Abstract
Previous research has suggested that dietary protein deficiency alters resistance to experimental pulmonary tuberculosis, in part, by affecting the distribution and trafficking of antigen-reactive T cells. In this study, guinea pigs were maintained on either a protein-deficient (10% ovalbumin) or control (30% ovalbumin) diet and infected 4 to 6 weeks later with a low dose of virulent Mycobacterium tuberculosis H37Rv by the respiratory route. Monoclonal antibodies directed against the CD4 or CD8 markers on guinea pig lymphocytes were used in a flow cytofluorometric assay to determine the proportion of each subset in the peripheral circulation, spleen, and bronchotracheal lymph nodes at 4 weeks after infection. In uninfected guinea pigs, only the spleen exhibited an effect of diet on T-cell distribution, with small but consistent reductions in the proportions of both CD4 and CD8 T lymphocytes. However, following infection, protein deficiency exerted a profound effect on T-cell distribution. Malnourished, tuberculous guinea pigs harbored only 20 and 60% of the T cells (as a proportion of total lymphoid cells) found in the spleen and blood, respectively, of their well-nourished counterparts. Normal relative proportions of CD4 and CD8 cells were observed, however. In striking contrast, the bronchotracheal lymph nodes of protein-deprived guinea pigs with tuberculosis contained more than twice the numbers of T cells of control guinea pigs, and the normal CD4-to-CD8 ratio was reversed. Peripheral T-cell function, as measured by the delayed hypersensitivity skin test to tuberculin, and antigen-induced lymphoproliferation in vitro were markedly suppressed in protein-malnourished animals. Conversely, purified protein derivative-induced (but not concanavalin A-induced) proliferation was significantly enhanced in cultures of lymph node cells from protein-deprived tuberculous animals. Taken together, these results suggest that immunological abnormalities and loss of antimycobacterial resistance in the lungs of protein-deficient guinea pigs may be explained, in part, by sequestration of antigen-reactive T cells in the lymph nodes draining the site of infection.
Chronic, moderate dietary protein deficiency in guinea pigs is associated with significant alterations in antigen-specific T-cell functions and vaccine-induced resistance following infection by the respiratory route with small numbers (10 to 20) of virulent Mycobacterium tuberculosis cells (4, 9, 11). Many of these observations have been confirmed recently in a mouse model of protein-calorie malnutrition in which the animals were infected by the intravenous route with extremely large inocula (104 to 106 cells) (3). Unresponsiveness to mycobacterial infection in protein-deprived guinea pigs and mice mimics the nonreactive pole of the clinical spectrum of tuberculosis (7, 9). Several mechanisms have been proposed to explain the lack of immunologic reactivity observed in some tuberculosis patients; these include alterations in the number, balance, reactivity, or distribution of thymus-dependent (T) cells and their helper-inducer (CD4) and suppressor-cytotoxic (CD8) subsets (5, 15, 21).
In previous work with this model of pulmonary tuberculosis, we have observed significant impairment of tuberculin-induced delayed-type hypersensitivity and of proliferation and interleukin-2 (IL-2) production by peripheral blood lymphocytes in protein-deficient animals (9, 11, 13). At the same time, the bronchotracheal lymph nodes draining the initial site of implantation of M. tuberculosis were found in low-protein guinea pigs to contain significantly increased proportions of rosette-forming (CD2-positive) T cells (2) and reduced levels of lymphocytes expressing the Fc receptor for immunoglobulin M (IgM) (FcμR+) (10). These data suggested that protein deficiency may alter the anatomical distribution of T cells in guinea pigs with pulmonary tuberculosis.
Immunological studies with this model have been hampered by the lack of readily available monoclonal antibodies directed against phenotypic markers on guinea pig lymphoid cells. Recently, a few such antibodies have been described (16, 17). In this study, monoclonal antibodies specific for the αβ T-cell receptor (αβTcR) and the CD4 and CD8 markers on guinea pig lymphocytes were used to test the hypothesis that dietary protein deficiency results in alterations in the distribution and relative proportions of T cells and their subsets in tuberculous guinea pigs.
MATERIALS AND METHODS
Experimental animals.
Male and female inbred strain 2 guinea pigs (NCI-Frederick Cancer Research Facility, Frederick, Md.), initially weighing 200 to 300 g, were used in these experiments. The animals were housed individually in polycarbonated cages with stainless-steel grid floors and feeders and were allowed food and water ad libitum. Once infected with virulent M. tuberculosis, the animals were moved into a BL3 biohazard suite and kept in individual stainless-steel cages with microisolator bonnets. Each animal was randomly assigned to an experimental diet.
Experimental diets.
Experimental diets were obtained from a commercial supplier (Dyets, Inc., Bethlehem, Pa.) and formulated to meet current recommended nutritional requirements for guinea pigs. The low-protein (LP) diet contained 10% ovalbumin, and the control (C) diet contained 30% ovalbumin. The two diets were isocaloric and identical with respect to all nutrients except protein. The precise composition of the diets has been published previously (14). The caloric contents of both the C and LP diets were identical, and food intake amounts between both groups of guinea pigs were not significantly different. Data on the impact of the LP diet on the status of other nutrients, such as zinc and iron, has been published previously (14). Animals were weaned from commercial chow to the experimental diets according to the protocol described earlier (4). After 4 weeks on the experimental diets, the animals were infected.
Pulmonary infection.
Virulent M. tuberculosis H37Rv (ATCC 27294), obtained from the American Type Culture Collection (Rockville, Md.), had been stored as a single-cell suspension at −70°C (6). Guinea pigs were infected via the respiratory route with an aerosol chamber, as described previously (4, 11, 22). Parameters of the infection procedure were adjusted empirically to result in the inhalation and retention of about 10 to 15 viable mycobacteria per animal.
Tuberculin skin test.
The delayed-type hypersensitivity response was evaluated by intradermal injection of 0.1 ml of purified protein derivative (PPD) containing 100 tuberculin units (PPD-RT 23; Statens Seruminstitut, Copenhagen, Denmark). Twenty-four hours later, the mean diameter of induration was measured, in millimeters.
Necropsy procedure and lymphocyte preparation.
Four weeks after infection, the animals were euthanized by the intraperitoneal injection of 1 to 2 ml of sodium pentobarbital (Fort Dodge Laboratories, Inc., Fort Dodge, Iowa). Whole blood was collected by intracardiac aspiration with a 10-ml heparinized syringe (heparin sodium; Sigma, St. Louis, Mo.). The spleen and bronchotracheal lymph nodes were removed aseptically. Peripheral blood lymphocytes were isolated by density gradient centrifugation with a mixture of Histopaque (Sigma) and lymphocyte separation medium (Organon Teknika Corp., Durham, N.C.) to achieve a final specific gravity of 1.107, as previously described (4). Lymphocytes were harvested and washed three times in phosphate-buffered saline. Single-cell suspensions of spleen and bronchotracheal lymph nodes were obtained by gently homogenizing the tissue in a sterile Ten Broeck homogenizer with tissue culture medium RPMI 1640 (Irving Scientific, Santa Ana, Calif.) supplemented with 10% fetal bovine serum (Sigma), penicillin (100 U/ml), streptomycin (100 μg/ml), 2-mercaptoethanol (10 μM), and l-glutamine (2 μM). The viability of the lymphocytes was determined by the trypan blue exclusion method.
Lymphoproliferation assay.
Mitogen- and antigen-induced lymphoproliferation was assessed in vitro by an established procedure (4). Lymphocytes from blood, spleen, and lymph nodes were suspended in the RPMI 1640 medium described above and placed in 96-well microtiter plates (2 × 105 cells per well) (Corning Glass Works, Corning, N.Y.). Triplicate cultures were stimulated with PPD (Statens Seruminstitut) at a final concentration of 12.5 μg/ml and with concanavalin A (Sigma) at a final concentration of 10 μg/ml. Control cultures received cells and medium alone. Following a 4-day incubation at 37°C in a 5% CO2 environment, 1.0 μCi of tritiated thymidine (6.7 Ci/mmol) (Dupont, NEN Research Products, Boston, Mass.) in a volume of 50 μl of medium was added to each well. Incubation was continued for an additional 6 h, and the cells were harvested onto fiberglass filter disks by a multiple automated sample harvester unit (MASH; Otto Hiller, Inc., Madison, Wis.). Results are expressed as net counts per minute, which is defined as counts per minute in antigen- or mitogen-stimulated wells minus the counts per minute in control (unstimulated) wells of the same cell population.
Monoclonal antibodies.
Monoclonal antibodies against phenotypic markers on the surfaces of guinea pig lymphocytes were obtained through collaboration with Reinhard Burger at the Robert Koch Institut, Berlin, Germany. The designation for each antibody is as follows: anti-αβTcR, H159 (16); anti-CD4, H155 (17); anti-CD8, Msgp6 (1). All three antibodies had been produced in rats and were provided as hybridoma culture supernatant fluids.
Flow cytometry.
The flow cytometry methodology was modified from a published procedure (17). Cells from the blood, spleen, and lymph nodes from each treatment group were washed three times with staining buffer (Hanks phosphate-buffered saline with 1% fetal bovine serum) and then resuspended in 300 μl of staining buffer. Aliquots (50 μl) of the three monoclonal antibodies listed above were added to 5 × 105 cells in separate tubes. For each cell source, negative controls consisted of 50 μl of staining buffer or normal rat IgG in place of the primary antibody. The cells were incubated with shaking for 1 h at 4°C; they were then washed three times with cold (4°C) staining buffer by centrifugation at 200 × g for 10 min at 4°C, and the supernatant was discarded. The pellet was resuspended in 300 μl of staining buffer, and 50 μl of diluted secondary antibody (fluorescein isothiocyanate-conjugated rabbit anti-rat IgG, lot no. 14978; Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.) was added and incubated on the shaker for 1 h at 4°C. The cells were washed three times, as described above, and 300 μl of cold 0.1% paraformaldehyde in staining buffer was added. The cells were stored at 4°C for 24 h until being analyzed by flow cytometry. Single-cell fluorescence was measured with an EPICS V flow cytometer (Coulter Electronics, Hialeah, Fla.), and the data were generated by flow cytofluorometric analysis of 10,000 events for each labeled cell population. Percentages were calculated from a one-parameter histogram. The cell populations were 90 to 95% viable when they were recovered and stained.
Statistics.
The influence of the independent variables (diet and cell source) on the dependent variables (proportions of total αβTcR, CD4+, and CD8+ lymphocytes) was determined by analysis of variance. Where indicated by a statistically significant main treatment effect, differences between individual group means were evaluated for significance by the new Duncan’s Multiple Range test (20). A level of probability of less than 5% was required for significance in all tests.
RESULTS
The LP diet exerted a significant influence on the growth of the guinea pigs. The mean body weight (± standard deviation) of protein-deprived animals was 332 ± 31 g at the time of infection, compared to a weight of 483 ± 63 g for animals fed the isocaloric control (C) diet (P < 0.05). Protein deficiency alone exerted little effect on the proportion of T cells in the spleen and blood, as shown in Table 1. The total numbers of viable cells recovered per milliliter of blood or per gram of spleen or lymph node were not different between diet groups (data not shown). Modest, statistically insignificant decreases in the proportions of total αβ T cells and CD4+ and CD8+ cells, expressed as percentages of total lymphocytes, were observed in both lymphoid sources after 8 weeks on the diet but without infection.
TABLE 1.
Influence of protein deficiency on T-cell subset distributions in uninfected guinea pigs
| Lymphocyte source | Dieta | % Total lymphocytesb
|
||
|---|---|---|---|---|
| αβTcR+ | CD4+ | CD8+ | ||
| Spleen | C | 40.0 ± 4.8 | 28.0 ± 4.0 | 15.2 ± 3.4 |
| LP | 38.5 ± 4.3 | 23.5 ± 3.8 | 8.3 ± 5.1 | |
| Blood | C | 42.3 ± 4.1 | 29.3 ± 4.3 | 8.2 ± 3.1 |
| LP | 32.4 ± 5.0 | 24.1 ± 3.8 | 6.0 ± 3.7 | |
C, 30% ovalbumin diet; LP, 10% ovalbumin diet. Guinea pigs were fed for 8 weeks.
Data are derived from flow cytofluorometric analysis of 10,000 events and are means ± standard errors of the means for three to five determinations.
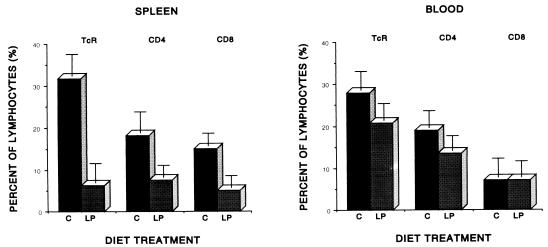
In contrast, dramatic and highly significant reductions in total αβ T cells and both subsets were observed in the spleen and blood at 4 weeks following pulmonary infection with virulent M. tuberculosis. Figure 1 illustrates the influence of diet on the relative proportions of T cells in the blood and spleen, expressed as percentages of total lymphocytes recovered. Protein-deprived (LP) guinea pigs harbored a significantly smaller (P < 0.001) population of αβ T cells in the spleen than did control animals following infection, with a slight but significant predominance of the CD4+ and CD8+ subset maintained in the control animals (P < 0.05). In the peripheral circulation, the decrease in total αβ T cells observed in LP guinea pigs was not statistically significant, although it was highly reproducible. This decrease occurred nearly completely at the expense of the CD4+ subset, while the proportions of CD8+ T cells were essentially identical in the two diet groups at 4 weeks after infection with virulent mycobacteria.
FIG. 1.
Effect of diet on the percentages of lymphocytes expressing αβTcR, CD4, or CD8 markers in the spleen and blood of strain 2 guinea pigs fed a C or LP diet for 4 weeks and infected for an additional 4 weeks with virulent M. tuberculosis H37Rv by the respiratory route. Data are based upon flow cytofluorometric analysis of 10,000 events and are means ± standard errors of the means for five to seven animals per treatment.
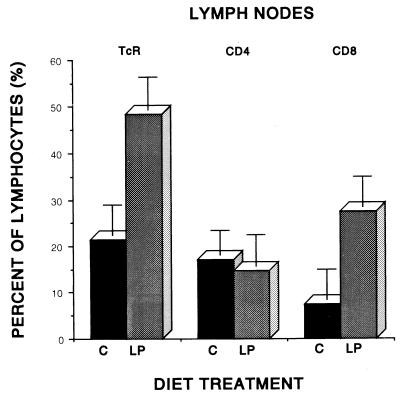
In contrast to the spleen and blood, the bronchotracheal lymph nodes revealed a very different combined effect of diet and infection (Fig. 2). The proportion of lymphocytes binding the anti-αβTcR antibody was more than twice as great in the lymph nodes of protein-deficient as in normally nourished tuberculous guinea pigs. This difference was highly statistically significant (P < 0.01). The CD4+-T-cell subset in this tissue was not influenced significantly by diet. However, the CD8+- T-cell subset was dramatically and significantly increased (P < 0.05) in the lymph nodes of LP animals infected with virulent M. tuberculosis, resulting in a reversal of the normal predominance of CD4+ T cells in the bronchotracheal lymph nodes of uninfected guinea pigs (24.2% ± 3.4% [CD4+] versus 12.8% ± 2.6% [CD8+]).
FIG. 2.
Influence of diet on the percentages of lymphocytes bearing αβTcR, CD4, or CD8 phenotypic markers in the bronchotracheal lymph nodes of strain 2 guinea pigs fed a C or LP diet for 4 weeks and infected by the pulmonary route with virulent M. tuberculosis H37Rv for an additional 4 weeks. Data are based upon flow cytofluorometric analysis of 10,000 events and are means ± standard errors of the means for five to seven animals per treatment.
As expected, malnourished guinea pigs exhibited a much less intense dermal reaction to tuberculin following infection. The diameter of induration was significantly reduced in protein-deficient guinea pigs (LP, 6.1 ± 3.6 mm; C, 15.2 ± 4.8 mm [P < 0.05]) despite both gross pathologic and bacteriologic evidence of extensive tuberculosis in the lungs, spleen, and bronchotracheal lymph nodes of the LP animals (data not shown). Although we did not perform a comprehensive set of bacteriological studies in these experiments, we have published extensively on the bacterial loads in the tissues of LP and C guinea pigs under identical circumstances, i.e., after 4 weeks of virulent infection (4, 11–13). Protein-deficient guinea pigs routinely have five- to eightfold-more bacilli in their lungs, spleens, and lymph nodes than do their well-nourished counterparts. While such a difference certainly could not be construed as evidence of “uncontrolled” growth (analogous to miliary tuberculosis in humans), the increased antigenic mass could have profound effects on both trafficking and local proliferation of lymphocytes. However, increased antigenic mass alone cannot account for the apparently selective accumulation of T cells in the lymph nodes, since the spleens of LP guinea pigs also contain more bacilli but actually have a significantly lower percentage of T cells.
Table 2 summarizes the lymphoproliferative responses of blood, spleen, and lymph node cells to PPD in vitro. The responses of blood and spleen lymphocytes from protein-deprived guinea pigs were significantly reduced (P < 0.05) compared to similar cell populations derived from normally nourished guinea pigs. In contrast, the proliferative response of lymph node lymphocytes from LP animals was significantly enhanced (P < 0.01) in response to PPD. All of the cultures contained the same number of viable lymphocytes.
TABLE 2.
Antigen-induced proliferation of lymphocytes recovered from blood, spleen, or bronchotracheal lymph nodes of normally nourished and protein-deficient guinea pigs at 4 weeks following pulmonary infection with virulent M. tuberculosis H37Rv
| Diet | Net uptake of [3H]thymidine (103 cpm)a
|
||
|---|---|---|---|
| Blood | Spleen | Lymph node | |
| C | 213 ± 16 | 32 ± 4 | 63 ± 7 |
| LP | 41 ± 8 | 8 ± 5 | 246 ± 9 |
Equal aliquots (2 × 105 cells per well) of viable lymphocytes were cultured with PPD (12.5 μg/ml) for 96 h. Net uptake = (cpm of stimulated cells) − (cpm of unstimulated cells) (from the same source). Data are means ± standard errors of the means for five to seven animals per treatment; all three pairs of values are significantly different by diet (P < 0.01).
DISCUSSION
Malnutrition, particularly dietary protein deficiency, is known to be accompanied by significant impairment of immune functions and resistance to many infectious diseases in humans and experimental animals (8). The precise mechanisms by which protein deficiency interferes with the immune response are essentially unknown. In previous work with a highly relevant guinea pig model of pulmonary tuberculosis, we have documented a series of immunologic abnormalities associated with protein deficiency which, viewed in a clinical context, mimic the nonresponder pole of human tuberculosis (7, 9). Some of these observations have been confirmed in a recent study of protein-calorie malnutrition in mice infected with M. tuberculosis by the intravenous route (3). Thus, several aspects of mycobacterial antigen-specific peripheral T-cell function are impaired; these include lymphoproliferation (4, 9), IL-2 production (13), expression of the CD2 marker (2), and the ability to mount a delayed hypersensitivity response in the skin (4, 9, 11). Data from earlier experiments had suggested that antigen-reactive T lymphocytes might be inappropriately sequestered in protein-deficient guinea pigs in the thoracic lymph nodes draining the lung fields where the M. tuberculosis cells were initially implanted and that this sequestration might occur at the expense of the recirculating pool of T cells (2, 10).
The results of the present study strongly implicate T-lymphocyte sequestration in bronchotracheal lymph nodes as one of the fundamental mechanisms by which protein deficiency impairs the antimycobacterial immune response in malnourished guinea pigs. An alternative hypothesis would be that the shifts in T-cell distribution result from enhanced local proliferation of antigen-reactive T cells in the lymph nodes of protein-deprived guinea pigs. The principal involvement of the bronchotracheal lymph nodes, and not the spleen, in this phenomenon is likely a result of the realistic route (pulmonary) and level (low dose) of infection with M. tuberculosis employed in these experiments. Thus, virulent mycobacteria reach the bronchotracheal lymph nodes much earlier than the spleen (several days) during the extrapulmonary phase of the disease following aerosol infection in guinea pigs, and the antigenic (i.e., bacterial) load and architectural changes are much more pronounced in the bronchotracheal nodes than in the spleen at the interval postinfection (4 weeks) at which these studies were performed (19).
Since protein malnutrition in humans and animals is essentially always accompanied by a profound proliferation defect in lymphocytes (4, 8, 9), at least in vitro, it seems much less likely that the accumulation of T cells in the lymph nodes of protein-deficient guinea pigs in our study was the result of local clonal expansion. Although our study did not directly demonstrate enhanced trafficking of lymphocytes from the circulation to the lymph nodes, it is quite likely that the pooling of antigen-reactive T lymphocytes in the lymph nodes of protein-deprived guinea pigs occurred at the expense of the circulating population. This may explain, in part, the reduced ability of peripheral lymphocytes to mediate a PPD-induced dermal hypersensitivity reaction in those animals and the impairment of PPD-driven proliferation (Table 2) and IL-2 production observed previously under identical experimental conditions in peripheral lymphocytes (13).
Peripheral anergy in a subset of patients with tuberculosis has been demonstrated to result from abnormalities in the ability of those infected individuals to mobilize protective T-cell populations, which apparently remain trapped in the lymph nodes (15). In that regard, the protein-deficient guinea pig demonstrates a similar defect, which increases our confidence that observations made in this model of pulmonary tuberculosis have relevance for human disease.
Severe protein deficiency is known to result in atrophy of lymphoid organs, although clinical observations in this regard have always been confounded by the stress and infection which coexist in many malnourished human populations (8). The moderate chronic protein deficiency produced in these experiments was not shown to result in decreased spleen size relative to body weight (4). Therefore, it was important to document that protein deprivation alone produced essentially no changes in the proportions of αβTcR+, CD4+, and CD8+ T cells in the spleens of our guinea pigs (Table 1). The superimposition of mycobacterial infection on the continued depletion of protein stores, however, resulted in a dramatic diminution of αβ T cells as a percentage of total lymphocytes recovered from the spleens of LP animals (Fig. 1). Thus, it appears that it is the combined effect of protein deficiency and infection which results in T-cell depletion in the spleen without an overall effect on spleen size or cellularity.
The relative paucity of appropriate reagents has severely hampered the study of guinea pig lymphocyte phenotypes in infectious disease models. In fact, the data presented here are the first to be reported on T-lymphocyte subsets in experimental tuberculosis in guinea pigs. It is clear from these results that the monoclonal antibodies employed, as well as those which have since become available commercially, will be extremely useful in future investigations. While the precise molecular targets of these antibodies have not been elucidated, sufficient characterization has been carried out to ensure that the anti-CD4 and anti-CD8 antibodies label mutually exclusive populations (1, 16, 17). The anti-αβTcR antibody (H159) is likely directed against epitopes on the guinea pig homolog of the αβ T-cell receptor (18). Thus, the H159 antibody may not truly represent a pan-T-cell marker and may underestimate the actual number of T cells present in a population.
It is important to point out that the marked increase in the proportion of αβ T cells in the lymph nodes of protein-deprived guinea pigs reflected exclusively a dramatic increase in the CD8+ subset (Fig. 2). Thus, the CD4-to-CD8 ratio in the lymph nodes was completely reversed, from approximately 2.1 in control diet animals to 0.5 in protein-deficient animals. A similar shift in T-cell ratios has been observed in this model in the proportions of T cells expressing Fc receptors for IgM (Tμ) or IgG (Tγ). Protein malnutrition resulted in a significant drop in the levels of Tμ in the lymph nodes of tuberculous guinea pigs, resulting in a reversal of the normal Tμ-to-Tγ ratio (10). The functional relationship between the expression of Fc receptors and CD phenotype expression in guinea pig lymphocytes has not been determined.
It is clear from studies of T lymphocytes in vitro that protein deprivation results in intrinsic alterations in function on a per cell basis in guinea pigs infected with M. tuberculosis (4, 9, 13). Therefore, the putative sequestration of T cells in lymph nodes draining the lung, as suggested in the present study, must be placed in the broader context of the overall impact of dietary protein deficiency on resistance to pulmonary tuberculosis in the guinea pig model (9). It is not unreasonable to hypothesize, however, that the inability of malnourished guinea pigs to mobilize T cells into the peripheral circulation and to other lymphoid organs (e.g., the spleen) or to the lungs would severely hamper the ability of the host to deal with systemic mycobacterial infections. The present observations suggest that studies on the impact of dietary protein and infection on the expression of integrins or other adhesion molecules would be warranted when reagents suitable for such studies become available for guinea pigs.
ACKNOWLEDGMENTS
We thank Susan Phalen and Pam Montgomery for excellent technical assistance and Jane Lantz for secretarial support in manuscript preparation.
This research was funded in part by NIH grants A1-15495 and A1-27204 from the USPHS.
REFERENCES
- 1.Baker D, Healy D G, Verghese S, Schäfer H, Turk J L. Phenotypic analysis of guinea pig Langerhans cells with antibodies directed against leucocyte surface antigens. Int Arch Allergy Appl Immunol. 1988;86:350–358. doi: 10.1159/000234596. [DOI] [PubMed] [Google Scholar]
- 2.Bartow R A, McMurray D N. Erythrocyte receptor (CD2)-bearing T lymphocytes are affected by diet in experimental pulmonary tuberculosis. Infect Immun. 1990;58:1843–1847. doi: 10.1128/iai.58.6.1843-1847.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J, Tian Y, Tanaka K E, Tsang M S, Yu K, Salgame P, Carroll D, Kress Y, Teitelbaum R, Bloom B R. Effects of protein calorie malnutrition on tuberculosis in mice. Proc Natl Acad Sci USA. 1996;93:14857–14861. doi: 10.1073/pnas.93.25.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen M K, Bartow R A, Mintzer C L, McMurray D N. Effects of diet and genetics on Mycobacterium bovis BCG vaccine efficacy in inbred guinea pigs. Infect Immun. 1987;55:314–319. doi: 10.1128/iai.55.2.314-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellner J J, Wallis R S. Immunologic aspects of mycobacterial infections. Rev Infect Dis. 1989;11:S455–S459. doi: 10.1093/clinids/11.supplement_2.s455. [DOI] [PubMed] [Google Scholar]
- 6.Grover A A, Kim H K, Wiegeshaus E H, Smith D W. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at −70°C. J Bacteriol. 1967;94:832–840. doi: 10.1128/jb.94.4.832-835.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenzini I, Rottoli R, Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977;27:230–237. [PMC free article] [PubMed] [Google Scholar]
- 8.McMurray D N. Cell-mediated immunity in nutritional deficiency. In: Chandra R K, editor. Progress in food and nutrition science. Vol. 8. New York, N.Y: Pergamon Press; 1984. pp. 193–228. [PubMed] [Google Scholar]
- 9.McMurray D N, Bartow R A. Immunosuppression and alteration of resistance to pulmonary tuberculosis in guinea pigs by protein undernutrition. J Nutr. 1992;122:738–743. doi: 10.1093/jn/122.suppl_3.738. [DOI] [PubMed] [Google Scholar]
- 10.McMurray D N, Bartow R A, Mintzer C L. Protein malnutrition alters the distribution of FcγR+ (Tγ) and FcμR+ (Tμ) T lymphocytes in experimental pulmonary tuberculosis. Infect Immun. 1990;58:563–565. doi: 10.1128/iai.58.2.563-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurray D N, Carlomagno M A, Mintzer C L, Tetzlaff C L. Mycobacterium bovis BCG vaccine fails to protect protein-deficient guinea pigs against respiratory challenge with virulent Mycobacterium tuberculosis. Infect Immun. 1985;50:555–559. doi: 10.1128/iai.50.2.555-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMurray D N, Kimball M S, Tetzlaff C L, Mintzer C L. Effects of protein deprivation and BCG vaccination on alveolar macrophage function in pulmonary tuberculosis. Am Rev Respir Dis. 1986;133:1081–1085. doi: 10.1164/arrd.1986.133.6.1081. [DOI] [PubMed] [Google Scholar]
- 13.McMurray D N, Mintzer C L, Bartow R A, Parr R L. Dietary protein deficiency and Mycobacterium bovis BCG affect interleukin-2 activity in experimental pulmonary tuberculosis. Infect Immun. 1989;57:2606–2611. doi: 10.1128/iai.57.9.2606-2611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurray D N, Yetley E A. Response to Mycobacterium bovis BCG vaccination in protein- and zinc-deficient guinea pigs. Infect Immun. 1983;39:755–761. doi: 10.1128/iai.39.2.755-761.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rook G A W, Carswell J W, Stanford J L. Preliminary evidence for the trapping of antigen specific lymphocytes in the lymphoid tissue of ‘anergic’ tuberculosis patients. Clin Exp Immunol. 1976;26:129–137. [PMC free article] [PubMed] [Google Scholar]
- 16.Schäfer H, Burger R. Identification and functional characterization of guinea pig CD4: antibody binding transduces a negative signal on T cell activation. Immunology. 1991;72:261–268. [PMC free article] [PubMed] [Google Scholar]
- 17.Schäfer H, Burger R. Analysis of mature guinea pig T cells with a monoclonal antibody directed against a framework determinant of the T cell receptor for antigen. Scand J Immunol. 1992;36:587–595. doi: 10.1111/j.1365-3083.1992.tb03227.x. [DOI] [PubMed] [Google Scholar]
- 18.Schenkel J, Schäfer H, Baron U, Müller B, Burger R. cDNA cloning of the constant region genes of the guinea pig α/β T cell receptor. Dev Comp Immunol. 1992;16:221–227. doi: 10.1016/0145-305x(92)90021-4. [DOI] [PubMed] [Google Scholar]
- 19.Smith D W, McMurray D N, Wiegeshaus E H, Grover A A, Harding G E. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am Rev Respir Dis. 1970;102:937–949. doi: 10.1164/arrd.1970.102.6.937. [DOI] [PubMed] [Google Scholar]
- 20.Steel R G D, Torrie J H. Principles and procedures of statistics. New York, N.Y: McGraw-Hill Book Co.; 1980. [Google Scholar]
- 21.Toosi Z, Edmonds K I, Tomford J W, Ellner J J. Suppression of purified protein derivative-induced interleukin-2 production by interaction of CD16 (Leu 11 reactive) lymphocytes and adherent mononuclear cells in tuberculosis. J Infect Dis. 1989;159:352–356. doi: 10.1093/infdis/159.2.352. [DOI] [PubMed] [Google Scholar]
- 22.Wiegeshaus E H, McMurray D N, Grover A A, Harding G E, Smith D W. Host-parasite relationships in experimental airborne tuberculosis. III. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis. 1970;102:422–429. doi: 10.1164/arrd.1970.102.3.422. [DOI] [PubMed] [Google Scholar]