Abstract
Background
A goal of gerontology is to discover phenotypes that reflect biological aging distinct from disease pathogenesis. Biomarkers that are strongly associated with mortality could be used to define such a phenotype. However, the relation of such an index with multiple chronic conditions warrants further exploration.
Methods
A biomarker index (BI) was constructed in the Cardiovascular Health Study (N = 3 197), with a mean age of 74 years. The BI incorporated circulating levels of new biomarkers, including insulin-like growth factor-1, interleukin-6, amino-terminal pro-B-type natriuretic peptide, cystatin-C, C-reactive protein, tumor necrosis factor-alpha soluble receptor 1, fasting insulin, and fasting glucose, and was built based on their relationships with mortality. Cox proportional hazards models predicting a composite of death and chronic disease involving cardiovascular disease, dementia, and cancer were calculated with 6 years of follow-up.
Results
The hazard ratio (HR, 95% CI) for the composite outcome of death or chronic disease per category of BI was 1.65 (1.52, 1.80) and 1.75 (1.58, 1.94) in women and men, respectively. The HR (95% CI) per 5 years of age was 1.57 (1.48, 1.67) and 1.55 (1.44, 1.67) in women and men, respectively. Moreover, BI could attenuate the effect of age on the composite outcome by 16.7% and 22.0% in women and men, respectively.
Conclusions
Biomarker index was significantly and independently associated with a composite outcome of death and chronic disease, and attenuated the effect of age. The BI that is composed of plasma biomarkers may be a practical intermediate phenotype for interventions aiming to modify the course of aging.
Keywords: Aging, Biomarker, Multimorbidity
One of the major goals of gerontology is to discover phenotypes that define and quantify aging-associated biological and physiological changes, while also reliably predicting outcomes relevant to older adults. Such phenotypes are needed to better define the heterogeneity of aging and lead to more precise, targeted, and effective therapies designed to slow or delay aging. Desirable phenotypes would reliably and inexpensively predict population-, clinician-, and patient-centric outcomes such as mortality, morbidity, and functional decline. Because primary and secondary prevention geroscience trials may target individuals with low to high risk, including people who have not yet manifested some diagnosed diseases, an ideal phenotype should also widely stratify aging-related outcome risk from low to high, rather than merely focusing on high-risk prediction like many clinical disease risk predictors (1). To that end, composite scores have the potential for improved risk stratification in older populations due to an inherently wider distribution and possibly by incorporating multiple biological processes (2,3).
Identifying blood-measured biomarkers that may be mediators not of a single disease process but of multiple chronic diseases is impactful. The Targeting Aging with Metformin (TAME) trial has been proposed as the first large-scale geroscience-based randomized, blinded, placebo-controlled clinical trial (4). Its goal is to test the efficacy of metformin in slowing the progression of varied age-related chronic diseases and the functional decline in older adults with potential comorbidities (5). The intermediate endpoint of TAME, therefore, should reflect the molecular aging pathways of various systems. Experts have proposed several candidate biomarkers that strongly correlate to the molecular aging process, which help to explain the biological mechanism for which metformin influences cardiovascular disease (CVD), cancer, cognition, and death (6). Such trials are essential in providing the context that is necessary for biomarker validation.
To help establish the predictive validity of intermediate biomarker phenotypes for future geroscience trials using multimorbidity endpoints, we constructed a biomarker index (BI) in the Cardiovascular Health Study (CHS) cohort and determined the strength of association of BI with incident CVD, dementia, cancer, or death. The BI incorporated circulating levels of new biomarkers, including insulin-like growth factor-1 (IGF)-1, interleukin-6 (IL-6), amino-terminal pro-B-type natriuretic peptide (NT-proBNP), cystatin-C, C-reactive protein (CRP), tumor necrosis factor-alpha soluble receptor 1 (TNFsR1), fasting insulin, and fasting glucose, and was built based on their relationships with mortality. We hypothesized that the BI would significantly and independently predict a composite outcome of death and chronic disease.
Method
Study Population
The CHS is an ongoing community-based study of cardiovascular risk in 5 888 men and women older than 65 years, from 4 regions of the United States (7,8). The cohort was enrolled in 1989–1990 and was supplemented with added recruitment of African Americans in 1992–1993. Participants and eligible household members were identified from Medicare eligibility lists. To be eligible, participants were ≥65 years old, did not have cancer under active treatment, could not be wheelchair- or bed-bound in the home, and did not plan to move out of the area within 3 years. The CHS is approved by the institutional review boards of all participating institutions and all participants gave informed consent. We used data from the 1992–1993 examination as baseline. Although follow-up is ongoing, we set the last observed follow-up time at the 6th year from baseline to align with the likely duration of geroscience prevention trials.
Candidate Biomarkers
Candidate biomarkers for inclusion in the BI were chosen for their documented association with aging-related processes in laboratory studies and outcomes in epidemiologic studies and their availability in the CHS database. We used the 8 biomarkers that were chosen by the expert panel of TAME, they are IGF-1, IL-6, NT-proBNP, cystatin-C, CRP, TNFsR1, fasting insulin, and fasting glucose. The TAME experts reviewed 258 candidate biomarkers and finally refined the list to 8 biomarkers because they fulfill all 4 of their criteria: (a) measurement reliability and feasibility; (b) relevance to aging; (c) robust and consistent ability to predict all-cause mortality, clinical and functional outcomes; and (d) responsiveness to intervention. Growth differentiation factor (GDF) 15 was not evaluated in CHS and therefore not included in this analysis. We included fasting glucose due to importance of glucose handling and nutrient regulation on aging biology and availability within the CHS cohort (6).
Fasting blood samples were collected at the 1992–1993 exam using standardized protocols and quality assurance (8,9). Insulin-like growth factor-1 was measured after an extraction step using enzyme-linked immunosorbent assays (ELISA; Diagnostics Systems Laboratory, Webster, TX) (10). The analytic coefficient of variation (CV) was 4%–6%. Interleukin-6 was measured by ultrasensitive ELISA (Quantikine HS Human IL-6 Immunoassay; RD Systems, Minneapolis, MN, USA); intra- and interassay CV were 2.9%–8.7% and 7.3%–9.0%, respectively (11). Amino-terminal pro-B-type natriuretic peptide was measured on the Elecsys 2010 system (Roche Diagnostics, Indianapolis, Indiana) with a CV of 2%–5%. Cystatin-C was measured by a BNII nephelometer (Dade Behring Inc., Deerfield, IL) that used a particle-enhanced immunephelometric assay (N Latex Cystatin-C; Dade Behring Inc., Newark, DE); intra- and interassay CV were 2.0%–2.8% and 2.3%–3.1%, respectively (12). C-reactive protein was measured using the BNII nephelometer (Dade Behring Inc.) with a CV of 5.0% (13). Fasting glucose was measured on a Kodak Ektachem 700 Analyzer (Ektachem Test Methodologies, Eastman Kodak, Rochester, NY) and assayed within 30 days, with an average monthly CV of 0.93% (14). Tumor necrosis factor-alpha soluble receptor 1 was measured using enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN), and the detectable limit was 3 pg/mL (using DRT100 kit) (15).
Composite Outcome of Death and Chronic Disease
The primary endpoint was the first on-study occurrence of the composite of new cancer, CVD, dementia or mild cognitive impairment (MCI), or death. The components were selected to represent a subset of major age-related chronic health conditions (16). Any event that happened before the 6th year of follow-up from baseline was considered an event. Because participants who have already had one event may still be eligible for one of the other composite events, we did not drop the participants who had prevalent diseases or disease histories. A second episode of the same disease was considered as a “nonevent” and did not count towards the composite outcome.
The timing of the composite outcome was based on the actual diagnosis date. For CVD, cancer, and death, the first occurrence was determined by the date of hospitalization or death certificate. On the other hand, dementia was assessed discreetly at regular intervals, every 6 months, noting the round when the condition was initially observed.
Cancer was defined using the International Classification of Diseases (ICD)-9 codes for all hospitalization, outpatient, or skilled nursing facility events. Nonmelanoma skin cancer was excluded from incident cancer. The time-to-cancer was the minimum of the time-to-incidence across all of the following cancer types: breast, colorectal, colon, rectum, ovarian, lung, buccal, digestive, esophagus, stomach, liver, pancreas, bladder, urinary bladder, kidney, cervical, uterine, melanoma, prostate, and lymphatic cancers. Any new cancer events that occurred before the 6-year follow-up were considered cancer events. Prevalent cancer under active treatment was an exclusion from CHS, but cancer history was not, so some participants were classified as prevalent cancer cases if the diagnosis date preceded enrollment in CHS or occurred before the 1992–1993 baseline.
Incident CVD was defined as the first occurrence of stroke or transient ischemic attack, coronary heart disease (CHD; myocardial infarction [MI] or a non-MI event, such as angina pectoris, or a revascularization procedure [coronary artery bypass grafting or percutaneous transluminal coronary angioplasty]), congestive heart failure (CHF), or claudication (17). Any CVD events that occurred before the 6-year follow-up were considered CVD events. Prevalent CVD was defined as the incidence before our 1992–1993 baseline or the diagnosis date preceded enrollment in CHS.
The diagnosis of dementia was based on a progressive or static cognitive deficit of sufficient severity to affect the subject’s cognition, activities of daily living, with a history of normal intellectual function before the onset of cognitive abnormalities (18). Individuals were classified as having dementia or MCI after adjudication based on the ICD-9 code (dementias, persistent mental disorders, or cerebral degeneration) or their results on the following examinations: modified Mini-Mental Status Exam (3MSE), Telephone Instrument of Cognitive Status (TICS), and Informant Questionnaire on Cognitive Decline (IQCODE). Neuropsychological evaluations and neurological exams were used as auxiliary, and the classification was done before and after reviewing the diamagnetic resonance imaging. In 3 other centers, only high-risk Whites but all Blacks were subjected to detailed evaluation for the diagnosis; in Pittsburgh, all participants were evaluated (18).
Mortality
Deaths were ascertained through participant surveillance every 6 months from study inception. Confirmation of deaths was conducted through reviews of obituaries, medical records, death certificates, the Centers for Medicare & Medicaid Services health care utilization database, and the National Death Index. Contacts and proxies were also interviewed for participants unavailable for follow-up. The follow-up for mortality was complete through December 1, 2019.
Potential Confounders
Age, sex, race, smoking status, alcohol consumption, and diet were determined by self-report (8). The Alternative Healthy Eating Index was used to account for dietary patterns (19). Anthropometrics (weight, height, and waist circumference) were measured with standard protocols and weight and height were used to calculate body mass index (BMI) in kg/m2. Physical activity was based on the Modified Minnesota Leisure Time Activities questionnaire that assessed frequency and duration of 18 activities in the prior week to calculate kilocalories of energy expended.
Statistical Analysis
From 5 888 subjects in the original cohort and the minority cohort, 5 534 were alive at the time of the 1992–1993 visit (baseline) and 4 777 had a blood draw done. We excluded people missing biomarkers: NT-proBNP (n = 630), cystatin-C (n = 21), IGF-1 (n = 564), IL-6 (n = 210), insulin (n = 18), CRP (n = 25), and TNFaR (n = 112). Thus, the final sample included a total of 3 197 participants.
We created BI by using a method that was used in our previous study, in which we created a BI with 5 biomarkers (5). We weighted the biomarkers used to create the BI based on the strength of their associations with mortality. We derived the weighting for the BI in a randomly selected training sample of two-thirds of the cohort. The BI was then validated in the remainder of the participants for its association with mortality, and when found consistent with those in the training sample, the validation and training samples were combined to derive estimates of mortality risk associated with BI in the full sample. The training sample had an area under the curve (AUC) value of 0.82, whereas the validation sample had an AUC value of 0.73. The steps to build BI were as follows: (a) We used sex-specific tertiles for cut points to score the level of each biomarker. Participants who were diagnosed with diabetes at baseline were assigned to the highest tertile of fasting glucose. (b) We performed gender-specific, age-adjusted multivariable Cox proportional hazards models for mortality risk up to year 6, including all 8 biomarkers with indicator variables for tertiles. We removed any biomarker with p value higher than .10 from the model, one at a time, until all biomarkers remained were statistically significant, resulting in the selection of 4 biomarkers for women (NT-proBNP, IL-6, cystatin-C, and fasting glucose) and 5 biomarkers for men (NT-proBNP, IGF-1, IL-6, TNFsR1, and fasting glucose; Table 2). (c) The coefficients from the models were used to compute points for each level of each biomarker, selecting the tertile with lowest risk as the reference group. Each coefficient was divided by 3 times the coefficient for age for scaling and rounded to produce a point score. Although 5 years of age has been used in other risk scores, notably the Framingham Risk Score, 3 years of age was selected as a reasonable comparison given the older ages of our cohort. (d) The sum of the points was defined as the BI score. (e) For convenience and approximate similarity of risk, we combined some levels of BI into larger summary categories, such as low (0–3), medium (4–5), and high (6–9) in men, and low (0–3), medium (4–5), and high (6–8) in women.
Table 2.
The Coefficient for Each Tertile of the Selected Biomarkers (N = 3 197)
| Women (N = 1 955) | Men (N = 1 242) | |||||
|---|---|---|---|---|---|---|
| Range | Coefficient | Point* | Range | Coefficient | Point | |
| NT-proBNP, pg/mL | ||||||
| 1st tertile | ≤94 | 0 | 0 | ≤74 | 0.00 | 0 |
| 2nd tertile | >94, ≤199 | 0.47 | 2 | >74, ≤201 | 0.19 | 1 |
| 3rd tertile | >199 | 0.82 | 3 | >201 | 0.84 | 3 |
| IGF, µg/L | ||||||
| 1st tertile | ≤77 | (not included in BI) | ≤91 | 0.00 | 0 | |
| 2nd tertile | >77, ≤101 | >91, ≤116 | 0.10 | 0 | ||
| 3rd tertile | >101 | >116 | 0.38 | 1 | ||
| IL-6, pg/mL | ||||||
| 1st tertile | ≤2.0 | 0 | 0 | ≤2.3 | 0.00 | 0 |
| 2nd tertile | >2.0, ≤3.3 | 0.16 | 1 | >2.3, ≤3.6 | 0.18 | 1 |
| 3rd tertile | >3.3 | 0.56 | 2 | >3.6 | 0.63 | 2 |
| Cystatin-C, mg/L | ||||||
| 1st tertile | ≤0.94 | 0.13 | 1 | ≤0.99 | (not included in BI) | |
| 2nd tertile | >0.94, ≤1.11 | 0.00 | 0 | >0.99, ≤1.17 | ||
| 3rd tertile | >1.11 | 0.30 | 1 | >1.17 | ||
| C-reaction protein, mg/L | ||||||
| 1st tertile | ≤1.6 | (not included in BI) | ≤1.4 | (not included in BI) | ||
| 2nd tertile | >1.6, ≤4.7 | >1.4, ≤3.5 | ||||
| 3rd tertile | >4.7 | >3.5 | ||||
| TNFsR1, pg/mL | ||||||
| 1st tertile | ≤1 212 | (not included in BI) | ≤1 259 | 0.03 | 0 | |
| 2nd tertile | >1 212, ≤1 522 | >1 259, ≤1 595 | 0.00 | 0 | ||
| 3rd tertile | >1 522 | >1 595 | 0.41 | 1 | ||
| Insulin, mIU/L | ||||||
| 1st tertile | ≤8 | (not included in BI) | ≤8 | (not included in BI) | ||
| 2nd tertile | >8, ≤12 | >8, ≤12 | ||||
| 3rd tertile | >12 | >12 | ||||
| Fasting glucose, mg/dL | ||||||
| 1st tertile | ≤93 | 0.14 | 1 | ≤95 | 0.00 | 0 |
| 2nd tertile | >93, ≤102 | 0.00 | 0 | >95, ≤107 | 0.28 | 1 |
| 3rd tertile† | >102 | 0.56 | 2 | >107 | 0.43 | 2 |
Notes: The Cox model employed an adjustment procedure that included all biomarkers and age. BI = biomarker index.
*The points are calculated as the ratio of the coefficient of the biomarker to the product of 3 and the coefficient of age in each gender. All points consolidated into the final BI.
†Subjects diagnosed with diabetes were allocated to the third category of fasting glucose levels.
The person-time of observation and the incidence rate of the composite outcome were calculated using Cox models with BI categories as indicator variables, in each gender. The proportional hazard assumption was confirmed based on Schoenfeld residuals and bivariate analysis of the BI and age showed a linear relationship. We obtained the hazard ratio (HR) for age and BI categories in these models. The models were built sequentially, with adjustment for the BI and age, race and health-related behaviors (current smoking, ever drinks alcohol, physical activity, and alternate healthy eating index), and body size (BMI and waist circumference). The AUC was used to estimate the effectiveness of BI categories in classifying those who had a composite outcome event versus those who remained outcome-free for more than 6 years of follow-up. Then, we repeated the above analyses replacing BI categories with the continuous BI, standardized and each individual biomarkers, adjusting for age and race. All analyses were done with Stata/SE 16.0 (College Station, TX) or RStudio Version 1.3.1056 (http://www.r-project.org).
Results
The mean age of the participants with all biomarkers (n = 3 197) was 74 years, and 15% were African American (Table 1). Women (n = 1 955) had lower levels of physical activity and waist circumference, and were less likely to drink alcohol or have a history of CHD, when compared with men (n = 1 242). Women were more likely to be of African American ancestry and to have a higher healthy eating index. As for biomarkers, women had higher levels of CRP and lower levels of NT-proBNP, IL-6, IGF-1, and fasting glucose than men (Table 1). In the overall sample, there were 1 267 (40%) composite outcomes of death and chronic diseases for more than6 years, among which there were 284 (9%) incident cancers, 464 (15%) incident cases of CVD, 281 (9%) cases of dementia/MCI, and 684 (21%) deaths. Women had a lower incidence of death and composite outcome than men (Table 1). When analyzing the occurrence of first events, it was observed that 21% of individuals experienced deaths as their initial event. Cardiovascular disease was the first event in 11% of individuals, whereas 7% experienced cancer as their first event, and 8% had their initial event as dementia/MCI.
Table 1.
Characteristics of Cardiovascular Health Study Cohort Study at Baseline (N = 3 197)
| All | Women | Men | |
|---|---|---|---|
| N = 3 197 | N = 1 955 | N = 1 242 | |
| Count (%) | |||
| African American | 476 (15%) | 311 (16%) | 165 (13%)* |
| Current smoker | 294 (9%) | 187 (10%) | 107 (9%) |
| Ever drinks alcohol | 1 458 (46%) | 773 (40%) | 685 (55%)* |
| History of coronary heart disease | 664 (21%) | 327 (17%) | 337 (27%)* |
| History of congestive heart failure | 152 (5%) | 91 (5%) | 61 (5%) |
| Frequency of each event type in the composite outcome | |||
| Cancer | 284 (9%) | 153 (8%) | 131 (11%) |
| CVD | 464 (15%) | 274 (14%) | 190 (15%) |
| Dementia/MCI | 281 (9%) | 181 (9%) | 100 (8%) |
| Death | 684 (21%) | 343 (18%) | 341 (27%)* |
| Composite of death and diseases† | 1 267 (40%) | 707 (36%) | 560 (45%)* |
| Mean (standard deviation) | |||
| Age, y | 74 (5) | 74 (5) | 75 (5) |
| Physical activity, kcal/d | 1 104 (1 571) | 831 (1 146) | 1 533 (1 997)* |
| Body mass index, kg/m2 | 27 (5) | 27 (5) | 27 (4) |
| Waist circumference, cm | 97 (13) | 96 (15) | 99 (10)* |
| Alternate Healthy Eating Index | 3.0 (1.3) | 3.0 (1) | 2.8 (1.3)* |
| NT-proBNP, pg/mL | 307 (752) | 277 (597) | 354 (944)* |
| IL-6, pg/mL | 3.3 (2.2) | 3.2 (2.1) | 3.6 (2.3)* |
| IGF-1, µg/L | 98 (33) | 93 (32) | 107 (35)* |
| Cystatin-C, mg/L | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.3) |
| Insulin, mg/dL | 14 (22) | 14 (25) | 14 (19) |
| Fasting glucose, mg/dL | 107 (32) | 106 (32) | 109 (33)* |
| CRP, mg/L | 4.8 (7.3) | 5.1 (7.9) | 4.2 (6.2)* |
| TNFsR1, pg/mL | 1 488 (533) | 1 455 (504) | 1 540 (573) |
Notes: CVD = cardiovascular disease; CRP = C-reactive protein; IGF-1 = insulin-like growth factor-1; IL-6 = interleukin-6; MCI = mild cognitive impairment; NT-proBNP = N-terminal-proB-type natriuretic peptide; TNFsR1 = tumor necrosis factor alpha soluble receptor 1.
*The difference was statistically significant at a level of .05.
†The first occurrence of the composite of cancer, CVD, dementia, or MCI, or death.
Scoring of each of the selected biomarkers as predictors of mortality is shown in Table 2. For the majority of the biomarkers, the points given to the biomarkers showed a monotonic upward trend with the concentration of biomarker. However, cystatin-C and fasting glucose in women and TNFsR1 in men show mild “U” shapes in risk across tertiles. The statistical significance of each biomarker is shown in Supplementary Table 1.
The incidence rate of composite outcome by BI category (low/medium/high) is shown in Table 3. There was a clear gradation of incidence rate across BI category. Supplementary Table 2 presents the incidence rate calculated using the continuous BI. The results in Table 4 show the strength of association of chronological age per 5 years in contrast to the BI categories with the composite outcome. Model 1 shows that the BI category was associated with a higher risk of the outcome in both women and in men. In model 2, which only included age as a variable, the HR for every 5-year increase in age as well as the AUCs were similar. After adjusting for the BI category, the HR for age decreased to 1.46 (95% CI = 1.37–1.56) in women and 1.41 (95% CI = 1.30–1.53) in men (model 3), indicating that the effect of age was attenuated by 16.7% and 22.0% in women and men, respectively, by the BI category. With both age and BI in the model, the AUC increased to 0.65 for women and 0.67 for men. The associations remained comparable after additional adjustment for health behaviors, body size, and race.
Table 3.
Incidence Rate of Composite Outcome for Each Biomarker Index Category (N = 3 197)
| Number of participants | Person-years (per 1 000) | Number of events | Incidence rate (per person-year) | 95% CI | |
|---|---|---|---|---|---|
| Women | |||||
| Low | 601 | 3 203 | 138 | 47.7 | 41.6, 54.6 |
| Medium | 799 | 3 859 | 298 | 76.8 | 68.5, 86.0 |
| High | 555 | 2 416 | 271 | 128.6 | 115.8, 142.8 |
| Men | |||||
| Low | 531 | 2 714 | 161 | 62.0 | 53.7, 71.6 |
| Medium | 398 | 1 737 | 191 | 120.0 | 105.0, 137.1 |
| High | 313 | 1 162 | 208 | 186.9 | 160.2, 217.9 |
Notes: The Cox model included age, race, current smoker, ever drinks alcohol, physical activity, healthy dietary score, body mass index, and waist circumference. BI = biomarker index.
The range of BI scores within the BI category: in women, low = 0–3, medium = 4–5; high = 6–8; in men, low = 0–3, medium = 4–5, high = 6–9.
Table 4.
Hazard Ratios (95% CI) of Composite Outcome for Biomarker Index Category and Age (N = 3 197)
| Women (N = 1 955) | Men (N = 1 242) | |||||
|---|---|---|---|---|---|---|
| Age (per 5 y) | BI category* | AUC | Age (per 5 y) | BI category | AUC | |
| Model 1: BI category | — | 1.65 (1.52, 1.80) | 0.63 (0.61, 0.64) | — | 1.75 (1.58, 1.94) | 0.66 (0.63, 0.68) |
| Model 2: Age | 1.57 (1.48, 1.67) | 1.57 (1.48, 1.67) | 0.64 (0.62, 0.65) | 1.55 (1.44, 1.67) | — | 0.66 (0.64, 0.68) |
| Model 3: BI Category + Age | 1.46 (1.37, 1.56) | 1.46 (1.37, 1.56) | 0.65 (0.63, 0.66) | 1.41 (1.30, 1.53) | 1.54 (1.38, 1.72) | 0.67 (0.65, 0.70) |
| Model 4: BI Category + Age + Covariates† | 1.57 (1.48, 1.67) | 1.57 (1.48, 1.67) | 0.63 (0.61, 0.64) | — | 1.50 (1.33, 1.68) | 0.66 (0.63, 0.68) |
Notes: AUC = area under receiver-operating characteristic curve; BI = biomarker index.
*The range of BI scores within the BI category: in women, low = 0–3, medium = 4–5; high = 6–8; in men, low = 0–3, medium = 4–5, high = 6–9.
†Covariates: race, current smoker, ever drinks alcohol, physical activity, healthy dietary score, body mass index, and waist circumference.
Table 5 shows the associations of the BI in standardized units versus the individual biomarkers (standardized) in men and in women, adjusted for age and race. Generally, the BI showed stronger associations with the composite outcome than any individual biomarker, except for NT-pro-BNP, which was more strongly related than the BI. We also examined the association of the BI with each component of the outcome separately in Supplementary Table 3. Here, we noted that the index was more strongly associated with cancer in men than in women, whereas it was more strongly associated with CVD and dementia in women than in men.
Table 5.
Hazard Ratios of Composite Outcome for Biomarker Index Score or Individual Biomarkers (N = 3 197)
| Women (N = 1 955) | Men (N = 1 242) | |||
|---|---|---|---|---|
| HR (95% CI) for BI Score (per SD) | AUC | HR (95% CI) for BI Score (per SD) | AUC | |
| BI score* | 1.45 (1.28, 1.59) | 0.64 (0.62, 0.67) | 1.52 (1.39, 1.66) | 0.68 (0.65, 0.70) |
| Individual biomarker† | HR (95% CI) for each biomarker (per SD) | AUC | HR (95% CI) for each biomarker (per SD) | AUC |
|---|---|---|---|---|
| NT-proBNP | 1.48 (1.35, 1.63) | 0.59 (0.57, 0.63) | 1.63 (1.46, 1.82) | 0.63 (0.61, 0.66) |
| IL-6 | 1.28 (1.16, 1.40) | 0.55 (0.53, 0.59) | 1.42 (1.28, 1.57) | 0.59 (0.57, 0.63) |
| Cystatin-C | 1.39 (1.27, 1.52) | 0.58 (0.55, 0.61) | (Cystatin-C not included in men’s BI) | |
| Fasting glucose | 1.15 (1.05, 1.26) | 0.53 (0.50, 0.56) | 1.22 (1.11, 1.35) | 0.58 (0.53, 0.64) |
| IGF | (IGF not included in women’s BI) | 0.97 (0.87, 1.07) | 0.48 (0.46, 0.51) | |
| TNFsR1 | (TNFsR1 not included in women’s BI) | 1.42 (1.30, 1.58) | 0.58 (0.56, 0.62) | |
Notes: AUC = area under receiver-operating characteristic curve; BI = biomarker index; CI = confidence interval; HR = hazard ratio; IGF = insulin-like growth factor; IL-6 = interleukin-6; NT-proBNP = amino-terminal pro-B-type natriuretic peptide; SD = standard deviation; TNFsR1 = tumor necrosis factor alpha soluble receptor 1.
*Model comprised of standardized BI, standardized age, and race.
†Models comprised of standardized single biomarker level, standardized age, and race. Standard deviations for each biomarker given in Table 1.
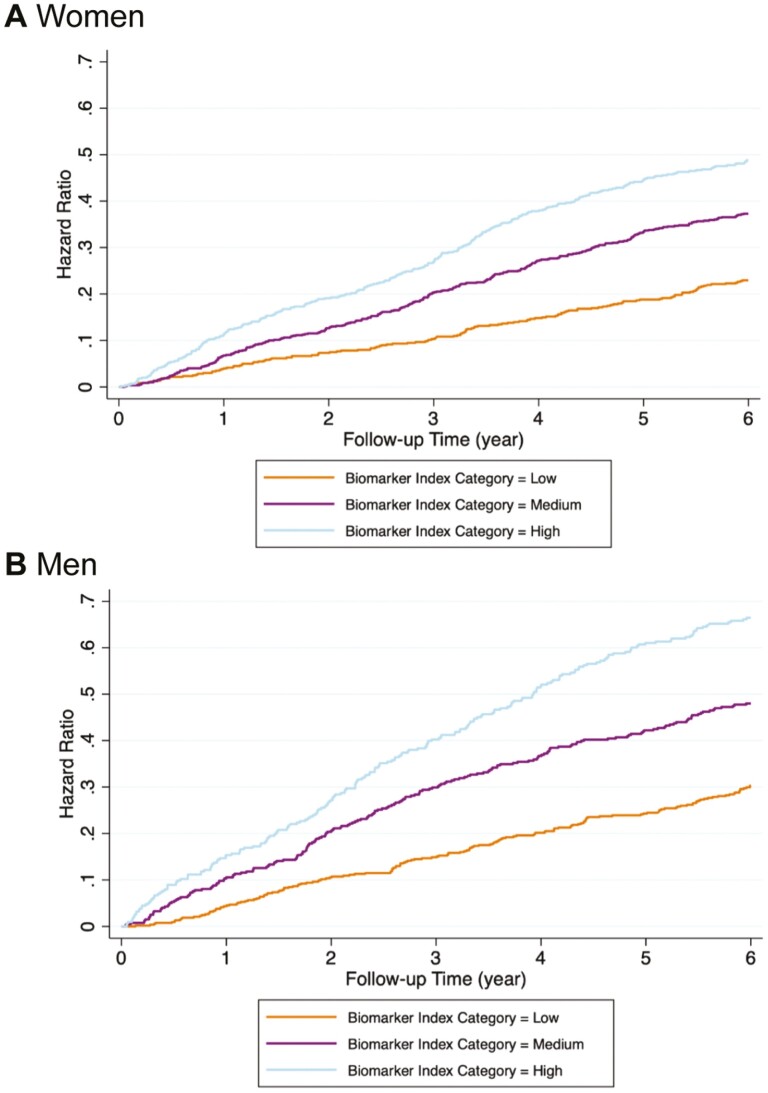
Figure 1 demonstrates that although men had higher HRs than women over the entire follow-up period, in both men and women, a higher BI category was associated with a higher hazard risk of the composite outcome in the age- and race-adjusted models. Similar results were observed when using BI score as the predictor.
Figure 1.
Hazard ratios for composite outcome by biomarker index (BI) category and by sex. (A) women, low BI category = BI score 0–3, medium BI category = BI score 4–5, high BI category = BI score 6–8. (B) men, low BI category = BI score 0–3, medium BI category = BI score 4–5, high BI category = BI score 6–9.
Discussion
The BI was developed as a summary of key blood-based biomarkers of aspects of biological aging and provided modest discrimination of risk for a composite outcome of mortality and incident chronic diseases (CVD, cancer, and dementia/MCI), as was shown in this sample of community-dwelling older adults. The BI was associated with the composite outcome similar to chronological age and could attenuate the association of the latter, indicating that BI is an independent predictor of multiple chronic conditions. Since the BI was composed of biomarkers and did not include chronological age, it is possibly capturing processes of biological aging that lead to disease risk. The difference in biomarkers related to the composite outcome between men and women is notable, raising the question as to whether one index will be suitable for both sexes.
The difference in BI related to the composite outcome of death and chronic diseases between men and women might be due to different mechanisms linking sex to individual biomarkers, the differences in sex-specific tertiles, or to the differences in the individual diseases comprising the outcome. There is sexual dimorphism in immune aging and endocrine aging, and even in late life when sex hormones decline (20–22). Older women had higher genomic activity for adaptive immune cells, whereas older men had higher activity for monocytes and inflammation, indicating greater immune-senescence in men (21,23). In addition, sex hormones regulate several key functions in nutrient sensing and metabolism of glucose, especially intracellular nutrient-sensing pathways (such as the IGF-1) (24). At the molecular level, women have lower fasting insulin and glucose levels, lower basal fat oxidation, and higher fat use (20). Consequently, men tend to have more visceral fat at the phenotypic level, which lead to a higher risk of cardiometabolic diseases than women (before menopause) (20). Finally, most cancers also have apparent sex-differentiated effects. In general, men have higher incidence rates and higher death rates in most cancers that are not related to reproduction (25). All the above calls for more research to better understand sex and its attributes that shape biological aging. Moreover, as CVD, dementia, and cancer are associated with systemic manifestations, there is likely a complex bidirectional interplay between the diseases and biological aging at the cellular level.
Multimorbidity is a recognized challenge to both the quality of life as well as to cost-containment efforts for the health care system (26–28). The definition presented in this article does not require the presence of multiple concurrent diseases. Our analysis addresses the onset of any one chronic disease regardless of the presence of others. Since the prevalence of many common chronic diseases increases significantly with age (29), it is critical to prevent the occurrence of any component of multimorbidity at an earlier age. However, the prevalence and degree of subclinical diseases can be substantial by the time of the clinical diagnosis of the diseases, as was suggested by epidemiologic studies using noninvasive tests of disease (30–33). Therefore, blood-based biomarkers are promising to quantify early pathology. In our study, the strong association between BI and the composite outcome of death and chronic diseases showed the potential that age-related comorbidity predictions could be improved with blood-based measurements.
Our present work expands on our previous study, which incorporated 5 blood-based biomarkers (5), including IGF-1, IGFBP3, NT-proBNP, dehydroepiandrosterone sulfate, and IL-6 and compared this to a physiologic index that combined measurements including carotid intima-media thickness, pulmonary vital capacity, serum cystatin-C, brain white matter grade, and fasting glucose. In the Cox models predicting 10-year mortality, the BI achieved 20% attenuation in age beta, whereas the physiologic index achieved 29% attenuation. Expanding on these prior analyses, we showed here that an expanded BI and variations thereof are associated with multiple chronic conditions in addition to mortality.
What are the underlying mechanisms relating each of the biomarkers to diseases? IL-6, CRP, and TNFsR1 are members of an integrated network of cytokines involved in inflammation and intercellular signaling. Cytokine dysregulation is both a consequence and driver of pathophysiologic processes leading to myriad health outcomes. IL-6 and TNFα rise with age in the absence of disease (34,35), have pleiotropic effects on various cell types, and induce synthesis of acute-phase proteins like CRP in response to infection or injury (36,37). Cohort studies have indicated the levels of IL-6, TNFsR1, and CRP are markers of morbidity, frailty, and mortality in the older subjects (38). In addition, low-grade inflammation accelerates the aging process if left untreated (39).
NT-proBNP may be a marker of undiagnosed or subclinical cardiovascular damage that occurs with aging and disease, as well as coexisting renal dysfunction. It is secreted by ventricular myocytes in response to cardiomyocyte stretching to decrease vascular resistance. NT-proBNP elevations occur in a number of heart disease phenotypes, renal dysfunction (40,41), and are independently associated with mortality (42,43).
IGF-1 and insulin both play the role of nutrient signaling. Ample evidence in animals indicates that IGF-1 signaling pathway is implicated in longevity (44). In humans, cross-sectional studies showed that older adults had lower IGF-1 levels compared with younger adults (45–49). However, cohort studies did not detect any inverse association of IGF-1 with mortality (10,50–52) except the Framingham Heart Study (53). In addition, the relationship may be U-shaped with both high and low levels of IGF-1 associated with higher mortality (50,54,55). Fasting insulin is responsive to caloric restriction in humans. Most age-related diseases have been associated with impaired insulin secretion, as it signifies the loss of function of the pancreas (56). In CHS, insulin was not included in the BI probably due to its strong correlation with fasting glucose.
Fasting glucose is a prominent biomarker of metabolic health and lack of glucoregulatory control with aging and is a standard measure for the diagnosis of diabetes (57–61). Though HbA1c may be a better indicator of metabolic aging compared with fasting glucose (62), HbA1c was not measured in the CHS. Lack of adequate glucoregulatory control suggested by high HbA1c and fasting glucose remains a leading cause of death, chronic disease, and functional decline in both nondiabetics and diabetic older adults (57–61).
Cystatin-C is an extracellular inhibitor of cysteine proteases and a marker of renal disease and aging (63,64). It is an independent risk factor for all-cause and CVD-related mortality, and higher levels are consistently associated with poor physical function and cognition (65–67).
We acknowledge several limitations in this analysis. First, we conducted these analyses based on a hypothesized set of biomarkers and were limited to those already measured in a cohort study with long-term follow-up. Future studies will hopefully find biomarkers that are more directly related to the underlying biology of aging. Alternative approaches are also possible. For example, one could develop a BI that was more disease-specific, or use an alternative outcome such as disability-free survival to develop a valid index. Second, whether sex specificity of an index is needed is debatable. The modeling approaches used here supported this, but other approaches need to be tested. Treatment of disease could also affect the biomarkers used in these analyses. Third, we acknowledge that although our method achieves some level of net discrimination over age, it may not meet the desired level of adequacy. Fourth, our approach does not provide sufficient evidence to use a BI as a surrogate outcome as surrogacy requires documentation of mediation of an outcome in the context of a clinical trial. Finally, it is important to consider that the values of biomarkers are specific to the distributions in the CHS cohort who were aged 65 and older; whether this can serve as a referent population requires further assessment in other age and demographic groups.
Conclusion
In conclusion, our index of 8 circulating biomarkers predicted a composite outcome comprised of death, CVD, cancer, and dementia/MCI and can explain some degree of the association of age with the composite outcome in older community-dwelling adults. Composed solely of markers readily measurable in clinical laboratories, this BI provides effective and more continuous discrimination of risk, hence promising great applicability at low expense. Future studies will hopefully refine the most critical biomarkers that reflect the underlying biology of aging.
Supplementary Material
Contributor Information
Xiao Zhang, Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA; Vanke School of Public Health, Tsinghua University, Beijing, China.
Jason L Sanders, Vertex Pharmaceuticals Inc., Boston, Massachusetts, USA.
Robert M Boudreau, Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Alice M Arnold, Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Jamie N Justice, Department of Internal Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Mark A Espeland, Department of Internal Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
George A Kuchel, UConn Center on Aging, UConn Health, Farmington, Connecticut, USA.
Nir Barzilai, Department of Medicine, Albert Einstein College of Medicine, Yeshiva University, Bronx, New York, USA.
Lewis H Kuller, Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Oscar L Lopez, Department of Neurology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Stephen B Kritchevsky, Department of Internal Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Anne B Newman, Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Funding
This work was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629, R01AG-15928, R01AG-20098, R01AG-027058, and R01AG-21332 from the National Institute on Aging (NIA), and an award from the Glenn Foundation for Medical Research. IGF-1 measurement was supported by 1R01AG031890 to R.K. A full list of principal CHS investigators and institutions can be found at https://chs-nhlbi.org/. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
A.B.N, J.L.S., X.Z., R.M.B., A.M.A., J.N.J., M.A.E., G.A.K., N.B., L.H.K., O.L.L., S.B.K., report no conflict of interest. JLS is an employee of Vertex Pharmaceuticals.
Author Contributions
J.L.S., A.B.N, X.Z., R.M.B. designed the study; R.M.B., X.Z. compiled and processed the data; X.Z. performed statistical analyses; A.M.A., J.N.J., M.A.E., G.A.K., N.B., L.H.K., O.L.L., S.B.K., A.B.N. interpreted the results and critically revised the manuscript; A.B.N. administered the original CHS; X.Z., J.L.S., R.M.B., A.B.N drafted the manuscript with input from all coauthors. All authors approved the final draft.
References
- 1. Sanders JL, Boudreau RM, Newman AB, Newman AB, Newman AB.. Understanding the aging process using epidemiologic approaches. In: Newman A, Cauley J, eds. The Epidemiology of Aging. Springer. doi: 10.1007/978-94-007-5061-6_12 [DOI] [Google Scholar]
- 2. Sanders JL, Boudreau RM, Penninx BW, et al. ; Health ABC Study. Association of a modified physiologic index with mortality and incident disability: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2012;67:1439–1446. doi: 10.1093/gerona/gls123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belsky DW, Moffitt TE, Cohen AA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2018;187:1220–1230. doi: 10.1093/aje/kwx346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The TAME Trial—Targeting the Biology of Aging. https://www.afar.org/tame-trial.
- 5. Sanders JL, Arnold AM, Boudreau RM, et al. Association of biomarker and physiologic indices with mortality in older adults: Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2019;74:114–120. doi: 10.1093/gerona/gly075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Justice JN, Ferrucci L, Newman AB, et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. Geroscience. 2018;40:419–436. doi: 10.1007/s11357-018-0042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort—the Cardiovascular Health Study All Stars Study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w [DOI] [PubMed] [Google Scholar]
- 9. Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP.. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. doi: 10.1093/clinchem/41.2.264 [DOI] [PubMed] [Google Scholar]
- 10. Kaplan RC, McGinn AP, Pollak MN, et al. Total insulinlike growth factor 1 and insulinlike growth factor binding protein levels, functional status, and mortality in older adults. J Am Geriatr Soc. 2008;56:652–660. doi: 10.1111/j.1532-5415.2007.01637.x [DOI] [PubMed] [Google Scholar]
- 11. Jenny NS, Tracy RP, Ogg MS, et al. In the elderly, interleukin-6 plasma levels and the− 174G> C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:2066–2071. doi: 10.1161/01.atv.0000040224.49362.60 [DOI] [PubMed] [Google Scholar]
- 12. Wasen E, Isoaho R, Mattila K, Vahlberg T, Kivelä SL, Irjala K.. Estimation of glomerular filtration rate in the elderly: a comparison of creatinine-based formulae with serum cystatin C. J Intern Med. 2004;256:70–78. doi: 10.1111/j.1365-2796.2004.01340.x [DOI] [PubMed] [Google Scholar]
- 13. Macy EM, Hayes TE, Tracy RP.. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. doi: 10.1093/clinchem/43.1.52 [DOI] [PubMed] [Google Scholar]
- 14. Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384 [DOI] [PubMed] [Google Scholar]
- 15. Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x [DOI] [PubMed] [Google Scholar]
- 16. Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK.. Defining and measuring chronic conditions: Imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:120239. doi: 10.5888/pcd10.120239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8 [DOI] [PubMed] [Google Scholar]
- 18. Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N.. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110 [DOI] [PubMed] [Google Scholar]
- 19. Del Gobbo LC, Kalantarian S, Imamura F, et al. Contribution of major lifestyle risk factors for incident heart failure in older adults: the Cardiovascular Health Study. JACC Heart Failure. 2015;3:520–528. doi: 10.1016/j.jchf.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Comitato R, Saba A, Turrini A, Arganini C, Virgili F.. Sex hormones and macronutrient metabolism. Crit Rev Food Sci Nutr. 2015;55:227–241. doi: 10.1080/10408398.2011.651177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Márquez EJ, Chung C-H, Marches R, et al. Sexual-dimorphism in human immune system aging. Nat Commun. 2020;11:1–17. doi: 10.1038/s41467-020-14396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jylhävä J, Pedersen NL, Hägg S.. Biological age predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bupp MRG. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015;294:102–110. doi: 10.1016/j.cellimm.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 24. Pignatti C, D’Adamo S, Stefanelli C, Flamigni F, Cetrullo S.. Nutrients and pathways that regulate health span and life span. Geriatrics. 2020;5:95. doi: 10.3390/geriatrics5040095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mauvais-Jarvis F, Merz NB, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396:565–582. doi: 10.1016/S0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee TA, Shields AE, Vogeli C, et al. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22:403–407. doi: 10.1007/s11606-007-0277-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fortin M, Bravo G, Hudon C, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006;15:83–91. doi: 10.1007/s11136-005-8661-z [DOI] [PubMed] [Google Scholar]
- 28. Health UDo, Services H. Multiple Chronic Conditions—A Strategic Framework: Optimum Health and Quality of Life for Individuals With Multiple Chronic Conditions. DC, USA: US Department of Health and Human Services; 2010:2. [Google Scholar]
- 29. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 30. Kuller L, Shemanski L, Psaty B, et al. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation. 1995;92:720–726. doi: 10.1161/01.cir.92.4.720 [DOI] [PubMed] [Google Scholar]
- 31. Enright PL, McBurnie MA, Bittner V, et al. ; Cardiovascular Health Study. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–398. doi: 10.1378/chest.123.2.387 [DOI] [PubMed] [Google Scholar]
- 32. Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737 [DOI] [PubMed] [Google Scholar]
- 33. Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB.. A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. doi: 10.1093/gerona/63.6.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh T, Newman AB.. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maggio M, Guralnik JM, Longo DL, Ferrucci L.. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Braegger CP, Nicholls S, Murch S, MacDonald T, Stephens S.. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j [DOI] [PubMed] [Google Scholar]
- 37. Sproston NR, Ashworth JJ.. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruunsgaard H, Andersen-Ranberg K, vB Hjelmborg J, Pedersen BK, Jeune B.. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003;115:278–283. doi: 10.1016/S0002-9343(03)00329-2 [DOI] [PubMed] [Google Scholar]
- 39. Jenny NS. Inflammation in aging: cause, effect, or both? Discov Med. 2012;13:451–460. [PubMed] [Google Scholar]
- 40. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC.. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4 [DOI] [PubMed] [Google Scholar]
- 41. Charloux A, Brandenberger G, Piquard F, Geny B.. Dysregulation of pulsatility in aging: IV. Pulsatile signaling and cardiovascular aging: functions and regulation of natriuretic peptide signaling. Ageing Res Rev. 2008;7:151–163. doi: 10.1016/j.arr.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 42. Witham MD, Gillespie ND, Hutcheon SD, Struthers AD, McMurdo ME.. B-Type natriuretic peptide is associated with mortality in older functionally impaired patients. J Am Geriatr Soc. 2005;53:1991–1995. doi: 10.1111/j.1532-5415.2005.53555.x [DOI] [PubMed] [Google Scholar]
- 43. Beleigoli AM, Boersma E, Diniz MFH, Vidigal PG, Lima-Costa MF, Ribeiro AL.. C-reactive protein and B-type natriuretic peptide yield either a non-significant or a modest incremental value to traditional risk factors in predicting long-term overall mortality in older adults. PLoS One. 2013;8:e75809. doi: 10.1371/journal.pone.0075809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 45. Landin-Wllhelmsen K, Wllhelmsen L, Lappast G, et al. Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf). 1994;41:351–357. doi: 10.1111/j.1365-2265.1994.tb02556.x [DOI] [PubMed] [Google Scholar]
- 46. Papadakis MA, Grady D, Tierney MJ, Black D, Wells L, Grunfeld C.. Insulin-like growth factor 1 and functional status in healthy older men. J Am Geriatr Soc. 1995;43:1350–1355. doi: 10.1111/j.1532-5415.1995.tb06613.x [DOI] [PubMed] [Google Scholar]
- 47. Goodman-Gruen D, Barrett-Connor E.. Epidemiology of insulin-like growth factor-I in elderly men and women: the Rancho Bernardo Study. Am J Epidemiol. 1997;145:970–976. doi: 10.1093/oxfordjournals.aje.a009065 [DOI] [PubMed] [Google Scholar]
- 48. Harris TB, Kiel D, Roubenoff R, et al. Association of insulin-like growth factor-I with body composition, weight history, and past health behaviors in the very old: the Framingham Heart Study. J Am Geriatr Soc. 1997;45:133–139. doi: 10.1111/j.1532-5415.1997.tb04497.x [DOI] [PubMed] [Google Scholar]
- 49. O’connor KG, Tobin JD, Harman SM, et al. Serum levels of insulin-like growth factor-I are related to age and not to body composition in healthy women and men. J Gerontol A Biol Sci Med Sci. 1998;53:M176–M182. doi: 10.1093/gerona/53a.3.m176 [DOI] [PubMed] [Google Scholar]
- 50. Cappola AR, Xue Q-L, Ferrucci L, Guralnik JM, Volpato S, Fried LP.. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694 [DOI] [PubMed] [Google Scholar]
- 51. Saydah S, Graubard B, Ballard-Barbash R, Berrigan D.. Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol. 2007;166:518–526. doi: 10.1093/aje/kwm124 [DOI] [PubMed] [Google Scholar]
- 52. Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D.. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–120. doi: 10.1210/jc.2003-030967 [DOI] [PubMed] [Google Scholar]
- 53. Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001 [DOI] [PubMed] [Google Scholar]
- 54. Burgers AMG, Biermasz NR, Schoones JW, et al. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96:2912–2920. doi: 10.1210/jc.2011-1377 [DOI] [PubMed] [Google Scholar]
- 55. Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167:2249–2254. doi: 10.1001/archinte.167.20.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bartke A. Insulin and aging. Cell Cycle. 2008;7:3338–3343. doi: 10.4161/cc.7.21.7012 [DOI] [PubMed] [Google Scholar]
- 57. Ashraf H, Boroumand MA, Amirzadegan A, Talesh SA, Davoodi G.. Hemoglobin A1C in non-diabetic patients: an independent predictor of coronary artery disease and its severity. Diabetes Res Clin Pract. 2013;102:225–232. doi: 10.1016/j.diabres.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 58. Palta P, Huang ES, Kalyani RR, Golden SH, Yeh H-C.. Hemoglobin A1c and mortality in older adults with and without diabetes: results from the National Health and Nutrition Examination Surveys (1988–2011). Diabetes Care. 2017;40:453–460. doi: 10.2337/dci16-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barzilay JI, Kronmal RA, Gottdiener JS, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults≥ 65 years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2236–2241. doi: 10.1016/j.jacc.2003.10.074 [DOI] [PubMed] [Google Scholar]
- 60. Gerstein HC, Swedberg K, Carlsson J, et al. ; CHARM Program Investigators. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med. 2008;168:1699–1704. doi: 10.1001/archinte.168.15.1699 [DOI] [PubMed] [Google Scholar]
- 61. El Assar M, Laosa O, Mañas LR.. Diabetes and frailty. Curr Opin Clin Nutr Metab Care. 2019;22:52–57. doi: 10.1097/MCO.0000000000000535 [DOI] [PubMed] [Google Scholar]
- 62. Dubowitz N, Xue W, Long Q, et al. Aging is associated with increased HbA1c levels, independently of glucose levels and insulin resistance, and also with decreased HbA1c diagnostic specificity. Diabet Med. 2014;31:927–935. doi: 10.1111/dme.12459 [DOI] [PubMed] [Google Scholar]
- 63. Wasén E, Isoaho R, Mattila K, Vahlberg T, Kivelä S-L, Irjala K.. Serum cystatin C in the aged: relationships with health status. Am J Kidney Dis. 2003;42:36–43. doi: 10.1016/s0272-6386(03)00406-2 [DOI] [PubMed] [Google Scholar]
- 64. Grubb A, Björk J, Nyman U, et al. Cystatin C, a marker for successful aging and glomerular filtration rate, is not influenced by inflammation. Scand J Clin Lab Invest. 2011;71:145–149. doi: 10.3109/00365513.2010.546879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shlipak MG, Fyr CLW, Chertow GM, et al. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/asn.2005050545 [DOI] [PubMed] [Google Scholar]
- 66. Odden MC, Chertow GM, Fried LF, et al. ; HABC Study. Cystatin C and measures of physical function in elderly adults: the Health, Aging, and Body Composition (HABC) Study. Am J Epidemiol. 2006;164:1180–1189. doi: 10.1093/aje/kwj333 [DOI] [PubMed] [Google Scholar]
- 67. Mathews PM, Levy E.. Cystatin C in aging and in Alzheimer’s disease. Ageing Res Rev. 2016;32:38–50. doi: 10.1016/j.arr.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.