, Di Lorenzo G, De Santo D, De Seta F, Maso G, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan DSA, Hamid LR, Ali A, Salam RA, Zuberi N, Lassi ZS, et al. Differences in pregnancy and perinatal outcomes among symptomatic versus asymptomatic COVID-19-infected pregnant women: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2021;21(1):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population-based cohort study BMJ 2020; 369: m2107. Accessed at 10.1136/bmj.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vouga M, Favre G, Martinez-Perez O, Pomar L, Acebal LF, Abascal-Saiz A, et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci Rep. 2021; 11(1):13898. doi: 10.1038/s41598-021-92357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirbeyk M, Saghazadeh A, Rezaei N. A systematic review of pregnant women with COVID-19 and their neonates. Archives of Gynecology and Obstetrics (2021) 304:5–38. Accessed at: 10.1007/s00404-021-06049-z [DOI] [PMC free article] [PubMed] [Google Scholar]


