Abstract
There is a need for more insight into the pathogenesis of Streptococcus pneumoniae pneumonia, as the fatality rate associated with this disease remains high despite appropriate antibiotherapy. The host response to pneumococci was investigated after intranasal inoculation of CD1 mice with 107 log-phase CFU of bacteria. We identified five major pathogenesis steps from initial infection to death. In step 1 (0 to 4 h), there was ineffective phagocytosis by alveolar macrophages, with concurrent release of tumor necrosis factor alpha (TNF), interleukin-6 (IL-6), and nitric oxide (NO) in bronchoalveolar lavage (BAL) fluid, TNF, IL-6, and interleukin-1 alpha (IL-1) in lung tissues, and IL-6 in serum, which were associated with tachypnea and hemoconcentration. In step 2 (4 to 24 h), bacterial growth in alveoli and polymorphonuclear cell recruitment from bloodstream to lung tissue (high myeloperoxidase levels) to alveoli were associated with high release of all three cytokines and leukotriene B4 (LTB4) in tissue and BAL fluid, as well as transient spillover of IL-1 in serum. In step 3 (24 to 48 h), despite downregulation of TNF and IL-1 in BAL fluid and lungs, there was appearance of injury to alveolar ultrastructure, edema to interstitium, and increase in lung weight as well as regeneration of type II pneumocytes and increased secretion of surfactant; bacteria progressed from alveoli to tissue to blood, and body weight loss occurred. In step 4 (48 to 72 h), strong monocyte recruitment from blood to alveoli was associated with high NO release in tissue and BAL fluid, but there was also noticeable lymphocyte recruitment and leukopenia; bacteremia was associated with TNF and IL-6 release in blood and thrombocytopenia. In step 5 (72 to 96 h), severe airspace disorganization, lipid peroxidation (high malondialdehyde release in BAL fluid), and diffuse tissue damage coincided with high NO levels; there was further increase in lung weight and bacterial growth, loss in body weight, and high mortality rate. Delineation of the sequential steps that contribute to the pathogenesis of pneumococcal pneumonia may generate markers of evolution of disease and lead to better targeted intervention.
The fatality rate associated with Streptococcus pneumoniae still approximates 23% despite the use of potent antibiotics and aggressive intensive-care support (57). Death can occur days after initiation of antibiotic therapy, when tissues are sterile and the pneumonia is clearing. There is growing evidence that aspects of the immune response greatly contribute to the high mortality rate: while immunosuppressed patients die as a consequence of poor host response, immunocompetent hosts face overwhelming inflammatory reactions that contribute to tissue injury, shock, and death (37, 69, 82, 88).
While most bacterium-induced pneumonia rodent models have been used to evaluate antibiotic pharmacokinetics and efficacy (7, 8, 50, 59, 67, 84, 87), various elements of the host response, including chemokines, pro- and anti-inflammatory cytokines, oxygen radicals, blood components, and immune and nonimmune cells, have also been characterized (10, 25, 45, 74, 77, 81, 86). Some pathogenesis studies have focused on interactions between bacterial or host factors, histological lesions, and edema (11, 19, 47, 78). However, thorough, detailed study of the inflammatory response to pneumococci in the lung over time is difficult to access from the diverse publications as a single time course evaluation of the infection. Although cytokines have been found in bronchoalveolar lavage (BAL) fluid or plasma of animals (77) or patients (20, 53, 61), little correlation has been made so far between cytokine levels within lung tissue, BAL fluid, and serum simultaneously, time course of the disease, and outcome of pneumonia. The chronology of leukotriene release and inflammatory cell recruitment has not been studied in association with kinetics of cytokines. In addition, nitric oxide (NO) release and its relationship to histopathology during pneumococcal pneumonia in mice have not been reported. This is the first pathogenesis study that addresses each of these concerns through extensive sets of data, thus providing new insights into the sequential pathogenesis of S. pneumoniae pneumonia which we hope will help establish guidelines for therapy with biological response modifiers.
(The results of this work have been presented in part elsewhere [9a, 9b, 20a, 55a]).
MATERIALS AND METHODS
Pneumococcal pneumonia model.
Female CD1 Swiss mice (20 to 22 g) were used for all experiments. Pneumonia was induced with a penicillin-susceptible clinical strain of S. pneumoniae serotype 3 originally isolated by blood culture, monthly passaged in mice for 1 year, and transparent in colonial morphology. The infection was as previously described (8), with minor modifications. Briefly, lightly anesthetized animals received an inoculum of 107 log-phase CFU of bacteria in 50 μl of phosphate-buffered saline (PBS) applied at the tip of the nose and involuntarily inhaled. To facilitate migration of the inoculum to the alveoli, mice were held in a vertical position for 2 min. They had free access to mouse chow and water throughout the experiment and were exposed to alternate standardized light/dark periods of 14 h/10 h/day.
Experimental protocol.
Each group consisted of 12 infected animals which were sacrificed at time zero (preinfection) and at 1, 2, 4, 12, 24, 48, 72, and 96 h postinfection. Blood, BAL fluid, and lung tissue were sampled to determine cellular response and to quantify inflammatory mediators. Microbiological counts were determined in blood and lung tissue. Histopathology of lung tissue was also done. Twelve additional unsacrificed infected animals were used as controls to monitor death rate.
Development of infection.
Body weight and mortality rate were recorded every day following infection. Lung weight was noted in every sacrificed animal. Piloerection, tachypnea, prostration, and morbidity were also noted when present. Bacterial growth in lungs was monitored by using a microbiological assay: lungs and heart were taken together and weighed before and after blood removal with 20 ml of sterile saline infused through the right ventricle until the effluent was clear; lungs were then homogenized with a Potter apparatus at a ratio of 1 g/10 ml of a 50 mM potassium phosphate buffer (pH 6.5); bacteria were quantified in 50 μl of this crude homogenate by plating 10-fold dilutions on sheep blood agar and incubating the plates for 18 h at 37°C in an atmosphere of 5% CO2. Homogenates were then frozen for subsequent detection of inflammatory mediators as described below. Progression of bacterial growth to the bloodstream was monitored by sampling blood from the retro-orbital sinus of the left eye with a heparinized capillary, followed by direct plating on sheep blood agar. Bacteremia was reported as the percentage of positive hemocultures after incubation for 18 h at 37°C in 5% CO2.
Hematological profile.
Blood was also deposited in heparinized Microvette (Sarstedt, Montreal, Quebec, Canada) tubes and analyzed with a Coulter counter for leukocytes, erythrocytes, hemoglobin, hematocrit, and platelets. Differentiation of cell populations was obtained after counting 100 leukocytes on a smear stained with Wright reagent.
Histology.
A scoring system for histopathology of lung tissue was derived from the work of Davis et al. (19). Briefly, whole lungs were fixed in formalin, embedded in paraffin, and then processed for light microscopy (10× objective) to quantify the percentage of lung involved in the inflammatory process. Tissue sections of inflamed areas were also fixed in glutaraldehyde followed by osmium tetroxide and then processed for light (40× objective) and electron microscopy according to standard methodology (9). A scoring grid (inflammatory cells + hemorrhage + edema + tissue injury + regeneration) was used to quantify inflammation (Table 1). The overall histopathologic score was calculated as percentage of lung involvement × scoring grid. Bacterial growth and phagocytosis were also estimated.
TABLE 1.
Scoring grid for histopathologic examination of lung tissue
| Parameter | Score | Interpretation |
|---|---|---|
| Inflammatory cells | 0 | No neutrophils, lymphocytes, or eosinophils in alveoli and lung tissue; normal amount of alveolar macrophages |
| 1 | Slight increase in the number of any inflammatory cell population | |
| 2 | Recruitment of a large number of inflammatory cells | |
| Hemorrhage | 0 | No erythrocytes in alveoli |
| 1 | Few erythrocytes in alveoli | |
| 2 | Numerous erythrocytes | |
| Edema | 0 | Absent |
| 1 | Minimal swelling of alveolar walls | |
| 2 | Widespread swelling of tissue interstitium | |
| Tissue injury | 0 | Absent |
| 1 | Minimal modification in alveolar architecture | |
| 2 | Widespread disorganization of lung tissue, including injuries to organelles | |
| Regeneration | 0 | Normal amount of type II pneumocytes and surfactant |
| 1 | Slight increase in the number of type II pneumocytes and surfactant secretion | |
| 2 | Marked increase in type II pneumocytes and surfactant |
Inflammatory cells.
Leukocyte recruitment in alveoli was determined by BAL. Briefly, animals were killed by decerebration, the trachea was exposed and intubated with a catheter, and then repeated 1-ml injections of PBS (without calcium and anticoagulant) were made until a total of 3 ml of BAL fluid was harvested. BAL fluid was centrifuged at 1,200 rpm for 10 min, and supernatant was frozen at −80°C for subsequent analysis of inflammatory mediators. Cells in the pellet were resuspended in PBS for quantification of leukocytes with a hemacytometer, and cell populations were enumerated from Diff-Quick (catalog no. B4132-1; Baxter, Pointe-Claire, Québec, Canada)-stained cytospin preparations. To avoid influence of a BAL on cytokine levels in lung homogenates and to allow rapid removal of blood from tissues for the dosage of myeloperoxidase (MPO), BALs were performed in six mice per group per time, while six of the infected mice were sacrificed for sampling of tissues.
Neutrophil (polymorphonuclear cell [PMN]) infiltration in lung tissue was quantified by the measurement of MPO as follows. Mice were sacrificed, and lungs were removed, cleared of blood, and homogenized in 50 mM potassium phosphate buffer (pH 6.5); to 100 μl of crude homogenate was added 100 μl of hexadecyltrimethylammonium bromide to achieve a final concentration of 0.5%; then homogenates were frozen at −80°C, thawed, sonicated for 30 s, and centrifuged at 6,000 rpm in a microcentrifuge for 30 min at 4°C. MPO was evaluated by adding 150 μl of homogenate supernatant to 825 μl of phosphate buffer, 75 μl of o-dianisidine at 1.25 mg/ml of distilled water, and 75 μl of hydrogen peroxide at 0.05%. The enzymatic reaction was stopped after 15 min by addition of 75 μl of 1% sodium azide, and absorbance was read at 450 nm against a standard curve made with commercial MPO (catalog no. M-6908; Sigma, Mississauga, Ontario, Canada).
Inflammatory mediators.
Tumor necrosis factor alpha (TNF), interleukin-1 alpha (IL-1), and IL-6 levels were detected in the supernatant of BAL fluid, in the supernatant of lung homogenates, and in sera of control and infected animals. Lung homogenates were prepared in potassium phosphate buffer as described above. To 600 μl of crude homogenate was added 600 μl of phosphate buffer containing aprotinin (20 U) and 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS; 0.2%), and samples were then frozen at −80°C. After thawing, samples were centrifuged at 6,000 rpm in a microcentrifuge for 30 min at 4°C, and mouse enzyme-linked immunosorbent assay kits were used to detect all cytokines in supernatants (TNF, IL-1, and IL-6; catalog no. 80-2802-00, 1900-01, and 80-3748-01, respectively; Genzyme Corporation, Cambridge, Mass.). Procedures for the assays were provided with the commercial kits. Serum was obtained by centrifuging blood for 10 min at 4°C in a microcentrifuge at maximal speed.
Leukotriene B4 (LTB4) assay was also performed in BAL supernatant, lung homogenate supernatant, and serum. LTB4 levels were quantified by radioimmunoassay kits after acidification of all samples to pH 3.5 with HCl, followed by extraction of lipids with ethyl acetate. LTB4 in extracted material was detected as instructed for the commercial product (catalog no. 8-6020; Cedarlane, Hornby, Ontario, Canada).
The release of NO in BAL supernatant, lung homogenate supernatant, and serum was evaluated through measurement of its oxidized nitrite and nitrate metabolites by the colorimetric method of Griess (28).
Biochemistry.
Injuries to cell membranes were monitored through the measurement of malondialdehyde (MDA), a metabolite resulting from lipid peroxidation, which we detected by the method of Ohkawa et al. (56) after slight modifications. Briefly, 0.2 ml of BAL supernatant, lung homogenate supernatant, or serum was added to 0.2 ml of 8.1% sodium dodecyl sulfate 1.5 ml of 20% acetic acid (pH 3.5), 1.5 ml of 0.8% thiobarbituric acid, and 0.6 ml of distilled water. The mixture was heated for 1 h at 100°C, and then 1 ml of water and 5 ml of butanol-pyridine (15:1) were added and the tubes were vigorously shaken. The upper organic phase obtained after centrifugation for 10 min at 4,000 rpm was read at an absorbance of 532 nm. We calculated the amount of MDA present in the samples after establishing a standard curve with 1,1,3,3-tetramethoxypropane which in the above experimental conditions yields MDA in a 1:1 molecular ratio.
Total protein content, an indirect test measuring vascular permeability and tissue injury which allow proteins to diffuse from capillaries to tissues, was verified in BAL supernatant and lung homogenate supernatant. Dosages were determined by the Bradford colorimetric method with Coomassie blue (catalog no. 23200; Pierce, Rockford, Ill.) as the reagent (42).
Statistical analysis.
All statistical analyses were performed with StatView SE+ Graphics (Abaccus Concepts Inc., Berkeley, Calif.). Statistical analysis of the difference between groups was performed by analysis of variance using a least-squares method. If the F test indicated a difference within groups (P < 0.05), group comparisons were performed by Fisher’s protected least significant difference test, and P < 0.05 was considered significant. All data are presented as means ± standard errors of the means (SEM).
RESULTS
Mouse infection model.
Inoculation of mice with 107 S. pneumoniae cells resulted in a transient hyperdynamic state of the animals, with tachypnea and piloerection observable 4 h after infection. Animals apparently recovered for a while but then developed a gradually pronounced hypodynamic state (lifeless behavior, prostration) which was associated with gradual loss in body weight from 24 h until death. Body weight fell from 20.0 g to 18.0, 16.8, 15.2, and 14.2 g at 0, 24, 48, 72, and 96 h, respectively. Although 21% of the animals died as early as 48 h after infection, death usually occurred at 72 h (50%) or later (29%). The early and late physiological manifestations were associated with hematological disorders such as hemoconcentration at 4 h (14.6% increase in hematocrit compared to controls at 0 h; P < 0.01) and thrombocytopenia at 72 h (21.5% decrease compared to controls; P < 0.05) but also with immunological and microbiological phenomena described below.
After an initial stabilization of bacterial growth over the first 4 h at 106 CFU/g, which may be related to mechanical and immune barriers against infection, bacterial counts in lung homogenates increased to 107 CFU/g and were maintained at that level for 60 h. They culminated at extremely high levels at 96 h (>108 CFU/g), which is quite comparable to the levels recovered by other investigators at the time of death (7). Hemocultures became positive at 24 h, with 83% of the animals developing bacteremia between 24 and 48 h and 100% demonstrating it between 72 and 96 h.
Histopathology.
Morphological examination of lungs showed progressive edema and hepatization of tissues; i.e., the gross appearance of the lungs resembled that of the liver due to passage of blood elements from capillaries to tissues. These modifications were associated with intense inflammatory response, tissue injury, and increased lung weight. In fact, lung weight increased from 183 ± 10 mg at time zero to 258 ± 11 mg at 24 h (P < 0.01); there was a plateau from 24 to 48 h, followed by an increase to 326 ± 9 mg at 72 h (P < 0.01 between 48 and 72 h) and a second plateau until death. Histopathological examination by light microscopy revealed progressive involvement of the whole lung surface by the inflammatory process (Table 2). Initial foci of inflammation (<24 h) were restricted to perivascular areas localized close to infected bronchioles. Progressive parenchymal involvement including interstitial edema, hemorrhage, modification in alveolar ultrastructure, and proliferation of type II pneumocytes appeared over the 24- to 48-h period in all lobes of the left and right lungs and progressed from inner to outer areas until 60 to 75% of the whole lung surface showed signs of alteration. Finally, widespread inflammation and tissue injury characterized the 72- to 96-h period, with 100% of the lung surface being involved (Fig. 1).
TABLE 2.
Histopathology of lung tissue after intranasal challenge with 107 CFU of S. pneumoniae
| Time (h) | % of lung involved | Score
|
Overall scorea | ||||
|---|---|---|---|---|---|---|---|
| Inflammatory cells | Hemorrhage | Interstitial edema | Tissue injury | Regeneration | |||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 15 | 1 | 0 | 0 | 0 | 0 | 15 |
| 12 | 30 | 1 | 0 | 0 | 0 | 0 | 30 |
| 24 | 60 | 1 | 1 | 1 | 1 | 1 | 300 |
| 48 | 75 | 2 | 1 | 2 | 2 | 2 | 675 |
| 72 | 100 | 2 | 2 | 2 | 2 | 1 | 900 |
| 96 | 100 | 2 | 2 | 2 | 2 | 2 | 1,000 |
Calculated as percentage of lung involvement × scoring grid (inflammatory cells + hemorrhage + edema + tissue injury + regeneration). See Table 1 for explanations of scoring grid.
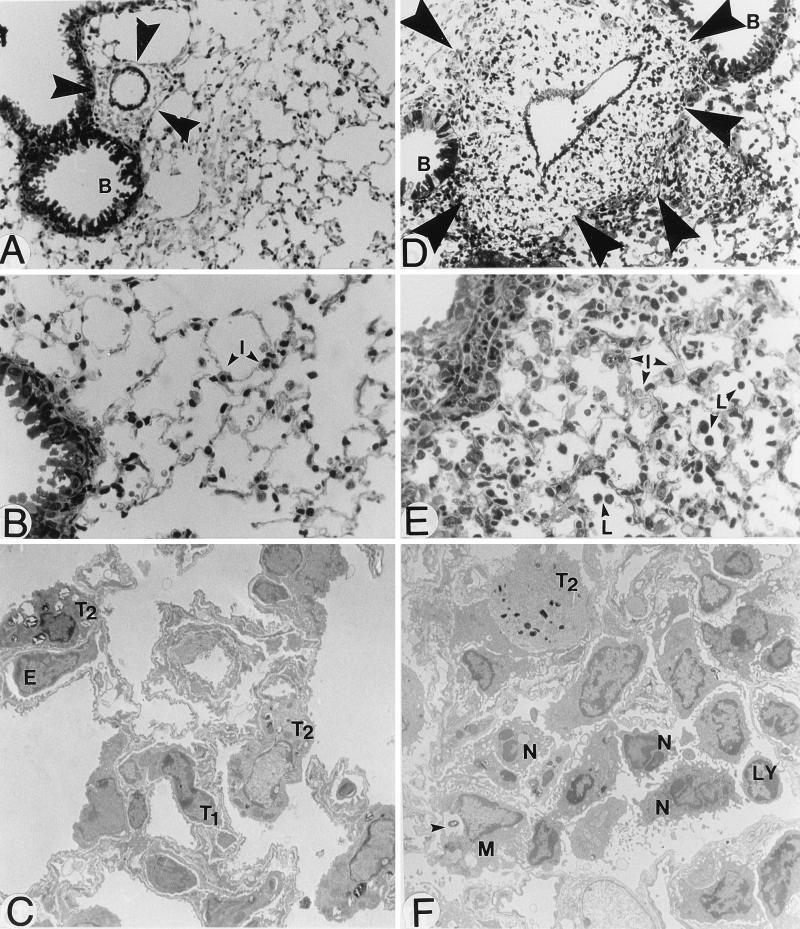
FIG. 1.
Light and electron microscopy of lung architecture of normal mice (A to C) and mice infected with 107 S. pneumoniae cells and sacrificed 72 h later (D to F). Perivascular areas (arrowheads) close to bronchioles (B) (A and D; magnification, ×400) were greatly enlarged after infection, due to edema and phagocyte recruitment. Interstitial tissues (I) in alveolar areas (B and E; ×400) were also enlarged, and leukocytes (L) could be seen in alveoli. Tissue injury characterized the infectious and inflammatory processes (C and F; ×5,000). Macrophages (M) containing ingested bacteria (arrowhead) were seen, as well as recruited neutrophils (N) and lymphocytes (LY). T1, type I pneumocyte; T2, type II pneumocyte; E, endothelial cell.
Electron microscopy confirmed the increasing recruitment of phagocytic cells in both tissue interstitium and alveoli over time, with PMNs being progressively replaced by monocytes and a few lymphocytes at 72 to 96 h. Erythrocytes in alveoli were so abundant at 72 to 96 h that they became visible in BAL fluid. Tissue edema became so intense at this same period that alveoli could hardly be distinguished in tissue sections, and breathing of the animals became laborious. Type II pneumocytes proliferated and secreted increased amount of surfactant in alveolar spaces (Fig. 2A and C). Widespread disorganization of lung architecture characterized the 72- to 96-h observations, and the presence of numerous collagen fibers possibly indicated a scarring process initiated by fibroblasts.
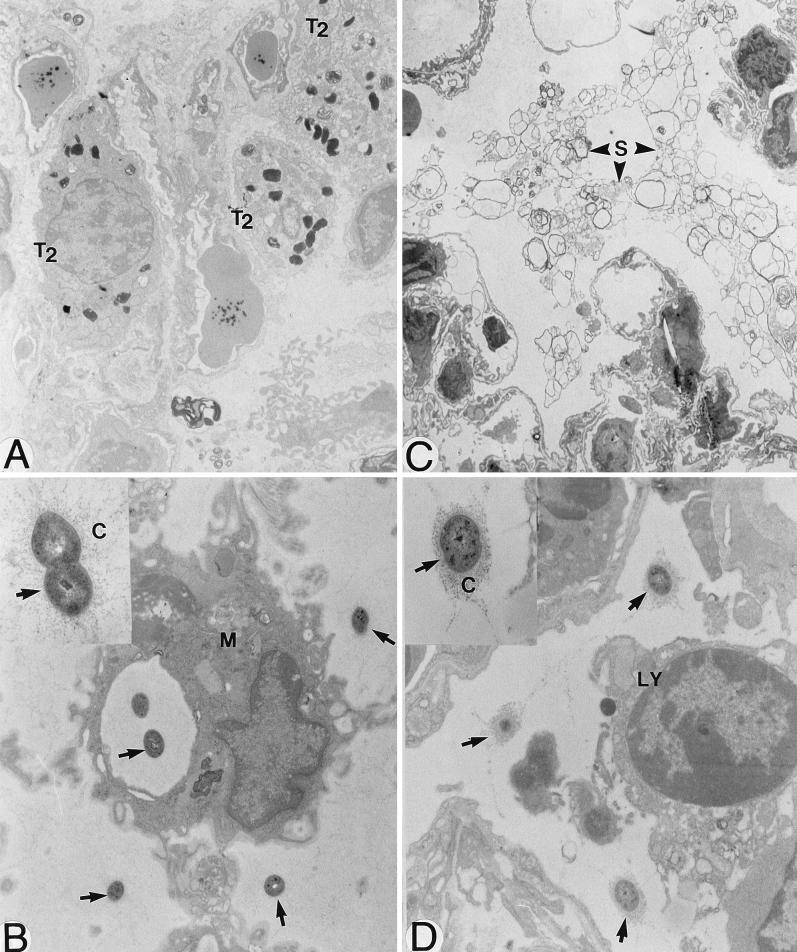
FIG. 2.
Electron microscopy of lungs of mice infected with 107 S. pneumoniae cells and sacrificed 72 h later. Type II pneumocytes (T2) proliferated after infection (A; magnification, ×9,600) and secreted abnormal amounts of surfactant (S) in alveoli (C; ×6,000). Although S. pneumoniae cells (arrows) were partly eradicated through phagocytosis (M, macrophage) (B; ×18,000), extracellular killing also seemed to occur, as the polysaccharide capsule (C) of bacteria localized outside phagocytes in areas of intense inflammation appeared more disaggregated (thinner and more diffuse) (B [arrows] and inset; ×44,000) than the capsule of bacteria localized in less severely inflamed areas (D [×18,000] and inset [×44,000]). LY, lymphocyte.
Phagocytosis of pneumococci both by macrophages and PMNs was noted. Moreover, extracellular killing due to release of toxic components by phagocytes or other host cells seemed to happen, as the polysaccharide capsule of many bacteria localized outside phagocytes in areas of intense inflammation appeared more disaggregated (thinner and more diffuse; Fig. 2B and inset) than the capsule of bacteria localized in less severely inflamed areas (Fig. 2D and inset).
Inflammatory cells.
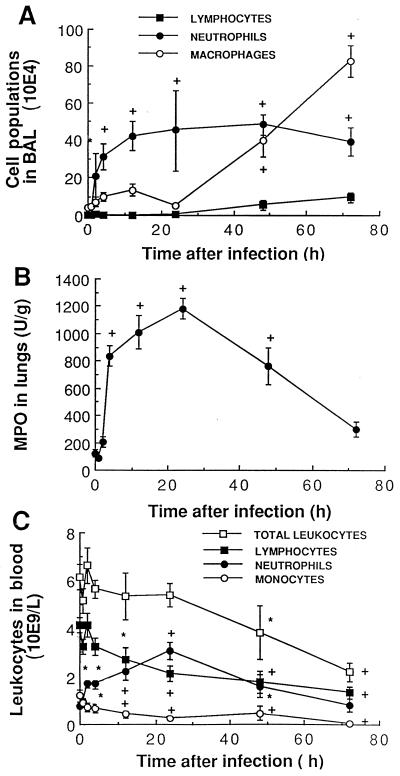
Recruitment of inflammatory cells from blood vessels to lung tissue and alveoli was monitored over time (Fig. 3). PMNs increased significantly in blood (Fig. 3C), lung tissue (Fig. 3B), and BAL fluid (Fig. 3A) as early as 2 to 4 h after infection and reached high levels at 24 h in all samples. Their numbers decreased gradually in blood and lung tissue thereafter while remaining elevated in BAL fluid until death of the animals. Monocyte/macrophage and lymphocyte levels decreased steadily in blood until death (Fig. 3C), as both populations moved to BAL fluid later (≥48 h) than PMNs (Fig. 3A). Therefore, strong variations in individual cell populations occurred over time, despite an apparent constant number of total leukocytes in blood samples over the first 24 h (Fig. 3C). When comparing data for mice and humans, one must remember that the blood of normal CD1 mice is composed of approximately 67% lymphocytes, 12% PMNs, and 19% monocytes.
FIG. 3.
Recruitment of inflammatory cells from blood vessels (C) to lung tissue (B) to BAL (A) as a function of time after intranasal infection with 107 S. pneumoniae cells. Cell populations (mean ± SEM) in blood and BAL fluid were counted, and neutrophils in lung homogenate were measured by quantifying MPO. ∗, P < 0.05 compared with preinfection values; +, P < 0.01 compared with preinfection values.
Inflammatory mediators.
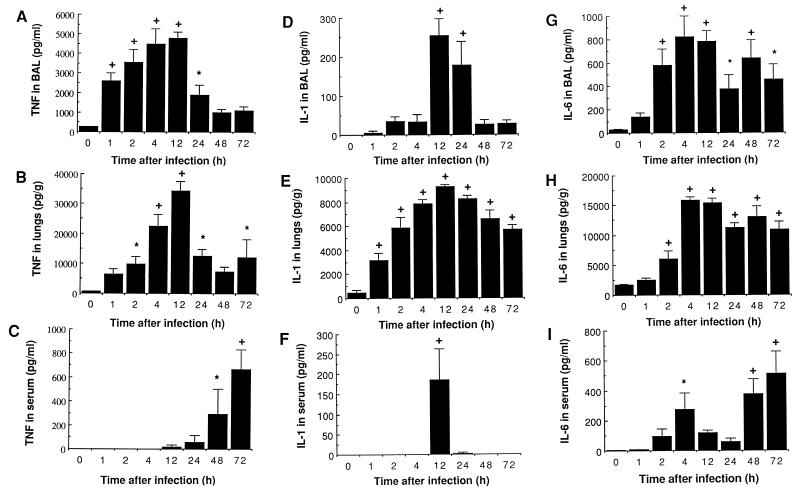
Cytokine levels are reported in Fig. 4. TNF was the first cytokine to be recovered in BAL fluid, with a significant increase from 1 to 12 h compared with preinfection values (Fig. 4A). The appearance of this cytokine in BAL fluid was transient despite progression of the infectious process, as TNF rapidly dropped to normal values after 12 h. TNF release in lung tissue reached extremely high levels (34,388 pg/g at 12 h), which may represent the participation of interstitial inflammatory cells as well as noninflammatory cells (Fig. 4B). TNF was absent or detected in very low levels in serum until 48 and 72 h, and then a rapid increase was noted (Fig. 4C). This increase closely coincided with the migration of bacteria to the bloodstream. Taken together, these results clearly demonstrate compartmentalization of TNF secretion to the site of infection, with successive appearances in BAL, lung tissue, and finally blood.
FIG. 4.
Mean (SEM) cytokine levels in cell-free BAL, lung homogenates previously cleared from blood, and serum of mice infected with 107 S. pneumoniae cells. TNF in BAL (A), lung (B), and serum (C), IL-1 in BAL (D), lung (E), and serum (F), and IL-6 in BAL (G), lung (H), and serum (I) are reported. ∗, P < 0.05 compared with preinfection values; +, P < 0.01 compared with preinfection values.
A sustained IL-1 release was observed in lung tissue throughout the experiment (Fig. 4E) combined with a very transient appearance of this cytokine in cell-free BAL and serum at 12 h (Fig. 4D and F). Both TNF and IL-1 peaked at 12 h in BAL and lungs (Fig. 4A, B, D, and E), a time that preceded bacterial dissemination to blood vessels, which might incriminate these cytokines in the breaking of the alveolo-capillary barrier.
IL-6 levels increased significantly very early in BAL and lung tissue after infection (2 h), with a peak at 4 h that also corresponds with partial release of IL-6 in serum (Fig. 4G to I). They remained elevated quite constantly throughout the experiment. In fact, serum levels indicated two episodes for circulating IL-6 (Fig. 4I): an early brief appearance at 4 h, and a late sustained production from 48 h until death which correlates with bacteremia.
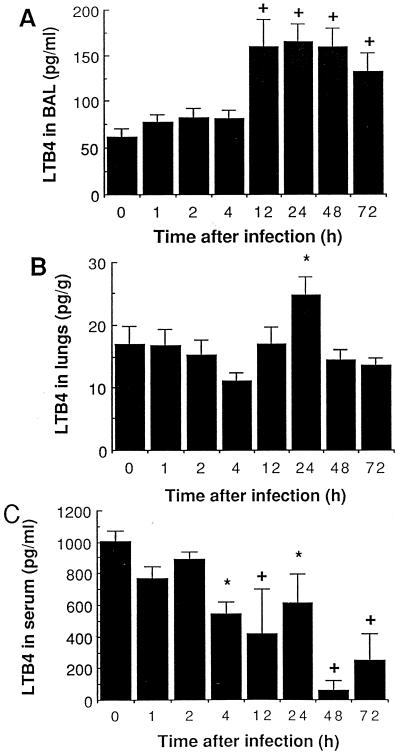
LTB4 levels, which were higher in BAL fluid than in the lung, increased in cell-free BAL fluid from 12 to 72 h and in lung tissue at 24 h (Fig. 5A and B), which accompanied and followed, rather than preceded, PMN recruitment. The amount of LTB4 recovered in serum decreased significantly over time (Fig. 5C), as did the number of monocytes and other secretory inflammatory cells in whole blood (Fig. 3C).
FIG. 5.
Mean (SEM) LTB4 levels in cell-free BAL (A), in lung homogenates previously cleared from blood (B), and in serum (C) of mice infected with 107 S. pneumoniae cells. ∗, P < 0.05 compared with preinfection values; +, P < 0.01 compared with preinfection values.
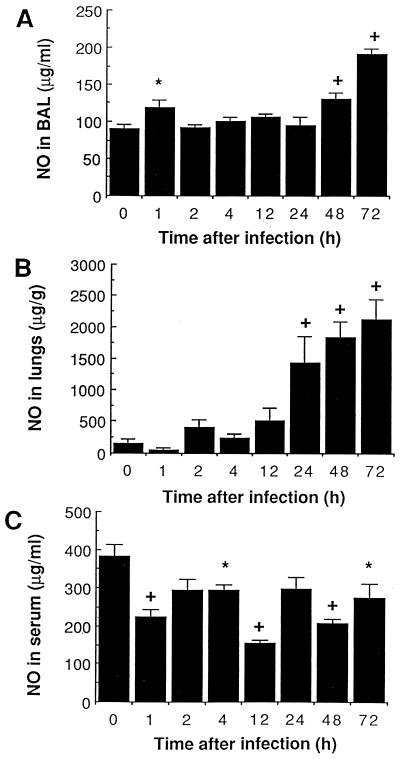
A reduction in NO release was observed in serum of infected animals throughout the experiment (Fig. 6C). By contrast, a brief spontaneous release of NO was noted in BAL shortly (1 h) after infection (Fig. 6A), at a time when only alveolar macrophages are harvested in BAL. A second sustained phase (mostly 48 to 72 h) of high NO secretion in lung tissue (Fig. 6B) and BAL (Fig. 6A) corresponded with the massive monocyte/macrophage recruitment period (Fig. 3A). Tissue injuries responsible for, or resulting from, these high NO levels were noticed during the same period (Fig. 1 and 2).
FIG. 6.
Mean (SEM) NO levels in cell-free BAL (A), in lung homogenates previously cleared from blood (B), and in serum (C) of mice infected with 107 S. pneumoniae cells. ∗, P < 0.05 compared with preinfection values; +, P < 0.01 compared with preinfection values.
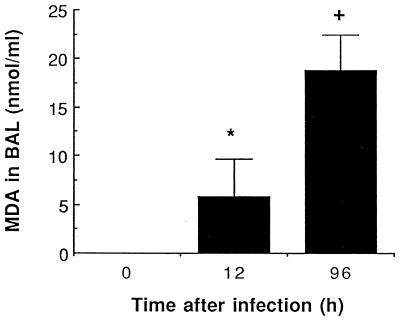
Although oxygen radicals and their oxidative potential for cell membranes were not directly measured, lipid metabolites resulting from membrane peroxidation were detected through MDA production. The release of large amounts of MDA in the alveolar environment was noted at 96 h (Fig. 7). No increase was noted in sera of infected and uninfected animals.
FIG. 7.
Mean (SEM) MDA levels in BAL 12 and 96 h after infection with 107 S. pneumoniae cells. ∗, P < 0.05 compared with preinfection values; +, P < 0.01 compared with preinfection values.
Protein levels increased significantly throughout the experiment, from 4 to 72 h in cell-free BAL fluid and from 12 to 96 h in lung homogenates (P < 0.05; data not shown).
DISCUSSION
Modern therapeutic approaches in infectious diseases have focused on modulation of the host response (63). However, extensive data which correlate histopathology and overall inflammatory response in BAL fluid, lung tissue, and blood of patients suffering from bacterial pneumonia cannot be easily obtained. To better characterize the chronology of events associated with fatal pneumococcal pneumonia, we developed a murine model which allowed us to identify five major pathogenesis steps from initial infection to death. The first two steps corresponded to pulmonary infection in the absence of bacteremia, the third step coincided with transition from pulmonary to systemic infection, and the last two steps were characterized by widespread overwhelming inflammatory reactions that led to severe tissue injuries, hematological and biochemical disorders, and death. In step 1 (between 0 and 4 h after infection), there was partial but ineffective bacterial clearance by resident alveolar macrophages, accompanied by the activation of cytokines (TNF and IL-6 in BAL fluid; TNF, IL-6, and IL-1 in lung tissues; IL-6 in serum) and transient NO release in BAL fluid, which led to physiological and hematological anomalies. In step 2 (between 4 and 24 h), there was bacterial growth in alveoli and recruitment of PMNs from bloodstream to lung tissue and alveoli, associated with high release of all three cytokines and LTB4 at the infected site and transient spillover of IL-1 in serum. In step 3 (between 24 and 48 h), there was downregulation of proinflammatory cytokines (TNF and IL-1) in BAL fluid and lungs, but tissue injuries became visible (loss of alveolar ultrastructure, edema to interstitium, increase in lung weight) and regeneration processes started (proliferation of type II pneumocytes, increased secretion of surfactant), concomitant with progression of bacteria to tissue and blood and noticeable loss in body weight. In step 4 (between 48 and 72 h), overall infection and inflammation were characterized by sharp reduction in blood leukocytes and gradual recruitment of monocytes and lymphocytes to BAL fluid; LTB4 and NO levels were very low in serum but increased in BAL fluid, thus corresponding to the monocyte migration; bacteremia was associated with activation of cytokines (TNF and IL-6) but also with a sharp decrease in platelet counts. In step 5 (between 72 and 96 h), pulmonary histopathological features included severe airspace disorganization with no remaining alveolar architecture and diffuse tissue damage that coincided with high NO and MDA levels recovered in BAL fluid; lung weight further increased; there was unrestrained bacterial growth, loss in body weight, and a high mortality rate.
In our study, resident alveolar macrophages and successive waves of PMNs, monocytes, and lymphocytes failed to eradicate S. pneumoniae. Pneumococci proliferated and induced bacteremia in addition to the initial pulmonary infection. In immunocompetent humans coincidence of pneumonia and bacteremia ranges from 30 to 50% in the absence of antibiotherapy, varying with serotype and the population studied (10). The clinically isolated virulent strain that we used allowed us to investigate both aspects of the infection, as 100% of the mice became bacteremic over time. The presepticemic and septicemic phases of infection were clearly manifested in the successive localization of TNF secretion to BAL fluid and blood, respectively. Confinement of TNF to the site of infection in rats after intratracheal or intravenous injection of endotoxin (55), and in patients suffering from unilobar pneumonia (20), has been reported. The downregulation of TNF in BAL fluid after 48 h despite sustained stimulation by bacterial components also corroborates other data from septicemic animals (70). In the context of pneumococcal pneumonia, TNF measurement may thus prove useful for monitoring the presepticemic and septicemic phases of pneumonia. Additional prognostic factors that appeared to behave similarly in mice and humans include thrombocytopenia and leukopenia, which coincided with bacteremia and death in our model and which are also considered useful negative prognostic factors in community-acquired pneumonia and sepsis (36, 54, 61). Although thrombocytopenia may have resulted from interactions of platelets with bacterial toxins, endogenous cytokines also contribute to coagulation anomalies and disseminated intravascular coagulation that sometimes accompany sepsis (2, 36).
In our model, blood levels of cytokines did not reflect appropriately tissue levels (and ongoing tissue injury), as the latter remained extremely high throughout the experiment while the former showed transient appearance only. The sporadic release of TNF, IL-1, and IL-6 in blood has been observed in other experimental models as well as in patients (20, 33, 53, 61). These observations might explain the discrepancies in the literature concerning the attempts to correlate outcome of pneumonia with cytokine levels. Blood analysis should be interpreted in terms of overall chronological events that mediate pathogenesis of pneumonia. As an example, high levels of TNF and IL-6 in blood at the same time in our model indicated fully developed infection with bacteremia (which possibly contributed to activating cytokines from blood elements); by contrast, increased IL-6 levels in blood in the absence of TNF indicated early disease with limited tissue injury and no bacteremia. According to Puren et al. (61), IL-6 probably reflects severity of stress rather than severity of infection during community-acquired pneumonia; the early high blood IL-6 level in our experiment (4 h) provides support for this hypothesis, as it coincides with early exposure of airways to pneumococci and with early physiological derangements including piloerection and tachypnea.
Monitoring of inflammatory mediators in BAL might also generate markers of evolution of disease, including cytokines, LTB4, and NO. While IL-6 in BAL fluid is most likely a good indicator of early infection, it did not reflect the evolution of infection and should not be considered in this fluid as a good indicator of the progression of the infectious process; the enhanced secretion of TNF, by contrast, correlated with the initial inflammatory response localized to lungs in the absence of systemic involvement. IL-1α, which exerts biologic activity primarily in a membrane-associated form compared to IL-1β or TNF (1), also transiently appeared in cell-free BAL and serum at 12 h postinfection (a time when tissue concentrations peaked at extremely high levels), suggesting a spillover of IL-1 from cells to fluids when overproduction occurred. Detectable levels of IL-1 in serum or BAL fluid may thus indicate very active inflammatory processes, mostly in tissues. Our data also show that low TNF and IL-1 levels recovered in BAL fluid do not necessarily indicate good health status, especially when LTB4 or NO is detected in BAL fluid, but rather signify transition from step 2 to steps 3 to 5 in the pathogenesis process and thus an evolution toward a more profound illness state. NO succeeded to TNF in BAL as infection and inflammation progressed. In fact, the combination of the profiles of TNF in BAL and blood and NO in BAL provided an accurate estimation of disease state which chronologically corresponded to worsening of pathological score. They could therefore be viewed as good biological markers for pneumonia, and antagonists should be investigated at appropriate stages of infection in the context of immunotherapy.
To our knowledge, this is the first study to demonstrate NO release in BAL fluid and tissue during pneumococcal pneumonia and to correlate it with tissue injury and monocyte recruitment in BAL fluid. NO has been reported to be secreted by alveolar and interstitial macrophages during endotoxemia and Pneumocystis carinii pneumonia (75, 90) and to be required for protective immunity against Klebsiella pneumoniae pneumonia (81). The monocyte migration from bloodstream to alveoli in our study was associated with decreased NO in blood and increased secretion in alveoli, which provides support for monocytes/macrophages as the main sources for NO during pneumococcal pneumonia. However, type II pneumocytes, endothelial cells, fibroblasts, and lymphocytes can also release NO (32, 48, 51, 60, 89). In fact, NO production in BAL was biphasic: an early but transient release (1 h) may have resulted from the activation of constitutive NO synthase by resident alveolar macrophages, but the late (48 to 72 h) massive and sustained release must have resulted from the activation of inducible NO synthase in recruited monocytes and other cells.
Although several animal models have been used to demonstrate a role for NO as an effector molecule for the killing of bacteria, parasites, and fungi (reviewed in references 27, 46, and 81), we could not demonstrate from this experiment a link between NO release and bacterial counts in tissue. However, both phagocytosis of bacteria and destruction of the pneumococcal capsule outside phagocytes were observed in areas of intense inflammation compared to less inflamed areas. Extracellular oxygen radicals (15, 22, 71) and NO may have helped restrain bacterial growth. On the other hand, leakage of serum proteins to alveoli most likely induced by NO may have served as a source of nutrients for bacteria as well as a vehicle for their dissemination.
NO possibly has a multifaceted role in our model, ranging from capillary leakage and edema (38, 43, 46) through modulation of leukocyte activity (23, 26, 44, 46) to tissue cytotoxicity (3, 6, 51, 52, 72). NO may affect cell activity by altering signal transduction, energy production, and DNA synthesis (48, 72). Reactive oxygen and nitrogen species also act in concert to induce lipid peroxidation, defective membrane permeability, and cell injury through the formation of peroxynitrites (31, 80). It is not surprising that in our experiment, NO overproduction was associated with high histopathologic score, lipid metabolite (MDA) release, and death. Blocking NO delays mortality in our model (unpublished data), and this therapeutic approach deserves further investigation. Antioxidants should also be investigated as potential adjuvants to antibiotherapy of pneumonia (6).
Other components of inflammatory response may have contributed to tissue injury. These include some virulence factors such as peptidoglycan-teichoic acid complex, which stimulates cytokine release and edema (16, 39), and pneumolysin, which can create transmembrane pores in lipid bilayers of virtually every type of cell in the lungs (10, 16, 64–66). Large numbers of immune and nonimmune cells as well as of cytokines and chemokines have been postulated to play important roles in the development or control of inflammation (45, 69, 73). Although minimal TNF and IL-1 secretion has been associated with protective immunity in a number of pulmonary disorders (4, 12, 18, 77, 85), their combination also mediates several hemodynamic manifestations and cell toxicity (24, 82), and they stimulate NO production (40, 44, 46, 90). Considering TNF- and IL-1-mediated epithelial cell toxicities and PMN-induced tissue injury (88, 91), their concomitant activity at 12 to 24 h after infection, in association with bacterial virulence factors, may have initiated membrane injury resulting thereafter in hemorrhage in BAL fluid, edema in tissue interstitium, bacterial growth inside pneumocytes (79) and further migration to bloodstream, and progressive loss in body weight. Proliferation of type II pneumocytes followed, as a repair process to regenerate both type II and type I epithelial cells (69). While type I pneumocytes are very sensitive to PMN-induced cytotoxicity (69), type II pneumocytes stimulated by IL-1 express surface receptors for pneumococci, which contributes to infection (16, 17). Increased type II/type I ratio might play a role in the outcome of pneumonia (62). As an example, the abundant surfactant release by type II cells possibly dampened inflammatory reactions, mostly by reducing proinflammatory cytokines levels in BAL fluid (69).
The inflammatory cell influx in our model closely paralleled the successive waves of PMNs, monocytes, and lymphocytes reported after intratracheal administration of endotoxin, IL-1, or TNF in rats (83). The kinetics of MPO in lung homogenates correlated with the observable PMNs on tissue sections. Also evidenced by our experiment is the fact that LTB4 cannot be accounted for as the primary chemotactic substance for PMNs, as its synthesis paralleled and followed, rather than preceded, PMN influx to alveoli. Although mouse and human PMNs show a high reactivity to LTB4 (76), and LTB4 is elevated in various pulmonary infections in animals and humans (35, 71, 75), we do not exclude that LTB4 release in BAL may have partly reflected an ongoing injury process to the alveolar barrier, as costimulation of pneumocytes with PMNs results in an amplification of LTB4 generation (30, 69).
Several lines of evidence suggest that C-X-C and C-C chemokines may be critically involved in PMN and monocyte recruitment in bacterial infections. IL-8, in particular, has been detected in patients with acute pulmonary infection (41, 74), and elevated BAL IL-8 levels correlated with fatal outcome (while plasma IL-8 levels did not) (14). Macrophage inflammatory protein 2 is the likely functional murine homologue of IL-8, as it performs the functions of human IL-8 in mice (11, 29, 68). It has been associated with lung PMN influx in K. pneumoniae pulmonary infection (74) and might play an important role in our model. The beneficial versus detrimental effects of pneumococcal-induced lung PMN influx in immunocompetent hosts, which may occur through CD18-independent mechanisms (21, 34, 49, 58, 82), remain to be better clarified.
Recruitment of monocytes to the lungs for clearance of debris usually occurs once PMN activity declines and infection subsides (5). Many chemokines increase chemotaxis and phagocytic activity of monocytes/macrophages (11, 74), and we do not exclude that PMNs may have secreted many of these cytokines (13). However, in our model of untreated fatal pneumonia, monocytes may have contributed to worsening tissue damage, as noted by the recrudescence of LTB4, IL-6, and NO secretion in BAL fluid and lung tissue and the increase in lung weight.
In conclusion, the five major pathogenesis steps that we identified from initial infection to death involved the successive recruitment of PMNs, monocytes, and lymphocytes, the pulmonary and/or systemic release of inflammatory mediators that characterized the presystemic and systemic phases of infection, and the participation of parenchymal cells in the host response. Although the kinetics of cytokines differed considerably from blood to lung tissue to alveoli and blood levels did not correlate with tissue levels, the kinetics of TNF and IL-6 in blood as well as TNF and NO in BAL were good indicators of the evolution of the disease. NO release was biphasic and corresponded mostly to monocyte recruitment in BAL and concomitant serious tissue injury. Pneumococci activated leukotriene release, but PMN recruitment was not primarily mediated by LTB4. Bacteremia, leukopenia, thrombocytopenia, and lipid peroxidation (MDA in BAL fluid) closely preceded death. Knowing the chronological inflammatory events that occur during pneumonia may help in designing appropriate diagnostic tests that could be used to monitor the evolution of this deadly infection. We are seeing an explosion of biological response modifiers which may be used to treat pneumonia. The proper use of these agents will require prior identification of biological markers in humans suffering from pneumonia.
ACKNOWLEDGMENTS
M.O. is a recipient of a Fonds de Recherche en Santé du Québec Junior II scholarship.
We thank Denis Beauchamp for his kind participation in the project.
REFERENCES
- 1.Abbas A K, Lichtman A H, Pober J S. Effector mechanisms of immune responses. Cytokines. In: Wonsiewicz M J, editor. Cellular and molecular immunology. W. B. Philadelphia, Pa: Saunders Company; 1991. pp. 232–235. [Google Scholar]
- 2.Aihara M, Nakazawa T, Dobashi K, Joshita T, Kojima M, Onai M, Mori M. A selective pulmonary thrombosis associated with sepsis-induced disseminated intravascular coagulation. Intern Med. 1997;36:97–101. doi: 10.2169/internalmedicine.36.97. [DOI] [PubMed] [Google Scholar]
- 3.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng Y M, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amura C R, Fontan P A, Sanjuan N, Sordelli D O. The effect of treatment with interleukin-1 and tumor necrosis factor on Pseudomonas aeruginosa lung infection in a granulocytopenic mouse model. Clin Immunol Immunopathol. 1994;73:261–266. doi: 10.1006/clin.1994.1196. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, V. M., and T. Turner. 1991. Histopathology of childhood pneumonia in developing countries. Rev. Infect. Dis. 13(Suppl. 6):S470–S476. [DOI] [PubMed]
- 6.Antonini J M, Van Dyke K, DiMatteo M, Reasor M J. Attenuation of acute inflammatory effects of silica in rat lung by 21-aminosteroid, U74389G. Inflammation. 1995;19:9–21. doi: 10.1007/BF01534376. [DOI] [PubMed] [Google Scholar]
- 7.Azoulay-Dupuis, E., J. P. Bedos, E. Vallée, and J. J. Pocidalo. 1991. Comparative activity of fluorinated quinolones in acute and subacute Streptococcus pneumoniae pneumonia models: efficacy of temafloxacin. J. Antimicrob. Chemother. 28(Suppl. C):45–53. [DOI] [PubMed]
- 8.Azoulay-Dupuis E, Vallée E, Bedos J P, Muffat-Joly M, Pocidalo J J. Prophylactic and therapeutic activities of azithromycin in a mouse model of pneumococcal pneumonia. Antimicrob Agents Chemother. 1991;35:1024–1028. doi: 10.1128/aac.35.6.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauchamp D, Gourde P, Simard M, Bergeron M G. Subcellular distribution of daptomycin given alone or with tobramycin in renal proximal tubular cells. Antimicrob Agents Chemother. 1994;38:189–194. doi: 10.1128/aac.38.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Bergeron Y, Ouellet N, Simard M, Olivier M, Bergeron M G. Abstracts of the 4th International Congress on Biological Response Modifiers. Alpharetta, Ga: The Inter-American Society for Chemotherapy, Imedex USA; 1997. Immunotherapy of pneumococcal pneumonia with cefodizime, abstr. 15. [Google Scholar]
- 9b.Bergeron, Y., A. M. Deslauriers, M. Olivier, M. Simard, D. Beauchamp, and M. G. Bergeron. 1995. Novel strategies to control the inflammatory response during lethal bacterial pneumonia. Can. J. Infect. Dis. 6(Suppl. C):418C. (Abstract.)
- 10.Boulnois G J. Pneumococcal proteins and the pathogenesis of disease caused by Streptococcus pneumoniae. J Gen Microbiol. 1992;138:249–259. doi: 10.1099/00221287-138-2-249. [DOI] [PubMed] [Google Scholar]
- 11.Broug-Holub E, Toews G B, Van Iwaarden J F, Strieter R M, Kunkel S L, Paine R, Standiford T J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buret A, Dunkley M L, Pang G, Clancy R L, Cripps A W. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats: roles of alveolar macrophages, tumor necrosis factor alpha, and interleukin-1 alpha. Infect Immun. 1994;62:5335–5343. doi: 10.1128/iai.62.12.5335-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 14.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts J M, Fagon J Y, Gougerot-Pocidalo M. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun. 1993;61:4553–4559. doi: 10.1128/iai.61.11.4553-4559.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coonrod J D, Varble R, Jarrells M C. Species variation in the mechanism of killing of inhaled pneumococci. J Lab Clin Med. 1990;116:354–362. [PubMed] [Google Scholar]
- 16.Cundell, D., H. R. Masure, and E. I. Tuomanen. 1995. The molecular basis of pneumococcal infection: a hypothesis. Clin. Infect. Dis. 21(Suppl. 3):S204–S212. [DOI] [PubMed]
- 17.Cundell D R, Tuomanen E I. Receptor specificity of adherence of Streptococcus pneumoniae to human type-II pneumocytes and vascular endothelial cells in vitro. Microb Pathog. 1994;17:361–374. doi: 10.1006/mpat.1994.1082. [DOI] [PubMed] [Google Scholar]
- 18.Czuprynski C J, Haak-Frendscho M, Maroushek N, Brown J F. Effects of recombinant human interleukin-6 alone and in combination with recombinant interleukin-1 alpha and tumor necrosis factor alpha on antibacterial resistance in mice. Antimicrob Agents Chemother. 1992;36:68–70. doi: 10.1128/aac.36.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis C C, Mellencamp M A, Preheim L C. A model of pneumococcal pneumonia in chronically intoxicated rats. J Infect Dis. 1991;163:799–805. doi: 10.1093/infdis/163.4.799. [DOI] [PubMed] [Google Scholar]
- 20.Dehoux M S, Boutten A, Ostinelli J, Seta N, Dombret M C, Crestani B, Deschenes M, Trouillet J L, Aubier M. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1994;150:710–716. doi: 10.1164/ajrccm.150.3.8087341. [DOI] [PubMed] [Google Scholar]
- 20a.Deslauries, A. M., Y. Bergeron, M. Olivier, D. Beauchamp, P. Gourde, and M. G. Bergeron. 1995. The role of host defense mechanisms in a murine model of Streptococcus pneumoniae pneumonia. Can. J. Infect. Dis. 6(Suppl. C):419C. (Abstract.)
- 21.Doerschuk C M, Winn R K, Coxson H O, Harlan J M. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990;144:2327–2333. [PubMed] [Google Scholar]
- 22.Dunne D W, Resnick D, Greenberg J, Krieger M, Joiner K A. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA. 1994;91:1863–1867. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elferink J G R, VanUffelen B E. The role of cyclic nucleotides in neutrophil migration. Gen Pharmacol. 1996;27:387–393. doi: 10.1016/0306-3623(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 24.Fahey T J, Tracey K J, Cerami A. Tumor necrosis factor (cachectin) and the adult respiratory distress syndrome. In: Fishman A P, editor. Update: pulmonary diseases and disorders. Montreal, Québec, Canada: McGraw-Hill; 1992. pp. 175–183. [Google Scholar]
- 25.Fox-Dewhurst R, Alberts M K, Kajikawa O, Caldwell E, Johnson M C, et al. Pulmonary and systemic inflammatory responses in rabbits with gram-negative pneumonia. Am J Respir Crit Care Med. 1997;155:2030–2040. doi: 10.1164/ajrccm.155.6.9196112. [DOI] [PubMed] [Google Scholar]
- 26.Gaboury J, Woodman R C, Granger D N, Reinhardt P, Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol. 1993;265(3 part 2):H862–H867. doi: 10.1152/ajpheart.1993.265.3.H862. [DOI] [PubMed] [Google Scholar]
- 27.Gosselin D, DeSanctis J, Boule M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–3278. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green L C, Tannenbaum S R, Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981;212:56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- 29.Greenberger M J, Streiter R M, Kunkel S L, Danforth J M, Laichalk L L, McGillicudy D C, Standiford T J. Neutralization of MIP-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–163. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 30.Grimminger F, Von Kurten I, Walmrath D, Seeger W. Type II alveolar epithelial eicosanoid metabolism: predominance of cyclooxygenase pathways and transcellular lipoxygenase metabolism in co-culture with neutrophils. Am J Respir Cell Mol Biol. 1992;6:9–16. doi: 10.1165/ajrcmb/6.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Gross S S, Wolin M S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez H H, Pitt B R, Schwarz M, Watkins S C, Lowenstein C, Caniggia I, Chumley P, Freeman B A. Pulmonary alveolar epithelial inducible NO synthase gene expression: regulation by inflammatory mediators. Am J Physiol. 1995;268(3 part 1):L501–L508. doi: 10.1152/ajplung.1995.268.3.L501. [DOI] [PubMed] [Google Scholar]
- 33.Hennet T, Ziltener H J, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149:932–939. [PubMed] [Google Scholar]
- 34.Hogg J C, Doerschuk C M. Leukocyte traffic in the lung. Annu Rev Physiol. 1995;57:97–114. doi: 10.1146/annurev.ph.57.030195.000525. [DOI] [PubMed] [Google Scholar]
- 35.Hopkins H, Stull T, Von-Essen S, Robbins R A, Rennard S I. Neutrophil chemotactic factors in bacterial pneumonia. Chest. 1989;95:1021–1027. doi: 10.1378/chest.95.5.1021. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs E R, Bone R C. Clinical indicators in sepsis and septic adult respiratory distress syndrome. Med Clin North Am. 1986;70:921–932. doi: 10.1016/s0025-7125(16)30932-4. [DOI] [PubMed] [Google Scholar]
- 37.Johnston, R. B. J. 1991. Pathogenesis of pneumococcal pneumonia. Rev. Infect. Dis. 13(Suppl. 6):S509–S517. [DOI] [PubMed]
- 38.Kageyama N, Miura M, Ichinose M, Tomaki M, Ishikawa J, Ohuchi Y, Endoh N, Shirato K. Role of endogenous nitric oxide in airway microvascular leakage induced by inflammatory mediators. Eur Respir J. 1997;10:13–19. doi: 10.1183/09031936.97.10010013. [DOI] [PubMed] [Google Scholar]
- 39.Keller R, Fischer W, Keist R, Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992;60:3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilbourn R G, Griffith O W. Overproduction of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Inst. 1992;84:827–831. doi: 10.1093/jnci/84.11.827. [DOI] [PubMed] [Google Scholar]
- 41.Krarup E, Vestbo J, Benfield T L, Lundgren J D. Interleukin-8 and leukotriene B4 in bronchoalveolar lavage fluid from HIV-infected patients with bacterial pneumonia. Respir Med. 1997;91:317–321. doi: 10.1016/s0954-6111(97)90036-6. [DOI] [PubMed] [Google Scholar]
- 42.Kruger N J. The Bradford method for protein quantitation. Methods Mol Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 43.Laszlo F, Whittle B J, Evans S M, Moncada S. Association of microvascular leakage with induction of nitric oxide synthase: effects of nitric oxide synthase inhibitors in various organs. Eur J Pharmacol. 1995;283:47–53. doi: 10.1016/0014-2999(95)00281-o. [DOI] [PubMed] [Google Scholar]
- 44.Liew F Y. Interactions between cytokines and nitric oxide. Adv Neuroimmunol. 1995;5:201–209. doi: 10.1016/0960-5428(95)00009-q. [DOI] [PubMed] [Google Scholar]
- 45.Lukacs N W, Ward P A. Inflammatory mediators, cytokines, and adhesion molecules in pulmonary inflammation and injury. Adv Immunol. 1996;62:257–291. doi: 10.1016/s0065-2776(08)60432-0. [DOI] [PubMed] [Google Scholar]
- 46.Lyons C R. The role of nitric oxide in inflammation. Adv Immunol. 1995;60:323–355. doi: 10.1016/s0065-2776(08)60589-1. [DOI] [PubMed] [Google Scholar]
- 47.Mellencamp M A, Preheim L C. Pneumococcal pneumonia in a rat model of cirrhosis: effects of cirrhosis on pulmonary defense mechanisms against Streptococcus pneumoniae. J Infect Dis. 1991;163:102–108. doi: 10.1093/infdis/163.1.102. [DOI] [PubMed] [Google Scholar]
- 48.Miles P R, Bowman L, Huffman L. Nitric oxide alters metabolism in isolated alveolar type II cells. Am J Physiol. 1996;271(1 part 1):L23–L30. doi: 10.1152/ajplung.1996.271.1.L23. [DOI] [PubMed] [Google Scholar]
- 49.Mizgerd J P, Meek B B, Kutkoski G J, Biullard D C, Beaudet A L, Doerschuk C M. Selectins and neutrophil traffic: margination and Streptococcus pneumoniae-induced emigration in murine lungs. J Exp Med. 1996;184:639–645. doi: 10.1084/jem.184.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moine P, Vallée E, Azoulay D E, Bourget P, Bedos J P, Bauchet J, Pocidalo J J. In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother. 1994;38:1953–1958. doi: 10.1128/aac.38.9.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 52.Moncada S, Higgs E A. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 53.Moussa K, Michie H J, Cree I A, McCafferty A C, Winter J H, Dhillon D P, Stephens S, Brown R A. Phagocyte function and cytokine production in community acquired pneumonia. Thorax. 1994;49:107–111. doi: 10.1136/thx.49.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musher D M. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin Infect Dis. 1992;14:801–807. doi: 10.1093/clinids/14.4.801. [DOI] [PubMed] [Google Scholar]
- 55.Nelson S, Bagby G J, Bainton B G, Wilson L A, Thompson J J, Summer W R. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammatory response. J Infect Dis. 1989;159:189–194. doi: 10.1093/infdis/159.2.189. [DOI] [PubMed] [Google Scholar]
- 55a.Ouellet N, Bergeron Y, Simard M, Duong M, Olivier M, Beauchamp D, Bergeron M G. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Beneficial immunomodulation of pneumococcal pulmonary infection with NG-monomethyl-l-arginine, abstr. G-56. [Google Scholar]
- 56.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 57.Pallares R, Linares J, Vadillo M, Cabellos C, Manresa F, Viladrich P F, Martin R, Gudiol F. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–486. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 58.Pilewski J M, Albelda S M. Adhesion molecules in the lung. An overview. Am Rev Respir Dis. 1993;148:S31–S37. doi: 10.1164/ajrccm/148.6_Pt_2.S31. [DOI] [PubMed] [Google Scholar]
- 59.Ponte C, Parra A, Nieto E, Soriano F. Development of experimental pneumonia by infection with penicillin-insensitive Streptococcus pneumoniae in guinea pigs and their treatment with amoxicillin, cefotaxime, and meropenem. Antimicrob Agents Chemother. 1996;40:2698–2702. doi: 10.1128/aac.40.12.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Punjabi C J, Laskin J D, Pendino K J, Goller N L, Durham S K, Laskin D L. Production of nitric oxide by rat type II pneumocytes: increased expression of inducible nitric oxide synthase following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol. 1994;11:165–172. doi: 10.1165/ajrcmb.11.2.7519435. [DOI] [PubMed] [Google Scholar]
- 61.Puren A J, Feldman C, Savage N, Becker P J, Smith C. Patterns of cytokine expression in community-acquired pneumonia. Chest. 1995;107:1342–1349. doi: 10.1378/chest.107.5.1342. [DOI] [PubMed] [Google Scholar]
- 62.Rhodes G C, Lykke A W, Tepsell J W, Smith L W. Abnormal alveolar epithelial repair associated with failure of resolution in experimental streptococcal pneumonia. J Pathol. 1989;159:245–253. doi: 10.1002/path.1711590312. [DOI] [PubMed] [Google Scholar]
- 63.Roilides E, Pizzo P A. Modulation of host defenses by cytokines: evolving adjuncts in prevention and treatment of serious infections in immunocompromised hosts. Clin Infect Dis. 1992;15:508–524. doi: 10.1093/clind/15.3.508. [DOI] [PubMed] [Google Scholar]
- 64.Rubins J B, Charboneau D, Paton J C, Mitchell T J, Andrew P W, Janoff E N. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J Clin Invest. 1995;95:142–150. doi: 10.1172/JCI117631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubins J B, Duane P G, Charboneau D, Janoff E N. Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect Immun. 1992;60:1740–1746. doi: 10.1128/iai.60.5.1740-1746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubins J B, Duane P G, Clawson D, Charboneau D, Young J, Niewoehner D E. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun. 1993;61:1352–1358. doi: 10.1128/iai.61.4.1352-1358.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sauve C, Azoulay-Dupuis E, Moine P, Darras-Joly C, Rieux V, Carbon C, Bedos J P. Efficacies of cefotaxime and ceftriaxone in a mouse model of pneumonia induced by two penicillin- and cephalosporin-resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2829–2834. doi: 10.1128/aac.40.12.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schall T J. The chemokines. In: Thomson A W, editor. The cytokine handbook. 2nd ed. San Diego, Calif: Academic Press; 1994. pp. 419–460. [Google Scholar]
- 69.Simon R H, Paine R. Participation of pulmonary alveolar epithelial cells in lung inflammation. J Lab Clin Med. 1995;126:108–118. [PubMed] [Google Scholar]
- 70.Simpson S Q, Casey L C. Role of tumor necrosis factor in sepsis and acute lung injury. Crit Care Clin. 1989;5:27–47. [PubMed] [Google Scholar]
- 71.Skerrett S J. Host defenses against respiratory infection. In: Niederman M S, editor. The medical clinics of North America. Pneumonia: pathogenesis, diagnosis and management. W. B. Montreal, Québec, Canada: Saunders Company; 1994. pp. 941–966. [DOI] [PubMed] [Google Scholar]
- 72.Snyder S H, Bredt D S. Biological roles of nitric oxide. Sci Am. 1992;266:68–77. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- 73.Standiford T J, Kunkel S L, Basha M A, Chensue S W, Lynch J P, Toews G B, Westwick J, Strieter R M. Interleukin-8 gene expression by a pulmonary epithelial cell line; a model for cytokine networks in the lung. J Clin Invest. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Standiford T J, Kunkel S L, Greenberger M J, Laichalk L L, Strieter R M. Expression and regulation of chemokines in bacterial pneumonia. J Leukocyte Biol. 1996;59:24–28. doi: 10.1002/jlb.59.1.24. [DOI] [PubMed] [Google Scholar]
- 75.Su T H, Martin W J. Pathogenesis and host response in Pneumocystis carinii pneumonia. Annu Rev Med. 1994;45:261–272. doi: 10.1146/annurev.med.45.1.261. [DOI] [PubMed] [Google Scholar]
- 76.Sugawara T, Miyamoto M, Takayama S, Kato M. Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J Pharmacol Toxicol Methods. 1995;33:91–100. doi: 10.1016/1056-8719(94)00062-9. [DOI] [PubMed] [Google Scholar]
- 77.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997;65:257–260. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takashima K, Tateda K, Matsumoto T, Ito T, Iizawa Y, Nakao M, Yamaguchi K. Establishment of a model of penicillin-resistant Streptococcus pneumoniae pneumonia in healthy CBA/J mice. J Med Microbiol. 1996;45:319–322. doi: 10.1099/00222615-45-5-319. [DOI] [PubMed] [Google Scholar]
- 79.Talbot U M, Paton A W, Paton J C. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect Immun. 1996;64:3772–3777. doi: 10.1128/iai.64.9.3772-3777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Terada L S, Repine J E. Oxygen radicals and lung disease. In: Fishman A P, editor. Update: pulmonary diseases and disorders. Montreal, Québec, Canada: McGraw-Hill; 1992. pp. 159–174. [Google Scholar]
- 81.Tsai W C, Strieter R M, Zisman D A, Wilkowski J M, Bucknell K A, Chen G H, Standiford T J. Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun. 1997;65:1870–1875. doi: 10.1128/iai.65.5.1870-1875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tuomanen E I, Austrian R, Masure H R. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 83.Ulich T R, Watson L R, Yin S, Guo K, Wang P, Thang H, Castillo J D. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1, and TNF-induced inflammatory infiltrate. Am J Pathol. 1991;138:1485–1496. [PMC free article] [PubMed] [Google Scholar]
- 84.Vallée E, Azoulay-Dupuis E, Pocidalo J J, Bergogne-Berezin E. Activity and local delivery of azithromycin in a mouse model of Haemophilus influenzae lung infection. Antimicrob Agents Chemother. 1992;36:1412–1417. doi: 10.1128/aac.36.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van der Poll T, Keogh C V, Buurman W A, Lowry S F. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 1997;155:603–608. doi: 10.1164/ajrccm.155.2.9032201. [DOI] [PubMed] [Google Scholar]
- 86.Van der Poll T, Marchant A, Keogh C V, Goldman M, Lowry S F. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 87.Veber B, Vallée E, Desmonts J M, Pocidalo J J, Azoulay D E. Correlation between macrolide lung pharmacokinetics and therapeutic efficacy in a mouse model of pneumococcal pneumonia. J Antimicrob Chemother. 1993;32:473–482. doi: 10.1093/jac/32.3.473. [DOI] [PubMed] [Google Scholar]
- 88.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 89.Willis R A, Nussler A K, Fries K M, Geller D A, Phipps R P. Induction of nitric oxide synthase in subsets of murine pulmonary fibroblasts: effect on fibroblast interleukin-6 production. Clin Immunol Immunopathol. 1994;71:231–239. doi: 10.1006/clin.1994.1077. [DOI] [PubMed] [Google Scholar]
- 90.Wizemann T M, Laskin D L. Effects of acute endotoxemia on production of cytokines and nitric oxide by pulmonary alveolar and interstitial macrophages. Ann N Y Acad Sci. 1994;730:336–337. doi: 10.1111/j.1749-6632.1994.tb44284.x. [DOI] [PubMed] [Google Scholar]
- 91.Worthen G S, Haslett C, Rees A J, Gumbay R S, Henson J E, Henson P M. Neutrophil-mediated pulmonary vascular injury. Am Rev Respir Dis. 1987;136:19–28. doi: 10.1164/ajrccm/136.1.19. [DOI] [PubMed] [Google Scholar]