Abstract
Background
Some groups of people have a greater risk of developing common non‐melanoma skin cancers (NMSC).
Objectives
To evaluate interventions for preventing NMSC in people at high risk of developing NMSC.
Search methods
We searched the Cochrane Skin Group Specialised Register (March 2007), the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 1, 2007, MEDLINE (from 2003 to March 2007), EMBASE (from 2005 to March 2007), the metaRegister of Controlled Trials (February 2007). References from trials and reviews were also searched. Pharmaceutical companies were contacted for unpublished trials.
Selection criteria
Randomised controlled trials of adults and children at high risk of developing NMSC.
Data collection and analysis
Two review authors independently selected studies and assessed their methodological quality.
Main results
We identified 10 trials (7,229 participants) that assessed a variety of interventions.
One trial found T4N5 liposome lotion significantly reduced the rate of appearance of new BCCs in people with xeroderma pigmentosum.
One of three trials of renal transplant recipients showed a significantly reduced risk of new NMSCs when acitretin was compared to placebo (relative risk (RR) 0.22 95% confidence interval (CI) 0.06 to 0.90) and no significant difference in risk of adverse events in two trials (RR 1.80, 95% CI 0.70 to 4.61).
In three trials conducted in people with a history of NMSC, the evidence was inconclusive for the development of BCCs for retinol or isoretinoin. However the risk of a new SCC in one trial (HR 1.79, 95% CI 1.16 to 2.76) and adverse events in another trial (RR 1.76, 95% CI 1.57 to 1.97) were significantly increased in the isotretinoin group compared with placebo.
In one trial selenium showed a reduced risk of other types of cancer compared with placebo (RR 0.65, 95% CI 0.50 to 0.85) but also a significantly elevated risk of a new NMSC (HR 1.17, 95% CI 1.02 to 1.34). The evidence for one trial of beta‐carotene was inconclusive; and there was a trend towards fewer new NMSC in a trial of a reduced fat diet (RR 0.16, 95% CI 0.02 to 1.31), p = 0.09.
Authors' conclusions
Some preventative treatments may benefit people at high risk of developing NMSC, but the ability to draw firm conclusions is limited by small numbers of trials, often with one trial per intervention or with inconsistent results between studies.
Keywords: Humans; Carcinoma, Basal Cell; Carcinoma, Basal Cell/etiology; Carcinoma, Basal Cell/prevention & control; Carcinoma, Squamous Cell; Carcinoma, Squamous Cell/etiology; Carcinoma, Squamous Cell/prevention & control; Neoplasms, Radiation‐Induced; Neoplasms, Radiation‐Induced/prevention & control; Randomized Controlled Trials as Topic; Risk Factors; Skin Neoplasms; Skin Neoplasms/etiology; Skin Neoplasms/prevention & control; Sunlight; Sunlight/adverse effects
Plain language summary
Interventions for preventing of non‐melanoma skin cancers in high‐risk groups
Non‐melanoma skin cancer is still the most common cancer in the UK, the United States and Australia. People at increased risk of getting non‐melanoma skin cancer include those with lowered immunity, a history of non‐melanoma skin cancer, rare inherited genetic skin disorders, trauma to the skin, exposure to arsenic, albinism or having had psoralen and ultraviolet A treatment. Very few studies have been conducted in people at increased risk of NMSC.
For people with Xeroderma pigmentosum (a rare inherited genetic skin disorder) topical application of T4N5 liposome lotion is beneficial in reducing the rate of appearance of new basal cell carcinomas, however it may increase the risk of a new squamous cell carcinoma. Acitretin in renal transplant recipients may be of some benefit, however, high doses of acitretin are associated with an increased number of adverse events. Retinol or a reduced fat diet may be worth trying for people with a history of non‐melanoma skin cancer. Further prevention studies for people at increased risk of non‐melanoma skin cancer are needed.
Background
Description of the condition
Skin cancer is the most common type of cancer in humans (Martinez 2001). Around 97% of skin cancers are epithelial in origin and are either basal cell carcinomas (BCCs) or squamous cell carcinomas (SCCs), collectively known as non‐melanoma skin cancer (NMSC). In this review we shall not address precursor lesions for NMSC, i.e. solar keratoses and Bowen's disease.
BCC is defined as a slow‐growing, locally invasive, malignant, epidermal skin tumour that mainly affects people with light coloured skins (Telfer 1999). BCCs are the most common malignant growth found in humans and originate from basal cells of the epidermis (Lang 1991; Telfer 1999). SCCs are generally more aggressive than BCCs and originate in skin cells that produce keratin. Unlike BCC, which has no reported precursor lesions, there are two principal precursors of SCC: actinic (solar) keratoses (AKs) and Bowen's disease (intra‐epidermal carcinoma; IEC), both of which are described as carcinoma‐in‐situ. SCC is distinguished from carcinoma‐in‐situ by having an invasive component (i.e. involving connective tissue and blood vessels in the dermis), which can be determined histologically (Goldman 1998).
Epidemiology
The incidence of NMSC is unclear but is known to increase the closer a person lives to the equator. Using data from 1947 to 1948, from 10 US cities, age‐adjusted rates of skin cancer were found to double for each 3°48' of latitude toward the equator (Auerbach 1961). In the year 2000 62,200 cases of NMSC were diagnosed in the UK (CRUK 2004). This is, however, likely to be an under‐estimate due to the incomplete registrations of these tumours and the fact that they very rarely lead to death. Increasing numbers of NMSCs are diagnosed and treated within family practice surgeries using destructive techniques such as cryotherapy (tissue destruction by freezing), which preclude histological confirmation of the lesion. Despite this conservative estimate, NMSC is still the most common cancer in the UK, United States and Australia (Alam 2001; Eedy 2000; Staples 1998; Stern 1999). NMSC accounts for 75% of all cancers in Australia and is approximately 30 times more prevalent than lung cancer among men, and 10 times more common than breast cancer in women (AIHW 2003). In the USA the incidence is estimated at over one million people per year, which means it is roughly five times more common than prostate and breast cancer (ACS 2003). In South Wales (United Kingdom) the incidence rate of NMSC between 1988 and 1998 rose from 173.5 to 265.4 per 100,000 per year, an overall increase of 16% for SCC and 66% for BCC (Holme 2000). In Australia, data from a population based study conducted between 1985 and 1995 showed that incidence rates for BCC increased by 19% to 788 per 100,000, and for SCC there was a 93% rise from 166 to 321 per 100,000 (Staples 1998).
Worldwide, the incidence of BCC shows a continued rise but for SCC there is a varied picture with increasing incidence in some countries and rates reaching a plateau in others (Harris 2001). This increase in incidence may be due to increased histopathological examination of suspicious lesions, an increased awareness of, and concern about these tumours and an increased number of dermatologists. SCC incidence rates may be levelling off in some countries because a larger number of precursor lesions are being removed by physicians before they can develop into SCCs (Harris 2001) and through the use of sunscreens, which are thought to be more protective for SCCs than BCCs (Green 1999).
Causes and risk factors
The five year recurrence rate of SCCs is influenced by the anatomical site, degree of differentiation and depth of tumour (Rowe 1992). SCCs greater than 2 cm in diameter have a 5 year recurrence rate which is double that of an SCC less than 2 cm (Rowe 1992). If depth of the lesion is greater than 4 mm, the 5 year recurrence rate is 17% (Rowe 1992), while lesions on the ears or lips (which are generally aggressive) have 2 to 3 times the 5 year recurrence rate of SCC of the same depth in other anatomical regions (Alam 2001).
The most important risk factors for NMSC are thought to be people's age, skin type and exposure to sunlight (ultraviolet (UV) radiation). UV radiation is subdivided, based on wavelength, into UVA (long wave), UVB (burning rays), and UVC (germicidal rays) and it plays a role in NMSC development through several mechanisms. People are not exposed to UVC since the ozone layer filters it out, preventing it from reaching the earth's surface. UVB is responsible for most skin burning after sun exposure. UVA has a longer wavelength and can therefore penetrate the skin more deeply. In addition to causing mutations in DNA, ultraviolet radiation can cause localised immune suppression (Grossman 1997). Human papillomavirus (HPV) infection is also thought to play a role in skin cancer carcinogenesis (Karagas 2006)
Normally, the immune system is able to detect early developing NMSCs and clear them effectively. Impairment of the immune system may allow the cancer to develop and prevent it from being rejected by the body.
High‐risk groups
There are subsets of people that are at greater risk of developing NMSC than the general population. The following is not an all inclusive list but covers a broad range of key groups as highlighted from the literature.
i) Individuals with precursor lesions
People with a precursor lesion are at more risk than the general population of developing a SCC. Not all precursor lesions, however, develop into SCCs. Only 4 to 6% of Bowen's disease transform to SCC (Eedy 2000) while the progression rate from AK to SCC ranges from 0.025% to 20% (Alam 2001).
ii) Individuals with a previous NMSC
The risk of developing a subsequent NMSC in people who have developed a first NMSC is not well defined. A critical review and meta‐analysis (Marcil 2000) has found that for people with fewer than 3 previous NMSCs the risk of developing another NMSC within the following 3 years is 38%. In people with 3 to 9 previous NMSCs this risk rises to 93%. One study found that individuals with more than nine prior NMSCs develop a new NMSC within two years (Marcil 2000).
iii) Lowered immunity
People who have had organ transplants (OTs) have a three to four fold increased risk of developing any cancer, over the general population. The risk of developing certain malignancies, including NMSC is dramatically higher in people with OTs. One study suggests that people who have had renal (kidney) transplants are 500 times more likely to develop NMSC than the general population (Hartevelt 1990). In Australia, incidence rates of NMSC in renal transplant recipients increases exponentially over time: 3% within the first year, 25% at 5 years and 44% at 9 or more years post transplant (Hardie 1980). As with the normal population tumour development is more likely with increased ultraviolet exposure, advancing age and fair skin.
Some people's immune systems do not function properly (i.e. they are immunocompromised) due to congenital disorders, viral infection or AIDS. Basal cell carcinoma is one of the most frequent malignant tumours among people with acquired immunodeficiency syndrome (AIDS) and its incidence appears to be higher than in the general population, although there is not enough epidemiological data to confirm this (Demopoulos 2003). People who are human immunodeficiency virus (HIV) positive develop NMSC at a significantly younger age than people who are HIV negative (Demopoulos 2003).
iv) Xeroderma pigmentosum
People with the rare inherited genetic (autosomal recessive) skin disorder, xeroderma pigmentosum (XP), have an abnormality in the ability to repair UV‐induced DNA damage. This results in the development of a large number of skin cancers from as early as two years of age. The median age of developing an NMSC in people with XP is 8 years old, compared to 60 years old in the general population (Kraemer 1980; Kraemer 1994). People with XP have over a 100 fold increased incidence of BCC or SCC than the general population, with 45% of them developing an NMSC (Kraemer 1980; Kraemer 1994). Photosensitivity begins in infancy, and freckles and keratoses (rough scaly patches) appear on exposed skin in childhood. SCCs, BCCs, keratoacanthomas and malignant melanomas subsequently develop in the UV‐damaged skin.
v) Albinism
Albinism describes people who lack skin pigment and hence the ability to tan. It is caused by a large group of genetic disorders. Albinos are at increased risk of all skin cancers especially SCC, since they lack the protective effects of melanin in the skin. In a study of 164 albinos in Tanzania, 91% of those who were over 20 years of age had AK, rising to 100% of those who were over 30 years old (Lookingbill 1995).
vi) Trauma and burns
SCC is uncommon in Blacks, Asians and Hispanics, however if SCCs occur they do so on sites of pre‐existing inflammatory skin conditions, burn injuries, or trauma.
vii) Basal cell naevus syndrome
Basal cell naevus syndrome (also known as Gorlin's syndrome) is a genetic autosomal dominant condition characterised by a range of skin and skeletal abnormalities and an increased occurrence of 2 or more BCC before the age of 30 (Johnson 1996). The syndrome is caused by mutations in the patched gene, present on chromosome 9, required for proper embryonic development and tumour suppression (Johnson 1996).
viii) Exposure to arsenic
Arsenic is the 20th most abundant element and exhibits both acute and chronic effects on humans (Neubauer 1947). It is ubiquitous in soil and is found in high concentrations in water wells in Taiwan, Argentina, Sweden and other regions where mining and smelting is prevalent (Neubauer 1947). It is also present in some forms of traditional Indian medicine and in illegally produced alcoholic beverages such as moonshine (Hughes 1983; Treleaven 1993). Many occupations involve exposure to arsenic including the agricultural industry. Arsenic is a carcinogen that is able to cause cancerous transformations of mammalian cells under laboratory conditions (Pershagen 1981). Three cutaneous cancers are associated with chronic exposure to arsenic: BCC and two precursors of SCC, Bowen's disease and arsenical keratosis. The distribution of lesions caused by arsenic exposure is not limited to parts of the body that have been exposed to sun or x‐rays. A scattering of neoplasms throughout the body are observed with the preferential formation of arsenical keratoses on the palms. It takes an average of 17.8 years from initial exposure to arsenic to developing cancer (Schwartz 1997). Chelation therapy, which removes arsenic, is available for people who have been exposed, however by the time cutaneous and other cancers develop there are likely to be no traces of arsenic left to eliminate (Heyman 1956).
ix) Recessive dystrophic epidermolysis bullosa (RDEB)
RDEB Hallopeau‐Siemens (RDEB‐HS), the most generalised subtype of RDEB, is thought to be one of the most devastating, chronic diseases known to human beings. It is due to an inherited defect in the type VII collagen gene, whereby there is either no collagen VII produced or very low levels. Collagen VII forms anchoring fibrils, crucial structures that "sew" the outer skin (epidermis) onto the inner skin (dermis). RDEB‐HS is characterised by repeated blister formation, leading to mechanical fragility of the skin. It can affect all tissues with an epithelial surface or lining. People who survive recurrent bacterial sepsis during infancy are at high‐risk of developing severe complications in later life including renal failure, corneal scarring and blindness, in addition to the progressive mutilation with eventual loss of their fingers and toes (Fine 2004) . The most severe complications are SCCs, which tend to arise within chronically eroded or hyperkeratotic skin lesions. Approximately 85% of all individuals with RDEB‐HS will have developed one cutaneous SCC by the age of 45 years and the risk is about 50 times the normal (Fine 1999). It is not yet understood why these people are at increased risk for SCC, other than having chronic non‐healing wounds for at least 14 years, when the incidence of SCCs starts to rise.
x) People treated using psoralen and ultraviolet A treatment (PUVA)
PUVA has been widely used as a treatment for psoriasis and other skin conditions since 1974 (Parrish 1974). Exposure to PUVA increases the risk of SCC in a dose dependent manner, while a substantial increase in the risk of BCC has not been observed (Stern 1998). In addition to being both mutagenic and carcinogenic (Dunnick 1991) PUVA is immunosuppressive in the skin. During active treatment it may therefore increase the risk of skin cancer in a pattern similar to that observed with people undergoing immunosuppressive therapy (e.g. transplant recipients). One study has found that people with at least 337 PUVA treatments had more than 100 fold increase in the risk of developing SCC, within 10 years of stopping treatment (Stern 1998), compared with that expected from population incidence rates. The risk of developing a SCC in the second decade after cessation of PUVA treatment was 70 fold (Stern 1998).
Clinical Features
BCCs exhibit several markedly different subtypes and occur at different places on the body (Wong 2003).
Eighty‐five percent appear on the head and neck region, while the rest are observed mainly on the trunk and lower limbs, especially in women (McCormack 1997).
Subtypes of BCC include:
superficial, which is a well‐demarcated, scaly, red minimally indurated plaque that can mimic a papulosquamous rash such as psoriasis
nodular
multifocal
morphoeic (scarring), which is the most invasive type
ulcerated
pigmented, which is often confused with melanoma
cystic
Sixty percent of the BCCs diagnosed in the UK are nodular, presenting as a pearly papule with telangiectasias (raised areas through which dilated vessels may show) throughout. In other countries, however, such as Australia, superficial BCC is the most common type (Staples 1998). Both nodular and superficial BCC usually exhibit a non infiltrative, superficial growth pattern and are therefore associated with low risk (Martinez 2001). The most important subtype clinically is morphoeic BCC, which has a more aggressive natural history and accounts for approximately 5% of all lesions (Wong 2003). Since they are difficult to diagnose, the tumours can be huge and devastating to the individual. Complete surgical excision is difficult under direct vision since morphoeic BCCs have ill defined borders (Wong 2003), and often lengthy plastic surgical reconstructions are required to correct cosmetic disfigurement (Wong 2003).
SCCs present clinically as nodular, superficial or as cutaneous horns (Dinehart 1996). Actinic keratoses (AKs), sometimes a precursor to SCC, appear as scaly pink patches located on sun exposed areas and are very common. Over 80% of fair skinned people aged 60 to 69 in the USA have at least one AK (Glass 1989). Most SCCs and AKs (about 70 to 80%) occur on the head and neck (Glass 1989; Gray 1997; Holme 2000; Iversen 1999). Bowen's disease presents as a red scaly or crusted plaque containing squamous cells and tends to be asymptomatic. Bowen's disease can affect any anatomical site, including the lower leg in women and the penis in men (Cox 1999).
There is a commonly held impression that SCC is a relatively benign form of cancer; however, the potential for metastasis can range from 0.5% to 40% depending on the subtype (Eedy 2000). SCCs developing from AKs have a 0.5 to 2 % rate of metastasis (Eedy 2000). If SCC evolves from Bowen's disease the metastatic rate soars to 33% (Cox 1999; Eedy 2000). Mortality due to NMSC is mainly due to metastasis of SCC to lymph nodes and other internal organs. NMSC have also been linked to second malignancies. Rosenberg 2004 found that women with a history of NMSC were 2.3 times more likely to report a history of another cancer, other than NMSC, compared with women who had no history of NMSC.
Diagnosis
The diagnosis of NMSC involves taking a medical history and a physical examination (NCCN 2004) where the whole body is examined for spots, bumps, sores and any other potential signs of skin cancer. If the physical examination and medical history suggest the possibility of a NMSC a skin biopsy is performed whereby a sample of skin is taken for microscopic analysis. Two of the most commonly used biopsy techniques are shave biopsy and punch biopsy. Both methods are able to determine the cancer type and pathological growth pattern.
Description of the intervention
Treatment
There are many options for the treatment of NMSC. These therapies are covered in two other Cochrane systematic reviews (Bath‐Hextall 2004; Westby 2004).
Prevention
Prevention is a major component in the management of NMSC.
Primary prevention
The best way to lower the risk of NMSC is to decrease skin exposure to sunlight, primarily by avoiding the peak hours of sunlight and avoiding deliberate sun tanning. Educating people, especially children, about the dangers of overexposure of their skin to sunlight is another way to reduce NMSC (Naldi 2004).
Secondary prevention
This has the aim of encouraging people to recognise skin changes and seek early diagnosis and treatment, as well as improving effective diagnosis.
Tertiary prevention
This involves extra interventions after treatment to reduce the risk of re‐occurrence or further development of the disease.
A combination of primary, secondary and tertiary prevention is particularly necessary for individuals who are at high risk of developing NMSC, as identified earlier.
Sunscreens
Sun protective products include sunscreens and sun blocks. Sunscreens of a chemical nature, (such as oxybenzone, avobenzone) work by absorbing UVR. Physical sunscreens contain titanium dioxide or zinc oxide, which scatter or block UVR. An in depth review on the use of sunscreens in the prevention of NMSC is given elsewhere (Gasparro 1998; Naylor 1997).
Retinoids
Retinoids are vitamin A derivatives. Experimental vitamin A deficiency, leading to cancer in rats, provided some of the earliest links between vitamin A and malignancies. Early studies, administering high dose retinoids for treating and preventing skin cancer in people considered to be at high‐risk of BCC, showed promising results (Peck 1982; Peck 1988). Although only a small number of studies have been reported, synthetic retinoids have shown the most promise for preventing NMSC in high‐risk groups such as those with XP (Lippman 1987) and people who have had renal transplants (Bavinck 1995). Retinoids work by controlling growth, death and differentiation of human cells. In malignant cell lines retinoids inhibit cell growth and induce normal differentiation of the cells (Lippman 1987).
Since vitamin A is stored in the liver, high doses may produce persistent side effects. Adverse effects include dryness and chapping of the lips, mucous membranes and skin in addition to skeletal toxicities. This has led to the development of synthetic retinoids, designed to have better therapeutic properties with lower toxicity. Osteoporosis, calcification of tendons and ligaments, osteophytes and bone spurs around joints may be accelerated by long‐term retinoid therapy (DiGiovanna 1995; DiGiovanna 2001).
Antioxidants
i) Selenium
Selenium is an essential trace element found in fish, shellfish and garlic. It is necessary for the functioning of the detoxifying enzyme glutathione peroxidase within cells. This enzyme helps to reduce the presence of highly reactive hydroxyl free radicals which are thought to attack DNA, inducing mutations (Buettner 1993) and thus its function is essential for a cell to remain damage free. Studies in mice have shown that increased levels of dietary selenium provide protection from UV induced skin tumours (Pence 1994).
ii) Beta‐carotene
Beta‐carotene (ß‐carotene) is the best characterised of a large group of carotenoid pigments that are widely distributed in vegetables and fruit. Although ß‐carotene has pro‐vitamin A (retinol) activity, it is possible that ingestion of ß‐carotene might prevent cancer without the involvement of retinol. It is an antioxidant that may reduce free radical damage of DNA after ultraviolet exposure. Some studies suggest that ß‐carotene supplementation works by reducing immunosuppression normally induced by ultraviolet radiation (Fuller 1992).
iii) Vitamin C
Vitamin C (ascorbic acid) is present in citrus fruits and potatoes. It has been shown that UV exposure in the epidermis and dermis in mice leads to the depletion of vitamin C (Shindo 1993). Vitamin C may play a role in preventing skin cancers by scavenging free radicals in cells, thus protecting the cells from DNA damage.
iv) Vitamin E
Vitamin E (present in vegetable oils, nuts and leafy green vegetables), like vitamin C, scavenges free radicals and protects cell membranes from damage. Topical vitamin E in mice has been shown to prevent UV induced immunosuppression (Gensler 1996), inhibit UV‐induced thymine dimer formation (McVean 1999) and inhibit absorption of UVB radiation (McVean 1999).
Dietary modifications
Reduction of fat intake
It has been noted that unsaturated fatty acids are a major target for free radical attack. It is therefore possible that decreased dietary fats could reduce free radical attack and carcinogenesis (Black 1998).
Complementary therapies
The use of complementary therapies for the treatment of a number of diseases, including cancer has increased. Their perceived lack of side effects has increased their popularity over conventional synthetic treatments. A literature review is available which outlines the variety of herbal therapies that have been tested to treat or prevent NMSC (Bialy 2002). Phytochemicals have been the focus of many studies in the last decade e.g. tea is thought to act by a variety of mechanisms to prevent NMSC, including the induction of apoptosis (cell death) in tumour cells (Alexis 1999).
Alternative immunosuppressive regimens
Reduction of immunosuppressive burden and shift to alternative immunosuppressive regimens may represent an option in transplanted patients with a first skin cancer. This will be covered in the updated review.
Why it is important to do this review
NMSC are not usually considered life‐threatening but they take a huge toll on health service budgets, as well as contributing to days lost in the workplace. Prevention is a better option than cure, especially given that preventative measures have a good chance of working. Many treatments have been described for the prevention of NMSC in high‐risk groups, but there are no evidence‐based guidelines. Literature reviews exist on the treatment and prevention of NMSC in the general population in addition to high‐risk groups, however, no systematic reviews exist on the topic.
Objectives
To evaluate interventions for the prevention of NMSC in people at high‐risk of developing NMSC.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials of interventions to prevent NMSC in people at high‐risk of developing NMSC. These will include any relevant study that compares any combination of interventions, any interventions compared to control (placebo/no treatment), or different dosages/durations of the same interventions.
Types of participants
Adults and children who are at high risk of developing NMSC. These are defined as people who:
have had a previous biopsy proven NMSC (BCC or SCC);
are organ transplant recipients;
have xeroderma pigmentosum;
have Gorlin's syndrome;
have been exposed to high levels of arsenic;
are immunocompromised due to disease (e.g. AIDS);
have albinism;
have precursors to SCC (Bowen's disease, solar keratoses);
are trauma patients (e.g. burns patients, large scars);
have RDEB (recessive dystrophic epidermolysis bullosa);
have been treated using PUVA (psoralen ultra violet A).
Types of interventions
(1) Topical therapies
(2) Retinoids
(3) Antioxidants
selenium
beta carotene
vitamin C
vitamin E
(4) Dietary modifications
reduction in fat intake
(5) Complementary therapies
phytochemicals e.g. green tea
Types of outcome measures
Primary outcomes
(i) The time from start of prevention to the development of a first NMSC, or in those with a previous NMSC, the development of a subsequent NMSC up until a maximum of five years.
Recurrence may either be a NMSC at another anatomical site or recurrence at the site of the primary NMSC.
Secondary outcomes
(i) Number of people with a new NMSC at two to five years from the start of treatment. (ii) Number of people with a new NMSC within the first year from the start of treatment. (iii) Mortality at the end of trial. (iv) Number of people with other cancers at the end of trial. (v) Adverse effects.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Skin Group's Specialised Register (March 2007) using the following search terms:
((non melanoma and skin and cancer) or (basal or squamous and (cell and carcinoma)) or 'BCC' or 'NMSC' or 'SCC' or (organ and transplant and recipient) or (xeroderma and pigmentosum) or (Gorlin* and syndrome) or arsenic or 'AIDS' or immunocompromis* or albinism or (Bowen* and disease) or (solar and keratos*) or burn* or scar* or 'RDEB' or (recessive and dystrophic and epidermolysis and bullosa) or 'PUVA' or (psoralen and ultra and violet)) AND (sunscreen* or antioxidant* or retinoid* or selenium or (beta and carotene) or (vitamin and ('A' or 'E')) or (diet* and (modification* or fat*)) or (complementary and (medicine* or therap*)) or phytochemical* or (green and tea*) or prevention*)
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2007) using the search strategy in Appendix 1.
We searched MEDLINE (OVID) (from 2003 to March 2007) using the search strategy in Appendix 2.
We searched EMBASE (from 2005 to March 2007) using the search strategy in Appendix 3.
Searching other resources
Pharmaceutical companies
Pharmaceutical companies were contacted where appropriate for information about unpublished trials.
Published and unpublished trials
References from included published studies were checked for further trials. The metaRegister of Controlled Trials, which includes the NHS Trusts Clinical Trials Register (www.controlled‐trials.com), was searched for ongoing trials (February 2007). Contact was made with specialists, such as clinicians and academics in the field, for information about ongoing or unpublished trials.
Language
No language restrictions were imposed and translations were obtained where necessary.
Adverse Effects
We looked at adverse events only in the included studies.
Data collection and analysis
Selection of studies
Two authors (FB‐H, NS) checked the titles and abstracts identified from the searches. The same two authors independently assessed the full text of all RCTs of possible relevance and decided on which trials fitted the inclusion criteria. Any disagreements were resolved by discussion between the authors. We contacted trial authors for clarification where ambiguities existed
Data extraction and management
Two authors (FB‐H, JL‐B) independantly performed data extraction, using a specially designed data extraction form, and discrepancies were resolved by a third author (AW or WP). Missing data were obtained from the trial authors where possible. One author (FB‐H) entered data into RevMan and this was double checked by JL‐B. Data recorded included: demographics, sites, clinical types, histological diagnosis, follow up period, number of previous NMSC and how they were treated, skin tone and loss to follow‐up, country of residence.
Assessment of risk of bias in included studies
The quality assessment included an evaluation of the following components for each included study, since there is some evidence that these are associated with biased estimates of treatment effect (Juni 2001):
(a) the method of generation of the randomisation sequence; (b) the method of allocation concealment ‐ it will be considered 'adequate' if the assignment cannot be foreseen; (c) who was blinded/ not blinded (participants clinicians, outcome assessors); (d) how many participants were lost to follow up in each arm and whether participants were analysed in the groups to which they were originally randomised (intention to treat).
The information was recorded in a table of quality criteria (Table 1) and a description of the quality of each study was given based on these components.
1. Quality components.
| Study | Allocation gen | Allocation conceal | Blinding | Loss to FU | Analysis method | Type of participant |
| Clark 1996 | yes | yes | Participant, physician | nine | PP | Previous BCC or SCC |
| Black 1995 | Yes | No | outcome assessor | 14 | PP | Previous NMSC |
| Bouwes Bavinck 1995 | Yes | Yes | Physician, participant | six | PP | RTR |
| de Sevaux 2003 | Yes | No | Outcome assessor: unclear | two withdrew | ITT | RTR |
| George 2002 | No | No | None | 12 withdrew | ITT | RTR |
| Greenberg 1990 | Yes | Yes | Physician, participant, outcome assessor | 93 + 178 | ITT | Previous NMSC |
| Levine 1997 | No | No | Physician, participant | 0 | ITT | Previous NMSC |
| Moon 1997 | Yes | No | Participant, outcome assessor | 334 | ITT | History of AKs, SCC or BCC |
| Tangrea 1992 | Yes | Yes | Physician, participants | 82 | ITT | Previous NMSC |
| Yarosh 2001 | Yes | Yes | Participant, physician, outcome assessor | two participants withdrawn | PP | Participants with Xeroderma pigmentosum and a history of AKs or other skin cancer |
Measures of treatment effect
We calculated a weighted pooled treatment effect across studies using a random effects model. We expressed the results as risk ratio (RR and 95% confidence intervals (CI) for dichotomous outcomes due to non rare expected events, and mean difference (MD and 95% CI) for continuous outcomes. The hazard ratio and associated statistics were calculated, where necessary, using and Excel spreadsheet developed by the Matthew Sydes (Cancer Division) in collaboration with the Meta‐analysis Group of the MRC Clinical Trials Unit, London.
Unit of analysis issues
Cross‐over studies were analysed using methods appropriate for such studies. We expressed the results as number needed to treat (NNT), where appropriate, with a 95% CI and the baseline risk to which it applies.
Assessment of heterogeneity
Heterogeneity in studies was explored using I2.
Subgroup analysis and investigation of heterogeneity
Where substantial heterogeneity existed between studies for the primary outcome (I2 > 50%) , we planned sensitivity analyses to examine the effects of excluding study subgroups, e.g. those studies with lower reported methodological quality (i.e. studies that did not clearly report randomisation, blinding and which do not have an 'intention to treat' analysis'), however the insufficient number of studies available precluded this. Where data were available, we performed a subgroup analysis if appropriate for BCCs versus SCCs.
Other
A consumer was consulted throughout, particularly for readability and understanding of the final review.
Results
Description of studies
Results of the search
We identified 10 fully published studies (7,229 participants). Three of the authors ( FB‐H, JL‐B, NS) independantly examined the full text of each study. All the studies were parallel group studies with the exception of one cross over study (George 2002). See 'Characteristics of included studies'. Seven of the studies were conducted in the USA (Black 1995; Clark 1996; Greenberg 1990; Levine 1997; Moon 1997; Tangrea 1992; Yarosh 2001). Two of the studies were conducted in the Netherlands (Bouwes Bavinck 1995; de Sevaux 2003), one in Australia (George 2002). Eight studies were multicentre and two were single centres (Black 1995; de Sevaux 2003). Only one study was conducted on participants with Xeroderma pigmentosum (Yarosh 2001), two studies included renal transplant recipients( RTR) and seven were on people with previous NMSC or precursors. In only one of the studies were the participants children (Yarosh 2001).
Included studies
(1) Topical therapies
One study was included.
T4N5 liposome
T4N5 liposome lotion contains the bacterial enzyme T4 endonuclease V encapsulated in a pH sensitive engineered liposome for delivery into the living cells of the skin. These liposomes are applied in a hydrogel lotion. T4N5 liposome lotion vs placebo (Yarosh 2001)
(2) Retinoids
Six studies were included:
acitretin vs placebo (Bouwes Bavinck 1995);
acitretin 0.4 mg/Kg/d for 1 yr vs 0.4 mg/Kg/d for 3 months then 0.2 mg/Kg/d of 9 months vs placebo (de Sevaux 2003);
acitretin vs placebo (cross‐over study) (George 2002);
oral retinol vs oral isotretinoin vs placebo (Levine 1997);
oral retinol vs placebo (Moon 1997);
oral isotretinoin vs placebo (Tangrea 1992).
(3) Antioxidants ‐ two studies
Selenium
Oral selenium vs placebo (Clark 1996).
Beta carotene
Beta carotene vs placebo (Greenberg 1990).
(4) Dietary modifications ‐one study
Reduction of fat in diet vs normal diet (Black 1995).
(5) Complementary therapies
No studies of complementary therapies were identified.
Excluded studies
Seven studies were excluded see Characteristics of excluded studies.
Risk of bias in included studies
Allocation
Nine of the studies were randomised controlled, parallel design and one was a randomised controlled cross‐over design (George 2002).
The randomisation process in general and concealment of allocation in particular are the most important and sensitive indicators that bias has been minimised in a clinical trial (Schulz 1995). Only five of the ten studies showed both clear randomisation and concealment of allocation ( Tangrea 1992; Yarosh 2001; Greenberg 1990; Clark 1996; Bouwes Bavinck 1995). However eight of the studies clearly described the method of randomisation.
Blinding
Only one study blinded participants, clinicians and outcome assessors (Yarosh 2001). Five studies blinded participants and clinicians (Clark 1996; Bouwes Bavinck 1995; Greenberg 1990; Levine 1997; Tangrea 1992). One study blinded the outcome assessor and participant (Moon 1997). In one study the blinding was unclear (de Sevaux 2003) and in another study there was no blinding (George 2002).
Incomplete outcome data
Handling of losses and attrition bias
Analysis should be performed according to intention‐to‐treat principle, thus avoiding bias (Altman 1991; May 1981; Sackett 1979). However, in three of the studies analysis of outcome was carried out only in those participants who completed the study (Black 1995; Bouwes Bavinck 1995; Yarosh 2001).
Other potential sources of bias
Baseline comparability of the participants for age, sex
For all studies the baseline differences were comparable between the treatment groups.
Effects of interventions
(1) Topical therapies
Only one study (Yarosh 2001) was identified that compared T4N5 liposome lotion vs placebo in 30 children and adults with xeroderma pigmentosum over a period of a year .
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
No data available
(ii) Recurrence
No data available
(b) Secondary outcomes
(i) Number of people with new NMSCs at two to five years from the start of treatment
No data available
(ii) Number of people with new NMSCs within the first year from the start of treatment
The study reported significantly fewer BCCs per year (mean difference 1.6 per year) in the treatment group (T4N5) as compared to the placebo group, (lesions/year MD ‐1.6, 95% CI ‐2.8 to ‐0.40; Analysis 1.4). There was no significant difference in the risk of getting a NMSC in the treatment group compared to placebo at the end of first year (RR 0.75, 95% CI 0.4 to 1.42; Analysis 1.1) , however there was a trend towards a significant 47% reduction in the risk of a new BCC, (RR 0.53, 95% CI 0.25 to 1.12; Analysis 1.2), p = 0.09. There was no significant difference in risk of a new SCC in the treatment group compared to the placebo group within the first year (RR 1.35, 95% CI 0.34 to 5.44; Analysis 1.3).
1.4. Analysis.
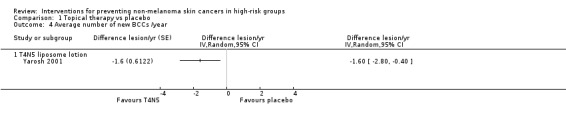
Comparison 1 Topical therapy vs placebo, Outcome 4 Average number of new BCCs /year.
1.1. Analysis.
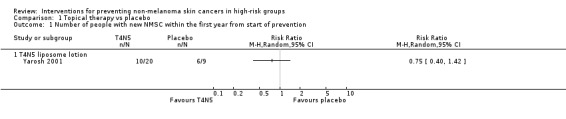
Comparison 1 Topical therapy vs placebo, Outcome 1 Number of people with new NMSC within the first year from start of prevention.
1.2. Analysis.
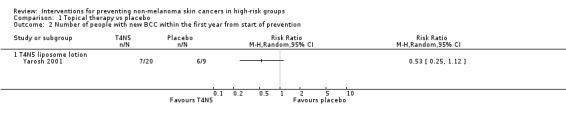
Comparison 1 Topical therapy vs placebo, Outcome 2 Number of people with new BCC within the first year from start of prevention.
1.3. Analysis.
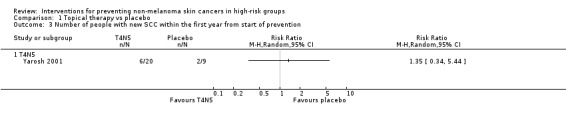
Comparison 1 Topical therapy vs placebo, Outcome 3 Number of people with new SCC within the first year from start of prevention.
(iii) Mortality at the end of trial follow up
No deaths were reported in either groups at end of study
(iv) Number of people with other cancers at the end of trial follow up
There were no significant differences in numbers of other cancers (RR 1.13, 95% CI 0.27 to 4.74; Analysis 1.5).
1.5. Analysis.
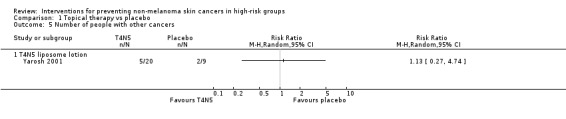
Comparison 1 Topical therapy vs placebo, Outcome 5 Number of people with other cancers.
(v) Adverse effects
No information was given on adverse events.
(2) Retinoids Acitretin versus placebo
Two studies compared acitretin versus placebo (one parallel study and one cross‐over) and one study compared two different doses of acitretin. All were conducted in renal transplant recipients (RTRs).
The first study (Bouwes Bavinck 1995) compared 30mg/d of oral acitretin to placebo in 115 RTRs over 6 months.
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
There were no significant differences in time to developing a new NMSC between the treatment groups within the first 6 months (HR 0.51, 95% CI 0.07 to 3.55; Analysis 2.1)
2.1. Analysis.
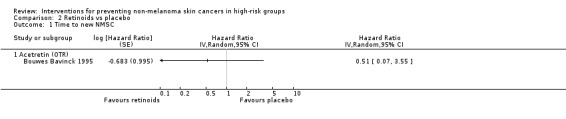
Comparison 2 Retinoids vs placebo, Outcome 1 Time to new NMSC.
(ii) Recurrence
No data available
(b) Secondary outcomes
(i) Number of people with new NMSCs at two to five years from the start of treatment
No data available
(ii) Number of people with new NMSCs within the first year from the start of treatment
A 78% significant reduction in the risk of NMSC was seen in the participants in the acitretin group as compared to placebo within first year (RR 0.22, 95% CI 0.06 to 0.90; Analysis 2.4).
2.4. Analysis.
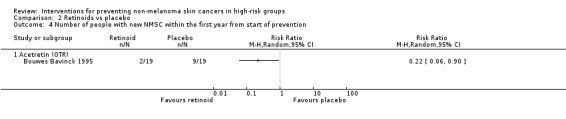
Comparison 2 Retinoids vs placebo, Outcome 4 Number of people with new NMSC within the first year from start of prevention.
(iii) Mortality at the end of trial follow up
No data on deaths were reported
(iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
There were no significant differences in risk of adverse events (RR 1.10, 95% CI 0.07 to 16.43; Analysis 2.9)
2.9. Analysis.
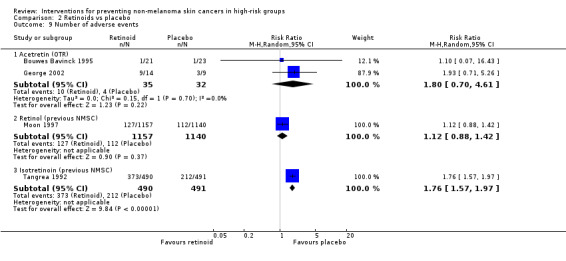
Comparison 2 Retinoids vs placebo, Outcome 9 Number of adverse events.
In a second study 23 participants were included in a two‐period crossover design of acitretin (25 mg/day) versus placebo (George 2002). It was not possible to extract data for many of the outcomes of interest.
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
No data available
(i) Recurrence
No data available
(b) Secondary outcomes
(i) Number of people with new NMSCs at two to five years from the start of treatment
No data available
(ii) Number of people with new NMSCs within the first year from the start of treatment
No data available
(iii) Mortality at the end of trial follow up
No data available
(iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
There was no significant reduction in risk of adverse events in the placebo group compared to the treatment group (RR 1.93, 95% CI 0.71 to 5.26; Analysis 2.9). Pooled data from two studies (Bouwes Bavinck 1995; George 2002) suggested no significant difference in the risk of adverse events in the acitretin group when compared to placebo (RR 1.80, 95% CI 0.70 to 4.61; Analysis 2.9). No heterogeneity was seen between the trial estimates (I2=0%).
A third study in 26 RTRs compared 0.4 mg/Kg/d acitretin for 12 months versus 0.4 mg/Kg/d for 3 months then 0.2 mg/Kg/d for 9 months (de Sevaux 2003).
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
No data available
(ii) Recurrence
No data available
(b) Secondary outcomes
Only overall numbers of new NMSC were given but no numbers for individual treatment groups.
(i) Number of people with a new NMSCs at two to five years from the start of treatment
No data available
(ii) Number of people with a new NMSCs within the first year from the start of treatment
No data available
(iii) Mortality at the end of trial follow up
No data available
(iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
There was no significant difference in risk of adverse events in the higher dose group as compared to the lower dose group, (RR 0.34, 95% CI 0.08 to 1.46; Analysis 4.1).
4.1. Analysis.
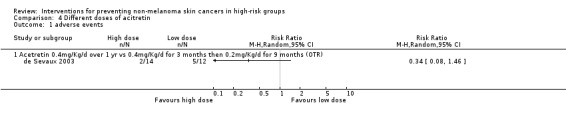
Comparison 4 Different doses of acitretin, Outcome 1 adverse events.
(2) Retinoids (B) Retinol versus placebo
Two studies compared retinol versus placebo (Levine 1997; Moon 1997) in people with a history of NMSC.
The first study (Moon 1997) compared oral retinol to placebo in 2297 adults over 5 years.
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
Retinol significantly reduced time to a first new SCC by 26% as compared to placebo (HR 0.74, 95% CI 0.55 to 0.99; Analysis 2.3). However, there was no significant difference in the risk of developing a new BCC (HR1.06, 95% CI 0.85 to 1.32; Analysis 2.2).
2.3. Analysis.
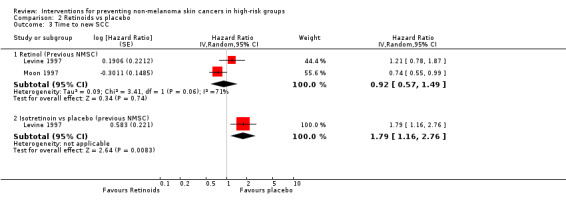
Comparison 2 Retinoids vs placebo, Outcome 3 Time to new SCC.
2.2. Analysis.
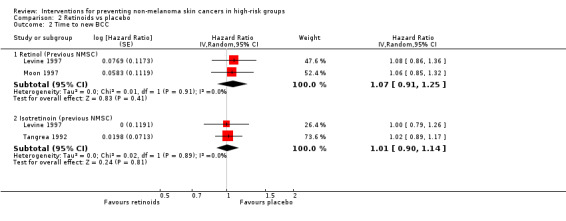
Comparison 2 Retinoids vs placebo, Outcome 2 Time to new BCC.
(b) Secondary outcomes
(i) Number of people with a new NMSCs at two to five years from the start of treatment
There was no significant difference in number of SCCs at two to five years from the start of the prevention (RR 0.82, 95% CI 0.65 to 1.04; Analysis 2.8)
2.8. Analysis.
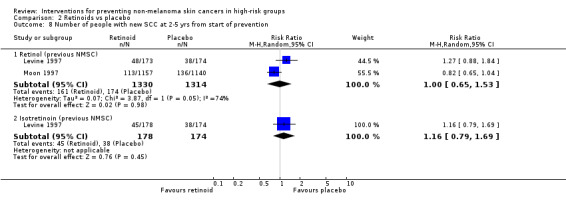
Comparison 2 Retinoids vs placebo, Outcome 8 Number of people with new SCC at 2‐5 yrs from start of prevention.
(ii) Number of people with a new NMSCs within the first year from the start of treatment
No data available
(iii) Mortality at the end of trial follow up
No significant difference in mortality at end of trial (RR 1.15, 95% CI 0.81 to 1.65; Analysis 2.10)
2.10. Analysis.

Comparison 2 Retinoids vs placebo, Outcome 10 Mortality end of study.
(iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
No significant difference in adverse events at the end of the study (RR 1.12, 95% CI 0.88 to 1.42; Analysis 2.9).
The second study compared retinol, isotretinoin and placebo, in 525 adults over 3 years (Levine 1997).
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
There was no significant difference in the time to the development of a new BCC in the retinol group as compared to placebo (HR 1.08, 95% CI 0.86 to 1.36; Analysis 2.2) or SCC (HR 1.21, 95% CI 0.78 to 1.87; Analysis 2.3), respectively
(b) Secondary outcomes
(i) Number of people with new NMSCs at two to five years from the start of treatment
There were no significant difference in the number of new BCCs at two to five years (RR 1.05, 95% CI 0.91 to 1.22 ; Analysis 2.7).There was no significant difference in number of people with SCC at two to five years RR 1.27, 95% CI 0.88 to 1.84; Analysis 2.8).
2.7. Analysis.

Comparison 2 Retinoids vs placebo, Outcome 7 Number of people with a new BCC at 2‐5 yrs from start of prevention.
(ii) Number of people with new NMSCs within the first year from the start of treatment
There was no significant difference in number of new BCCs within the first year when retinol was compared to placebo (RR 1.12, 95% CI 0.88 to 1.43; Analysis 2.5). There was a trend towards fewer number of people with SCC in the placebo group compared to retinol group within the first year, (RR 1.53, 95% CI 0.92 to 2.55, p = 0.1, Analysis 2.6)
2.5. Analysis.
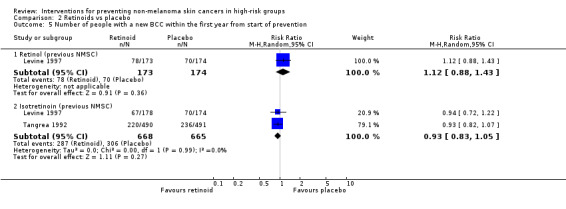
Comparison 2 Retinoids vs placebo, Outcome 5 Number of people with a new BCC within the first year from start of prevention.
2.6. Analysis.

Comparison 2 Retinoids vs placebo, Outcome 6 Number of people with a new SCC within the first year from start of prevention.
(iii) Mortality at the end of trial follow up
No data available
(iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
No data available
In a pooled analysis of the two trials there was no significant difference in the time to a new BCC or SCC when retinol was compared to placebo (pooled data: HR 1.07, 95% CI 0.91 to 1.25; Analysis 2.2; and HR 0.92, 95% CI 0.57 to 1.49; Analysis 2.3 respectively). High levels of heterogeneity were seen between the two trials (I2 = 70.6%).
In a pooled analysis, no significant difference in risk was seen for the number of people with a new SCC at two to five years from the start of prevention treatment (RR 1.00, 95% CI 0.65 to 1.53, Analysis 2.8) when retinol was compared to placebo. High levels of heterogeneity were also seen between the trial results for this comparison (I2 = 74.2%).
(2) Retinoids (C) Isotretinoin versus placebo
Two studies compared isotretinoin versus placebo (Levine 1997; Tangrea 1992) in people with a history of NMSC ‐ the second study is also covered under retinols versus placebo. The first study (Tangrea 1992) compared isotretinoin versus placebo in 981 participants over 3 years and followed‐up for a further 3 years.
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
There was no significant difference in time to a new BCC in participants receiving isotretinoin and those receiving placebo (HR 1.02, 95% CI 0.89 to 1.17; Analysis 2.2).
(b) Secondary outcomes
(i) Number of people with new NMSCs at two to five years from the start of treatment
No significant difference in the number of people with a new BCC at two to five years from start of prevention (RR 1.00, 95% CI 0.92 to 1.08; Analysis 2.7).
(ii) Number of people with new NMSCs within the first year from the start of treatment
No significant differences in the number of people with a new BCC in first year (RR 0.93, 95% CI 0.82 to 1.07; Analysis 2.5).
(iii) Mortality at the end of trial follow up
No data available
(iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
Significantly more adverse events were reported in the isotretinoin group as compared to placebo (RR 1.76, 95% CI 1.57 to 1.97; Analysis 2.9). Most of the adverse events were mucocutaneous.
The second study (Levine 1997) compared retinol, isotretinoin and placebo in 525 participants over 3 years.
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
Participants in the isotretinoin group were 79% significantly more likely to develop a new SCC during the trial (HR 1.79, 95% CI 1.16 to 2.76; Analysis 2.3) compared to the placebo group. However, there was no significant difference in time to developing a new BCC between the isotretinoin and placebo groups (HR 1.00, 95% CI 0.79 to 1.26; Analysis 2.2).
(b) Secondary outcomes
(i) Number of people with new NMSCs at two to five years from the start of treatment
There was no significant differences in the number of people with a new BCC (RR 1.00, 95% CI 0.86 to 1.17; Analysis 2.7) or SCC (RR 1.16, 95% CI 0.79 to 1.69; Analysis 2.8) between two to five years from start of prevention.
(ii) Number of people with new NMSCs within the first year from the start of treatment
There was no significant difference in the number of participants with a new BCC or SCC within the first year from start of prevention (RR 0.94, 95% CI 0.72 to 1.22, Analysis 2.5; RR 0.88, 95% CI 0.49 to 1.59; Analysis 2.6, respectively).
(iii) Mortality at the end of trial follow up
No data available
(iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
No data available since numbers cannot be added up i.e. one participant may experience more than one event.
In a pooled analysis of the two trials which assessed isotretinoin as compared to placebo (Levine 1997; Tangrea 1992), no significant differences were seen for the time to a new BCC (HR 1.01, 95% CI 0.90 to 1.14; Analysis 2.2), the number of people with a new BCC within the first year (RR 0.93, 95% CI 0.83 to 1.05, Analysis 2.5) or two to five years (RR 1.00, 95% CI 0.93 to1.08; Analysis 2.7) from start of prevention.
(3) Antioxidants (A) Selenium vs placebo
Only one study was identified (Clark 1996) which was conducted in people with a history of NMSC and a second paper by (Duffield‐Lillico) gave further follow up data on secondary outcomes. This study compared oral administration of 200 ug/day of selenium to placebo in 1312 participants for a mean of 4.5 years.
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
Participants in the selenium group were 17% more likely to develop a new NMSC during the trial (HR 1.17, 95% CI 1.02 to 1.34; Analysis 3.1) and this was statistically significant and appeared to be primarily related to the development of SCCs (HR 1.25, 95% CI 1.03 to 1.51; Analysis 3.3) rather than the development of BCCs (HR 1.09, 95% CI 0.94 to 1.26; Analysis 3.2).
3.1. Analysis.
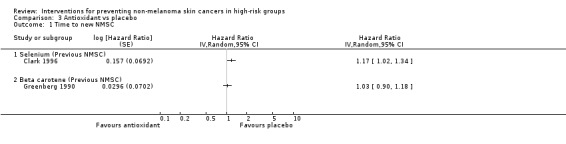
Comparison 3 Antioxidant vs placebo, Outcome 1 Time to new NMSC.
3.3. Analysis.
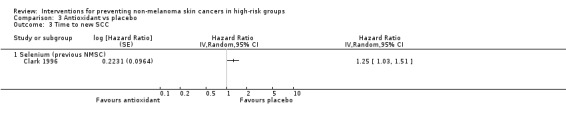
Comparison 3 Antioxidant vs placebo, Outcome 3 Time to new SCC.
3.2. Analysis.
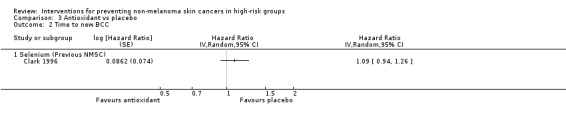
Comparison 3 Antioxidant vs placebo, Outcome 2 Time to new BCC.
Secondary outcomes
(a) Number of people with new NMSCs at two to five years from the start of treatment
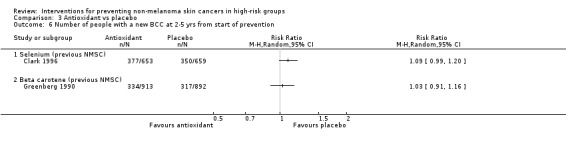
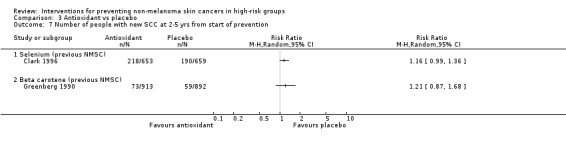
There was no significant difference in number of people with a new BCC or SCC two to five years from start of prevention treatment in the selenium group as compared to placebo (RR 1.09, 95% CI 0.99 to 1.20, Analysis 3.6; RR 1.16, 95% CI 0.99 to 1.36, Analysis 3.7, respectively).
3.6. Analysis.
Comparison 3 Antioxidant vs placebo, Outcome 6 Number of people with a new BCC at 2‐5 yrs from start of prevention.
3.7. Analysis.
Comparison 3 Antioxidant vs placebo, Outcome 7 Number of people with new SCC at 2‐5 yrs from start of prevention.
(ii) Number of people with new NMSCs within the first year from the start of treatment
No data available
(iii) Mortality at the end of trial follow up
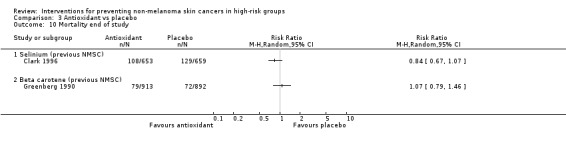
No significant difference in mortality at end of trial (RR 0.84, 95% CI 0.67 to 1.07; Analysis 3.10).
3.10. Analysis.
Comparison 3 Antioxidant vs placebo, Outcome 10 Mortality end of study.
iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
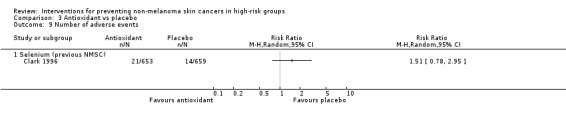
No significant differences in the risk of adverse events (RR 1.51, 95% CI 0.78 to 2.95; Analysis 3.9) or mortality at the end of trial (RR 0.84, 95% CI 0.67 to 1.07; Analysis 3.10). A 35% significant reduction in the risk of other cancers was seen in the selenium group as compared to the placebo group at the end of the trial (RR 0.65, 95% CI 0.50 to 0.85; Analysis 3.8).
3.9. Analysis.
Comparison 3 Antioxidant vs placebo, Outcome 9 Number of adverse events.
3.8. Analysis.
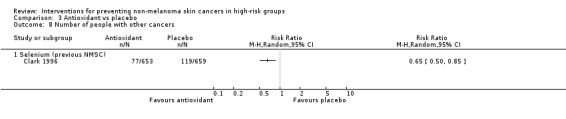
Comparison 3 Antioxidant vs placebo, Outcome 8 Number of people with other cancers.
(3) Antioxidants (B) Beta carotene vs placebo
Only one study was identified (Greenberg 1990) which assessed the treatment in people with a history of NMSC. This study compared 50 mg beta carotene versus placebo over 5 years in 1805 participants.
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
There was no significant difference in time to a new NMSC (HR 1.03,95% CI 0.90 to1.18; Analysis 3.1)
Secondary outcomes
(i) Number of people with a new NMSCs at two to five years from the start of treatment
There was no significant difference in number of participants with a new NMSC at two to five years (RR 1.04, 95% CI 0.93 to 1.17; Analysis 3.5), number of new BCCs (RR 1.03, 95% CI 0.91 to 1.16; Analysis 3.6) or SCCs (RR 1.21, 95% CI 0.87 to 1.68; Analysis 3.7).
3.5. Analysis.
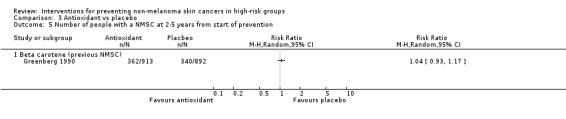
Comparison 3 Antioxidant vs placebo, Outcome 5 Number of people with a NMSC at 2‐5 years from start of prevention.
(ii) Number of people with a new NMSCs within the first year from the start of treatment
There was no significant difference in the number of participants with a new NMSC in first year (RR 1.03, 95% CI 0.84 to 1.27; Analysis 3.4)
3.4. Analysis.
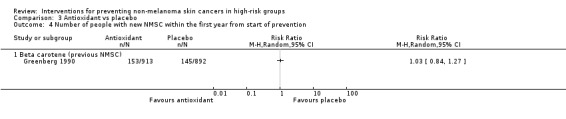
Comparison 3 Antioxidant vs placebo, Outcome 4 Number of people with new NMSC within the first year from start of prevention.
(iii) Mortality at the end of trial follow up
No significant difference in mortality at the end of trial (RR 1.07, 95% CI 0.79 to 1.46; Analysis 3.10)
(iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
No data available
(4) Dietary modifications
Only one study was identified (Black 1995) which assessed the treatment in people with a history of NMSC.
One study compared a diet of reduced fat to a normal diet over 24 months in 115 adults with a history of NMSC. In a separate paper the authors (Jaax 1997) compare the rate of occurrence of NMSC by dividing the study into 8 month periods. The results of this paper are highlighted in the discussion.
(a) Primary outcome
(i) Time from start of prevention to the development of a first NMSC
No data were available for the primary outcome.
(b) Secondary outcomes
(i) Number of people with a new NMSCs at two to five years from the start of treatment
There was a trend towards a significant reduction in number of people with a new NMSC at two to five years (RR 0.16, 95% CI 0.02 to 1.31, p=0.09; Analysis 5.2).
5.2. Analysis.
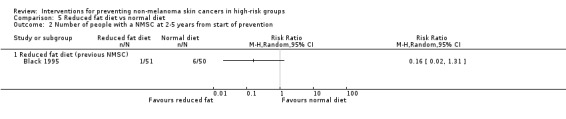
Comparison 5 Reduced fat diet vs normal diet, Outcome 2 Number of people with a NMSC at 2‐5 years from start of prevention.
(ii) Number of people with a new NMSCs within the first year from the start of treatment
There were no significant differences in number of people with a new NMSC in the first year (RR 1.31, 95% CI 0.49 to 3.50; Analysis 5.1)
5.1. Analysis.
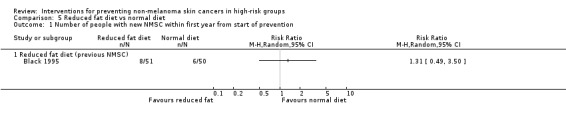
Comparison 5 Reduced fat diet vs normal diet, Outcome 1 Number of people with new NMSC within first year from start of prevention.
(iii) Mortality at the end of trial follow up
No significant differences in risk of mortality were seen between the reduced fat and normal diets at the end of the trial (RR 0.49, 95% CI 0.05 to 5.27; Analysis 5.3).
5.3. Analysis.
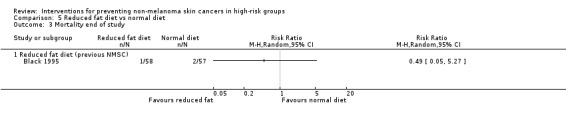
Comparison 5 Reduced fat diet vs normal diet, Outcome 3 Mortality end of study.
(iv) Number of people with other cancers at the end of trial follow up
No data available
(v) Adverse effects
No data available
Discussion
Summary of main results
The studies identified in this review fell into three main categories of people who are at a high risk of NMSC. These were people with Xeroderma pigmentosum, renal transplant recipients and people with a history of NMSC. The conclusions from the review are presented based on these high risk groups.
People with Xeroderma pigmentosum
One study, of good methodological quality (Yarosh 2001) found that topical application of T4N5 liposome lotion significantly reduced the rate of appearance of new BCCs and there was a trend for reducing the risk of getting a new BCC in the treatment group compared to placebo.
Renal transplant recipients (RTRs)
Three studies included RTRs and these were found to be of very different methodological quality. Two studies were of acitretin versus placebo and one study compared high and low doses of acitretin. Only one study (Bouwes Bavinck 1995) assessed the primary outcome of the review but found no significant difference in time to developing a new NMSC in either the acitretin group or the placebo group within the first six months of prevention treatment. Pooled data from the two studies (Bouwes Bavinck 1995; George 2002) found no significant difference in adverse events in the acitretin group compared to the placebo group, and one study (de Sevaux 2003) found no significant difference in adverse events when lower dose acitretin group was compared to the higher dose group.
People with a history of NMSC
Several RCTs of preventive treatments for people with a history of NMSC have been conducted; these include retinols, isotretinoin, selenium, beta carotene, and a low fat diet.
Two studies of retinol included participants with a history of NMSC (Levine 1997; Moon 1997). Although one of the studies (Moon 1997) found that retinol significantly reduced the risk of a first new SCC compared to placebo, pooled data from both studies (Levine 1997; Moon 1997) showed no significant difference in time to first SCC or BCC. Heterogeneity between the two studies was detected, which could possibly be due to the poorer quality of the Levine study as compared to the Moon study. Only one of the studies (Moon 1997) reported adverse events and death data, however there were no statistically significant differences in the risks of these two outcomes between the treatment groups. Two studies (Levine 1997; Tangrea 1992) compared isotretinoin versus placebo and included participants with a history of previous NMSC. The methodological quality of one of the studies was very good (Tangrea 1992) and the other was categorised as poorer quality (Levine 1997). Although pooled data from the two trials suggested no significant difference in time to a new BCC or the risk of a new BCC, the trial by Levine 1997 found that there was an increased risk in the time to a new SCC in the isotretinoin group as compared to the placebo group. One of the studies reported a significant increase in the risk of adverse events in the isotretinoin group compared to placebo. When comparing numbers of participants with new NMSC in the respective groups between the first and last month periods, it should be noted that noncompliance in a large percentage of participants enrolled in retinol chemoprevention studies has been attributed to symptoms associated with vitamin A ingestion (Cartmel 2000)
One good quality study assessed the effect of selenium as compared to placebo in participants with a history of NMSC (Clark 1996). There was a significant increased risk of a new NMSC in participants in the treatment group and this seemed to be primarily related to the development of SCCs in particular. Additionally, selenium was also associated with significantly fewer participants in the treatment group developing other cancers at the end of the study. There were no significant differences in adverse events.
One good quality study assessed the effects of beta carotene as compared to placebo in people with a history of NMSC (Greenberg 1990). They found no significant difference in the number of participants with a new NMSC in first year or at two to five years from the start of the prevention treatment. Additionally, when the analysis was grouped into the type of NMSC, no significant differences were seen in the number of new SCCs or BCCs. Reassuringly, no significant difference was seen in the risk of death at the end of the study.
Only one study was identified which compared a diet of reduced fat and a normal diet over 24 months in 115 people with a history of no more than 2 previous NMSC (Black 1995). Very little data were available from this study for the outcomes of the review. A diet of reduced fat did not significantly affect the number of NMSCs in the first year as compared to placebo; however there were fewer skin cancers in the reduced fat diet group at the end of the study. There was no significant difference in the risk of death at the end of trial. In an additional paper the authors divided the study into eight month periods and when they compared numbers of patients with new NMSC in the respective groups between the first and last month periods they found a significant improvement in the low‐fat diet compared to the normal diet (Jaax 1997) .
Authors' conclusions
Implications for practice.
It should be borne in mind that reduction in sun exposure through seeking shade, clothing protection and wearing sunscreens are all of key importance in the prevention of non melanoma skin cancer. Sun avoidance and protection are not part of the scope of this review as they have been considered elsewhere.
Xeroderma pigmentosum is a rare inherited genetic skin disorder. The frequency of all forms of skin cancer is higher in these people than in the general population. Topical application of T4N5 appears to significantly reduce the rate of appearance of new BCCs and possibly the risk of new BCCs. However these results should not be taken in isolation since this is based on one small study.
Renal transplant recipients are at a significantly increased risk of developing skin cancer compared to immunocompetent individuals. The number of solid organ transplants continue to rise and survival time continues to improve; however with increased survival times comes an increased risk of developing skin cancer.
One study (Bouwes Bavinck 1995) found significantly fewer participants in the acitretin group developed NMSCs within six months, however this is based on one small study. It is possible that altering the immunosuppresive regimens may have an important effect in reducing NMSC risk and this aspect will be considered in the next update of this review.
People with a history of NMSC are at increased risk of developing further NMSC. There is insufficient evidence to support the use of retinol however only two small studies have been done. Isotretinoin increases the risk of developing a new SCC. No difference in the effectiveness of beta carotene, selenium or a reduced fat diet have been seen in this review, however data are from single studies.
Implications for research.
There is an urgent need for more research since the incidence of NMSC is increasing year on year and the number of transplant recipients is also on the increase. Prevention of skin cancers in these groups should be a priority, not only from the patient perspective, but also in terms of financial savings for the Health Services. Further randomised controlled studies of the interventions identified in this review should be done. Further research in people with Xeroderma pigmentosum is needed since this disorder of the skin has a huge impact on the quality of life of these often very young people whose life expectancy may be shortened by over 30 yrs.
Additionally, this systematic review identified no randomised controlled trials for the prevention of non melanoma skin cancer for people with albinism; people with trauma or burns; people with basal cell naevus syndrome; people exposed to arsenic; people with RDEB; or those treated using PUVA. All of these groups of people are at a high risk of NMSC and therefore high quality trials of prevention treatment should be implemented.
What's new
| Date | Event | Description |
|---|---|---|
| 18 February 2015 | Amended | This review is going to be updated. We have written a published note to say that the original review is being updated by way of 2 new titles. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 23 May 2008 | Amended | Converted to new review format. |
Notes
This review is being updated by way of 2 new titles (Interventions for preventing keratinocyte carcinoma (non‐melanoma skin cancer) in solid organ transplant recipients and Interventions for preventing keratinocyte cancer in high‐risk groups not receiving immunosuppressive therapy), because transplant patients are a distinct group.
Acknowledgements
The authors would like to thank the Cochrane Skin Group team and Sue Jessop for all their hard work.
The editorial base would like to thank the following people who were the external referees for this review: Homer Black and Jan N. Bouwes Bavinck (content experts) and Shirley Manknell (consumer).
Appendices
Appendix 1. Cochrane Library (CLIB) search strategy
| #1 non‐melanoma skin cancer in Abstract or NMSC in Abstract or basal cell carcinoma in Abstract or BCC in Abstract or squamous cell carcinoma in Abstract in all products #2 SCC in Abstract or organ transplant recipient in Abstract or xeroderma pigmentosum in Abstract or gorlin* syndrome in Abstract or arsenic in Abstract in all products #3 AIDS or (acquired immunodeficiency syndrome) in Abstract or immunocompromis* in Abstract or albinism in Abstract or bowen* disease in Abstract or solar keratos* in Abstract in all products #4 burn* or scar* in Abstract or recessive dystrophic epidermolysis bullosa in Abstract or PUVA in Abstract or psoralen ultra violet in Abstract in all products #5 sunscreen* in Abstract or antioxidant* in Abstract or retinoid* in Abstract or selenium in Abstract or beta carotene in Abstract in all products #6 vitamin A in All Fields or vitamin E in All Fields or diet* NEAR/2 modification* in Abstract or diet* NEAR/2 fat* in Abstract or complementary NEAR/2 therap* in Abstract in all products #7 complementary NEAR/2 medicine* in Abstract or phytochemical* in All Fields or green tea* in All Fields in all products #8 (#1 OR #2 OR #3 OR #4) #9 (#5 OR #6 OR #7) #10 (#8 AND #9) #11 SR‐SKIN in All Fields in all products #12 (#10 AND NOT #11) |
Appendix 2. MEDLINE (OVID) search strategy
| 1. RANDOMIZED CONTROLLED TRIAL.pt. 2. CONTROLLED CLINICAL TRIAL.pt. 3. RANDOMIZED CONTROLLED TRIALS.sh. 4. RANDOM ALLOCATION.sh. 5. DOUBLE BLIND METHOD.sh. 6. SINGLE‐BLIND METHOD.sh. 7. or/1‐6 8. animal/ not human/ 9. 7 not 8 10. CLINICAL TRIAL.pt. 11. exp CLINICAL TRIALS/ 12. (clin$ adj25 trial$).ti,ab. 13. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 14. PLACEBOS.sh. 15. placebo$.ti,ab. 16. random$.ti,ab. 17. RESEARCH DESIGN.sh. 18. or/10‐17 19. 18 not 8 20. 19 not 9 21. COMPARATIVE STUDY.pt. 22. exp EVALUATION STUDIES/ 23. FOLLOW UP STUDIES.sh. 24. PROSPECTIVE STUDIES.sh. 25. (control$ or prospectiv$ or volunteer$).ti,ab. 26. or/21‐25 27. 26 not 8 28. 27 not (9 or 20) 29. 9 or 20 or 28 30. *Carcinoma, Squamous Cell/ or *Skin Neoplasms/ or non‐melanoma skin cancer.mp. or *Carcinoma, Basal Cell/ 31. *Xeroderma pigmentosum/ 32. *Precancerous Conditions/ 33. *Neoplasms, Radiation‐Induced/ 34. *Organ Transplantation/ or organ transplant recipients.mp. 35. *Immunosuppression/ 36. *Immunocompromised Host/ 37. *Acquired Immunodeficiency Syndrome/ 38. *Arsenic Poisoning/ or *Arsenic/ 39. *Albinism, Ocular/ or albinism.mp. or *Albinism/ or *Albinism, Oculocutaneous/ 40. gorlin$ syndrome.mp. or *Basal Cell Nevus Syndrome/ 41. *epidermolysis bullosa/ or *epidermolysis bullosa dystrophica/ or skin diseases, genetic/ 42. recessive dystrophic epidermolysis bullosa.mp. 43. RDEB‐HS.mp. 44. *PUVA Therapy/ 45. *burns/ or *sunburn/ 46. previous nmsc.mp. 47. *retinoids/ or *vitamin a/ 48. *Selenium/ 49. *beta Carotene/ 50. vitamin C.mp. or *Ascorbic Acid/ 51. *Vitamin E/ 52. exp Antioxidants/ 53. *Isoniazid/ 54. *Food Habits/ 55. *obesity/ or *dietary fats/ 56. exp Complementary Therapies/ 57. *flavonoids/ or *catechin/ or *phenols/ 58. *phytochemicals/ or *tea/ or *plant extracts/ 59. *Drugs, Chinese Herbal/ 60. *Medicine, Herbal/ 61. *Medicine, Traditional/ 62. green tea.mp. 63. *acupuncture therapy/ or *homeopathy/ or *holistic health/ 64. *musculoskeletal manipulations/ or *natural childbirth/ 65. *Relaxation Techniques/ or mind‐body relaxation techniques.mp. 66. *reflexology/ or *rejuvenation/ or *sensory art therapies/ or exp spiritual therapies/ 67. exp Primary Prevention/ 68. exp Chemoprevention/ 69. 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 70. 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 71. 29 and 69 and 70 72. limit 71 to yr="2003 ‐ 2007" |
Appendix 3. EMBASE (OVID) search strategy
| 1. random$.mp. 2. factorial$.mp. 3. crossover$.mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. assign$.mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. *Carcinoma, Squamous Cell/ or *Skin Neoplasms/ or non‐melanoma skin cancer.mp. or *Carcinoma, Basal Cell/ 15. *Xeroderma pigmentosum/ 16. *Precancerous Conditions/ 17. *Neoplasms, Radiation‐Induced/ 18. *Organ Transplantation/ or organ transplant recipients.mp. 19. *Immunosuppression/ 20. *Immunocompromised Host/ 21. *Acquired Immunodeficiency Syndrome/ 22. *Arsenic Poisoning/ or *Arsenic/ 23. *Albinism, Ocular/ or albinism.mp. or *Albinism/ or *Albinism, Oculocutaneous/ 24. gorlin$ syndrome.mp. or *Basal Cell Nevus Syndrome/ 25. *epidermolysis bullosa/ or *epidermolysis bullosa dystrophica/ or skin diseases, genetic/ 26. recessive dystrophic epidermolysis bullosa.mp. 27. RDEB‐HS.mp. 28. *PUVA Therapy/ 29. *burns/ or *sunburn/ 30. previous nmsc.mp. 31. *retinoids/ or *vitamin a/ 32. *Selenium/ 33. *beta Carotene/ 34. vitamin C.mp. or *Ascorbic Acid/ 35. *Vitamin E/ 36. exp Antioxidants/ 37. *Isoniazid/ 38. *Food Habits/ 39. *obesity/ or *dietary fats/ 40. exp Complementary Therapies/ 41. *flavonoids/ or *catechin/ or *phenols/ 42. *phytochemicals/ or *tea/ or *plant extracts/ 43. *Drugs, Chinese Herbal/ 44. *Medicine, Herbal/ 45. *Medicine, Traditional/ 46. green tea.mp. 47. *acupuncture therapy/ or *homeopathy/ or *holistic health/ 48. *musculoskeletal manipulations/ or *natural childbirth/ 49. *Relaxation Techniques/ or mind‐body relaxation techniques.mp. 50. *reflexology/ or *rejuvenation/ or *sensory art therapies/ or exp spiritual therapies/ 51. exp Primary Prevention/ 52. exp Chemoprevention/ 53. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 54. 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 55. 13 and 53 and 54 56. limit 55 to yr="2005 ‐ 2007" |
Data and analyses
Comparison 1. Topical therapy vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people with new NMSC within the first year from start of prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 T4N5 liposome lotion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of people with new BCC within the first year from start of prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 T4N5 liposome lotion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of people with new SCC within the first year from start of prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 T4N5 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Average number of new BCCs /year | 1 | Difference lesion/yr (Random, 95% CI) | Totals not selected | |
| 4.1 T4N5 liposome lotion | 1 | Difference lesion/yr (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of people with other cancers | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 T4N5 liposome lotion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Retinoids vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to new NMSC | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 1.1 Acetretin (OTR) | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Time to new BCC | 3 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Retinol (Previous NMSC) | 2 | Hazard Ratio (Random, 95% CI) | 1.07 [0.91, 1.25] | |
| 2.2 Isotretinoin (previous NMSC) | 2 | Hazard Ratio (Random, 95% CI) | 1.01 [0.90, 1.14] | |
| 3 Time to new SCC | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Retinol (Previous NMSC) | 2 | Hazard Ratio (Random, 95% CI) | 0.92 [0.57, 1.49] | |
| 3.2 Isotretinoin vs placebo (previous NMSC) | 1 | Hazard Ratio (Random, 95% CI) | 1.79 [1.16, 2.76] | |
| 4 Number of people with new NMSC within the first year from start of prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Acetretin (OTR) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of people with a new BCC within the first year from start of prevention | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Retinol (previous NMSC) | 1 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.88, 1.43] |
| 5.2 Isotretinoin (previous NMSC) | 2 | 1333 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.05] |
| 6 Number of people with a new SCC within the first year from start of prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Retinol (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Isotretinoin (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of people with a new BCC at 2‐5 yrs from start of prevention | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Retinol (previous NMSC) | 1 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.91, 1.22] |
| 7.2 Isotretinoin (previous NMSC) | 2 | 1333 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.08] |
| 8 Number of people with new SCC at 2‐5 yrs from start of prevention | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Retinol (previous NMSC) | 2 | 2644 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.65, 1.53] |
| 8.2 Isotretinoin (previous NMSC) | 1 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.79, 1.69] |
| 9 Number of adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Acetretin (OTR) | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.70, 4.61] |
| 9.2 Retinol (previous NMSC) | 1 | 2297 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.88, 1.42] |
| 9.3 Isotretinoin (previous NMSC) | 1 | 981 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.57, 1.97] |
| 10 Mortality end of study | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Retinol (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Antioxidant vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to new NMSC | 2 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 1.1 Selenium (Previous NMSC) | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Beta carotene (Previous NMSC) | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Time to new BCC | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 2.1 Selenium (Previous NMSC) | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Time to new SCC | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 3.1 Selenium (previous NMSC) | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of people with new NMSC within the first year from start of prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Beta carotene (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of people with a NMSC at 2‐5 years from start of prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Beta carotene (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of people with a new BCC at 2‐5 yrs from start of prevention | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Selenium (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Beta carotene (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of people with new SCC at 2‐5 yrs from start of prevention | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Selenium (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Beta carotene (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of people with other cancers | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Selenium (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number of adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Selenium (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Mortality end of study | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Selinium (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Beta carotene (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Different doses of acitretin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Acetretin 0.4mg/Kg/d over 1 yr vs 0.4mg/Kg/d for 3 months then 0.2mg/Kg/d for 9 months (OTR) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Reduced fat diet vs normal diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people with new NMSC within first year from start of prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Reduced fat diet (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of people with a NMSC at 2‐5 years from start of prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Reduced fat diet (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mortality end of study | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Reduced fat diet (previous NMSC) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Black 1995.
| Methods | D: parallel group, single centre, USA AC: unclear RS: list of randomly generated numbers B: outcome assessor PP | |
| Participants | Inc: participants with max of two previously pathologically proven NMSCs Excl: Asian, black, Hispanic or American Indian; genetically predisposed to skin cancer; > two previous skin cancers; cancer; received photochemotherapy for psoriasis within past five yrs; treatment with antimetabolites, systemic glucocorticoid, tretinoin or isotretinoin;received X‐ray treatment for acne; taking megavitamin or mineral supplements; eating therapeutic diet requiring fat intake > 20% of total calories; diabetic. Age mean/SD: (T1:51+‐9, T2:54+‐13) Duration: two years Randomised: 115 m/f: (T1: 30/21, T2:36/14) Evaluable: 101 | |
| Interventions | T1: reduction of fat in diet to 20% of caloric intake through 8 week nutrition classes, T2: continue with normal diet. | |
| Outcomes | FU: four month intervals for two year period. Number of new confirmed skin cancers per participant totalled at eight month interval over two year study period. | |
| Notes | 14 participants lost to FU (T1:7,T2:7), including 3 deaths (T1:1,T2:2), 4 withdrawals due to illness or hospitalisation (T1:3, T2:1), relocation (T2:2) failure to attend hospital visits (T1:3,T2:2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bouwes Bavinck 1995.
| Methods | D: parallel group, multicentre, Netherlands. AC: list provided by pharmaceutical company RS: Randomisation list provided by pharmaceutical company. Participants grouped in blocks of six. Participants were stratified according to presence or absence of a history of SCCor BCC or both B: participants, clinicians, outcome assessor. PP | |
| Participants | Incl: renal transplant recipients with at least ten keratotic skin lesions localised on the forearms and hands. Excl: creatine clearance < 20 mL/min, serum cholesterol > 9 mmol/L, serum triglyceride > 10 mmol/L, disturbed liver function, women wanting to bear children. Set: Netherlands. Mean age T1: 52.5, T2: 50.6 Randomised: 44 Evaluable: 38 m/f: 23/15 T1: 19, T2: 19 | |
| Interventions | T1: 30 mg/d oral acitretin for 6 months T2: placebo for six months | |
| Outcomes | FU: month 1,2,3,4.5,6 during tx. Skin was checked for SCC and BCC all lesions had to be histologically confirmed. Number of keratotic lesions counted. Side effects ‐ measurement of 24 hr urinary creatinine clearance every 3 months, serum creatinine, cholesterol and triglyceride levels and liver function every FU visit. | |
| Notes | Withdrawn: 6 ‐ before first FU (T1:2, T2:4) 1 ‐ CVA due to benign tumour (T1) 1‐ palpitations (T24 ‐ no reason given (T1:1,T2:3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Clark 1996.
| Methods | D: Parallel group, multicentre, (USA) AC: treatment group assignment made centrally using sealed pill bottles distributed at the clinic RS: blocked on time and stratified on clinic ITT | |
| Participants | Inc: history of two or more BCCs or one SCC of the skin with one of these carcinomas occurring within the prior year. Excl: history of sig liver or kidney disease. T1: 653, T2: 659. Mean age (SD), T1: 63.4 (10.2), T2: 63 (10). Randomised 1312 from 1983 to 1990 and were followed with regular dermatologic examinations through, 1993 for a total of 8269 person‐years of observation. At end of study period (1993) 43.6% of participants were still on treatment. Range of active treatment was 0 to 10.3 yrs. Evaluable: 1303 | |
| Interventions | T1: 200ug oral selenium, daily in capsule. T2: placebo identical in appearance. | |
| Outcomes | Fu: 0,6,12,18,24,30,36,42,48,53 months. Participants were treated for a mean (SD) of 4.5 (2.8) yrs and had a total FU of 6.4 (2) years. Primary outcome was incidence of BCC and SCC. Secondary outcomes were all cause mortality and total cancer mortality. | |
| Notes | 82% of participants in both groups had missed taking a pill less than twice a month. Thirty‐five participants experienced adverse side effects (mainly gastrointestinal upset) and withdrew (T1:21, T2:14). All cause mortality (T1: 108, T2: 129). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
de Sevaux 2003.
| Methods | D: parallel, single centre, Netherlands. AC: unclear RS: sealed envelope with lowest available study number B: outcome assessor unclear ITT | |
| Participants | Inc: RTR with history of at least one SCC and > ten AKs, of which one was histologically proven. Excl: participants with nephrotic syndrome, hypercholesterolemia , hypertriglyceridemia, elevated transaminase levels, excessive alcohol intake; who were pregnant or wishing to become pregnant; using antiepileptic drugs. No oral retinoids were allowed for one yr before study. Set: Mean age T1: 54+‐11, T2: 57+‐8 Duration: Randomised: T1: 14, T2: 12 m/f: T1: 8/6; T2: 5/7 Evaluable: 24 | |
| Interventions | T1: acitretin 0.4 mg/kg/d for 1 yr T2: acitretin 0.4 mg/kg/d for 3 months then 0.2 mg/kg/d for 9 months. | |
| Outcomes | FU: week 2; months 1,2,3,4,5,6,9 and 12. Number of new NMSC compared with total number of tumours in the 12 months before acitretin treatment. | |
| Notes | Two participants discontinued acitretin (T1:1, T2:1) due to adverse events. Treatment dose was lowered for five patients (T1: 1, T2:4) in 4 of them due to mucocutaneous side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
George 2002.
| Methods | D: cross over , multicentre, Australia. AC: unclear RS: unclear B: unclear ITT | |
| Participants | Inc: RTR participants with > three SCC pr BCC in previous five yrs or > ten AKs Excl: cholesterol > 7 mmol/L, triglyceride > 3 mmol/L, normal liver function, > 10 g of alcohol /day, pregnant. Set: Mean Age (SD): T1: 56.7(9.5), T2: 52.2(0.2) Duration: two yrs Randomisation: 23 participants m/f: T1: 11/3, T2: 7/2 Evaluable: 23 | |
| Interventions | T1: acitretin in first year then none, T2: none in first year then acitretin in second year. | |
| Outcomes | Fu: weeks 2,4,8,12; then 3 monthly until end of study period. Participants on drug free period followed up every 12 weeks using same protocol. | |
| Notes | withdrawn: nine participants due to side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Greenberg 1990.
| Methods | D:Parallel, Multicentre, USA AC: participants assigned to treatment by computer RS: randomisation in blocks of 16 with no stratification B: patient and physician ITT | |
| Participants | 1805 participants Incl: at least 1 biopsy proven SCC. Excl: xeroderma pigmentosum, basal cell nevus syndrome, cancer other than skin, liver disease. Age <65 yrs (T1:472, T2:457), >65 yrs (T1:441, T2:435). Previous skin cancers 1‐3 (T1:688, T2:691), 4‐5(T1:106, T2:92), 6‐9 (T1:62,T2:54), >10 (T1:51,T2:51). Randomised: 1805 Evaluable: 1805 | |
| Interventions | T1:50mg oral B carotene capsule daily, T2: placebo identical in appearance. Duration of treatment five yrs | |
| Outcomes | FU: 4 monthly questionnaire by post, 12 monthly visits to clinic over a 5 yr period. Outcome ‐ occurrence of new BCC or SCC. | |
| Notes | 271 lost to FU (T1:93, T2:178). 151 deaths (T1:79, T2:72). 80 participants treatment was stopped or altered due to adverse effects (T1: 49, T2:31). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Levine 1997.
| Methods | D: three arms, multicentre, USA. AC: unclear RS: unclear B: physician and patient ITT | |
| Participants | 525 participants Incl: history of 4 or more pathologically confirmed BCCs or SCCs. Exc: xeroderma pigmentosum, basal cell naevus syndrome, abnormal liver function. Age < 66 yrs (T1:76, T2:75, T3:87), age > 66 yrs (T1:97, T2:103, T3:87). 4 to 10 previous NMSC (T1:118, T2:116, T3:113), >10 prior NMSC (T1:47, T2:49, T3:55). Randomised: 525 Evaluable: 525 | |
| Interventions | T1: 25000 IU oral retinol (capsule) daily, T2: 10 mg oral isotretinoin (capsule) daily, T3: placebo capsule identical in appearance. Duration of treatment three yrs | |
| Outcomes | FU: at one month and then every 6 months for 3 yrs. Outcomes: time to first BCC or SCC | |
| Notes | No loss to FU. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Moon 1997.
| Methods | D: parallel group, multicentre, USA. AC: unclear RS: randomised by permuted blocks of size 4. B: participants, outcome assessor ITT | |
| Participants | 2297 participants. Incl: history of > ten AKs and at most two SCC or BCCs. Excl: xeroderma pigmentosum or basal cell nevus syndrome. Set: USA Age < 63 T1: 584, T2: 558 Age > 63 T1: 573, T2: 582 Randomised: T1: 1157, T2: 1140. m/f , T1: 823/334 T2: 795/345 Evaluable: | |
| Interventions | T1: 25,000IU oral retinol daily in capsule. T1: placebo capsule identical in appearance. Duration of treatment five yrs. | |
| Outcomes | FU: month 1 and then every 6 months while on treatment (5 yrs). No FU after cessation of treatment. Time to first new occurrence of SCC and time to first new occurrence of BCC | |
| Notes | 334 participants lost to Fu (T1:169,T2:165). 239 participants withdrew from trial due to adverse effects (T1: 127, T2:112). 115 deaths (T1:62,T2:53) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tangrea 1992.
| Methods | Multicenter (USA) D: parallel AC: Central data coordination center RC: telephone randomisation B: physician and patient ITT | |
| Participants | 981 participants with 2 or more biopsy proven BCC within the last 5 years (T1:490,T2:491). Two prior BCCs T1:183,T2:193. Three to four prior BCCs T1:167, T2:166. >4 BCCs T1:140, T2: 132. Excl: basal cell naevus syndrome, xeroderma pigmentosum, active malignancy, current evidence of hyperlipidaemia Mean age T1: 61, T2: 61 yrs Randomised: 981d Evaluable: 899 | |
| Interventions | T1: 2x5 mg isotretinoin capsules daily, T2: placebo identical in appearance. | |
| Outcomes | FU: week two, months three, six and then every six months for three yrs whilst on treatment . After treatment six monthly for years four and five. Outcomes: Number of new BCCs and time to first appearance of new BCC. | |
| Notes | 82 participants lost to FU (T1:43, T2:39). Less than 20% of participants treatment regimen altered due to adverse reactions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Yarosh 2001.
| Methods | D: parallel, multicentre, USA, Europe. AC: clear RS: generated by quality assurance department B: participants, clinician, outcome assessor PP | |
| Participants | Incl: Xeroderma pigmentosum participants with a history of AK or other skin cancer. Excl: treatment within previous 30 days with drugs that would interfere with examination of skin lesions; pregnancy; breast feeding; inadequate contraception methods in women of childbearing age. Set: Median age: T1: 19.5, T2: 16 Duration: one yr Randomised: 30 (T1:20, T2:10) m/f: T1: 14/16, T2: 4/5 Evaluable: 29 | |
| Interventions | T1: 1 mg/L T4 endonuclease V encapsulated in liposomes in a 1% hydrogel lotion T2: placebo lotion consisted of equivalent empty liposomes, without enzyme, formulated in same lotion. liposome lotion applied daily for one yr. | |
| Outcomes | FU: 3 monthly visits for 1 year, then 13 and 18 months. New AKs, BCC | |
| Notes | withdrawn: one placebo participants before treatment and one at nine months with progressive disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
D: design AC: method of allocation concealment RS: method of generating randomisation sequence B: blinding (participant, clinician, outcome assessment) tx: treatment CVA cerebrovascular accident RTR: renal transplant recipients T1: treatment one T2: treatment two or placebo or control AK: actinic keratosis BCCs: basal cell carcinomas SCC: squamous cell carcinoma IU: international units M/f: males/females ITT: intention to treat PP: per protocol
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dantal 1998 | Looking at optimum doses of cyclosporin as used in maintenance therapy for kidney‐transplant recipients. |
| de Graaf 2006 | PDT is not an intervention that this review is considering |
| Einspahr 2002 | This study looked at reductions of AK and not numbers of BCC or SCC |
| Frieling 2001 | Study was to test whether supplementation with beta carotene reduces risk for development of a first NMSC in healthy males |
| Green 1994 | Use of unselected adult population in Australia |
| Neale 2002 | Sun protective products including sunscreens and sun blocks have been covered in another review and therefore not included here. |
| Shigaki 2002 | Looking at sun protection habits |
Characteristics of ongoing studies [ordered by study ID]
Pittelkow.
| Trial name or title | Randomised study of acitretin in patients with multiple prior skin cancers who received solid organ transplantation. |
| Methods | |
| Participants | Solid organ recipient receiving immunosuppressive agents, with two prior BCC or SCC respected |
| Interventions | Oral acitretin or placebo daily for two years. |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Contributions of authors
Link with editorial base and co‐ordinate contributions from co‐authors (FB‐H) Draft Protocol (FB‐H, NS) Consumer input to protocol (JD) Advice on transplant patients (AW) Run search (FB‐H, NS) Identify relevant titles and abstracts from searches (FB‐H, J L‐B, NS) Obtain copies of trials ( FB‐H, NS, J L‐B) Selection of trials (FB‐H, NS) Extract data from trials (FB‐H, J L‐B, NS) Enter data into RevMan (FB‐H, J L‐B) Carry out analysis (FB‐H, J L‐B) Interpret data (FB‐H, J L‐B, WP)
Sources of support
Internal sources
University Nottingham, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Black 1995 {published data only}
- Black HS, Thornby JI, Wolf JE, Goldberg LH, Herd A, Rosen T, et al. Evidence that a low‐fat diet reduces the occurrence of non‐melanoma skin cancer. International Journal of Cancer 1995;62:165‐9. [DOI] [PubMed] [Google Scholar]
Bouwes Bavinck 1995 {published data only}
- Bouwes Bavinck JN, Tieben LM, Woude FJ, Tegzess AM, Hermans J, Schegget JT, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: A double‐blind , placebo‐controlled study. Journal of Clinical Oncology 1995;13:1933‐8. [DOI] [PubMed] [Google Scholar]
Clark 1996 {published data only}
- Clark LC, Combs GF, Turnbull BW, Slate EH for the Nutritional Prevention of Cancer Study Group. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. JAMA 1996;276(24):1957‐1963. [PubMed] [Google Scholar]
- Combs GF, Clark LC, Turnbull BW. Reduction of cancer risk with an oral supplement of selenium. Biomedical and Environmental Sciences 1997;10(2‐3):227‐34. [PubMed] [Google Scholar]
- Duffield‐Lillico AJ, Slate EH, Reid ME, Turnbull BW. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomised trial. Journal of the National Cancer Institute 2003;95(19):1477‐81. [DOI] [PubMed] [Google Scholar]
de Sevaux 2003 {published data only}
- Sevaux RGL, Smit JV, Jong EMGJ, Kerkhof PCM, Hoitsma AJ. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. Journal of the American Academy of Dermatology 2003;49:407‐12. [DOI] [PubMed] [Google Scholar]
George 2002 {published data only}
- George R, Weightman W, Russ GR, Bannister KM, Mathew TH. Acitretin for chemoprevention of non‐melanoma skin cancers in renal transplant recipients. Australasian Journal of Dermatology 2002;43:269‐73. [DOI] [PubMed] [Google Scholar]
Greenberg 1990 {published data only}
- Greenberg ER, Baron JA, Stevens MM, Skukel TA, and the skin cancer prevention study group. The skin cancer prevention study: design of a clinical trial of beta‐carotene among persons at high risk for non melanoma skin cancer. Controlled Clinical Trials 1989;10:153‐66. [DOI] [PubMed] [Google Scholar]
- Greenberg ER, Baron JA, Stukel TA, Stevens MM, Mandel JS, Spencer SK, et al. A clinical trial of beta carotene to prevent basal‐cell and squamous‐cell cancers of the skin. New England Journal of Medicine 1990;323:789‐95. [DOI] [PubMed] [Google Scholar]
Levine 1997 {published data only}
- Levine N, Moon TE, Cartmel B, Bangert JL, for the Southwest Skin Cancer Prevention Study Group. Trial of retinol and isotretinoin in skin cancer prevention: a randomized, double‐blind, controlled trial. Cancer Epidemiology, Biomarkers and Prevention 1997;6:957‐61. [PubMed] [Google Scholar]
- Moon TE, Levine N, Cartmel B, Bangert J, Rodney S, Schreiber M, et al. Design and recruitment for retinoid skin cancer prevention (SKICAP) trials. Cancer Epidemiology, Biomarkers and Prevention 1995;4:661‐9. [PubMed] [Google Scholar]
Moon 1997 {published data only}
- Moon TE, Levine N, Cartmel B, Bangert J, Rodney S, Schreiber M, et al. Design and recruitment for retinoid skin cancer prevention (SKICAP) trials. Cancer Epidemiology, Biomarkers and Prevention 1995;4:661‐9. [PubMed] [Google Scholar]
- Moon TE, Levine N, Cartmel B, Bangert JL, Rodney S, Dong Q, et al. Effect of retinol in preventing squamous cell skin cancer in moderate‐risk subjects: A randomized, double‐blind, controlled trial. Cancer Epidemiology, Biomarkers and Prevention 1997;6:949‐56. [PubMed] [Google Scholar]
Tangrea 1992 {published data only}
- Tangrea J, Edwards B, Hartman A, Taylor P, and the ISO‐BCC Study Group. Isotretinoin‐Basal Cell Carcinoma Prevention Trial. Controlled Clinical Trials 1990;11(6):433‐50. [DOI] [PubMed] [Google Scholar]
- Tangrea JA, Edwards BK, Taylor PR, Hartmen AM, and the other members of the Isotretinoin‐Basal Cell Carcinoma Study Group. Long‐term therapy with low‐dose isotretinoin for prevention of basal cell carcinoma: A multicenter clinical trial. Journal of the National Cancer Institute 1992;84:328‐32. [DOI] [PubMed] [Google Scholar]
- Trangrea JA, Adrianza E, Helsel WE, Taylor PR, Hartman AM, Peck GL, et al. Clinical and Laboratory adverse effects associated with long‐term, low‐dose isotretinoin: incidence and risk factors. The Isotretinoin‐Basal Cell Carcinoma study Group. American Society of Preventive Oncology 1993;2(4):375‐80. [PubMed] [Google Scholar]
Yarosh 2001 {published data only}
- Yarosh D, Klein J, O'Connor A, Hawk J, Rafal E, Wolf P, for the Xeroderma Pigmentosum Study Group. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. The Lancet 2001;357:926‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dantal 1998 {published data only}
- Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, et al. Effect of long‐term immunosuppression in kidney‐graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet 1998;351:623‐8. [DOI] [PubMed] [Google Scholar]
de Graaf 2006 {published data only}
- Graaf YGL, Kennedy C, Wolterbeek R, Collen AFS, Willemze R, Bouwes Bavinck JN. Photodynamic therapy does not prevent cutaneous squamous cell carcinoma in organ ‐ transplant recipients: results of a randomized‐controlled trial. Journal of Investigative Dermatology 2006;126:569‐74. [DOI] [PubMed] [Google Scholar]
Einspahr 2002 {published data only}
- Einspahr JG, Nelson MA, Saboda K, Warneke J, Bowden GT, Alberts DS. Modulation of biologic endpoints by topical difluoromethylornithine (DFMO), in subjets at high‐risk of nonmelanoma skin cancer. Clinical Cancer Research 2002;8:149‐55. [PubMed] [Google Scholar]
Frieling 2001 {published data only}
- Frieling UM, Schaumberg DA, Kupper TS, Muntwyler J, Hennekens CH. A randomized, 12‐ year primary‐prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the Physicians' Health Study. Archives of Dermatology 2000;136(2):179‐84. [DOI] [PubMed] [Google Scholar]
Green 1994 {published data only}
- Green A, Battistutta D, Hart V, Leslie D, Marks G, Williams G, et al. The Nambour Skin Cancer and Actinic Eye Disease Prevention Trial: design and baseline characteristics of participants. Controlled Clinical Trials 1994;15(6):512‐22. [DOI] [PubMed] [Google Scholar]
Neale 2002 {published data only}
- Neal R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Archives of Dermatology 2002;138:1319‐25. [DOI] [PubMed] [Google Scholar]
Shigaki 2002 {published data only}
- Shigaki D, Maddock JE, Isnec MR. A randomized trial of skin cancer prevention in aquatics settings: The Pool Cool Program. Health Psychology 2002;21(6):579‐87. [PubMed] [Google Scholar]
References to ongoing studies
Pittelkow {published data only (unpublished sought but not used)}
- Randomised study of acitretin in patients with multiple prior skin cancers who received solid organ transplantation.. Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
ACS 2003
- ACS. Cancer facts and figures 2003. American Cancer Society.
AIHW 2003
- AIHW. Media release: higher risk of cancer for men. Australian Health and Welfare Institute.
Alam 2001
- Alam M, Ratner D. Cutaneous squamous‐cell carcinoma. New England Journal of Medicine 2001;344(13):975‐83. [DOI] [PubMed] [Google Scholar]
Alexis 1999
- Alexis AF, Jones VA, Stiller MJ. Potential therapeutic applications of tea in dermatology. International Journal of Dermatology 1999;38(10):735‐43. [DOI] [PubMed] [Google Scholar]
Altman 1991
- Altman DG. Practical statistics for medical research. London: Chapman and Hall, 1991. [Google Scholar]
Auerbach 1961
- Auerbach H. Geographic variation in incidence of skin cancer in the United States. Public Health Reports 1961;76:345‐8. [PMC free article] [PubMed] [Google Scholar]
Bath‐Hextall 2004
- Bath‐Hextall F, Bong J, Perkins W, Williams HC. Interventions for basal cell carcinoma of the skin: systematic review. BMJ 2004;329:705‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bavinck 1995
- Bavinck JN, Tieben LM, Woude FJ, Tegzess AM, Hermans J, ter Schegget J, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double‐blind, placebo‐controlled study. Journal of Clinical Oncology 1995;13(8):1933‐8. [DOI] [PubMed] [Google Scholar]
Bialy 2002
- Bialy T, Rothe M, Grant‐Kels J. Dietary factors in the prevention and treatment of nonmelanoma skin cancer and melanoma. Dermtological Surgery 2002;28(12):1143‐52. [DOI] [PubMed] [Google Scholar]
Black 1998
- Black HS. Influence of dietary factors on actinically‐induced skin cancer. Mutation Research 1998;422(1):185‐90. [DOI] [PubMed] [Google Scholar]
Buettner 1993
- Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha‐tocopherol, and ascorbate. Archives of Biochemistry and Biophysics 1993;300(2):535‐43. [DOI] [PubMed] [Google Scholar]
Cartmel 2000
- Cartmel B, Moon TE, Levine N, Rodney S, Alberts D. Predictors of inactivation and reasons for participant inactivation during a skin cancer chemoprevention study. Cancer Epidemiology Biomarkers Prevention 2000;9:999‐1002. [PubMed] [Google Scholar]
Cox 1999
- Cox NH, Eedy DJ, Morton CA. Guidelines for management of Bowen's disease. British Association of Dermatologists. British Journal of Dermatology 1999;141(4):633‐41. [DOI] [PubMed] [Google Scholar]
CRUK 2004
- CRUK. Skin cancer – non‐melanoma. http://www.cruk.org.uk 2004.
Demopoulos 2003
- Demopoulos BP, Vamvakas E, Ehrlich JE, Demopoulos R. Non‐acquired immunodeficiency syndrome‐defining malignancies in patients infected with human immunodeficiency virus. Archives of Pathology and Laboratory Medicine 2003;127(5):589‐92. [DOI] [PubMed] [Google Scholar]
DiGiovanna 1995
- DiGiovanna JJ, Sollitto RB, Abangan DL, Steinberg SM, Reynolds JC. Osteoporosis is a toxic effect of long‐term etretinate therapy. Archives of Dermatology 1995;131(11):1263‐7. [PubMed] [Google Scholar]
DiGiovanna 2001
- DiGiovanna JJ. Isotretinoin effects on bone. Journal of the American Academy of Dermatology 2001;45(5):S176‐82. [DOI] [PubMed] [Google Scholar]
Dinehart 1996
- Dinehart SM, Jansen GT. Cancer of the skin. In: Myers EN, Suen JY editor(s). Cancer of the Head and Neck. 3rd Edition. WB Saunders Company, 1996:143‐159. [Google Scholar]
Duffield‐Lillico
- Duffield‐Lillico AJ, Slate EH, Reid ME, Turnbull BW. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomised trial. Journal of the National Cancer Institute 2003;95(19):1477‐81. [DOI] [PubMed] [Google Scholar]
Dunnick 1991
- Dunnick JK, Forbes PD, Eustis SL, Hardisty JF, Goodman DG. Tumors of the skin in the HRA/Skh mouse after treatment with 8‐methoxypsolaren and UVA radiation. Fundamental and Applied Toxicology 1991;16:92‐102. [DOI] [PubMed] [Google Scholar]
Eedy 2000
- Eedy DJ. Non‐melanoma skin cancer and the 'new National Health Service': implications for U.K. dermatology?. British Journal of Dermatology 2000;142(3):397‐9. [DOI] [PubMed] [Google Scholar]
Fine 1999
- Fine JD, Johnson LB, Suchidran C, Bauer EA, Carter DM, McGuire J. Cancer and inherited epidermolysis bullosa registry study population. In: Fine JD, Bauer EA, McGuire J, Moshell A editor(s). Epidermolysis bullosa: clinical, epidemiologic, and laboratory advances, and the findings of the national epidermolysis bullosa registry. Baltimore: John Hopkins University Press, 1999:175‐92. [Google Scholar]
Fine 2004
- Fine J, Johnson LB, Weiner M, Stein A, Suchindran C. Chemoprevention of squamous cell carcinoma in recessive dystrophic epidermolysis bullosa: Results of a phase 1 trial of systemic isotretinoin. Journal of the American Academy of Dermatology 2004;50(4):563‐71. [DOI] [PubMed] [Google Scholar]
Fuller 1992
- Fuller CJ, Faulkner H, Bendich A, Parker RS, Roe DA. Effect of beta‐carotene supplementation on photosuppression of delayed‐type hypersensitivity in normal young men. American Journal of Clinical Nutrition 1992;56(4):684‐90. [DOI] [PubMed] [Google Scholar]
Gasparro 1998
- Gasparro FP, Mitchnick M, Nash JF. A review of sunscreen safety and efficacy. Photochemistry and photobiology 1998;68(3):243‐56. [PubMed] [Google Scholar]
Gensler 1996
- Gensler HL, Aickin M, Peng YM, Xu M. Importance of the form of topical vitamin E for prevention of photocarcinogenesis. Nutrition and Cancer 1996;26(2):183‐91. [DOI] [PubMed] [Google Scholar]
Glass 1989
- Glass AG, Hoover RN. The emerging epidemic of melanoma and squamous cell skin cancer. Journal of the American Medical Association 1989;262(15):2097‐100. [PubMed] [Google Scholar]
Goldman 1998
- Goldman GD. Squamous cell cancer: a practical approach. Seminars in Cutaneous Medicine and Surgery 1998;17(2):80‐95. [DOI] [PubMed] [Google Scholar]
Gray 1997
- Gray DT, Suman VJ, Su WP, Clay RP, Harmsen WS, Roenigk RK. Trends in the population‐based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992. Archives in Dermatology 1997;133(6):735‐40. [PubMed] [Google Scholar]
Green 1999
- Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal‐cell and squamous‐cell carcinomas of the skin: a randomised controlled trial. Lancet 1999;354(9180):723‐9. [DOI] [PubMed] [Google Scholar]
Grossman 1997
- Grossman D, Leffell DJ. The molecular basis of nonmelanoma skin cancer: new understanding. Archives in Dermatology 1997;133(10):1263‐70. [PubMed] [Google Scholar]
Hardie 1980
- Hardie IR, Strong RW, Hartley LC, Woodruff PW, Clunie GJ. Skin cancer in Caucasian renal allograft recipients living in a subtropical climate. Surgery 1980;87(2):177‐83. [PubMed] [Google Scholar]
Harris 2001
- Harris RB, Griffith K, Moon TE. Trends in the incidence of nonmelanoma skin cancers in southeastern Arizona, 1985‐1996. Journal of the American Academy of Dermatology 2001;45(4):528‐36. [DOI] [PubMed] [Google Scholar]
Hartevelt 1990
- Hartevelt MM, Bavinck JN, Kootte AM, Vermeer BJ, Vandenbroucke JP. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation 1990;49(3):506‐9. [DOI] [PubMed] [Google Scholar]
Heyman 1956
- Heyman A, Pfeiffer JB Jr, Willett RW, Taylor HM. Peripheral neuropathy caused by arsenical intoxication; a study of 41 cases with observations on the effects of BAL (2, 3, dimercapto‐propanol). New England Journal of Medicine 1956;254(9):401‐9. [DOI] [PubMed] [Google Scholar]
Holme 2000
- Holme SA, Malinovszky K, Roberts DL. Changing trends in non‐melanoma skin cancer in South Wales, 1988‐98. British Journal of Dermatology 2000;143(6):1224‐9. [DOI] [PubMed] [Google Scholar]
Hughes 1983
- Hughes GS, Jr. Davis L. Variegate porphyria and heavy metal poisoning from ingestion of "moonshine". Southern Medical Journal 1983;76(8):1027‐9. [DOI] [PubMed] [Google Scholar]
Iversen 1999
- Iversen T, Tretli S. Trends for invasive squamous cell neoplasia of the skin in Norway. British Journal of Cancer 1999;81(3):528‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaax 1997
- Jaxx S, Scott LW, Wolf JE, Thornby JL, Black HS. General guidelinesfor a low‐fat diet effective in the management and prevention of nonmelanoma skin cancer. Nutrition and Cancer 1997;27:150‐6. [DOI] [PubMed] [Google Scholar]
Johnson 1996
- Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 1996;272(5268):1668‐71. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Assessing the qualtity of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karagas 2006
- Karagas MR, Nelson HH, Sehr P, Waterboer T, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. Journal of the National Cancer Institute 2006;98(6):389‐95. [DOI] [PubMed] [Google Scholar]
Kraemer 1980
- Kraemer KH. Xeroderma pigmentosum. A prototype disease of environmental‐genetic interaction. Archives in Dermatology 1980;116(5):541‐2. [DOI] [PubMed] [Google Scholar]
Kraemer 1994
- Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Archives in Dermatology 1994;130(8):1018‐21. [PubMed] [Google Scholar]
Lang 1991
- Lang PG, Maize JC. Basal Cell Carcinoma. In: Friedman RJ, Rigel, Kopf AW, Harris MN, Baker D editor(s). Cancer of the skin. WB Saunders Company, 1991:35‐73. [Google Scholar]
Lippman 1987
- Lippman SM, Kessler JF, Meyskens FL Jr. Retinoids as preventive and therapeutic anticancer agents (Part I). Cancer Treatment Reports 1987;71(4):391‐405. [PubMed] [Google Scholar]
Lookingbill 1995
- Lookingbill DP, Lookingbill GL, Leppard B. Actinic damage and skin cancer in albinos in northern Tanzania: findings in 164 patients enrolled in an outreach skin care program. Journal of the American Academy of Dermatology 1995;32(4):653‐8. [DOI] [PubMed] [Google Scholar]
Marcil 2000
- Marcil I, Stern R. Risk of developing a subsequent non‐melanoma skin cancer in patients with a history of non‐melanoma skin cancer: A critical review of the literature and meta analysis. Archives of Dermatology 2000;136:1524. [DOI] [PubMed] [Google Scholar]
Martinez 2001
- Martinez JC, Otley CC. The management of melanoma and nonmelanoma skin cancer: a review for the primary care physician. Mayo Clinic Proceedings 2001;76(12):1253‐65. [DOI] [PubMed] [Google Scholar]
May 1981
- May GS, Demets DL, Friedman LM, Furberg C, Passamani E. The randomized clinical trial:bias in analysis. Circulation 1981;64:669‐73. [DOI] [PubMed] [Google Scholar]
McCormack 1997
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of the histological subtypes of basal cell carcinoma. A possible indicator of differing causes. Archives of Dermatology 1997;133(5):593‐6. [PubMed] [Google Scholar]
McVean 1999
- McVean M, Liebler DC. Prevention of DNA photodamage by vitamin E compounds and sunscreens: roles of ultraviolet absorbance and cellular uptake. Molecular Carcinogenesis 1999;24(3):169‐76. [DOI] [PubMed] [Google Scholar]
Naldi 2004
- Naldi L, Buzzetti R, Cecchi C, Baldwin L, Battistutta D, Benvenuto C, et al. Educational programmes for the skin cancer prevention (Protocol for a Cochrane Review). Cochrane Database of Systematic Reviews 2004, Issue 2. [Google Scholar]
Naylor 1997
- Naylor MF, Farmer KC. The case for sunscreens: a review of their use in preventing actinic damage and neoplasia. Archives of Dermatology 1997;133(9):1146‐54. [DOI] [PubMed] [Google Scholar]
NCCN 2004
- National Comprehensive Cancer Network (NCCN). Guidelines for the treatment of basal and squamous skin cancers. http://www.nccn.org/physician_gls/f_guidelines.html Accessed 13 July 2004.
Neubauer 1947
- Neubauer O. Arsenical Cancer: a review. British Journal of Cancer 1947;1:192‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parrish 1974
- Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA. Photochemotherapy of psoriasis with oral methoxsalen and long wave ultraviolet light. New England Journal of Medicine 1974;291:1207‐11. [DOI] [PubMed] [Google Scholar]
Peck 1982
- Peck GL, Gross EG, Butkus D, DiGiovanna JJ. Chemoprevention of basal cell carcinoma with isotretinoin. Journal of the American Academy of Dermatology 1982;6(4 Pt 2 Suppl):815‐23. [DOI] [PubMed] [Google Scholar]
Peck 1988
- Peck GL, DiGiovanna JJ, Sarnoff DS, Gross EG, Butkus D, Olsen TG, et al. Treatment and prevention of basal cell carcinoma with oral isotretinoin. Journal of American Academy of Dermatology 1988;19 (1Pt 2):176‐85. [DOI] [PubMed] [Google Scholar]
Pence 1994
- Pence BC, Delver E, Dunn DM. Effects of dietary selenium on UVB‐induced skin carcinogenesis and epidermal antioxidant status. Journal of Investigative Dermatology 1994;102(5):759‐61. [DOI] [PubMed] [Google Scholar]
Pershagen 1981
- Pershagen G. The carcinogenicity of arsenic. Environmental Health Perspectives 1981;40:93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenberg 2004
- Rosenberg C, Greenland P, Khandekar J, Loar A, Ascensao J, Lopez A. Association of nonmelanoma skin cancer with second malignancy. Cancer 2004;100(1):130‐8. [DOI] [PubMed] [Google Scholar]
Rowe 1992
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. Journal of the American Academy of Dermatology 1992;26(6):976‐90. [DOI] [PubMed] [Google Scholar]
Sackett 1979
- Sackett DL, Gent M. Controversy in counting and attributing events in clinical trials. New England Journal of Medicine 1979;301:1410‐12. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ. Empirical evidence of bias. Journal of the American Medical Academy 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schwartz 1997
- Schwartz RA. Arsenic and the skin. International Journal of Dermatology 1997;36(4):241‐50. [DOI] [PubMed] [Google Scholar]
Shindo 1993
- Shindo Y, Witt E, Packer L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. Journal of Investigative Dermatology 1993;100(3):260‐5. [DOI] [PubMed] [Google Scholar]
Staples 1998
- Staples M, Marks R, Giles G. Trends in the incidence of non‐melanocytic skin cancer (NMSC) treated in Australia 1985‐1995: are primary prevention programs starting to have an effect?. International Journal of Cancer 1998;78(2):144‐8. [DOI] [PubMed] [Google Scholar]
Stern 1998
- Stern RS, Liebman EJ, Vakeva L. Oral psolaren and ultraviolet‐A Light (PUVA) treatment of psoriasis and persistent risk of nonmelanoma skin cancer. Journal of the National Cancer Institute 1998;90(17):1278‐84. [DOI] [PubMed] [Google Scholar]
Stern 1999
- Stern RS. The mysteries of geographic variability in nonmelanoma skin cancer incidence. Archives of Dermatology 1999;135(7):843‐4. [DOI] [PubMed] [Google Scholar]
Telfer 1999
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. British Journal of Dermatology 1999;141(3):415‐23. [DOI] [PubMed] [Google Scholar]
Treleaven 1993
- Treleaven J, Meller S, Farmer P, Birchall D, Goldman J, Piller G. Arsenic and ayurveda. Leukemia and Lymphoma 1993;10(4‐5):343‐5. [DOI] [PubMed] [Google Scholar]
Westby 2004
- Westby M, Bath FJ, Herd R, Macnell SJ. Photodynamic therapy for the localised squamous cell carcinoma of the skin (Protocol for a Cochrane Review). Cochrane Database of Systematic Reviews 2003, Issue 2. [Google Scholar]
Wong 2003
- Wong C, Strange R, Lear J. Basal cell carcinoma. BMJ 2003;327:794. [DOI] [PMC free article] [PubMed] [Google Scholar]


