Abstract
Clusia is the only genus containing dicotyledonous trees with a capacity to perform crassulacean acid metabolism (CAM). Since the discovery of CAM in Clusia 40 years ago, several studies have highlighted the extraordinary plasticity and diversity of life forms, morphology and photosynthetic physiology of this genus. In this review, we revisit aspects of CAM photosynthesis in Clusia and hypothesize about the timing, the environmental conditions and potential anatomical characteristics that led to the evolution of CAM in the group. We discuss the role of physiological plasticity in influencing species distribution and ecological amplitude in the group. We also explore patterns of allometry of leaf anatomical traits and their correlations with CAM activity. Finally, we identify opportunities for further research on CAM in Clusia, such as the role of elevated nocturnal accumulation of citric acid, and gene expression in C3–CAM intermediate phenotypes.
Keywords: CAM photosynthesis, trees, plasticity, carbon, Neotropics
INTRODUCTION
Crassulacean acid metabolism (CAM) is a photosynthetic mode found in ~7 % of all vascular land plants, commonly as an adaptation to water-limited environments. Unlike C3 species, typical CAM plants fix atmospheric CO2 predominantly at night, leading to the synthesis of malate and its overnight storage as malic acid in the vacuoles of photosynthetic cells. The following day, malic acid is decarboxylated, thereby regenerating CO2, which is then refixed and reduced in the Calvin–Benson–Bassham cycle (Osmond, 1978). Thus, during the day (when the air is hottest and driest), CAM allows photosynthesis to occur behind closed stomata, resulting in less water being lost to the atmosphere, in comparison to C3 plants, over a 24 h period. CAM has evolved convergently in ≥37 families of vascular plants, with multiple independent origins within most families (Winter and Smith, 1996; Winter et al., 2021). However, despite CAM having evolved so many times, the Neotropical genus Clusia is thus far considered the only group of dicotyledonous trees in which some species show CAM (Lüttge, 2008).
Clusia is composed of ~321 species of terrestrial and hemiepiphytic woody plants distributed across the Neotropics (POWO, 2022). The plants also occur as epiphytes, although it is often not clear whether epiphytic individuals observed in the field eventually become hemiepiphytes. The plants occupy a wide range of habitats, from lowland forests to montane forests, in addition to high-elevation páramos (Fig. 1). Furthermore, Clusia is found in dry scrubs in interandean valleys, sandstone and granite rock outcrops, and open coastal sands (restingas), where they can often be pioneer species and function as nurse plants (Dias and Scarano, 2007; Gustafsson et al., 2007). The terrestrial habit of Clusia species is most common in high-elevation montane forests and in seasonally dry scrubland, where some species can have relatively high abundance [e.g. Clusia trochiformis Vesque in the Venezuelan Andes (Kelly et al., 1994) and Clusia flava Jacq in some areas of the Yucatán peninsula (Fig. 2)]. In contrast, the hemiepiphytic habit of Clusia is common in lower montane forests and lowland wet forests, where Clusia species grow mostly as woody shrubs on top of the mid to high branches of phorophytes [e.g. Clusia uvitana Pittier (previously described as Clusia odorata Seem.) and Clusia flavida (Benth.) Pipoly on Barro Colorado Island in Panama (Todzia, 1986); Clusia spp. in rainforests in Jamaica (Kelly, 1985)].
Fig. 1.

Habitats of selected Clusia species. (A) Clusia multiflora Kunth in cloud forest in the Central Cordillera in Colombia. (B) Clusia uvitana growing as a hemiepiphytic shrub in lowland wet forest in Panama. (C) Clusia pringlei Lundell growing in dry scrublands in central Mexico. (D) Clusia grandiflora Splitg. growing in swamp forests in the Orinoco Delta in Venezuela.
Fig. 2.

Habit of selected Clusia species, showing epiphytic and terrestrial plants with mature adventitious roots. (A, B) Epiphytic forms of Clusia massoniana Lundell growing on a phorophyte (oak trees). (C) Clusia rosea. (D) Clusia pringlei. (E) Clusia flava. Most mature roots in C and D are apparently developed from the same terrestrial individual plant, which produces adventitious roots that auto-strangle the main parental stem.
The earliest physiological observations on Clusia were probably made by Alexander von Humboldt during his travels in Venezuela, where he noticed that leaves of Clusia rosea Jacq., unlike those of other species, did not produce bubbles when submerged in water under direct sunlight (Lüttge, 2007). The lack of bubbles probably occurred because this CAM plant had closed its stomata during the day. However, Humboldt could not interpret his observations further owing to the technical limitations of his time, and it was not until the late 1940s when the CAM cycle was described formally (Bonner and Bonner, 1948; Thomas and Beevers, 1949). The presence of CAM activity in Clusia was reported properly, for the first time, by Tinoco-Ojanguren and Vázquez-Yanes (1983) and Ting et al. (1985), who measured titratable acidity and gas exchange in Clusia lundellii Standl and Clusia rosea, respectively. Subsequent physiological studies found that some Clusia species can shift between C3 and CAM depending on environmental conditions. This photosynthetic flexibility might be part of the reason why species of Clusia occupy such a wide range of habitats across the Neotropics (Franco et al., 1990; Lüttge, 1996).
PECULIARITIES OF THE CRASSULACEAN ACID METABOLISM CYCLE BIOCHEMISTRY IN CLUSIA
The CAM plants can be divided into two major groups regarding their mode of malate decarboxylation during the diurnal cycle: those that use mainly NADP- and NAD-malic enzyme (NAD-ME), and those that use predominantly phosphoenolpyruvate carboxykinase (PEPCK). In CAM-performing Clusia, PEPCK appears to be the predominant enzyme catalysing the decarboxylation reaction (Borland et al., 1998). However, in one of the few attempts to study gene expression in Clusia thus far, RNA-seq analysis found that both PEPCK and NAD-ME were upregulated when Clusia pratensis Seem. switched from C3 to CAM, suggesting that NAD-ME might also be contributing to malate decarboxylation (A. Leverett, unpublished data).
A peculiarity of Clusia species with CAM is the nocturnal accumulation of citric acid. When nocturnal H+ accumulation is measured across phylogenetically diverse CAM taxa, Clusia often exhibits the greatest dawn–dusk difference in titratable acidity (Winter and Smith, 2022). This is largely attributable to the accumulation of citric acid, which adds to the proton pool in the vacuole. Citric acid accumulation also occurs in other CAM species, such as Talinum triangulare (Jacq.) Willd., Ananas comosus (L.) Merr. and Nidularium billbergioides (Schult. and Schult.f.) L.B.Sm. However, the magnitude of citric acid accumulation in Clusia appears to be much greater than in any other taxa (Osmond et al., 1996). It is unclear whether citric acid accumulation is a direct consequence of CO2 assimilation. Citrate can be formed by converting malate to oxaloacetate, then adding a carbon atom via citrate synthase. However, this reaction requires acetyl-CoA, which is generated by the decarboxylation of pyruvate. Consequently, the net carbon balance of this reaction is neutral, meaning that nocturnal citrate accumulation would have no impact on CO2 assimilation (Lüttge, 1988). In contrast, if mitochondrial isocitrate dehydrogenase were to carboxylate α-ketoglutarate, in the reverse direction to its typical function, the product of this reaction, d-isocitrate, could be converted to citrate. If this were the case, citric acid would be the direct consequence of CO2 assimilation. Although this scenario has been simulated using flux balance analysis modelling, experimental evidence is lacking (Töpfer et al., 2020; Winter and Smith, 2022). Clusia is an ideal model in which to test the metabolic origin of citric acid, because this genus accumulates this molecule in abundance during the night.
Finally, CAM plants in the genus Clusia appear to exhibit elevated nocturnal respiratory rates, in comparison to C3 species. A comparison of four Venezuelan species of Clusia reported nocturnal oxygen uptake rates that were more than twice as large in constitutive CAM species than in weak CAM species (Ting et al., 1987). Likewise, the drought-induced switch from C3 to CAM in Clusia pratensis is accompanied by a 1.6-fold increase in oxygen consumption. In contrast, the same drought treatment applied to obligate C3Clusia species does not cause a change in nocturnal oxygen consumption, suggesting that this switch in respiratory physiology is a requirement of CAM and not simply a more general drought response (A. Leverett, unpublished data). Recent flux balance analysis modelling of the CAM cycle (Shameer et al., 2018) has suggested that CAM requires elevated nocturnal respiratory rates, in large part to drive the ATP-dependent import of malate into the vacuole. Clusia appears to corroborate the predictions from this model. However, it remains unclear whether elevated nocturnal respiratory rates are a peculiarity, unique to Clusia, or if other CAM taxa share this trait.
LARGE PHOTOSYNTHETIC CELLS AND CRASSULACEAN ACID METABOLISM: A FUNCTIONAL RELATIONSHIP
In most leaf-succulent lineages that perform CAM, such as Kalanchoë, Crassula and Yucca, the mesophyll is composed of relatively uniform, spherical chlorenchyma cells (Abdel-Raouf, 2012; Heyduk et al., 2016; Fradera-Soler et al., 2021). In contrast, Clusia leaves contain well-defined palisade and spongy mesophyll layers, the latter with large intercellular spaces (Fig. 3; Popp et al., 1987; Borland et al., 1998). Relationships between CAM and anatomy have probably been studied more comprehensively in Clusia than in any other genus (Popp et al., 1987; Borland et al., 1998, 2018; Barrera-Zambrano et al., 2014; Luján et al., 2022; Leverett et al., 2023a). Investigations into both glasshouse- and field-grown Clusia species have demonstrated that CAM species have larger palisade cells and thicker palisade tissue than C3 relatives (Barrera-Zambrano et al., 2014; Luján et al., 2022). Development of large photosynthetic tissues within leaves of CAM species is believed to provide overnight storage space for malic acid. Although interspecific comparative studies show that CAM is associated with large photosynthetic cells, these relationships remain correlative (Barrera-Zambrano et al., 2014; Males, 2018). This is problematic, because it is unclear whether greater volume in the palisade truly exists to aid the storage of nocturnal malic acid or whether large photosynthetic cells are, in fact, an indirect consequence of maximizing leaf water-storage capabilities, which would also be beneficial in water-limited niches (Edwards, 2019). Large cells are often thought to increase hydraulic capacitance, which allows leaves to mitigate reductions in water potential during dehydration, thereby protecting the mesophyll and vascular tissue from mechanical damage (Ogburn and Edwards, 2010, 2012). However, recent work has shown that interspecific variation in hydraulic capacitance is not driven by differences in palisade cell size or thickness in Clusia (Leverett et al., 2023a). Instead, variation in the size of specialized water-storage hypodermis tissue (which is itself independent of CAM) determines hydraulic capacitance. Therefore, the relationship between palisade cell size and CAM is not complicated by the confounding variable, hydraulic capacitance. Eliminating this potentially confounding relationship increases the confidence that large palisade cells have evolved specifically for the purpose of malic acid storage, rather than to buffer leaf water potentials (Leverett et al., 2023a). Therefore, rather than being merely correlative, there appears to be a truly functional relationship between CAM and large photosynthetic cells in Clusia.
Fig. 3.
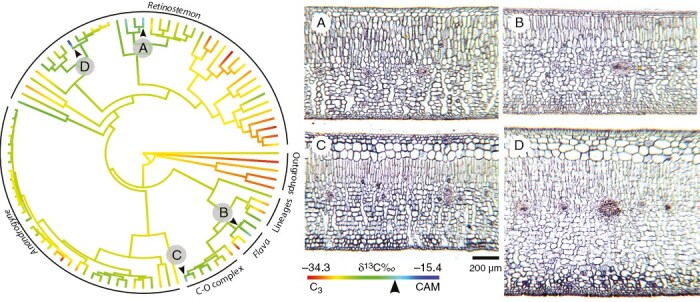
Ancestral state reconstruction of δ13C values on a maximum likelihood phylogenetic tree. Arrowheads indicate δ13C less negative than −20 ‰. (A–D) Transverse sections of leaves of plants with strong CAM. (A) Clusia uvitana. (B) Clusia lundellii. (C) Clusia rosea. (D) Clusia firmifolia Cuatrec. Modified from Luján et al. (2022).
CRASSULACEAN ACID METABOLISM AFFECTS MESOPHYLL ALLOMETRY IN CLUSIA LEAVES
In Clusia, there is evidence that palisade cells, and not the spongy mesophyll cells, are the main location of the CAM cycle. The abundance of PEPC in the palisade is substantially higher in leaves of Clusia species with CAM than in those doing C3, whereas abundance of this enzyme in the spongy mesophyll is comparable between species with contrasting photosynthetic modes (Barrera-Zambrano et al., 2014). Furthermore, anatomical studies have found that correlations between palisade cell size and CAM are far stronger than correlations between spongy mesophyll dimensions and CAM (Barrera-Zambrano et al., 2014; Borland et al., 2018; Luján et al., 2022). In contrast, Clusia species living in high-elevation cloud forests have thicker spongy mesophyll tissues. This might confer greater leaf longevity, as is seen in other taxa (Cordell et al., 1998). It is also possible that C3Clusia species living in moist, high-elevation montane forests develop thick spongy mesophyll layers to maximize absorption of light, which is often obscured by cloud cover and algal mats. Hence, different environmental stresses might affect the development of each tissue layer: low water availability has led to large palisade cells to facilitate CAM, and cloud cover might have led to thick spongy mesophyll to maximize C3 photosynthesis in low-light conditions. A consequence of this anatomical specialization in Clusia leaves is that CAM affects the allometry of mesophyll layers. In other taxa, palisade and spongy mesophyll thickness and cell sizes scale tightly with each other, such that differences in palisade thickness will result in proportional differences in the spongy mesophyll (John et al., 2013). In Clusia, although palisade and spongy mesophyll thickness are correlated, the scaling factor that drives this relationship is affected by CAM. The contribution of CAM to mesophyll allometry can be observed, using published data (Luján et al., 2022) to build a multiple linear regression model, with palisade thickness as the dependent variable and both spongy mesophyll thickness and δ13C as predictive independent variables. This model shows that spongy mesophyll thickness is a significant predictor of palisade thickness (P = 0.02). However, the predictive correlation between spongy mesophyll thickness and palisade thickness is different depending on δ13C, which has a significant interaction effect on this relationship (P = 0.04). Likewise, these same anatomical data can be split into three groups (in which δ13C is <−30, −30 to −25 or >−25 ‰) and linear regressions built for each subset of data (Fig. 4). When this is done, the slope of the line describing the correlation between palisade and spongy mesophyll thickness differs; steeper slopes are seen when δ13C values are less negative (i.e. when there is a greater investment in CAM). Put simply, the presence of CAM affects the scaling relationship between spongy and palisade mesophyll thickness, such that CAM is associated with thicker palisade forming without proportional spongy mesophyll thickness. Interestingly, a recent study on the genus Cymbidium (Orchidaceae) suggests CAM might have had a similar effect on anatomical allometry to that observed in Clusia (Yamaga-Hatakeyama et al., 2022). In Cymbidium, CAM is associated with larger adaxial palisade cells, whereas the relationship between CAM and spongy mesophyll size is less pronounced. It would be intriguing to compare any climatic factors that have led to allometric differences between C3 and CAM plants in both Clusia and Cymbidium, to determine whether the anatomy of these genera has undergone parallel evolutionary trajectories.
Fig. 4.
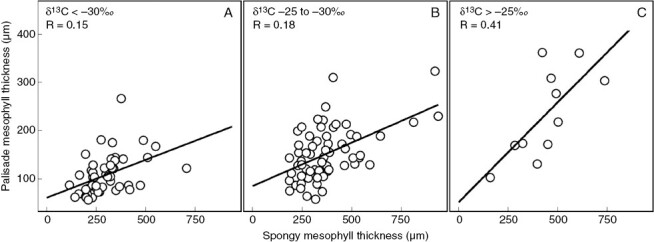
CAM affects the allometry of mesophyll tissues in Clusia leaves. Anatomical data were grouped into three bins: (A) δ13C < −30 ‰; (B) δ13C values between −30 and −25 ‰; and (C) δ13C > −25 ‰. The correlation between palisade and spongy mesophyll tissue thickness was estimated.
INTERNAL AIR SPACE IN CLUSIA LEAVES
In addition to large photosynthetic cells, the leaves of CAM plants are often characterized by low fractional internal air space (% IAS), because the development of tightly packed cells will maximize the space available for the storage of malic acid within a given volume of leaf (Nelson et al., 2005; Males, 2018). However, the relationship between CAM and % IAS is called into question in Clusia. Analysis of 64 field-grown species found that δ13C was negatively correlated with % IAS, although the effect size was weak (Fig. 5A). However, this trend did not hold true when these data were recalculated to estimate the % IAS in the spongy mesophyll. If porosity in the epidermis, hypodermis and palisade are assumed to be negligible, then the % IAS of the spongy mesophyll can be estimated by dividing the total % IAS by the fraction of leaf thickness composed of spongy mesophyll. This approach found no correlation between spongy mesophyll % IAS and δ13C (Fig. 5B). Hence, correlations between total % IAS and CAM in Clusia are likely to be the consequence of thicker palisade tissues, rather than developmental differences in the spongy mesophyll. It seems that, in Clusia, the requisite anatomical configurations required by CAM can evolve in the palisade without affecting % IAS in the spongy mesophyll. Being able to evolve CAM without decreasing spongy mesophyll % IAS could be beneficial, because it would allow relatively high rates of mesophyll conductance, which would be likely to aid assimilation rates during phases I, II and IV of the CAM cycle (Owen and Griffiths, 2013).
Fig. 5.
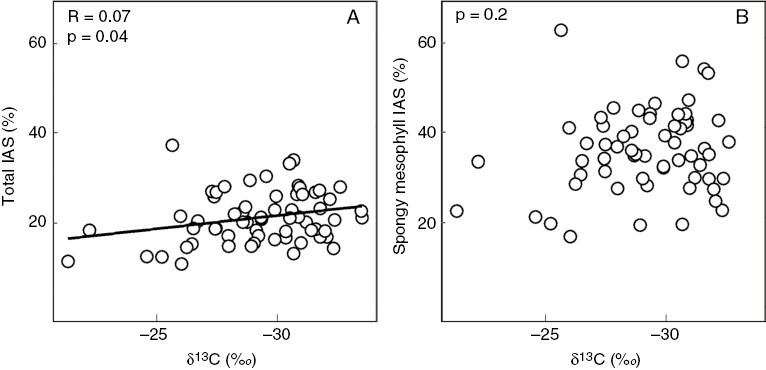
Linear regression between δ13C and the percentage of total intercellular air space (A) and the percentage intercellular air space of the spongy mesophyll (B).
LEAF VASCULAR ARCHITECTURE IS COORDINATED WITH CRASSULACEAN ACID METABOLISM
In addition to studies on mesophyll anatomy, the relationship between CAM and leaf vascular anatomy has recently been analysed in Clusia (Leverett et al., 2023b). Across ten photosynthetically diverse species, leaves with constitutive CAM phenotypes developed lower vein length per area (VLA) and a lower vein termini density (VTD) compared with facultative CAM or obligate C3 species (Leverett et al., 2023b). The reduced transpiration rates in constitutive CAM species result in lower fluxes of water across the leaf (Winter et al., 2005; Leverett, et al., 2023a). Hence, constitutive CAM species can stay hydrated with lower leaf vein densities, because less water needs to flow through the xylem. In contrast, in facultative CAM species, VLA and VTD values closely resemble those seen in obligate C3 species (Leverett et al., 2023b). In Clusia species in Panama, outside the dry season, facultative CAM species predominantly assimilate CO2 via C3 photosynthesis, which is associated with higher transpiration rates than those seen in CAM leaves. Given that facultative CAM species spend most of the year doing C3, they appear to optimize their VLA and VTD to allow elevated hydraulic conductance when plants are engaged in C3 photosynthesis, rather than when plants are performing CAM.
In addition to VLA, CAM is linked to differences in vascular allometry in Clusia leaves (Leverett et al., 2023b). In almost all angiosperm species, intervein distance (IVD) is coordinated with the distance from veins to the lower epidermis (VED), such that IVD ≈ VED (Zwieniecki and Boyce, 2014). If IVD:VED ratios are higher than one, insufficient veins are present to maintain mesophyll hydration, whereas IVD:VED ratios less than one would often result in superfluous veins that do not add substantial hydraulic benefit to the leaf (Noblin et al., 2008). However, in some CAM taxa, the allometry between vein and mesophyll dimensions varies from that seen in most angiosperms (Males, 2017). In Clusia, C3 species have IVD:VED ratios of approximately one. However, species that engage more in CAM have thicker leaves, without equivalently lower vein densities, meaning that these species have IVD:VED ratios less than one. It is likely that the thick mesophyll tissue of CAM species is associated with greater mesophyll resistance, because water must travel a greater distance to move from the xylem conduits to stomata. The apparent ‘overinvestment’ in veins (i.e. IVD:VED ratios less than one) could act as an adaptation to overcome this apoplastic hydraulic resistance, thereby allowing the thick palisade tissue needed for CAM to remain hydrated when leaves are transpiring (de Boer et al., 2016). Overall, VLA, VTD and IVD:VED ratios all appear to be coordinated with CAM across Clusia, highlighting that this metabolic adaptation does not only affect photosynthetic cells but also has a considerable impact on the hydraulic architecture of leaves (Males and Griffiths, 2018).
PHOTOSYNTHETIC PATHWAY DIVERSITY IN CLUSIA: C3 VERSUS CRASSULACEAN ACID METABOLISM PHOTOSYNTHESIS
According to the survey by Pachon et al. (2022), photosynthetic pathway information is available for 156 of the 321 currently accepted species of Clusia (POWO, 2022). For ≥35 species, there is evidence of CAM, although the nature and magnitude of CAM expression vary widely between species (Lüttge, 2006; Barrera-Zambrano et al., 2014). Clusia species with CAM typically occur in lowland habitats and belong to section Retinostemon (which corresponds, in part, to the Clusia minor group sensuHammel, 1986) and to a clade that includes the Clusia flava group and the Omphalanthera–Chlamydoclusia complex (Luján et al., 2022). In contrast, C3Clusia species are predominantly found at higher elevations and typically belong to the species-rich section Anandrogyne (similar to the Clusia multiflora group in the study by Hammel, 1986).
Examples of species in which the CAM pathway is the principal contributor to carbon gain are the drought-tolerant Clusia hilariana Schltdl. and Clusia flava Jacq. Clusia hilariana grows in the edaphically and climatically harsh restingas of eastern Brazil (Franco et al., 1996), and Clusia flava is well known from the seasonally dry Yucatan Peninsula in Mexico. Thirty-eight of 39 specimens of Clusia flava collected at various sites in Mexico had δ13C values less negative than −20 ‰, indicative of strong CAM (−15.6 ± 2.9 ‰, mean ± s.d., n = 39; Vargas-Soto et al., 2009). At the other end of the CAM spectrum are species with low-level CAM, such as those with only small nocturnal increases in leaf tissue acidity. In these species, C3-photosynthetic CO2 uptake during the daytime remains the main route of carbon acquisition during the annual cycle, as indicated by their C3-type δ13C values (Holtum et al., 2004). In addition, some species display pronounced periodic, facultative CAM, such as the closely related Clusia minor L. and Clusia pratensis. Potted plants of these species, when well watered, fix CO2 mainly through the C3 pathway, although some weak CAM can also occur. When irrigation is withheld, C3 photosynthetic CO2 uptake during the daytime declines, and nocturnal net CO2 via CAM is induced or strongly upregulated. Plants revert to a largely C3-type pattern of diel net CO2 exchange upon rewatering (e.g. Borland et al., 1998; Winter et al., 2008; Winter and Holtum, 2014). Individual attached leaves of Clusia pratensis have the capacity to switch between C3 and CAM reversibly, at least four times during successive wet–dry–wet cycles (K. Winter, unpublished data).
Consistent with field studies by Borland et al. (1992) on Clusia minor in Trinidad during the transition from the wet season to the dry season and consistent with facultative CAM, nocturnal increases in leaf-tissue acidity were restricted to the 4-month-long dry season, from mid-December to mid-April, in 3-m-tall plants of Clusia minor and Clusia pratensis growing at the forest edge at the Smithsonian Tropical Research Institute research facilities in Gamboa, Panama (K. Winter, unpublished data). Nocturnal acidification was not detectable during the remaining wet months of the year. In contrast, Clusia rosea at the same site exhibited nocturnal acidification throughout the seasons. The iconic Clusia rosea is one of the best-known and best-studied species of Clusia and generally considered a species with strong obligate CAM. Indeed, δ13C values as high as −14.5 ‰ have been recorded for plants from Mexico, northern Venezuela and southern Florida (Popp et al., 1987; Sternberg et al., 1987; Vargas-Soto et al., 2009), although δ13C values in the C3 range were also noted. For example, in a Panamanian field study of Clusia rosea, δ13C values ranged from −16.7 to −27.5 ‰ (Holtum et al., 2004), and in a recent Colombian survey, δ13C values were entirely within the C3 range (−22.2 to −26.5 ‰; Pachon et al., 2022), suggesting that Clusia rosea has greater photosynthetic pathway plasticity than previously thought.
One of the most remarkable studies on C3–CAM physiology in Clusia is probably the detailed work by Gerhard Zotz on epiphytic and hemiepiphytic individuals of Clusia uvitana Pittier in the crown of a 47-m-tall kapok tree [Ceiba pentandra (L.) Gaertn.] on Barro Colorado Island, Panama. In agreement with laboratory studies of potted Clusia uvitana exhibiting clear features of facultative CAM on top of a low- to medium-level obligate CAM background (Winter et al., 1992), Zotz’s in situ gas-exchange measurements revealed increased dark CO2 fixation and greater nocturnal acidification of Clusia uvitana during the dry season compared with the wet season (Zotz and Winter, 1993, 1994a, 1994b, 1996; Zotz et al., 1995). Besides these long-term seasonal trends, extremely rapid alterations in CAM activity were also evident in situ. On days without precipitation, Clusia uvitana showed uptake of atmospheric CO2 at night, a feature of CAM, and the early morning and late afternoon, whereas during 36 h of almost continuous rainfall the nocturnal net CO2 uptake stopped, and the diel pattern of net CO2 exchange became like that of a C3 plant (Zotz and Winter, 1993). The rapid switching between CAM- and C3-type carbon fixation occurred because decarboxylation was repressed in low-light conditions, thereby saving malic acid reserves until environmental conditions warranted the use of CAM.
Despite the extraordinary photosynthetic flexibility of some species of Clusia, gene-expression studies are lacking that track the up- and downregulation of enzymes during transitions from C3 to CAM and vice versa. We suggest this to be a top priority of future Clusia research.
CARBON ISOTOPE RATIOS IN CLUSIA
Lineages with large numbers of C3 and CAM species, such as Bromeliaceae, Orchidaceae or the genus Euphorbia (Euphorbiaceae), show a distinct bimodal distribution of δ13C values, with a pronounced frequency peak around −27 ‰, typical of species with C3 photosynthesis, and a second peak around −15 ‰, typical of species with strong CAM (Silvera et al., 2010; Horn et al., 2014; Crayn et al., 2015). In contrast, only a few Clusia species, such as Clusia flava, are known to exhibit δ13C values consistently within the strong CAM range (Vargas-Soto et al., 2009), and in frequency histograms of Clusia isotope surveys, the CAM peak, if one exists, is very small (Holtum et al., 2004; Pachon et al., 2022). The δ13C values of the majority of Clusia species, including many isotope values of Clusia species with facultative CAM, contribute to a large cluster of values consistent with predominantly C3 photosynthetic CO2 fixation (Winter and Holtum, 2002). In other lineages containing annual facultative CAM species, such as Mesembryanthemum crystallinum L., CAM is induced in the middle of the lifespan of the plant, with onset of the dry season. At the end of the lifespan, leaves from this species will exhibit δ13C values common to CAM (Winter et al., 1978). In contrast, the situation in Clusia species with facultative CAM is completely different, because leaf longevity significantly exceeds 1 year. Although species such as Clusia minor and Clusia pratensis can show substantial CAM activity during a 3-month dry season in Panama (K. Winter, unpublished data), for most of the year the leaves perform predominantly C3 photosynthesis, which strongly dilutes the isotopic signal of periodically occurring CAM. In addition, it is possible that growth rates are substantially slower in the dry season, when water availability becomes limiting. Such a scenario would mean that even if a quarter of the year was spent doing CAM, this would be disproportionately underrepresented in the δ13C value of leaves. Consequently, in Clusia, the value of δ13C in characterizing photosynthetic pathway diversity and predicting the ability of a species to perform CAM is clearly limited, unless δ13C values are in the −15 ‰ range consistent with strong sustained CAM.
EVOLUTIONARY TRAJECTORIES OF CRASSULACEAN ACID METABOLISM IN CLUSIA
It is unclear whether C3–CAM phenotypes are evolutionary transitional stages towards strong CAM or stable states that have been selected during the evolutionary history of various plant lineages (Edwards, 2019). The significant number of C3–CAM phenotypes in Clusia might indicate that these intermediate forms have been stably selected. There are multiple advantages of maintaining C3–CAM phenotypes, because the optional use of CAM during the dry season improves water-use efficiency (Winter and Holtum, 2014). It stands to reason that these plastic phenotypes might be favoured by stabilizing selection, although the role of phenotypic plasticity remains a controversial topic in evolutionary biology, because conflicting predictions exist about whether plasticity constrains or facilitates adaptive evolution (Paenke et al., 2007; Ghalambor et al., 2015). Describing the genetic mechanisms involved in upregulating CAM activity in C3–CAM phenotypes might allow testing for potential correlations between allele frequencies and environmental variables. Such studies might identify patterns of genomic variation that are indicative of natural selection. Furthermore, given that CAM has evolved multiple times independently within Clusia (Luján et al., 2022), it would be valuable to test whether the genetic mechanisms involved in the upregulation of CAM activity are comparable across different CAM lineages. In this context, the role of phenotypic plasticity, and in particular the patterns of how plasticity might be inherited over successive generations, deserves further attention (Charmantier et al., 2008; Fox et al., 2019). Cross-disciplinary approaches, including phylogenomic reconstructions based on transcriptomic data of CAM-related genes coupled with experimental physiological studies, are warranted to understand the role of photosynthetic plasticity in the evolution of Clusia.
EVOLUTIONARY HISTORY OF CRASSULACEAN ACID METABOLISM IN CLUSIA
Early phylogenetic reconstructions based on plastid (rbcL) and nuclear (ITS) markers found Clusia to be a well-supported monophyletic group (Gustafsson et al., 2002; Gustafsson and Bittrich, 2003). At least two independent origins of CAM photosynthesis have been suggested in the evolutionary history of Clusia (Vaasen et al., 2002; Gehrig et al., 2003); although multiple origins of CAM and no fewer than nine reversals have also been reconstructed (Gustafsson et al., 2007), indicating that CAM photosynthesis is a highly homoplasious trait. Recent multi-locus phylogenies have confirmed the monophyly of the genus and supported multiple independents origins of CAM in Clusia (Fig. 3). Although a dated phylogeny is still needed to describe the timing of the evolution of CAM in Clusia, preliminary time-calibrated phylogenetic analyses suggest that CAM is a relatively recent innovation in the group (Luján et al., 2022).
Reproductive morphology of the fossil species Paleoclusia chevalieri Crepet and Nixon suggest that Clusiaceae was well differentiated and a highly diversified group as early as the Turonian (i.e. 93.9–89.8 Ma; Crepet and Nixon, 1998; Schönenberger et al., 2020). Furthermore, Clusia fossilia Berry, a fossil species from the later Miocene (23.03–5.3 Ma) from Trinidad, displays close morphological resemblance to Clusia rosea (Berry, 1925), a species capable of strong CAM, suggesting that CAM capacity was likely developed in Clusia by that time. Major changes in terrestrial ecosystems, including the reduction of closed forests and expansion of grasslands, marked the transition between late Oligocene and early Miocene (30 Ma). These changes can be linked to fluctuations in global atmospheric CO2. Atmospheric CO2 directly affected tree cover by modifying water relationships in herbaceous vs. woody plants (Polley et al., 2002) and by altering plant growth rates (Bond et al., 2003). A significant drawdown of CO2 might have influenced the proliferation of open grassland habitat and opening of the forest vegetation during the early Miocene (Kürschner et al., 2008). Although explicit ancestral state reconstruction modelling is still necessary, it is likely that CAM capacity in Clusia evolved ~30 Ma as a carbon-concentration mechanism in response to the abrupt decline in global atmospheric CO2 that occurred in the Oligocene to Miocene transition.
The evolution of CAM in the clusioid clade (i.e. Clusiaceae and its allied families within Malpighiales; Ruhfel et al., 2011) seems to be exclusive to Clusia, because CAM has not been reported in any other clusioid genus. In the Neotropics, Clusia is the most species-rich lineage within the clusioid clade, followed by Garcinia (~105 Neotropical species). Given its relatively high species richness, it is tempting to propose that the presence of CAM might be correlated with increased speciation rates in Clusia. Nonetheless, CAM activity has not been found in the most species-rich clade within Clusia (section Anandrogyne; Fig. 3). Plants in this group are mostly trees from montane cloud forests in the Andes and Central America, habitats where terrestrial plants rarely experience severe soil water deficits (Bruijnzeel and Veneklaas, 1998). The increased species richness seen in the Anandrogyne clade might not be related to photosynthetic diversity but could instead have resulted from adaptative changes in reproductive biology (discussed in more detail below) linked to the Andean orogeny, a pattern seen in many other Neotropical plant lineages (Schwery et al., 2015; Lagomarsino et al., 2016). Relatively lower extinction rates in Clusia section Anandrogyne might also explain higher species richness observed in this group, although this remains to be tested.
THE ROLE OF LIFE HISTORY AND CLIMATE IN THE EVOLUTION OF CRASSULACEAN ACID METABOLISM IN CLUSIA
In some plant lineages, such as orchids, CAM is strongly associated with epiphytism. Species with a constitutive epiphytic life habit are considerably more likely to have CAM capacity, because the increased water-use efficiency conferred from this metabolic adaptation is advantageous to plants living with no access to soil water (Lüttge, 2004; Heyduk et al., 2019). However, in Clusia, the association between CAM and epiphytism is far less clear. No analysis has been able to show directly that epiphytic plants do more CAM. This might be, in part, because the life history of Clusia is highly plastic, given that many species can survive as epiphytes, hemiepiphytes or free-standing shrubs or trees (Figs 1 and 2). The plasticity in life history across Clusia is so pronounced that it is unclear whether any species of Clusia live exclusively as epiphytic plants. Likewise, photosynthetic physiology is extremely plastic in Clusia, because some species can upregulate carbon flux facultatively through the CAM cycle when subjected to drought. High plasticity, both in the predisposition for epiphytism–hemiepiphytism and in the strength of CAM, makes it challenging to determine the character states of species clearly (i.e. epiphyte, hemiepiphyte or terrestrial; or strong CAM, C3–CAM or C3, etc.). Therefore, it is extremely difficult to determine whether these characters are correlated across the genus. For example, extensive sampling of 909 herbaria samples found no relationship between epiphytism and δ13C ratios within Clusia (Pachon et al., 2022). It is also important to consider that although phenotypic plasticity might obscure any relationship between epiphytism and photosynthetic physiology, other traits, such as the predisposition to form adventitious aerial roots, might be more important than CAM in driving differences in growth habit. More comprehensive data on the extent of epiphytism across different species are required for a full understanding of the role (if any) that CAM plays in the life habit of Clusia species.
Although relationships between life habit and photosynthetic mode remain elusive, a clear pattern can be observed between CAM, climate and elevation within Clusia. In a Panamanian study, C3–CAM species have not been reported in species living >1689 m above sea level, and species with strong CAM were not collected at >680 m above sea level (Holtum et al., 2004). It appears that C3 species dominate montane cloud forests, possibly because these environments are less water limited and have less severe dry seasons than low-elevation ecosystems (Bruijnzeel and Veneklaas, 1998). Phylogenetic comparative analyses have established a link between CAM and water availability across Clusia species. For example, a study of 64 species found that species with lower δ13C values live in habitats characterized by a more severe dry season and greater precipitation seasonality, suggesting that constitutive CAM has evolved in response to precipitation deficits (Luján et al., 2022). In addition, species that can switch facultatively from C3 to CAM were found to live in climatic niches characterized by relatively high annual precipitation, but strong acute dry seasons (Leverett et al., 2021). Therefore, facultative CAM appears to be an adaptation to fluctuating precipitation availability; this adaptation allows plants to make use of higher growth rates associated with C3 when water is available, whilst also benefitting from the water-conserving nature of CAM during the dry season.
In addition to CAM and plant habit, reproductive biology might have also played an important role in the ecological distributions of some Clusia species. Although links between CAM and climate can be established over regional distributions (see Luján et al., 2022), these links are less clear at local scales. For example, no relationships were found between photosynthetic physiology and water availability at different local microsites in two locations in Southeast Brazil (Lüttge et al., 2015). The authors suggest that aspects of the reproductive biology of the species might be more relevant in explaining differences in plant distribution at local scales. Furthermore, two of the more widespread Clusia species (i.e. Clusia flavida and Clusia minor; Fig. 6), are terrestrial or hemiepiphytic shrubs with facultative CAM activity (Ting et al., 1987; Zotz et al., 1999). It would be intuitive to assume that CAM capacity might increase the ability of species to occupy diverse habitats. However, Clusia flavida and Clusia minor have smaller fruits than most facultative CAM Clusia species, which have narrower geographical ranges and larger fruits (e.g. Clusia lundellii and Clusia uvitana; Fig. 6). A correlation between fruit size and species distribution has been observed in other tropical woody plant groups, in which smaller-fruited species have wider distribution ranges than species with larger fruits (e.g. Lauraceae;[Rossetto et al., 2015). Therefore, although CAM has probably played a role determining the distribution of Clusia species, aspects of reproductive biology, particularly related to fruit morphology and seed dispersion, might also be important. Potential correlations between fruit size and geographical range are not straightforward in Clusia, because there are widespread species (Fig. 7) with relatively large fruits, such as Clusia rosea (a species capable of strong CAM). Phylogenetic analyses that explicitly test potential correlations between CAM activity and speciation rates, reproductive morphology and geographical distribution are now needed to further our understanding of the evolutionary history of Clusia.
Fig. 6.
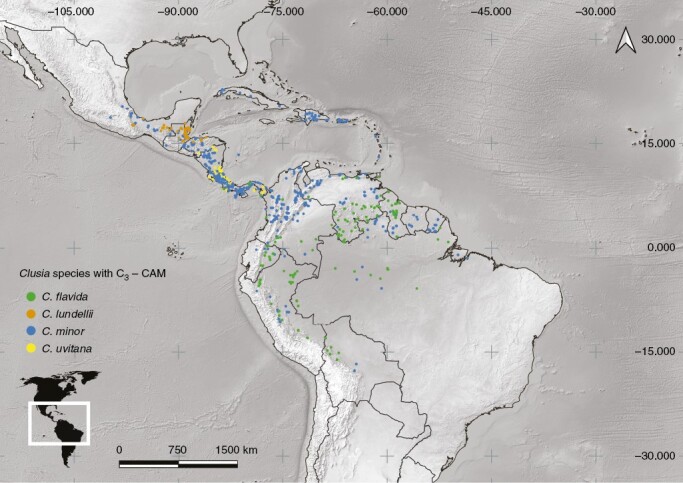
Geographical distribution map of selected species with intermediate C3–CAM, including Clusia flavida, C. minor, C. lundellii and C. uvitana.
Fig. 7.
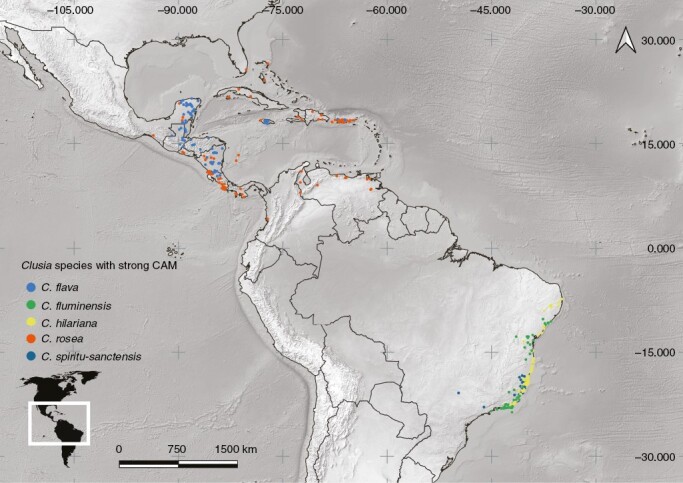
Geographical distribution map of selected species with strong CAM, including Clusia flava, C. fluminensis Planch. & Triana, C. hilariana, C. rosea and C. spiritu-sanctensis G. Mariz & B. Weinberg.
CLUSIA: A GENUS WITH TREES CAPABLE OF CRASSULACEAN ACID METABOLISM
Carbon-concentrating mechanisms appear to be very rare in tree species. Although some attention has been given to explaining the scarcity of C4 trees (Sage, 2014; Young et al., 2020, 2022), far less consideration has been given to understanding why there are so few trees capable of CAM (Borland et al., 2015). Clusia is the only known genus of trees (sensu stricto) with CAM, although woody arborescent CAM plants do exist; for example, the North American cactus Carnegiea gigantea (Engelm.) Britton and Rose and the African Euphorbia tirucalli L. (Hastilestari et al., 2013; Drezner, 2014). In addition, woody monocot CAM plants, such as Yucca filifera Chabaud and Aloidendron dichotomum (Masson) Klopper and Gideon F.Sm., can grow to many metres in height (González-Salvatierra, 2019; Grey et al., 2022). The existence of these species demonstrates that CAM is not incompatible with an arborescent life form, although CAM trees are the exception rather than the norm. The scarcity of the arborescent life form among CAM plants might have two explanations. Maximum rates of CO2 uptake per unit surface area are generally lower in CAM plants than in C3 and C4 plants, but in typical CAM plants this is offset, at least in part, by the fact that nearly the entire shoot surface is photosynthetic, as in Agaves and cacti. This is not the case in a tree, where substantial quantities of carbon are needed to support a largely non-photosynthetic woody trunk. Moreover, the high construction cost of succulent CAM leaves with much higher leaf masses per area than those of C3 tree leaves further limits overall plant growth rates compared with non-CAM trees, especially under a closed or partly closed canopy. It is therefore not surprising that although Clusia species with CAM can be important components of closed tropical forests, they are typically not found as free-standing trees on the ground of forests with a high leaf area index, but rather as epiphytes and hemiepiphytes high up in the canopy, where their foliage is closer to the sun. For example, in Panama, Clusia plants will often appear along forest edges and clearings, only to disappear when the forest canopy closes. Terrestrial CAM trees in Clusia are common in fully exposed, high-light environments, such as Clusia flava in open dry forest in the Yucatan and Clusia hilariana in dry Southeast Brazil. It is likely that environments with high light exposure might be a requirement for CAM trees, because they cannot sustain sufficient growth rates to become trees in low-light conditions.
CONCLUSIONS AND FUTURE DIRECTIONS
The study of CAM photosynthesis in Clusia continues to be a fascinating topic even after four decades of research on this system. The fact that some members of this group of tropical trees display such an extraordinary photosynthetic plasticity in response to rapid environmental change is relevant more than ever in the face of current global anthropogenic climatic change and in the context of bioengineering efforts to increase drought tolerance of useful plants. For instance, there is evidence that presence of Clusia species with CAM activity positively affects soil conditions in restinga vegetation in Southeast Brazil (Dias and Scarano, 2007), in addition to seed dispersion in forest edges and fragments of moist lowland forests in Colombia (Aide and Cavelier, 1994). If CAM capacity could be enhanced in C3 species of Clusia and other groups of trees growing in different types of vegetation, it would potentially enable short-term survival for more severe or extended drought periods, a desirable adaptation in plants used in forestry and ecological restoration across tropical regions.
Given that Clusia includes species with leaf architecture typical of C3 metabolism, in addition to species with relatively enlarged cells and tissues that allow strong CAM capacity, comparative analyses between C3 and CAM species have the potential to identify the necessary changes in critical enzymes and transporters needed for establishment of CAM. A complex network of interactions between genotypes, phenotypes and environmental conditions influences the expression of CAM. Integrative approaches that consider anatomical constraints in addition to gene expression patterns have been useful in disentangling the biochemical photosynthetic pathways of CAM (e.g. Heyduk et al., 2022; Moreno-Villena et al., 2022). Having a clearer understanding of the biochemical and genetic factors governing photosynthetic plasticity in Clusia could be instrumental for engineering CAM into C3 plants, allowing safer agriculture and forestry in a warming world with more unpredictable weather.
ACKNOWLEDGEMENTS
M.L. and A.L. are thankful to Klaus Winter for being a generous collaborator and an inspiring scientist; both authors were hosted by Klaus at the Smithsonian Institution in Panama through short-term fellowships to work on various aspects of CAM in Clusia. Klaus and his research team at STRI, including Aurelio Virgo and Jorge Aranda, fostered productive and collegial relationships that continue to support our research careers, for which we are extremely grateful. M.L. thanks Ivalú Cacho, Victor Steinmann, Iván Tamayo and Gabriella Ghiselli for their valuable companionship during fieldwork.
Contributor Information
Manuel Luján, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AE, UK.
Alistair Leverett, School of Life Sciences, University of Essex, Colchester, Essex CO4 3SQ, UK.
Klaus Winter, Smithsonian Tropical Research Institute, PO Box 0843-03092, Balboa, Ancón, Republic of Panama.
LITERATURE CITED
- Abdel-Raouf HS. 2012. Anatomical traits of some species of Kalanchoe (Crassulaceae) and their taxonomic value. Annals of Agricultural Sciences 57: 73–79. doi: 10.1016/j.aoas.2012.03.002. [DOI] [Google Scholar]
- Aide TM, Cavelier J.. 1994. Barriers to lowland tropical forest restoration in the Sierra Nevada de Santa Marta, Colombia. Restoration Ecology 2: 219–229. doi: 10.1111/j.1526-100x.1994.tb00054.x. [DOI] [Google Scholar]
- Barrera-Zambrano VA, Lawson T, Olmos E, Fernández-García N, Borland AM.. 2014. Leaf anatomical traits which accommodate the facultative engagement of crassulacean acid metabolism in tropical trees of the genus Clusia. Journal of Experimental Botany 65: 3513–3523. doi: 10.1093/jxb/eru022. [DOI] [PubMed] [Google Scholar]
- Berry E. 1925. A Pleistocene flora from the island of Trinidad. Proceedings of the United States National Museum 66: 1–9. doi: 10.5479/si.00963801.66-2558.1. [DOI] [Google Scholar]
- Bond WJ, Midgley GF, Woodward FI.. 2003. The importance of low atmospheric CO2 and fire in promoting the spread of grasslands and savannas. Global Change Biology 9: 973–982. doi: 10.1046/j.1365-2486.2003.00577.x. [DOI] [Google Scholar]
- Bonner W, Bonner J.. 1948. The role of carbon dioxide in acid formation by succulent plants. American Journal of Botany 35: 113–117. doi: 10.1002/j.1537-2197.1948.tb05194.x. [DOI] [Google Scholar]
- Borland AM, Griffiths H, Maxwell C, Broadmeadow MSJ, Griffiths NM, Barnes JD.. 1992. On the ecophysiology of the Clusiaceae in Trinidad: expression of CAM in Clusia minor L. during the transition from wet to dry season and characterization of three endemic species. New Phytologist 122: 349–357. doi: 10.1111/j.1469-8137.1992.tb04240.x. [DOI] [PubMed] [Google Scholar]
- Borland AM, Técsi LI, Leegood RC, Walker RP.. 1998. Inducibility of crassulacean acid metabolism (CAM) in Clusia species; physiological/biochemical characterisation and intercellular localization of carboxylation and decarboxylation processes in three species which exhibit different degrees of CAM. Planta 205: 342–351. doi: 10.1007/s004250050329. [DOI] [Google Scholar]
- Borland AM, Wullschleger SD, Weston DJ, et al. 2015. Climate-resilient agroforestry: physiological responses to climate change and engineering of crassulacean acid metabolism (CAM) as a mitigation strategy. Plant, Cell & Environment 38: 1833–1849. doi: 10.1111/pce.12479. [DOI] [PubMed] [Google Scholar]
- Borland AM, Leverett A, Hurtado-Castaño N, Hu R, Yang X.. 2018. Functional anatomical traits of the photosynthetic organs of plants with crassulacean acid metabolism. In: Adams WW III, Terashima I. eds. The leaf : a platform for performing photosynthesis, 1st edn. Gewerbestrasse, Switzerland: Springer Nature. 281–305. doi: 10.1007/978-3-319-93594-2_10 [DOI] [Google Scholar]
- Bruijnzeel LA, Veneklaas EJ.. 1998. Climatic conditions and tropical montane forest productivity: the fog has not lifted yet. Ecology 79: 3–9. doi: 10.1890/0012-9658(1998)079[0003:ccatmf]2.0.co;2. [DOI] [Google Scholar]
- Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LE, Sheldon BC.. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320: 800–803. doi: 10.1126/science.1157174. [DOI] [PubMed] [Google Scholar]
- Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM.. 1998. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia 113: 188–196. doi: 10.1007/s004420050367. [DOI] [PubMed] [Google Scholar]
- Crayn DM, Winter K, Schulte K, Smith JA.. 2015. Photosynthetic pathways in Bromeliaceae: phylogenetic and ecological significance of CAM and C3 based on carbon isotope ratios for 1893 species. Botanical Journal of the Linnean Society 178: 169–221. doi: 10.1111/boj.12275. [DOI] [Google Scholar]
- Crepet WL, Nixon KC.. 1998. Fossil Clusiaceae from the Late Cretaceous (Turonian) of New Jersey and implications regarding the history of bee pollination. American Journal of Botany 85: 1122–1133. doi: 10.2307/2446345. [DOI] [PubMed] [Google Scholar]
- de Boer HJ, Drake PL, Wendt E, et al. 2016. Apparent overinvestment in leaf venation relaxes leaf morphological constraints on photosynthesis in arid habitats. Plant Physiology 172: 2286–2299. doi: 10.1104/pp.16.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias ATC, Scarano FR.. 2007. Clusia as nurse plant. In: Lüttge U, ed. Clusia: a woody Neotropical genus of remarkable plasticity and diversity. Berlin, Heidelberg: Springer, 55–71. doi: 10.1007/978-3-540-37243-1_5 [DOI] [Google Scholar]
- Drezner TD. 2014. How long does the giant saguaro live? Life, death and reproduction in the desert. Journal of Arid Environments 104: 34–37. doi: 10.1016/j.jaridenv.2014.01.013. [DOI] [Google Scholar]
- Edwards EJ. 2019. Evolutionary trajectories, accessibility, and other metaphors: the case of C4 and CAM photosynthesis. New Phytologist 223: 1742–1755. doi: 10.1111/nph.15851. [DOI] [PubMed] [Google Scholar]
- Fox RJ, Donelson JM, Schunter C, Ravasi T, Gaitán-Espitia JD.. 2019. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Philosophical Transactions of the Royal Society B: Biological Sciences 374: 20180174. doi: 10.1098/rstb.2018.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradera-Soler M, Rudall PJ, Prychid CJ, Grace OM.. 2021. Evolutionary success in arid habitats: morpho-anatomy of succulent leaves of Crassula species from southern Africa. Journal of Arid Environments 185: 104319. doi: 10.1016/j.jaridenv.2020.104319. [DOI] [Google Scholar]
- Franco AC, Ball E, Lüttge U.. 1990. Patterns of gas exchange and organic acid oscillations in tropical trees of the genus Clusia. Oecologia 85: 108–114. doi: 10.1007/BF00317350. [DOI] [PubMed] [Google Scholar]
- Franco AC, Haag-Kerwer A, Herzog B, et al. 1996. The effect of light levels on daily patterns of chlorophyll fluorescence and organic acid accumulation in the tropical CAM tree Clusia hilariana. Trees 10: 359–365. doi: 10.1007/BF02185639. [DOI] [Google Scholar]
- Gehrig HH, Aranda J, Cushman MA, et al. 2003. Cladogram of Panamanian Clusia based on nuclear DNA: implications for the origins of crassulacean acid metabolism. Plant Biology 5: 59–70. doi: 10.1055/s-2003-37983. [DOI] [Google Scholar]
- Ghalambor CK, Hoke KL, Ruell EW, Fischer EK, Reznick DN, Hughes KA.. 2015. Non-adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature. Nature 525: 372–375. doi: 10.1038/nature15256. [DOI] [PubMed] [Google Scholar]
- González-Salvatierra C. 2019. Water-shortage tolerance and recovery after rehydration in the Chihuahuan Desert plant Yucca filifera (Asparagaceae). The Journal of the Torrey Botanical Society 146: 128–137. doi: 10.3159/torrey-d-18-00001.1. [DOI] [Google Scholar]
- Grey KA, Foden WB, Midgley GF.. 2022. Bioclimatic controls of CO2 assimilation near range limits of the CAM succulent tree Aloidendron dichotomum. Journal of Experimental Botany 73: 7434–7449. doi: 10.1093/jxb/erac343. [DOI] [PubMed] [Google Scholar]
- Gustafsson MH, Bittrich V.. 2003. Evolution of morphological diversity and resin secretion in flowers of Clusia (Clusiaceae): insights from ITS sequence variation. Nordic Journal of Botany 22: 183–203. doi: 10.1111/j.1756-1051.2002.tb01364.x. [DOI] [Google Scholar]
- Gustafsson MH, Bittrich V, Stevens PF.. 2002. Phylogeny of Clusiaceae based on rbcL sequences. International Journal of Plant Sciences 163: 1045–1054. doi: 10.1086/342521. [DOI] [Google Scholar]
- Gustafsson MH, Winter K, Bittrich V.. 2007. Diversity, phylogeny and classification of Clusia. In: Lüttge U, ed. Clusia: a woody Neotropical genus of remarkable plasticity and diversity. Heidelberg: Springer, 95–116. doi: 10.1007/978-3-540-37243-1_7 [DOI] [Google Scholar]
- Hammel BE. 1986. New species of Clusiaceae from Central America with notes on Clusia and synonymy in the tribe Clusieae. Selbyana 1: 112–120. doi: https://journals.flvc.org/selbyana/article/view/120799. [Google Scholar]
- Hastilestari BR, Mudersbach M, Tomala F, et al. 2013. Euphorbia tirucalli L.–comprehensive characterization of a drought tolerant plant with a potential as biofuel source. PLoS One 8: e63501. doi: 10.1371/journal.pone.0063501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, Burrell N, Lalani F, Leebens-Mack J.. 2016. Gas exchange and leaf anatomy of a C3–CAM hybrid Yucca gloriosa (Asparagaceae). Journal of Experimental Botany 67: 1369–1379. doi: 10.1093/jxb/erv536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, Moreno-Villena JJ, Gilman IS, Christin PA, Edwards EJ.. 2019. The genetics of convergent evolution: insights from plant photosynthesis. Nature Reviews Genetics 20: 485–493. doi: 10.1038/s41576-019-0107-5. [DOI] [PubMed] [Google Scholar]
- Heyduk K, McAssey EV, Leebens-Mack J.. 2022. Differential timing of gene expression and recruitment in independent origins of CAM in the Agavoideae (Asparagaceae). New Phytologist 235: 2111–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtum JA, Aranda J, Virgo A, Gehrig HH, Winter K.. 2004. δ13C values and crassulacean acid metabolism in Clusia species from Panama. Trees 18: 658–668. doi: 10.1007/s00468-004-0342-y. [DOI] [Google Scholar]
- Horn JW, Xi Z, Riina R, et al. 2014. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 68: 3485–3504. doi: 10.1111/evo.12534. [DOI] [PubMed] [Google Scholar]
- John GP, Scoffoni C, Sack L.. 2013. Allometry of cells and tissues within leaves. American Journal of Botany 100: 1936–1948. doi: 10.3732/ajb.1200608. [DOI] [PubMed] [Google Scholar]
- Kelly DL. 1985. Epiphytes and climbers of a Jamaican rain forest: vertical distribution, life forms and life histories. Journal of Biogeography 12: 223–241. doi: 10.2307/2844997. [DOI] [Google Scholar]
- Kelly DL, Tanner EVJ, Lughadha EN, Kapos V.. 1994. Floristics and biogeography of a rain forest in the Venezuelan Andes. Journal of Biogeography 21: 421–440. doi: 10.2307/2845760. [DOI] [Google Scholar]
- Kürschner WM, Kvacek Z, Dilcher DL.. 2008. The impact of Miocene atmospheric carbon dioxide fluctuations on climate and the evolution of terrestrial ecosystems. Proceedings of the National Academy of Sciences of the United States of America 105: 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagomarsino LP, Condamine FL, Antonelli A, Mulch A, Davis CC.. 2016. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae). New Phytologist 210: 1430–1442. doi: 10.1111/nph.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverett A, Hurtado Castaño N, Ferguson K, Winter K, Borland AM.. 2021. Crassulacean acid metabolism (CAM) supersedes the turgor loss point (TLP) as an important adaptation across a precipitation gradient, in the genus Clusia. Functional Plant Biology 48: 703–716. doi: 10.1071/fp20268. [DOI] [PubMed] [Google Scholar]
- Leverett A, Ferguson K, Winter K, Borland AM.. 2023b. Leaf vein density correlates with crassulacean acid metabolism, but not hydraulic capacitance, in the genus Clusia. Annals of Botany 132: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverett A, Hartzell S, Winter K, Garcia M, Aranda J, Virgo A, Smith A, Focht P, Rasmussen-Arda A, Willats WGT, Cowan-Turner D, Borland AM.. 2023a. Dissecting succulence: crassulacean acid metabolism and hydraulic capacitance are independent adaptations in Clusia leaves. Plant, Cell & Environment 1–17. doi: 10.1111/pce.14539 [DOI] [PubMed] [Google Scholar]
- Luján M, Oleas NH, Winter K.. 2022. Evolutionary history of CAM photosynthesis in Neotropical Clusia: insights from genomics, anatomy, physiology and climate. Botanical Journal of the Linnean Society 199: 538–556. doi: 10.1093/botlinnean/boab075. [DOI] [Google Scholar]
- Lüttge U. 1988. Day‐night changes of citric‐acid levels in crassulacean acid metabolism: phenomenon and ecophysiological significance. Plant, Cell & Environment 11: 445–451. doi: 10.1111/j.1365-3040.1988.tb01782.x. [DOI] [Google Scholar]
- Lüttge U. 1996. Clusia: plasticity and diversity in a genus of C3/CAM intermediate tropical trees. In: Winter K. and Smith JAC, eds. Crassulacean acid metabolism. Ecological studies, Vol. 114. Berlin, Heidelberg: Springer, 296–311. doi: 10.1007/978-3-642-79060-7_20 [DOI] [Google Scholar]
- Lüttge U. 2004. Ecophysiology of crassulacean acid metabolism (CAM). Annals of Botany 93: 629–652. doi: 10.1093/aob/mch087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U. 2006. Photosynthetic flexibility and ecophysiological plasticity: questions and lessons from Clusia, the only CAM tree, in the neotropics. New Phytologist 171: 7–25. doi: 10.1111/j.1469-8137.2006.01755.x. [DOI] [PubMed] [Google Scholar]
- Lüttge U. 2007. Historical recollections. In: Lüttge U, ed. Clusia. A woody neotropical genus of remarkable plasticity and diversity. Ecological Studies, Vol. 194. Berlin: Springer, 3–9. doi: 10.1007/978-3-540-37243-1_1 [DOI] [Google Scholar]
- Lüttge U. 2008. Clusia: holy grail and enigma. Journal of Experimental Botany 59: 1503–1514. doi: 10.1093/jxb/ern006. [DOI] [PubMed] [Google Scholar]
- Lüttge U, Scarano FR, de Mattos EA, et al. 2015. Does ecophysiological behaviour explain habitat occupation of sympatric Clusia species in a Brazilian Atlantic rainforest? Trees 29: 1973–1988. doi: 10.1007/s00468-015-1277-1. [DOI] [Google Scholar]
- Males J. 2017. Adaptive variation in vein placement underpins diversity in a major Neotropical plant radiation. Oecologia 185: 375–386. doi: 10.1007/s00442-017-3956-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Males J. 2018. Concerted anatomical change associated with crassulacean acid metabolism in the Bromeliaceae. Functional Plant Biology 45: 681–695. doi: 10.1071/fp17071. [DOI] [PubMed] [Google Scholar]
- Males J, Griffiths H.. 2018. Economic and hydraulic divergences underpin ecological differentiation in the Bromeliaceae. Plant, Cell & Environment 41: 64–78. doi: 10.1111/pce.12954. [DOI] [PubMed] [Google Scholar]
- Moreno-Villena JJ, Zhou H, Gilman IS, Tausta SL, Cheung CYM, Edwards EJ.. 2022. Spatial resolution of an integrated C4+CAM photosynthetic metabolism. Science Advances 8: eabn2349. doi: 10.1126/sciadv.abn2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EA, Sage TL, Sage RF.. 2005. Functional leaf anatomy of plants with crassulacean acid metabolism. Functional Plant Biology 32: 409–419. doi: 10.1071/fp04195. [DOI] [PubMed] [Google Scholar]
- Noblin X, Mahadevan L, Coomaraswamy IA, Weitz DA, Holbrook NM, Zwieniecki MA.. 2008. Optimal vein density in artificial and real leaves. Proceedings of the National Academy of Sciences of the United States of America 105: 9140–9144. doi: 10.1073/pnas.0709194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogburn RM, Edwards EJ.. 2010. The ecological water-use strategies of succulent plants. In Kader J-C, Delseny M, eds. Advances in botanical research, Chapter 4, Vol. 55. Burlington, Massachusetts, USA: Academic Press, 179–225. doi: 10.1016/B978-0-12-380868-4.00004-1 [DOI] [Google Scholar]
- Ogburn RM, Edwards EJ.. 2012. Quantifying succulence: a rapid, physiologically meaningful metric of plant water storage. Plant, Cell & Environment 35: 1533–1542. doi: 10.1111/j.1365-3040.2012.02503.x. [DOI] [PubMed] [Google Scholar]
- Osmond CB. 1978. Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology 29: 379–414. doi: 10.1146/annurev.pp.29.060178.002115. [DOI] [Google Scholar]
- Osmond CB, Popp M, Robinson SA.. 1996. Stoichiometric nightmares: studies of photosynthetic O2 and CO2 exchanges in CAM plants. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism biochemistry, ecophysiology and evolution, 1st edn, Vol. 1. Berlin, Heidelberg: Springer, 19–30. doi: 10.1007/978-3-642-79060-7_2 [DOI] [Google Scholar]
- Owen NA, Griffiths H.. 2013. A system dynamics model integrating physiology and biochemical regulation predicts extent of crassulacean acid metabolism (CAM) phases. New Phytologist 200: 1116–1131. doi: 10.1111/nph.12461. [DOI] [PubMed] [Google Scholar]
- Pachon P, Winter K, Lasso E.. 2022. Updating the occurrence of CAM (crassulacean acid metabolism) in the genus Clusia through carbon isotope analysis of species from Colombia. Photosynthetica 60: 304147–304322. doi: 10.32615/ps.2022.018. [DOI] [Google Scholar]
- Paenke I, Sendhoff B, Kawecki TJ.. 2007. Influence of plasticity and learning on evolution under directional selection. The American Naturalist 170: E47–E58. doi: 10.1086/518952. [DOI] [PubMed] [Google Scholar]
- Polley HW, Johnson HB, Tischler CR.. 2002. Woody invasion of grasslands: evidence that CO2 enrichment indirectly promotes establishment of Prosopis glandulosa. Plant Ecology 164: 85–94. doi: 10.1023/A:1021271226866. [DOI] [Google Scholar]
- Popp M, Kramer D, Lee H, Diaz M, Ziegler H, Lüttge U.. 1987. Crassulacean acid metabolism in tropical dicotyledonous trees of the genus Clusia. Trees 1: 238–247. doi: 10.1007/bf01816822. [DOI] [Google Scholar]
- POWO: Plants of the World Online. Royal Botanic Gardens, Kew. Retrieved 2 September 2022. https://powo.science.kew.org/.
- Rossetto M, Kooyman R, Yap JYS, Laffan SW.. 2015. From ratites to rats: the size of fleshy fruits shapes species’ distributions and continental rainforest assembly. Proceedings of the Royal Society B: Biological Sciences 282: 20151998. doi: 10.1098/rspb.2015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhfel BR, Bittrich V, Bove CP, et al. 2011. Phylogeny of the clusioid clade (Malpighiales): evidence from the plastid and mitochondrial genomes. American Journal of Botany 98: 306–325. doi: 10.3732/ajb.1000354. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2014. Stopping the leaks: new insights into C4 photosynthesis at low light. Plant, Cell & Environment 37: 1037–1041. doi: 10.1111/pce.12246. [DOI] [PubMed] [Google Scholar]
- Schönenberger J, von Balthazar M, López Martínez A, et al. 2020. Phylogenetic analysis of fossil flowers using an angiosperm-wide data set: proof-of-concept and challenges ahead. American Journal of Botany 107: 1433–1448. doi: 10.1002/ajb2.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwery O, Onstein RE, Bouchenak‐Khelladi Y, Xing Y, Carter RJ, Linder HP.. 2015. As old as the mountains: the radiations of the Ericaceae. New Phytologist 207: 355–367. doi: 10.1111/nph.13234. [DOI] [PubMed] [Google Scholar]
- Shameer S, Baghalian K, Cheung CYM, Ratcliffe RG, Sweetlove LJ.. 2018. Computational analysis of the productivity potential of CAM. Nature Plants 4: 165–171. doi: 10.1038/s41477-018-0112-2. [DOI] [PubMed] [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC.. 2010. Evolution along the crassulacean acid metabolism continuum. Functional Plant Biology 37: 995–1010. doi: 10.1071/fp10084. [DOI] [Google Scholar]
- Sternberg L da SL, Ting IP, Price D, Hann J.. 1987. Photosynthesis in epiphytic and rooted Clusia rosea Jacq. Oecologia 72: 457–460. doi: 10.1007/BF00377579. [DOI] [PubMed] [Google Scholar]
- Thomas M, Beevers H.. 1949. Physiological studies on acid metabolism in green plants. II. Evidence of CO2 fixation in Bryophyllum and the study of diurnal variation of acidity in this genus. The New Phytologist 48: 421–447. doi: 10.1111/j.1469-8137.1949.tb05134.x. [DOI] [Google Scholar]
- Ting IP, Lord EM, Sternberg LDS, DeNiro MJ.. 1985. Crassulacean acid metabolism in the strangler Clusia rosea Jacq. Science 229: 969–971. doi: 10.1126/science.229.4717.969. [DOI] [PubMed] [Google Scholar]
- Ting IP, Hann J, Holbrook NM, et al. 1987. Photosynthesis in hemiepiphytic species of Clusia and Ficus. Oecologia 74: 339–346. doi: 10.1007/BF00378927. [DOI] [PubMed] [Google Scholar]
- Tinoco-Ojanguren C, Vázquez-Yanes C.. 1983. Especies CAM en la selva húmeda tropical de Los Tuxtlas, Veracruz. Boletín de la Sociedad Botánica de México 45: 150–153. doi: 10.17129/botsci.1309. [DOI] [Google Scholar]
- Todzia C. 1986. Growth habits, host tree species, and density of hemiepiphytes on Barro Colorado Island, Panama. Biotropica 18: 22–27. doi: 10.2307/2388357. [DOI] [Google Scholar]
- Töpfer N, Braam T, Shameer S, Ratcliffe RG, Sweetlove LJ.. 2020. Alternative crassulacean acid metabolism modes provide environment-specific water-saving benefits in a leaf metabolic model. Plant Cell 32: 3689–3705. doi: 10.1105/tpc.20.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaasen A, Begerow D, Lüttge U, Hampp R.. 2002. The genus Clusia L.: molecular evidence for independent evolution of photosynthetic flexibility. Plant Biology 4: 86–93. doi: 10.1055/s-2002-20440. [DOI] [Google Scholar]
- Vargas-Soto JG, Andrade JL, Winter K.. 2009. Carbon isotope composition and mode of photosynthesis in Clusia species from Mexico. Photosynthetica 47: 33–40. doi: 10.1007/s11099-009-0007-6. [DOI] [Google Scholar]
- Winter K, Holtum JA.. 2002. How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiology 129: 1843–1851. doi: 10.1104/pp.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM.. 2014. Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. Journal of Experimental Botany 65: 3425–3441. doi: 10.1093/jxb/eru063. [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC.. 1996. An introduction to crassulacean acid metabolism. Biochemical principles and ecological diversity. In Winter K, Smith JAC, eds. Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Berlin: Springer, 1–13. doi: 10.1007/978-3-642-79060-7_1 [DOI] [Google Scholar]
- Winter K, Smith JAC.. 2022. CAM photosynthesis: the acid test. New Phytologist 233: 599–609. doi: 10.1111/nph.17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Lüttge U, Winter E, Troughton JH.. 1978. Seasonal shift from C3 photosynthesis to crassulacean acid metabolism in Mesembryanthemum crystallinum growing in its natural environment. Oecologia 34: 225–237. doi: 10.1007/BF00345168. [DOI] [PubMed] [Google Scholar]
- Winter K, Zotz G, Baur B, Dietz KJ.. 1992. Light and dark CO2 fixation in Clusia uvitana and the effects of plant water status and CO2 availability. Oecologia 91: 47–51. doi: 10.1007/BF00317239. [DOI] [PubMed] [Google Scholar]
- Winter K, Aranda J, Holtum JAM.. 2005. Carbon isotope composition and water-use efficiency in plants with crassulacean acid metabolism. Functional Plant Biology 32: 381–388. doi: 10.1071/fp04123. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Holtum JA.. 2008. On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. Journal of Experimental Botany 59: 1829–1840. doi: 10.1093/jxb/ern080. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Virgo A, Smith JAC.. 2021. Low-level CAM photosynthesis in a succulent-leaved member of the Urticaceae, Pilea peperomioides. Functional Plant Biology 48: 683–690. doi: 10.1071/fp20151. [DOI] [PubMed] [Google Scholar]
- Yamaga-Hatakeyama Y, Okutani M, Hatakeyama Y, Yabiku T, Yukawa T, Ueno O.. 2022. Photosynthesis and leaf structure of F1 hybrids between Cymbidium ensifolium (C3) and C. bicolor subsp. pubescens (CAM). Annals of Botany (In press). doi: 10.1093/aob/mcac157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SNR, Sack L, Sporck-Koehler MJ, Lundgren MR.. 2020. Why is C4 photosynthesis so rare in trees? Journal of Experimental Botany 71: 4629–4638. doi: 10.1093/jxb/eraa234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SNR, Dunning LT, Liu H, Stevens CJ, Lundgren MR.. 2022. C4 trees have a broader niche than their close C3 relatives. Journal of Experimental Botany 73: 3189–3204. doi: 10.1093/jxb/erac113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz G, Winter K.. 1993. Short-term regulation of crassulacean acid metabolism activity in a tropical hemiepiphyte, Clusia uvitana. Plant Physiology 102: 835–841. doi: 10.1104/pp.102.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz G, Winter K.. 1994a. A one‐year study on carbon, water and nutrient relationships in a tropical C3‐CAM hemi‐epiphyte, Clusia uvitana Pittier. New Phytologist 127: 45–60. doi: 10.1111/j.1469-8137.1994.tb04258.x. [DOI] [PubMed] [Google Scholar]
- Zotz G, Winter K.. 1994b. Annual carbon balance and nitrogen-use efficiency in tropical C3 and CAM epiphytes. New Phytologist 126: 481–492. doi: 10.1111/j.1469-8137.1994.tb04245.x. [DOI] [PubMed] [Google Scholar]
- Zotz G, Winter K.. 1996. Seasonal changes in daytime versus nightime CO2 fixation of Clusia uvitana in situ. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Ecological studies, Vol. 114. Berlin, Heidelberg: Springer, 312–323. doi: 10.1007/978-3-642-79060-7_21 [DOI] [Google Scholar]
- Zotz G, Harris G, Königer M, Winter K.. 1995. High rates of photosynthesis in a tropical pioneer tree, Ficus insipida. Flora 190: 265–272. doi: 10.1016/s0367-2530(17)30663-1. [DOI] [Google Scholar]
- Zotz G, Reichling P, Krack S.. 1999. Another woody hemiepiphyte with CAM: Havetiopsis flexilis Spruce ex Planch. Et Tr. (Clusiaceae). Flora 194: 215–220. doi: 10.1016/s0367-2530(17)30899-x. [DOI] [Google Scholar]
- Zwieniecki MA, Boyce CK.. 2014. Evolution of a unique anatomical precision in angiosperm leaf venation lifts constraints on vascular plant ecology. Proceedings of the Royal Society B: Biological Sciences 281: 20132829. doi: 10.1098/rspb.2013.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]


