Abstract
This study investigates the effects of peroxisome proliferator-activated receptor gamma (PPARγ) inhibition on bone and immune cell profiles in aged female mice, as well as in vitro stromal stem cell osteogenic differentiation and inflammation gene expression. The hypothesis was that inhibition of PPARγ would increase bone mass and alter immune and other cellular functions. Our results showed that treatment with PPARγ antagonist GW9662 for 6 weeks reduced bone volume and trabecular number and increased trabecular spacing. However, inhibition of PPARγ had no significant effect on marrow and spleen immune cell composition in aged female mice. In vitro experiments indicated that GW9662 treatment increased the expression of osteogenic genes but did not affect adipogenic genes. Additionally, GW9662 treatment decreased the expression of several inflammation-related genes. Overall, these findings suggest that PPARγ inhibition may have adverse effects on bone in aged female mice.
Keywords: Aging, Bone loss, Hematopoietic skew, PPARγ
Aging is a complex process characterized by various physiological changes that lead to a decline in overall health. Chronic inflammation (1–3), impaired immune function (4–7), and bone loss with marrow fat accumulation (8–10) are among the hallmarks of aging. Peroxisome proliferator-activated receptor gamma (PPARγ) is a transcription factor that plays an essential role in adipocyte differentiation (11,12) and is involved in inflammation, immune cell differentiation, and activation (13–15). Targeting PPARγ has been suggested as a potential therapeutic approach for managing age-related bone loss due to the reciprocal relationship between osteoblasts and adipocytes (16–18) and the fact that bone marrow stromal stem cells (SSCs) are a common precursor for these 2 cell lineages (19–21). Although previous studies have shown that haploinsufficiency in PPARγ results in high bone mass and low marrow adipocytes (22), it is unclear whether PPARγ in mesenchymal lineage cells is critical for aging-induced bone loss. To address this question, we and others generated mesenchymal lineage cell PPARγ knockout mice (Dermo1-Cre:PPARγfl/fl and Prx1-Cre:PPARγfl/fl mice) (23,24). Although our data showed that deletion of PPARγ in Dermo1-expressing mesenchymal lineage cells increased cortical bone thickness in aged mice (23), deletion of PPARγ in Prx1-expressing cells, unexpectedly, increased cortical bone porosity (24). Both Dermo1- and Prx1-Cre PPARγ cKO mice showed no trabecular bone phenotype. As a complementary approach, aged female C57BL/6 mice were treated with a selective PPARγ antagonist, GW9662, and the effects on bone, immune cell composition, and the expression of inflammatory genes are reported here in this study. Understanding the role of PPARγ in bone, immune function, and inflammation in aging may provide new insights into the development of effective therapies for age-related bone loss and associated complications. In this study, female mice are included as subjects due to significant gender disparities in osteoporosis prevalence, etiology, and disease progression. Prior research has predominantly concentrated on male mice, necessitating a more balanced investigation of both sexes to achieve a comprehensive understanding of the condition.
Materials and Methods
Animals
Female C57BL/6 mice were obtained from the National Institute on Aging (NIA) aged rodent colonies. After a week of recovery, the mice were randomly divided into 2 groups (n = 10 mice per group): a control group (vehicle injection) and a treatment group (GW9662, 1 mg/kg body weight, daily IP injection for 6 weeks). The dosage and duration of treatment were based on several studies investigating the effect of GW9662 in mice (25,26). The mice were group housed (4–5 mice per cage) in the Augusta University Laboratory Animal Service facility under a 12-hour dark-light cycle and fed with standard rodent chow (Teklad, cat# 2918) and water ad libitum. One of the mice in the treatment group died unexpectedly soon after the initiation of the treatment (n = 9). All animal procedures were performed in accordance with a protocol (#2008 ± 0302) approved by the Augusta University Institutional Animal Care and Use Committee.
Microcomputed Tomography and Histology Analysis
Bone density and structural parameters were measured by microcomputed tomography (μCT) scan (μCT-40; Scanco Medical AG, Bassersdorf, Switzerland) as previously described (27,28). Bone tissues for histologic and histomorphometric analyses were collected, processed, and analyzed following our previously described procedures (27).
Flow Cytometry
Blood was obtained by making a small incision at the tip of the mouse tail and collected in heparinized capillary tubes. Bone marrow cells were collected by flushing long bones with RPMI 1640 medium + 10% fetal bovine serum (FBS) as previously described (29). Splenocytes were prepared by mashing the spleen with plunger end through a cell strainer. After rinsing the strainer, cells were centrifuged at 800×g for 3 minutes, resuspended in 1 mL ACK lysis buffer, and incubated at RT for 5–10 minutes before adding 9 mL RPMI media with 10% FBS and centrifuging again. The cell pellet was resuspended in 3 mL of media. The cells were then incubated with indicated antibodies (CD3-FITC, CD4-APC, CD8-FITC, CD11b-APC, Ly6c-PE, and Ly6g-FITC, all from Biolegend) and analyzed on a 4-color flow cytometer (FACSCalibur, BD Biosciences, San Diego, CA). Data were collected using CellQuest software (BD Biosciences) as described previously (30). Cells expressing specific markers were reported as a percentage of the number of gated events.
Isolation of Bone Marrow Mesenchymal Stem Cells and Differentiation
Bone marrow stromal stem cells (SSCs) were obtained from the Mouse Stem Cell Core Facility, Augusta University. Briefly, the SSCs were isolated from the long bones of 23-month-old female C57BL/6 mice using a negative immunodepletion procedure (Lineage Cell Depletion Kit, Miltenyi Biotec) with biotinylated CD5, CD45R/B220, CD11b, Ly-6G/C, 7-4, and Ter-119 antibodies, followed by positive immunoselection with anti-Sca-1 beads, as previously described (31). The purified cells were cultured in standard osteogenic differentiation media for 7 to 15 days for ALP or alizarin red S staining assays. The stained plates were scanned, and results were quantified using NIH Image J software.
Real-Time qRT-PCR Analysis
For qRT-PCR analysis, total cellular RNAs were collected from cells treated with or without 5 µM GW9662 for 48 hours. An equal amount of RNA samples (0.5 µg) was reverse transcribed (Bio-Rad iScript cDNA Synthesis Kit, Bio-Rad Laboratories, Hercules, CA), and PCR reactions performed in quintuplicate or sextuplicate using a StepOnePlus Real-Time qPCR System (Thermo Fisher Scientific, Waltham, MA). The mRNA levels were normalized to β-actin (internal controls). Results are presented as fold changes (delta-delta CT) from 2 to 3 independent experiments. The primer sequences used in qRT-PCR are listed in Supplementary Table 1.
Statistical Analysis
Data were analyzed by unpaired t test (Mann-Whitney) or one-way ANOVA, where appropriate, using Prism GraphPad software version 9.4.1. A p value less than .05 was considered significant.
Results
Inhibition of PPARγ on Bone in Aged Female Mice
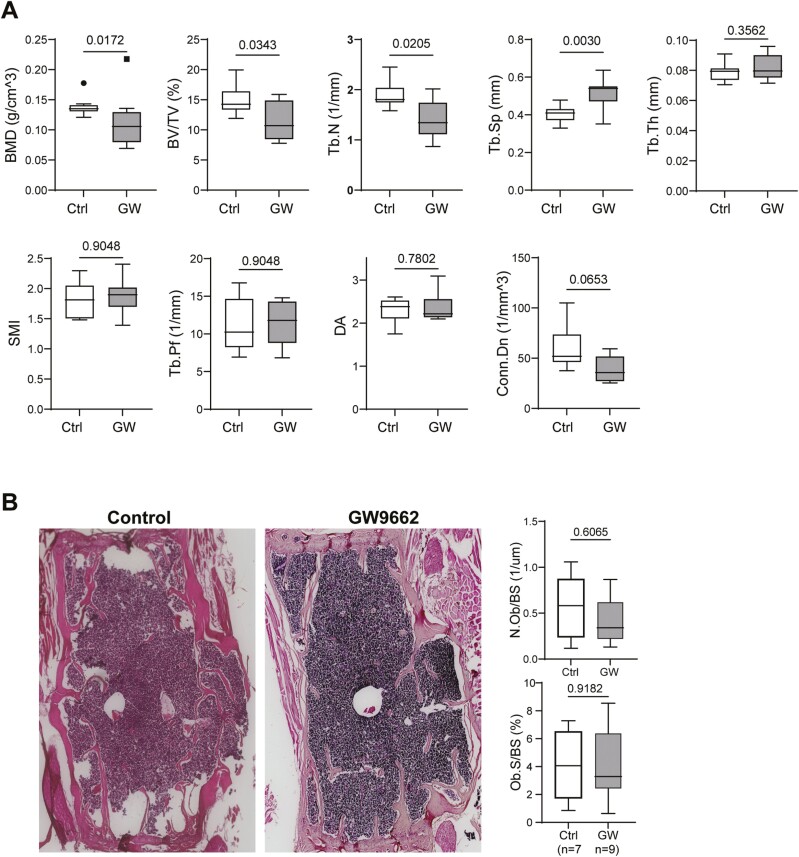
To determine the effect of PPARγ inhibition on bone, we injected aged (23.5 months) female C57BL/6 mice with a selective PPARγ antagonist GW9662 (1 mg/kg daily IP injection) for 6 weeks. At the end of treatment (aged 25 months), mice were sacrificed, and bones were analyzed by microcomputed tomography (µCT). Results showed that GW9662 treatment significantly decreased vertebrae (L4) bone mineral density (BMD), bone volume (BV/TV), trabecular number (Tb.N) and increased trabecular spacing (Tb.Sp; Figure 1A). The other bone parameters such as trabecular thickness (Tb.Th), structure model index (SMI), trabecular pattern factor (Tb.Pf), and degree of anisotropy (DA) showed no difference between the 2 groups. The connectivity density (Conn.Dn) decreased but this decrease fell short to reach statistical significance (p = .0653). However, these adverse bone effects were not observed in mature adult female mice (8-month-old) treated with GW9662 (Supplementary Figure 1). As expected, major bone architecture parameters indicative of healthy bone (BMD, BV/TV, Tb.Th, Tb.N) are significantly lower and the parameters indicative of unhealthy bone (Tb.Sp and Conn.Dn) are significantly higher in aged mice than that in young mice (Supplementary Figure 2). Hematoxylin and eosin (H&E) staining of decalcified bone sections showed that GW9662 treatment had no effects on osteoblast numbers (N. Ob/BS) or osteoblast surface (Ob. S/BS) compared to control mice (Figure 1B). Quantitative results are shown in graphs. Together, these results indicate that systemic inhibition of PPARγ may, in fact, have an adverse effect on bone architecture in aged female mice.
Figure 1.
(A) Microcomputed tomography (μCT) data showing bone mineral density (BMD) and bone structural parameters of vertebrae from 25-month-old female C57BL/6 mice treated without or with GW9662 for 6 weeks. Values are given as mean ± SD; n = 9–10 mice per group. Unpaired t test, p values are indicated. Symbols (dot and square, included in data set) denote outliers. BV/TV = bone volume/tissue volume; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular spacing; SMI = structure model index, a parameter indicating the plate- or rod-like geometry of trabecular structures; Tb.Pf = trabecular pattern factor; DA = degree of anisotropy; Conn.Dn. = connectivity density. (B) Histology and histomorphometry analyses. Representative hematoxylin and eosin (H&E) stained vertebrae sections from 25-month-old female C57BL/6 mice treated without or with GW9662 for 6 weeks. Quantitative results for osteoblast number and osteoblast-covered bone surface area are shown in graphs. Data are shown as mean ± SD, n = 7–9 mice per group. Unpaired t test and p values are indicated.
Inhibition of PPARγ on SSC Osteogenic Differentiation
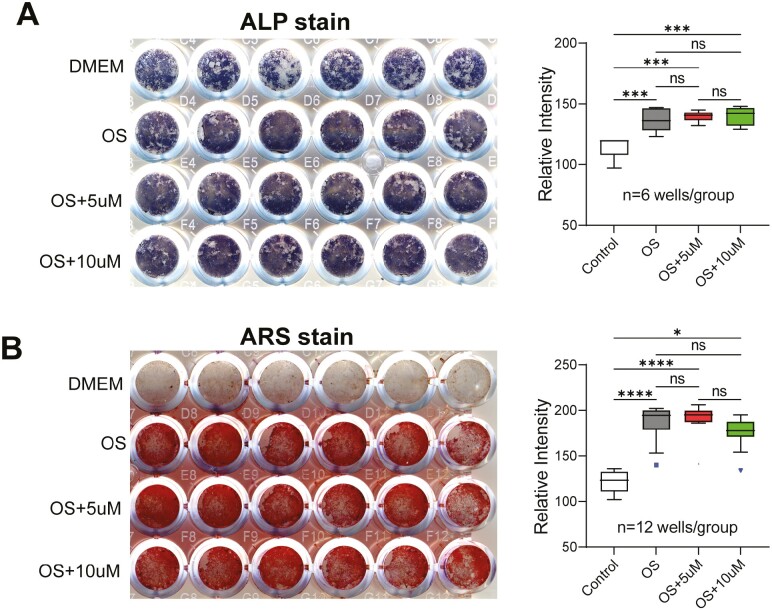
To confirm the in vivo results presented above, we induced SSCs isolated from aged female mice (23-month-old C57BL/6) with osteogenic differentiation media supplemented with GW9662 (5 or 10 µM) and performed alkaline phosphatase (ALP, Figure 2A) and alizarin red S (ARS, Figure 2B) assays. The results showed that inhibition of PPARγ had no effect on osteogenic differentiation of aged SSCs in vitro. As expected, the osteogenic cocktail enhanced ALP and induced mineralization compared with the cells cultured in regular growth medium (Dulbecco’s Modified Eagle Medium (DMEM)). The purified SSCs express high levels of ALP but they do not mineralize without induction (top rows in A and B). Quantitative results are shown in graphs.
Figure 2.
Effect of GW9662 on osteogenic differentiation of stromal stem cells (SSCs). SSCs isolated from 23-month-old female mice were cultured in standard osteogenic induction media supplemented with 5 or 10 µM GW9662 for 7 or 15 days. (A) After 7 days of treatment, cells were fixed and stained with FAST BCIP/NBT buffered substrate to detect ALP activity. (B) After 15 days of treatment, cells were fixed and stained with alizarin red S to visualize mineralized nodules. Quantitative results for A and B, performed using ImageJ software, are shown on the right. Data are shown as mean ± SD. Ordinary one-way ANOVA analysis. n = number of wells per treatment (indicated). *p = .0171; ***p ≤ .0006; ****p < .0001. Symbols (square and triangle, included in data set) denote outliers.
Inhibition of PPARγ on Gene Expression
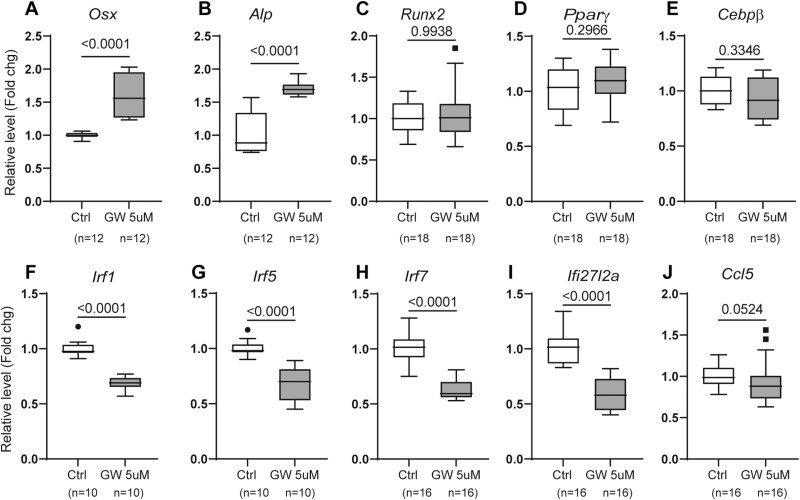
We treated SSCs with GW9662 (5 µM for 48 hours) and performed qRT-PCR analysis. The results showed that GW9662 treatment significantly increased the expression levels of osteogenic Osx and Alp, but not Runx2 or adipogenic Pparγ and C/ebpβ (Figure 3A–E). However, despite the increased expression of Osx and Alp, there was no corresponding enhancement in osteogenic differentiation (Figure 2). It is possible that the enhanced gene expression by GW9662 seen in qPCR was outweighed by the presence of strong osteoblast differentiation stimulating agents, such as dexamethasone, β-glycerophosphate, and ascorbic acid, which are present in the osteogenic media in these in vitro assays.
Figure 3.
qRT-PCR analysis of gene expression. Stromal stem cells isolated from 23-month-old female mice were treated without or with 5 µM GW9662 for 48 hours and harvested for total RNA isolation. The mRNA levels of indicated genes were analyzed. Data are shown as mean ± SD from 2–3 independent experiments. PCR reactions were performed in quadruplicate or sextuplicate for each sample, and data presented as fold changes. Unpaired t test, sample size (number of wells) and p values are indicated. Symbols (dots and squares, included in data set) represent outliers.
Previously, we demonstrated that deletion of PPARγ in mesenchymal lineage cells (Dermo1-Cre: PPARγfl/fl mice) reduced expression of inflammation-associated genes in cultured bone marrow cells, including a class of genes involved in interferon signaling (23). To determine if inhibition of PPARγ would have the same effect on SSCs, we also analyzed the expression of several of these genes in GW9662-treated cells, including interferon regulatory factors (Irf1, -5, -7), interferon alpha-inducible protein 27-like 2A (Ifi27l2a), and C-C motif chemokine ligand 5 (Ccl5). Similar to PPARγ-deficient bone marrow stromal cells (23), results showed that the expression levels of all these genes were significantly decreased in GW9662-treated SSCs (Figure 3F–J).
Inhibition of PPARγ on Immune Cell Reconstitution
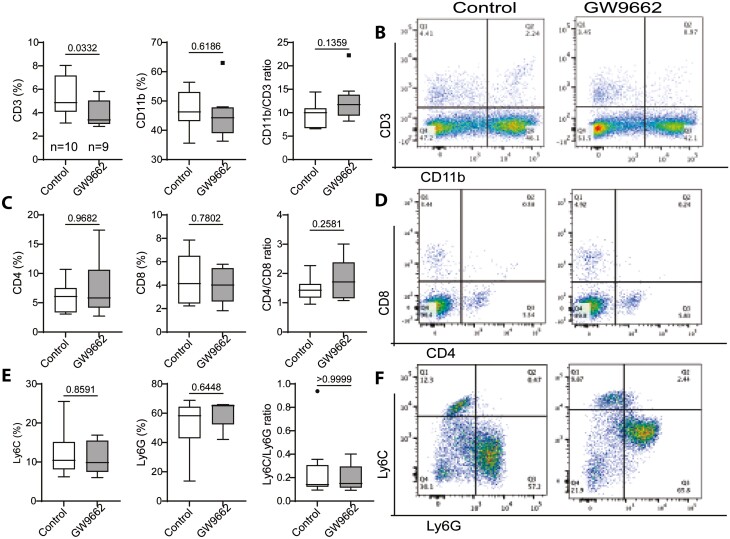
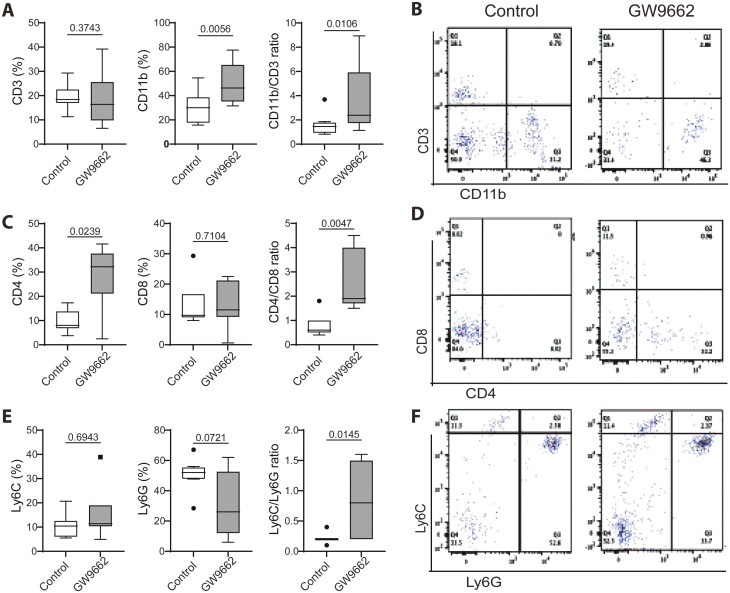
The immune function declines with aging due to the decline of hematopoietic stem cells (HSCs) and progenitor cells, as well as the shifting of the HSC compartment from a predominant lymphoid-biased (Ly-bi) to a myeloid-biased (My-bi) proportion (4–6,30,32–34). To determine if inhibition of PPARγ can alter this shift or change the composition within the myeloid and lymphoid subpopulation, we examined bone marrow and splenocytes by Fluorescence-activated cell sorting (FACS) analysis. The results showed that GW9662 treatment significantly reduced the population of lymphoid lineage (CD3+) cells but also slightly reduced myeloid lineage (CD11b+) cells, as a result it did not alter the balance (CD11b/CD3 ratio) between these 2 lineage cells (Figure 4A). To further delineate the changes within the lymphoid and myeloid compartments, we analyzed CD4 and CD8 (lymphoid) and Ly6c and Ly6g (myeloid) subpopulations. The results showed that GW9662 treatment had no effects on these subpopulations or changed the ratio of CD4/CD8 and Ly6C/Ly6G (Figure 4C and E). Representative flow cytometry dot plots are shown (Figure 4B, D, and F). As the spleen is a site for the activation and proliferation of immune cells, we also examined the composition of these cells. Like in bone marrow, the results from splenocytes showed no differences between control and GW9662 treatment groups in lymphoid (CD3) or myeloid (CD11b) populations, or in the composition of subpopulations (Supplementary Figure 3). These findings suggest that systemic inhibition of PPARγ has no or very limited effect on immune cell production or proliferation in aged female mice. However, upon monitoring the blood 3 weeks into the treatment for the drug effect, the data indicated that GW9662 treatment resulted in a significant increase in CD11b and CD4 cells, while reducing Ly6G cells. This led to an imbalance between the lymphoid and myeloid (CD11b/CD3 ratio) lineage cells, as well as subpopulations (CD4/CD8 and Ly6C/Ly6G ratios) within each lineage (Figure 5). These results indicated that systemic inhibition of PPARγ may have certain effects on the balance of lymphoid and myeloid cells as well as on the subpopulations within each lineage in peripheral blood, but these changes seemed to have no influence on bone in aged female mice. Clearly, the hematopoietic skew occurs in peripheral blood with age as demonstrated by significantly decreased CD3 and CD4 cell populations and resulted in an imbalance between lymphoid and myeloid cells (CD11b/CD3 ratio) when compared with young mice (Supplementary Figure 4).
Figure 4.
FACS analysis showing changes in bone marrow cells in 25-month-old female C57BL/6 mice treated without or with GW9662 for 6 weeks. (A, C, E) Bar graphs showing percentages of immune cells positive for indicated cell surface makers. (B, D, F) Representative flow cytometry dot plots show the distribution of cells positive for the markers detected. Values are given as mean ± SD; n = 9–10 mice per group. Unpaired t test, p values are indicated. Symbols (dots and squares, included in data set) denote outliers.
Figure 5.
FACS analysis of blood in 25-month-old female C57BL/6 mice treated without or with GW9662 for 3 weeks. The experiment was performed as described in Figure 4. Values are given as mean ± SD; n = 9–10 mice per group. Unpaired t test and p values are indicated. Symbols (dots and squares, included in data set) denote outliers.
Discussion
We previously reported that mice lacking PPARγ in mesenchymal lineage cells (Dermo1-Cre:PPARfl/fl) exhibited increased cortical bone thickness while displaying no alterations in trabecular bone in aged mice (23). In concurrence with our findings, a study by Almeida et al. using Prx1-Cre:PPARfl/fl mice demonstrated that PPARγ deficiency led to an augmented cortical bone area in young mice (6-month-old). Intriguingly, in contrast to its negligible impact on trabecular bone, PPARγ deficiency resulted in elevated cortical bone porosity in aged female mice within the same study (24). Almeida et al. also established that enhanced marrow adipogenesis did not contribute to age-related bone loss in female mice (24). Furthermore, a study authored by Beekman et al. demonstrated that administration of GW9662 (at a dosage of 1 mg/kg per day for 3 weeks) yielded no discernible impact on bone marrow adipose tissue or bone volume. This outcome was evident in female C3H/HeJ mice, both those subjected to a sham procedure and those undergoing ovariectomy, at 16 weeks of age upon experiment conclusion (26). Our µCT data echoed the findings of Beekman et al., revealing analogous trends: reduced BV/TV and Tb.N, alongside elevated Tb.Sp within the GW9662-treated group. However, these changes did not attain statistical significance in the context of C3H/HeJ mice. Moreover, other parameters pertinent to bone health, such as MAR, BFR/BS, Oc.S/BS, and MS/BS, exhibited no substantial alterations in GW-treated C3H/HeJ mice.
Activation of PPARγ by its agonist rosiglitazone is known to induce bone loss and marrow adipocyte accumulation (35–37). Thus, the counterintuitive findings of bone deterioration following PPARγ deletion (24) or systemic inhibition (as indicated in the present study) raise questions regarding the widely accepted hypothesis regarding PPARγ’s role in bone formation or its protective effects against age-related bone loss (38). Presently, the reasons behind the inability of PPARγ deletion in bone progenitor cells to shield mice from age-associated bone loss remain elusive.
It is worth noting that our result is inconsistent with a previous study on mature male mice that showed that inhibition of PPARγ with bisphenol‐A‐diglycidyl ether (BADGE), another PPARγ antagonist, increased bone volume, and improved bone quality in 9-month-old male C57BL/6 mice (39). This discrepancy appears to stem from variations in the age and sex of the mice under scrutiny (young males vs old females), both of which significantly influence bone density and quality (40), as well as discrepancies in the administered drugs and dosages (30 mg/kg per day BADGE vs 1 mg/kg per day GW9662).
To summarize, the function of PPARγ in the skeletal system is intricate, displaying diverse outcomes contingent upon the specific experimental model employed, as well as the gender and age of the subjects under investigation. Our previous research findings highlighted that the removal of PPARγ from type I collagen-expressing cells (PPARfl/fl:Col3.6-Cre) resulted in a moderate bone mass alteration, varying by skeletal site, observed in 6-month-old mice (41). In a parallel study, He et al. demonstrated that the absence of PPARγ (PPARγfl/fl:Sox2-Cre) led to augmented BMD solely in vertebrae, without affecting long bones (42). A separate study by Sun et al. uncovered yet another dimension. Their work, centered on the ablation of PPARγ in osteoblasts through an inducible Osx promoter-driven Cre (PPARγfl/fl:Sp7-tTA, tetO-EGFP/Cre), yielded increased Tb.N and decreased Tb.Sp in 6-month-old mice (43). Intriguingly, this effect did not extend to femur BMD, which pertains to long bones. The authors of this study concluded that PPARγ suppression fosters osteogenesis through the activation of mTOR signaling (43). Furthermore, the investigation by Baroi et al. contributed another layer of insight. Their study involving the deletion of PPARγ in osteocytes (DMP1-Cre:PPARγfl/fl), demonstrated an increase in bone mass, attributed to a reduction in SOST expression (44).
The precise function of PPARγ in osteoclasts remains contentious: while one study showed that PPARγ deletion by Tie2Cre in myeloid lineage cells impeded osteoclast differentiation, culminating in osteopetrosis (45), another study demonstrated that PPARγ deletion by LysM-Cre exerted no discernible influence on osteoclastogenesis or skeletal architecture (46).
To conclude, the role of PPARγ in the skeleton is complex, with outcomes shaped by specific circumstances and cellular contexts. Exploring the potential effects of PPARγ inhibition at earlier stages of aging, when accelerated bone loss commences, could offer valuable insights. This could extend to evaluating its impact on bone health, the equilibrium of marrow immune cells, and inflammatory processes. As age-related bone loss is influenced by a myriad of factors, these considerations merit attention, given their known contributions to this process (47–49).
Supplementary Material
Contributor Information
Xingming Shi, Department of Neuroscience and Regenerative Medicine, Augusta University, Augusta, Georgia, USA.
Kehong Ding, Department of Neuroscience and Regenerative Medicine, Augusta University, Augusta, Georgia, USA.
Raysa Rosario, Department of Neuroscience and Regenerative Medicine, Augusta University, Augusta, Georgia, USA.
Ashwin Ajith, Center for Molecular Chaperone/Radiobiology and Cancer Virology, Augusta University, Augusta, Georgia, USA.
Yun Su, Department of Neuroscience and Regenerative Medicine, Augusta University, Augusta, Georgia, USA.
Sean Shaw, Department of Neuroscience and Regenerative Medicine, Augusta University, Augusta, Georgia, USA.
Meghan McGee-Lawrence, Department of Cellular Biology and Anatomy, Augusta University, Augusta, Georgia, USA.
Xin-Yun Lu, Department of Neuroscience and Regenerative Medicine, Augusta University, Augusta, Georgia, USA.
Anatolij Horuzsko, Center for Molecular Chaperone/Radiobiology and Cancer Virology, Augusta University, Augusta, Georgia, USA.
Carlos M Isales, Department of Neuroscience and Regenerative Medicine, Augusta University, Augusta, Georgia, USA; Department of Medicine, Augusta University, Augusta, Georgia, USA.
Gustavo Duque, (Biological Sciences Section).
Funding
The research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award numbers R01 AG046248 and R56 AG066052.
Conflict of Interest
None.
Author Contributions
X.S., C.M.I., A.H., and X.-Y.L. designed the experiments and edited the manuscript. X.S. analyzed data and wrote the manuscript. A.H. and A.A. performed FACS analysis. K.D. performed bone density analysis. M.M.-L. performed bone histomorphometry analysis. R.R. injected animals and collected cells for FACS analysis. Y.S. isolated SSCs. S.S. performed qRT-PCR and cell differentiation assays.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908(1):244–254. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 2. Ginaldi L, Di Benedetto MC, De Martinis M.. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2. 10.1186/1742-4933-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lencel P, Magne D.. Inflammaging: the driving force in osteoporosis? Med Hypotheses. 2011;76(3):317–321. 10.1016/j.mehy.2010.09.023 [DOI] [PubMed] [Google Scholar]
- 4. Waterstrat A, Van Zant G.. Effects of aging on hematopoietic stem and progenitor cells. Curr Opin Immunol. 2009;21(4):408–413. 10.1016/j.coi.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 5. Vas V, Senger K, Dorr K, Niebel A, Geiger H.. Aging of the microenvironment influences clonality in hematopoiesis. PLoS One. 2012;7(8):e42080. 10.1371/journal.pone.0042080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA.. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201. 10.1371/journal.pbio.0050201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stowe RP, Goodwin JS.. Effects of aging on immune function. In: Rosenthal R, Zenilman M, Katlic M, eds. Principles and Practice of Geriatric Surgery. Springer, New York, NY. 2011. 10.1007/978-1-4757-3432-4_4 [DOI] [Google Scholar]
- 8. Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ.. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55(9):693–698. 10.1136/jcp.55.9.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meunier P, Aaron J, Edouard C, Vignon G.. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. 10.1097/00003086-197110000-00021 [DOI] [PubMed] [Google Scholar]
- 10. Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M.. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. 10.1023/a:1011513223894 [DOI] [PubMed] [Google Scholar]
- 11. Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4(4):585–595. 10.1016/s1097-2765(00)80209-9 [DOI] [PubMed] [Google Scholar]
- 12. Rosen ED, Sarraf P, Troy AE, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4(4):611–617. 10.1016/s1097-2765(00)80211-7 [DOI] [PubMed] [Google Scholar]
- 13. Jiang C, Ting AT, Seed B.. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. 10.1038/34184 [DOI] [PubMed] [Google Scholar]
- 14. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK.. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. 10.1038/34178 [DOI] [PubMed] [Google Scholar]
- 15. Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, Evans RM.. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93(2):241–252. 10.1016/s0092-8674(00)81575-5 [DOI] [PubMed] [Google Scholar]
- 16. Minguell JJ, Erices A, Conget P.. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001;226(6):507–520. 10.1177/153537020122600603 [DOI] [PubMed] [Google Scholar]
- 17. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- 18. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. 10.1126/science.276.5309.71 [DOI] [PubMed] [Google Scholar]
- 19. Ahdjoudj S, Lasmoles F, Oyajobi BO, Lomri A, Delannoy P, Marie PJ.. Reciprocal control of osteoblast/chondroblast and osteoblast/adipocyte differentiation of multipotential clonal human marrow stromal F/STRO-1(+) cells. J Cell Biochem. 2001;81(1):23–38. [DOI] [PubMed] [Google Scholar]
- 20. Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME.. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102(Pt 2):341–351. 10.1242/jcs.102.2.341 [DOI] [PubMed] [Google Scholar]
- 21. Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF.. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275(13):9645–9652. 10.1074/jbc.275.13.9645 [DOI] [PubMed] [Google Scholar]
- 22. Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113(6):846–855. 10.1172/JCI19900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao J, Ding K, Pan G, et al. Deletion of PPARγ in mesenchymal lineage cells protects against aging-induced cortical bone loss in mice. J Gerontol A Biol Sci Med Sci. 2020;75(5):826–834. 10.1093/gerona/glaa049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Almeida M, Kim HN, Han L, et al. Increased marrow adipogenesis does not contribute to age-dependent appendicular bone loss in female mice. Aging Cell. 2020;19(11):e13247. 10.1111/acel.13247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sato K, Feng X, Chen J, et al. PPARγ antagonist attenuates mouse immune-mediated bone marrow failure by inhibition of T cell function. Haematologica. 2016;101(1):57–67. 10.3324/haematol.2014.121632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beekman KM, Veldhuis-Vlug AG, van der Veen A, et al. The effect of PPARγ inhibition on bone marrow adipose tissue and bone in C3H/HeJ mice. Am J Physiol Endocrinol Metab. 2019;316(1):E96–E105. 10.1152/ajpendo.00265.2018 [DOI] [PubMed] [Google Scholar]
- 27. Pan G, Cao J, Yang N, et al. Role of glucocorticoid-induced leucine zipper (GILZ) in bone acquisition. J Biol Chem. 2014;289:19373–19382. 10.1074/jbc.m113.535237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cao JJ, Gregoire BR, Shen CL.. A high-fat diet decreases bone mass in growing mice with systemic chronic inflammation induced by low-dose, slow-release lipopolysaccharide pellets. J Nutr. 2017;147:1909–1916. 10.3945/jn.117.248302 [DOI] [PubMed] [Google Scholar]
- 29. Yang N, Baban B, Isales CM, Shi XM.. Crosstalk between bone marrow-derived mesenchymal stem cells and regulatory T cells through a glucocorticoid-induced leucine zipper/developmental endothelial locus-1-dependent mechanism. FASEB J. 2015;29(9):3954–3963. 10.1096/fj.15-273664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gazit R, Weissman IL, Rossi DJ.. Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol. 2008;45(4):218–224. 10.1053/j.seminhematol.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 31. Zhang W, Ou G, Hamrick M, et al. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J Bone Miner Res. 2008;23(7):1118–1128. 10.1359/jbmr.080304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swierczek SI, Agarwal N, Nussenzveig RH, et al. Hematopoiesis is not clonal in healthy elderly women. Blood. 2008;112(8):3186–3193. 10.1182/blood-2008-03-143925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vas V, Wandhoff C, Dorr K, Niebel A, Geiger H.. Contribution of an aged microenvironment to aging-associated myeloproliferative disease. PLoS One. 2012;7(2):e31523. 10.1371/journal.pone.0031523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Linton PJ, Dorshkind K.. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. 10.1038/ni1033 [DOI] [PubMed] [Google Scholar]
- 35. Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL.. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146(3):1226–1235. 10.1210/en.2004-0735 [DOI] [PubMed] [Google Scholar]
- 36. Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B.. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148(6):2669–2680. 10.1210/en.2006-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B.. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145(1):401–406. 10.1210/en.2003-0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pei L, Tontonoz P.. Fat’s loss is bone’s gain. J Clin Invest. 2004;113(6):805–806. 10.1172/JCI21311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duque G, Li W, Vidal C, Bermeo S, Rivas D, Henderson J.. Pharmacological inhibition of PPARγ increases osteoblastogenesis and bone mass in male C57BL/6 mice. J Bone Miner Res. 2013;28(3):639–648. 10.1002/jbmr.1782 [DOI] [PubMed] [Google Scholar]
- 40. Alswat KA. Gender disparities in osteoporosis. J Clin Med Res. 2017;9(5):382–387. 10.14740/jocmr2970w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cao J, Ou G, Yang N, et al. Impact of targeted PPARgamma disruption on bone remodeling. Mol Cell Endocrinol. 2015;410:27–34. 10.1016/j.mce.2015.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He F, Dominique P, Anne W, Serge F, Beatrice D.. Bone marrow adiposity affects osteoclastogenesis by modulating the bone marrow niche. In: ASBMR 2011 Annual Meeting Abstracts; September 16–20, 2011; San Diego. Washington, DC: JBMR; 2011. [Google Scholar]
- 43. Sun H, Kim JK, Mortensen R, Mutyaba LP, Hankenson KD, Krebsbach PH.. Osteoblast-targeted suppression of PPARγ increases osteogenesis through activation of mTOR signaling. Stem Cells. 2013;31(10):2183–2192. 10.1002/stem.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baroi S, Czernik PJ, Chougule A, Griffin PR, Lecka-Czernik B.. PPARG in osteocytes controls sclerostin expression, bone mass, marrow adiposity and mediates TZD-induced bone loss. Bone. 2021;147:115913. 10.1016/j.bone.2021.115913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wan Y, Chong LW, Evans RM.. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13(12):1496–1503. 10.1038/nm1672 [DOI] [PubMed] [Google Scholar]
- 46. Zou W, Rohatgi N, Chen TH-P, Schilling J, Abu-Amer Y, Teitelbaum SL.. PPAR-γ regulates pharmacological but not physiological or pathological osteoclast formation. Nat Med. 2016;22(11):1203–1205. 10.1038/nm.4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang X, Young HA.. PPAR and immune system—what do we know? Int Immunopharmacol. 2002;2(8):1029–1044. 10.1016/s1567-5769(02)00057-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Le Menn G, Neels JG.. Regulation of immune cell function by PPARs and the connection with metabolic and neurodegenerative diseases. Int J Mol Sci. 2018;19(6):1575. 10.3390/ijms19061575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clark RB. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71(3):388–400. 10.1189/jlb.71.3.388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.