Abstract
Background
Little is known about how depressive symptoms and glial fibrillary acid protein (GFAP) concentrations taken together may influence cognitive functioning. Understanding this relationship may inform strategies for screening and early intervention to decrease the rate of cognitive decline.
Methods
This study sample includes 1 169 participants from the Chicago Health and Aging Project (CHAP), consisting of 60% Black participants and 40% White participants, and 63% female participants and 37% male participants. CHAP is a population-based cohort study of older adults with a mean age of 77 years. Linear mixed-effects regression models tested the main effects of depressive symptoms and GFAP concentrations and their interactions on baseline cognitive function and cognitive decline over time. Models included adjustments for age, race, sex, education, chronic medical conditions, body mass index, smoking status, alcohol use, and their interactions with time.
Results
The interaction of depressive symptomology and GFAP (β = −0.105 [standard error = 0.038], p = .006) on global cognitive function was statistically significant. Participants with depressive symptoms including and above the cutoff and high log of GFAP concentrations had more cognitive decline over time, followed by participants with depressive symptoms below the cutoff and high log of GFAP concentrations, depressive symptom scores including and above the cutoff and low log of GFAP concentrations, and depressive symptom scores below the cutoff and low log of GFAP concentrations.
Conclusions
Depressive symptoms have an additive effect on the association between the log of GFAP and baseline global cognitive function.
Keywords: Biomarkers, Cognitive aging, Depression
Approximately 25% of older adults experience both depression and cognitive impairment. These 2 conditions taken together may be indicative of another condition, such as hypothyroidism or vascular dementia. In other instances, they may be fairly independent but co-occurring conditions. Further, individuals with depression may have cognitive difficulty as a symptom of depression. Individuals with dementia may experience both cognitive and physical symptoms that are similar to those who have depression. Cognitive difficulty may also point to dementia due to pathophysiological variations which occur with depression (1). However, it is somewhat unclear whether or not depression magnifies the risk of dementia or is an early indicator of variations in the brain related to dementia (2). This study aims to increase the understanding of the role of depression or whether it is a prodrome, comorbidity, or risk factor for cognitive function and cognitive decline by examining the association between baseline depression and cognitive function and between experiencing depression initially and subsequent cognitive decline over time.
Greater levels of glial fibrillary acidic protein (GFAP) measured in cerebrospinal fluid have been found in participants with unipolar depression (3). GFAP measured in serum may be used to determine the severity of depression and enhance diagnostic procedures for major depressive disorder (4). Higher serum-measured GFAP levels are associated with cognitive decline and brain structure alterations (5). GFAP measured in plasma may identify Alzheimer’s disease (AD) pathology in individuals with mild cognitive impairment (MCI) (6). Concentrations of plasma-measured GFAP may be higher in individuals with greater amyloid-β (Aβ) in the brain (7). Increased levels of plasma-measured GFAP are related to poor memory and microstructure of white matter (8). Although we found studies examining associations between depression and cognitive decline, GFAP and depression, and GFAP and cognitive decline, we did not find papers that evaluated the relationship between all 3. It is important to understand this relationship longitudinally to determine strategies for early detection and intervention. We hypothesize that depression is an effect modifier in the association between GFAP and cognitive decline. Does the number of depressive symptoms differentially affect the relationship between GFAP and cognitive decline?
Method
This study is a secondary analysis of a portion of data collected in the Chicago Health and Aging Project (CHAP). CHAP is a population-based cohort study focused on AD and other prevalent conditions in older adults. Study participants were recruited via door-to-door census in 4 Chicago area neighborhoods. Data were collected every 3 years from 1993 to 2012. Home interviews were conducted, and a clinical evaluation was implemented with a stratified random sample of home interview participants. Clinical evaluation participants were asked to provide blood samples (5,9,10). Eligible study participants for this analysis had 1 blood sample assayed which is the baseline for this study sample and completed a minimum of 2 cognitive function assessments. The measures of focus for this study are described later.
Depressive Symptoms
Depressive symptoms were determined using an adapted, 10-item version of the Center for Epidemiologic Studies—Depression (CES-D). For each of the 10 items, participants respond to the following question: Have you felt this way much of the time during the past week? with 1 = Yes and 2 = No. The items consist of the following statements: I was happy; I enjoyed life; I felt sad; I felt lonely; I felt depressed; I could not get going; My sleep was restless; I felt like everything I did was an effort; I felt that people disliked me; People were unfriendly. A score was calculated summing responses which were indicative of depressive symptoms, ranging from 0 to 10, with 0–2 or <3 or below the cutoff symptoms and 3–0 or ≥3 defined as including and above the cutoff (11–13).
Covariates
Demographic characteristics, alcohol use in grams per day, current and former smoking status, and presence or absence of chronic conditions were collected through self-report. Body mass index (BMI) was calculated. Demographic characteristics are defined as date of birth/age, sex, race, and education, and chronic medical conditions include diabetes, cardiovascular conditions, stroke, fractured or broken hip, hypertension, and cancer (14–16).
Serum-Measured GFAP
A single blood draw was conducted in the home. Samples were then transferred within a 2-hour window to a biorepository freezer (−80°C) at Rush University Medical Center. Samples that were not thawed were submitted to Quanterix Corporation. GFAP was assayed in duplicates. The average of duplicate measurements had a coefficient of variation of 3.0%. The lower and upper limits of quantification were 28.1 and 6411.5 pg/mL, respectively (5,17).
Cognitive Function
Global cognitive function was determined using a summary score of the East Boston Memory Test: Immediate Recall and Delayed Recall, which measures episodic memory; the Mini-Mental State Examination; and the Symbol Digit Modalities Test (modified, oral version), which measures perceptual speed (16, 18–21). z-Scores were calculated from the baseline means and standard deviations of each of the 4 measures. The mean of the z-scores for all 4 measures was computed to develop the global cognitive function score (15,16). Scores were standardized by mean and standard deviation of the complete CHAP population at baseline and not the subset in this study.
Statistical Analyses
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). We conducted descriptive statistics to examine the baseline sample. We implemented collinearity diagnostics including correlation and variance inflation factor (VIF) between GFAP and depression. Separate linear mixed-effects regression models were conducted to test the associations between the log of GFAP (continuous variable) and global cognitive function and global cognitive decline and depressive symptom status (categorical variable) and global cognitive function and global cognitive decline. This was done to establish whether or not either predicts global cognitive function and decline as expected. Another model was implemented with both the log of GFAP and depressive symptoms as main effects to determine if each is independently associated with global cognitive function and decline. A separate model was conducted to examine the interaction of depression and the log of GFAP on the baseline level of global cognitive function and on global cognitive decline. Similar models were conducted examining associations with episodic memory and with perceptual speed at baseline and over time. For stratified analysis by depressive symptom status, linear mixed-effects regression models were conducted to examine the associations between the log of GFAP on the same cognitive function outcomes and the decline in these outcomes. All models were adjusted for age, race, sex, education, BMI, current and former smoking, alcohol use, chronic medical conditions, and each of their interactions with time. Models included person-specific intercepts and slopes with an unstructured correlation matrix structure. For regression analyses, we use an adjusted p value for multiple comparisons of p = .05/2 primary predictors *3 outcomes = .008. R version 4.0.3 was used to create a figure which shows a global cognitive decline over time for 4 groups: low CES-D, low GFAP; low CES-D, high GFAP; high CES-D, low GFAP; and high CES-D, high GFAP. Low GFAP was defined by the 10th percentile and high GFAP was defined by the 90th percentile for the figure. CES-D was defined the same as for the models: <3 or >=3.
Results
Baseline Descriptive Characteristics
The CHAP study consisted of 10 802 participants. A subset of 5 565 participants had serum samples, and 1 327 participants had samples that were assayed. Of these, 1 169 participants completed 2 or more cognitive assessments after the blood draw, which is the total number of participants in this study. Table 1 describes the baseline sample characteristics in total and by CES-D/depressive symptom status: below the cutoff (<3) versus including and above the cutoff (≥3). The geometric mean of GFAP for participants with more depressive symptoms is higher than for participants with scores below the cutoff. Individuals with more depressive symptoms are more likely to be female, Black, have diabetes, and have hypertension compared to participants with fewer depressive symptoms. The mean follow-up period for participants in this study is 10.1 ± 4.9 years. Collinearity diagnostics between baseline CES-D and the log of GFAP showed a correlation of r = 0.0145 and VIF = 1.07 for baseline CES-D and VIF = 1.30 for the log of GFAP, suggesting that collinearity is less likely between baseline CES-D and the log of GFAP. Supplementary Table 1 compares the baseline characteristics of the 1 327 participants with biomarker measurement to the 9 475 participants without biomarker measurement. There were statistically significant differences with the following characteristics: education, chronic conditions, smoking status, CES-D, GFAP, and cognitive measures.
Table 1.
Baseline Characteristics: Total Sample and by CES-D Symptom Status
| Variable | Overall, N = 1 169* | CES-D <3, N = 925 | CES-D ≥3, N = 244 |
|---|---|---|---|
| Age, mean (SD) | 77.4 (6.0) | 77.2 (6.0) | 78.4 (6.1) |
| Education, mean (SD) | 12.6 (3.5) | 12.8 (3.5) | 11.8 (3.5) |
| Female, n (%) | 734 (63) | 556 (60) | 178 (73) |
| Black, n (%) | 704 (60) | 530 (57) | 174 (71) |
| Chronic conditions, mean (SD) | 1.31 (1.02) | 1.26 (1.01) | 1.50 (1.00) |
| Diabetes, n (%) | 259 (22) | 182 (20) | 77 (32) |
| Cardiovascular conditions, n (%) | 164 (14) | 133 (14) | 31 (13) |
| Stroke, n (%) | 120 (10) | 90 (9.7) | 30 (12) |
| Fractures or broken hip, n (%) | 47 (4.0) | 34 (3.7) | 13 (5.3) |
| Hypertension, n (%) | 694 (59) | 532 (58) | 162 (66) |
| Cancer, n (%) | 246 (21) | 192 (21) | 54 (22) |
| BMI, mean (SD) | 27.5 (5.6) | 27.5 (5.5) | 27.8 (5.8) |
| Alcohol (grams), mean (SD) | 5.0 (13.7) | 5.8 (14.8) | 2.0 (7.6) |
| Former smokers, n (%) | 457 (39) | 365 (39) | 92 (38) |
| Current smokers, n (%) | 106 (9.1) | 86 (9.3) | 20 (8.2) |
| CES-D, mean (SD) | 1.43 (1.90) | 0.61 (0.75) | 4.54 (1.69) |
| GFAP (pg/mL), median (IQR) | 224 (157–326) | 222 (155–322) | 250 (169–351) |
| GFAP > 232 pg/mL, n (%) | 562 (48) | 431 (47) | 131 (54) |
| Global cognitive function, mean (SD) | 0.21 (0.68) | 0.28 (0.64) | −0.08 (0.73) |
| Episodic memory, mean (SD) | 0.19 (0.83) | 0.25 (0.81) | −0.06 (0.86) |
| MMSE, mean (SD) | 26.7 (3.6) | 27.1 (3.2) | 25.2 (4.5) |
| Perceptual speed, mean (SD) | 0.24 (0.90) | 0.34 (0.88) | −0.14 (0.87) |
| Time in study, mean (SD) | 6.4 (3.7) | 6.6 (3.7) | −5.8 (3.5) |
*Mean (SD); n (%); Median (IQR). BMI = body mass index; CES-D = Center for Epidemiologic Studies—Depression; GFAP = glial fibrillary acid protein; IQR = interquartile range; MMSE = Mini-Mental State Examination; SD = standard deviation.
Baseline and Longitudinal Associations Main Effects With Global Cognition
Table 2 shows statistically significant associations between the log of GFAP (β = −0.312; standard error [SE] = 0.080; p = .000) and global cognitive function and depressive symptoms (β = −0.049; SE = 0.009; p = .000) and global cognitive function. There is a statistically significant association between the log of GFAP (β = −0.072; SE = 0.016; p = .000) and global cognitive decline but not with depressive symptoms (β = −0.005; SE = 0.002; p = .015) and global cognitive decline. The model that includes both main effects of the log of GFAP and depressive symptoms showed statistically significant associations. These results indicate that the log of GFAP (β = −0.298; SE = 0.080; p = .000) and depressive symptoms (β = −0.047; SE = 0.009; p = .000) are independently associated with global cognitive function. This model also shows a statistically significant association between the log of GFAP (β = −0.071; SE = 0.016; p = .000) and global cognitive decline but not for depressive symptoms (β = −0.004; SE = 0.002; p = .018) and global cognitive decline.
Table 2.
Associations Between Depressive Symptoms, Log of GFAP, and Baseline Cognitive Function and Cognitive Decline
| Global cognition | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | 1 160 | 1 162 | 1 159 | 1 159 | ||||||||
| Total observations | 3 505 | 3 509 | 3 500 | 3 500 | ||||||||
| β | SE | p Value | β | SE | p Value | β | SE | p Value | β | SE | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log GFAP | −0.313 | 0.080 | .000 | −0.298 | 0.079 | .000 | −0.144 | 0.097 | .136 | |||
| Log GFAP × time | −0.072 | 0.016 | .000 | −0.071 | 0.016 | .000 | −0.052 | 0.019 | .007 | |||
| CES-D | −0.049 | 0.009 | .000 | −0.047 | 0.009 | .000 | 0.206 | 0.092 | .025 | |||
| CES-D × time | −0.046 | 0.002 | .015 | −0.004 | 0.002 | .018 | 0.026 | 0.019 | .172 | |||
| CES-D × Log GFAP | −0.105 | 0.038 | .006 | |||||||||
| CES-D × Log GFAP × time | −0.013 | 0.008 | .111 | |||||||||
| Episodic memory | ||||||||||||
| Subjects | 1 151 | 1 153 | 1 150 | 1 150 | ||||||||
| Total observations | 3 457 | 3 461 | 3 452 | 3 452 | ||||||||
| β | SE | p Value | β | SE | p Value | β | SE | p Value | β | SE | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log GFAP | −0.305 | 0.104 | .003 | −0.288 | 0.103 | .005 | −0.126 | 0.126 | .319 | |||
| Log GFAP × time | −0.068 | 0.020 | .001 | −0.068 | 0.020 | .001 | −0.055 | 0.024 | .023 | |||
| CES-D | −0.050 | 0.012 | .000 | −0.048 | 0.012 | .000 | 0.219 | 0.120 | .067 | |||
| CES-D × time | −0.002 | 0.002 | .399 | −0.002 | 0.002 | .417 | 0.021 | 0.024 | .367 | |||
| CES-D × Log GFAP | −0.111 | 0.050 | .026 | |||||||||
| CES-D × Log GFAP × time | −0.010 | 0.010 | .325 | |||||||||
| Perceptual speed | ||||||||||||
| Subjects | 1 122 | 1 124 | 1 121 | 1 121 | ||||||||
| Total observations | 3 370 | 3 374 | 3 365 | 3 365 | ||||||||
| β | SE | p Value | β | SE | p Value | β | SE | p Value | β | SE | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log GFAP | −0.270 | 0.101 | .008 | −0.265 | 0.100 | .008 | −0.200 | 0.123 | .103 | |||
| Log GFAP × time | −0.040 | 0.016 | .012 | −0.041 | 0.016 | .011 | −0.049 | 0.020 | .014 | |||
| CES-D | −0.051 | 0.012 | .000 | −0.050 | 0.012 | .000 | 0.062 | 0.121 | .606 | |||
| CES-D × time | −0.004 | 0.002 | .043 | −0.004 | 0.002 | .043 | −0.018 | 0.020 | .375 | |||
| CES-D × Log GFAP- | −0.047 | 0.051 | .350 | |||||||||
| CES-D × Log GFAP × time | 0.006 | 0.009 | .488 |
Notes: Models were adjusted for age, race, sex, education, BMI, current and former smoking, alcohol use, chronic medical conditions, and each of their interactions with time for the longitudinal models. BMI = body mass index; CES-D = Center for Epidemiologic Studies—Depression; GFAP = glial fibrillary acid protein; SE = standard error.
Testing Interactions at Baseline and Longitudinally
Table 2 also indicates the results of models which tested for the interaction of depression symptoms and the log of GFAP on cognitive function outcomes and the interaction of depression and the log of GFAP and time on the decline in cognitive outcomes. The interaction of CES-D and the log of GFAP on baseline level of global cognitive function (β = −0.105; SE = 0.038; p = .006) was statistically significant but this interaction was not statistically significant (β = −0.013; SE = 0.008; p = .111) on global cognitive decline. This interaction was also not statistically significant for episodic memory or perceptual speed at baseline or over time.
Stratified Analyses by CES-D Cutoff at Baseline and Longitudinally
Table 3 describes the results of the stratified analysis categorized by depressive symptoms status: below CES-D score cutoff versus including and above cutoff and examines the associations between the log of GFAP and cognitive function outcomes. For participants with CES-D scores including and above the cutoff (β = −0.781; SE = 0.198; p = .000), there is a statistically significant association between the log of GFAP and global cognitive function at baseline. The association between the log of GFAP and global cognitive decline is statistically significant for participants with CES-D scores below the cutoff (β = −0.069; SE = 0.017; p = .000). Among participants with depressive symptoms including and above the cutoff, the association between the log of GFAP and baseline episodic memory is statistically significant (β = −0.924; SE = 242; p = .000). For participants with depressive symptoms below the cutoff, the association between the log of GFAP and decline in episodic memory is statistically significant (β = −0.071; SE = 0.021; p = .001). Participants with CES-D scores below the cutoff showed a statistically significant association between the log of GFAP and baseline perceptual speed (β = −0.300; SE = 0.111; p = .007).
Table 3.
Associations Between Log GFAP and Baseline Cognitive Function and Cognitive Decline, Stratified by Depressive Symptom Status
| Outcome | Global Cognition | Episodic Memory | Perceptual Speed | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low CES-D | High CES-D | Low CES-D | High CES-D | Low CES-D | High CES-D | |||||||||||||
| Subjects | 919 | 240 | 914 | 236 | 897 | 224 | ||||||||||||
| Total observations | 2 825 | 675 | 2792 | 660 | 2743 | 622 | ||||||||||||
| β | SE | p Value | β | SE | p Value | β | SE | p Value | β | SE | p Value | β | SE | p Value | β | SE | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log GFAP | −0.183 | 0.086 | .033 | −0.729 | 0.195 | .000 | −0.134 | 0.114 | .238 | −0.877 | 0.241 | .000 | −0.289 | 0.113 | .010 | −0.305 | 0.228 | .182 |
| Log GFAP × time | −0.069 | 0.017 | .000 | −0.097 | 0.040 | .015 | −0.072 | 0.021 | .001 | −0.066 | 0.049 | .174 | −0.043 | 0.018 | .014 | −0.030 | 0.043 | .481 |
Notes: Models were adjusted for age, race, sex, education, BMI, current and former smoking, alcohol use, chronic medical conditions and each of their interactions with time for the longitudinal models. BMI = body mass index; CES-D = Center for Epidemiologic Studies—Depression; GFAP = glial fibrillary acid protein; SE = standard error.
Longitudinal Trend
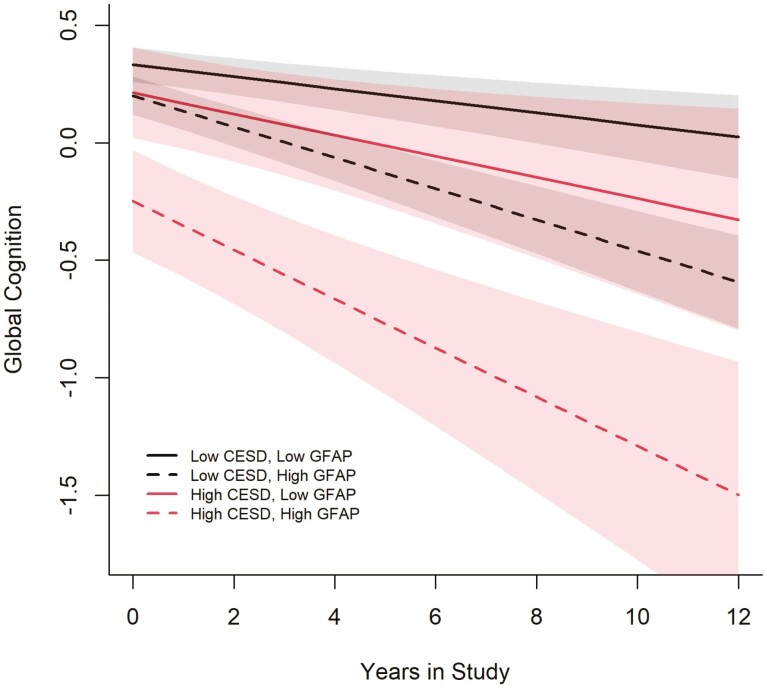
Figure 1 plots the log of GFAP concentrations in pg/mL categorized as low at the 10th percentile and high at the 90th percentile and depressive symptom status of below versus including and above cutoff. Participants with CES-D scores including and above the cutoff and high log of GFAP concentrations experienced more cognitive decline over time, followed by participants with CES-D scores below the cutoff and high log of GFAP concentrations, CES-D scores including and above the cutoff and low log of GFAP concentrations, and CES-D scores below the cutoff and low log of GFAP concentrations. The shading represents changing confidence intervals over time.
Figure 1.
Global cognitive decline over time by Center for Epidemiologic Studies—Depression (CES-D) and glial fibrillary acid protein (GFAP).
Discussion
Study findings indicate an additive effect of depressive symptomology in the association between the log of GFAP and global cognitive function. However, we did not find that the interaction between depressive symptoms and the log of GFAP to be statistically significant over time. Our results suggest that depressive comorbidity may worsen cognitive function. These results suggest that, in addition to screening for cognitive impairment, screening for depression and evaluating blood biomarker levels may inform individualized AD risk and the determination of early intervention strategies to slow the disease course. Blood biomarker assessment is relatively inexpensive (5), and depression screening is often part of standard clinical practice, which supports the feasibility of implementation as part of risk assessment.
Study results did not show statistically significant associations between the interaction of the log of GFAP and CES-D on decline in cognitive outcomes. This may be because GFAP is driving the association over time, as suggested by the figure. This might also be because the sample of participants with CES-D scores including and above the cutoff is smaller than the sample of participants with CES-D scores above the cutoff. Timing of onset of depression: late life versus early life might explain study findings, as timing of onset could mean different pathogenesis, although we do not have access to onset time (22). There is a mechanistic relationship between depression and cognitive difficulty. Individuals with the comorbid conditions, MCI, and major depressive disorder show volumetric reductions in several brain regions including the amygdala, thalamus, superior temporal gyrus, hippocampus, and inferior temporal gyrus (23).
Additional factors to consider in the associations between GFAP concentrations, depression, and cognitive functioning include depression severity and duration. However, the one longitudinal study found did not report a relationship between depression severity or duration and decline in global cognition. The results of this study did indicate that depression that is chronic and decline in cognition may have similar brain pathology in the subcortical region (24). Our findings suggest that the addition of a blood biomarker profile may offer clinical insight into the relationship between depression and cognitive impairment and inform the prediction of cognitive decline. Evaluating GFAP concentrations may be both diagnostically important and a way to determine suitable early interventions (5).
Study Limitations
This study has several limitations. We used an adapted version of the CES-D to measure depressive symptoms and there are other more robust ways to assess for depression. Our study sample was limited to 2 racial groups in a specified area of the United States. The sample size for the group with CES-D scores including and above the cutoff is smaller than the sample size of the group with CES-D scores below the cutoff. Time of onset of depression is not taken into account in examining associations in this study. Finally, the global cognitive function measure does not include all cognitive domains. Elevated GFAP concentrations have been linked to depression, so there is a possibility of collinearity (3). However, collinearity diagnostics indicate that this possibility is unlikely.
Strengths
This may be the first study to examine the role of depression in the relationship between GFAP and cognitive decline. This analysis leverages a population-based, bi-racial cohort of older adult participants with multiple measurement points of cognitive performance.
Future Directions
Future research should focus on examining specific individual depressive symptoms, as well as severity and timing of onset of depression, in the relation between GFAP and global cognitive decline and individual tests of cognitive decline. Additional work should evaluate demographic differences by race, ethnicity, sex, and socioeconomic status when exploring depressive symptoms, GFAP, and cognitive decline, which will improve the generalizability of study findings.
Supplementary Material
Acknowledgments
We would like to extend our thanks to the study participants, Chicago communities that engaged in the study, and the study team.
Contributor Information
Pankaja Desai, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, Illinois, USA.
Kristin R Krueger, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, Illinois, USA.
Carlos Mendes de Leon, School of Medicine, Georgetown University, Washington, District of Columbia, USA.
Robert S Wilson, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA.
Denis A Evans, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, Illinois, USA.
Kumar B Rajan, Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, Illinois, USA; Department of Neurology, University of California at Davis, Davis, USA.
Funding
This work is supported by National Institutes of Health (grant numbers: R01AG051635, RF1AG057532, R01AG073627, and R01AG058679).
Conflict of Interest
There are no conflicts of interest, except for National Institutes of Health funding received by some of the authors.
References
- 1. Morimoto SS, Kanellopoulos D, Alexopoulos GS.. Cognitive impairment in depressed older adults: implications for prognosis and treatment. Psychiatr Ann. 2014;44(3):138–142. doi: 10.3928/00485713-20140306-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H.. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 3. Michel M, Fiebich BL, Kuzior H, et al. Increased GFAP concentrations in the cerebrospinal fluid of patients with unipolar depression. Transl Psychiatry. 2021;11(1):308. doi: 10.1038/s41398-021-01423-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steinacker P, Al Shweiki MR, Oeckl P, et al. Glial fibrillary acidic protein as blood biomarker for differential diagnosis and severity of major depressive disorder. J Psychiatr Res. 2021;144:54–58. doi: 10.1016/j.jpsychires.2021.09.012 [DOI] [PubMed] [Google Scholar]
- 5. Rajan KB, Aggarwal NT, McAninch EA, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020;88(6):1065–1076. doi: 10.1002/ana.25874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cicognola C, Janelidze S, Hertze J, et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res Ther. 2021;13(1):68. doi: 10.1186/s13195-021-00804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatterjee P, Pedrini S, Stoops E, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry. 2021;11(1):27. doi: 10.1038/s41398-020-01137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bettcher BM, Olson KE, Carlson NE, et al. Astrogliosis and episodic memory in late life: higher GFAP is related to worse memory and white matter microstructure in healthy aging and Alzheimer’s disease. Neurobiol Aging. 2021;103:68–77. doi: 10.1016/j.neurobiolaging.2021.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60(2):185–189. doi: 10.1001/archneur.60.2.185 [DOI] [PubMed] [Google Scholar]
- 10. Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA.. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis. 2003;5(5):349–355. doi: 10.3233/jad-2003-5501 [DOI] [PubMed] [Google Scholar]
- 11. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 12. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J.. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- 13. McDowell I., Newell C. (1996). Measuring Health: A Guide to Rating Scales and Questionnaires (2nd. ed.). Oxford. [Google Scholar]
- 14. Cornoni-Huntley J, Brock D, Ostfeld A, Taylor J, Wallace R, eds. Established Populations for Epidemiologic Studies of the Elderly Resource Data Book. Government Printing Office; 1986. [Google Scholar]
- 15. Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54(3):P155–P160. doi: 10.1093/geronb/54b.3.p155 [DOI] [PubMed] [Google Scholar]
- 16. Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA.. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61(6):812–816. doi: 10.1212/01.wnl.0000083989.44027.05 [DOI] [PubMed] [Google Scholar]
- 17. Quanterix. Simoa Assay Kits: TAU. Accessed October 20, 2021. https://www.quanterix.com/simoa-assay-kits/tau/#:~:text=The%20Simoa%E2%84%A2%20Human%20Total,assay%20recognizes%20all%20tau%20isoforms
- 18. Folstein MF, Folstein SE, McHugh PR.. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 19. Smith A. Symbol Digit Modalities Test Manual. Western Psychological Services; 1982. [Google Scholar]
- 20. Scherr PA, Albert MS, Funkenstein HH, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128(5):1084–1101. doi: 10.1093/oxfordjournals.aje.a115051 [DOI] [PubMed] [Google Scholar]
- 21. Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10(4):578–589. doi: 10.1037//0882-7974.10.4.578 [DOI] [PubMed] [Google Scholar]
- 22. Invernizzi S, Simoes Loureiro I, Kandana Arachchige KG, Lefebvre L.. Late-life depression, cognitive impairment, and relationship with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2021;50(5):414–424. doi: 10.1159/000519453 [DOI] [PubMed] [Google Scholar]
- 23. Zacková L, Jáni M, Brázdil M, Nikolova YS, Marečková K.. Cognitive impairment and depression: meta-analysis of structural magnetic resonance imaging studies. Neuroimage Clin. 2021;32:102830. doi: 10.1016/j.nicl.2021.102830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Comijs HC, van Tilburg T, Geerlings SW, et al. Do severity and duration of depressive symptoms predict cognitive decline in older persons? Results of the Longitudinal Aging Study Amsterdam. Aging Clin Exp Res. 2004;16(3):226–232. doi: 10.1007/BF03327388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.