Abstract
As the most common cause of sexually transmitted disease in women, chlamydial infections can lead to pelvic inflammatory disease, infertility, and ectopic pregnancy. To better understand the role played by sex hormones in modulating the immune response of the genital tract to microbial infections, we have developed a rat model to study Chlamydia trachomatis infection. Inbred female Lewis rats were primed with progesterone and inoculated by intrauterine instillation of C. trachomatis (mouse pneumonitis strain MoPn) into each uterine horn. When infected animals were examined for the presence of chlamydial antigens 14 days postinfection, both the uterus and vagina were found to be positive compared to those of saline-treated animals, which did not show specific staining. The involvement of local and systemic immune systems following chlamydial infection was determined by analyzing major histocompatibility complex (MHC) class II expression in the reproductive tract and lymphocyte proliferation in response to mitogenic and chlamydia-specific stimulation of cells from the spleen and lymph nodes (LN) draining the reproductive tract. Enhanced proliferation was observed in LN following mitogenic but not antigenic (MOMP [major outer membrane protein]) stimulation. In contrast, spleen cell proliferation was lower in chlamydia-infected rats than in saline-treated controls. MHC class II expression, an indicator of inflammatory responses, was upregulated in the uterus, on glandular epithelial cells, and adjacent to glands in response to chlamydial infection. In other experiments, when rats were infected at estrus and diestrus without prior progesterone priming, chlamydial inclusions were not detected in either the uterus or vagina. However, enhanced lymphocyte proliferation was observed in response to mitogenic and MOMP stimulation in the reproductive tract-draining LN from estrous and diestrous animals. These findings indicate that under appropriate endocrine conditions, the rat uterus is susceptible to C. trachomatis infection and that immune responses to this pathogen can be detected locally and systemically. Further, they suggest that clearance of the infection from the reproductive tract involves immune cells from the LN draining the reproductive tract.
Chlamydia trachomatis is an obligate intracellular gram-negative bacteria that is known to infect the genital tract and ocular epithelium. Disease caused by this organism can range from acute self-limiting infection to chronic inflammatory conditions, which can result in pelvic inflammatory disease, infertility, and ectopic pregnancy (12). Chlamydial infection of the urogenital tract is currently the leading cause of sexually transmitted disease in adolescents and women in the United States and Europe (11). Despite the recognition of chlamydial infection as a major public health concern, immune responses to human chlamydial infection are not well understood. Both T-cell-mediated and humoral responses are known to be involved in resolution of chlamydial infection. However, protective immunity developed as a result of chlamydial infection is transient, and reinfections are common (12).
Animal models to study chlamydial infections have been developed in the mouse, guinea pig, marmoset, and nonhuman primate (13, 15). In mice, for example, inoculations of chlamydiae via intravaginal and intrauterine routes are known to cause cervicitis or salpingitis, respectively (17, 18). In this species, intravaginal infection with human serovars of chlamydiae was dependent on pretreatment with progesterone (18). In the guinea pig, however, pretreatment with estradiol markedly enhanced the course of infection with guinea pig inclusion conjunctivitis (16). In all cases, endocrine balance at the time of exposure to chlamydiae appeared to play a crucial role for host susceptibility.
Previous work from our laboratory has demonstrated that sex hormones regulate the mucosal immune response in the female reproductive tract. Using the rat as a model system, we and others have shown that stage of the estrous cycle and treatment with sex hormones, specifically estradiol and progesterone, influence both the afferent and efferent arms of the immune system (for a review see reference 19). For example, antigen presentation, immunoglobulin A (IgA) transport, immune cell traffic into the reproductive tract, and cytokine production in the uterus and vagina have been shown to be under hormonal control. The aims of the present study were (i) to determine if infection could be successfully established in rats following intrauterine exposure to chlamydiae with or without progesterone treatment and (ii) to determine if immune responses occur locally and at sites distal to the reproductive tract in response to chlamydial infection.
MATERIALS AND METHODS
General methods and inoculation.
Adult female Lewis rats (Charles River Breeding Laboratories, Kingston, N.Y.) weighing 150 to 200 g were maintained under standard temperature-controlled conditions with 12-h intervals of light and dark. Progesterone was purchased from Calbiochem (La Jolla, Calif.), suspended in saline by glass-glass homogenization, and administered by subcutaneous injection. Stages of the estrous cycle were determined by daily vaginal smears. C. trachomatis (mouse pneumonitis nigg-II strain, MoPn) was purchased from the American Type Culture Collection (catalog no. VR-123). Females were administered 10 mg of progesterone 7 days before infection with 1 × 104 to 5 × 104 50% tissue culture infective doses (TCID50)/40 μl/uterus. A second 10-mg progesterone injection was given on the day of infection. Control animals received progesterone treatment followed by intrauterine instillation of sterile 0.15 M saline (40 μl). Animals at estrous and diestrous stages of the cycle received intrauterine inoculations of 1 × 104 to 5 × 104 TCID50 of MoPn. Control animals received saline instead of MoPn at the same stage of the estrous cycle. In all studies, animals were sacrificed 14 days postinfection. For intrauterine instillation of MoPn, rats were anesthetized, a midventral incision was made, uteri were exposed, and MoPn in saline or saline alone was injected with a 30-gauge needle into the uterus at the oviductal end.
Immunohistochemical analysis.
For immunohistochemical analysis, uteri and vaginas were excised and rinsed in cold saline (0.9%) prior to processing with acetone, methyl alcohol, and xylene as previously described (8). Briefly, 6- to 8-μm sections were cut with a microtome and placed on slides coated with 1.5% bovine serum albumin. Sections were deparaffinized in xylene, rehydrated, and washed in 0.01 M phosphate-buffered saline–bovine serum albumin (1 mg/ml). Nonspecific binding was blocked by incubating sections with 1% rabbit serum for 20 min at room temperature. To detect chlamydial infection, sections were stained with rabbit anti-C. trachomatis (Biodesign Int., Kennebunk, Maine) polyclonal antisera (1:200) for 60 min, rinsed, and incubated with goat anti-rabbit Ig (1:200 dilution) for an additional 30 min. Antiserum from normal rabbits was substituted for primary antibody at an equivalent concentration for control staining. Avidin-biotin coupled to alkaline phosphatase (ABC Elite kit; Vector Laboratories, Burlingame, Calif.) followed by Vector Red (alkaline phosphatase substrate kit; Vector Laboratories) was used to reveal antigen localization. To stain for major histocompatibility complex (MHC) class II antigens, a mouse monoclonal antibody to rat Ia (OX-6) was used as the primary antibody, and the second antibody was horse anti-mouse Ig coupled to biotin. Slides were counterstained with methyl green, dehydrated in alcohol, and mounted in Permount medium prior to microscopic examination.
LN and spleen cell proliferation.
To measure lymph node (LN) and spleen cell proliferation, para-aortic LN draining the genital tract and spleens were removed aseptically from animals. Single-cell suspensions were prepared by teasing with sterile forceps. Debris was allowed to settle for 2 min, and the supernatant containing single cells was spun down at 500 × g for 10 min. Spleen cells were treated with NH4Cl solution for 10 min to lyse the erythrocytes as previously described (14). Cells were washed three times with RPMI 1640 medium containing 10% fetal bovine serum and plated in 96-well chambers at a concentration of 5 × 105 cells per well. A final concentration of 1 μg of concanavalin A (ConA) per ml, 5 μg of phytohemagglutinin (PHA) per ml, 10 μg of lipopolysaccharide (LPS) per ml, or 1 and 5 μg of major outer membrane protein (MOMP) per ml was added to each well. Proliferative responses were measured by uptake of 1 μCi of [3H]thymidine per well for the last 24 h of a 3-day culture. Results are reported as mean counts per minute ± standard error of triplicate cultures. Each experiment was repeated at least two times. Data were analyzed by using Student’s t test.
RESULTS
Intrauterine chlamydial infection under the influence of progesterone.
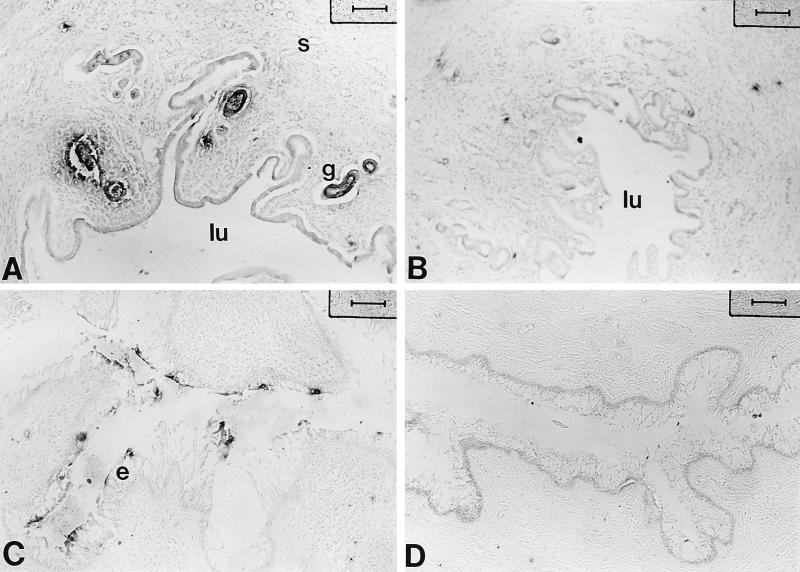
Chlamydial infection is initiated when the infectious form of C. trachomatis, the elementary body, attaches to the lining of the genital tract and invades the epithelial cells. Once internalized, the elementary body avoids phagolysosomal fusion and differentiates into the reticulate body, which multiplies by binary fission inside the endophagosome, forming a large inclusion body inside the epithelial cell. The presence of inclusion bodies is a characteristic feature of chlamydial infection. In this study, uteri and vaginas from progesterone-treated animals exposed to chlamydiae or saline were examined 14 days postinfection for the presence of chlamydial inclusions by immunohistochemistry, using a polyclonal rabbit antibody to chlamydial inclusions. As shown in Fig. 1A, positive staining with antichlamydia antibody was observed in the uteri of animals which had received an intrauterine inoculum of MoPn. Chlamydial inclusions were localized in glandular epithelial cells in the uterus. Saline-injected control animals did not show any positive staining (Fig. 1B). Among the total of 14 animals infected with chlamydiae in the uterus, infection was also detected in the vaginas of 5 animals. As seen in Fig. 1C, chlamydia-specific staining was observed in cervicovaginal epithelia of infected animals. Infection was confined to localized areas of the epithelium, as determined by positive staining for chlamydial antigen and not present throughout the epithelial lining of the cervicovaginal tissue. No specific staining was detected in saline controls (Fig. 1D). As a part of these studies, uteri and vaginal tissues stained with a monoclonal antibody specific for chlamydiae showed a staining pattern similar to that seen in Fig. 1 with polyclonal sera (data not shown).
FIG. 1.
Localization of chlamydial antigen by immunochemistry in uteri and vaginas of progesterone-treated rats inoculated with chlamydiae. Polyclonal antichlamydia antibody (1:200) was used to detect chlamydia-specific staining. Positive staining is seen in the epithelium of both uterus (A) and vagina (C). Controls shown are uterus (B) and vagina (D) from saline-treated animals stained with the same antibody. s, stroma; g, gland; lu, lumen; e, epithelium. Bars, 80 μm.
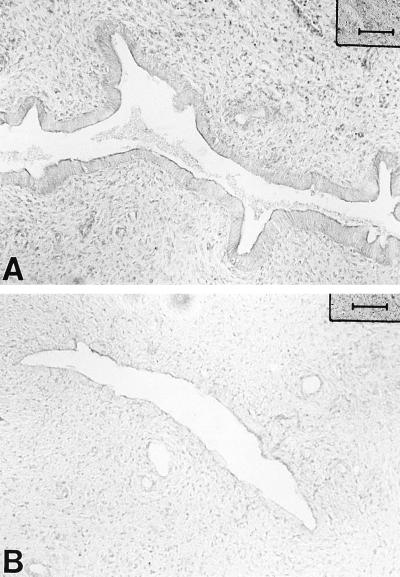
When immune markers for host inflammatory response were analyzed following chlamydial infection, uteri from chlamydia-infected animals showed enhanced MHC class II (Ia) expression compared to saline-treated animals, indicating that chlamydial infection increases the number of Ia-positive cells in uteri of infected rats. As seen in Fig. 2, Ia-positive staining was observed in the glandular epithelium, although a few positive cells were seen adjacent to the glands as well as interspersed in the stroma (Fig. 2A). In contrast, very few MHC class II-positive cells were localized in the uteri of control animals exposed to saline instead of chlamydia (Fig. 2B).
FIG. 2.
MHC class II-positive cells in uteri of progesterone-treated rats inoculated with chlamydiae (A) and saline (B). Class II expression is upregulated in the uteri of chlamydia-treated animals compared to saline-treated rats. lu, lumen. Bars, 80 μm.
LN and spleen responses of progesterone-treated infected rats to mitogens and MOMP.
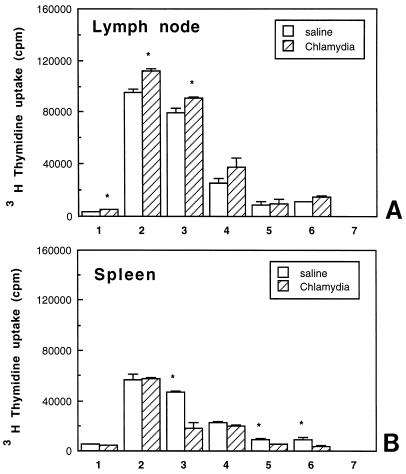
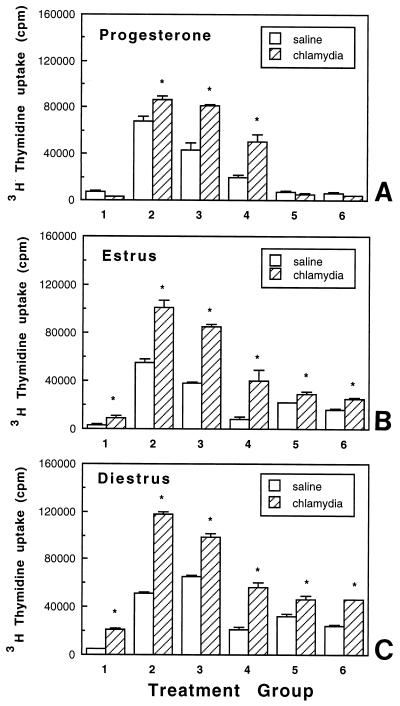
To examine whether immune responses to chlamydial infection in progesterone-treated animals extended beyond the reproductive tract, cells from the LN draining the reproductive tract (para-aortic LN) and spleen were cultured either alone or in the presence of known mitogens or MOMP (a chlamydia-specific antigen) to measure cell proliferation. As seen in Fig. 3A, LN cells from chlamydia-infected animals, in the absence of mitogenic stimulation, showed proliferation two to threefold higher than that seen in saline controls. In response to ConA and PHA, LN cells from chlamydia-infected rats had a significantly higher proliferative response than did LN cells from control animals. The chlamydia-specific response indicated by MOMP-induced proliferation was higher in progesterone-treated, chlamydia-infected animals than in saline controls. In contrast, spleen cell proliferation, in the absence of mitogens (control), was lower in infected animals than in saline controls (Fig. 3B). In response to mitogen (PHA) and MOMP, proliferation of spleen cells from chlamydia-infected animals was significantly lower than that seen in saline-treated animals (Fig. 3B) (the results are representative of four separate experiments).
FIG. 3.
LN (A) and spleen (B) cell proliferation on day 14 in response to mitogens and MOMP in progesterone-treated, control (saline-inoculated), and chlamydia-infected animals. Three to five animals were used for each treatment group. Results shown are representative of four separate experiments. Experimental groups consist of LN or spleen cells incubated in the presence of control (medium alone) (bar 1), ConA (bar 2), PHA (bar 3), LPS (bar 4), and MOMP at 1 (bar 5), 5 (bar 6), and 10 (bar 7) μg/ml. ∗, P < 0.05.
Intrauterine chlamydial infection in animals at normal stages of the estrous cycle.
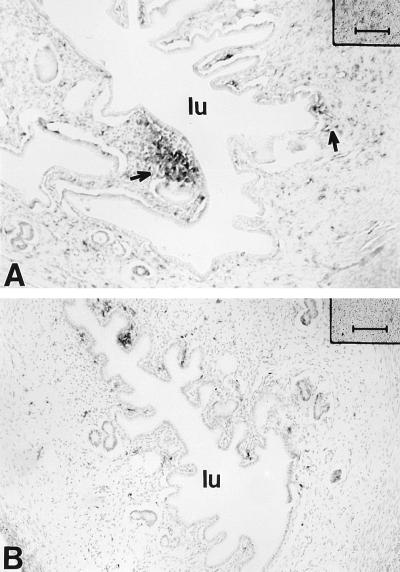
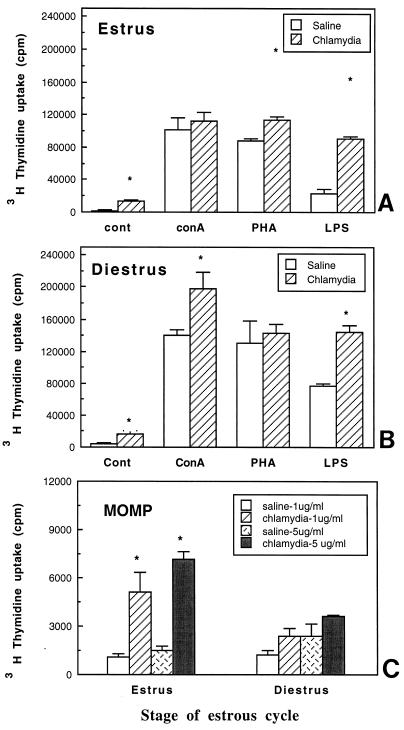
In an attempt to determine if progesterone pretreatment is essential for successful chlamydial infection, normal rats at specific stages of the estrous cycle were infected without prior progesterone treatment. Female rats were infected with 1 × 104 to 5 × 104 TCID50 of MoPn/uterine horn at either the estrous or diestrous stage of the reproductive cycle. Control animals at the same stage of the cycle received 40 μl of saline/uterus. Estrous and diestrous stages were selected because estradiol and progesterone, respectively, are known to be the predominant hormones in the circulation at these stages of the reproductive cycle. Animals were sacrificed 14 days postinfection, and uteri and vaginas were examined for chlamydial inclusions, using a polyclonal antichlamydia antibody. As seen in Fig. 4, no positive staining could be detected in the uteri of chlamydia-infected animals at either estrus or diestrus. Irrespective of whether animals were infected at estrus or diestrus, vaginas were also negative for any signs of infection (data not shown).
FIG. 4.
Localization of chlamydial antigen by immunochemistry in uteri of rats inoculated with chlamydiae at estrus (A) or diestrus (B). No positive staining could be detected in the uterus at either stage with a chlamydia-specific polyclonal antibody. Bars, 80 μm.
To examine whether lymph node proliferation was influenced by uterine chlamydia infection in rats infected at estrus or diestrus, para-aortic lymph nodes were cultured in the presence or absence of mitogens. As seen in Fig. 5, in the absence of mitogens, LN cells from chlamydia-infected animals at estrus and diestrus had levels of proliferation significantly higher (four- to sixfold) than that seen with LN cells from saline-treated animals. Shown in Fig. 5A and B are the responses of LN cells to mitogenic stimulation. Compared to estrous cycle-matched controls, LN cells from chlamydia-infected animals had significantly enhanced proliferation at estrus (PHA and LPS) and diestrus (ConA and LPS). When chlamydia-specific proliferation was examined following in vitro stimulation with MOMP (1 and 5 μg), significantly enhanced proliferation was seen in LN cells from rats infected with chlamydia at estrus compared to saline controls (Fig. 5C). LN from animals infected at diestrus also had higher proliferation compared to their saline controls, although this difference was not found to be statistically significant. These results indicate that intrauterine exposure of intact animals to chlamydiae results in enhanced LN proliferation in response to mitogens and chlamydia-specific antigen 14 days postinoculation under conditions in which no evidence of chlamydial infection was detected in the uterus or vagina.
FIG. 5.
(A and B) LN proliferation on day 14 in response to mitogens in animals treated with chlamydiae at estrus (A) and diestrus (B). (C) MOMP-specific proliferation in rats exposed to saline or chlamydiae at estrus or diestrus. Three to five animals were used for each treatment group. Results shown are representative of two separate experiments. ∗, P < 0.05.
Time course of immune response to chlamydial infection following intrauterine exposure.
In an attempt to understand the kinetics of infection and immune response to intrauterine chlamydia instillation, we performed a time course study where rats were infected with MoPn (i) at estrus, (ii) at diestrus, and (iii) following progesterone pretreatment and sacrificed 3, 7, or 14 days postinfection. Shown in Fig. 6 are LN proliferation responses at the earliest time point (day 3) at which LN proliferation was observed in all three experimental groups, indicating an early immune response to the presence of chlamydiae. LN proliferation on day 3 varied with hormonal balance. Enhanced mitogenic responses were seen with LN cells from all three experimental groups. Higher LN proliferation in response to MOMP was observed in animals infected at estrus and diestrus than in progesterone-pretreated animals. In the absence of any stimulation, LN from progesterone-pretreated animals had lower proliferation (Fig. 6A). On the other hand, significantly higher proliferation was seen in LN cells from animals infected at both estrus (twofold) and diestrus (fourfold) in the absence of any other stimulation (Fig. 6B and C). These results indicate that LN immune responses of intact rats (estrus and diestrus) are more pronounced in response to infection than those of progesterone-treated rats.
FIG. 6.
LN proliferation on day 3 in response to mitogens and MOMP in animals infected with chlamydiae following progesterone pretreatment (A), at estrus (B), and at diestrus (C). Three to five animals were used for each treatment group. Results shown are representative of two separate experiments. Experimental groups consist of LN cells incubated in the presence of control (medium alone) (bar 1), ConA (bar 2), PHA (bar 3), LPS (bar 4), and MOMP at 1 (bar 5) and 5 (bar 6) μg/ml. ∗, P < 0.05.
In other studies (not shown), infection, determined by the presence of inclusion bodies, was not observed in intact animals infected during the estrous cycle (estrus and diestrus) at any of the time points analyzed. In progesterone-pretreated animals, no infection was observed in the uterus on day 3, uteri of four of eight animals were found sporadically positive on day 7 around the glandular epithelium, and uteri of all animals were positive on day 14 (data not shown).
DISCUSSION
Collectively these data show that the rat can be used to study infectivity of the female reproductive tract and immune responses to C. trachomatis. Upon progesterone treatment followed by MoPn exposure, chlamydial inclusions were found in the epithelial cells of the uteri and vaginas of infected rats 14 days postinfection. This study indicates that under appropriate hormonal conditions, C. trachomatis is able to establish a prolonged, low-grade infection in the rat uterus which may descend into the vagina. When non-progesterone-treated intact rats were exposed to C. trachomatis at estrous and diestrous stages of the reproductive cycle, chlamydial inclusions were not detected in either the uterus or vagina 14 days after infection. One likely explanation for this is that in these animals, chlamydial infection was cleared within 14 days, indicating an acute, self-limiting infection. That the immune system responded to chlamydial challenge is supported by the observation that lymph node cells were still in a state of activation in animals infected at both estrus and diestrus, despite the absence of chlamydial inclusions in the reproductive tract tissue. Enhanced proliferation of LN cells from animals infected at estrus and diestrus was seen, both in response to nonspecific mitogenic stimulation and following in vitro challenge with the chlamydia-specific antigen MOMP. These results indicate that the infected animals exhibited a specific immune response to chlamydial infections.
A comparison of proliferative responses on day 3 following infection of rats pretreated with progesterone as well as those infected at estrus and diestrus indicated that immune responses to chlamydia vary with endocrine balance at the time of infection. Progesterone-treated animals did not show any chlamydia-specific responses and modestly enhanced mitogenic stimulation. In contrast, animals infected at estrus and diestrus, without prior progesterone treatment, had dramatically enhanced LN cell proliferation responses in the absence of any stimulation, following mitogenic stimulation, and in response to chlamydia-specific antigenic challenge. These results indicate that early immune responses may play an important role in the clearance of infection in this animal model.
Unlike exposure to other intracellular pathogens such as Mycobacteria avium and Toxoplasma gondii (5, 20), exposure to C. trachomatis does not appear to suppress the immune system in these studies. This was indicated by the enhanced LN cell proliferation observed in progesterone-treated rats following infection, as well as the four- to sixfold-higher proliferation observed in the LN of intact rats exposed to chlamydiae at estrus and diestrus. Unexpectedly, we found that the mitogenic response of spleen cells was suppressed in MoPn-infected animals coincident with enhanced proliferation in local draining LN. In other studies, we have found that gamma interferon placed in the uteri of rats increased spleen cell mitogenic responses (14). Also in response to gamma interferon deposited in the uterus, there was an infiltration of polymorphonuclear cells and intraepithelial lymphocytes into the uterine tissue. When considered in context with the present studies, these findings suggest that immune cells may migrate from the spleen into the local LN in response to the infection in the reproductive tract. Whether chlamydial infection leads to enhanced leukocytic traffic into the reproductive tract directly from the spleen or via the LN remains to be determined.
As a part of this study, we observed a marked upregulation of MHC class II expression in the uteri of infected animals compared to that seen in saline controls, indicating a host inflammatory response to chlamydial infection. In other studies, expression of MHC class II antigens has been observed in the rat reproductive tract during the estrous cycle (6, 7, 9). Levels of class II antigens were decreased in the uterus at diestrus and following administration of progesterone along with estradiol in ovariectomized animals. The results of this study suggest that chlamydial infection overrides the effect of progesterone in the uteri of infected animals. This may have important implications in light of a recent hypothesis that chlamydiae can lead to chronic inflammation in the uterus, which may lead to embryo loss at the time of implantation (11). Chronic elevation of class II antigen expression following chlamydial infection may cause recurrent abortions (1, 4). This may be another possible cause of infertility in women suffering from chronic chlamydial infection beyond the known pathologic sequelae including cervicitis and salpingitis.
Others have shown that sex hormones and their analogs influence susceptibility to a variety of infections in the female reproductive tract. For example, the incidence of vaginal candidiasis increases with the use of oral contraceptives (3). In animal models, progesterone increases the mortality rate of mice infected intravaginally with herpes simplex virus type 2 (2). In contrast, mice are more susceptible to genital infection with Neisseria gonorrhoeae at proestrus, when estrogen levels are highest (10). Similarly, estradiol treatment prior to chlamydial infection increases susceptibility to infection in female guinea pigs (16). In contrast, others have shown that mice are readily infected only after treatment with progesterone (18). Our studies indicate that progesterone pretreatment is required to establish persistent uterine infection in rats when the reproductive tract is exposed to low doses of chlamydiae. Why progesterone pretreatment increases susceptibility to local infection needs to be investigated. One possibility is that progesterone treatment interrupts the normal reproductive cyclicity, which may enhance the attachment of chlamydiae and establishment of infection in uterine epithelial cells. It is also possible that progesterone causes immunosuppression, decreasing innate immunity to chlamydial infection. However, normal mitogenic responses of spleen and LN cells were observed in progesterone-treated females exposed to MoPn or saline. Previous studies by us and others have shown that progesterone may reverse immunity-enhancing effects of estradiol in the reproductive tract, on polymeric immunoglobulin receptor levels, on antigen presentation, and on the number of immune cells present in the uterus (19).
In conclusion, in addition to identifying a new animal model, our studies indicate that endocrine balance influences local immune responses which may result in either enhanced or compromised immune protection. The rat uterus may be a particularly useful model for defining immune responses to reproductive tract infections in humans because of the close functional similarities between the reproductive tracts of the two species. Future studies with this rat model should provide insight into the role of ovarian hormones in regulating the immune response to chlamydial infections in the genital tract.
ACKNOWLEDGMENTS
We thank Richard Rossoll for excellent technical assistance and Christopher Wira for assistance with immunohistochemistry.
Photomicroscopy was done in the Herbert C. Englert Cell Analysis Laboratory, which is supported in part by a core grant from Norris Cotton Cancer Center (CA 23108) and by equipment grants from the Fannie E. Rippel Foundation. This work was supported by research grants AI-13541 from NIH and CA-23108 from NCI.
REFERENCES
- 1.Athanassakis I, Grigoriou M, Galanopoulos V, Papamatheakis J. Induction of class II MHC antigens expression on murine placenta by 5-AzaC correlates with fetal abortion. Cell Immunol. 1990;128:438–449. doi: 10.1016/0008-8749(90)90039-t. [DOI] [PubMed] [Google Scholar]
- 2.Baker D A, Plotkin S A. Enhancement of vaginal infection in mice by herpes simplex virus type Ii with progesterone. Proc Soc Exp Biol Med. 1978;158:131–134. doi: 10.3181/00379727-158-40156. [DOI] [PubMed] [Google Scholar]
- 3.Catterall R D. Influence of gestogenic contraceptive pills on vaginal candidiasis. Br J Vener Dis. 1971;47:45–47. doi: 10.1136/sti.47.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crainie M, Semeluk A, Lee K C, Wegmann T G. Regulation of constitutive and lymphokine-induced Ia expression by murine alpha-fetoprotein. Cell Immunol. 1989;118:41–52. doi: 10.1016/0008-8749(89)90356-0. [DOI] [PubMed] [Google Scholar]
- 5.Haque S, Khan I, Haque A, Kasper L. Impairment of cellular immune response in acute murine toxoplasmosis: regulation of interleukin-2 production and macrophage-mediated inhibitory effects. Infect Immun. 1994;62:2908–2916. doi: 10.1128/iai.62.7.2908-2916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Head J R, Gaede S D. Ia antigen expression in the rat uterus. J Reprod Immunol. 1986;9:137–153. doi: 10.1016/0165-0378(86)90007-0. [DOI] [PubMed] [Google Scholar]
- 7.Kaushic, C., E. Frauendorf, R. M. Rossoll, J. M. Richardson, and C. R. Wira. Influence of estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am. J. Reprod. Biol., in press. [DOI] [PubMed]
- 8.Kaushic C, Richardson J M, Wira C R. Regulation of polymeric immunoglobulin A receptor messenger ribonucleic acid expression in rodent uteri: effect of sex hormones. Endocrinology. 1995;136:2836–2844. doi: 10.1210/endo.136.7.7789308. [DOI] [PubMed] [Google Scholar]
- 9.Kaushic C, Wira C. Effect of sex hormones on immune cells and cytokines in the female reproductive tract. Clin Immunol Pathol. 1995;76:S57. [Google Scholar]
- 10.Kita E, Matsuura H, Kashiba S. A mouse model for the study of gonococcal genital infection. J Infect Dis. 1981;143:67–70. doi: 10.1093/infdis/143.1.67. [DOI] [PubMed] [Google Scholar]
- 11.Morell V. Attacking the causes of “silent” infertility. Science. 1995;269:775–777. doi: 10.1126/science.7638588. [DOI] [PubMed] [Google Scholar]
- 12.Morrison R P, Manning D S, Caldwell H D. Immunology of chlamydia trachomatis infections. In: Quinn T C, editor. Sexually transmitted diseases. New York, N.Y: Raven Press, Ltd.; 1992. pp. 57–84. [Google Scholar]
- 13.Patton D L, Rank R G. Animal model for study of pelvic inflammatory disease. In: Quinn T C, editor. Sexually transmitted diseases. New York, N.Y: Raven Press, Ltd.; 1992. pp. 85–111. [Google Scholar]
- 14.Prabhala R H, Wira C R. Cytokine regulation of the mucosal immune system: in vivo stimulation by interferon-γ of secretory component and immunoglobulin A in uterine secretions and proliferation of lymphocytes from spleen. Endocrinology. 1991;129:2915–2923. doi: 10.1210/endo-129-6-2915. [DOI] [PubMed] [Google Scholar]
- 15.Rank R G. Animal models for urogenital infections. Methods Enzymol. 1994;235:83–93. doi: 10.1016/0076-6879(94)35133-3. [DOI] [PubMed] [Google Scholar]
- 16.Rank R G, White H J, Hough A J, Jr, Pasley J N, Barron A L. Effect of estradiol on chlamydial genital infection of female guinea pigs. Infect Immun. 1982;38:699–705. doi: 10.1128/iai.38.2.699-705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swenson C E, Donegan E, Schachter J. Chlamydia trachomatis-induced salpingitis in the mouse. J Infect Dis. 1983;148:1101–1107. doi: 10.1093/infdis/148.6.1101. [DOI] [PubMed] [Google Scholar]
- 18.Tuffrey M, Falder P, Taylor-Robinson D. Genital-tract infection and disease in nude and immunologically competent mice after inoculation of a human strain of chlamydia trachomatis. Br J Exp Pathol. 1982;63:539–546. [PMC free article] [PubMed] [Google Scholar]
- 19.Wira C R, Kaushic C. Mucosal immunity in the female reproductive tract: effect of sex hormones on immune recognition and responses. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines: new trends in immunization. New York, N.Y: Academic Press; 1996. pp. 375–388. [Google Scholar]
- 20.Zychlinsly A, Karim M, Nonacs R, Young D-E J. A homogenous population of lymphokine-activated killer (LAK) cells is incapable of killing virus-, bacteria- or parasite-infected macrophages. Cell Immunol. 1990;125:261–267. doi: 10.1016/0008-8749(90)90080-b. [DOI] [PubMed] [Google Scholar]