Abstract
Background and Aims
Bulnesia retama is a drought-deciduous, xerophytic shrub from arid landscapes of South America. In a survey of carbon isotope ratios (δ13C) in specimens from the field, B. retama exhibited less negative values, indicative of CAM or C4 photosynthesis. Here, we investigate whether B. retama is a C4 or CAM plant.
Methods
Gas-exchange responses to intercellular CO2, diurnal gas-exchange profiles, δ13C and dawn vs. afternoon titratable acidity were measured on leaves and stems of watered and droughted B. retama plants. Leaf and stem cross-sections were imaged to determine whether the tissues exhibited succulent CAM or C4 Kranz anatomy.
Key Results
Field-collected stems and fruits of B. retama exhibited δ13C between −16 and −19 ‰. Plants grown in a glasshouse from field-collected seeds had leaf δ13C values near −31 ‰ and stem δ13C values near −28 ‰. The CO2 response of photosynthesis showed that leaves and stems used C3 photosynthesis during the day, while curvature in the nocturnal response of net CO2 assimilation rate (A) in all stems, coupled with slightly positive rates of A at night, indicated modest CAM function. C4 photosynthesis was absent. Succulence was absent in all tissues, although stems exhibited tight packing of the cortical chlorenchyma in a CAM-like manner. Tissue titratable acidity increased at night in droughted stems.
Conclusions
Bulnesia retama is a weak to modest C3 + CAM plant. This is the first report of CAM in the Zygophyllaceae and the first showing that non-succulent, xerophytic shrubs use CAM. CAM alone in B. retama was too limited to explain less negative δ13C in field-collected plants, but combined with effects of low stomatal and mesophyll conductance it could raise δ13C to observed values between −16 and −19 ‰. Modest CAM activity, particularly during severe drought, could enable B. retama to persist in arid habitats of South America.
Keywords: Bulnesia retama, CAM photosynthesis, carbon isotope ratio, retamo, stem photosynthesis, xerophyte, Zygophyllaceae
INTRODUCTION
The identification of species with photosynthetic carbon-concentrating mechanisms remains an ongoing task decades after the discovery of C4 and crassulacean acid metabolism (CAM) photosynthesis (Frohlich et al., 2022; Gilman et al., 2023). Surveys of carbon isotope ratios (δ13C) using live plants or herbarium specimens readily identify C3 and C4 species, allowing C4 clades to be mapped onto their respective phylogenies (Sage et al., 2007; Lauterbach et al., 2016, 2019). C3 plants typically show δ13C values between −21 and −32 ‰, whereas C4 plants show values between −10 and −16 ‰ (Vogel, 1993; Cerling et al., 1997; Sage et al., 2007). A few species in lineages containing both C3 and C4 plants exhibit δ13C values between −21 and −16 ‰ and are termed C4-like species because they are evolutionary intermediates that operate a C4 photosynthetic pathway but with incomplete enzyme compartmentalization (for example, Flaveria brownii, Monson et al., 1988; and Alloteropsis semialata, Lundgren et al., 2019). The other group of terrestrial plants with less negative δ13C values typical of C4 or C4-like plants are CAM plants, which assimilate CO2 at night via PEP carboxylase (PEPC) and use Rubisco to refix it during the day when stomata are closed (Kluge and Ting, 1978; Winter and Smith, 1996a; Winter and Holtum, 2002). Obligate CAM plants often exhibit a similar range of δ13C values as C4 plants, because most of the carbon in the plants enters at night via PEP carboxylation, during phase I of the CAM cycle (Osmond, 1978; Winter and Holtum, 2002; Edwards, 2019). Alternatively, many CAM species exhibit δ13C values that overlap with typical C3 values, and some exhibit δ13C values of −16 to −20 ‰ that fall between the range of C3 and C4 values (Silvera et al., 2010; Winter et al., 2015; Messerschmid et al., 2021). These species show intermediate contributions of PEP carboxylation to the overall pool of carbon in the plant, indicating co-function of a C3 mode of photosynthesis and a CAM mode. The PEP carboxylation step discriminates less against 13CO2 than does Rubisco carboxylation; hence, as the PEPC contribution to the carbon pool in a plant increases, its δ13C value become less negative (Farquhar and Lloyd, 1993). Based on empirical relationships between δ13C and the fraction of daily carbon fixed at night by PEPC, Winter and Holtum (2002) predicted that plants having δ13C values less negative than −20 ‰ acquire >50 % of their photosynthate via CAM. The term ‘strong CAM’ refers to these plants (Edwards, 2019; Hancock et al., 2019; Sage et al., 2023). Strong CAM plants also have pronounced CAM anatomical traits, notably high leaf succulence, whereby chloroplast-containing cells (chlorenchyma) have large vacuoles, tight cell packing and low intercellular air space (Nelson and Sage, 2008; Borland et al., 2018; Edwards, 2019; Luján et al., 2022). Weak CAM plants are largely C3 in function, with low levels of succulence and low CAM activity (<5 % of daily C is acquired by CAM; Winter, 2019). Plants classified as C3 + CAM obtain 5–50 % of their C from nocturnal CO2 assimilation by PEPC and generally exhibit low to intermediate levels of succulence (Edwards, 2019; Winter, 2019; Luján et al., 2022).
To identify species using C4 or CAM photosynthesis, researchers in our laboratory, in collaboration with others, have been surveying δ13C values in plant families common to arid and semi-arid landscapes (for example, Boraginaceae, Frohlich et al., 2022; Nyctaginaceae, Khoshravesh et al., 2020). In recent years, we have surveyed the photosynthetic pathway in the Zygophyllaceae, where two distinct C4 lineages are identified, one in Tetreana simplex (= Zygophyllum simplex of the Zygophylloideae subgenus; Lauterbach et al., 2016) and the other in the Tribuloideae subfamily (Kallstroemia, Tribulus and Tribulopsis; Lauterbach et al., 2019). Results of our δ13C survey for subfamilies Larreoideae, Seetzenioideae and Morkillioideae of the Zygophyllaceae are presented here for the first time.
In this survey, we observed mostly C3-like δ13C values; however, leaf and stem samples from herbarium specimens of one Larreoideae species, Bulnesia retama (Gillies ex Hook. & Arn.) Griseb., consistently showed C4-like δ13C values between −16 and −19 ‰. Ecological studies also noted δ13C values between −17 and −24 ‰ in B. retama (Gatica et al., 2017). Bulnesia retama (common name retamo) is a xerophytic drought-deciduous shrub from dry areas of Argentina and Peru (Fig. 1A; Ribas-Fernández et al., 2009; Biruk et al., 2022). It forms green stems and is common if not dominant across its geographical range and produces a flush of small compound leaves during the summer rainy season (Crisci et al., 1979; Godoy-Bürki et al., 2018; Biruk et al., 2022). The leaves are deciduous, senescing at the end of the rainy season to yield an aphyllous stem canopy that persists throughout the dry season and is the source of retamo polishing and ink wax (Warth, 1956; Debandi et al., 2002). This habit represents an interesting variation in the function of xerophytic shrubs, in that it allows for relatively high rates of photosynthesis in the moist season, while continuing carbon assimilation into the dry season using water use-efficient stems (Nilsen, 1995; Smith et al., 1997). Smith et al. (1997) describe drought-deciduous shrubs (and trees) with green stems as being functionally intermediate between drought-evading deciduous shrubs and drought-resisting evergreen shrubs. They also note that deciduous xerophytic shrubs are largely C3 unless they exhibit succulence, in which case they may be CAM.
Fig. 1.
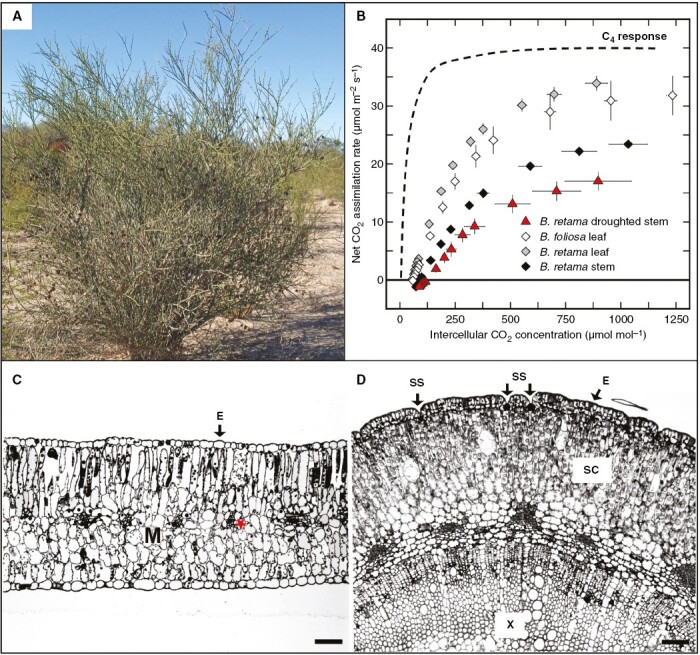
(A) A photograph of Bulnesia retama in its native habitat in La Rioja, northwestern Argentina, showing mostly leafless green stems (photograph by Peter Searles). (B) The net CO2 assimilation rate (A) vs. intercellular CO2 concentration (Ci) of well-watered Bulnesia foliosa leaves (white diamonds; C3), well-watered B. retama leaves and stems (grey and black diamonds, respectively) and moderately droughted B. retama stems (red triangles). Values are means ± s.e.m. Moderately droughted stems had a of −2.5 ± 0.1 MPa. Droughted leaves are not shown because B. retama is aphyllous under drought. The dashed line represents a well-watered C4A/Ci response from Tribulus cistoides. (C, D) Cross-sections of a B. retama leaf (C) and stem (D). Scale bars: 50 µm. Abbreviations: E, epidermis; M, mesophyll; red asterisk, bundle sheath cell; SC, stem cortex; SS, sunken stomata; X, secondary xylem.
The best-known species in the Larreoideae is the xerophytic evergreen shrub Larrea tridentata (creosote bush), an often-dominant C3 plant from the warm deserts across southwestern North America (Rundel and Sharifi, 1993). It is recognized as having some of the lowest water potentials measured in any drought-tolerant species and is considered a flagship species of the Mojave, Chihuahuan and Sonoran deserts (Smith et al., 1997; Supplementary Data Table S1). In South America, B. retama occupies many of the same habitats that L. tridentata does in North America, and it often co-occurs with other Larrea species, notably, the Southern Hemisphere counterpart to the creosote bush, termed Larrea divaricata (common name, jarillo or chaparral).
Less negative δ13C values, approaching −21 ‰, are often observed in C3 plants from dry climates (Ehleringer and Cooper, 1988; Ehleringer et al., 1998; Sage et al., 2007; Frohlich et al., 2022). In the Larreiodeae, Gatica et al. (2017) observed δ13C values in xerophytic Larrea and Prosopis shrubs in the B. retama habitat between −21 and −27 ‰. In L. tridentata, large variation in δ13C can occur, from −32 ‰ in wet soil to −21 ‰ in very dry conditions (Rundel and Sharifi, 1993). In C3 plants, the increase in δ13C values is largely attributable to high diffusive resistance between the atmosphere and the chloroplast stroma caused by low stomatal (gs) and mesophyll conductance (gm, the diffusive conductance between the intercellular air space and the chloroplast interior) relative to net CO2 assimilation rate (Farquhar et al., 1989; Seibt et al., 2008). Lower diffusive conductance relative to CO2 assimilation rate allows for proportionally more 13C to be fixed by Rubisco relative to 12C, increasing δ13C in leaf and stem tissue (Farquhar et al., 1989; Seibt et al., 2008). However, low conductance is rarely known to produce δ13C values less negative than −20 ‰ in C3 plants, and thus, such values commonly indicate the operation of a carbon-concentrating mechanism (Monson et al., 1988; Cerling et al., 1997; Winter and Holtum, 2002). For plants such as B. retama, δ13C values between −16 and −19 ‰ are strong indicators that either C4-like or CAM-like photosynthesis is a major source of carbon in the plant.
There are currently no experimental data evaluating the photosynthetic pathway of B. retama and no known instance of CAM within the Zygophyllaceae (Winter and Smith, 1996b; Gilman et al., 2023). In this study, we first present δ13C values from a survey of the Larreoideae, Morkillioideae and Seetzenioideae, then examine whether B. retama uses the C4 or CAM photosynthetic pathway by assessing leaf and stem gas exchange, leaf and stem anatomy, and dawn vs. dusk acid titrations of leaves and stems.
MATERIALS AND METHODS
Growth conditions
We grew six B. retama plants in 20 L pots filled with a sandy loam in the glasshouse at the University of Toronto. Plants grew from seed collected in April 2015 at a xeric site in La Rioja, Argentina (28°48ʹS, 66°56ʹW; elevation 1325 m; 150 mm annual rainfall) dominated mostly by xerophytic shrubs (B. retama, Larrea cuneifolia, Parkinsonia praecox and Senna aphylla) with interspersed Prosopis trees. The shrubs in the glasshouse were trimmed to remain ~1 m tall. Beginning in the spring of 2023, we also grew six Bulnesia foliosa plants from seed that was collected from plants in an abandoned roadside lot in La Rioja, Argentina; these plants were grown in 5 L pots of sandy loam. During the study period between July 2022 and July 2023, all plants were maintained in a glasshouse or plant growth chamber at approximately 30 °C day–25 °C night temperatures and with photosynthetic photon flux densities (PPFD) reaching 1600 µmol photons m−2 s−1 on sunny days (in the glasshouse), or 800 µmol m−2 s−1 at the top of the plant canopy (in the growth chamber). High-pressure sodium lamps in the glasshouse supplemented daily light intensities to maintain a constant photoperiod of 13 h over the year and a minimum PPFD of 250 µmol m−2 s−1 on cloudy days. Plants were fertilized weekly with a 50:50 mix of two commercial fertilizers (Plant-Products 21-7-7 Acid and 20-20-20 Classic; www.plantprod.com) supplemented with calcium nitrate and magnesium sulfate at Hoagland solution concentrations (Epstein, 1972). Three B. retama plants were drought treated and three served as well-watered controls.
Drought treatment and water potential measurements
The study consisted of two drought trials. In the first, pre-drought measurements started on 1 August and drought treatments began on 30 September 2022, using plants from the glasshouse. The water potential (Ψw) of stems with leaves (well-watered controls) or without leaves (drought treatments) was measured with a PMS model 600 pressure chamber (www.PMSinstruments.com) immediately before the start of the experimental dawn at 07:00 h. Experimental dawn was controlled by removing opaque tents over individual plants in the glasshouse at night, or by switching on lamps to begin the diurnal light cycle if plants were in growth chambers or the gas-exchange laboratory. The rate of pressure increase was approximately 0.5 MPa min−1, which avoided overshoot of the end point. The drought treatment initially consisted of withholding water for 2 weeks, during which shoot Ψw declined to −5.5 to −6 MPa. Gas exchange and titratable acidity were determined after 14 days of no water, when plants had a mean Ψw at dawn of −5.7 MPa. Because the plants exhibited drought injury (darkening and wilting of stems) and had minimal gas exchange at this time, we designated the measurements as the ‘extreme’ drought set and instituted a limited water regime of 500 mL applied to each pot every other day, with a 500 mL fertilizer solution added weekly. On the 21st day after initiation of the drought treatment (20 October 2022), we reassessed gas exchange and titratable acidity, when plants had a mean Ψw of −3.8 MPa. This was designated our ‘severe’ drought stress. Thereafter, the limited watering regime stabilized the plant Ψw between −2 and −3 MPa, and the plants appeared healthy in terms of having visibly turgid, green stems. This became our ‘moderate’ drought treatment and was designated as the principal drought stress for the duration of the study. Gas exchange and titratable acidity were re-measured on glasshouse-grown plants on 3 or 4 November 2022. Given that gas-exchange rates declined with the arrival of the short, cloudy days of winter, we moved the plants to a Biochamber GC-20 growth chamber (www.Biochambers.com) in January 2023 and repeated the diurnal measurements after the plants had acclimated to the growth chamber and exhibited net CO2 assimilation rates (A) similar to those observed in the glasshouse during the summer of 2022. The control and moderate drought-treated plants were maintained in the growth chamber. Growth chamber daylength was 13 h day–11 h night, with 30 °C days and 25 °C nights. After 14 days of acclimation in the chamber and full recovery of A, gas-exchange and titratable acidity measurements were repeated until the experiment ended on 2 March 2023, at which point all plants were fully rewatered and returned to the glasshouse.
The second drought trial began on 1 June 2023, by initiating a 15-day diurnal gas-exchange measurement on stems of two B. retama plants that were previously part of the non-droughted treatment. For the first 2 days, 1.5 L of water was supplied to maintain high Ψw. For the next 5 days (days 3–7), daily water was reduced in 250 mL increments per day from 1.5 L to 250 mL, which allowed pre-dawn Ψw of the plants to drop below −4 MPa by day 7. On day 7, daily water was increased to between 500 and 750 mL to bring Ψw to between −2 and −3 MPa until the end of the trial. After the 15-day diurnal measurements, we measured a 48-h diurnal response of stem gas exchange on droughted and well-watered stems of B. retama at 25 °C day–20 °C night to determine whether CAM increased in cooler temperatures. We also measured the CO2 assimilation rate (A), net intercellular CO2 partial pressure (Ci) and diurnal gas-exchange responses of non-droughted B. foliosa plants. Given that no C4 or CAM activity was evident, B. foliosa served as our C3 comparison.
Gas exchange
Gas-exchange measurements were conducted on leaves and stems using a LI-6400XT system with a 6 cm2 leaf chamber (LI-COR Biosciences, Lincoln, NE, USA; www.licor.com). About 1.5 cm2 of recent, fully expanded leaves, or two to three stems of 2 cm length were measured, with measured stem regions being two to three nodes below the shoot apex. Leaves were removed when measuring stem photosynthesis, and stems were excluded from the gas-exchange chamber during leaf measurements. All daytime gas-exchange measurements were conducted at light saturation (1000 µmol m−2 s−1). Before and during drought, the response of net CO2 assimilation rate (A) to variation in intercellular CO2 partial pressure (Ci) was measured as described by Adachi et al. (2023). The diurnal response of gas exchange was measured at 400 µmol CO2 mol−1 air and 30 °C over a 13-h day period and 25 °C over an 11-h night period. Measurements began at noon and continued for 24 h in trial 1, and for 15 days in drought trial 2. To simulate dusk during drought trial 1, we decreased PPFD by 200 µmol m−2 s−1 and temperature by 1 °C every 30 min in a ramp down to darkness. At the simulated dawn, temperature and light were increased at the same rate. In trial 2, the end-of-day ramp to darkness was changed to a 200 μmol m−2 s−1 and 1 °C decrease every 15 min, and the rate of ramp to full illumination in the morning was changed to a 200 μmol m−2 s−1 and 1 °C increase every 15 min. Leaf and stem area used for gas exchange were digitally imaged, with the projected area in two dimensions being determined using ImageJ image analysis software (Schneider et al., 2012; https://imagej.net/).
Titratable acidity
We sampled ~1.5 cm2 of leaf or 2 cm stem segments at 16:00 h (when tissue acidity was minimal) and at the experimental dawn (07:00 h, when tissue acidity would be at its peak) for determination of titratable acidity (Heyduk et al., 2019). Tissue sampling followed criteria used for selecting gas-exchange samples. After sampling, the tissue was frozen in liquid N2 and stored at −80 °C until assay. Immediately upon removal from the freezer and before tissue could thaw, we weighed the samples for fresh weight. Samples were then placed in 60 mL of 20 % EtOH, boiled to half volume, returned to 60 mL with dH2O, boiled to half volume again, then returned to 60 mL with dH2O again and allowed to cool to room temperature. Once cooled, we titrated the solution to pH 7.0 using 2 mm NaOH and calculated titratable acidity as follows:
Anatomy
Recently matured leaf and stem tissue was sampled for light microscopy between 08:00 and 10:00 h. Tissue was fixed in a 2 % glutaraldehyde solution in 0.2 m sodium cacodylate buffer (pH 7.4) and post-fixed in a 2 % osmium tetroxide solution. Samples were then dehydrated in 10 % EtOH increments and embedded in Araldite 502 resin (https://www.emsdiasum.com/). Embedded tissues were sectioned at 3 µm, stained with Toluidine Blue (O’Brien and McCully, 1981), and imaged using a Zeiss Axioplan microscope (https://www.zeiss.com/) with an image analysis system (model DP71, Olympus: EMPIX Imaging). The planar cross-sectional cell area of stem cortical or leaf mesophyll cells was quantified, as was the percentage of intercellular air space (% IAS) of leaf mesophyll or stem cortical tissue. For cross-sectional cell area, ten cells per leaf were measured randomly from palisade and spongy mesophyll from four leaves of each of four plants and from ten cortex cells of six stems from each of six plants. The % IAS was estimated using a point intercept method (Parkhurst, 1982). Images were analysed using a Cintiq graphics tablet (https://www.wacom.com/) and ImageJ software (Schneider et al., 2012; https://imagej.net/).
δ13C assays
For δ13C assays, herbarium specimens of the Larreoideae were sampled at Kew Gardens or the Missouri Botanical Gardens (for voucher specimens and raw δ13C values, see Supplementary Data Table S2). We also sampled live tissue from our plants grown in the glasshouse in Toronto and fruits with seeds of the source material collected in La Rioja, Argentina. Typically, δ13C values were assayed on 0.5–2 mg leaf or stem tissue using mass spectroscopy at the University of California, Davis stable isotope facility (before 2015, Stable Isotope Facility (ucdavis.edu)) or the Washington State University stable isotope facility (after 2014; https://labs.wsu.edu/isotopecore/). None of the species assayed for δ13C other than B. retama and B. foliosa was grown for physiological and anatomical analyses.
RESULTS
δ13C survey
The δ13C values for 70 specimens of 30 Larreoideae, Morkillioideae and Seetzenioideae species showed that all but one of the species exhibited C3-like mean δ13C values (Table 1; Supplementary Data Table S2). The exception is B. retama, for which herbarium specimens (largely stems) from five distinct B. retama collections and from the fruits and seeds from plants collected near La Rioja, Argentina, had δ13C values between −16.1 and −18.9 ‰ (mean ± s.e.m. of −17.4 ± 0.4 ‰). All other Bulnesia species had δ13C values ranging between −23.0 and −27.3 ‰. Notably, B. retama leaf and stem samples produced in the glasshouse in Toronto had C3-like δ13C values between −27 and −31.5 ‰ (Table 1), in contrast to the material collected in the native habitat, including the seeds from which these plants were grown. Of the non-Bulnesia species, two from the Larreoideae, Pintoa chilensis and Porlieria arida, had mean δ13C values of −22.3 and −22.5 ‰, respectively, which are at the least negative end of the C3 range.
Table 1.
δ13C values from species within Zygophyllaceae subfamilies Larreoideae, Seetzenioideae and Morkillioideae.
| Taxa | δ13C (‰) | Taxa | δ13C (‰) |
|---|---|---|---|
| Larreoideae | |||
| Porlieria clade (6/6) | |||
| Porlieria | Bulnesia retama | ||
| angustifolia | −24.3 ± 1.1, n = 2 | Glasshouse-grown leaf | −31.4 ± 0.3, n = 10 |
| arida | −22.5 ± 1.2, n = 2 | Glasshouse-grown stem (pre-drought) | −28.0 ± 0.2, n = 5 |
| chilensis | −27.3, n = 1 | Glasshouse-grown stem (droughted) | −28.5, n = 1 |
| hygrometra | −25.9 ± 1.8, n = 2 | Glasshouse-grown stem (control plants, no drought) | −27.5, n = 1 |
| lorentzii | −26.0 ± 0.6, n = 2 | ||
| microphylla | −28.0 ± 0.5, n = 2 | ||
| Guaiacum clade (1/5) | B. schickendanzii | −23.0 ± 0.05, n = 2 | |
| Guaiacum coulteri | −27.7, n = 1 | Pintoa chilensis | −22.3 ± 1.0, n = 2 |
| Plectrocarpa clade (2/2) | Larrea clade (1/5) | ||
| Plectrocarpa | Larrea nitida | −24.6 ± 0.5, n = 2 | |
| rougesii | −23.6, n = 1 | ||
| tetracantha | −25.7, n = 1 | ||
| Gonopterodendron clade (3/4) | |||
| Gonopterodendron | Note: Gatica et al. (2017) show that L. cuneifolia and L. divaricata plants in the field have C3-like δ13C values between −22 and −27 ‰ over a year. Larrea tridentata show a C3-like δ13C of −21 to −31 ‰ (Philpottt and Troughton, 1974; Ehleringer and Cooper, 1988; Rundel and Sharifi, 1993). | ||
| aboreum | −25.1 ± 0.7, n = 4 | ||
| carrapo | −27.6, n = 1 | ||
| sarmientoi | −25.5 ± 0.7, n = 3 | ||
| Bulnesia s.s. Pintoa–Metharme clade (5/6) | Seetzenioideae (2/2) | ||
| Bulnesia | Seetzenia | ||
| chilensis | −23.3, n = 1 | lanata | −27.2 ± 0.4, n = 2 |
| foliosa field-collected fruit | −26.5 ± 0.4, n = 3 | orientalis | −27.0 ± 0.1, n = 2 |
| foliosa glasshouse-grown leaf | −30.5 ± 0.7, n = 3 | Morkillioideae (3/5) | |
| foliosa glasshouse-grown stem | −30.2 ± 0.1, n = 2 | Morkillia mexicana | −24.4, n = 1 |
| retama from herbaria | −17.5 ± 0.7, n = 5 | Sericodes greggi | −24.2, n = 1 |
| retama fruit wing (field collected) | −17.3 ± 0.5, n = 3 | Viscainoa geniculata | −24.4 ± 0.02, n = 2 |
Taxonomy follows Godoy-Bürki et al. (2018) and Tropicos (www.Tropicos.com). Numbers in parentheses following a clade name indicate the number of species sampled/present in the clade. In total, 23 of 35 species (66 %) in these three subfamilies are sampled. For sample source and voucher information, see Supplementary Data Table S2.
Leaf and stem anatomy
Bulnesia retama leaves and stems lack anatomical traits associated with C4 or strong CAM photosynthesis (Fig. 1C, D; Table 2). Ground tissue of the dorsiventral leaves was composed of elongate adaxial and abaxial palisade chlorenchyma, with the abaxial cells being shorter and wider. Numerous chlorenchyma cells were between bundle sheath cells, which lacked prominence in cross-section, with each bundle sheath cell having chloroplasts along its outer periphery as occurs in C3 species (Fig. 1C; Dengler and Nelson, 1999; Stata et al., 2014, 2016). Cylindrical stems of B. retama were composed of an epidermis with a thick waxy cuticle and sunken stomata, a cortex of chlorenchyma cells, and secondary xylem and phloem (Fig. 1D). Leaf mesophyll and stem cortex cells were not obviously succulent, being smaller than chlorenchyma cells of C3 plants and well below the cross-sectional area of CAM chlorenchyma cells (Table 2; Nelson et al., 2005). Stems exhibited a % IAS that was 7.5 % of the planar area of the cortex tissue in cross-section, below the mean % IAS of photosynthetic tissues in CAM plants (Fig. 1D; Table 2). In contrast, the % IAS of B. retama leaf mesophyll was similar to C3 values in the study by Nelson et al. (2005; Table 2).
Table 2.
The size of photosynthetic cells and relative intercellular air space in Bulnesia retama.
| Organ | Tissue region | Area per cell (µm2) | Relative IAS (%) |
|---|---|---|---|
| Leaf | Palisade | 1311 ± 68a | 26.0 ± 2.3a |
| Spongy | 1161 ± 79a | ||
| Stem | Cortex | 597 ± 31b | 8.2 ± 0.6b |
| Mean cell size and relative IAS (±s.e.m.) from Nelson et al. (2005) | |||
| Organ | Tissue region | Area per cell (µm2) | Relative IAS (%) |
| CAM leaf (n = 18 species) | Mesophyll | 3260 ± 550 | 14.8 ± 1.7 |
| C3 leaf (n = 6 species) | Mesophyll | 840 ± 230 | 31.6 ± 4.9 |
| C4 leaf (n = 4 species) | Mesophyll | 620 ± 150 | 28.7 ± 5.5 |
Cell size is estimated as cross-sectional area in leaf or stem. The percentage of intercellular air space (% IAS) is the percetnage area of the IAS in planar cross-sections divided by the corresponding area of leaf mesophyll or stem cortical tissue. Values are the mean ± s.e.m. Superscripted letters indicate statistically different groups at P < 0.05 via one-way ANOVA and a Tukey's post-hoc test. For comparison, leaf mesophyll cell area in cross-section and % IAS are shown for CAM, C3 and C4 values from Nelson et al. (2005).
Gas-exchange measurements
Drought-treated B. retama plants lost all leaves 7–10 days after water cessation and relied completely on stem photosynthesis in the droughted state. Plants exhibited symptoms of severe drought stress at a Ψw near −4 MPa, such as darkening of stems and desiccation of stem tips, but appeared healthy, with green, turgid stems at a Ψw of −2 to −3 MPa, which we designated as our moderate drought treatment. Under moderate drought, B. retama did not grow new shoots.
Before and during the drought treatment, the responses of net CO2 assimilation rate (A) vs. intercellular CO2 (Ci) in leaves and stems of B. retama were assessed to determine whether any tissue conducted C4 photosynthesis. No evidence of C4 photosynthetic behaviour was detected, and all A/Ci responses were typical of C3 photosynthesis (Fig. 1B). CO2 compensation points (Γ) were 55 ± 1 µmol mol−1 (n = 5) in leaves and 90 ± 4 µmol mol−1 (n = 6) in stems, rather than near 0 µmol mol−1 as they would be if C4 photosynthesis occurred. The A/Ci response of moderately droughted B. retama stems was also C3-like (Γ = 126 ± 16 μmol mol−1, n = 3), although A was reduced by the stress (Fig. 1B). Stem Γ is likely to be high owing to a large respiratory signal from heterotrophic vascular tissue (Kocurek et al., 2020). The initial slope of the A/Ci responses in B. retama stems and leaves was well below the initial slope of the C4 plant Tribulus cistoides, as is typical for C3 vs. C4 photosynthesis (Sage and Pearcy, 2000). In common with A/Ci responses of C3 plants, the A/Ci response of B. retama leaves and stems and B. foliosa leaves did not show saturation until high Ci values >600 µmol mol−1 (Fig. 1B).
In B. retama leaves, diurnal gas-exchange responses showed no net nocturnal CO2 fixation; however, weak CAM activity was indicated by a slight curvature in the nocturnal response of A, whereby A became less negative in the middle of the night (Fig. 2A; Winter and Holtum, 2015). Daytime A in B. retama leaves exceeded 12 µmol m−2 s−1 , with typical C3-like Ci/Ca ratios between 0.7 and 0.8 (Fig. 2A–C; Table 3). These responses are similar to those observed in the C3 species B. foliosa, except that its nocturnal A response was C3-like, with little curvature (Fig. 2G–I).
Fig. 2.
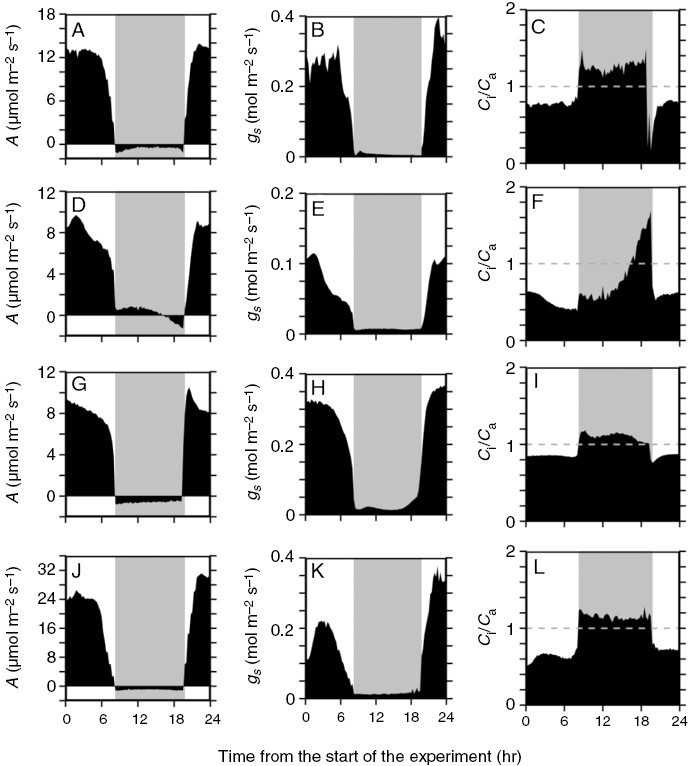
Diurnal gas-exchange responses of well-watered Bulnesia retama leaves and stems, and from representative C3 and C4 species grown in the University of Toronto glasshouse facility. Plots show representative 13 h light and 11 h dark measurements of net CO2 assimilation rate (A; A, D, G, J); stomatal conductance (gs; B, E, H, K) and the ratio of intercellular to ambient CO2 partial pressure (Ci/Ca; C, F, I, L) in a well-watered B. retama leaf (A–C) and stems (D–F), a well-watered Bulnesia foliosa leaf (C3; G–I) and a well-watered Tribulus cistoides leaf (C4; J–L). Experiments began at 12:00 h. Note dissimilar y-axes and positive nocturnal CO2 assimilation rates in B. retama stems (panel D). The grey dashed line in the Ci/Ca plots indicates 1.0. Grey shading on each panel indicates the nocturnal period.
Table 3.
Maximum day and night values of net CO2 assimilation rate (A) in Bulnesia retama, with the corresponding stomatal conductance (gs) and Ci/Ca plus late afternoon (18:00) values of A, gs and Ci/Ca.
| Plant | Maximum values | Values 6 h after start of experiment (18:00 h) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | Night | ||||||||
|
A
(μmol m−2 s−1) |
g
s
(mol m−2 s−1) |
C i/Ca |
A
(μmol m−2 s−1) |
g
s
(mol m−2 s−1) |
C i/Ca |
A
(µmol m−2 s−1) |
g
s
(mol m−2 s−1) |
C i/Ca | |
| From Fig. 2 (glasshouse) | |||||||||
| Bulnesia retama leaf (Fig. 2A–C) |
13.92 | 0.38 | 0.82 | −0.26 | 0.06 | 1.15 | 11.84 | 0.25 | 0.77 |
| Bulnesia retama stem (Fig. 2D–F) |
9.71 | 0.11 | 0.61 | 0.87 | 0.08 | 0.52 | 6.84 | 0.05 | 0.42 |
| Bulnesia foliosa leaf (C3) Fig. 2G–I) |
10.51 | 0.37 | 0.88 | −0.41 | 0.04 | 1.18 | 7.61 | 0.26 | 0.84 |
| Tribulus cistoides (C4) (Fig. 2J–L) |
31.18 | 0.28 | 0.41 | −0.75 | 0.02 | 1.10 | 19.23 | 0.14 | 0.34 |
| From Fig. 3 (growth chamber) | |||||||||
| Well-watered B. retama stem (Fig. 3A, C, E) | 8.04 ± 2.33 | 0.11 ± 0.02 | 0.65 ± 0.31 | 0.42 ± 2.32 | 0.01 ± 0.02 | 0.77 ± 0.32 | 7.43 ± 0.52 | 0.07 ± 0.02 | 0.52 ± 0.05 |
| Moderate drought B. retama stem (Fig. 3B, D, F) | 8.21 ± 2.07 | 0.09 ± 0.02 | 0.58 ± 0.45 | 0.60 ± 1.99 | 0.01 ± 0.02 | 0.71 ± 0.45 | 8.04 ± 0.56 | 0.08 ± 0.02 | 0.56 ± 0.002 |
| From Fig. 4 (glasshouse) | |||||||||
| Well-watered B. retama stem (Fig. 4A–C) | 7.83 | 0.10 | 0.67 | −0.14 | 0.003 | 1.18 | 4.19 | 0.04 | 0.52 |
| Moderate drought B. retama stem (Fig. 4D–F) | 8.97 | 0.13 | 0.67 | −0.13 | 0.001 | 0.34 | 5.83 | 0.04 | 0.40 |
| Severe drought B. retama stem (Fig. 4G–I) | 0.50 | 0.01 | 0.71 | 0.68 | 0.002 | – | 0.22 | 0.01 | 0.80 |
| Severe drought B. retama stem (Fig. 4J–L) | −0.01 | 0.01 | 0.97 | −0.09 | 0.004 | 1.06 | −0.33 | 0.004 | 1.33 |
In the well-watered state, B. retama stems from the first drought trial often showed slight positive A early in the dark period, which became negative towards the end of the night (Figs 2B and 3A). Other stems exhibited slightly negative values of A in the early to middle of the night, after which A drifted more negative as dawn approached (Fig. 4A). In moderately droughted stems, A peaked near 8 µmol m−2 s−1 during the daytime in plants of the first drought trial (Figs 3B and 4D; Table 3). At night, A in these stems was slightly positive (Fig. 3B) or near zero (Fig. 4D) in the early to the middle of the night, after which it drifted negative. In severely stressed stems (Ψw of −3.4 to −4.5 MPa), daytime A fell to near 0 μmol m−2 s−1, whereas nocturnal A showed initial positive rates which became negative late in the night (Fig. 4G, H). During extreme stress (Ψw of −5.9 MPa), B. retama stems showed negative A and minimal gs in both day and night; however, the night response of A was curved, rising to near 0 µmol m−2 s−1 at ~20.00 h, then declining after midnight (Fig. 4J, K).
Fig. 3.
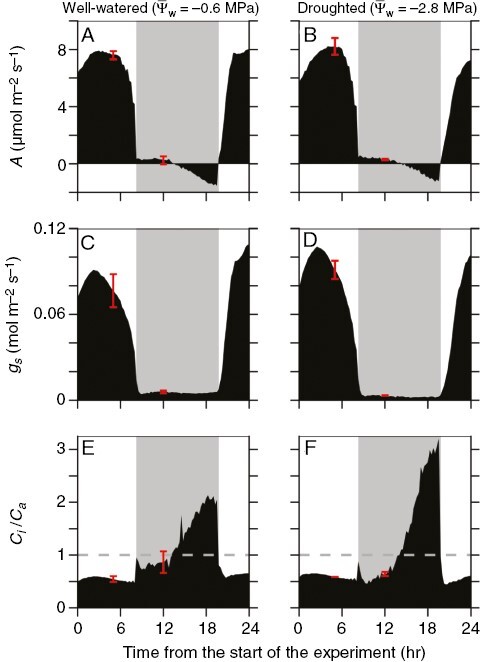
Diurnal gas-exchange response of well-watered and moderately droughted Bulnesia retama stems grown in a plant growth chamber at the University of Toronto growth facility. The plots show mean net CO2 assimilation rate (A; A, B), stomatal conductance (gs; C, D) and the ratio of intercellular to ambient CO2 partial pressure (Ci/Ca; E, F) during a 13 h light–11 h dark diurnal cycle for well-watered (A, C, E) and moderately droughted (B, D, F) B. retama stems from a growth chamber. Experiments began at 12:00 h. Mean value of well-watered plants is −0.6 ± 0.03 MPa and for droughted plants −2.8 ± 0.3 MPa (n = 3). Red bars indicate representative s.e.m. values. The grey dashed line in the Ci/Ca panels indicates 1.0. Grey shading in each panel indicates the nocturnal period.
Fig. 4.
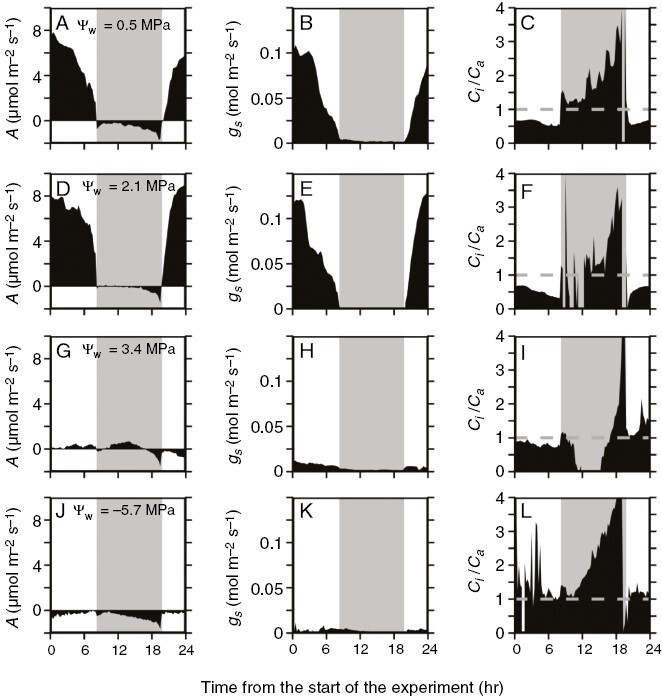
Diurnal gas-exchange responses of glasshouse-grown Bulnesia retama stems experiencing varying degrees of drought. Plots show responses over a 13 h light–11 h dark period in well-watered and droughted B. retama stems from glasshouse-grown plants. The responses shown are net CO2 assimilation rate (A; A, D, G, J), stomatal conductance (gs; B, E, H, K) and the ratio of intercellular to ambient partial pressure of CO2 (Ci/Ca; C, F, I, L) in stems of glasshouse-grown B. retama plants. (A–C) Responses of well-watered plants. (D–F) Responses of moderately droughted stems. (G–I) Responses of severely droughted plants. (J–L) Responses during extreme drought. Experiments began at 12:00 h. Each response is from one representative plant. The grey dashed line in the Ci/Ca plots indicates 1.0. Grey shading in each panel indicates the nocturnal period.
In the 15-day diurnal response of drought trial 2, A peaked near 8 µmol m−2 s−1, then declined following water restriction, with low rates (nil to 1.5 µmol m−2 s−1) present during severe drought on days 5–7 and with rates between 1 and 4 µmol m−2 s−1 on days 9–11 during moderate drought (Fig. 5A; Supplementary Data Table S3). Despite variation in daytime A, stems exhibited curved nocturnal responses of A as observed in watered to droughted plants of trial 1, in that A values were slightly positive or, if negative, were near 0 µmol m−2 s−1 in the early to middle of the night, then declined late in the dark period (Fig. 5A). On some nights, particularly when A was positive, the negative drift in A as dawn approached appeared pronounced (days 11–15 in Fig. 5A).
Fig. 5.
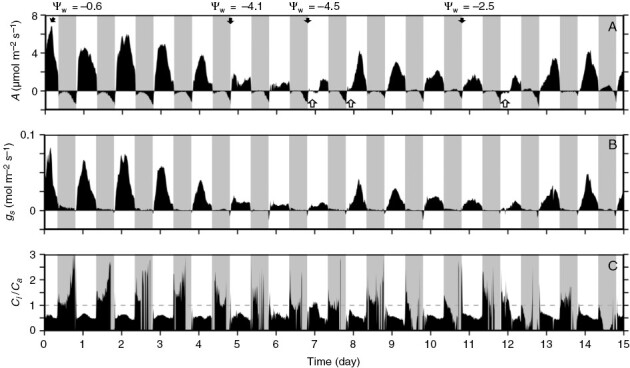
Diurnal gas-exchange responses of Bulnesia retama stems over a 15-day period, during which severe and then moderate drought treatments were imposed on initially well-watered plants. Diurnal net CO2 assimilation rate (A), stomatal conductance (gs) and the ratio of intercellular to ambient CO2 partial pressure (Ci/Ca) over a 15-day period of controlled water stress with 13 h light–11 h dark photoperiod from one representative glasshouse-grown B. retama plant in drought trial 2. The experiment began at 12:00 h on day 0. The plant was given water each day at 10:00 h; 1.5 L of water on the first 2 days, decreasing to 250 mL over 5 days in 250 mL increments on each day until day 7, and 500–750 mL thereafter. White arrows indicate days when the plants received an additional 250 mL above the regular 500 mL of water owing to low photosynthetic rates indicative of drought stress. Black arrows represent plant values measured at 07:00 h, which are indicated in megapascals. The grey dashed line in the Ci/Ca panel indicates 1.0. See Supplementary Data Table S3 for summaries of gas-exchange values at 0:00, 10:00, 12:00, 14:00 and 18:00 h. Grey shading indicates the mocturnal periods.
CAM activity did not increase in B. retama stems in cooler temperatures of 25°C day and 20°C night temperature (Supplementary Data Fig. S1), as occurs in some CAM species (Lüttge, 2004).
We estimated the contribution of nocturnal CO2 fixation to the daily carbon budget in B. retama stems, as described in the legend to Table 4, to provide a rough idea of CAM contributions to daily carbon gain (Table 4). In well-watered stems, 12–14 % of the daily carbon gain is estimated to result from nocturnal carbon acquisition, whereas in moderately droughted stems, 13–25 % of the daily carbon gain is estimated to be acquired by CAM at night. In the severe or extremely droughted stems, the gross carbon assimilation at night shows little change compared with moderately droughted stems, but with the large reduction in carbon gain during the day, the nocturnal contribution by CAM rises to an estimated 42–49 % of the daily carbon gain (Table 4).
Table 4.
Estimations of diurnal and nocturnal net and gross CO2 assimilation in Bulnesia retama stems, and the proportion of gross CO2 assimilation acquired at night.
| Condition | Net CO2 assimilation | |||||
|---|---|---|---|---|---|---|
| Integrated net CO2 assimilation (mmol m−2 day−1) |
Integrated gross CO2 assimilation (mmol m−2 day−1) |
|||||
| Light period (13 h) |
Dark period (11 h) |
Light period (13 h) |
Dark period (11 h) |
%D | Estimated respiration rate (µmol m−2 s−1) |
|
| Growth chamber, Fig. 3 | ||||||
| Well watered | 300 ± 18 | −10 ± 11 | 371 ± 10 | 52 ± 15 | 12 | −1.6 ± 0.3 |
| Moderate drought | 282 ± 31 | −3 ± 3 | 344 ± 32 | 52 ± 4 | 13 | −1.4 ± 0.04 |
| Glasshouse, Fig. 4 | ||||||
| Well watered | 213 | −21 | 290 | 47 | 14 | −1.7 |
| Moderate drought | 292 | −7 | 358 | 52 | 13 | −1.5 |
| Severe drought | −0.3 | 6 | 67 | 65 | 49 | −1.5 |
| Extreme drought | −13 | −22 | 61 | 44 | 42 | −1.7 |
| Glasshouse, Fig. 5 | ||||||
| Well watered, day 1 | 187 | −17 | 245 | 39 | 14 | −1.4 |
| Moderate drought, day 10 | 58 | −2 | 91 | 31 | 25 | −0.8 |
Growth chamber values are means ± s.e.m. Glasshouse values are derived from individual plants. Daily CO2 assimilation was calculated as the area under the curve from measurements of net CO2 assimilation rate in Figs 3A, B, 4A, D, G, J and 5A. To estimate gross CO2 assimilation, dark respiration rate was estimated at the end of the dark period and was assumed to be constant over day and night intervals. The respiration rate was then integrated over the 13 h day period and 11 h night periods and added to the day and night integrated CO2 assimilation values. %D is the proportion of 24 h gross CO2 assimilation acquired during the dark period and is calculated as: .
In well-watered and moderately droughted stems of trials 1 and 2, pronounced declines in gs began in the early to middle of the afternoon (Figs 2E, 3C, D, 4B, E and 5B). This decline was associated with a reduction in Ci/Ca values from >0.6 to ≤0.5 (Figs 2F, 3E, F, 4C, F and 5C; Table 3; Supplementary Data Table S3). For example, in moderately droughted B. retama stems grown in the glasshouse, the midday Ci/Ca of 0.67 declined to 0.42 at 18.00 h. The decline in Ci/Ca indicates greater stomatal limitation reduces A in the later afternoon.
Titratable acidity
Nocturnal acid accumulation (ΔH+) in B. retama stems was modest in stems of well-watered plants [mean of 22.4 ± 10.6 μmol H+ g−1 fresh weight (FW)] and increased to a mean of 98.6 ± 23.7 μmol H+ g−1 FW during moderate drought (Fig. 6; Table 5). In severe to extreme drought, ΔH+ in stems was not significant. Values of ΔH+ became negative below a Ψw of −4 MPa (Fig. 6). Leaf ΔH+ was insignificant in well-watered and moderately droughted plants, although there was near significance in the ΔH+ of droughted leaves (P = 0.8), suggesting an increase in titratable acidity at night immediately before their senescence (Table 5).
Fig. 6.
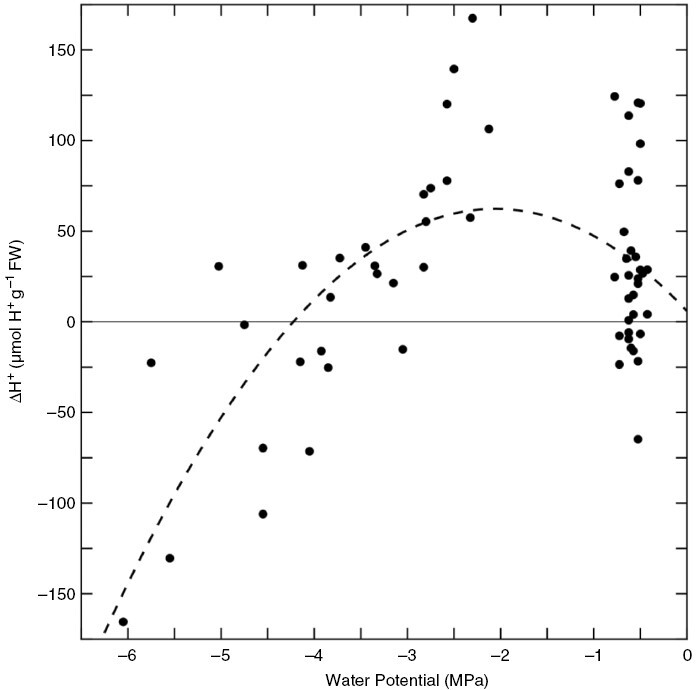
The difference in titratable acidity (ΔH+) of stem tissue sampled at 07:00 and 14:00 h vs. water potential at 07:00 h in Bulnesia retama stems. Each point is a single ΔH+ for paired stems sampled on the same day. The dashed line represents a best-fitting polynomial regression (y = −13.3 x2 − 54.8x + 5.9; R2 = 0.41, P < 0.001) for the slope value.
Table 5.
Titratable acidity in Bulnesia retama plants at varying drought intensity.
| Organ | Water potential | Titratable acidity (µmol H+ g−1 FW) |
|||
|---|---|---|---|---|---|
| w (MPa) | Afternoon | Dawn | ΔH+ | P-value | |
| Measured values - l eaf | |||||
| Well watered | −0.6 ± 0.01 | 89.1 ± 6.6 | 86.3 ± 6.1 | −2.8 ± 8.9 | P = 0.62 |
| Moderate drought | −2.6 ± 0.1 | 91.3 ± 7.4 | 138.1 ± 15.0 | 46.9 ± 22.2 | P = 0.08 |
| Measured values - s tem | |||||
| Well watered | −0.6 ± 0.01 | 114.6 ± 11.4 | 137.0 ± 21.8 | 22.4 ± 10.6 | P = 0.046 |
| Moderate drought | −2.4 ± 0.1 | 131.0 ± 20.9 | 229.7 ± 43.0 | 98.6 ± 23.7 | P = 0.013 |
| Severe drought | −3.5 ± 0.2 | 148.5 ± 21.4 | 159.2 ± 26.6 | 10.6 ± 12.2 | ns |
| Extreme drought | −5.0 ± 0.3 | 224.2 ± 35.4 | 154.9 ± 21.3 | −69.3 ± 41.3 | ns |
| Literature values | Mean ± s.d. | ||||
| Agave americana | – | 18.1 ± 4.6 | 331.1 ± 28.9 | 313 | – |
| Calandrinia flava | |||||
| Watered | – | – | – | 13.1 ± 2.0 | – |
| Droughted | – | – | – | 27.6 ± 2.2 | – |
| Re-watered | – | – | – | 24.3 ± 7.0 | – |
| Calandrinia volubilis | |||||
| Watered | – | – | – | 3.2 ± 1.9 | – |
| Droughted | – | – | – | 82.2 ± 11.6 | – |
| Re-watered | – | – | – | 23.8 ± 2.7 | – |
Titratable acidity of leaf or stem tissue was determined in the afternoon (16:00 h) and at dawn (07:00 h). Values are the mean ± s.e.m. (n = 3 plants per treatment). Differences between ΔH+ means were tested using Student’s one-tailed t-test, where all ΔH+ values from a given plant were pooled to give one value per plant for the category of water status shown. For comparison, values from Winter and Smith (2022) for strong CAM Agave americana (Asparagaceae) and from Hancock et al. (2019) for C3 + CAM Calandrinia flava and C. volubilis (Montiaceae) are shown. FW is fresh weight.
DISCUSSION
Our results demonstrate that B. retama stems largely use C3 photosynthesis during the day but exhibit a weak CAM cycle at night that is strengthened during drought. Leaves largely function in a C3 mode but might have weak CAM as indicated by slight curvature in the nocturnal response of A (Winter and Holtum, 2015), with a near-significant enhancement of nocturnal acidity when droughted. Because the leaves senesce when droughted, any enhancement of leaf CAM as soils dry would probably be of little consequence to annual carbon gain. There is no evidence of C4 photosynthesis in B. retama, because plants had high Γ values and relatively low initial slopes of the A/Ci response and showed no evidence of C4 anatomy, such as enlarged, organelle-enriched bundle sheath cells (Dengler and Nelson, 1999; Voznesenskaya et al., 2005; Sage et al., 2013). Previously, δ13C values between −17 and −24 ‰ were observed at the beginning and end of the growing season in stems of B. retama located in the Monte Desert of Argentina, suggesting a variable photosynthetic strategy over the growing season (Gatica et al., 2017). We also found evidence of a variable strategy, because leaves and stems from well-watered plants grown in Toronto exhibited C3 δ13C values between −27.5 and −31.5 ‰, while the fruits produced by the parents of these plants in their Argentinian habitat had a δ13C average of −17.4 ‰. Large shifts in δ13C are documented in non-succulent, xerophytic C3 shrubs grown in moist vs. dry conditions; for example, in L. tridentata, δ13C values range between −32 and −21 ‰; however, it is rare for δ13C to be less negative than −21 ‰ in C3 species (Philpott and Troughton, 1974; Rundel and Sharifi, 1993; Ehleringer et al., 1998; Gatica et al., 2017). We therefore suspected facultative CAM in stems as a possible explanation for the high δ13C in field-grown B. retama. In the discussion below, we consider the evidence for CAM photosynthesis in B. retama along with other mechanisms that could explain its less negative δ13C in the field.
We observed that net CO2 assimilation rate (A) in B. retama stems is often positive early in the night, consistent with a weak CAM phase I. It drifted negative later during the dark period in a pattern observed in weaker C3 + CAM species, such as those in Calandrinia, Cistanthe, Pilea and Jatropha (Winter and Holtum, 2015; Holtum et al., 2017, 2021; Winter et al., 2020). We also observed cases where A was slightly negative early in the night in B. retama stems, in which case the values of A also drifted more negative towards the end of the night. This is a pattern known as cryptic CAM and is observed in species such as Jatropha curcas and Pilea peperomioides (Winter and Holtum, 2015; Winter et al., 2020). In B. retama, this pattern was typical in well-watered stems, indicating weak CAM activity. Our estimated gross CO2 fixation contribution at night of 12–25 % in watered to moderately droughted plants (Table 4) qualifies B. retama as a weak to moderate C3 + CAM species rather than a cryptic CAM species (Winter, 2019). Low nocturnal CO2 fixation in B. retama stems could be explained, in part, by low tissue succulence of the cortical cells, which would not provide much volume for storing malate (Borland et al., 2018). CAM plants with low storage potential tend to fill their vacuoles with organic acids earlier in the night, at which time the nocturnal rate of CO2 fixation by PEP carboxylase begins to decline, and the estimated A value drops as stem respiration increasingly dominates the net flux of CO2 (Winter and Holtum, 2015; Holtum et al., 2017, 2021; Borland et al., 2018). Such a decline was observed commonly in the nocturnal gas exchange of B. retama.
Titratable acidity in well-watered stems of B. retama is greater at dawn relative to late afternoon, with moderate drought increasing nocturnal acid accumulation (ΔH+). In a survey of CAM in Calandrinia, well-watered plants exhibited ΔH+ < 20 µmol H+ g−1 FW, slightly below the mean ΔH+ of 22 µmol H+ g−1 FW we observed in well-watered B. retama stems. In droughted leaves of Calandrinia volubulis, ΔH+ rose to a mean of 82 µmol H+ g−1 FW, leading to its designation as a facultative CAM plant (Table 5; Hancock et al., 2019). By this criterion, droughted B. retama stems would approximate facultative CAM behaviour, given that their mean ΔH+ was 99 µmol H+ g−1 FW. This shift in ΔH+ was often associated either with less negative values of A at night or with the appearance of positive values of A during the early to mid-nocturnal period, in droughted relative to well-watered stems of B. retama. Consistently, when integrated over the dark period, the net C flux was less negative in moderately droughted compared with well-watered B. retama stems and became positive in the severely droughted stems during trial 1 (Table 4). Owing to variable dark respiration estimates, we could not detect consistent patterns in the gross CO2 assimilation rate at night. By assuming that the stem respiration rate measured immediately before dawn was constant throughout the diurnal cycle, we estimated that dark CO2 fixation contributed 13–25 % of the daily carbon gain (Table 4), leading us to consider B. retama a moderate C3 + CAM plant when its CAM potential is induced fully. Consistent with a moderate CAM designation, the relative CAM contribution increased to near 50 % in severely droughted plants; however, this was attributable to large reductions in daytime A, rather than a strengthening of CAM activity.
The results presented here are the first to document CAM in Bulnesia, the Larreoideae, the Zygophyllaceae and the order Zygophyllales. This discovery raises the number of CAM plant families to 38 in 17 orders of terrestrial plants (Gilman et al., 2023). We suspect that CAM is more widespread in the Zygophyllaceae, because several species have δ13C values less negative than −23 ‰. We observed that the Larreoideae species Pintoa chilensis had a mean δ13C of −22.3 ‰ and that in Porlieria arida it was −22.5 ‰. The Zygophylloideae, in particular, have numerous species with δ13C values less negative than −21 ‰ (in addition to the C4 plants in the Tetraena simplex complex). Lauterbach et al. (2016) lists five specimens from the Zygophylloideae with δ13C values between −19 and −21 ‰, and one specimen of Tetraena bucharica was −18.8 ‰, which falls outside of the general C3 range. The Tribuloideae species Sisyndite spartea also exhibits a δ13C near −21 ‰ (Lauterbach et al., 2019). Although Zygophyllaceae species represent some of the most extreme xerophytes known, with leathery to fleshy leaves, tolerance of low water potentials and repeated reliance on stem photosynthesis (Smith et al., 1997; Gibson, 1996; Porter, 2016; Godoy-Bürki et al., 2018), the only definitive evidence for CAM in the family is from B. retama. We encourage wider investigation before ruling out weak CAM or C3 + CAM in other species of the family.
Possible mechanisms for less negative δ13C in B. retama
The δ13C values of −30 to −32 ‰ observed in B. retama and B. foliosa plants grown in the Toronto glasshouse reflect a 2–3 ‰ more negative δ13C CO2 source in the urban Toronto air relative to mid-20th century non-urban atmospheres (Graven et al., 2020), coupled with strong C3 contributions over the lifetime of the plant, when they were generally well watered and leafy. In the field, where there is no urban source of CO2, hypothetical B. retama plants without CAM and sufficiently watered could be expected to exhibit δ13C values near −27 ‰, as indicated by the mean δ13C of −26.5 ‰ of field-collected B. foliosa fruits. Winter and Holtum (2002) used −27 ‰ as their pure C3 reference value in modelling the response of δ13C to increasing CAM strength. Based on their model, for a C3 + CAM plant to exhibit δ13C values near −17 ‰, 60 % of the daily CO2 uptake must happen at night (Winter and Holtum, 2002; Hancock et al., 2019; Winter, 2019). Hence, the level of CAM in well-watered to moderately droughted B. retama stems is insufficient to explain δ13C values observed in field-collected material. Instead, the model described by Winter and Holtum (2002) predicts that the 13–25 % nocturnal C contribution estimated here for well-watered to moderately droughted stems of B. retama would increase δ13C by ~1–4 ‰ units, to stem values of −26 to −23 ‰, assuming that δ13C is −27 ‰ in purely C3B. retama (Winter and Holtum, 2002). The values of approximately −28 ‰ observed in stems of glasshouse-grown B. retama in Toronto are consistent with a 2–3 ‰ unit increase attributable to CAM, assuming non-CAM values of −30 to −31 ‰ occur in the functionally C3 leaves of B. retama and B. foliosa.
One possible cause of less negative δ13C in field B. retama samples is that the plants exist in a severely droughted state for enough of the year that the CAM contribution dominates the annual carbon budget. In severely droughted B. retama stems, we estimated that the CAM contribution rose to 49 % of the daily C uptake. This increase in the CAM contribution was not attributable to increased CAM activity in an absolute sense, but rather, from a large drop in the C3 contribution during the day. Although severe drought over a lengthy dry season could increase the CAM contribution to the annual carbon budget, we do not anticipate that the increase would approach values required to explain field δ13C values in B. retama. In their native habitat of the Monte desert, Argentina, the growing season of B. retama ranges from October to March, when summer rains occur and minimal to moderate drought can be expected (Gatica et al., 2017). This length, coupled with the much higher daytime rates of carbon gain relative to nocturnal CAM activity that would occur on moist to moderately dry soils, does not indicate that CAM would dominate the yearly carbon budget. We therefore hypothesize that a mechanism other than CAM contributes to the less negative δ13C values in B. retama plants from the field. With C4 photosynthesis unlikely, this mechanism is probably low diffusive conductance in B. retama stems.
To evaluate diffusion limitations in stems, we modelled the relationship between δ13C and stem Ci/Ca (the ratio of intercellular CO2 concentration to atmospheric CO2 concentration) using eqn (10) in the paper by Cernusak et al. (2013):
| (1) |
where a is diffusional fractionation and b is carboxylation fractionation by Rubisco. The term a is typically 4.4 ‰, b is 27 ‰, and the source air around the time when the herbarium specimens of B. retama were collected would have been near −7.5 ‰. The Ci/Ca ratio reflects the balance between leaf or stem conductance and A; as it increases, δ13C declines in a linear relationship in C3 plants (Farquhar et al., 1989). Equation 1 is a simplified relationship that incorporates effects of mesophyll diffusion limitations into the b term, which is commonly taken as 27 ‰ when carbon isotopic discrimination is modelled as a function of term Ci/Ca for a typical C3 leaf (Cernusak et al., 2013). At a typical Ci/Ca for C3 leaves of 0.75 and b = 27 ‰, the predicted δ13C is −28.9 ‰ (or −30.9 ‰, assuming that the δ13C for Toronto source CO2 is −9.5 ‰). Tight cell packing in cortical tissue and the associated low % IAS is a common feature in photosynthetic stems of xerophytes (Gibson, 1983, 1996; Nilsen, 1995), which reduces mesophyll conductance (Maxwell et al., 1997) and, in turn, the value of b (Seibt et al., 2008). Other features of B. retama that are common to xerophytes include sunken stomata, low stomatal density, thick waxy cuticles and small stomatal aperture (Biruk et al., 2022). These xeromorphic features act in concert with low gs to reduce stem conductance (Šantrůček, 2022), and are accounted for in eqn (1) as a lower Ci/Ca.
We observed daytime Ci/Ca in watered and moderately droughted B. retama stems to be between 0.67 and 0.4 (Table 3), which, using eqn (1), would correspond to modelled δ13C of −27.0 to −20.9 ‰, respectively. If we assume that b is 26 ‰ to account for reduced mesophyll conductance from tight cell packing, the predicted range of δ13C becomes −26.4 to −20.5 ‰. Our measurements, however, were conducted at vapour pressure deficits of 1–2 kPa, not at the values of >3 kPa common in the native habit of B. retama (Biruk, 2021). High vapour pressure deficit normally reduces gs and Ci/Ca, with the integrated effect being a lower daily mean Ci/Ca because early-day maxima are reduced and afternoon declines begin earlier (Schulze and Hall, 1982; Cernusak et al., 2019; Grossiod et al., 2020). A mean field Ci/Ca of 0.4 would thus seem reasonable for B. retama stems, such that the modelled δ13C of −20.5 ‰ is plausible. If we then add a 4 ‰ increase owing to modest nocturnal CAM activity, the stem δ13C estimate is −16.5 ‰, which is consistent with the least negative δ13C values observed in field-collected B. retama here and by Gatica et al. (2017).
The above exercise demonstrates how less negative δ13C values in B. retama might be explained by a plausible combination of modest CAM and diffusion limitations. Follow-up research is needed to show how B. retama achieves its high δ13C values, using real-time isotopic determinations (via starch or online discrimination measurements), accounting for effects of gs, mesophyll conductance, drought and vapour pressure deficit in the B. retama habitat.
Conclusion: the role of CAM in B. retama
Although thousands of xerophytic plant species are known, it is a rarity to observe δ13C values less negative than −20 ‰ in C3 plants lacking CAM or C4-like photosynthesis. Because of this, the δ13C values of −16 to −19 ‰ observed in field-grown B. retama led us to hypothesize CAM or C4 function. Modest C3 + CAM function was observed, but this was too little to explain field δ13C values unless we accounted for high diffusive limitations common in xerophytic shrubs, including B. retama. These results represent the first evidence of CAM in the non-succulent, xerophytic shrub functional type that is common to dominant across the arid and semi-arid zones of the Earth. Although some succulent, xerophytic shrubs use C4 photosynthesis (mainly in the Amaranthaceae family or the Polygonaceae genus Calligonum; Sage and Pearcy, 2000), they are largely considered as being C3, with a range of fitness strategies for xeric environments including thermodynamic drought tolerance, tight stomatal control, waxy cuticles with sunken stomata, drought deciduousness and photosynthetic stems (Gibson, 1996; Smith et al., 1997). In B. retama, CAM photosynthesis can now be added to the mix of strategies conferring fitness in xeric environments in non-succulent, xerophytic shrubs. As with other weak to modest CAM plants (Griffiths, 1989; Holtum et al., 2017), B. retama shrubs rely predominantly on C3 photosynthesis in moist to moderately dry soils for the bulk of their carbon, but with the contributions of CAM, they are well poised to maintain a positive carbon balance and thus viability when extreme drought curtails C3 carbon gain. As a result, CAM might allow them to occupy drier sites in the arid landscapes of South America, such as the Monte desert of Argentina, where they persist on extreme sandy soils and where their roots do not reach the water table (Ribas-Fernández et al., 2009; Gatica et al., 2017; Biruk, 2021; Biruk et al., 2022).
We close with a reminder. Delineations of CAM often depend upon δ13C surveys, with −20 ‰ commonly taken as the threshold between strong and modest CAM (Winter and Holtum, 2002). If low conductance produces δ13C values approaching −21 ‰, weak to modest CAM could be interpreted erroneously as strong CAM. Such possibilities should be kept in mind when assessing CAM using δ13C alone.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following:
Table S1: the Larrea pledge, which illustrates the significance of Larrea tridentata to ecologists in the American Southwest. Table S2: the source information and raw δ13C values for 70 specimens of 30 species from the Larreoideae, Morkillioideae and Seetzenioideae subfamilies of the Zygophyllaceae. Table S3: gas-exchange values at key times during the 15-day diurnal trial shown in Fig. 5. Figure S1: 48-h response of A for well-watered and moderately droughted B. retama stems at 25 and 20 °C night.
ACKNOWLEDGEMENTS
We thank Ria Patel for assistance with training D. Mok in microscopy methods. We also thank Bruce Hall, Bill Cole and Tom Gludovacz for taking care of the B. retama plants.
Contributor Information
Daniel Mok, Department of Ecology and Evolutionary Biology, University of Toronto, 25 Wilcocks Street, Toronto, Ontario M5R3C6, Canada.
Arthur Leung, Department of Ecology and Evolutionary Biology, University of Toronto, 25 Wilcocks Street, Toronto, Ontario M5R3C6, Canada.
Peter Searles, Centro Regional de Investigaciones Científicas y Transferencia Tecnológica de La Rioja (CRILAR-CONICET), Entre Ríos y Mendoza s/n, Anillaco (5301), La Rioja, Argentina.
Tammy L Sage, Department of Ecology and Evolutionary Biology, University of Toronto, 25 Wilcocks Street, Toronto, Ontario M5R3C6, Canada.
Rowan F Sage, Department of Ecology and Evolutionary Biology, University of Toronto, 25 Wilcocks Street, Toronto, Ontario M5R3C6, Canada.
FUNDING
This research was supported by NSERC Discovery grant RGPIN-2017-06476 to R.F.S., RGPIN-2020-05925 to T.L.S., and a Queen Elizabeth II/Charles E. Eckenwalder Scholarship in Science and Technology to A.L.
LITERATURE CITED
- Adachi S, Stata M, Martin DG, et al. 2023. The evolution of C4 photosynthesis in Flaveria (Asteraceae): insights from the Flaveria linearis complex. Plant Physiology 191: 233–251. doi: 10.1093/plphys/kiac467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biruk LN. 2021. Ecofisiología de especies leñosas del Monte Central: aportes para la selección de especies y métodos de cultivo para la restauración de tierras secas. Doctoral dissertation, University of Buenos Aires, Buenos Aires. [Google Scholar]
- Biruk LN, Fernández ME, González CV, Guevara A, Rovida-Kojima E, Giordano CV.. 2022. High and diverse plastic responses to water availability in four desert woody species of South America. Trees 36: 1881–1894. doi: 10.1007/s00468-022-02335-8. [DOI] [Google Scholar]
- Borland AM, Leverett A, Hurtado-Castano N, Hu R, Yang X.. 2018. Functional anatomical traits of the photosynthetic organs of plants with crassulacean acid metabolism In: Adams WW III, Terashima I, eds. Advances in photosynthesis and respiration, Vol. 44. The leaf: a platform for performing photosynthesis. Berlin: Springer International, 281–305. doi: 10.1007/978-3-319-93594-2_10 [DOI] [Google Scholar]
- Cerling TE, Harris JM, MacFadden BJ, et al. 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature 389: 153–158. doi: 10.1038/38229. [DOI] [Google Scholar]
- Cernusak LA, Ubierna N, Winter K, Holtum JAM, Marshall JD, Farquhar GD.. 2013. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytologist 200: 950–965. doi: 10.1111/nph.12423. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Goldsmith GR, Arend M, Siegwolf RTW.. 2019. Effect of vapor pressure deficit on gas exchange in wild-type and abscisic acid-insensitive plants. Plant Physiology 181: 1573–1586. doi: 10.1104/pp.19.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisci JV, Hunziker JH, Palacios RA, Naranjo CA.. 1979. A numerical–taxonomic study of the genus Bulnesia (Zygophyllaceae): cluster analysis, ordination and simulation of evolutionary trees. American Journal of Botany 66: 133–140. doi: 10.1002/j.1537-2197.1979.tb06205.x. [DOI] [Google Scholar]
- Debandi G, Rossi B, Araníbar J, Ambrosetti JA, Peralta IE.. 2002. Breeding system of Bulnesia retama (Gillies ex Hook & Arn.) Gris. (Zygophyllaceae) in the Central Monte Desert (Mendoza, Argentina). Journal of Arid Environments 51: 141–152. doi: 10.1006/jare.2001.0924. [DOI] [Google Scholar]
- Dengler NG, Nelson T.. 1999. Leaf structure and development in C4 plants In: Sage RF, Monson RK, eds. C4plant biology. San Diego: Academic Press, 133–172. doi: 10.1016/B978-012614440-6/50006-9 [DOI] [Google Scholar]
- Edwards EJ. 2019. Evolutionary trajectories, accessibility and other metaphors: the case of C4 and CAM photosynthesis. New Phytologist 223: 1742–1755. doi: 10.1111/nph.15851. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Cooper TA.. 1988. Correlations between carbon isotope ratio and microhabitat in desert plants. Oecologia 76: 562–566. doi: 10.1007/BF00397870. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Rundel PW, Palma B, Mooney HA.. 1998. Carbon isotope ratios of Atacama desert plants reflect hyperaridity of region in Northern Chile. Revista Chilena de Historia Natural 71: 79–86. [Google Scholar]
- Epstein E. 1972. Mineral Nutrition of plants: principles and perspectives. New York: Wiley and Sons. [Google Scholar]
- Farquhar GD, Lloyd J.. 1993. Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere In: Ehleringer JR, Hall AE, Farquhar GD, eds. Stable isotopes and plant carbon-water relations. San Diego: Academic Press, 47–70. doi: 10.1016/B978-0-08-091801-3.50011-8. [DOI] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT.. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40: 503–537. doi: 10.1146/annurev.pp.40.060189.002443. [DOI] [Google Scholar]
- Frohlich MW, Sage RF, Craven LA, et al. 2022. Molecular phylogenetics of Euploca (Boraginaceae): homoplasy in many characters, including the C4 photosynthetic pathway. Botanical Journal of the Linnean Society 199: 497–537. doi: 10.1093/botlinnean/boab082. [DOI] [Google Scholar]
- Gatica MG, Aranibar JN, Pucheta E.. 2017. Environmental and species-specific controls on δ13C and δ15N in dominant woody plants from central-western Argentinian drylands. Austral Ecology 42: 533–543. doi: 10.1111/aec.12473. [DOI] [Google Scholar]
- Gibson AC. 1983. Anatomy of photosynthetic old stems of nonsucculent dicotyledons from North American deserts. Botanical Gazette 144: 347–362. doi: 10.1086/337383. https://www.jstor.org/stable/2474431. [DOI] [Google Scholar]
- Gibson AC. 1996. Structure-function relations of warm desert plants. Berlin: Springer, 91–116. doi: 10.1007/978-3-642-60979-4 [DOI] [Google Scholar]
- Gilman IS, Smith JAC, Holtum JAM, Sage RF, Winter K, Edwards EJ.. 2023. The CAM lineages of planet Earth. Annals of Botany (submitted, this volume). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy-Bürki AC, Acosta JM, Aagesen L.. 2018. Phylogenetic relationships within the New World subfamily Larreoideae (Zygophyllaceae) confirm polyphyly of the disjunct genus Bulnesia. Systematics and Biodiversity 16: 453–468. doi: 10.1080/14772000.2018.1451406. [DOI] [Google Scholar]
- Graven H, Keeling RF, Rogelj J.. 2020. Changes to carbon isotopes in atmospheric CO2 over the industrial era and into the future. Global Biogeochemical Cycles 34: e2019GB006170. doi: 10.1029/2019GB006170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H. 1989. Carbon dioxide concentrating mechanisms and the evolution of CAM in vascular epiphytes. In: Lüttge U, ed. Vascular plants as epiphytes: evolution and ecophysiology. Berlin: Springer-Verlag, 42–86. [Google Scholar]
- Grossiod C, Buckley TN, Cernusak LA, et al. 2020. Plant responses to rising vapor pressure deficit. New Phytologist 226: 1550–1566. doi: 10.1111/nph.16485. [DOI] [PubMed] [Google Scholar]
- Hancock LP, Holtum JAM, Edwards EJ.. 2019. The evolution of CAM photosynthesis in Australian Calandrinia reveals lability in C3+CAM phenotypes and a possible constraint to the evolution of strong CAM. Integrative and Comparative Biology 59: 517–534. doi: 10.1093/icb/icz089. [DOI] [PubMed] [Google Scholar]
- Heyduk K, Hwang M, Albert V, et al. 2019. Altered gene regulatory networks are associated with the transition from C3 to crassulacean acid metabolism in Erycina (Oncidiinae: Orchidaceae). Frontiers in Plant Science 9: 1–15. doi: 10.3389/fpls.2018.02000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K.. 2017. Facultative CAM photosynthesis (crassulacean acid metabolism) in four species of Calandrinia, ephemeral succulents of arid Australia. Photosynthesis Research 134: 17–25. doi: 10.1007/s11120-017-0359-x. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K.. 2021. CAM photosynthesis in desert blooming Cistanthe of the Atacama, Chile. Functional Plant Biology 48: 691–702. doi: 10.1071/fp20305. [DOI] [PubMed] [Google Scholar]
- Khoshravesh R, Stata M, Adachi S, Sage TL, Sage RF.. 2020. Evolutionary convergence of C4 photosynthesis: a case study in the Nyctaginaceae. Frontiers in Plant Science 11: 1–21. doi: 10.3389/fpls.2020.578739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge M, Ting IP.. 1978. Crassulacean acid metabolism. Berlin: Springer-Verlag. doi: 10.1007/978-3-642-67038-1_5 [DOI] [Google Scholar]
- Kocurek M, Kornas A, Wierzchnicki R, Lüttge U, Miszalski Z.. 2020. Importance of stem photosynthesis in plant carbon allocation of Clusia minor. Trees 34: 1009–1020. doi: 10.1007/s00468-020-01977-w. [DOI] [Google Scholar]
- Lauterbach M, van der Merwe PDW, Keßler L, Pirie MD, Bellstedt DU, Kadereit G.. 2016. Evolution of leaf anatomy in arid environments – a case study in southern African Tetraena and Roepera (Zygphyllaceae). Molecular Phylogenetics and Evolution 97: 129–144. doi: 10.1016/j.ympev.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Lauterbach M, Zimmer R, Alexa AC, et al. 2019. Variation in leaf anatomical traits relates to the evolution of C4 photosynthesis in Tribuloideae (Zygophyllaceae). Perspectives in Plant Ecology, Evolution and Systematics 39: 125463. doi: 10.1016/j.ppees.2019.125463. [DOI] [Google Scholar]
- Luján M, Oleas NH, Winter K.. 2022. Evolutionary history of CAM photosynthesis in neotropical Clusia: insights from genomics, anatomy, physiology and climate. Botanical Journal of the Linnean Society 199: 538–556. doi: 10.1093/botlinnean/boab075. [DOI] [Google Scholar]
- Lundgren MR, Dunning LT, Olofsson JK, et al. 2019. C4 anatomy can evolve via a single developmental change. Ecology Letters 22: 302–312. doi: 10.1111/ele.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U. 2004. Ecophysiology of crassulacean acid metabolism (CAM). Annals of Botany 93: 629–652. doi: 10.1093/aob/mch087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, von Caemmerer S, Evans JR.. 1997. Is a low internal conductance to CO2 diffusion a consequence of succulence in plants with Crassulacean acid metabolism? Australian Journal of Plant Physiology 24: 777–786. doi: 10.1071/PP97088. [DOI] [Google Scholar]
- Messerschmid TFE, Wehling J, et al. 2021. Carbon isotope composition of plant photosynthetic tissues reflects a crassulacean acid metabolism (CAM) continuum in the majority of CAM lineages. Perspectives in Plant Ecology, Evolution and Systematics 51: 125619. doi: 10.1016/j.ppees.2021.125619. [DOI] [Google Scholar]
- Monson RK, Teeri JA, Ku MSB, Gurevitch J, Mets LJ, Dudley S.. 1988. Carbon-isotope discrimination by leaves of Flaveria species exhibiting different amounts of C3- and C4-cycle co-function. Planta 174: 145–151. doi: 10.1007/BF00394765. [DOI] [PubMed] [Google Scholar]
- Nelson EA, Sage RF.. 2008. Functional constraints of CAM leaf anatomy: tight cell packing is associated with increased CAM function across a gradient of CAM expression. Journal of Experimental Botany 59: 1841–1850. doi: 10.1093/jxb/erm346. [DOI] [PubMed] [Google Scholar]
- Nelson EA, Sage TL, Sage RF.. 2005. Functional leaf anatomy of plants with crassulacean acid metabolism. Functional Plant Biology 32: 409–419. doi: 10.1071/FP04195. [DOI] [PubMed] [Google Scholar]
- Nilsen ET. 1995. Stem photosynthesis: extent, patterns, and role in plant carbon economy In: Gartner BL, ed. Plant stems: physiology and functional morphology. San Diego: Academic Press, 223–240. DOI: 10.1016/B978-012276460-8/50012-6 [DOI] [Google Scholar]
- O’Brien TP, McCully ME.. 1981. The study of plant structure: principles and selected methods. Melbourne: Termarcarphi. ISBN: 0959417400 [Google Scholar]
- Osmond CB. 1978. Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology 29: 379–414. doi: 10.1146/annurev.pp.29.060178.002115. [DOI] [Google Scholar]
- Parkhurst DF. 1982. Stereological methods for measuring internal leaf structure variables. American Journal of Botany 69: 31–39. doi: 10.1002/j.1537-2197.1982.tb13233.x. [DOI] [Google Scholar]
- Philpott J, Troughton JH.. 1974. Photosynthetic mechanisms and leaf anatomy of hot desert plants. Carnegie Institute of Washington Yearbook 73: 790–793. [Google Scholar]
- Porter DM. 2016. Zygophyllaceae R. Brown. In: Flora of North America Editorial Committee, eds. Flora of North America, Vol. 3. Oxford: Oxford University Press, 28–42. ISBN: 9780190643720 [Google Scholar]
- Ribas-Fernández Y, Quevedo-Robledo L, Pucheta E.. 2009. Pre- and post-dispersal seed loss and soil seed dynamics of the dominant Bulnesia retama (Zygophyllaceae) shrub in a sandy Monte desert of western Argentina. Journal of Arid Environments 73: 14–21. doi: 10.1016/j.jaridenv.2007.12.001. [DOI] [Google Scholar]
- Rundel PW, Sharifi MR.. 1993. Carbon isotope discrimination and resource availability in the desert shrub Larrea tridentata. In: Ehleringer JR, Hall AE, Farquhar GD, eds. Stable isotopes and plant carbon-water relations. San Diego: Academic Press, 47–70. doi: 10.1016/B978-0-08-091801-3.50019-2 [DOI] [Google Scholar]
- Sage RF, Pearcy RW.. 2000. The physiological ecology of C4 photosynthesis In: Leegood RC, Sharkey TD, von Caemmerer S, eds. Advances in photosynthesis and respiration. Photosynthesis: physiology and metabolism. Dordrecht: Springer Netherlands, 497–532. doi: 10.1007/0-306-48137-5_21 [DOI] [Google Scholar]
- Sage RF, Sage TL, Pearcy RW, Borsch T.. 2007. The taxonomic distribution of C4 photosynthesis in Amaranthaceae sensu stricto. American Journal of Botany 94: 1992–2003. doi: 10.3732/ajb.94.12.1992. [DOI] [PubMed] [Google Scholar]
- Sage TL, Busch FA, Johnson DC, et al. 2013. Initial events during the evolution of C4 photosynthesis in C3 species of Flaveria. Plant Physiology 163: 1266–1276. doi: 10.1104/pp.113.221119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Gilman IS, Smith JAC, Edwards EJ, Silvera K.. 2023. Atmospheric CO2 decline and the timing of CAM plant evolution. Annals of Botany (submitted this volume). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šantrůček J. 2022. The why and how of sunken stomata: does the behaviour of encrypted stomata and the leaf cuticle matter? Annals of Botany 130: 285–300. doi: 10.1093/aob/mcac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheider CA, Rasband WS, Eliceiri KW.. 2012. NIH image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E-D, Hall AE.. 1982. Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Encyclopedia of plant physiology new series. Physiological plant ecology II: water relations and carbon assimilation. Berlin: Springer-Verlag, 181–230. doi: 10.1007/978-3-642-68150-9_8 [DOI] [Google Scholar]
- Seibt U, Rajabi A, Griffiths H, Berry J.. 2008. Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia 155: 441–454. doi: 10.1007/s00442-007-0932-7. [DOI] [PubMed] [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman J.. 2010. Evolution along the crassulacean acid metabolism continuum. Functional Plant Biology 37: 995–1010. doi: 10.1071/FP10084. [DOI] [Google Scholar]
- Smith SD, Monson RK, Anderson JE.. 1997. Physiological ecology of North American desert plants. Berlin: Springer. doi: 10.1007/978-3-642-59212-6 [DOI] [Google Scholar]
- Stata M, Sage TL, Rennie TD, et al. 2014. Mesophyll cells of C4 plants have fewer chloroplasts than those of closely related C3 plants. Plant, Cell & Environment 37: 2587–2600. doi: 10.1111/pce.12331. [DOI] [PubMed] [Google Scholar]
- Stata M, Sage TL, Hoffmann N, Covshoff S, Wong GKS, Sage RF.. 2016. Mesophyll chloroplast investment in C3, C4 and C2 species of the genus Flaveria. Plant and Cell Physiology 57: 904–918. doi: 10.1093/pcp/pcw015. [DOI] [PubMed] [Google Scholar]
- Vogel JG. 1993. Variability of carbon isotope fractionation during photosynthesis. In Ehleringer JR, Hall AE, Farquhar GD, eds. Stable isotopes and plant carbon-water relations. San Diego: Academic Press, 29–46. [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Chuong SDX, Akhani H, Edwards GE, Franceschi VR.. 2005. Differentiation of cellular and biochemical features of the single-cell C4 syndrome during leaf development in Bienertia cycloptera (Chenopodiaceae). American Journal of Botany 92: 1784–1795. doi: 10.3732/ajb.92.11.1784. [DOI] [PubMed] [Google Scholar]
- Warth AH. 1956. The chemistry and technology of waxes, 2nd edn. New York: Reinhold. [Google Scholar]
- Winter K. 2019. Ecophysiology of constitutive and facultative CAM photosynthesis. Journal of Experimental Botany 70: 6495–6508. doi: 10.1093/jxb/erz002. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM.. 2002. How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiology 129: 1843–1851. doi: 10.1104/pp.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM.. 2015. Cryptic crassulacean acid metabolism (CAM) in Jatropha curcas. Functional Plant Biology 42: 711–717. doi: 10.1071/FP15021. [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC.. 1996a. An introduction to crassulacean acid metabolism. Biochemical principles and ecological diversity In: Klaus W, Smith JAC, eds. Crassulacean acid metabolism. Berlin: Springer, 1–13. doi: 10.1007/978-3-642-79060-7_1 [DOI] [Google Scholar]
- Winter K, Smith JAC.. 1996b. Crassulacean acid metabolism: current status and perspectives In: Klaus W, Smith JAC, eds. Ecological studies. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 389–426. doi: 10.1007/978-3-642-79060-7_26 [DOI] [Google Scholar]
- Winter K, Smith JAC.. 2022. CAM photosynthesis: the acid test. New Phytologist 233: 599–609. doi: 10.1111/nph.17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM, Smith JAC.. 2015. Crassulacean acid metabolism: a continuous or discrete trait? New Phytologist 208: 73–78. doi: 10.1111/nph.13446. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Virgo A, Smith JAC.. 2020. Low-level CAM photosynthesis in a succulent-leaved member of the Urticaceae, Pilea peperomioides. Functional Plant Biology 48: 683–690. doi: 10.1071/fp20151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


