Key Points
Question
What is the long-term risk of disabilities among individuals diagnosed with bacterial meningitis in childhood?
Findings
In a cohort study of 36 230 participants with up to 35 years of follow-up, those diagnosed with bacterial meningitis in childhood between 1987 and 2021 had a significantly higher risk of developing 7 neurological disabilities (cognitive disabilities, seizures, hearing loss, motor function disorders, visual disturbances, behavioral and emotional disorders, and intracranial structural injuries) relative to general population controls matched on age, sex, and place of residence.
Meaning
These findings suggest that individuals in Sweden diagnosed with bacterial meningitis in childhood have an increased risk of long-term disabilities.
This cohort study estimates the risk of disabilities among individuals in Sweden who were diagnosed with bacterial meningitis during childhood compared with matched controls from the general population.
Abstract
Importance
Few studies have examined the incidence of long-term disabilities due to bacterial meningitis in childhood with extended follow-up time and a nationwide cohort.
Objective
To describe the long-term risks of disabilities following a childhood diagnosis of bacterial meningitis in Sweden.
Design, Setting, and Participants
This nationwide retrospective registry-based cohort study included individuals diagnosed with bacterial meningitis (younger than 18 years) and general population controls matched (1:9) by age, sex, and place of residence. Data were retrieved from the Swedish National Patient Register from January 1, 1987, to December 31, 2021. Data were analyzed from July 13, 2022, to November 30, 2023.
Exposure
A diagnosis of bacterial meningitis in childhood recorded in the National Patient Register between 1987 and 2021.
Main Outcomes and Measures
Cumulative incidence of 7 disabilities (cognitive disabilities, seizures, hearing loss, motor function disorders, visual disturbances, behavioral and emotional disorders, and intracranial structural injuries) after bacterial meningitis in childhood.
Results
The cohort included 3623 individuals diagnosed with bacterial meningitis during childhood and 32 607 controls from the general population (median age at diagnosis, 1.5 [IQR, 0.4-6.2] years; 44.2% female and 55.8% male, median follow-up time, 23.7 [IQR, 12.2-30.4] years). Individuals diagnosed with bacterial meningitis had higher cumulative incidence of all 7 disabilities, and 1052 (29.0%) had at least 1 disability. The highest absolute risk of disabilities was found for behavioral and emotional disorders, hearing loss, and visual disturbances. The estimated adjusted hazard ratios (HRs) showed a significant increased relative risk for cases compared with controls for all 7 disabilities, with the largest adjusted HRs for intracranial structural injuries (26.04 [95% CI, 15.50-43.74]), hearing loss (7.90 [95% CI, 6.68-9.33]), and motor function disorders (4.65 [95% CI, 3.72-5.80]). The adjusted HRs for cognitive disabilities, seizures, hearing loss, and motor function disorders were significantly higher for Streptococcus pneumoniae infection (eg, 7.89 [95% CI, 5.18-12.02] for seizure) compared with Haemophilus influenzae infection (2.46 [95% CI, 1.63-3.70]) or Neisseria meningitidis infection (1.38 [95% CI, 0.65-2.93]). The adjusted HRs for cognitive disabilities, seizures, behavioral and emotional disorders, and intracranial structural injuries were significantly higher for children diagnosed with bacterial meningitis at an age below the median.
Conclusions and Relevance
The findings of this cohort study of individuals diagnosed with bacterial meningitis during childhood suggest that exposed individuals may have had an increased risk for long-term disabilities (particularly when diagnosed with pneumococcal meningitis or when diagnosed at a young age), highlighting the need to detect disabilities among surviving children.
Introduction
Bacterial meningitis is an inflammation of the fluid and membranes surrounding the brain and spinal cord due to a bacterial infection in the central nervous system. Despite the availability of antibiotic therapy, bacterial meningitis in children is associated with high mortality and a high risk of long-term disabilities such as seizure disorders, focal neurological deficits, hearing loss, and impaired cognitive functioning.1,2 The limited ability of antibiotics to cross the blood-brain barrier and the clinical problem of antibiotic resistance contribute to these risks.3 Among survivors, the occurrence of permanent disabilities has been reported to range from 20% to 50%.1,4,5,6
Studies investigating risks of long-term disabilities after childhood bacterial meningitis are limited.5,7,8,9,10 Existing studies tend to focus on well-known disabilities (hearing loss) and may therefore miss or underestimate risks of less visible disabilities such as behavioral disorders or psychiatric disease.2,5,11 In addition, most existing studies do not include a comparison group, making it difficult to determine the actual risk increase due to bacterial meningitis. The objective of this study was to estimate the risk of disabilities among individuals diagnosed with bacterial meningitis in childhood in Sweden compared with matched controls from the general population, by type of disability, by 3 major causes of bacterial meningitis (Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae), and by age at diagnosis, with up to 35 years of follow-up.
Methods
This nationwide matched cohort study includes individuals diagnosed with childhood bacterial meningitis in Sweden from January 1, 1987, to December 31, 2021, and their matched controls. Using the Swedish unique national registration number assigned to each resident, data from the National Patient Register12 were linked to data from the Total Population Register, the Medical Birth Register, the Cancer Register, the Multigeneration Register, and the Longitudinal Integration Database for Health Insurance and Labor Market studies. The study was approved by the regional ethics review board in Stockholm, Sweden; the requirement for informed consent was waived owing to the use of deidentified data. This cohort study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Inclusion and Exclusion Criteria
The study population consisted of individuals who were born in Sweden, had a diagnosis of bacterial meningitis (International Classification of Diseases, Ninth Revision [ICD-9], codes 036A and 320 and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10], codes G00 and A390) in the National Patient Register from 1987 to 2021 (nationwide inpatient data from 1987; hospital-based outpatient data from 2001), were younger than 18 years at diagnosis (index date), lived at least 1 day after the index date, and had no infection of the central nervous system (ICD-9 and ICD-10 codes in eTable 1 in Supplement 1) recorded in the National Patient Register before the index date. To each case we matched 9 controls who were born in Sweden, were the same sex as the case, lived in the same geographic area (parish, municipality, or county) at the index date, were born in the same calendar year, were younger than 18 years at the index date, lived at least 1 day after the index date, and had no diagnosis for bacterial meningitis or infection of the central nervous system recorded in the National Patient Register before the index date (eFigure 1 in Supplement 1).
Outcomes
Outcome data were retrieved from the National Patient Register. Individuals were followed up from the index date until death, emigration, or end of follow-up (a maximum of 35 years; completed on December 31, 2021). Matched controls who were diagnosed with bacterial meningitis after the index date were censored at the diagnosis date.
In the primary analysis, we examined the risk of postmeningitis functional disabilities in 6 categories (cognitive disabilities, seizures, hearing loss, motor function disorders, visual disturbances, and behavioral and emotional disorders), and 1 type of postmeningitis complication (intracranial structural injuries), all adapted from a review article (ICD-9 and ICD-10 codes in eTable 2 in Supplement 1).1 For brevity, we refer to these outcomes (functional disabilities and complications) as disabilities. The risks of postmeningitis disabilities were further stratified by 3 major causes of bacterial meningitis (S pneumoniae, N meningitidis, and H influenzae) and by age at diagnosis (below or above the median age). In the secondary analysis, we examined the number of disabilities across 7 categories (a minimum of 0 and a maximum of 7 disabilities). A patient with cognitive disabilities and motor function disorders, for example, was treated as having 2 disabilities.
Covariates
Data on age and sex were collected from the Total Population Register. Data on biological parents’ educational attainment (missing, primary school, high school, or university) were retrieved from the Multigeneration Register (parenthood) and the Longitudinal Integration Database for Health Insurance and Labor Market studies (educational attainment). Data on birth characteristics were obtained from the Medical Birth Register (5-minute Apgar score [missing, 0-6, 7, 8, 9, or 10], birth weight [missing or 1st to 10th decile], and head circumference [missing or 1st to 4th quartile]). Data on 7 preexisting conditions (malignant neoplasms, perinatal complications, mental and behavioral disorders, diseases of the nervous system, congenital disabilities, traumatic injuries, and operations in the nervous system),9 selected as potential risk factors for bacterial meningitis and postmeningitis disabilities, were retrieved from the National Patient Register and the Cancer Register (eTables 3 and 4 in Supplement 1). Data on index year were coded in 7 categories (1987-1991, 1992-1996, 1997-2001, 2002-2006, 2007-2011, 2012-2016, and 2017-2021). All variables were retrieved from national registers, resulting in little missing data (0.6% for mother’s educational level, 1.7% for father’s educational level, 4.3% for Apgar score, 1.8% for birth weight, and 4.7% for head circumference). All observations were used in the statistical analysis.
Statistical Analysis
Data were analyzed from July 13, 2022, to November 30, 2023. In the primary analysis of disabilities, we described the risk of events using estimated Kaplan-Meier cumulative incidence curves. Hazard ratios (HRs) for disabilities, comparing cases and controls, were estimated using Cox proportional hazards regression models, adjusting for age at the index date, sex, maternal and paternal educational level, birth characteristics (5-minute Apgar score, birth weight, and head circumference), 7 dummy variables for each of the preexisting conditions, and the index year. As a first sensitivity analysis, we estimated the relative risk of postmeningitis disabilities among cases and controls with no preexisting conditions. As a second sensitivity analysis, we rematched cases and controls 1:1 on year of diagnosis and preexisting conditions (exact match), sex, age at diagnosis, parents’ educational attainment, and birth characteristics (propensity score match) and estimated the risk of events in this alternative matched cohort.
We performed subgroup analyses for the 3 major causes of bacterial meningitis (S pneumoniae, N meningitidis, and H influenzae), estimating the HRs for disabilities (cases vs controls) by type of infection (eTable 5 in Supplement 1). Tests of pairwise differences between types of infections were conducted with interaction terms in the Cox proportional hazards regression for bacterial meningitis status and type of infection. Due to few events with intracranial structural injuries among the controls, the subgroup analysis by type of infection could not be performed for this event. As a sensitivity analysis, we investigated differences between types of infections using cases and controls diagnosed during the first half of our study period (before 2005), ensuring at least 17 years of (potential) follow-up regardless of bacterial pathogen.
We also performed subgroup analyses by age at diagnosis, estimating the HRs for disabilities among cases and controls below or above the median age at diagnosis conditional on cause of bacterial meningitis (ICD-9 and ICD-10 codes in eTable 5 in Supplement 1). Conditioning on type of infection reduces confounding due to variations in the age when different infections occur. Tests of pairwise differences between age groups were conducted with an interaction term in the Cox proportional hazards regression for bacterial meningitis status and age at diagnosis.
Hazard ratios for disabilities were estimated for the whole follow-up period (constant risk coefficients) and for different periods using time-varying risk coefficients for 0 to 1, more than 1 to 3, more than 3 to 5, and more than 5 years after the index date. The proportional hazards assumption was tested with an interaction term between follow-up time and bacterial meningitis status.
In the secondary analysis, we computed the mean number of postmeningitis disabilities and the distribution of the number of disabilities among cases and controls. The incidence rate ratio of number of disabilities, comparing cases and controls, was estimated using negative binomial regression with the same adjustment as in the primary analysis and an offset for observation time.
All analyses were performed using SAS, version 9.4 (SAS Institute Inc), and Stata, version 17.0 (StataCorp LLC). Statistical significance was defined as a 2-sided P < .05.
Results
Study Population
The study population comprised 36 230 participants (20 200 male [55.8%] and 16 030 female [44.2%]). The matched cohort included 3623 individuals diagnosed with bacterial meningitis (cases) (median age at diagnosis, 1.5 [IQR, 0.4-6.2] years; 1451 [40.0%] younger than 1 year; 2020 male [55.8%] and 1603 female [44.2%]) and 32 607 general population controls (12 435 [38.1%] younger than 1 year; 18 180 male [55.8%] and 14 427 female [44.2%]) (Table 1). Individuals with bacterial meningitis had similar birth characteristics as the controls, and parents of cases and controls had similar educational attainment, but children with bacterial meningitis had more preexisting conditions. The median follow-up time overall was 23.7 (IQR, 12.2-30.4) years, with 23.1 (IQR, 10.9-30.3) years for cases and 23.8 (IQR, 12.4-30.4) years for controls (eTable 6 in Supplement 1). During follow-up, 150 cases (4.1%) and 209 controls (0.6%) died, whereas 183 cases (5.1%) and 2211 controls (6.8%) emigrated.
Table 1. Participant Characteristics.
| Characteristic | Participant groupa | |
|---|---|---|
| Bacterial meningitis (n = 3623) | Controls (n = 32 607) | |
| Sex | ||
| Female | 1603 (44.2) | 14 427 (44.2) |
| Male | 2020 (55.8) | 18 180 (55.8) |
| Age at diagnosis of bacterial meningitis, y | ||
| Mean (SD) | 4.1 (5.1) | 4.1 (5.1) |
| Median (IQR) | 1.5 (0.4-6.2) | 1.5 (0.5-6.2) |
| Age group | ||
| 0 | 1451 (40.0) | 12 435 (38.1) |
| 1 | 595 (16.4) | 5697 (17.5) |
| 2-4 | 548 (15.1) | 5204 (16.0) |
| 5-17 | 1029 (28.4) | 9271 (28.4) |
| Educational attainment of mother | ||
| Primary school | 425 (11.7) | 3572 (11.0) |
| High school | 1639 (45.2) | 14 800 (45.4) |
| University | 1537 (42.4) | 14 044 (43.1) |
| Missing | 22 (0.6) | 191 (0.6) |
| Educational attainment of father | ||
| Primary school | 627 (17.3) | 5515 (16.9) |
| High school | 1750 (48.3) | 15 722 (48.2) |
| University | 1185 (32.7) | 10 811 (33.2) |
| Missing | 61 (1.7) | 559 (1.7) |
| Birth characteristics | ||
| 5-min Apgar score, mean (SD) | 9.6 (1.0) | 9.7 (0.7) |
| Birth weight, mean (SD), g | 3430 (716) | 3515 (573) |
| Head circumference, mean (SD), cm | 34.5 (2.2) | 34.8 (1.7) |
| Preexisting conditions | ||
| Malignant neoplasms | 81 (2.2) | 19 (0.1) |
| Perinatal complications | 418 (11.5) | 2264 (6.9) |
| Mental and behavioral disorders | 70 (1.9) | 363 (1.1) |
| Diseases of the nervous system | 296 (8.2) | 564 (1.7) |
| Congenital disabilities | 254 (7.0) | 1073 (3.3) |
| Traumatic injuries | 70 (1.9) | 358 (1.1) |
| Operation in nervous system | 395 (10.9) | 1265 (3.9) |
Unless otherwise indicated, data are expressed as No. (%) of participants. Percentages have been rounded and may not total 100.
Primary Outcome
Individuals with bacterial meningitis had a higher cumulative incidence of all disabilities compared with the general population controls (Figure). The highest absolute risk of disabilities (rate of events or cumulative incidence) (Table 2 and eTable 7 in Supplement 1) was found for behavioral and emotional disorders, hearing loss, and visual disturbances.
Figure. Cumulative Incidence of Disabilities by Type of Disability .
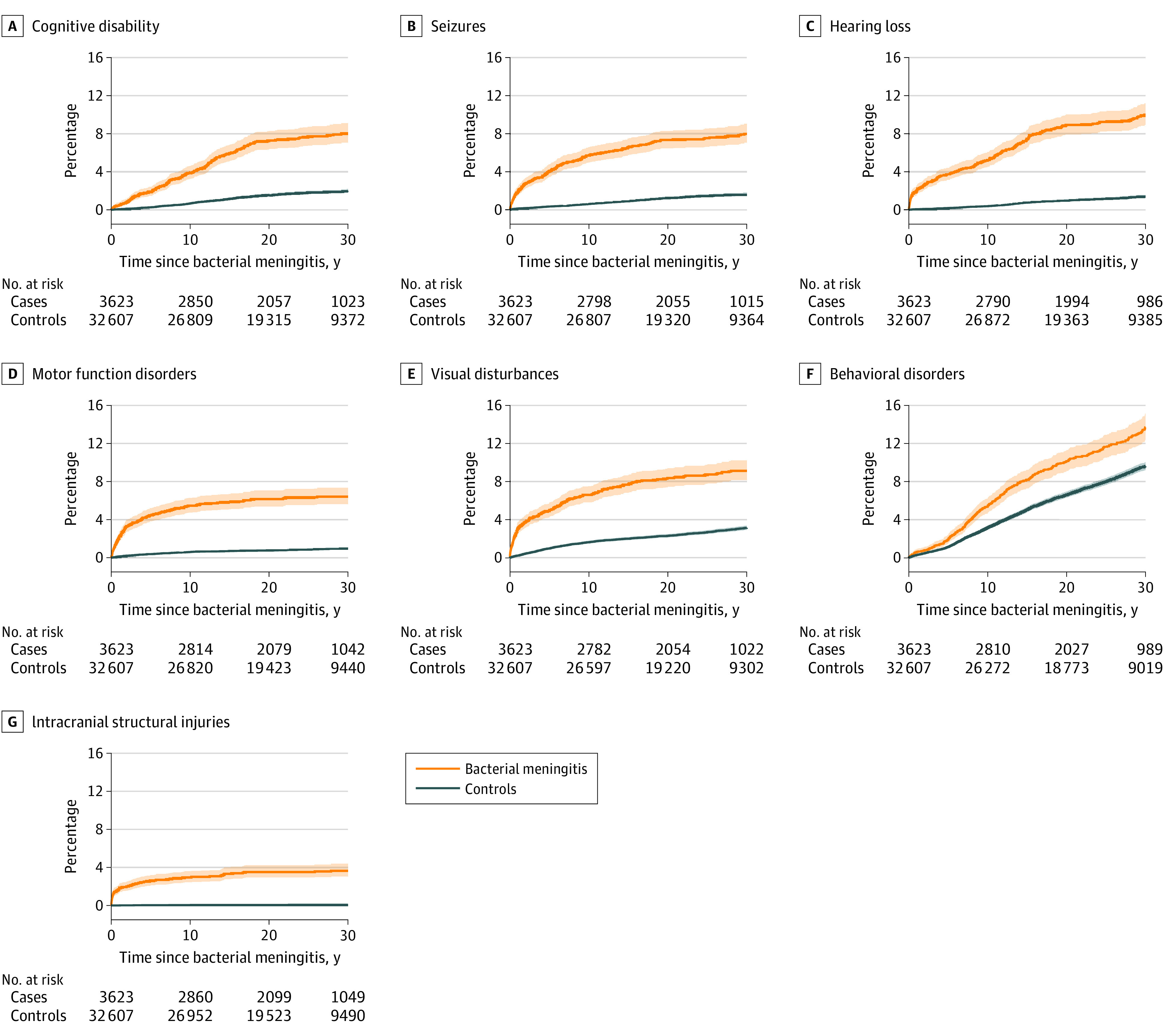
Shading represents 95% CIs.
Table 2. Risk of Disabilities.
| Disability | Participant group events, No. | Participant group rate per 100 000 person-years | Adjusted HR (95% CI)a | Participant group 30-y cumulative hazard | |||
|---|---|---|---|---|---|---|---|
| Bacterial meningitis (n = 3623) | Controls (n = 32 607) | Bacterial meningitis (n = 3623) | Controls (n = 32 607) | Bacterial meningitis (n = 3623) | Controls (n = 32 607) | ||
| Cognitive disability | 233 | 486 | 307.7 | 68.8 | 3.23 (2.71-3.85) | 8.01 | 1.99 |
| Seizures | 249 | 398 | 332.1 | 56.4 | 4.14 (3.46-4.96) | 8.00 | 1.62 |
| Hearing loss | 294 | 331 | 397.6 | 46.8 | 7.90 (6.68-9.33) | 10.00 | 1.40 |
| Motor function disorders | 214 | 257 | 283.5 | 36.3 | 4.65 (3.72-5.80) | 6.42 | 0.95 |
| Visual disturbances | 292 | 819 | 391.0 | 116.7 | 2.37 (2.04-2.74) | 9.12 | 3.15 |
| Behavioral and emotional disorders | 370 | 2315 | 494.2 | 334.6 | 1.42 (1.26-1.59) | 13.70 | 9.61 |
| Intracranial structural injury | 120 | 22 | 157.2 | 3.1 | 26.04 (15.50-43.74) | 3.66 | 0.08 |
Adjusted for age at the index date, the index year, sex, maternal and paternal educational attainment, birth characteristics (5-minute Apgar score, birth weight, head circumference), and 7 preexisting conditions (malignant neoplasms, perinatal complications, mental and behavioral disorders, diseases of the nervous system, congenital disabilities, traumatic injuries, and operations in the nervous system). P < .001 for all comparisons.
The estimated HRs for disabilities showed a significantly elevated risk for cases relative to general population controls for all disabilities (Table 2). The estimated adjusted HRs were 3.23 (95% CI, 2.71-3.85) for cognitive disabilities, 4.14 (95% CI, 3.46-4.96) for seizures, 7.90 (95% CI, 6.68-9.33) for hearing loss, 4.65 (95% CI, 3.72-5.80) for motor function disorders, 2.37 (95% CI, 2.04-2.74) for visual disturbances, 1.42 (95% CI, 1.26-1.59) for behavioral and emotional disorders, and 26.04 (95% CI, 15.50-43.74) for intracranial structural injuries, implying the largest relative risk for intracranial structural injuries, hearing loss, and motor function disorders.
Removing cases (912 [25.2%]) and controls with preexisting conditions resulted in a reduced sample with 2711 cases (74.8%) and 27 959 controls (85.7%). Confining the analysis to this subgroup had little effect on the estimated adjusted HRs for disabilities (eTable 8 in Supplement 1). The alternative 1:1 match of cases and controls on preexisting conditions and other covariates resulted in a smaller cohort with 3228 cases and controls (eTable 9 in Supplement 1), and similar estimated adjusted HRs for disabilities (eTable 8 in Supplement 1).
The classification by type of bacterial infection resulted in 888 cases of bacterial meningitis (24.5%) due to H influenzae (median year of diagnosis, 1990), 571 (15.8%) due to S pneumoniae (median year of diagnosis, 2000), 497 (13.7%) due to N meningitidis (median year of diagnosis, 1997), and the remainder (1667 [46.0%]) due to other specified bacteria (423 [11.7%]) or undetermined bacteria (1244 [34.3%]) (eTables 10 and 11 in Supplement 1). The adjusted HRs for cognitive disabilities, seizures, hearing loss, and motor function disorders were significantly higher for S pneumoniae infection (eg, 7.89 [95% CI, 5.18-12.02] for seizure) compared with H influenzae (eg, 2.46 [95% CI, 1.63-3.70] for seizure) and N meningitidis (eg, 1.38 [95% CI, 0.65-2.93] for seizure) infections (Table 3 and eTable 12 and eFigure 2 in Supplement 1). Apart from cognitive disabilities, there was no significant difference in the adjusted HRs for disabilities for H influenzae compared with N meningitidis infection (adjusted HR for cognitive disability, 2.25 [95% CI, 1.50-3.39] vs 1.00 [95% CI, 0.45-2.21]). Results were similar using only cases and controls diagnosed during the first half of the study period (eFigure 3 in Supplement 1).
Table 3. Adjusted HR for Disabilities by Type of Bacterial Pathogen.
| Disability | Causative pathogen, HR (95% CI)a | ||
|---|---|---|---|
| Haemophilus influenzae | Streptococcus pneumoniae | Neisseria meningitidis | |
| Cognitive disability | 2.25 (1.50-3.39) | 5.47 (3.76-7.95) | 1.00 (0.45-2.21) |
| Seizures | 2.46 (1.63-3.70) | 7.89 (5.18-12.02) | 1.38 (0.65-2.93) |
| Hearing loss | 9.08 (6.59-12.51) | 27.13 (18.87-39.00) | 4.75 (2.84-7.93) |
| Motor function disorders | 2.37 (1.39-4.05) | 7.85 (4.51-13.64) | 1.09 (0.32-3.67) |
| Visual disturbances | 1.31 (0.87-1.96) | 2.30 (1.64-3.23) | 1.83 (1.11-3.01) |
| Behavioral and emotional disorders | 1.07 (0.81-1.40) | 1.23 (0.93-1.63) | 1.58 (1.17-2.12) |
Adjusted for age at the index date, the index year, sex, parental educational attainment, birth characteristics (5-minute Apgar score, birth weight, head circumference), and 7 preexisting conditions (malignant neoplasms, perinatal complications, mental and behavioral disorders, diseases of the nervous system, congenital disabilities, traumatic injuries, and operations in the nervous system).
In the subgroup analysis by age at diagnosis, the adjusted HRs (comparing cases and controls) for cognitive disabilities, seizures, behavioral and emotional disorders, and intracranial structural injuries were significantly higher for children diagnosed with bacterial meningitis at a young age (below median age conditional on cause of bacterial meningitis) (eTable 13 in Supplement 1). For example, the adjusted HR for seizures was 5.43 (95% CI, 4.32-6.83) for those diagnosed below the median age compared with 2.87 (95% CI, 2.15-3.83) for those diagnosed above the median age. The median follow-up time was similar across age groups (eTable 14 in Supplement 1).
The proportional hazards assumption was violated for all disabilities (P < .01) when the Cox proportional hazards regression model was estimated with a constant risk. The estimated Cox proportional hazards regression model with time-varying risks (0 to 1, >1 to 3, >3 to 5, and >5 years after the index date) showed a significantly elevated relative risk for cases vs controls for all disabilities and all time periods (eg, hearing loss at >3 to 5 years, with an adjusted HR of 9.11 [95% CI, 5.12-16.22]) but 2 (behavioral and emotional disorders at >1 to 3 years and visual disturbances at >3 to 5 years) (Table 4). The relative risk of disabilities was highest during an acute phase (0 to 1 or >1 to 3 years), but the risk remained significantly higher than 1.00 for all categories of disabilities during the period starting over 5 years after the index date.
Table 4. Time-Varying Adjusted HR for Disabilities.
| Disability | Adjusted HR (95% CI)a | P value |
|---|---|---|
| Cognitive disability | ||
| 0 to 1 y | 3.52 (1.78-6.96) | <.001 |
| >1 to 3 y | 6.05 (3.45-10.59) | <.001 |
| >3 to 5 y | 2.38 (1.27-4.48) | .007 |
| >5 y | 3.08 (2.54-3.73) | <.001 |
| Seizures | ||
| 0 to 1 y | 7.86 (5.19-11.91) | <.001 |
| >1 to 3 y | 9.42 (5.83-15.21) | <.001 |
| >3 to 5 y | 4.91 (2.98-8.10) | <.001 |
| >5 y | 2.85 (2.25-3.59) | <.001 |
| Hearing loss | ||
| 0 to 1 y | 26.73 (16.35-43.71) | <.001 |
| >1 to 3 y | 22.92 (12.07-43.54) | <.001 |
| >3 to 5 y | 9.11 (5.12-16.22) | <.001 |
| >5 y | 5.53 (4.53-6.74) | <.001 |
| Motor function disorders | ||
| 0 to 1 y | 7.82 (5.10-11.99) | <.001 |
| >1 to 3 y | 7.25 (4.77-11.01) | <.001 |
| >3 to 5 y | 4.33 (2.50-7.51) | <.001 |
| >5 y | 2.90 (2.10-4.00) | <.001 |
| Visual disturbances | ||
| 0 to 1 y | 8.83 (6.41-12.17) | <.001 |
| >1 to 3 y | 2.11 (1.47-3.02) | <.001 |
| >3 to 5 y | 1.41 (0.89-2.23) | .14 |
| >5 y | 1.73 (1.40-2.12) | <.001 |
| Behavioral and emotional disorders | ||
| 0 to 1 y | 1.88 (1.17-3.03) | .009 |
| >1 to 3 y | 1.20 (0.72-1.99) | .50 |
| >3 to 5 y | 1.56 (1.03-2.36) | .04 |
| >5 y | 1.40 (1.23-1.59) | <.001 |
| Intracranial structural injuries | ||
| 0 to 1 y | 59.15 (20.13-173.80) | <.001 |
| >1 to 3 y | 22.07 (7.24-67.26) | <.001 |
| >3 to 5 y | 20.61 (5.63-75.44) | <.001 |
| >5 y | 16.67 (8.13-34.20) | <.001 |
Adjusted for age at the index date, the index year, sex, parental educational attainment, birth characteristics (5-minute Apgar score, birth weight, head circumference), and 7 preexisting conditions (malignant neoplasms, perinatal complications, mental and behavioral disorders, diseases of the nervous system, congenital disabilities, traumatic injuries, and operations in the nervous system).
Secondary Outcome
During the whole follow-up time, cases with bacterial meningitis had a mean (SD) of 0.49 (0.96) disabilities (0.14 [0.42] for controls), and 1052 cases (29.0%) had 1 or more disabilities (compared with 3935 [12.1%] controls) (eTable 15 in Supplement 1). The estimated negative binomial regression model gave an adjusted incidence rate ratio for additional disabilities of 2.77 (95% CI, 2.58-2.97; P < .001) for cases relative to controls.
Discussion
To our knowledge, this study is the first to describe long-term risks of disabilities after bacterial meningitis in childhood with nationwide data, a control group, several decades of follow-up time, and stratification by the causative pathogen and age at diagnosis. Individuals who had bacterial meningitis during childhood had a higher incidence of all types of disabilities compared with general population controls. The largest relative risk was found for intracranial structural injuries, hearing loss, and motor function disorders. There was also an increased risk of cognitive disabilities as well as behavioral and emotional disorders, 2 disabilities with a high impact on quality of life.
The relative risk of disabilities tended to be highest during the first years after a diagnosis of bacterial meningitis but remained higher during the period starting over 5 years after diagnosis, suggesting that bacterial meningitis has both acute and long-term consequences. The young age at diagnosis in this study (median, 1.5 years) imposes clinical difficulties on detection of disabilities after an episode of bacterial meningitis, which could explain why there was no significantly elevated risk of behavioral and emotional disorders during the first 1 to 3 years of follow-up (these disabilities are difficult to detect at an early age).11
Streptococcus pneumoniae as the causative pathogen was a strong risk factor in our study. The adjusted HRs for cognitive disabilities, seizures, hearing loss, and motor function disorders were significantly higher for S pneumoniae compared with H influenzae or N meningitidis, consistent with results in previous studies.13,14 Our work strengthens these earlier findings by considering a wider range of disabilities and by adjusting for several individual and parental characteristics.
Age also appears to be an important risk factor. When we compared the risk of long-term disabilities among children diagnosed at a young (below median) vs older (above median) age conditional on type of infection, the relative risk of disabilities was numerically higher for children diagnosed at an early age for all types of disabilities and significantly higher for cognitive disabilities, seizures, behavioral and emotional disorders, and intracranial structural injuries. Our interpretation is that the damage to the brain and nervous system that can follow an episode of bacterial meningitis is more detrimental for young children who are at a sensitive stage in their physical and mental development.15
Compared with previous studies reporting disabilities following bacterial meningitis,1 our study documents a higher risk of disabilities, potentially due to our long follow-up period and extensive data on a wide range of disabilities. The review article by Edmond et al1 included 59 studies with follow-up data after hospital discharge for all-cause meningitis and reported a median risk of at least 1 disability of 19.9%. In our study, the corresponding fraction with at least 1 disability is 29.0%. It should be noted that Edmond et al1 found the highest risk of sequelae in low-income countries and a risk of major sequelae that was twice as high in Africa and southeast Asia relative to European countries. Considering these geographic differences, the results of this study on the risk of disabilities in a European country are substantially higher than previous results described by Edmond et al.1
There are 2 main clinical implications of our findings. First, as long-term disabilities are more common than previously reported, and young age and bacterial meningitis due to S pneumoniae appear to be risk factors, our results underscore the importance of protecting children against bacterial meningitis and the benefits of childhood pneumococcal vaccinations. Second, given the high risk of disabilities that are less noticeable (cognitive disabilities, behavioral and emotional disorders, and others), recommendations for follow-up after bacterial meningitis should include strategies to detect such disabilities, as they otherwise could remain undetected for several years.16
Strengths and Limitations
This study using Swedish national registers has several strengths, including the large sample size, a nationwide design, comparable controls, background information on family characteristics, the near-complete and long-term follow-up with minimal missing data, and the linkage to several national registries, which contributes to the richness of the data. This study also has several limitations, including possible coding errors in the National Patient Register that can lead to misclassification of bacterial meningitis. We did not have access to clinical and laboratory data to verify diagnoses, and diagnostic limitations in earlier years may reduce the quality of diagnosis codes for the causative pathogen. Another weakness is that we did not have information on caregivers other than biological parents, which is why we adjust for the characteristics of biological parents.
It is difficult to adjust for all comorbidities and preexisting conditions in a study of children who tend to be young at diagnosis and have a short medical history. The 2 sensitivity analyses in which we removed all children with preexisting conditions (912 cases [25.2%]) or rematched cases and controls on preexisting conditions generated similar estimated risks of disabilities as our main analysis. These additional analyses suggest that, although potential confounding is a limitation in this and other observational studies, the adjusted risks of disabilities that we present are primarily due to bacterial meningitis in childhood and not induced by other confounding factors.
Conclusion
Bacterial meningitis diagnosed in childhood can have a severe impact that extends into adulthood through long-term disabilities. Children diagnosed with meningitis due to S pneumoniae and children diagnosed with bacterial meningitis at a young (below median) age face the highest risk of developing such disabilities. The results of this cohort study highlight the need for preventive measures against bacterial meningitis and efforts to detect disabilities among surviving children (especially behavioral and emotional disorders, hearing loss, and visual disturbances).
eFigure 1. Flowchart (Sequential Exclusions)
eFigure 2. Differences in Risk of Disabilities by Type of Infection
eFigure 3. Differences in Risk of Disabilities by Type of Infection (Year of Diagnosis Before 2005)
eTable 1. ICD Codes for Preexisting Infections of the Central Nervous System
eTable 2. ICD Codes for Disabilities
eTable 3. ICD Codes for Preexisting Conditions
eTable 4. Medical Procedure Codes for Operations in the Nervous System
eTable 5. ICD Codes for Types of Infection
eTable 6. Follow-Up Time
eTable 7. Unadjusted Cumulative Incidence of Disabilities at 15 and 30 Years of Follow-Up
eTable 8. Adjusted Hazard Ratios With No Preexisting Conditions and in Alternative Matched Cohort
eTable 9. Participant Characteristics Alternative Matched Cohort
eTable 10. Frequency of Infections by Type of Bacterial Pathogen
eTable 11. Year of Diagnosis by Type of Bacterial Pathogen
eTable 12. P Values for Pairwise Comparison of Adjusted Hazard Ratios by Type of Infection
eTable 13. Adjusted Hazard Ratios for Disabilities by Age at Diagnosis
eTable 14. Median Follow-Up Time for Cases and Controls by Age at Diagnosis
eTable 15. Number of Disabilities
Data Sharing Statement
References
- 1.Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317-328. doi: 10.1016/S1473-3099(10)70048-7 [DOI] [PubMed] [Google Scholar]
- 2.Lukšić I, Mulić R, Falconer R, Orban M, Sidhu S, Rudan I. Estimating global and regional morbidity from acute bacterial meningitis in children: assessment of the evidence. Croat Med J. 2013;54(6):510-518. doi: 10.3325/cmj.2013.54.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi: 10.1128/CMR.00007-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed AS, Khan NZ, Hussain M, et al. Follow-up of cases of Haemophilus influenzae type B meningitis to determine its long-term sequelae. J Pediatr. 2013;163(1)(suppl):S44-S49. doi: 10.1016/j.jpeds.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 5.Chandran A, Herbert H, Misurski D, Santosham M. Long-term sequelae of childhood bacterial meningitis: an underappreciated problem. Pediatr Infect Dis J. 2011;30(1):3-6. doi: 10.1097/INF.0b013e3181ef25f7 [DOI] [PubMed] [Google Scholar]
- 6.Viner RM, Booy R, Johnson H, et al. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol. 2012;11(9):774-783. doi: 10.1016/S1474-4422(12)70180-1 [DOI] [PubMed] [Google Scholar]
- 7.Gustafsson N, Stallknecht SE, Skovdal M, Poulsen PB, Østergaard L. Societal costs due to meningococcal disease: a national registry-based study. Clinicoecon Outcomes Res. 2018;10:563-572. doi: 10.2147/CEOR.S175835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickering L, Jennum P, Ibsen R, Kjellberg J. Long-term health and socioeconomic consequences of childhood and adolescent onset of meningococcal meningitis. Eur J Pediatr. 2018;177(9):1309-1315. doi: 10.1007/s00431-018-3192-0 [DOI] [PubMed] [Google Scholar]
- 9.Roed C, Omland LH, Skinhoj P, Rothman KJ, Sorensen HT, Obel N. Educational achievement and economic self-sufficiency in adults after childhood bacterial meningitis. JAMA. 2013;309(16):1714-1721. doi: 10.1001/jama.2013.3792 [DOI] [PubMed] [Google Scholar]
- 10.Snoek L, Gonçalves BP, Horváth-Puhó E, et al. Short-term and long-term risk of mortality and neurodevelopmental impairments after bacterial meningitis during infancy in children in Denmark and the Netherlands: a nationwide matched cohort study. Lancet Child Adolesc Health. 2022;6(9):633-642. doi: 10.1016/S2352-4642(22)00155-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson Kostenniemi U, Bazan A, Karlsson L, Silfverdal SA. Psychiatric disabilities and other long-term consequences of childhood bacterial meningitis. Pediatr Infect Dis J. 2021;40(1):26-31. doi: 10.1097/INF.0000000000002908 [DOI] [PubMed] [Google Scholar]
- 12.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedford H, de Louvois J, Halket S, Peckham C, Hurley R, Harvey D. Meningitis in infancy in England and Wales: follow up at age 5 years. BMJ. 2001;323(7312):533-536. doi: 10.1136/bmj.323.7312.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter JA, Neville BG, Newton CR. Neuro-cognitive impairment following acquired central nervous system infections in childhood: a systematic review. Brain Res Brain Res Rev. 2003;43(1):57-69. doi: 10.1016/S0165-0173(03)00192-9 [DOI] [PubMed] [Google Scholar]
- 15.Verger K, Junqué C, Jurado MA, et al. Age effects on long-term neuropsychological outcome in paediatric traumatic brain injury. Brain Inj. 2000;14(6):495-503. doi: 10.1080/026990500120411 [DOI] [PubMed] [Google Scholar]
- 16.Johansson Kostenniemi U, Silfverdal SA. Predictive scores failing at identifying psychiatric disabilities following childhood bacterial meningitis calls for revision of current follow-up guidelines. Infect Dis (Lond). 2022;54(7):514-521. doi: 10.1080/23744235.2022.2050942 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart (Sequential Exclusions)
eFigure 2. Differences in Risk of Disabilities by Type of Infection
eFigure 3. Differences in Risk of Disabilities by Type of Infection (Year of Diagnosis Before 2005)
eTable 1. ICD Codes for Preexisting Infections of the Central Nervous System
eTable 2. ICD Codes for Disabilities
eTable 3. ICD Codes for Preexisting Conditions
eTable 4. Medical Procedure Codes for Operations in the Nervous System
eTable 5. ICD Codes for Types of Infection
eTable 6. Follow-Up Time
eTable 7. Unadjusted Cumulative Incidence of Disabilities at 15 and 30 Years of Follow-Up
eTable 8. Adjusted Hazard Ratios With No Preexisting Conditions and in Alternative Matched Cohort
eTable 9. Participant Characteristics Alternative Matched Cohort
eTable 10. Frequency of Infections by Type of Bacterial Pathogen
eTable 11. Year of Diagnosis by Type of Bacterial Pathogen
eTable 12. P Values for Pairwise Comparison of Adjusted Hazard Ratios by Type of Infection
eTable 13. Adjusted Hazard Ratios for Disabilities by Age at Diagnosis
eTable 14. Median Follow-Up Time for Cases and Controls by Age at Diagnosis
eTable 15. Number of Disabilities
Data Sharing Statement


