Abstract
Background and Scope
The epiphytic life form characterizes almost 10 % of all vascular plants. Defined by structural dependence throughout their life and their non-parasitic relationship with the host, the term epiphyte describes a heterogeneous and taxonomically diverse group of plants. This article reviews the importance of crassulacean acid metabolism (CAM) among epiphytes in current climatic conditions and explores the prospects under global change.
Results and Conclusions
We question the view of a disproportionate importance of CAM among epiphytes and its role as a ‘key innovation’ for epiphytism but do identify ecological conditions in which epiphytic existence seems to be contingent on the presence of this photosynthetic pathway. Possibly divergent responses of CAM and C3 epiphytes to future changes in climate and land use are discussed with the help of experimental evidence, current distributional patterns and the results of several long-term descriptive community studies. The results and their interpretation aim to stimulate a fruitful discussion on the role of CAM in epiphytes in current climatic conditions and in altered climatic conditions in the future.
Keywords: Carbon isotopes, crassulacean acid metabolism, disturbance, dry forest, epiphytes, functional traits, germination, global change, key innovation, plant–water relationships
A BRIEF HISTORY OF OUR VIEW OF CAM IN EPIPHYTES
Vascular epiphytes differ from ground-rooted terrestrial autotrophs by their structural dependence on a host, typically a tree. They germinate, grow and reproduce on a host tree without establishing contact to the soil at any stage of their life cycle, which sets them apart from other structurally dependent forms, such as climbers or hemiepiphytes (Zotz, 2016). Importantly, in contrast to co-occurring mistletoes, they do not tap the host directly for resources. The epiphytic lifestyle is common; the most recent tally of the number of vascular plant species that primarily occur epiphytically reported >30 000 species, i.e. ~10 % of the global flora (Zotz et al., 2021b). A rather precarious supply of water is typically seen as a major consequence of epiphytic growth in trees.
Considering that a key benefit of crassulacean acid metabolism (CAM) is the economical use of water (Winter, 2019), it is unsurprising that researchers investigated the possibility that CAM might be used by vascular epiphytes. [‘CAM plants’ obtain the majority of their carbon through the CAM pathway throughout their lives, typically deduced from a δ13C value of leaf tissue > −20 ‰ (Winter, 2019).] This was shown, for the first time, ~60 years ago (Nuernbergk, 1961, 1963). From the 1960s until the early 1980s, it was possible to publish reports with the basic discovery that an epiphyte was capable of nocturnal CO2 uptake, e.g. ‘Algumas informações sôbre a capacidade rítmica diária da fixação e acumulação de CO2 no escuro em epífitas e erbáceas terrestres da mata pluvial’ by Coutinho (1964), ‘Two types of carbon fixation in tropical orchids’ by Neales and Hew (1975), or ‘Water relations of tropical epiphytes. III. Evidence for CAM’ by Sinclair (1984). Starting out as a curiosity, more and more studies documented the occurrence of CAM among epiphytes not only in dry forests, but also in moist habitats (Winter et al., 1983; Earnshaw et al., 1987; Holthe et al., 1992; Kluge et al., 1995; Zotz and Ziegler, 1997). A pattern that was noted early on (Nuernbergk, 1961, 1963) was a correlation between leaf thickness and the presence of CAM. Once C4 is excluded by way of inspection of leaf anatomy, species with thin leaves are typically C3 and those with thicker leaves are associated with CAM. Although later studies largely confirmed this general pattern (e.g. Teeri et al., 1981; Winter et al., 1983; Earnshaw et al., 1987; Zotz and Ziegler, 1997), there are also obvious deviations from this rule, e.g. in the case of species with thin leaves and with a carbon isotope ratio indicative of CAM (Zotz and Ziegler, 1997; Herrera, 2020). Hence, leaf thickness is a rough, but not totally reliable proxy for the photosynthetic pathway of epiphytes.
Summarizing the knowledge on CAM among epiphytes after more than three decades of research, Winter and Smith (1996) came up with the following calculation: with a then known number of 20 000 orchid species, more than two-thirds of which are epiphytes and an estimated 50 % of which were considered likely to use the CAM metabolic pathway (Winter and Smith, 1996), there could be as many as 7000 epiphytic orchid species with CAM. The number of CAM taxa in the global flora apart from orchids was estimated as 9000 species by the same authors (Smith and Winter, 1996; Winter and Smith, 1996). Given that many of these CAM species are also epiphytic, most importantly in Bromeliaceae (e.g. in genera such as Aechmea and Tillandsia), Cactaceae (e.g. in Rhipsalis and Epiphyllum) or Apocynaceae (e.g. in Hoya and Dischidia), epiphytic species would thus account for the majority of an estimated 16 000 CAM species globally, exceeding by far the number of terrestrial CAM taxa.
During the last two decades it has become increasingly clear that the bimodal distribution of δ13C values commonly found in screening studies (e.g. Winter, 1979; Winter et al., 1983; Earnshaw et al., 1987; Zotz and Ziegler, 1997) with a ‘gap’ at ~−20 ‰ does not delineate two distinct groups with and without the capacity for nocturnal acidification. Rather, there is a linear relationship between the proportion of nocturnally fixed carbon and δ13C values (Winter and Holtum, 2002), with a value of −20 ‰ being indicative of ~50% nocturnal uptake. Not surprisingly then, there is an ongoing debate over whether CAM should be seen as a discrete or a continuous trait (e.g. Zotz, 2002; Winter et al., 2015; Messerschmid et al., 2021; Winter and Smith, 2022). However, such a conceptual debate is by no means idiosyncratic for this photosynthetic pathway. ‘Halophyte’ vs. ‘glycophyte’ is another example of seemingly binary categories that will probably remain an issue of rather arbitrary delimitations (Grigore et al., 2014), as is the case for ‘succulent’ (‘Succulence is not a binary trait’; Ogburn and Edwards 2010) or ‘pseudobulb’ vs. ‘normal’ stem in Orchidaceae (Göbel et al., 2020). In the following treatise, we do not ignore cases with rather modest nocturnal activity, frequently called ‘weak CAM’ (Winter, 2019), but reserve the term ‘CAM species’ or ‘CAM plant’ for those cases in which the majority of carbon uptake occurs nocturnally (Winter, 2019).
RECENT INSIGHTS: HOW IMPORTANT IS CAM IN EPIPHYTES?
Regular water shortage is usually seen as one of the few challenges that most epiphytes share. Considering that the water-use efficiency of CAM plants is much higher than that of C3 plants (Winter, 2019), suggesting a high proportion of epiphyte species with CAM seems very reasonable, but has the estimate of Winter and Smith (1996) stood the test of time? The answer is clearly no.
Further exploration in the following decades until today has led to a strong decrease in our current estimate of the numbers of epiphytes using CAM. In one of the most ambitious studies to date, covering >1000 orchid species from Panama and Costa Rica, Silvera et al. (2010) detected δ13C values indicative of CAM (δ13C > −20 ‰) in only ~10 % of all epiphytic species. Although the proportion of CAM species is much lower among terrestrial orchids (4 of 121 species, i.e. 3 %), this proportion among epiphytes falls short by far of the 50 % estimate of Winter and Smith (1996). Another, similarly extensive survey among Colombian orchids by Torres-Morales et al. (2020) reported a comparable figure for epiphytic CAM species (9.5 %, or 76 of 805 species), but relatively more CAM species among terrestrials (7.3 %, or 19 of 260 species).
A recent, comprehensive analysis of numerous functional traits of vascular epiphytes by Hietz et al. (2022) revealed that CAM is found in ~10 % of the studied epiphyte species. This proportion is similar to the global estimate of 6–7 % for all vascular plants (Winter and Smith, 1996), particularly when considering that the latter group includes >70 000 species of trees (Gatti et al., 2022), all of which are C3 (with the rare exception of a few CAM trees in the genus Clusia; Luján et al., 2021) and a strong taxonomic and geographical bias in the epiphyte data (details in the study by Hietz et al., 2022). Note that bromeliads are excluded from this calculation because this family stands out with a much higher proportion of CAM species (Crayn et al., 2015). In the subfamily Bromelioideae, CAM is highly dominant (~90 %), but this high proportion is not related to life form; epiphytic and terrestrial taxa do not differ in that regard. In the subfamily Tillandsioideae, which is dominated by epiphytes, CAM is also common; 28 % of all species show CAM, but again without a life form bias. Hietz et al. (2022) pointed out that many, if not most, aspects of the functional ecology of vascular epiphytes are understudied, hampering generalizations. However, this is not true for CAM; there is a solid database available. Thus, we can dismiss with considerable certainty previous assertions that CAM is ‘common’ among epiphytes (e.g. Zotz and Hietz, 2001) or speculations that the majority of all CAM taxa globally are epiphytic (e.g. Winter and Smith, 1996; Lüttge, 2004), and as discussed below, we conclude that the large majority of epiphytes are not CAM plants. This statement does not ignore that in some taxonomic groups CAM species can be very common (e.g. in Bromeliaceae) or even ubiquitous (e.g. in Cactaceae). However, these are family characteristics irrespective of terrestrial or epiphytic growth. Neither is this a statement about the occurrence of limited nocturnal acidification in epiphytes. There are simply not enough data to conclude whether such ‘weak’ CAM is particularly common among epiphytic compared with terrestrial plants.
In the following, we quantify the relative importance of CAM in epiphyte communities with data from three published studies (Einzmann et al., 2015; Einzmann and Zotz, 2017; Wagner et al., 2021). When possible, we perform a separate analysis for epiphytes in tree crowns, excluding trunk epiphytes. In the first of these studies, in emergent trees on Barro Colorado Island (BCI), the number of epiphyte individuals with CAM is clearly highest on drought-deciduous tree species, such as Cavanillesia platanifolia (Table 1), whereas such a trend is not observed with regard to species numbers. As expected, the proportion of CAM species is higher in tree crowns compared with that on the trunk or that of the epiphyte community of the entire tree (Wilcoxon signed rank tests, P < 0.01).
Table 1.
Occurrence (abundance and species) of epiphytes with crassulacean acid metabolism (CAM) on five emergent tree species of varying phenology on Barro Colorado Island, Panama. Of each tree species, five individuals were studied, except Brosimum alicastrum with only four individuals. The ‘Total’ column gives the number of all individuals and species, respectively, across all 24 studied trees (or crowns, respectively) and the proportion of individuals/species with CAM. Percentage data for the individual tree species are given as the mean ± s.d. Data are from Einzmann et al. (2015). Barro Colorado Island is located in the Panamanian lowlands and experiences a pronounced 4-month dry season and ~2700 mm yr−1 rainfall
| Host tree species | ||||||
|---|---|---|---|---|---|---|
| Epiphyte | Total | Anacardium excelsum | Brosimum alicastrum | Ceiba pentandra | Pseudobombax septenatum | Cavanillesia platanifolia |
| Individuals | 25 592 | 10 963 | 3432 | 8826 | 2296 | 75 |
| Species | 87 | 65 | 49 | 50 | 20 | 7 |
| CAM individuals (%) | 4.9 | 1.2 ± 1.1 | 9.3 ± 6.4 | 8.1 ± 4.6 | 15.3 ± 19.7 | 24.6 ± 33.9 |
| CAM species (%) | 16 | 7.1 ± 5.5 | 17.0 ± 10.0 | 21.3 ± 9.7 | 21.4 ± 8.0 | 16.7 ± 23.6 |
| Crown individuals | 24 420 | 10 458 | 2755 | 8290 | 1763 | 14 |
| Crown species | 79 | 60 | 43 | 47 | 7 | 7 |
| Crown CAM individuals (%) | 4.6 | 1.2 ± 1.1 | 8.3 ± 8.1 | 8.0 ± 5.3 | 15.7 ± 20.5 | 16.7 ± 33.3 |
| Crown CAM species (%) | 15.2 | 6.6 ± 5.5 | 18.5 ± 15.7 | 22.6 ± 12.0 | 31.3 ± 6.4 | 8.3 ± 16.7 |
The relative importance of CAM in the epiphyte communities of four evergreen emergents in the moister forest of the San Lorenzo crane site in Panama is similar in terms of both individual numbers and species (Table 2). However, in this case the differences between the proportion of CAM species in tree crowns vs. tree trunks or that of the epiphyte community of the entire tree are not significant (Wilcoxon signed rank test, P > 0.2).
Table 2.
Occurrence (abundance and species) of epiphytes with crassulacean acid metabolism (CAM) on four tree species in the forest of San Lorenzo, Panama. Eight emergent trees of Aspidosperma spruceanum, three trees of Brosimum utile, eight trees of Calophyllum longifolium and four trees of Manilkara bidentata were studied. Only species for which δ13C information was available were included. Hemiepiphytes were excluded. The ‘Total’ column gives the number of all individuals and species, respectively, across all 24 studied trees (or crowns, respectively) and the proportion of individuals/species with CAM. Percentage data for the individual tree species are given as the mean ± s.d. Data are from Wagner et al. (2021). The forest is tropical moist lowland forest that experiences a 3- to 4-month-long dry season each year and ~3300 mm yr−1 of rainfall
| Host tree species | |||||
|---|---|---|---|---|---|
| Epiphyte | Total | Aspidosperma spruceanum | Brosimum utile | Calophyllum longifolium | Manilkara bidentata |
| Individuals | 4655 | 824 | 807 | 1516 | 1508 |
| Species | 66 | 39 | 39 | 53 | 33 |
| CAM individuals (%) | 3.0 | 10.1 ± 12.6 | 1.6 ± 1.9 | 5.1 ± 7.0 | 1.6 ± 1.3 |
| CAM species (%) | 11.9 | 10.3 ± 10.7 | 5.1 ± 4.5 | 8.7 ± 5.6 | 7.7 ± 6.2 |
| Crown individuals | 4080 | 672 | 713 | 1307 | 1388 |
| Crown species | 57 | 32 | 33 | 46 | 29 |
| Crown CAM individuals (%) | 2.8 | 10.3 ± 15.1 | 1.9 ± 2.0 | 4.8 ± 9.6 | 6.1 ± 9.4 |
| Crown CAM species (%) | 15.1 | 11.1 ± 13.9 | 7.1 ± 6.3 | 6.5 ± 5.3 | 13.7 ± 14.1 |
The third data set represents epiphytes on pasture trees along a rainfall gradient in western Panama (Table 3). Here, CAM species account for a remarkable 57 % of all individuals (and 33 % of the species) even at the wet end, where rainfall is similar to that received by the forest in San Lorenzo. This reinforces the notion that the spatial context of host trees is highly important, and not only large-scale climatic conditions; the microclimate in the crowns of widely spaced trees differs substantially from those in tree crowns in a closed forest. At the dry end of the gradient, with <1500 mm yr−1 of rain, almost all epiphytes use CAM (Einzmann and Zotz, 2017).
Table 3.
Occurrence of crassulacean acid metabolism (CAM) in epiphytes on 425 pasture trees with epiphytes in Panama, 2013. Only species for which δ13C information was available were included, excluding five species. CAM was much more prominent at the dry end of the rainfall gradient of this study (Einzmann and Zotz, 2017). The study was conducted in the south-western Panamanian lowlands, spanning a rainfall gradient from ~1100 to 4200 mm yr−1. The region experiences a 3- to 4-month dry season each year
| Epiphyte | Census 2013 | Gradient dry end, 1100–1500 mm yr−1 |
Gradient wet end, >3500 mm yr−1 |
|---|---|---|---|
| Individuals | 60 878 | 2576 | 21 282 |
| Species | 81 | 9 | 57 |
| CAM individuals (%) | 53.2 | 99.6 | 57.2 |
| CAM species (%) | 34.6 | 66.7 | 33.3 |
The last observation is fully in line with reports from dry tropical forest habitats. There, CAM seems to be (almost) indispensable for epiphytic existence. Relevant studies from dry tropical forests in Mexico consistently show that the proportion of epiphytes with CAM reaches 100 % (Mooney et al., 1989; Reyes-García et al., 2008; Cach-Pérez et al., 2018), whereas the results of a study from Brazil (Fontoura and Reinert, 2009) are more in line with observations by Einzmann and Zotz (2017) in pasture trees in lowland Panama (Table 3). In both cases, CAM is clearly dominant, but there is a considerable proportion of species that use C3.
The previous comparisons of species numbers assessed the relative importance of CAM primarily in a biodiversity context. To evaluate the importance of CAM in an ecosystem context, carbon flux or biomass is the more appropriate currency. Using this rationale, Zotz (2004) compared the relative contribution of CAM epiphytes to the total species count, total number of individuals and total biomass in a moist lowland forest. Given that CAM species tended to be rarer and smaller than C3 species, the relative importance of CAM at the community level was much smaller when expressed on an individual and on a biomass basis (Fig. 1). Whether this is a general pattern remains to be assessed in future studies.
Fig. 1.
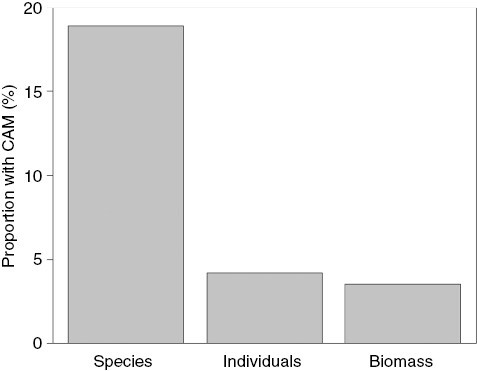
Relative importance of crassulacean acid metabolism (CAM) in a vascular epiphyte community in the lowland forest of San Lorenzo (Panama) expressed as the proportion of species with CAM, the proportion of individuals with CAM and the contribution of these individuals to total biomass. For details, see Zotz (2004).
Along elevational gradients, such as the one that Hietz et al. (1999) studied, from dry premontane forest to montane cloud forest, the proportion of CAM in epiphyte communities declines. In humid upper montane forests with particularly lush epiphyte vegetation, the proportion of CAM species is low (Earnshaw et al., 1987; Hietz et al., 1999; Torres-Morales et al., 2020; Guzmán-Jacob et al., 2022) and CAM biomass may account for only 2–3 % of the total epiphyte biomass (Hietz et al., 1999). Thus, although the global database is still rather limited and future studies might lead to some adjustments of these figures, current evidence from studies of elevational patterns does not support the notion that CAM is particularly common in these epiphyte-rich habitats.
Finally, Zotz (2016) compiled biomass data for epiphytic and terrestrial CAM plants at a global scale. Available data suggest that the biomass of CAM epiphytes in tropical lowland forests is typically <10 kg ha−1, with much higher values for so-called ‘atmospheric’ epiphytic bromeliads in semi-desert environments (>100 kg ha−1;Flores-Palacios et al., 2015). Terrestrial CAM biomass in arid systems in southern Peru, North America or Southern Africa can exceed 1000 kg ha−1 (Zotz, 2016). Thus, CAM in epiphytes does not rival the importance of CAM in terrestrial plants from an ecosystem perspective either.
To summarize, CAM is not (as frequently claimed) generally predominant in the epiphytic habitat, whether analysed as a proportion of species or individuals or as the contribution of CAM species to total biomass. However, at least in two Neotropical families (Cactaceae and Bromeliaceae), the presence of CAM has been called a key factor for the evolutionary radiation of plant lineages into tree crowns (e.g. Benzing, 1989; Givnish et al., 2014). Is this a valid argument?
CAM AND THE EVOLUTION OF EPIPHYTISM
A key evolutionary innovation is a morphological or physiological change in an individual trait that leads to an increase in diversification rate (Hunter, 1998). CAM is an excellent candidate for the study of such a potential key innovation because both the adaptive advantages and the metabolic costs of CAM are well characterized and because this trait has arisen multiple times, which allows the comparative study of the ecological and evolutionary implications of a transition from C3 to CAM and vice versa in many independent plant groups. Another aspect of considerable interest in the context of this review is the possible correlation of the evolution of CAM with other aspects of the biology of a plant group, such as particular morphological features (e.g. a tank in bromeliads or a pseudobulb in orchids) or habitat preferences, i.e. a possible link between epiphytism and CAM.
There are a number of studies that suggest increased diversification rates in CAM clades compared with C3 lineages, e.g. in Euphorbiaceae (Horn et al., 2014), Bromeliaceae (Givnish et al. 2014; Silvestro et al., 2014) or Orchidaceae (Silvera et al., 2009; Givnish et al., 2015), irrespective of a preference for terrestrial or epiphytic growing sites. In contrast, other studies have found no such differences. For example, diversification rates of CAM and C3 species of Afro-Malagasy Eulophiinae orchids did not differ (Bone et al., 2015), and a recent study with >100 Bulbophyllum species (Hu et al., 2022) suggested that a stimulation of speciation processes by CAM did happen initially, but was only temporary (i.e. during a low-CO2 period in the Miocene). In the long run, CAM lineages in that genus had ten times higher extinction rates than C3 lineages.
The empirical support for a link between CAM and epiphytism is also mixed. There is, for example, a study with the genus Cymbidium, in which CAM is restricted to epiphytic and lithophytic taxa, whereas all terrestrial Cymbidium species use C3 photosynthesis (Motomura et al., 2008b). Givnish et al. (2014) reported a very strong link between the evolution of epiphytism and both the possession of a water-impounding tank and entangling seeds in Bromeliaceae, but they were unable to detect a correlated evolution between the epiphytic habit and CAM. They observed, however, that CAM photosynthesis evolved more frequently overall in epiphytic than in terrestrial clades. An earlier analysis of the same family had found the opposite trend; transitions to CAM photosynthesis were much more common in terrestrial clades (Quezada and Gianoli, 2011). Even if there are methodological reasons for such disparate results for the same family, neither study provides strong support for a link between CAM and epiphytism.
In summary, evidence that CAM is a key innovation that leads to higher diversification in plants in general is mixed. Likewise, evidence for correlated evolution of CAM and epiphytism is also varied, which is not very surprising, given that previous estimates of a disproportionate number of CAM species among epiphytes seem to be exaggerated.
PARTICULAR ASPECTS OF CAM IN EPIPHYTES
Epiphytes might still be special in the use of CAM because this photosynthetic pathway can be found in any vegetative and reproductive organ, particularly noteworthy in roots. Roots of terrestrial plants typically grow underground, which precludes any photosynthetic activity, but at least the roots of bark epiphytes are exposed to sunlight. In some cases, such as many species in the genera Campylocentrum, Dendrophylax or Chiloschista, roots even serve as the only photosynthetically active organ (Benzing and Ott, 1981). All the tested leafless orchids have roots that use CAM (Benzing and Ott, 1981; Cockburn et al., 1985; Winter et al., 1985). In most other studied epiphytic orchids, however, roots fix carbon via the C3 pathway, even in species in which leaves engage in CAM (Motomura et al., 2008a; Moreira et al., 2009; Martin et al., 2010). In most cases, the contribution of roots to the carbon budget of the entire plant is probably moderate; Benzing and Ott (1981) found constant release of CO2 during both day and night in eight of nine tested species. No data are available about possible photosynthetic activity of roots of non-orchid epiphytes.
CAM is found in the stems of all epiphytic (as in terrestrial) cacti, and there is also evidence for CAM in pseudobulbs of many Orchidaceae (Rodrigues et al., 2013; Tay et al., 2019). There are a few leafless orchids, such as Bulbophyllum minutissimum, in which the pseudobulb is even the primary photosynthetic organ. Unlike most other pseudobulbs (Göbel et al., 2020), these organs possess not only a well-developed succulent chlorenchyma, but also stomata, and a δ13C value of −17 ‰, which is clear evidence for CAM (Winter et al., 1983). CAM activity has also been documented in reproductive organs. Nocturnal CO2 fixation in petals was already reported >50 years ago by Dueker and Arditti (1968) for two Cymbidium hybrids, and CAM in the fruits of two orchid species has been reported decades later (Zotz et al., 2003).
In most of the cases described, CAM activity in non-foliar organs does not seem to lead to a net uptake of CO2 but rather allows the recovery of some otherwise lost respiratory CO2 and an increase in water retention across the entire plant body. This might be highly relevant in epiphytes that can invest up to 30 % of plant biomass into reproductive structures. As in foliar organs, CAM can be constitutive or inducible (sensuWinter, 2019). For example, no CAM activity was detected in leaves, pseudobulbs and roots of well-watered Oncidium sp., but under drought stress the pseudobulbs and roots switched to CAM (Rodrigues et al., 2013).
All these observations lead to the conclusion that the photosynthetic pathways of different plant organs can be independent. Thus, a ‘CAM plant’, typically identified by the analysis of the photosynthetic pathway of its primary photosynthetically active organ, can have roots with C3 photosynthesis, such as in a Paphiopedilum cultivar (Martin et al., 2010), whereas a ‘C3 plant’, such as Dimerandra emarginata (Zotz and Tyree, 1996), can have fruits that engage in CAM (Zotz et al., 2003).
CAM AND EPIPHYTES IN THE FUTURE
Human activities have led to unprecedented changes in the biosphere and continue to do so (IPCC, 2021). At a global level, CO2 concentrations and air temperatures are on the rise, climatic extremes are increasing in frequency and severity, and all these changes are accompanied by changes in land use and increased nutrient depositions (Sala et al., 2000). In the following, we review experiments and other evidence that shed light on the effects of several aspects of global change: elevated CO2, increased temperature, altered water supply and extreme weather events, always with particular emphasis on epiphytes with CAM. We note from the start that the data basis is generally thin, and conclusions must therefore be considered preliminary.
Effects of elevated CO2
Numerous gas exchange studies have investigated the effect of varying CO2 concentrations on plants. Generally, an increase in atmospheric CO2 concentration stimulates leaf photosynthesis, water-use efficiency and growth in C3 and C4 plants (Drake et al., 1997; Ward et al., 1999; Winter et al., 2001; Cernusak et al., 2013). Although CAM plants use phosphoenolpyruvate carboxylase (PEPCase) for the initial CO2 fixation at night, in well-watered conditions they can assimilate CO2 directly by ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) during the daytime (phases II and IV of CAM; Nobel and Bobich, 2002; Winter and Smith, 2022). Then, the response of net CO2 uptake to increasing atmospheric CO2 concentrations of CAM species resembles that of C3 species and exceeds that of C4 species (Drennan and Nobel, 2000). Terrestrial CAM plants generally have higher maximal instantaneous rates of net CO2 uptake (3–18 μmol m−2 s−1) than epiphytic CAM plants (2–3 μmol m−2 s−1) (Nobel and Bobich, 2002). Does this translate into relative differences in the responses to increasing atmospheric CO2 concentrations?
Few studies have investigated the effect of elevated CO2 on vascular epiphytes, and most of them have used in vitro plantlets (Gouk et al., 1997, 1999) or ornamental plants (Li et al., 2002a; Croonenborghs et al., 2009; Monteiro et al., 2009). Furthermore, some results from studies with epiphytic bromeliads are inconsistent (Monteiro et al., 2009; Zotz et al., 2010). Among the expected physiological responses of C3 and CAM epiphytes to elevated CO2 are increases in photosynthesis, growth and dark respiration, as observed in gametophytes of the C3 fern Pyrrosia piloselloides (Ong et al., 1998), reduced stomatal conductance in phase I of CAM, that led to a reduction of ~20% in water loss in Tillandsia brachycaulos, but an increase in stomatal conductance and water loss by ~130% in the C3 fern Phlebodium aureum in low-light conditions (Batke et al., 2018). However, there are also unexpected growth responses (Croonenborghs et al., 2009; Monteiro et al., 2009). For example, Croonenborghs et al. (2009) found that elevated CO2 treatments had adverse effects on the ornamental value of three bromeliads, producing shorter plants with paler green leaves than control plants.
Laboratory studies on the effect of doubled CO2 concentrations on C3 and CAM epiphytes report an average increase in relative growth rate of ~20 %, with little difference in growth stimulation between C3 species and CAM species (Table 4). The median increase of ~16 % in CAM epiphytes is less than half of the reported increase in terrestrial CAM plants. There, increases in shoot dry mass average 35 % under similar CO2 enrichment (Drennan and Nobel, 2000).
Table 4.
The effect of elevated CO2 on growth of vascular epiphyte species in five published studies. Given are the average relative growth rates (RGR, in mg g−1 d−1) at low and high CO2, the absolute (in mg g−1 d−1) and relative effect size (as a %). CO2 concentrations were 280/560 µL L−1 (Monteiro et al., 2009), 350/700 µL L−1 (Zotz et al., 2010), 380/760 µL L−1 (Li et al., 2002a; Croonenborghs et al., 2009) and 400/800 µL L−1 (Wagner and Zotz, 2018). The median absolute and relative effect sizes are 0.4 mg g−1 d−1 and 19 %, respectively, with no differences between CAM and C3 plants (Wilcoxon test, P > 0.5)
| Species | Family | C3/CAM | RGR low | RGR high | Effect size (absolute) | Effect size (relative) | Source |
|---|---|---|---|---|---|---|---|
| Aechmea bracteata | Bromeliaceae | CAM | 1.1 | 3.9 | 2.8 | 250 | Wagner and Zotz (2018) |
| Aechmea fasciata | Bromeliaceae | CAM | 11.8 | 10.6 | −1.2 | −10.2 | Croonenborghs et al. (2009) |
| Aechmea fasciata | Bromeliaceae | CAM | 2.1 | 2.9 | 0.7 | 33.6 | Monteiro et al. (2009) |
| Aechmea ‘Maya’ | Bromeliaceae | CAM | 3.6 | 3.2 | −0.3 | −8.7 | Croonenborghs et al. (2009) |
| Aechmea mexicana | Bromeliaceae | CAM | 7.1 | 10 | 2.9 | 43 | Wagner and Zotz (2018) |
| Aechmea veitchii | Bromeliaceae | CAM | 5.9 | 7.0 | 1.1 | 19 | Wagner and Zotz (2018) |
| Catopsis juncifolia | Bromeliaceae | C3 | 3.8 | 4.3 | 0.5 | 11.8 | Monteiro et al. (2009) |
| Catopsis nitida | Bromeliaceae | C3 | 6.5 | 6.1 | −0.5 | −7.1 | Zotz et al. (2010) |
| Guzmania ‘Hilda’ | Bromeliaceae | C3 | 10.5 | 12.9 | 2.4 | 23.1 | Croonenborghs et al. (2009) |
| Guzmania monostachya | Bromeliaceae | C3 | 1.4 | 2.0 | 0.6 | 39.0 | Zotz et al. (2010) |
| Racinea contorta | Bromeliaceae | C3 | 0.4 | 2.0 | 1.6 | 440 | Zotz et al. (2010) |
| Tillandsia elongata | Bromeliaceae | CAM | 3.6 | 4.0 | 0.4 | 11.1 | Zotz et al. (2010) |
| Tillandsia fasciculata | Bromeliaceae | CAM | 2.8 | 2.4 | −0.4 | −14.2 | Monteiro et al. (2009) |
| Tillandsia fasciculata | Bromeliaceae | CAM | 2.0 | 2.3 | 0.3 | 14.0 | Zotz et al. (2010) |
| Tillandsia heterophylla | Bromeliaceae | C3 | 0.1 | 2.2 | 2.2 | 4300 | Zotz et al. (2010) |
| Tillandsia juncea | Bromeliaceae | C3 | 2.3 | 2.7 | 0.5 | 20.7 | Zotz et al. (2010) |
| Tillandsia subulifera | Bromeliaceae | CAM | 1.8 | 1.0 | −0.8 | −44.6 | Zotz et al. (2010) |
| Tillandsia viridiflora | Bromeliaceae | C3 | 0.4 | 0.8 | 0.4 | 88.6 | Zotz et al. (2010) |
| Vriesea ‘Splenriet’ | Bromeliaceae | C3 | 4.4 | 4.6 | 0.2 | 3.8 | Monteiro et al. (2009) |
| Werauhia laxa | Bromeliaceae | C3 | 8.6 | 8.7 | 0.1 | 1.3 | Zotz et al. (2010) |
| Werauhia sanguinolenta | Bromeliaceae | C3 | 4.2 | 5.5 | 1.4 | 32.8 | Zotz et al. (2010) |
| Bulbophyllum longissimum | Orchidaceae | CAM | 0.9 | 1.0 | 0.2 | 20.0 | Monteiro et al. (2009) |
| Mokara ‘Yellow’ | Orchidaceae | CAM | 6.8 | 8.5 | 1.7 | 25.3 | Li et al. (2002a) |
| Oncidium enderianum | Orchidaceae | C3 | 1.2 | 1.1 | −0.1 | −8.2 | Monteiro et al. (2009) |
In several CAM epiphytes, an increase of photosynthetic and sugar production enzymes, titratable acidity (Li et al., 2002b) and exceptional root growth (Gouk et al., 1999; Li et al., 2002b) have been found. Such increased root growth in elevated CO2 conditions has also been described in terrestrial CAM species (Cui et al., 1993; Zhu et al., 1997).
What is the physiological basis of increased growth? Higher carbon gain during the light period under elevated CO2, compared with current CO2 conditions (mostly in phase IV of CAM), has been reported for terrestrial CAM plants (Graham and Nobel, 1996; Winter et al., 2014). Indeed, many CAM plants will take up substantial amounts of CO2 during the daytime given favourable environmental conditions (Nobel, 2003). This is also true for CAM epiphytes. For instance, two epiphytic bromeliad species took up ~20 % of the daily total CO2 during the light period, mostly in phase IV, in well-watered conditions (Graham and Andrade, 2004). Further evidence that an increase in nocturnal CO2 uptake might play hardly any role in a growth stimulation of epiphytic CAM plants comes from an experimental study with three Aechmea species (Wagner and Zotz, 2018): doubling CO2 from 400 to 800 µL L−1 increased their relative growth rate by 61 % in well-watered conditions, but nocturnal acidification remained unchanged. Thus, this increase in growth resulted entirely from higher assimilation during the light period.
The expected mitigating effect of elevated CO2 under low water supply was not found in a study with several epiphytic bromeliad species (Zotz et al., 2010); however, it was noted that species from the lowlands were more drought tolerant than those from montane areas. Likewise, Wagner and Zotz (2018), studying three Aechmea species, found that elevated CO2 did not compensate for the negative effect of low water supply. In contrast, Batke et al. (2018) reported a strong reduction in 24 h water loss for Tillandsia brachycaulos in high-light and elevated CO2 conditions, but no changes in water relationships between CO2 treatments. Yet, in this last study, the high-light treatment (650 mmol m−2 s−1) was unrealistic, because in situ such light conditions occur only during the dry season in the driest forest habitat of this species (González-Salvatierra et al., 2021), whereas the experiment was conducted with well-watered plants. Moreover, although T. brachycaulos inhabits forests where high light and low water availability are usually accompanied by high vapour pressure deficits and high temperature, this species shows modest seasonal changes in leaf water relationships within the forest canopy (Hernández-Robinson et al., 2020).
Increased temperature
Temperature affects all aspects of the biology of plants, from germination to growth and reproduction. In a study with a focus on epiphytic bromeliads, Müller et al. (2017) observed that the expected temperature increase in tropical latitudes of 3 °C by the end of this century would stimulate germination in most cases. This positive effect warns against sweeping predictions of generally negative effects of climate change. However, conditions in nature are complex, and any positive temperature effect in laboratory conditions might be irrelevant ecologically if in situ low and more erratic water availability precludes germination. Using the data of Müller et al. (2017), we tested whether a temperature rise of 3 °C would affect germination in C3 and CAM species differently. Specifically, we analysed whether the experimentally determined optimal temperature for germination would fall within the predicted mean annual temperature ranges of each species at the end of this century. This is the case for a majority of C3 species (14 of 20 species), but for only eight of the 21 CAM species in that particular study. However, this difference was not significant (χ2 = 2.6, P = 0.11). If anything, the trend indicates an advantage of C3 species.
At a leaf physiological level, the main problem for CAM plants should be an increase in nocturnal temperatures, because daytime temperatures hardly affect net CO2 uptake in terrestrial CAM plants (Nobel, 2003). In contrast, there is evidence for many terrestrial CAM plants that high night-time temperatures (>30 °C) affect stomatal aperture and reduce net CO2 uptake (Nobel, 2003). Certainly, the optimal mean nocturnal temperature for most of these terrestrial CAM plants is relatively low, ~15 °C (Nobel and Bobich, 2002; Nobel, 2003), because PEPCase and NAD(P)-malate dehydrogenase have optimal mean temperatures reflecting the conditions of cooler nights (Yamori et al., 2014). Indeed, substantial net CO2 uptake occurs at a nocturnal temperature of as low as 0 °C for Opuntia ficus-indica (Cui et al., 1993). In tropical lowland areas, CAM epiphytes grow at higher mean nocturnal temperatures for most of the year, and their PEPCase activity can acclimatize to higher stem temperatures. Thus, CAM epiphytes can be expected to have an optimal night temperature for CO2 uptake like that of the hemiepiphytic cactus Hylocereus undatus, which is ~20–25 °C (Raveh et al., 1995; Nobel and de la Barrera, 2004).
Owing to the lack of experimental data for temperature optima in CAM epiphytes in fully controlled laboratory conditions, we focus on data from field studies. Epiphytic CAM orchids growing in the open in Singapore experience a nocturnal minimum night temperature of ~22 °C (Neales and Hew, 1975). The epiphytic CAM bromeliad Tillandsia usneoides shows an optimum CO2 assimilation between temperatures of 15 and 20 °C (Medina, 1987). Epiphytic orchids from dry forests have higher increases in titratable tissue acidity (ΔH+) during the early dry season, when leaf nocturnal temperatures are ~22 ° C or less, compared with those of the rainy and late dry seasons (de la Rosa-Manzano et al., 2014). Also, some Tillandsia spp. increase ΔH+ in cooler months in a tropical dry forest of the Pacific coast of Mexico (e.g. T. eistetteri and T. ionantha; Reyes-García et al., 2008). However, in another tropical dry forest in Yucatan, Mexico, plants of T. brachycaulos have greater ΔH+ during the warmer nights of the rainy season than during the cooler early dry season nights (González-Salvatierra et al., 2021).
High temperatures have relatively little influence on growth and survival of terrestrial CAM plants; apparently, daytime air and leaf or stem temperatures are not critical for photosynthesis, with the tolerated temperature extreme reaching 68 °C (Nobel and Bobich, 2002). In general, however, tolerance limits are certainly lower for tropical CAM plants. For example, hemiepiphytic H. undatus and terrestrial Mammillaria gaumeri cannot tolerate temperatures >50 °C (Nobel et al., 2002; Cervera et al., 2006). Tillandsia species from tropical dry forests tolerate air temperatures of ≤42 °C (Cervantes et al., 2005; Hernández-Robinson et al., 2020; Rosado-Calderón et al., 2020). Furthermore, a study applying different day/night temperature regimes found that most of the 17 tested species of epiphytic bromeliads (C3 and CAM) performed well at temperatures predicted for the end of this century (Müller et al., 2018). More field and laboratory studies are clearly required, with information on the abiotic conditions of epiphytic microhabitats, to understand the effect of high temperatures on growth and survival of CAM epiphytes and of epiphytes in general.
Climatic extremes
Apart from changing long-term averages in temperature and precipitation, an increased likelihood of extreme events in the future is a major concern (Castillo et al., 2021). For example, climate change might lead to more and stronger cyclonic storms (Landsea et al., 2006; Elsner et al., 2008; Sobel et al., 2016). In combination with the ongoing fragmentation of tropical forests, strong wind (of increased intensity or otherwise) will affect epiphyte communities in addition to the habitat loss they experience. Fragmented forests are more vulnerable to wind disturbance (Laurance et al., 1997; Laurance and Curran, 2008), with the epiphytic component in the upper strata of the forest being particularly vulnerable (Tay et al., 2021; Einzmann et al., 2022a). However, CAM epiphytes might be less affected if such disturbance leads mainly to increased exposure, but overall, the photosynthetic pathway used by epiphytes is likely to be largely irrelevant in the context of such disturbance.
As another consequence of global change, there seems to be an increase in the frequency of Central Pacific El Niño events in recent decades (Freund et al., 2019). For one such event in 2015–16, the effect on an epiphyte community in small-statured Annona glabra trees growing along the shoreline of BCI has been documented (Einzmann et al., 2022b). During this very strong El Niño event, annual rainfall in central Panama was reduced from the average 2600 mm yr−1 to only 1800 mm yr−1 (Paton, 2022), following 2 years with already markedly below-average rainfall. Annona glabra has a relatively open crown, and growth along the shore means that epiphytes experience conditions resembling the upper crown in the forest despite the small stature of the host tree. If CAM were of major advantage, C3 species should be much more affected than CAM species. However, no difference in their change in abundance was found (Einzmann et al., 2022b). In the reference period, 2002–15, both CAM and C3 species growing on the very same trees consistently increased in abundance, while individual numbers decreased in similar proportion irrespective of photosynthetic pathway from 2015 to 2016 (Table 5).
Table 5.
Proportions of epiphytes with crassulacean acid metabolism (CAM) and C3 growing on the Annona glabra trees along the shoreline of Barro Colorado Island (BCI), Panama. About 50 % of the trees growing around BCI form the basis for a long-term study that encompasses three censuses: 1994, 2002 and 2015. Included here is a subset of 145 trees that were studied before and after a very strong El Niño event (Einzmann et al., 2022b)
| Epiphyte | 2002 | 2015 | 2016 |
|---|---|---|---|
| Individuals | 4298 | 8074 | 7895 |
| Species | 42 | 52 | 52 |
| CAM individuals | 2032 | 3457 | 3340 |
| CAM species | 11 | 14 | 14 |
| CAM individuals (%) | 47.3 | 42.8 | 42.3 |
| CAM species (%) | 26.2 | 26.9 | 26.9 |
Long-term variation in the proportion of CAM species
Gradual changes in climate could affect C3 and CAM species differentially. Increasing temperatures and changes in precipitation could lead to a drier climate, in which the CAM species among epiphytes might be favoured. We searched for possible evidence in long-term studies of epiphyte assemblages. We found only three data sets that cover a longer time period, from 8 years (Einzmann and Zotz, 2017) and ~11 years (Mendieta-Leiva et al., 2022) to a maximum of 21 years (Einzmann et al., 2021). Given that all these studies were performed in Panama, where neither annual precipitation nor dry season length has changed over the last decades (Paton, 2022), and where changes in precipitation are not predicted for the future (Kusunoki et al., 2019), one would not expect any directional change in community composition. However, we consider it important to document these unique time series of ‘normal’ fluctuations as essential background information for future studies designed to detect any directional changes in the proportion of CAM species elsewhere.
Over 21 years and three censuses, epiphytes growing on Annona glabra around BCI did not show a consistent pattern regarding CAM species (Table 6; Einzmann et al., 2021). In a first census interval (1994–2002), there was a relative decrease in abundance of CAM species, whereas in the second census interval (2002–15) there was a relative increase in CAM species. Overall, in the long-term data set the proportion of CAM species varied little (~20–26 %), with a similarly constant contribution of ~47 % of CAM individuals to total epiphyte abundance (Table 6). Compared with the proportion of CAM species and individuals from forest trees (Tables 1 and 2), CAM species were of much greater importance in Annona glabra.
Table 6.
Epiphytes growing on Annona glabra trees along the shoreline of Barro Colorado Island, Panama, were followed over 21 years (Einzmann et al., 2021)
| Epiphyte | 1994 | 2002 | 2015 |
|---|---|---|---|
| Individuals | 14 920 | 23 674 | 30 734 |
| Species | 58 | 69 | 72 |
| CAM individuals | 6913 | 11 497 | 14 419 |
| CAM species | 15 | 14 | 17 |
| CAM individuals (%) | 46.3 | 48.6 | 46.9 |
| CAM species (%) | 25.9 | 20.3 | 23.6 |
There were no larger changes in the proportion of CAM epiphytes in the other two studies either (Fig. 2). The maximum difference was observed in epiphytes in pasture trees, with a decrease in abundance of CAM species of ~10 % over 8 years.
Fig. 2.
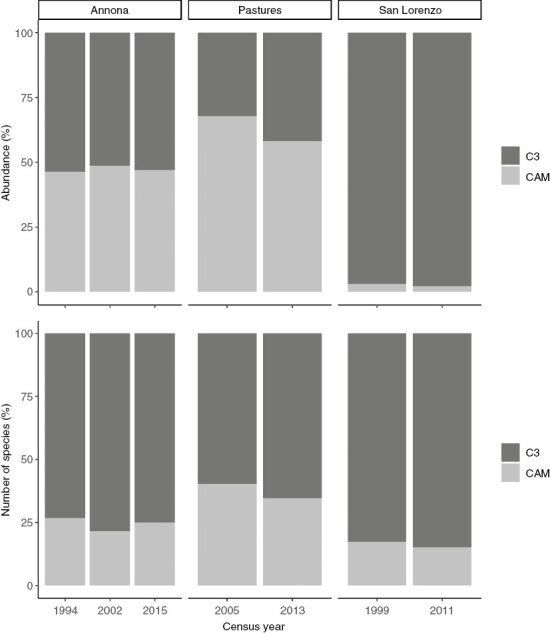
Temporal changes in the percentage of abundance and number of species of epiphytic C3 species and species with crassulacean acid metabolism (CAM) in three different systems. Annona, epiphyte assemblages censused three times on Annona glabra trees growing along the shoreline of Barro Colorado Island (Einzmann et al., 2021); Pastures, epiphyte assemblages censused twice on pasture trees growing along a rainfall gradient in western Panama (Einzmann and Zotz, 2017); San Lorenzo, epiphyte assemblages censused twice on all trees within the San Lorenzo crane site (Mendieta-Leiva et al., 2022).
Land use change
The process euphemistically termed ‘land use change’ is still the largest threat for tropical epiphytes compared with other drivers of global change identified by Sala et al. (2000), such as direct effects of CO2, altered rainfall patterns or climatic extremes. Although the photosynthetic pathway is clearly irrelevant in the case of complete deforestation, it seems to play a role given more moderate conversions, e.g. to secondary forests or agricultural landscapes with pasture trees. In the case of more isolated trees and more fragmented forest patches, the microclimate is typically drier, which should favour CAM species. Indeed, the proportion of CAM species is much higher in pasture trees (Table 3) than in forest trees (Table 2) under otherwise comparable precipitation regimes. The same is true for the number of individuals.
However, historically, epiphytic CAM species might have suffered much more from previous human activities than C3 species, because tropical dry forests, in which CAM epiphytes dominate, have been transformed to a larger degree than any other type of tropical forest for hundreds of years (Portillo-Quintero et al., 2015; Sunderland et al., 2015; Siyum, 2020). Some attention has been paid to dry forest recovery. For instance, the long history of the transformation of tropical dry deciduous forest of northern Yucatan has resulted in the impoverishment of tree species, because succession has favoured species that sprout after perturbation (González-Iturbe et al., 2002). In other areas with less perturbation and with remnants of old forest, recovery of canopy height, plant density and crown cover occured in ~20 years, but species richness and biomass continue to increase after >80 years (Lebrija-Trejos et al., 2008; Dupuy et al., 2012; Guerra-Martínez et al., 2021). Nevertheless, the recovery of epiphyte assemblages has been almost entirely ignored in successional studies of tropical dry forests and tropical forests in general. In one of the few exceptional studies with a focus on epiphytes, Woods and DeWalt (2013) argued that it takes almost two centuries for epiphyte species composition in secondary lowland forests of central Panama to recover. Comparing this number with the estimated 120 years for secondary tropical forest to recover other forest attributes (Poorter et al., 2021), epiphytes might take particularly long to recover from such disturbances.
WHY IS CAM NOT MORE COMMON AMONG VASCULAR EPIPHYTES?
Although CAM is key at the dry end of current epiphytic existence (Mooney et al., 1989; Reyes-García et al., 2008; Fontoura and Reinert, 2009; Cach-Pérez et al., 2018), current evidence does not suggest that, compared with vascular plants in general, this metabolic pathway is disproportionately common among epiphytes. If water scarcity is genuinely the defining feature of the epiphytic habitat, as often suggested (e.g. Benzing, 1990; Zotz and Hietz, 2001; Lüttge, 2008), this is somewhat surprising. So, what did we miss?
We see a number of explanations for this seeming discrepancy. We know that at the regional scale the epiphytic life form responds more strongly than others (e.g. trees or climbers) to differences in water supply, as demonstrated in the classic study by Gentry and Dodson (1987). However, by far the highest numbers of species and the highest abundances are found at the wet end, where epiphytes are not much affected by water scarcity. In such ecosystems, CAM would primarily incur a metabolic cost, while possible benefits would be restricted to rare drought events. The impact of such occasional periods of water scarcity on cloud forest epiphytes has been studied in detail by Gotsch and collaborators (e.g. Gotsch et al., 2015, 2018; Williams et al., 2020). These authors highlighted the importance of water storage. The need to develop a more differentiated view of the importance of the factor water for epiphytes was also emphasized by Zotz et al. (2021a) in a recent review of epiphyte ecophysiology. Here are a few observations that contradict the arguably simplistic view that epiphytes, as a group, are generally more affected by water stress than ground-rooted plants. First, δ13C values of C3 epiphytes do not differ from those of terrestrial herbs in the TRY database (Kattge et al., 2020), which suggests similar broad-scale stomatal limitations (Hietz et al., 2022). Second, water content per dry mass or leaf area is substantially lower in epiphytic bromeliads compared with terrestrials, in both C3 and CAM species (Males and Griffiths, 2017). As the samples for that study came from well-watered collections in botanical gardens, this finding should primarily reflect genetic differences, which makes it even more surprising. Finally, in forests at Mount Kilimanjaro, tree leaves stored three times as much water as foliage of co-occurring epiphytes (Schellenberger Costa et al., 2018). Taken together, epiphytes might be less special in terms of their water relationships than previously thought, which should also lead to an adjustment of our expectation about the prevalence of CAM among epiphytes.
Following Winter (2019), we reserved the term ‘CAM plant’ to those species that ‘throughout their lives, obtain the majority of their carbon through the CAM pathway’, which is typically deduced from a δ13C value of leaf tissue higher than −20 ‰, which is indicative of ≥50 % nocturnal CO2 uptake. This approach ignores the possibility that CAM might affect whole plant carbon budgets via reducing carbon losses by other organs (petals, fruit, stems and roots; see above) and also ignores the possible importance of limited nocturnal acidification without uptake of external CO2, i.e. ‘weak CAM’ sensuWinter (2019), as found in the leaves of many orchids, bromeliads and ferns (Zotz and Tyree, 1996; Holtum and Winter, 1999; Schmidt and Zotz, 2001; Silvera et al., 2005). Naturally, all species estimates would change if all taxa that show small but detectable nocturnal acidification at least occasionally were to be included as ‘C3–CAM plants’ or ‘weak CAM plants’. Given that most of the relevant studies were performed with epiphytes, there is a largely unknown number of terrestrial equivalents, which does not allow us to provide a reasonable estimate for the relative occurrence of this phenomenon among epiphytic vs. terrestrial taxa. However, we would argue that rather than juggling with the number of species with ‘weak CAM’, it is more important to study the ecological relevance of such small nocturnal acidification in either life form. The few detailed analyses of such ‘weak CAM’ activity in vascular epiphytes suggest a rather moderate contribution to water and carbon budgets. In the tank bromeliad Werauhia sanguinolenta (Schmidt and Zotz, 2001) and the orchid Dimerandra emarginata (Zotz and Tyree, 1996) maximum nocturnal acidification under drought amounted to the equivalent of net CO2 fixation rates of, respectively, 0.1 and 0.2 µmol m−2 s−1 during the night. This is less than what one would expect from the complete recycling of respiratory CO2. Currently, it is unclear whether such weak ‘CAM activity’ in C3 plants is ecologically important and/or more or less idiosyncratic for epiphytes; we would need information on the frequency of ‘weak CAM’ in epiphytic vs. terrestrial taxa to address this question.
It is important to emphasize that CAM is not the only option for plants to deal with drought. Sinclair (1984), for example, stated that the most important drought-related strategy in the studied ferns is the tolerance to very large reductions in leaf relative water content rather than CAM. Other ways to bridge periods of water scarcity among epiphytic taxa include internal water storage in leaves, roots and shoots (Gessner, 1956; Yang et al., 2016; Hernández-Robinson et al., 2020), external water storage in phytotelmata (Zotz and Thomas, 1999; Zotz et al., 2020), strongly reduced cuticular water loss (Benzing and Burt, 1970; Helbsing et al., 2000), drought-deciduousness (Benzing et al., 1982; Mehltreter, 2008) and desiccation tolerance (Stuart, 1968; Proctor, 2012), while highly effective water uptake via roots (Biebl, 1964; Zotz and Winkler, 2013; Leroy et al., 2019) and leaves (Gotsch et al., 2015; Darby et al., 2016) allows epiphytes to replenish stores fast as part of a general recovery after stress events. Interestingly, the C4 pathway, which is common in many arid terrestrial biomes (Sage et al., 2018), is apparently not used by any epiphyte (Zotz, 2016).
Although our view of the importance of CAM among epiphytes has possibly been exaggerated in the past, there are recurring patterns in epiphyte community structure that demonstrate an important role of CAM, not only at the very dry end of forest habitats. Similar to the results shown for epiphytes on emergent trees in central Panama (Table 1), a number of studies have found a clear stratification, with CAM species typically being dominant or at least relatively abundant in the upper parts of moist tropical forests (Griffiths and Smith, 1983; Zotz and Ziegler, 1997; Zotz, 2004). Again, such a pattern should not be confused with the notion that CAM is indispensable in the most exposed growing sites. Numerous C3 species can be found there, with well-studied examples in the upper crown of large, drought-deciduous emergents in the lowland forest of BCI being Dimerandra emarginata (Einzmann et al., 2015), Niphidium crassifolium and Catasetum viridiflavum (Zotz and Winter, 1994). Likewise, Fontoura and Reinert (2009), studying epiphytic bromeliads in a dry forest in Southeastern Brazil, found that most species used CAM, but at the most exposed microsites a C3 bromeliad (Vriesea procera) dominated, and not a CAM species. A similar pattern was decribed by Zotz (1997); the large C3 tank bromeliad Werauhia sanguinolenta was more common in the exposed parts of Annona glabra crowns than a co-occurring CAM (Tillandsia fasciculata) and a C3–CAM species (Guzmania monostachia). This again highlights that CAM can be important among epiphytes, but is still but one of several functional options allowing growth in demanding epiphytic situations.
CONCLUSION
This paper reviews the importance of CAM among epiphytes in general in current climatic conditions and explores future prospects under global change. We aimed to stimulate a discussion by questioning the frequently expressed view of a disproportionate importance of CAM among vascular epiphytes compared with ground-rooted plants. Although there is little doubt that at the dry end of the ecological spectrum, epiphytic growth is often difficult or impossible for C3 plants, in general CAM species are not overly common among epiphytes. Thus, we should also reconsider claims that CAM is a key innovation for epiphytism as such and narrow any evolutionary importance down to specific ecological scenarios, such as growth in dry forests. An answer to the question regarding whether global change might lead to a shift in the relative importance of CAM among epiphytes is again facing complexity. Historically, the almost complete destruction of tropical dry forests has probably affected CAM epiphytes more than C3 species, but depending on the particular driver (temperature, precipitation, CO2 or disturbance), future effects of human activities might be neutral with regard to photosynthetic pathway, or CAM species might possibly be less affected, e.g. by drought, and thus benefit relatively, as long as trees are available as hosts.
ACKNOWLEDGEMENTS
G.Z. wants to thank his former PhD advisor for many stimulating conversations on CAM and other topics over more than three decades.
Contributor Information
Gerhard Zotz, Functional Ecology Group, Institute of Biology and Environmental Sciences, Carl von Ossietzky University Oldenburg, Box 5634, D-26046 Oldenburg, Germany; Smithsonian Tropical Research Institute, Box 0843-03092, Panama, Republic of Panama.
José Luis Andrade, Unidad de Recursos Naturales, Centro de Investigación Científica de Yucatán, Calle 43 No. 130, Chuburná de Hidalgo, Mérida, Yucatán, Mexico.
Helena J R Einzmann, Functional Ecology Group, Institute of Biology and Environmental Sciences, Carl von Ossietzky University Oldenburg, Box 5634, D-26046 Oldenburg, Germany.
LITERATURE CITED
- Batke SP, Holohan A, Hayden R, Fricke W, Porter AS, Evans-Fitz.Gerald CM.. 2018. The pressure is on – epiphyte water-relations altered under elevated CO2. Frontiers in Plant Science 9: 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing DH. 1989. The evolution of epiphytism. In: Lüttge U, ed. Vascular plants as epiphytes: evolution and ecophysiology. Heidelberg: Springer, 15–41. [Google Scholar]
- Benzing DH. 1990. Vascular epiphytes. General biology and related biota. Cambridge: Cambridge University Press. [Google Scholar]
- Benzing DH, Bent A, Moscow D, Peterson G, Renfrow A.. 1982. Functional correlates of deciduousness in Catasetum integerrimum (Orchidaceae). Selbyana 7: 1–9. [Google Scholar]
- Benzing DH, Burt KM.. 1970. Foliar permeability among twenty species of the Bromeliaceae. Bulletin of the Torrey Botanical Club 97: 269–279. doi: 10.2307/2483646. [DOI] [Google Scholar]
- Benzing DH, Ott DW.. 1981. Vegetative reduction in epiphytic Bromeliaceae and Orchidaceae: its origin and significance. Biotropica 13: 131–140. doi: 10.2307/2387715. [DOI] [Google Scholar]
- Biebl R. 1964. Zum Wasserhaushalt von Tillandsia recurvata L. und Tillandsia usneoides L. auf Puerto Rico. Protoplasma 58: 345–368. doi: 10.1007/bf01253007. [DOI] [Google Scholar]
- Bone RE, Smith JAC, Arrigo N, Buerki S.. 2015. A macro-ecological perspective on crassulacean acid metabolism (CAM) photosynthesis evolution in Afro-Madagascan drylands: Eulophiinae orchids as a case study. New Phytologist 208: 469–481. doi: 10.1111/nph.13572. [DOI] [PubMed] [Google Scholar]
- Cach-Pérez MJ, Andrade JL, Reyes-García C.. 2018. Morphophysiological plasticity in epiphytic bromeliads across a precipitation gradient in the Yucatan peninsula, Mexico. Tropical Conservation Science 11: 1940082918781926. doi: 10.1177/1940082918781926. [DOI] [Google Scholar]
- Castillo F, Wehner M, Stone DA.. 2021. Extreme events and climate change: a multidisciplinary approach. New York: John Wiley & Sons. [Google Scholar]
- Cernusak LA, Winter K, Dalling JW, et al. 2013. Tropical forest responses to increasing atmospheric CO2: current knowledge and opportunities for future research. Functional Plant Biology 40: 531–551. doi: 10.1071/fp12309. [DOI] [PubMed] [Google Scholar]
- Cervantes SE, Graham EA, Andrade JL.. 2005. Light microhabitats, growth and photosynthesis of an epiphytic bromeliad in a tropical dry forest. Plant Ecology 179: 107–118. doi: 10.1007/s11258-004-5802-3. [DOI] [Google Scholar]
- Cervera JC, Andrade JL, Simá JL, Graham EA.. 2006. Microhabitats, germination, and establishment for Mammillaria gaumeri (Cactaceae), a rare species from Yucatan. International Journal of Plant Sciences 167: 311–319. doi: 10.1086/498650. [DOI] [Google Scholar]
- Cockburn W, Goh CJ, Avadhani PN.. 1985. Photosynthetic carbon assimilation in a shootless orchid, Chiloschista usneoides (DON) LDL: a variant on crassulacean acid metabolism. Plant Physiology 77: 83–86. doi: 10.1104/pp.77.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho LM. 1964. Algumas informações sôbre a capacidade rítmica diária da fixação e acumulação de CO2 no escuro em epífitas e erbáceas terrestres da mata pluvial. Botânica 21: 397–408. [Google Scholar]
- Crayn DM, Winter K, Schulte K, Smith JAC.. 2015. Photosynthetic pathways in Bromeliaceae: phylogenetic and ecological significance of CAM and C3 based on carbon isotope ratios for 1893 species. Botanical Journal of the Linnean Society 178: 169–221. [Google Scholar]
- Croonenborghs S, Ceusters J, Londers E, De Proft MP.. 2009. Effects of elevated CO2 on growth and morphological characteristics of ornamental bromeliads. Scientia Horticulturae 121: 192–198. doi: 10.1016/j.scienta.2009.01.018. [DOI] [Google Scholar]
- Cui M, Miller PM, Nobel PS.. 1993. CO2 exchange and growth of the crassulacean acid metabolism plant Opuntia ficus-indica under elevated CO2 in open-top chambers. Plant Physiology 103: 519–524. doi: 10.1104/pp.103.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby A, Draguljić D, Glunk A, Gotsch SG.. 2016. Habitat moisture is an important driver of patterns of sap flow and water balance in tropical montane cloud forest epiphytes. Oecologia 182: 357–371. [DOI] [PubMed] [Google Scholar]
- Drake BG, Gonzàlez-Meler MA, Long SP.. 1997. More efficient plants: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology 48: 609–639. doi: 10.1146/annurev.arplant.48.1.609. [DOI] [PubMed] [Google Scholar]
- Drennan PM, Nobel PS.. 2000. Responses of CAM species to increasing atmospheric CO2 concentrations. Plant, Cell & Environment 23: 767–781. [Google Scholar]
- Dueker J, Arditti J.. 1968. Photosynthetic 14CO2 fixation by green Cymbidium (Orchidaceae) flowers. Plant Physiology 43: 130–132. doi: 10.1104/pp.43.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy JM, Hernández-Stefanoni JL, Hernández-Juárez RA, et al. 2012. Patterns and correlates of tropical dry forest structure and composition in a highly replicated chronosequence in Yucatan, Mexico. Biotropica 44: 151–162. [Google Scholar]
- Earnshaw MJ, Winter K, Ziegler H, et al. 1987. Altitudinal changes in the incidence of crassulacean acid metabolism in vascular epiphytes and related life forms in Papua New Guinea. Oecologia 73: 566–572. doi: 10.1007/bf00379417. [DOI] [PubMed] [Google Scholar]
- Einzmann HJR, Beyschlag J, Hofhansl F, Wanek W, Zotz G.. 2015. Host tree phenology affects vascular epiphytes at the physiological, demographic and community level. AoB Plants 7: plu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einzmann HJR, Tay J, Zotz G.. 2022a. What happens to epiphytic bromeliads in a windy spot? Journal of Tropical Ecology 38: 158–163. [Google Scholar]
- Einzmann HJR, Weichgrebe L, Zotz G.. 2021. Long-term community dynamics in vascular epiphytes on Annona glabra along the shoreline of Barro Colorado Island, Panama. Journal of Ecology 109: 1931–1946. doi: 10.1111/1365-2745.13618. [DOI] [Google Scholar]
- Einzmann HJR, Weichgrebe L, Zotz G.. 2022b. The impact of a severe El Niño event on vascular epiphytes in lowland Panama. Diversity 14: 325. doi: 10.3390/d14050325. [DOI] [Google Scholar]
- Einzmann HJR, Zotz G.. 2017. ‘No signs of saturation’: long-term dynamics of vascular epiphyte communities in a human-modified landscape. Biodiversity and Conservation 26: 1393–1410. doi: 10.1007/s10531-017-1306-z. [DOI] [Google Scholar]
- Elsner JB, Kossin JP, Jagger TH.. 2008. The increasing intensity of the strongest tropical cyclones. Nature 455: 92–95. doi: 10.1038/nature07234. [DOI] [PubMed] [Google Scholar]
- Flores-Palacios A, García-Franco JG, Capistrán-Barradas A.. 2015. Biomass, phorophyte specificity and distribution of Tillandsia recurvata in a tropical semi-desert environment (Chihuahuan Desert, Mexico). Plant Ecology and Evolution 148: 68–75. [Google Scholar]
- Fontoura T, Reinert F.. 2009. Habitat utilization and CAM occurrence among epiphytic bromeliads in a dry forest from southeastern Brazil. Revista Brasileira de Botânica 32: 521–530. [Google Scholar]
- Freund MB, Henley BJ, Karoly DJ, McGregor HV, Abram NJ, Dommenget D.. 2019. Higher frequency of Central Pacific El Niño events in recent decades relative to past centuries. Nature Geoscience 12: 450–455. doi: 10.1038/s41561-019-0353-3. [DOI] [Google Scholar]
- Gatti RC, Reich PB, Gamarra JGP, et al. 2022. The number of tree species on Earth. Proceedings of the National Academy of Sciences of the United States of America 119: e2115329119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry AH, Dodson CH.. 1987. Diversity and biogeography of Neotropical vascular epiphytes. Annals of the Missouri Botanical Garden 74: 205–233. doi: 10.2307/2399395. [DOI] [Google Scholar]
- Gessner F. 1956. Der Wasserhaushalt der Epiphyten und Lianen. In: Stocker O, ed. Pflanze und Wasser. Berlin: Springer, 915–950. [Google Scholar]
- Givnish TJ, Barfuss MHJ, Van Ee B, et al. 2014. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution 71: 55–78. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Spalink D, Ames M, et al. 2015. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proceedings of the Royal Society B: Biological Sciences 282: 20151553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel CY, Schlumpberger BO, Zotz G.. 2020. What is a pseudobulb? Toward a quantitative definition. International Journal of Plant Sciences 181: 686–696. [Google Scholar]
- González-Iturbe JA, Olmsted I, Tun-Dzul F.. 2002. Tropical dry forest recovery after long term Henequen (sisal, Agave fourcroydes Lem.) plantation in northern Yucatan, Mexico. Forest Ecology and Management 167: 67–82. doi: 10.1016/s0378-1127(01)00689-2. [DOI] [Google Scholar]
- González-Salvatierra C, Peña-Rodríguez LM, Reyes-García C, de la Barrera E, Andrade JL.. 2021. Seasonal changes in photosynthesis for the epiphytic bromeliad Tillandsia brachycaulos in a tropical dry deciduous forest. Botanical Sciences 99: 850–862. [Google Scholar]
- Gotsch SG, Dawson TE, Draguljić D.. 2018. Variation in the resilience of cloud forest vascular epiphytes to severe drought. New Phytologist 219: 900–913. [DOI] [PubMed] [Google Scholar]
- Gotsch SG, Nadkarni NM, Darby A, et al. 2015. Life in the treetops: ecophysiological strategies of canopy epiphytes in a tropical montane cloud forest. Ecological Monographs 85: 393–412. doi: 10.1890/14-1076.1. [DOI] [Google Scholar]
- Gouk SS, He J, Hew CS.. 1999. Changes in photosynthetic capability and carbohydrate production in an epiphytic CAM orchid plantlet exposed to super-elevated CO2. Environmental and Experimental Botany 41: 219–230. doi: 10.1016/s0098-8472(99)00006-4. [DOI] [Google Scholar]
- Gouk SS, Yong JWH, Hew CS.. 1997. Effects of super-elevated CO2 on the growth and carboxylating enzymes in an epiphytic CAM orchid plantlet. Journal of Plant Physiology 151: 129–136. doi: 10.1016/s0176-1617(97)80144-7. [DOI] [Google Scholar]
- Graham EA, Andrade JL.. 2004. Drought tolerance associated with vertical stratification of two co-occurring epiphytic bromeliads in a tropical dry forest. American Journal of Botany 91: 699–706. doi: 10.3732/ajb.91.5.699. [DOI] [PubMed] [Google Scholar]
- Graham EA, Nobel PS.. 1996. Long-term effects of a doubled atmospheric CO2 concentration on the CAM species Agave deserti. Journal of Experimental Botany 47: 61–69. doi: 10.1093/jxb/47.1.61. [DOI] [Google Scholar]
- Griffiths H, Smith JAC.. 1983. Photosynthetic pathways in the Bromeliaceae of Trinidad: relations between life-forms, habitat preference and the occurrence of CAM. Oecologia 60: 176–184. doi: 10.1007/bf00379519. [DOI] [PubMed] [Google Scholar]
- Grigore M-N, Ivanescu L, Toma C.. 2014. Halophytes: an integrative anatomical study. Switzerland: Springer International Publishing. [Google Scholar]
- Guerra-Martínez F, García-Romero A, Martínez-Morales MA, López-García J.. 2021. Resiliencia ecológica del bosque tropical seco: recuperación de su estructura, composición y diversidad en Tehuantepec, Oaxaca. Revista Mexicana de Biodiversidad 92: 923422. doi: 10.22201/ib.20078706e.2021.92.3422. [DOI] [Google Scholar]
- Guzmán-Jacob V, Guerrero Ramirez N, Craven D, et al. 2022. Broad- and small-scale environmental gradients drive variation in chemical, but not morphological, leaf traits of vascular epiphytes. Functional Ecology 36: 1858–1872. doi: 10.1111/1365-2435.14084. in press. [DOI] [Google Scholar]
- Helbsing S, Riederer M, Zotz G.. 2000. Cuticles of vascular epiphytes: efficient barriers for water loss after stomatal closure? Annals of Botany 86: 765–769. [Google Scholar]
- Hernández-Robinson S, Graham EA, Hernáández-González O, et al. 2020. Hot but not dry: Modest changes in water relations for an epiphytic bromeliad in a tropical dry deciduous forest. International Journal of Plant Sciences 181: 945–954. [Google Scholar]
- Herrera A. 2020. Are thick leaves, large mesophyll cells and small intercellular air spaces requisites for CAM? Annals of Botany 125: 859–868. doi: 10.1093/aob/mcaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietz P, Wagner K, Nunes Ramos F, et al. 2022. Putting vascular epiphytes on the traits map. Journal of Ecology 110: 340–358. [Google Scholar]
- Hietz P, Wanek W, Popp M.. 1999. Stable isotopic composition of carbon and nitrogen, and nitrogen content in vascular epiphytes along an altitudinal transect. Plant, Cell & Environment 22: 1435–1443. [Google Scholar]
- Holthe PA, Patel A, Ting IP.. 1992. The occurrence of CAM in Peperomia. Selbyana 13: 77–87. [Google Scholar]
- Holtum JAM, Winter K.. 1999. Degrees of crassulacean acid metabolism in tropical epiphytic and lithophytic ferns. Australian Journal of Plant Physiology 26: 749–757. [Google Scholar]
- Horn JW, Xi Z, Riina R, et al. 2014. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 68: 3485–3504. doi: 10.1111/evo.12534. [DOI] [PubMed] [Google Scholar]
- Hu AQ, Gale SW, Liu ZJ, Fischer GA, Saunders RMK.. 2022. Diversification slowdown in the Cirrhopetalum alliance (Bulbophyllum, Orchidaceae): insights from the evolutionary dynamics of crassulacean acid metabolism. Frontiers in Plant Science 13: 794171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JP. 1998. Key innovations and the ecology of macroevolution. Trends in Ecology & Evolution 13: 31–36. [DOI] [PubMed] [Google Scholar]
- IPCC. 2021. Climate change 2021: the physical science basis. contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press. [Google Scholar]
- Kattge J, Bönisch G, Díaz S, et al. 2020. TRY plant trait database – enhanced coverage and open access. Global Change Biology 26: 119–188. [DOI] [PubMed] [Google Scholar]
- Kluge M, Brulfert J, Rauh W, Ravelomanana D, Ziegler H.. 1995. Ecophysiological studies on the vegetation of Madagascar: a δ13C and δD survey for incidence of crassulacean acid metabolism (CAM) among orchids from montane forests and succulents from the xerophytic thorn-bush. Isotopes in Environmental and Health Studies 31: 191–210. doi: 10.1080/10256019508234018. [DOI] [Google Scholar]
- Kusunoki S, Nakaegawa T, Pinzón R, Sanchez-Galan JE, Fábrega JR.. 2019. Future precipitation changes over Panama projected with the atmospheric global model MRI-AGCM3.2. Climate Dynamics 53: 5019–5034. [Google Scholar]
- Landsea CW, Harper BA, Hoarau K, Knaff JA.. 2006. Can we detect trends in extreme tropical cyclones? Science 313: 452–454. doi: 10.1126/science.1128448. [DOI] [PubMed] [Google Scholar]
- Laurance WF, Curran TJ.. 2008. Impacts of wind disturbance on fragmented tropical forests: a review and synthesis. Australian Ecology 33: 399–408. [Google Scholar]
- Laurance WF, Laurance SG, Ferreira LV, Rankin de Merona JM, Gascon C, Lovejoy TE.. 1997. Biomass collapse in Amazonian forest fragments. Science 278: 1117–1118. [Google Scholar]
- Lebrija-Trejos E, Bongers F, Pérez-García EA, Meave JA.. 2008. Successional change and resilience of a very dry tropical deciduous forest following shifting agriculture. Biotropica 40: 422–431. doi: 10.1111/j.1744-7429.2008.00398.x. [DOI] [Google Scholar]
- Leroy C, Gril E, Si Ouali L, et al. 2019. Water and nutrient uptake capacity of leaf-absorbing trichomes vs. roots in epiphytic tank bromeliads. Environmental and Experimental Botany 163: 112–123. [Google Scholar]
- Li CR, Gan LJ, Xia K, Zhou X, Hew CS.. 2002a. Responses of carboxylating enzymes, sucrose metabolizing enzymes and plant hormones in a tropical epiphytic CAM orchid to CO2 enrichment. Plant, Cell & Environment 25: 369–377. [Google Scholar]
- Li CR, Liang YH, Hew CS.. 2002b. Responses of Rubisco and sucrose-metabolizing enzymes to different CO2 in a C3 tropical epiphytic orchid Oncidium Goldiana. Plant Science 163: 313–320. doi: 10.1016/s0168-9452(02)00100-0. [DOI] [Google Scholar]
- Luján M, Oleas NH, Winter K.. 2021. Evolutionary history of CAM photosynthesis in Neotropical Clusia: insights from genomics, anatomy, physiology and climate. Botanical Journal of the Linnean Society 199: 538–556. doi: 10.1093/botlinnean/boab075. [DOI] [Google Scholar]
- Lüttge U. 2004. Ecophysiology of crassulacean acid metabolism (CAM). Annals of Botany 93: 629–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U. 2008. Physiological ecology of tropical plants. Berlin: Springer. [Google Scholar]
- Males J, Griffiths H.. 2017. Functional types in the Bromeliaceae: relationships with drought resistance traits and bioclimatic distributions. Functional Ecology 31: 1868–1880. doi: 10.1111/1365-2435.12900. [DOI] [Google Scholar]
- Martin CE, Mas EJ, Lu C, Ong BL.. 2010. The photosynthetic pathway of the roots of twelve epiphytic orchids with CAM leaves. Photosynthetica 48: 42–50. doi: 10.1007/s11099-010-0007-6. [DOI] [Google Scholar]
- Medina E. 1987. Aspectos ecofisiológicos de plantas CAM en los trópicos. Revista de Biología Tropical 35(Suppl. 1): 55–70. [Google Scholar]
- Mehltreter K. 2008. Phenology and habitat specificity of tropical ferns. In: Ranker TA, Haufler CH, eds. Biology and evolution of ferns and lycophytes. New York: Cambridge University Press, 201–221. [Google Scholar]
- Mendieta-Leiva G, Buckley HL, Zotz G.. 2022. Directional changes over time in the species composition of tropical vascular epiphyte assemblages. Journal of Ecology 110: 553–568. [Google Scholar]
- Messerschmid TFE, Wehling J, Bobon N, et al. 2021. Carbon isotope composition of plant photosynthetic tissues reflects a Crassulacean Acid Metabolism (CAM) continuum in the majority of CAM lineages. Perspectives in Plant Ecology, Evolution and Systematics 51: 125619. [Google Scholar]
- Monteiro JAF, Zotz G, Körner C.. 2009. Tropical epiphytes in a CO2-rich atmosphere. Acta Oecologica 35: 60–68. doi: 10.1016/j.actao.2008.08.001. [DOI] [Google Scholar]
- Mooney HA, Bullock SH, Ehleringer JR.. 1989. Carbon isotope ratios of plants of a tropical forest in Mexico. Functional Ecology 3: 137–142. doi: 10.2307/2389294. [DOI] [Google Scholar]
- Moreira ASP, de Lemos Filho JP, Zotz G, Isaias RMS.. 2009. Anatomy and photosynthetic parameters of roots and leaves of two shade adapted orchids, Dichaea cogniauxiana Shltr. and Epidendrum secundum Jacq. Flora – Morphology, Distribution, Functional Ecology of Plants 204: 604–611. [Google Scholar]
- Motomura H, Ueno O, Kagawa A, Yukawa T.. 2008a. Carbon isotope ratios and the variation in the diurnal pattern of malate accumulation in aerial roots of CAM species of Phalaenopsis (Orchidaceae). Photosynthetica 46: 531–536. [Google Scholar]
- Motomura H, Yukawa T, Ueno O, Kagawa A.. 2008b. The occurrence of crassulacean acid metabolism in Cymbidium (Orchidaceae) and its ecological and evolutionary implications. Journal of Plant Research 121: 163–177. doi: 10.1007/s10265-007-0144-6. [DOI] [PubMed] [Google Scholar]
- Müller L-LB, Albach D, Zotz G.. 2017. ‘Are 3°C too much?’: thermal niche breadth in Bromeliaceae and global warming. Journal of Ecology 105: 507–516. [Google Scholar]
- Müller L-LB, Albach D, Zotz G.. 2018. Growth responses to elevated temperatures and the importance of ontogenetic niche shifts in Bromeliaceae. New Phytologist 217: 127–139. [DOI] [PubMed] [Google Scholar]
- Neales TF, Hew CS.. 1975. Two types of carbon fixation in tropical orchids. Planta 123: 303–306. doi: 10.1007/bf00390710. [DOI] [PubMed] [Google Scholar]
- Nobel PS. 2003. Environmental biology of agaves and cacti. New York: Cambridge University Press. [Google Scholar]
- Nobel PS, de la Barrera E, Beilman DW, Doherty JH, Zutta BR.. 2002. Temperature limitations for cultivation of edible cacti in California. Madroño 49: 228–236. [Google Scholar]
- Nobel PS, Bobich EG.. 2002. Environmental biology. In: Nobel PS, ed. Cacti: biology and uses. Berkeley: University of California Press, 57–74. [Google Scholar]
- Nobel PS, de la Barrera E.. 2004. CO2 uptake by the cultivated hemiepiphytic cactus, Hylocereus undatus. Annals of Applied Biology 144: 1–8. [Google Scholar]
- Nuernbergk EL. 1961. Endogener Rhythmus und CO2-Stoffwechsel bei Pflanzen mit diurnalem Säurerhythmus. Planta 56: 28–70. [Google Scholar]
- Nuernbergk EL. 1963. On the CO2 metabolism of orchids and its ecological aspects. Proceedings of 4th World Orchid Conference, Singapore. Singapore: Straits Times Press, 158–169. [Google Scholar]
- Ogburn RM, Edwards EJ.. 2010. The ecological water-use strategies of succulent plants. In: Kader JC, Delseny M, eds. Advances in Botanical Research, Vol. 55. Burlington: Academic Press, 179–225. [Google Scholar]
- Ong BL, Koh CK-K, Wee YC.. 1998. Effects of CO2 on growth and photosynthesis of Pyrrosia piloselloides (L.) price gametophytes. Photosynthetica 35: 21–27. [Google Scholar]
- Paton S. 2022. Yearly reports Barro Colorado Island. Smithsonian Tropical Research Institute. Data set. 10.25573/data.11799111.v3 (18 March 2022, date last accessed). [DOI] [Google Scholar]
- Poorter L, Craven D, Jakovac CC, et al. 2021. Multidimensional tropical forest recovery. Science 374: 1370–1376. [DOI] [PubMed] [Google Scholar]
- Portillo-Quintero C, Sanchez-Azofeifa A, Calvo-Alvarado J, Quesada M, do Espirito Santo MM.. 2015. The role of tropical dry forests for biodiversity, carbon and water conservation in the neotropics: lessons learned and opportunities for its sustainable management. Regional Environmental Change 15: 1039–1049. [Google Scholar]
- Proctor MCF. 2012. Light and desiccation responses of some Hymenophyllaceae (filmy ferns) from Trinidad, Venezuela and New Zealand: poikilohydry in a light-limited but low evaporation ecological niche. Annals of Botany 109: 1019–1026. doi: 10.1093/aob/mcs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada IM, Gianoli E.. 2011. Crassulacean acid metabolism photosynthesis in Bromeliaceae: an evolutionary key innovation. Biological Journal of the Linnean Society 104: 480–486. doi: 10.1111/j.1095-8312.2011.01713.x. [DOI] [Google Scholar]
- Raveh E, Gersani M, Nobel PS.. 1995. CO2 uptake and fluorescence responses for a shade-tolerant cactus Hylocereus undatus under current and doubled CO2 concentrations. Physiologia Plantarum 93: 505–511. doi: 10.1111/j.1399-3054.1995.tb06850.x. [DOI] [Google Scholar]
- Reyes-García C, Griffiths H, Rincón E, Huante P.. 2008. Niche differentiation in tank and atmospheric epiphytic bromeliads of a seasonally dry forest. Biotropica 40: 168–175. [Google Scholar]
- Rodrigues MA, Matiz A, Cruz AB, et al. 2013. Spatial patterns of photosynthesis in thin- and thick-leaved epiphytic orchids: unravelling C3–CAM plasticity in an organ-compartmented way. Annals of Botany 112: 17–29. doi: 10.1093/aob/mct090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa-Manzano E, Andrade JL, Zotz G, Reyes-García C.. 2014. Respuestas fisiológicas a la sequía de cinco especies de orquídeas epífitas en dos selvas secas de la península de Yucatán. Botanical Sciences 92: 607–616. doi: 10.17129/botsci.139. [DOI] [Google Scholar]
- Rosado-Calderón AT, Tamayo-Chim M, de la Barrera E, et al. 2020. High resilience to extreme climatic changes in the CAM epiphyte Tillandsia utriculata L. (Bromeliaceae). Physiologia Plantarum 168: 547–562. [DOI] [PubMed] [Google Scholar]
- Sage RF, Monson RK, Ehleringer JR, Adachi S, Pearcy RW.. 2018. Some like it hot: the physiological ecology of C4 plant evolution. Oecologia 187: 941–966. doi: 10.1007/s00442-018-4191-6. [DOI] [PubMed] [Google Scholar]
- Sala OE, Chapin FS 3rd, Armesto JJ, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1774. [DOI] [PubMed] [Google Scholar]
- Schellenberger Costa D, Zotz G, Hemp A, Kleyer M.. 2018. Trait patterns of epiphytes compared to other plant life forms along a tropical elevation gradient. Functional Ecology 32: 2073–2084. [Google Scholar]
- Schmidt G, Zotz G.. 2001. Ecophysiological consequences of differences in plant size: in situ carbon gain and water relations of the epiphytic bromeliad, Vriesea sanguinolenta. Plant, Cell & Environment 24: 101–112. [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K.. 2009. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiology 149: 1838–1847. doi: 10.1104/pp.108.132555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K.. 2010. The incidence of crassulacean acid metabolism in Orchidaceae derived from carbon isotope ratios: a checklist of the flora of Panama and Costa Rica. Botanical Journal of the Linnean Society 163: 194–222. doi: 10.1111/j.1095-8339.2010.01058.x. [DOI] [Google Scholar]
- Silvera K, Santiago LS, Winter K.. 2005. Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Functional Plant Biology 32: 397–407. doi: 10.1071/fp04179. [DOI] [PubMed] [Google Scholar]
- Silvestro D, Zizka G, Schulte K.. 2014. Disentangling the effects of key innovations on the diversification of Bromelioideae (Bromeliaceae). Evolution 68: 163–175. [DOI] [PubMed] [Google Scholar]
- Sinclair R. 1984. Water relations of tropical epiphytes. III. Evidence for CAM. Journal of Experimental Botany 35: 1–7. doi: 10.1093/jxb/35.1.1. [DOI] [Google Scholar]
- Siyum ZG. 2020. Tropical dry forest dynamics in the context of climate change: syntheses of drivers, gaps, and management perspectives. Ecological Processes 9: 25. [Google Scholar]
- Smith JAC, Winter K.. 1996. Taxonomic distribution of crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Berlin: Springer, 427–436. [Google Scholar]
- Sobel AH, Camargo SJ, Hall TM, Lee C-Y, Tippett MK, Wing AA.. 2016. Human influence on tropical cyclone intensity. Science 353: 242–246. doi: 10.1126/science.aaf6574. [DOI] [PubMed] [Google Scholar]
- Stuart TS. 1968. The revival and respiration and photosynthesis in dried leaves of Polypodium polypodioides. Planta 83: 185–206. doi: 10.1007/bf00385023. [DOI] [PubMed] [Google Scholar]
- Sunderland TCH, Apgaua D, Baldauf C, et al. 2015. Global dry forests: a prologue. International Forestry Review 17: 1–9. doi: 10.1505/146554815815834813. [DOI] [Google Scholar]
- Tay S, He J, Yam TW.. 2019. CAM plasticity in epiphytic tropical orchid species responding to environmental stress. Botanical Studies 60: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay J, Zotz G, Pusczylowski J, Einzmann HJR.. 2021. Go with the flow: the extent of drag reduction as epiphytes reorient in wind. PLoS One 16: e0252790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeri JA, Tonsor SJ, Turner M.. 1981. Leaf thickness and carbon isotope composition in the Crassulaceae. Oecologia 50: 367–369. doi: 10.1007/bf00344977. [DOI] [PubMed] [Google Scholar]
- Torres-Morales G, Lasso E, Silvera K, Turner BL, Winter K.. 2020. Occurrence of crassulacean acid metabolism in Colombian orchids determined by leaf carbon isotope ratios. Botanical Journal of the Linnean Society 193: 431–477. doi: 10.1093/botlinnean/boaa027. [DOI] [Google Scholar]
- Wagner K, Wanek W, Zotz G.. 2021. Functional traits of a rainforest vascular epiphyte community: trait covariation and indications for host specificity. Diversity 13: 97. doi: 10.3390/d13020097. [DOI] [Google Scholar]
- Wagner K, Zotz G.. 2018. Epiphytic bromeliads in a changing world: the effect of elevated CO2 and varying water supply on growth and nutrient relations. Plant Biology 20: 636–640. doi: 10.1111/plb.12708. [DOI] [PubMed] [Google Scholar]
- Ward JK, Tissue DT, Thomas RB, Strain BR.. 1999. Comparative responses of model C3 and C4 plants to drought in low and elevated CO2. Global Change Biology 5: 857–867. [Google Scholar]
- Williams CB, Murray JG, Glunk A, Dawson TE, Nadkarni NM, Gotsch SG.. 2020. Vascular epiphytes show low physiological resistance and high recovery capacity to episodic, short-term drought in Monteverde, Costa Rica. Functional Ecology 34: 1537–1550. doi: 10.1111/1365-2435.13613. [DOI] [Google Scholar]
- Winter K. 1979. δ13C values of some succulent plants from Madagascar. Oecologia 40: 103–112. doi: 10.1007/bf00388814. [DOI] [PubMed] [Google Scholar]
- Winter K. 2019. Ecophysiology of constitutive and facultative CAM photosynthesis. Journal of Experimental Botany 70: 6495–6508. doi: 10.1093/jxb/erz002. [DOI] [PubMed] [Google Scholar]
- Winter K, Aranda J, Garcia M, Virgo A, Paton SR.. 2001. Effect of elevated CO2 and soil fertilization on whole-plant growth and water use in seedlings of a tropical pioneer tree, Ficus insipida Willd. Flora 196: 458–464. doi: 10.1016/s0367-2530(17)30087-7. [DOI] [Google Scholar]
- Winter K, Garcia M, Holtum JAM.. 2014. Nocturnal versus diurnal CO2 uptake: how flexible is Agave angustifolia? Journal of Experimental Botany 65: 3695–3703. doi: 10.1093/jxb/eru097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM.. 2002. How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiology 129: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM, Smith JAC.. 2015. Crassulacean acid metabolism: a continuous or discrete trait? New Phytologist 208: 73–78. doi: 10.1111/nph.13446. [DOI] [PubMed] [Google Scholar]
- Winter K, Medina E, García V, Mayoral ML, Muniz R.. 1985. Crassulacean acid metabolism in roots of the leafless orchid, Campylocentrum tyrridion Garay & Dunsterv. Journal of Plant Physiology 118: 73–78. [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC.. 1996. An introduction to crassulacean acid metabolism: biochemical principles and biological diversity. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Berlin: Springer, 1–13. [Google Scholar]
- Winter K, Smith JAC.. 2022. CAM photosynthesis: the acid test. New Phytologist 233: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Wallace BJ, Stocker GC, Roksandic Z.. 1983. Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia 57: 129–141. doi: 10.1007/bf00379570. [DOI] [PubMed] [Google Scholar]
- Woods CL, DeWalt SJ.. 2013. The conservation value of secondary forests for vascular epiphytes in Central Panama. Biotropica 45: 119–127. [Google Scholar]
- Yamori W, Hikosaka K, Way DA.. 2014. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynthesis Research 119: 101–117. [DOI] [PubMed] [Google Scholar]
- Yang S-J, Sun M, Yang Q-Y, Ma R-Y, Zhang J-L, Zhang S-B.. 2016. Two strategies by epiphytic orchids for maintaining water balance: thick cuticles in leaves and water storage in pseudobulbs. AoB Plants 8: plw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Bartholomew DP, Goldstein G.. 1997. Effect of elevated carbon dioxide on the growth and physiological responses of pineapple, a species with crassulacean acid metabolism. Journal of the American Society of Horticultural Science 122: 233–237. [Google Scholar]
- Zotz G. 1997. Substrate use of three epiphytic bromeliads. Ecography 20: 264–270. doi: 10.1111/j.1600-0587.1997.tb00370.x. [DOI] [Google Scholar]
- Zotz G. 2002. Categories and CAM – blurring divisions, increasing understanding? New Phytologist 156: 4–6. doi: 10.1046/j.1469-8137.2002.00511.x. [DOI] [Google Scholar]
- Zotz G. 2004. How prevalent is crassulacean acid metabolism among vascular epiphytes? Oecologia 138: 184–192. doi: 10.1007/s00442-003-1418-x. [DOI] [PubMed] [Google Scholar]
- Zotz G. 2016. Plants on plants. The biology of vascular epiphytes. Cham: Springer International Publishing Switzerland. [Google Scholar]
- Zotz G, Bogusch W, Hietz P, Ketteler N.. 2010. Growth of epiphytic bromeliads in a changing world: the effect of elevated CO2 and varying water and nutrient supply. Acta Oecologica 36: 659–665. doi: 10.1016/j.actao.2010.10.003. [DOI] [Google Scholar]
- Zotz G, Hietz P, Einzmann HJR.. 2021a. Functional ecology of vascular epiphytes. Annual Plant Reviews Online 4: 869–905. [Google Scholar]
- Zotz G, Leja M, Aguilar-Cruz Y, Einzmann HJR.. 2020. How much water is in the tank? An allometric analysis with 205 bromeliad species. Flora – Morphology, Distribution, Functional Ecology of Plants 264: 151557. doi: 10.1016/j.flora.2020.151557. [DOI] [Google Scholar]
- Zotz G, Hietz P.. 2001. The physiological ecology of vascular epiphytes: current knowledge, open questions. Journal of Experimental Botany 52: 2067–2078. doi: 10.1093/jexbot/52.364.2067. [DOI] [PubMed] [Google Scholar]
- Zotz G, Thomas V.. 1999. How much water is in the tank? Model calculations for two epiphytic bromeliads. Annals of Botany 83: 183–192. [Google Scholar]
- Zotz G, Tyree MT.. 1996. Water stress in the epiphytic orchid, Dimerandra emarginata (G. Meyer) Hoehne. Oecologia 107: 151–159. doi: 10.1007/bf00327898. [DOI] [PubMed] [Google Scholar]
- Zotz G, Winkler U.. 2013. Aerial roots of epiphytic orchids: the velamen radicum and its role in water and nutrient uptake. Oecologia 171: 733–741. doi: 10.1007/s00442-012-2575-6. [DOI] [PubMed] [Google Scholar]
- Zotz G, Winter K.. 1994. Annual carbon balance and nitrogen use efficiency in tropical C3 and CAM epiphytes. New Phytologist 126: 481–492. doi: 10.1111/j.1469-8137.1994.tb04245.x. [DOI] [PubMed] [Google Scholar]
- Zotz G, Vollrath B, Schmidt G.. 2003. Carbon relations of fruits of epiphytic orchids. Flora 198: 98–105. [Google Scholar]
- Zotz G, Weigelt P, Kessler M, Kreft H, Taylor A.. 2021b. EpiList 1.0: a global checklist of vascular epiphytes. Ecology 102: e03326. [DOI] [PubMed] [Google Scholar]
- Zotz G, Ziegler H.. 1997. The occurrence of crassulacean acid metabolism among vascular epiphytes from Central Panama. New Phytologist 137: 223–229. doi: 10.1046/j.1469-8137.1997.00800.x. [DOI] [PubMed] [Google Scholar]


