Abstract
Background
Osteoarthritis is a prevalent condition in frail older adults that requires hip or knee replacement in many patients. The aim of the study was to determine the impact of hip and knee arthroplasty on frailty.
Methods
In this prospective short-term study, we used data from 101 participants of the ongoing Special Orthopaedic Geriatrics (SOG) trial, funded by the German Federal Joint Committee (GBA). Frailty, measured by Fried’s Physical Frailty Phenotype (PFP), was assessed preoperatively, 7 days postoperatively, 4–6 weeks and 3 months after hip and knee arthroplasty. ANOVA with repeated measures and post-hoc tests for the subgroups were used for the statistical analysis.
Results
Of the 101 participants, 50 were pre-frail (1–2 PFP criteria) and 51 were frail (≥ 3 PFP criteria) preoperatively. In the pre-frail group, the PFP score decreased from 1.56 ± 0.50 (median 2) preoperatively to 0.53 ± 0.73 (median 0) 3 months after surgery (p < 0.001). The PFP score in the frail cohort decreased from 3.39 ± 1.45 (median 3) preoperatively to 1.27 ± 1.14 (median 1) 3 months postoperatively (p < 0.001). While the PFP score of the pre-frail participants increased 7 days after surgery, the PFP score of the frail group decreased significantly.
Conclusion
Pre-frail individuals often regain robustness and patients with frailty are no longer assessed as frail after surgery. Joint replacement is an effective intervention to improve frailty in hip and knee osteoarthritis.
Trial registration
This study is part of the Special Orthopaedic Geriatrics (SOG) trial, German Clinical Trials Register DRKS00024102. Registered on 19 January 2021.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-024-07210-w.
Keywords: Orthogeriatric, Frailty, Hip arthroplasty, Knee arthroplasty, Older people
Background
Frailty is a multidimensional geriatric syndrome characterised by loss of individual reserve capacity and increased vulnerability to internal and external stressors [1]. A frail person is at increased risk of adverse outcomes such as falls, disability, hospitalisation and mortality [2, 3]. Therefore, frailty is an emerging global health burden that has significant implications for clinical practice and public health [2, 4]. A commonly used tool for assessing frailty is the Fried Frailty Phenotype [3]. This clinical phenotype of frailty manifests as multi-system pathology characterised by low physical activity, global weakness with low muscle strength, exhaustion, reduced walking speed and weight loss. Pre-frailty occurs at an earlier stage of the frailty spectrum and is associated with the later development of frailty [3, 5].
So far, many influencing factors have been identified that can promote the development of frailty. These include risk factors such as high age, multimorbidity, obesity, polypharmacy, low physical activity, and inflammation [1]. There is also an association of osteoarthritis (OA) with frailty and pre-frailty in older adults [6]. OA is considered the most prevalent chronic joint disease in the world [7]. Chronic comorbidities can lead to a progression of frailty [8]. However, little is known about the regression or reversibility of frailty. Interventions have so far been limited to physical training, high-protein diets, or a combination of both [9].
In Germany, total hip (THA) and knee arthroplasty (TKA) are among the 20 most common surgical procedures for hospitalised patients overall [10]. The aim of the study was to determine the impact of primary total hip and knee arthroplasty in elderly patients with OA on the Fried Frailty Phenotype. We hypothesised that hip and knee replacement would correlate with a decrease in frailty and pre-frailty as assessed by Fried’s phenotype criteria. The association of hip and knee OA with frailty has been clearly demonstrated and was also reconfirmed by the European Project on OSteoArthritis (EPOSA) in 2015 [6]. There is also no doubt that frailty can have an impact. Regression has been shown multiple times in studies involving physical activity and a high-protein diet or a combination of both [9]. It therefore stands to reason that if the trigger OA for the frailty is removed, i.e. the degenerated hip or knee joint is replaced and thus the cause eliminated, a regression or reversibility of the pre-frailty/frailty can also occur. The reason is to restore joint function and thus build muscle. As frailty is a multidimensional syndrome, factors such as improvement in pain and walking problems must also be considered. However, these are assessed directly or indirectly by the frailty phenotype criteria [3].
The baseline characteristics listed in Table 1 were selected because they are commonly used assessment tools in geriatrics, both clinically and scientifically, and are well descriptive of the population studied.
Table 1.
Baseline characteristics
| Characteristics | Total (n = 101) | Pre-frail (n = 50) | Frail (n = 51) |
|---|---|---|---|
| Female n (%) | 71 (70.30) | 29 (58.00) | 42 (82.35) |
| Age y, mean ± SD | 78.52 ± 4.51 | 76.98 ± 4.20 | 80.04 ± 4.33 |
| BMI kg/m² mean ± SD | 28.99 ± 4.74 | 28.10 ± 4.14 | 29.86 ± 5.16 |
| Medication n mean ± SD | 7.70 ± 3.78 | 7.10 ± 3.82 | 8.29 ± 3.67 |
| Comorbidities n mean ± SD | 7.53 ± 3.13 | 7.50 ± 2.98 | 7.57 ± 3.31 |
| CCI mean ± SD | 5.45 ± 1.94 | 5.16 ± 1.96 | 5.73 ± 1.89 |
| NRS score (0–7) mean ± SD | 1.28 ± 0.78 | 1.20 ± 0.61 | 1.35 ± 0.91 |
| Barthel Index (0-100) mean ± SD | 92.03 ± 12.47 | 95.90 ± 5.41 | 88.24 ± 15.90 |
| IADL score (0–8) mean ± SD | 6.69 ± 1.67 | 7.26 ± 1.24 | 6.14 ± 1.85 |
| SPPB score (0–12) mean ± SD | 6.66 ± 2.64 | 8.36 ± 2.01 | 5.00 ± 2.08 |
| MMSE score (0–30) mean ± SD | 26.95 ± 2.34 | 27.22 ± 2.25 | 26.69 ± 2.42 |
| GDS-15 score (0–15) mean ± SD | 3.49 ± 2.92 | 2.90 ± 2.87 | 4.06 ± 2.89 |
BMI, Body Mass Index; CCI, Charlson Comorbidity Index; NRS, Nutritional Risk Screening; IADL, Instrumental Activities of Daily Living; SPPB, Short Physical Performance Battery; MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale.
Methods
Study design
This study is part of the ongoing Special Orthopaedic Geriatrics (SOG) trial (German Clinical Trials Register, 19/01/2021, DRKS00024102). The SOG study is a monocentric, prospective, randomised controlled trial funded by the German Federal Joint Committee (GBA). The original study aimed to investigate a specially developed multimodal care model (SOG care model) for orthogeriatric patients with total hip and total knee arthroplasty compared to usual orthopaedic care without orthogeriatric co-management. Frailty was a secondary outcome measure. A detailed description of the study can be found elsewhere [11]. The current study enrolled all 125 patients who underwent surgery in the SOG trial between 01 April 2021 and 30 November 2022. This additional analysis was not planned when the original study was designed.
Data collection
In the Orthopaedic Department of the University Hospital of Regensburg, about 18,000 patients are treated annually in the university outpatient clinic and more than 1500 endoprosthetic procedures on hip and knee joints are performed each year. Participants were recruited at the university outpatient clinic if they were diagnosed with primary hip or knee osteoarthritis and had an indication for THA or TKA. The study data were collected preoperatively, on the 7th day after surgery before discharge, 4–6 weeks, and 3 months after surgery.
Study population
Eligibility criteria included: primary hip or knee osteoarthritis, age 70 years and older with multimorbidity or age 80 years and older, indication for elective unilateral hip or knee replacement and pre-frailty or frailty according to Fried’s criteria [3]. Exclusion criteria were age under 70 years, previous bony surgery or tumour in the area of the joint to be treated, acute infection, robustness (0 criteria according to Fried’s Frailty Phenotype) [3] and increased need for care (care level ≥ 4; severe impairment of independence, need for help with basic care 24 h a day).
Out of a total of 125 subjects in the SOG study, there were 7 drop-outs. The reasons were cancellation of surgery or refusal to participate in the study. Another 17 patients were excluded due to robustness (0 criteria). As a result, the number of people included in the analysis was 101. The number of patients lost to follow-up was 3 at 4–6 weeks (follow-up 1) and 1 at 3 months (follow-up 2).
Surgical techniques and implants
All operations were performed in a single Department of Orthopaedic Surgery of a University Medical Centre. The lateral decubitus position was used for the cementless THA. A minimally invasive anterolateral approach was chosen [12]. Press-fit acetabular components and cementless stems from a single manufacturer (Pinnacle cup, Corail or Trilock stem; DePuy, Warsaw, IN) were used in all THAs. The cemented TKA was performed via a medial parapatellar approach. Cemented components from a single manufacturer (PFC Sigma; DePuy) were used in all TKAs. Patella resurfacing was not performed.
Assessment of frailty
Frailty was measured based on the five criteria of the Physical Frailty Phenotype (PFP) proposed by Fried [3, 13], adapted as follows: shrinking (self-reported unintentional weight loss of more than 4,5 kg in the past year), exhaustion (self-reported using the CES-D depression scale), slowed walking speed (walking time of 5 m below an adjusted cut-off by gender and height), weakness [grip strength below an established cut-off based on gender and body mass index (BMI) measured on the dominant hand using a dynamometer (Jamar® Hydraulic Hand Dynamometer; Performance Health, Wisconsin)] and low physical activity (kilocalories per week below an established gender-specific cut-off using self-reported frequency and duration of walking or cycling based on activity level according to the Swiss Health Observatory). Each component or question was given a score of 0 or 1, depending on whether it was present or not. Robust patients were defined with a score of 0, pre-frail with a score of 1–2 and frail with a score of 3 and higher.
Additional study variables
The Charlson Comorbidity Index (CCI) predicts the mortality of a patient who has a number of comorbidities, such as heart disease, AIDS or cancer (taking into account a total of 19 diseases). A value of zero means that no comorbidities were found; the higher the value, the higher the predicted mortality rate [14].
The purpose of the Nutritional Risk Screening (NRS) system is to detect the presence of malnutrition and the risk of developing malnutrition in the hospital setting. It includes four questions as a pre-screening. If one of these is answered positively, a screening follows which includes surrogate measures of nutritional status, with static and dynamic parameters and data on the severity of the disease (stress metabolism). For each parameter, a score from 0 to 3 can result. Age over 70 years is considered as a risk factor, and is included in the screening tool as well, giving 1 point. A total score of 3 or more points means that the patient is at risk of malnutrition or already malnourished and therefore a nutritional therapy is indicated [15].
The Barthel Scale/Index is an ordinal scale used to measure performance in activities of daily living (ADL). Ten variables describing ADL and mobility are scored, a higher number reflecting greater ability to function independently following hospital discharge [16].
The Lawton & Brody Instrumental Activities of Daily Living Scale (IADL) is an appropriate instrument to assess independent living skills. These skills are considered more complex than the basic activities of daily living as measured by the Barthel Index. There are eight domains of function measured with the Lawton IADL scale. Participants are scored according to their highest level of functioning in that category. A summary score ranges from 0 (low function, dependent) to 8 (high function, independent) [17].
Short Physical Performance Battery (SPPB) is a measure of physical functioning. SPPB evaluates balance, mobility, and muscle strength by examining an individual’s ability to stand in different positions, time to walk 4 m, and time to rise up from and sit down on a chair 5 times. The tests are scored between 0 and 4, leaving a maximum score of 12 [18].
The Mini Mental State Examination (MMSE) is a 30-point questionnaire that is used extensively in clinical and research settings to measure cognitive impairment. The test examines functions such as registration (repeating named prompts), attention and calculation, recall, language, ability to follow simple commands and orientation. Any score of 24 or more (out of 30) indicates a normal cognition. Below this, scores can indicate severe (≤ 9 points), moderate (10–18 points) or mild (19–23 points) cognitive impairment [19].
The 15-item Geriatric Depression Scale (GDS-15) is a short form of GDS and is used to screen, diagnose, and evaluate depression in elderly individuals. In scoring the GDS, 1 point is awarded for each answer that indicates depression. If a person scores more than 5 on the 15-question assessment, this may indicate the presence of depression [20].
Statistical analysis
Descriptive information including demographic and morbidity-related characteristics were calculated for the whole sample. As a core statistical method one-way ANOVA with repeated measures was employed, to test whether there are significant differences between the four times of measurement in the study population, with the Fried Frailty Phenotype being the variable of interest and our primary outcome. If necessary, Greenhouse-Geisser correction was applied, to adjust for violation of the sphericity assumption. For this the R-package “afex” was used. If the repeated measures ANOVA yielded significant results and to examine differences between each pair of time of measurement post-hoc tests were performed using the “emmeans” R-package. In this procedure Bonferroni-correction was used to reduce the risk of a type I error. Further analyses included repeated measures ANOVA as well as post-hoc tests for the subgroups of pre-frail (Score 1–2) and frail patients (Score ≥ 3) and for each of the five subdomains (weight loss, exhaustion, low physical activity, slowness, weakness) of the Fried frailty phenotype as outcome variable. To examine whether certain patient characteristics can serve as predictors for improvement in frailty after joint replacement, we performed a logistic regression. For this a dichotomous variable which indicated whether a patient experienced any improvement in frailty after joint replacement was used as the dependent variable. For independent variables, we included age, gender, BMI, SPPB-Score, GDS-Score, and NRS-Score. All analyses were conducted in R version 4.2.1. P-values p < 0.05 were considered statistically significant.
Results
Baseline characteristics
Of a total of 101 participants, 50 were pre-frail (1–2 PFP criteria) and 51 were frail (≥ 3 PFP criteria) preoperatively. The female gender was much more prevalent in the total population at 70.30% and most prevalent in the frail cohort at 82.35%. Mean age at 80.04 ± 4.33 years, mean BMI at 29.86 ± 5.16 kg/m², mean number of medications at 8.29 ± 3.67, mean Charlson Comorbidity Index at 5.73 ± 1.89 and mean GDS-15 score at 4.06 ± 2.89 were higher in the frail participants. Mobility (mean SPPB score 5.00 ± 2.08) and (instrumental) activities of daily living (mean IADL score 6.14 ± 1.85, mean Barthel Index 88.24 ± 15.90) were most clearly reduced in the frail group (Table 1).
General improvement in frailty after THA/TKA
Hip or knee replacement improved PFP at 3 months in 80 patients (80%) from a pre-frail or frail status before surgery. In 14 participants (14%), PFP remained unchanged 3 months after joint replacement and in 6 patients (6%) PFP worsened.
Frailty scores at the different measurement times
Table 2 demonstrates the total PFP scores for the whole sample (THA/TKA), the THA subgroup and the TKA subgroup, as well as the PFP scores of the prefrail and frail of the corresponding cohort.
Table 2.
PFP score (mean ± SD) at different study time points of 101 participants
| Total sample (Hip and knee patients) | Total ( n = 101) | Pre-frail ( n = 50) | Frail ( n = 51) |
| Frailty score pre-op (t0) | 2.49 ± 1.05 | 1.56 ± 0.50 | 3.39 ± 1.45 |
| Frailty score d 7 post-op (t1) | 2.12 ± 1.43 | 1.90 ± 1.40 | 2.33 ± 1.44 |
| Frailty score 4–6 wk follow-up (t2) | 1.39 ± 1.39 | 0.88 ± 1.17 | 1.78 ± 1.45 |
| Frailty score 12 wk follow-up (t3) | 0.91 ± 1.17 | 0.53 ± 0.73 | 1.27 ± 1.14 |
| Hip patients | Total ( n = 65) | Pre-frail ( n = 26) | Frail ( n = 39) |
| Frailty score pre-op (t0) | 2.63 ± 1.02 | 1.54 ± 0.51 | 3.36 ± 0.49 |
| Frailty score d 7 post-op (t1) | 2.15 ± 1.46 | 2.11 ± 1.56 | 2.18 ± 1.41 |
| Frailty score 4–6 wk follow-up (t2) | 1.44 ± 1.44 | 1.08 ± 1.44 | 1.71 ± 1.39 |
| Frailty score 12 wk follow-up (t3) | 0.85 ± 1.09 | 0.50 ± 0.86 | 1.08 ± 1.18 |
| Knee patients | Total ( n = 36) | Pre-frail ( n = 24) | Frail ( n = 12) |
| Frailty score pre-op (t0) | 2.22 ± 1.05 | 1.58 ± 0.50 | 3.50 ± 0.52 |
| Frailty score d 7 post-op (t1) | 2.06 ± 1.39 | 1.67 ± 1.20 | 2.83 ± 1.47 |
| Frailty score 4–6 wk follow-up (t2) | 1.11 ± 1.28 | 0.65 ± 0.71 | 2.00 ± 1.65 |
| Frailty score 12 wk follow-up (t3) | 1.03 ± 1.32 | 0.57 ± 0.73 | 1.92 ± 1.73 |
PFP, Physical Frailty Phenotype; SD, Standard deviation; wk, week.
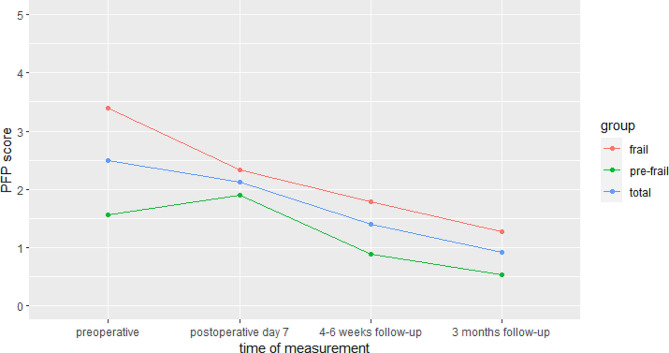
The mean PFP score of the total populations and the frail groups decreased continuously after surgery. In the pre-frail participants, the PFP score initially increased on postoperative day 7 before decreasing from 4 to 6 weeks follow-up. Frail patients benefited most from hip or knee replacements. Here, the mean PFP score decreased from 3.39 ± 1.45 preoperatively to 1.27 ± 1.14 3 months after surgery (p < 0.001) ). The course of the frailty scores of the total study group is shown graphically in Fig. 1.
Fig. 1.
Physical Frailty Phenotype scores (means) of the total study group (n = 101), the pre-frail group (n = 50) and the frail group (n = 51) at the four different measurement points of the study
Results of rmANOVA tests
Table 3 shows the time points between which there were statistically significant differences in the PFP scores of THA/TKA patients combined. In the total population and in the pre-frail group, there were no significant differences in PFP score preoperatively compared to 7 days postoperatively (hospital discharge) (p = 0.135 and p = 0.749). In addition, there was no significant difference in the PFP score between 4 and 6 weeks and 3 months follow-up in the pre-frail group (p = 0.136). There were significant differences between all other measures of PFP score in the rmANOVA tests in the total population as well as in the pre-frail and frail groups. As a result, the PFP score was significantly reduced in the pre-frail and frail groups 3 months after surgery (p < 0.001). In contrast to the pre-frail participants, the frail cohort benefited significantly from joint replacement as early as 7 days after surgery (p = 0.749 vs. p < 0.001). Separate repeated measures ANOVAs were also conducted for the THA and TKA subgroups. These were further subdivided into pre-frail and frail (Additional file 2).
Table 3.
Results of rmANOVA tests for the four measurement points (t0-t3) of the Fried Frailty Phenotype
| Total sample | |||||
| Effect | df | MSE | F | ges | p .value |
| Time | 2.61, 255.95 | 0.99 | 58.78 | 0.193 | < 0.001 |
| contrast | estimate | SE | df | t.ratio | p .value |
| pre-op (t0) - d7 post-op (t1) | 0.364 | 0.157 | 98 | 2.317 | 0.135 |
| pre-op (t0) − 4–6 wk post-op (t2) | 1.152 | 0.136 | 98 | 8.484 | < 0.001 |
| pre-op (t0) − 12 wk post-op (t3) | 1.566 | 0.124 | 98 | 12.663 | < 0.001 |
| d7 post-op (t1) − 4–6 wk post-op (t2) | 0.788 | 0.124 | 98 | 6.367 | < 0.001 |
| d7 post-op (t1) − 12 wk post-op (t3) | 1.202 | 0.143 | 98 | 8.415 | < 0.001 |
| 4–6 wk post-op (t2) − 12 wk post-op (t3) | 0.414 | 0.103 | 98 | 4.039 | < 0.001 |
| P value adjustment: bonferroni method for 6 tests | |||||
| Pre-frail | |||||
| Effect | df | MSE | F | ges | p .value |
| Time | 2.31, 110.87 | 0.95 | 25.36 | 0.215 | < 0.001 |
| contrast | estimate | SE | df | t.ratio | p .value |
| pre-op (t0) - d7 post-op (t1) | -0.327 | 0.209 | 48 | -1.562 | 0.749 |
| pre-op (t0) − 4–6 wk post-op (t2) | 0.673 | 0.183 | 48 | 3.680 | 0.004 |
| pre-op (t0) − 12 wk post-op (t3) | 1.020 | 0.135 | 48 | 7.549 | < 0.001 |
| d7 post-op (t1) − 4–6 wk post-op (t2) | 1.000 | 0.152 | 48 | 6.600 | < 0.001 |
| d7 post-op (t1) − 12 wk post-op (t3) | 1.347 | 0.197 | 48 | 6.844 | < 0.001 |
| 4–6 wk post-op (t2) − 12 wk post-op (t3) | 0.347 | 0.147 | 48 | 2.354 | 0.136 |
| P value adjustment: bonferroni method for 6 tests | |||||
| Frail | |||||
| Effect | df | MSE | F | ges | p .value |
| Time | 2.71, 132.75 | 0.92 | 49.06 | 0.286 | < 0.001 |
| contrast | estimate | SE | df | t.ratio | p .value |
| pre-op (t0) - d7 post-op (t1) | 1.04 | 0.192 | 49 | 5.429 | < 0.001 |
| pre-op (t0) − 4–6 wk post-op (t2) | 1.62 | 0.178 | 49 | 9.092 | < 0.001 |
| pre-op (t0) − 12 wk post-op (t3) | 2.10 | 0.177 | 49 | 11.884 | < 0.001 |
| d7 post-op (t1) − 4–6 wk post-op (t2) | 0.58 | 0.192 | 49 | 3.023 | 0.024 |
| d7 post-op (t1) − 12 wk post-op (t3) | 1.06 | 0.207 | 49 | 5.125 | < 0.001 |
| 4–6 wk post-op (t2) − 12 wk post-op (t3) | 0.48 | 0.144 | 49 | 3.344 | 0.010 |
| P value adjustment: bonferroni method for 6 tests | |||||
df, degrees of freedom; MSE, mean squared error; SE, standard error; ges, generalised eta squared.
Impact on frailty stages
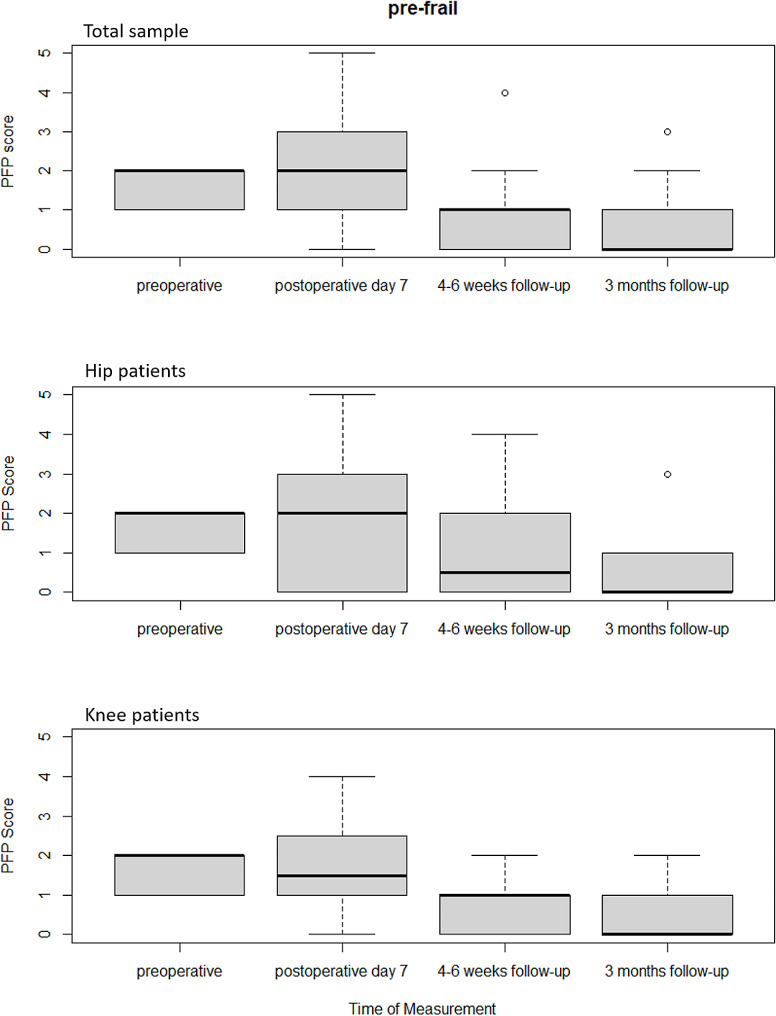
Changes in frailty stages according to Fried can be well assessed by comparing the median values. Before hip or knee replacement, the median PFP score of the total sample in the pre-frail group was 2. Already 4–6 weeks postoperatively, the PFP score decreased to a median value of 1. 3 months after joint replacement, the median was 0. According to Fried’s criteria, pre-frailty was no longer present (Fig. 2).
Fig. 2.
Box plots of the frailty scores according to Fried’s phenotype of the pre-frail groups pre- and postoperatively
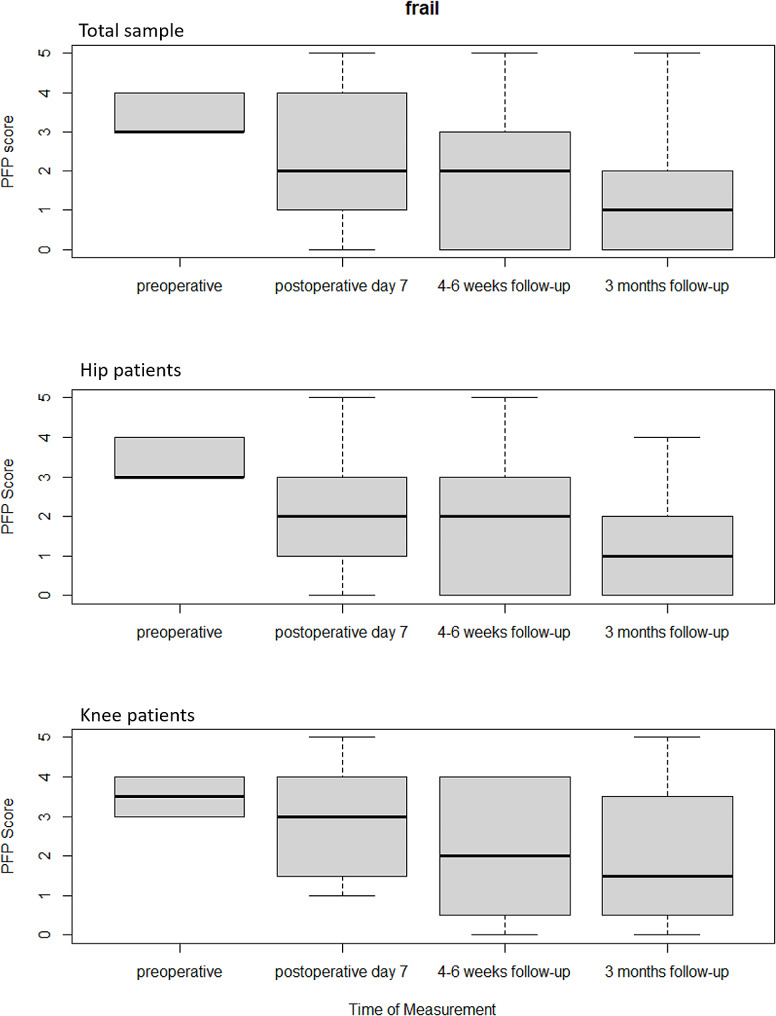
The median PFP score in the frail cohort of the total sample before surgery was 3. Here, there was already a decrease in the median PFP score to 2 on postoperative day 7. 3 months after hip or knee replacement, the median PFP score was only 1, so that there were no longer any criteria for frailty according to Fried’s Phenotype (Fig. 3).
Fig. 3.
Box plots of the frailty scores according to Fried’s Phenotype of the frail groups pre- and postoperatively
Fried frailty phenotype subscores for the total study group (THA/TKA)
The results of the repeated measures ANOVA tests for each of the five subscores of the Fried Frailty Phenotype (weight loss, exhaustion, slowness, weakness, low physical activity) of the total population, the prefrail and frail group can be found in Additional file 1. While no significant differences were found for the two criteria weight loss and weakness (grip strength), significant differences were shown in the subscores exhaustion, slow walking speed and low physical activity between different time points of the measurement. This applies to the total population, but also to pre-frail and frail cohorts.
Exclusion of confounding factors in the improvement of frailty
Logistic regression analysis (univariate and multivariate) was used to determine whether the variables “Age”, “Gender”, “BMI”, “SPPB”, “GDS” and “NRS” had an influence on the improvement in frailty between t0 (pre-OP) and t3 (12 wk follow-up). As can be seen in Table 4, this was not the case for any of the variables included. Thus, we assume that there were no confounding factors. It can therefore be concluded that joint replacement can improve frailty in patients independent of the analysed characteristics.
Table 4.
Logistic Regression on predictors of improvement in frailty after THA/TKA
| Univariate | Multivariate | |
|---|---|---|
| (Intercept) | 1.65 *** | |
| [0.99, 2.31] | ||
| Age | -0.10 | -0.04 |
| [-0.60, 0.39] | [-0.60, 0.52] | |
| Gender (0 = female) | -0.56 | -0.65 |
| [-1.58, 0.46] | [-1.78, 0.49] | |
| BMI | 0.13 | -0.03 |
| [-0.38, 0.63] | [-0.60, 0.54] | |
| SPPB-Score | 0.10 | 0.04 |
| [-0.39, 0.60] | [-0.60, 0.68] | |
| GDS-Score | -0.38 | -0.38 |
| [-0.83, 0.07] | [-0.90, 0.14] | |
| NRS-Score | -0.03 | 0.01 |
| [-0.51, 0.45] | [-0.52, 0.54] |
The outcome was a dichotomous variable indicating whether there was an improvement in frailty between t0 and t3. *** p < 0.001; ** p < 0.01; * p < 0.05.
Discussion
Purpose of the study
Frailty has mainly been considered as a predictor of adverse events after surgery. There are several studies on this in the field of arthroplasty [21–23]. For example, Meyer et al. described a higher rate of reoperations, hospital readmissions, surgical and non-surgical complications, and blood transfusions in elderly frail patients [21]. In Johnson et al. frailty was associated with increased perioperative complication rates and mortality [23]. This should be considered before indicating THA/TKA, especially if geriatric co-management is not possible. Risk stratification is of great importance here. However, little is known about the impact of hip and knee replacement in OA on frailty. OA is considered the most prevalent chronic joint disease in the world and has a particularly high mortality rate when combined with frailty [7, 24]. Some publications have shown a strong, independent association between OA and pre-frailty/frailty in people aged 65 years and older [6, 25, 26]. The purpose of this prospective study was to show that in elderly patients with OA, primary hip or knee replacement can have a significant impact on the regression or reversibility of frailty. The results suggest that THA and TKA are effective interventions for the prevention and treatment of pre-frailty and frailty in older patients with osteoarthritis. Participants with pre-frailty and a median PFP score of 2 preoperatively had a median PFP score of 0 3 months after THA/TKA and were robust. In the frail patients with a preoperative medial PFP score of 3, the medial PFP score decreased to 1 after surgery. The patients were no longer in a frail condition.
Interventions for frailty
Due to the high prevalence of OA and frailty in people aged 65 years and older, the strong association between the two processes, and the fact that frailty is a predictor of increased mortality in people with OA [24], there is an international call for preventive and therapeutic interventions [6]. However, little is known about the reversibility of pre-frailty and frailty. So far, interventions have mainly been limited to physical training, high-protein diets, or a combination of both [9]. These conservative interventions have been studied and applied in pre-frail/frail patients without the context of OA and indicated THA or TKA. However, this type of intervention may play a role in the future before surgery (prehabilitation) to reduce the risk of surgery. Frailty can be influenced and is therefore a modifiable preoperative risk factor that is associated with some adverse events [21, 23]. Currently, there is only good evidence for physical training and the combination of physical training and high-protein diet [9].
The results of previous interventions to delay the progression of frailty or improve frailty are sometimes very limited [9]. Also, many studies focus on pre-frail patients. In 2018, Gené Huguet et al. achieved a significant return from pre-frailty to robustness through a six-month interdisciplinary intervention based on physical activity, Mediterranean dietary counselling, an assessment of inappropriate prescribing in patients with polypharmacy and a social assessment. Frail patients were not included [27]. This result could also be achieved in our study by a THA or TKA. However, hip or knee replacement surgery has also been shown to significantly reduce the stage of existing frailty.
Reversing frailty in older adults
Frailty probably seems to be more influenceable than previously assumed. However, further studies are certainly needed on this. Factors that may trigger or exacerbate frailty, but also lead to regression or even reversibility, should be explored. The recently published study by Kolle et al. emphasises the topicality and relevance of the study results in the context of reversing frailty. The approach in our work is in line with the current understanding of the reversal of frailty [28]. Despite the growing importance of frailty, there is still no international consensus on a uniform definition and assessment. The frailty score proposed by Fried et al. [9] is suitable for prospective studies and intervention evaluation. In addition, PFP has biological validity and is easy and inexpensive to measure [29].
Limitations
This study has some limitations. The study is part of the ongoing SOG trial and the analyses presented here were not originally planned. For this reason, there is no control group. Although the participants had exhausted all conservative measures (analgesics including opioids, physiotherapy and often rehabilitation) as a prerequisite for the surgery, there are always circumstances that could have influenced the frailty even without special intervention. This could include, for example, psychosocial aspects or the treatment of comorbidities. However, as previous studies have shown, interventions are usually needed to achieve regression or even reversibility of pre-/frailty. Spontaneous improvements are hardly to be expected [9]. This is a single-centre study with possible limitations in the heterogeneity of the study population and potential ‘centre bias’. Whether and to what extent geriatric co-management provides additional benefits in improving frailty, especially after 3 months, is unknown. No data are available on this. This is being investigated for the first time in the still ongoing SOG study. Strictly considered, there are some factors postoperatively that could possibly play a role beyond that, e.g. surgery according to the fast-track principle, duration and type of rehabilitation or postoperative complications. Here, however, one comes up against ethical limits. It will not be possible to refuse a patient geriatric co-management or a certain rehabilitation if it is necessary. On the contrary, these procedures are necessary to regain joint function.
The fact that preoperatively all conservative treatments (physical training, etc.) were exhausted and after 3 months postoperatively the frailty status improved in 80% of the participants strongly suggests the benefit of hip/knee replacement.
Strengths
A major strength of this study is its prospective design with 101 participants. Studies on interventions for frailty often have a small number of participants. It is very difficult to recruit older people with frailty to take part in a trial. In previous studies, the proportion of participants with pre-frailty often predominated. Many trials included people who were either pre-frail or frail. In this study, even slightly more patients with frailty could be included. Separate analyses were performed for total, pre-frail and frail participants. The study population has a variety of risk factors for frailty such as advanced age, female gender, obesity, multimorbidity, malnutrition, polypharmacy, and reduced mobility according to Hoogendijk et al. [1] (Table 1). There were few drop-outs and few patients were lost to follow-up. Data analysis was performed externally and independently by the Department of Health Economics at the Technical University of Munich.
Identifying frailty as a predictor of adverse events is important. But pre-frailty and frailty should also be perceived as a risk factor that can be modified. With appropriate interventions, both can be improved or reversed. It may be possible not only to achieve functional improvement, but also to improve prognosis and reduce mortality. Therefore, further studies on interventions for frailty are needed.
Conclusion
Primary total hip and knee arthroplasty results in a significant decrease in frailty score in older patients with OA as measured by the Fried Frailty Phenotype. Pre-frail participants were often robust after joint replacement. Frailty could be improved by THA or TKA to the pre-frailty stage, so that no further frailty was present. In conclusion, joint replacement can be seen as an effective intervention for the prevention and treatment of frailty in patients with hip and knee osteoarthritis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1. Repeated measures ANOVA for the 5 subscores of the Fried Frailty Phenotype
Additional file 2. Repeated measures ANOVA for the THA and TKA subgroups
Acknowledgements
Not applicable.
Abbreviations
- ANOVA
Analysis of variance
- BMI
Body Mass Index
- CCI
Charlson Comorbidity Index
- df
degrees of freedom
- GDS
Geriatric Depression Scale
- ges
generalised eta squared
- IADL
Instrumental Activities of Daily Living
- MMSE
Mini-Mental State Examination
- MSE
Mean squared error
- NRS
Nutritional Risk Screening
- OA
Osteoarthritis
- PFP
Physical Frailty Phenotype
- SD
Standard deviation
- SE
Standard error
- SOG
Special Orthopaedic Geriatrics
- SPPB
Short Physical Performance Battery
- THA
Total hip arthroplasty
- TKA
Total knee arthroplasty
- wk
week
Author contributions
MM, GM and TK originated the idea for the study and led on its design. MM and JG supervised the project. GM, FG, LP, DH, AS, JG, KM and TK participated in the design of the study and were responsible for data acquisition. PB, MM and TK contributed to analysis and interpretation of data. PB provided statistical consultation. TK drafted the manuscript. MM, GM and PB revised the manuscript critically for important intellectual content. All authors read and approved the final version of the manuscript.
Funding
This study used data from participants in the SOG trial. The SOG study was fully funded by the German Federal Joint Committee - GBA (Grant no. 01VSF19030 (SOG)) and the funding management was carried out by the German Aerospace Center. The funder played no role in the conception or execution of the study, data analyses or results reporting. The authors and their contributions to the manuscript are independent from the funder.
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data are available on reasonable request from the corresponding author.
Declarations
Ethics approval and consent to participate
The study is part of the SOG trial approved by the Ethics Committee of the University of Regensburg (2020/06/24, No. 20-1837-101) and was conducted in accordance with the guidelines of the Declaration of Helsinki. All participants provided written informed consent. No financial compensation was offered for participation in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–75. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 4.Cesari M, Prince M, Thiyagarajan JA, De Carvalho IA, Bernabei R, Chan P, Gutierrez-Robledo LM, Michel JP, Morley JE, Ong P, Rodriguez Manas L, Sinclair A, Won CW, Beard J, Vellas B. Frailty: an emerging Public Health Priority. J Am Med Dir Assoc. 2016;17(3):188–92. doi: 10.1016/j.jamda.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. doi: 10.1093/gerona/59.3.M255. [DOI] [PubMed] [Google Scholar]
- 6.Castell MV, van der Pas S, Otero A, Siviero P, Dennison E, Denkinger M, Pedersen N, Sanchez-Martinez M, Queipo R, van Schoor N, Zambon S, Edwards M, Peter R, Schaap L, Deeg D. Osteoarthritis and frailty in elderly individuals across six European countries: results from the European Project on OSteoArthritis (EPOSA) BMC Musculoskelet Disord. 2015;16:359. doi: 10.1186/s12891-015-0807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Wei X, Zhou J, Wei L. The age-related changes in cartilage and osteoarthritis. Biomed Res Int. 2013;2013:916530. doi: 10.1155/2013/916530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, Mohammed MA, Parry J, Marshall T. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–60. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–86. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 10.Statistisches Bundesamt (Destatis). Germany: The 20 most frequent surgeries of full inpatient hospital patients together. https://www.destatis.de/EN/Themes/Society-Environment/Health/Hospitals/Tables/drg-surgeries-together.html (2023). Accessed 19 Jan 2023.
- 11.Kappenschneider T, Maderbacher G, Weber M, Greimel F, Holzapfel D, Parik L, Schwarz T, Leiss F, Knebl M, Reinhard J, Schraag AD, Thieme M, Turn A, Götz J, Zborilova M, Pulido LC, Azar F, Spörrer JF, Oblinger B, Pfalzgraf F, Sundmacher L, Iashchenko I, Franke S, Trabold B, Michalk K, Grifka J, Meyer M. Special orthopaedic geriatrics (SOG) - a new multiprofessional care model for elderly patients in elective orthopaedic surgery: a study protocol for a prospective randomized controlled trial of a multimodal intervention in frail patients with hip and knee replacement. BMC Musculoskelet Disord. 2022;23(1):1079. doi: 10.1186/s12891-022-05955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel MC, Witschger P, MicroHip A minimally invasive procedure for total hip replacement surgery A modified Smith-Petersen approach. Hip Int. 2006;3:40–7. doi: 10.1177/112070000601603S07. [DOI] [PubMed] [Google Scholar]
- 13.Braun T, Grüneberg C, Thiel C. German translation, cross-cultural adaptation and diagnostic test accuracy of three frailty screening tools: PRISMA-7, FRAIL scale and Groningen Frailty Indicator. Z Gerontol Geriatr. 2018;51(3):282–92. doi: 10.1007/s00391-017-1295-2. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–36. doi: 10.1016/S0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 16.Mahony FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 17.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer O. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 21.Meyer M, Parik L, Leiß F, Renkawitz T, Grifka J, Weber M. Hospital Frailty risk score predicts adverse events in primary total hip and knee arthroplasty. J Arthroplasty. 2020;35(12):3498–504. doi: 10.1016/j.arth.2020.06.087. [DOI] [PubMed] [Google Scholar]
- 22.Meyer M, Schwarz T, Renkawitz T, Maderbacher G, Grifka J, Weber M. Hospital Frailty risk score predicts adverse events in revision total hip and knee arthroplasty. Int Orthop. 2021;45(11):2765–72. doi: 10.1007/s00264-021-05038-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RL, Abdel MP, Frank RD, Chamberlain AM, Habermann EB, Mantilla CB. Impact of Frailty on outcomes after primary and revision total hip arthroplasty. J Arthroplasty. 2019;34(1):56–64. doi: 10.1016/j.arth.2018.09.078. [DOI] [PubMed] [Google Scholar]
- 24.Cacciatore F, Della-Morte D, Basile C, Mazzella F, Mastrobuoni C, Salsano E, Gargiulo G, Galizia G, Rengo F, Bonaduce D, Abete P. Long-term mortality in frail elderly subjects with osteoarthritis. Rheumatology (Oxford) 2014;53(2):293–99. doi: 10.1093/rheumatology/ket348. [DOI] [PubMed] [Google Scholar]
- 25.Wise BL, Parimi N, Zhang Y, Cawthon PM, Barrett-Connor E, Ensrud KE, Lane NE. Frailty and hip osteoarthritis in men in the MrOS cohort. J Gerontol A Biol Sci Med Sci. 2014;69(5):602–8. doi: 10.1093/gerona/glt126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra D, Felson DT, Silliman RA, Nevitt M, Lewis CE, Torner J, Neogi T. Knee osteoarthritis and frailty: findings from the Multicenter Osteoarthritis Study and Osteoarthritis Initiative. J Gerontol A Biol Sci Med Sci. 2015;70(3):339–44. doi: 10.1093/gerona/glu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gené Huguet L, Navarro González M, Kostov B, Ortega Carmona M, Colungo Francia C, Carpallo Nieto M, Hervás Docón A, Vilarrasa Sauquet R, García Prado R, Sisó-Almirall A. Pre Frail 80: multifactorial intervention to prevent progression of Pre-frailty to Frailty in the Elderly. J Nutr Health Aging. 2018;22(10):1266–74. doi: 10.1007/s12603-018-1089-2. [DOI] [PubMed] [Google Scholar]
- 28.Kolle AT, Lewis KB, Lalonde M, Backman C. Reversing frailty in older adults: a scoping review. BMC Geriatr. 2023;23(1):751. doi: 10.1186/s12877-023-04309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cesari M, Leeuwenburgh C, Lauretani F, Onder G, Bandinelli S, Maraldi C, Guralnik JM, Pahor M, Ferrucci L. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83(5):1142–48. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Repeated measures ANOVA for the 5 subscores of the Fried Frailty Phenotype
Additional file 2. Repeated measures ANOVA for the THA and TKA subgroups
Data Availability Statement
The data are available on reasonable request from the corresponding author.