Abstract
Background and Scope
The growth of experimental studies of crassulacean acid metabolism (CAM) in diverse plant clades, coupled with recent advances in molecular systematics, presents an opportunity to re-assess the phylogenetic distribution and diversity of species capable of CAM. It has been more than two decades since the last comprehensive lists of CAM taxa were published, and an updated survey of the occurrence and distribution of CAM taxa is needed to facilitate and guide future CAM research. We aimed to survey the phylogenetic distribution of these taxa, their diverse morphology, physiology and ecology, and the likely number of evolutionary origins of CAM based on currently known lineages.
Results and Conclusions
We found direct evidence (in the form of experimental or field observations of gas exchange, day–night fluctuations in organic acids, carbon isotope ratios and enzymatic activity) for CAM in 370 genera of vascular plants, representing 38 families. Further assumptions about the frequency of CAM species in CAM clades and the distribution of CAM in the Cactaceae and Crassulaceae bring the currently estimated number of CAM-capable species to nearly 7 % of all vascular plants. The phylogenetic distribution of these taxa suggests a minimum of 66 independent origins of CAM in vascular plants, possibly with dozens more. To achieve further insight into CAM origins, there is a need for more extensive and systematic surveys of previously unstudied lineages, particularly in living material to identify low-level CAM activity, and for denser sampling to increase phylogenetic resolution in CAM-evolving clades. This should allow further progress in understanding the functional significance of this pathway by integration with studies on the evolution and genomics of CAM in its many forms.
Keywords: crassulacean acid metabolism, nocturnal acidification, vascular plants, C3 photosynthesis, C3 + CAM, C4 + CAM, strong CAM, photosynthetic pathway evolution
INTRODUCTION
Crassulacean acid metabolism (CAM) is now recognized as a key ecological adaptation to water and CO2 limitation. From the outset, this metabolism was noted for its distinctive association with succulent plants. De Saussure (1804), in the course of extensive manometric measurements of gas exchange by plants, made the seminal observation that Opuntia and several other succulent plants were able to show net uptake of CO2 at night. Also, Heyne (1815) detected the rhythmic nocturnal acidification of Kalanchoë leaves by the simple expedient of comparing their taste in the morning and afternoon. By the end of the 19th century, these characteristic day–night changes in acidity had become recognized as a well-known phenomenon of succulent plants. Since then, a full description of CAM, from biochemistry to ecophysiology, has involved researchers from around the globe and continues into the genomics age.
Research into CAM has been spurred by advances in technology that have enabled precise biochemical and physiological descriptions of the marked differences between succulent and non-succulent plants. Mayer (1875), Kraus (1883) and Warburg (1886) appreciated that the metabolism of succulents involved a specific acid, malic acid, and that carbohydrate concentrations showed reciprocal, diel cycling. However, it was to be another half-century before the biochemical mechanism of nocturnal CO2 fixation was established. The experiments of Thurlow and Bonner (1948), Thomas (1949) and Thomas and Beevers (1949) showed that nocturnal synthesis of malic acid could be explained by direct fixation of CO2 via the Wood–Werkman reaction, first discovered in bacteria and now known to be catalysed (using HCO3− as the true substrate) by the enzyme phosphoenolpyruvate (PEP) carboxylase (PEPC). Work at the Connecticut Agricultural Experiment Station and elsewhere around the same time confirmed the primary role of malic acid and the relationship between organic acids and the major carbohydrates involved in the CAM pathway (Pucher and Vickery, 1942; Pucher et al., 1947). The development of diffusion resistance analysis and infrared gas analysers allowed precise quantification of stomatal conductance and gas exchange in natural and experimental settings, leading to the direct demonstration of nocturnal opening of stomata associated with uptake of atmospheric CO2, the sensitivity of the gas-exchange cycle to photoperiod, and the endogenous rhythmicity of dark CO2 fixation (Gregory et al., 1954; Wilkins, 1959; Nuernbergk, 1961; Warren and Wilkins, 1961; Nishida, 1963). Following the discovery of C4 photosynthesis (Hatch and Slack, 1966), the advances in biochemistry and other aspects of photosynthesis became canonized in a four-stage model of CAM (Osmond, 1978) (Fig. 1), which provides a useful framework for considering the characteristics of carbon metabolism in these plants over the 24 h cycle.
Fig. 1.
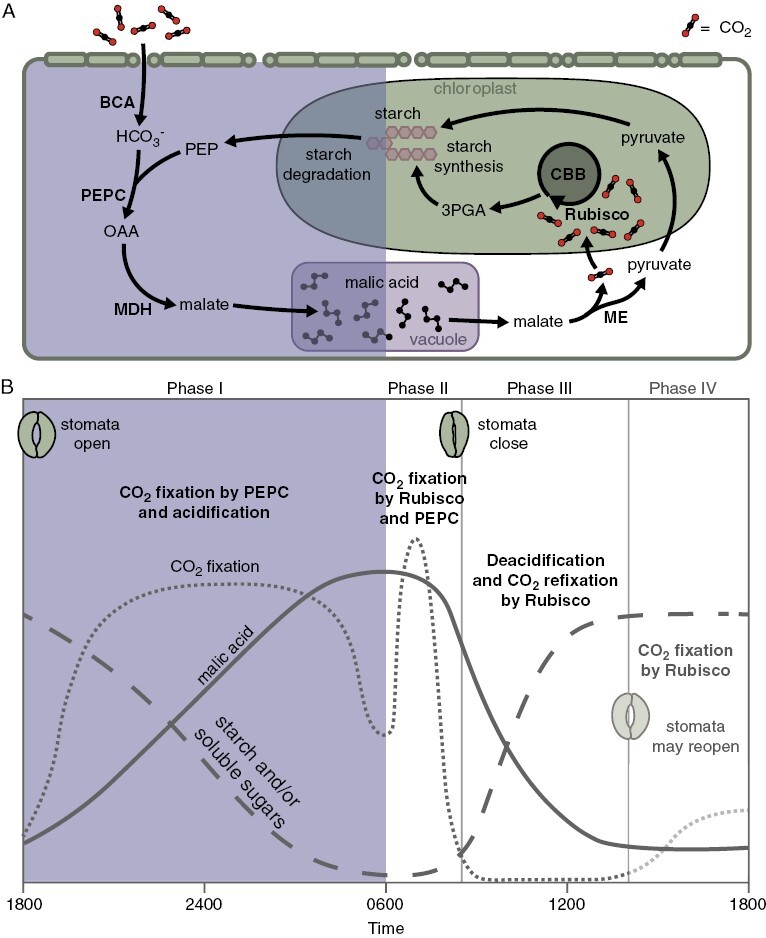
Simplified overview of biochemistry (A) and four phases of CAM (B); phases in (B) are adapted from Osmond (1978). During phase I of CAM, atmospheric CO2 is captured in a series of steps involving phosphoenolpyruvate (PEP) carboxylase (PEPC) to form malate, which is stored as malic acid in the vacuole overnight. In phase II, stomata remain open, and both Rubisco and PEPC fix atmospheric CO2. Stomatal closure marks the beginning of phase III, in which malate is released from the vacuole and decarboxylated by malic enzyme (NADP- or NAD-ME) as shown (or PEP carboxykinase, not shown), releasing CO2 to be re-fixed by Rubisco. Finally, if phase IV occurs, stomata reopen, and atmospheric CO2 is fixed predominantly by Rubisco. If phase IV does not occur, stomatal opening is delayed until phase I begins again. Abbreviations: 3PGA, 3-phosphoglycerate; BCA, β-carbonic anhydrase; CBB, Calvin–Benson–Bassham cycle; MDH, malate dehydrogenase; OAA, oxaloacetate.
Phase I occurs in the dark period, when stomata are open, CO2 is taken up from the atmosphere, and PEP supplied by glycolysis is carboxylated by PEPC to produce malate, which is then stored overnight as malic acid in the central vacuole of chlorenchymatous mesophyll cells. Phase II is characterized by a short burst of CO2 fixation at the beginning of the light period, initially catalysed mainly by PEPC but later increasingly by Rubisco, while stomata remain open. Malic acid is then released from the vacuole and decarboxylated during the central part of the day in phase III, raising intercellular CO2 dramatically to supply Rubisco and causing stomatal closure, and yielding a three-carbon moiety (pyruvate or PEP) that is recycled via gluconeogenesis back to storage carbohydrate. Finally, when the deacidification phase is completed, stomata may reopen in phase IV in the late afternoon (environmental conditions permitting), and CO2 is fixed directly from the atmosphere by Rubisco.
Species such as Kalanchoë daigremontiana Raym.-Hamet & H.Perrier are generally well described by this canonical four-phase model of CAM, but in many species the magnitude of the CAM cycle (and its individual phases) is variously expressed and can be phenotypically plastic over short time scales and throughout the life history (see ‘The landscape of CAM phenotypes and CAM evolution’, below). Regardless of individual variation, life history variation and environmentally induced variation, CAM is most broadly defined by the cyclic diel rhythm of: (1) nocturnal fixation of CO2 into malate, catalysed by PEPC; (2) storage of malate as malic acid overnight in the vacuole; and (3) efflux from the vacuole and decarboxylation of the malate during the daytime to release CO2, which is re-fixed by Rubisco. The formalization of this framework coincided with the recognition of the adaptive significance of CAM: fixing carbon at night while closing stomata for much of the day reduces transpirational water loss, and the two-stage carbon-concentrating mechanism increases the efficiency of Rubisco CO2 fixation when stomata are closed (Neales et al., 1968; Kluge and Ting, 1978; Osmond, 1978; Cockburn et al., 1979).
Concurrent with this synthesis of CAM, it was recognized that C4 and CAM plants could be distinguished from C3 species by their lesser discrimination against 13C relative to 12C (Vogel and Lerman, 1969; Bender, 1971; Smith and Epstein, 1971; Bender et al., 1973; Osmond et al., 1973) (Box 1). Although it took almost another decade to describe the mechanisms behind carbon isotope discrimination fully (O’Leary, 1981; Farquhar et al., 1982), the realization that photosynthetic types could be discerned with relative ease from small samples of plant tissue (including desiccated and dead tissues, such as those in herbarium collections) was an impetus for broad ecological and taxonomic surveys using stable-isotope analysis. Great strides were made in major CAM clades, including the Bromeliaceae (Coutinho, 1969; Medina and Troughton, 1974; Medina et al., 1977; Griffiths and Smith, 1983; Crayn et al., 2004, 2015), Orchidaceae (Coutinho, 1969; Winter et al., 1983; Kluge et al., 1995; Silvera et al., 2005, 2010a; Torres-Morales et al., 2020), Clusiaceae (Holtum et al., 2004; Lüttge, 2007; Pachon et al., 2022), Crassulaceae (Osmond et al., 1975; Rundel et al., 1979; Teeri et al., 1981; Tenhunen et al., 1982; Pilon-Smits et al., 1991, 1992; Kluge et al., 1993) and Aizoaceae (Mooney et al., 1977; Rundel et al., 1999; Messerschmid et al., 2021). Ecologically, carbon isotope surveys were conducted primarily in semi-arid regions, such as Baja California and Chile (Mooney et al., 1974; Arroyo et al., 1990), Southern Africa and Madagascar (Mooney et al., 1977; Winter, 1979; Rundel et al., 1999), North Africa and the Middle East (Winter, 1981; Ziegler et al., 1981) and Mexico (Mooney et al., 1989), and in tropical ecosystems rich in epiphytes, including South America (Medina and Troughton, 1974; Medina et al., 1977), the Caribbean and Central America (Griffiths and Smith, 1983; Zotz and Ziegler, 1997), Papua New Guinea (Earnshaw et al., 1987), Australia (Winter et al., 1983) and Madagascar (Kluge et al., 1998).
Box 1. Diagnosing CAM in the field and laboratory.
There are various methods for identifying CAM, with trade-offs between ease of sampling and what types of CAM expression they can capture (for a detailed review of experimental methods, see Osmond et al., 1989).
Stable carbon isotope ratios [δ13C, expressed in parts per thousand (‰)] reflect the ratio of 13C to 12C in plant tissues relative to Pee Dee belemnite limestone or a secondary standard. Rubisco discriminates against 13C more strongly than does PEPC; thus, the more CO2 initially fixed by PEPC (during CAM) relative to Rubisco, the less negative the resulting δ13C value. Experiments demonstrate a linear relationship between δ13C and the proportion of CO2 fixed in the dark: purely light and dark CO2 assimilation translate to δ13C values of about −27 and −8 ‰, respectively, with −18 ‰ reflecting roughly equal light and dark assimilation (Winter and Holtum, 2002). Plants that assimilate less than one-third of their CO2 in the dark have δ13C values indistinguishable from C3 species; therefore, δ13C values can be used as positive evidence for strong CAM but are, at best, suggestive of C3 + CAM. Given the bimodality of δ13C values across most clades containing C3 and CAM species (see ‘The landscape of CAM phenotypes and CAM evolution’), δ13C > −20 ‰ is typically indicative of strong CAM. Two strengths of isotopic analysis are the small amount of tissue needed (~1 mg) and that it does not require living tissue and can therefore be assessed from herbarium and other non-living historical specimens, including fossils.
Diel changes in titratable acidity [ΔH+, typically expressed in millimoles of H+ per kilogram of fresh mass (FM)] measure the change in acid content in photosynthetic tissues and can confirm the presence of CAM. Calculating ΔH+ requires measurement of titratable acidity or malate concentration at dusk (phase IV), when malic acid storage is expected to be at a minimum, and subtracting that value from the expected maximum at dawn (phase I). Statistically significant ΔH+ greater than zero indicates CAM activity; strong-CAM species can have ΔH+ > 200 mmol H+ kg−1 FM, whereas most C3 + CAM and C4 + CAM species have ΔH+ < 200 mmol H+ kg−1 FM (Holtum et al., 2017; Winter and Smith, 2022), and ΔH+ can be < 10 mmol H+ kg−1 FM in species with very low CAM activity (Fig. 3; Hancock et al., 2019); the lowest differences in ΔH+ that can currently be resolved experimentally are ~1 mmol H+ kg−1 FM. A thorough test for CAM requires measurement of ΔH+ in stressful conditions to assess the capacity of a plant for facultative CAM, including both stem and leaf tissues. Appropriate degrees of stress must be ascertained for each species. Too much stress can lead to the physiology of the tissues shutting down, resulting in little acid shift or nighttime CO2 fixation and precluding the evaluation of CAM behaviour.
Gas-exchange curves show net CO2 exchange over 24 h periods, with dark-period net CO2 uptake providing evidence of CAM. However, many C3 + CAM (and C4 + CAM) species exhibit net negative CO2 exchange during the dark period. In these taxa, dark-period CO2 loss is typically reduced towards the middle of the night while CO2 fixation by PEPC occurs (phase I) (e.g. Pilea peperomioides; Winter et al., 2021a). In contrast, C3 (and C4) species show relatively constant dark-period CO2 loss rates, representing background respiration. As in measurements of ΔH+ or malate, facultative-CAM species require observations of gas exchange during normal and stressful conditions. Portable equipment to monitor photosynthesis has facilitated field use and enhanced screening potential; however, challenges to be addressed in gas-exchange assessments are sufficiently sized chambers for succulent leaf and/or stem tissue and sufficient battery power for lengthy gas-exchange runs over ≥24 h in remote field sites.
Enzymatic activity and transcript and protein abundance can be used to measure the presence and expression status of key CAM enzymes, such as PEPC, and to diagnose carboxylation and decarboxylation enzyme subtypes used in CAM. Increased abundance and activity of key enzymes (e.g. PEPC) in biochemical assays, in combination with physiological data, can provide supportive evidence for CAM (and rule out C4 photosynthesis). Both whole-transcriptome sequencing and quantitative PCR allow powerful and high-throughput analysis of hundreds to thousands of genes at once, but as proxies for enzymatic activity they must be used in conjunction with other CAM assays. Measurement of protein amount/activity and transcript abundance is still mostly restricted to the laboratory to avoid sample degradation.
When combined, the CAM-identification methods discussed here [along with ancillary methods, such as 14C pulse–chase experiments, online stable-isotope discrimination techniques (Griffiths et al., 2007; Barbour, 2017) and metabolic flux analysis following stable-isotope labelling (Szecowka et al., 2013)] can illustrate how CAM is used day-to-day and throughout the life history.
These broad surveys and subsequent physiological studies revealed a diversity of CAM physiology, associated morphology and ecological contexts throughout vascular plants (Fig. 2). CAM is not only common in succulent xeromorphic terrestrial and epiphytic plants, which experience frequent water-deficit stress, but is also present in a handful of aquatic lineages (Keeley, 1998). As isotope data accumulated, C3 and CAM species in many clades separated largely into two distinct groups, showing a bimodal distribution of δ13C values, with a gap or minimum around −20 ‰ (Medina et al., 1977; Griffiths and Smith, 1983; Winter et al., 1983; Winter and Holtum, 2002; Crayn et al., 2015; Messerschmid et al., 2021; Orlov et al., 2022). However, multiple species showed substantial intraspecific variation in carbon isotopic ratios (δ13C) and gas-exchange patterns indicating that, unlike C4 species, CAM plants could regulate their use of CAM relative to the C3 pathway depending on environmental conditions (Bender et al., 1973; Osmond et al., 1973; Black and Williams, 1976).
Fig. 2.
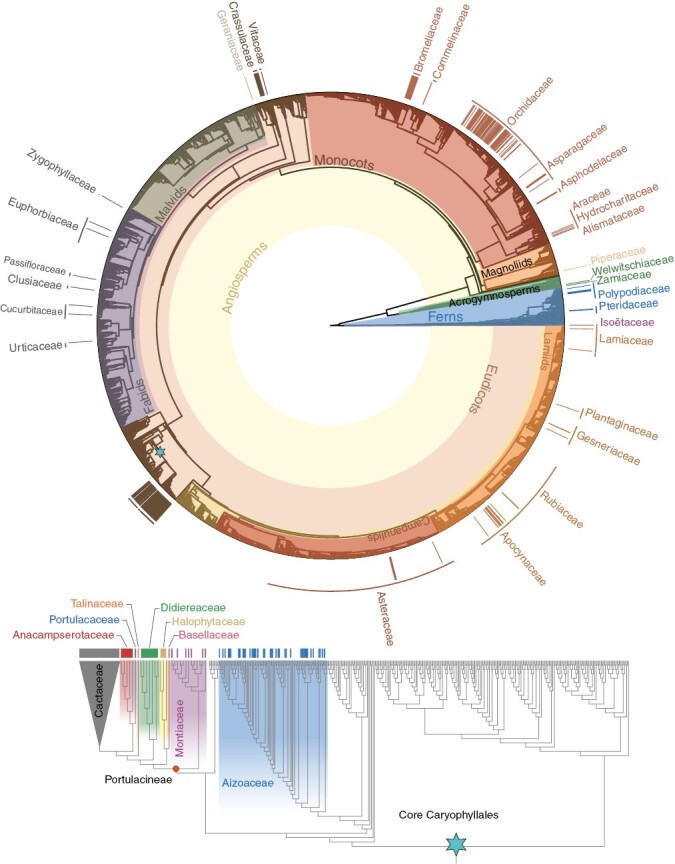
The distribution of crassulacean acid metabolism among vascular plant genera and families. The topology is adapted from Hinchliff and Smith (2014), with an updated topology of some lineages in the core Caryophyllales following Moore et al. (2018); the core Caryophyllales (blue star) are expanded below, with the Cactaceae collapsed because all genera are assumed to be CAM. Only families with CAM genera are labelled, and bars above each tip indicate genera known to have one or more species capable of CAM, regardless of CAM phenotype. Branches are not to scale and have been adjusted for visualization.
The variation in δ13C also reflects different degrees of CAM throughout ontogeny and seasonally. It was a landmark experiment by Winter and von Willert (1972) on Mesembryanthemum crystallinum L. that first demonstrated rapid induction of CAM in response to environmental stress (high salinity in this case). Since their discovery, subsequent research has uncovered diverse CAM phenotypes in dozens of lineages, ranging from ferns and lycophytes to cycads, gnetales and even C4 angiosperms (see ‘The phylogenetic diversity of CAM plants’, below). Unlike C4 photosynthesis (Sage et al., 2011), the cataloguing of CAM species (e.g. Szarek and Ting, 1977; Szarek, 1979; Winter and Smith, 1996; Sayed, 2001) has not yet been coupled with an attempt to estimate the number of evolutionary origins of CAM across the plant tree of life. Here, leveraging advances in molecular phylogenetics and expanded surveys for CAM, we provide an updated occurrence record of the genera of vascular plants in which CAM activity has been detected (Table 1; a fully referenced list is presented in Supplementary Data Table S1), speculate about the number of independent evolutionary origins of CAM, discuss the challenges of this type of evolutionary accounting, and highlight outstanding questions in CAM evolution that we hope this review will facilitate in addressing (Box 2).
Table 1.
List of genera containing species capable of CAM photosynthesis; major clades are ordered following the linear classification system of APG IV (2016). Where species names have subsequently been synonymized or segregated into other genera, the original taxonomy is indicated in parentheses after the currently accepted name. Relevant phylogenetic studies are cited for clades with multiple putative origins of CAM. A fully referenced list with citations to initial and subsequent reports of CAM activity in these taxa, together with details of names reduced to synonymy, is provided as Supplementary Data (Table S1).
| Major clade | Genus | Putative CAM origins |
|---|---|---|
| Isoëtales | ||
| Isoëtaceae | Isoëtes (including Stylites) | 1 |
| Polypodiales | ||
| Polypodiaceae | ||
| Microsoroideae | Lecanopteris; Microsorum | 2; 1 each in Lecanopteris and Microsorum (Chen et al., 2020) |
| Loxogrammoideae | Dictymia | 1 |
| Polypodioideae | Niphidium | 1 |
| Platycerioideae | Platycerium; Pyrrosia (including Drymoglossum) | 1 or 2; 1 in the ancestor of Platycerium and Pyrrosia or 1 in each clade (Wei et al., 2017) |
| Pteridaceae | ||
| Vittarioideae | Haplopteris; Anetium; Vittaria | 2; 1 in the ancestor of Anetium and Vittaria and 1 in Haplopteris (Schuettpelz et al., 2016) |
| Cycadales | ||
| Zamiaceae | Dioon | 1 |
| Welwitschiales | ||
| Welwitschiaceae | Welwitschia | 1 |
| Piperales | ||
| Piperaceae | ||
| Piperoideae | Peperomia | 5–12 (Frenzke et al., 2016; Lim et al., 2019) |
| Alismatales | ||
| Alismataceae | Sagittaria | 1 |
| Araceae | Zamioculcas | 1 |
| Hydrocharitaceae | Ottelia; Vallisneria | 2; 1 each in Ottelia and Vallisneria (Chen et al., 2022) |
| Asparagales | ||
| Orchidaceae | ||
| Epidendroideae | Acianthera; Aerangis; Aeranthes; Anathallis; Angraecum; Arachnis; Aspasia; Barkeria; Bogoria; Brassavola; Brassia; Bryobium; Bulbophyllum; Campylocentrum; Cattleya (including Sophronitis); Caularthron; Caluera; Capanemia; Chiloschista; Cischweinfia; Coelogyne (including Pholidota); Comparettia (including Scelochilus); Coryanthes; Cymbidium; Cyrtopodium; Dendrobium (including Cadetia, Dockrillia, Flickingeria and Grastidium); Dendrophylax; Didymoplexis; Domingoa; Dimerandra; Echinosepala (including Brenesia); Elleanthus; Encyclia; Epidendrum (including Lanium and Oerstedella); Eriopsis; Erycina (including Psygmorchis); Eulophia (including Acrolophia, Lissochilus, Oeceoclades and Orthochilus); Gomesa; Gongora; Guarianthe; Hintonella; Ionopsis; Jacquiniella; Laelia (including Schomburgkia); Leochilus; Lockhartia; Luisia; Lycaste; Macradenia; Macroclinium; Maxillaria (including Camaridium, Heterotaxis, Ornithidium and Trigonidium); Meiracyllium; Microcoelia (including Gussonea); Micropera; Mobilabium; Mormodes; Myoxanthus; Myrmecophila; Notylia; Oberonia; Oeonia; Oncidium; Ornithocephalus; Pabstiella; Peristeria; Phalaenopsis (including Sedirea); Platyrhiza; Plectorrhiza; Plectrophora; Pleurothallis; Pomatocalpa; Prosthechea; Psychilis; Psychopsis; Pterostemma; Quekettia; Rhinerrhiza; Robiquetia; Rodriguezia; Rossioglossum (including Chelyorchis); Saccolabiopsis; Saccolabium; Sarcochilus; Scaphyglottis; Schoenorchis; Sobralia; Solenidium; Stanhopea ; Stelis; Taeniophyllum; Tetramicra; Thrixspermum; Tolumnia; Trachoma; Trichocentrum (including Cohniella and Lophiaris); Trichoglottis; Trichopilia; Trichotosia; Trizeuxis; Vanda (including Ascocentrum); Warmingia; Zygostates | 5–9 (Silvera et al., 2009) |
| Vanilloideae | Vanilla | 1 |
| Asphodelaceae | 1; in the ancestor of Alooideae and Bulbine | |
| Alooideae | Aloe; Aloidendron; Aristaloe; Astroloba (including Poellnitzia); Gasteria; Gonialoe; Haworthia; Haworthiopsis; Tulista | |
| ‘Asphodeloideae’1 | Bulbine | |
| Asparagaceae | ||
| Agavoideae | Agave (including Manfreda and Polianthes); Beschorneria; Furcraea; Hesperaloe; Yucca | 3; 1 in the ancestor of Agave, Beschorneria and Furcraea, 1 in Yucca sect. sarcocarpa, and 1 in Hesperaloe (Heyduk et al., 2022) |
| Nolinoideae | Beaucarnea, Sansevieria2 | 2; 1 each in Beaucarnea and Sansevieria (Meng et al., 2021) |
| Commelinales | ||
| Commelinaceae | ||
| Commelinoideae | Callisia; Cyanotis; Tradescantia; Tripogandra | 2; 1 in the ancestor of Callisia, Tradescantia and Tripogandra; and 1 in Cyanotis (Lee et al., 2021) |
| Poales | ||
| Bromeliaceae | ||
| Bromelioideae | Acanthostachys; Aechmea (including Streptocaylx); Ananas; Androlepis; Araeococcus; Billbergia; Bromelia; Canistropsis; Canistrum; Cryptanthus; Deinacanthon; Disteganthus; Edmundoa; Eduandrea; Forzzaea; Hohenbergia; Hohenbergiopsis; Hylaeaicum; Karawata; Lymania; Neoglaziovia; Neoregelia; Nidularium; Ochagavia; Orthophytum; Portea; Pseudananas; Pseudaraeococcus; Quesnelia; Ronnbergia; Sincoraea; Ursulaea; Wittrockia | 2–5 in Bromelioideae (Givnish et al., 2014) |
| Hechtioideae | Hechtia | 1 |
| Pitcairnioideae | Deuterocohnia; Dyckia; Encholirium | 1 |
| Puyoideae | Puya | 1–3 within Puya (Givnish et al., 2014) |
| Tillandsioideae | Guzmania; Josemania; Lemeltonia; Tillandsia; Werauhia | 1–5 (Barfuss et al., 2016) |
| Saxifragales | ||
| Crassulaceae | 1; CAM assumed ancestral to the Crassulaceae | |
| Crassuloideae | Crassula (including Rochea) | |
| Kalanchoideae | Adromischus; Cotyledon; Kalanchoë; Tylecodon | |
| Sempervivoideae | Aeonium (including Greenovia); Aichryson; Cremnophila; Dudleya; Echeveria; Graptopetalum; Hylotelephium; Lenophyllum; Monanthes; Orostachys; Pachyphytum; Rosularia; Sedum (including Diamorpha); Sempervivum; Umbilicus; Villadia | |
| Vitales | ||
| Vitaceae | ||
| Vitoideae | Cissus; Cyphostemma | 2; 1 each in Cissus and Cyphostemma (Wen et al., 2018) |
| Zygophyllales | ||
| Zygophyllaceae | ||
| Larreoideae | Bulnesia | 1 |
| Cucurbitales | ||
| Cucurbitaceae | Seyrigia; Xerosicyos | 2; 1 each in Seyrigia and Xerosicyos (Guo et al., 2020) |
| Rosales | ||
| Urticaceae | Pilea | 1 |
| Malphighiales | ||
| Clusiaceae | Clusia | 1–4 within Clusia (Luján et al., 2022) |
| Passifloraceae | Adenia | 1 |
| Euphorbiaceae | ||
| Euphorbiodeae | Euphorbia (including Monadenium, Pedilanthus and Synadenium) | 1–13 within Euphorbia (Horn et al., 2014) |
| Crotonoideae | Jatropha | 1 |
| Geraniales | ||
| Geraniaceae | Monsonia (including some members of Sarcocaulon); Pelargonium | 2–8; 1 in Monsonia and 1–7 in Pelargonium (Jones et al., 2003; García-Aloy et al., 2017; van de Kerke et al., 2019) |
| Caryophyllales | ||
| Aizoaceae | 1–4; CAM might be ancestral to Aizoaceae or has evolved independently in Aizooideae, Mesembryanthemoideae, Ruschioideae and Sesuvioideae, perhaps twice in Sesuvioideae (Klak et al., 2004, 2017a; Valente et al., 2014) | |
| Aizooideae | Tetragonia | |
| Mesembryanthemoideae | Mesembryanthemum (including Aptenia, Aridaria, Aspazoma, Brownanthus, Opophytum, Phyllobolus, Prenia, Psilocaulon, Sceletium, Sphalmanthus and Synaptophyllum) | |
| Ruschioideae | Antimima; Argyroderma; Astridia; Bergeranthus; Carpobrotus; Carruanthus; Cephalophyllum; Chasmatophyllum; Cheiridopsis; Conophytum; Delosperma; Disphyma; Dracophilus; Drosanthemopsis (including Anisocalyx); Drosanthemum Eberlanzia; Erepsia; Faucaria; Fenestraria; Glottiphyllum; Hartmanthus; Hereroa; Jacobsenia; Jordaaniella; Lampranthus; Lithops; Malephora; Meyerophytum; Mitrophyllum; Monilaria; Pleiospilos; Prepodesma; Psammophora; Rabiea; Rhinephyllum; Ruschia; Sarcozona; Schlechteranthus; Stoeberia; Titanopsis; Trichodiadema; Vanheerdea | |
| Sesuvioideae | Sesuvium; Trianthema | |
| Portulacineae | 1; CAM likely to be ancestral to the Portulacineae (Goolsby et al., 2018) | |
| Montiaceae | Australian Calandrinia3; Calyptridium; Cistanthe; Claytonia; Lewisia; Phemeranthus | |
| Didiereaceae | ||
| Didiereoideae | Alluaudia; Alluaudiopsis; Decarya; Didierea | |
| Portulacarioideae | Portulacaria (including Ceraria) | |
| Basellaceae | Anredera; Basella | |
| Halophytaceae | Halophytum | |
| Talinaceae | Talinum (including Talinella) | |
| Portulacaceae | Portulaca | |
| Anacampserotaceae | Anacampseros; Grahamia; Talinopsis | |
| Cactaceae 4 | ||
| Cactoideae | Acanthocereus (including some members of Peniocereus); Bergerocactus; Carnegiea; Cephalocereus (including Neobuxbaumia) Cereus (including Subpilocereus); Chamaecereus; Cleistocactus; Cochemiea (including some members of Mammillaria); Consolea; Copiapoa (including Pilocopiapoa); Disocactus; Echinocactus; Echinocereus; Echinopsis; Epiphyllum; Eriosyce; Eulychnia; Ferocactus; Haageocereus; Hatiora; Leucostele; Lobivia; Lophocereus; Lophophora; Mammillaria; Melocactus; Myrtillocactus; Oreocereus; Oroya; Pachycereus; Parodia; Pelecyphora; Pilosocereus; Polaskia; Rhipsalis; Schlumbergera (including Zygocactus); Sclerocactus; Selenicereus (including Hylocereus); Stenocereus (including Ritterocereus); Stetsonia; Trichocereus; Turbinicarpus | |
| Opuntioideae | Austrocylindropuntia; Cylindropuntia; Grusonia; Maihueniopsis; Opuntia (including Nopalea); Pereskiopsis; Pterocactus; Quiabentia; Tephrocactus | |
| Pereskioideae | Maihuenia; Pereskia5 | |
| Gentianales | ||
| Rubiaceae | ||
| Rubioideae | Hydnophytum; Myrmecodia; Squamellaria | 1–3; either 1 in the ancestor of Hydnophytineae or 1 in each genus (Chomicki and Renner, 2016) |
| Apocynaceae | ||
| Apocynoideae | Pachypodium | 1 |
| Asclepiadoideae | Apteranthes; Boucerosia (including Frerea); Caralluma; Caudanthera; Ceropegia6; Cynanchum (including Folotsia and Sarcostemma); Desmidorchis; Dischidia; Duvalia; Hoodia (including Trichocaulon); Hoya; Huernia; Orbea; Quaqua; Stapelia | 3; 1 each in Marsdenieae, Asclepiadeae and Ceropegieae (Wanntorp et al., 2014; Bruyns et al., 2017; Liede-Schumann et al., 2022) |
| Lamiales | ||
| Plantaginaceae | Littorella | 1 |
| Gesneriaceae | ||
| Didymocarpoideae | Haberlea; Ramonda | 1 |
| Gesnerioideae | Codonanthopsis | 1 |
| Lamiaceae | ||
| Lamioideae | Marrubium | 1 |
| Nepetoideae | Coleus (including some members of Plectranthus) | 1 |
| Asterales | ||
| Asteraceae | ||
| Asteroideae | Baculellum; Caputia; Crassothonna; Curio; Kleinia; Othonna; Senecio | 2 or 3; 1 in the Gynuroid clade and 1 or 2 in the Faujasia–Bethencourtia clade (Pelser et al., 2007; Ozerova et al., 2017) |
| Total known origins: 66–114+ |
1Asphodeloideae is polyphyletic, with Bulbine generally sister to Alooideae, as summarized by Smith and Figueiredo (2020).
2 Sansevieria has been proposed to be subsumed within Dracaena (Takawira-Nyenya et al., 2018).
3 Calandrinia is not monophyletic (Hancock et al., 2019), and CAM has been observed only in the monophyletic clade inclusive of all Australian members of Calandrinia sensu lato.
4All cacti are assumed CAM; the genera presented here are those with published data confirming CAM activity.
5We do not include Leuenbergeria but recognize that Pereskia is non-monophyletic.
6 Ceropegia has recently been shown to be polyphyletic, with Brachystelma and the stem-succulent stapeliads (including Apteranthes, Caralluma, Caudanthera, Boucerosia, Duvalia, Hoodia, Huernia, Orbea, Quaqua and Stapelia) nested within it. These genera have been proposed to be subsumed within an expanded and recircumscribed Ceropegia (Bruyns et al., 2017) but are still recognized by Kew (POWO, 2023) and other authorities (Endress et al., 2018),.
Box 2. Outstanding questions in CAM evolution.
Although CAM evolution has long been an area of CAM research, phylogenetic analyses of CAM have been published largely within the last two decades. These include the reconstruction of ancestral character states (Crayn et al., 2004; Edwards and Donoghue, 2006; Bone et al., 2015; Heyduk et al., 2016; Hancock et al., 2019; Luján et al., 2022) and tests of the association between CAM and net diversification rates (Givnish et al., 2014, 2015; Horn et al., 2014); but many open questions remain, ripe for investigation with recently developed models of trait evolution, denser and broad phylogenies, and increased CAM-associated trait data.
The timing of CAM evolution
Carbon-concentrating mechanisms are generally believed to have evolved in terrestrial species post-Oligocene, following a reduction in atmospheric CO2 to near current values (Edwards and Ogburn, 2012), but few clades have been evaluated for the timing of CAM origins. Those that have been examined are generally consistent with the C4 pattern of post-Oligocene evolution followed by extensive diversification (Sage et al., 2023). Some large CAM clades, however, such as the Portulacineae and Crassulaceae, might have ancient origins, pre-dating the late-Oligocene CO2 decline (Wang et al., 2019a; Messerschmid et al., 2020), implying CAM evolution in higher-CO2 settings than C4 evolution. These hypotheses will be re-evaluated as phylogenomic data, paired with new phylogenetic comparative analyses, produce the more detailed, CAM-specific phylogenies needed to identify CAM origins on time-calibrated phylogenies.
The earliest steps in CAM evolution
Research efforts towards the engineering of CAM have sharpened our understanding that CAM evolution is likely to involve both discrete and continuous changes (Borland et al., 2014; Bräutigam et al., 2017; Edwards, 2019, 2023; Winter and Smith, 2022). However, we lack the comparative genomic studies between very recently diverged CAM and non-CAM species or populations needed to identify protein and regulatory changes that enabled the emergence of CAM in a non-CAM ancestor.
CAM-associated traits
The evolution of CAM has been associated with many traits (recently reviewed by Niechayev et al., 2019), but quantitative or statistical tests of these relationships are generally lacking. The link between CAM and succulence has been apparent from the earliest studies of CAM, but explicit relationships have not been described. Likewise, we lack quantitative analyses of CAM–environment associations at evolutionary scales. The development of climate-dependent trait evolution models (Clavel and Morlon, 2017) might facilitate such investigations, and the creation of CAM physiological models (Shameer et al., 2018; Töpfer et al., 2020) might help to identify potential hotspots of CAM evolution in deep time, as has been done for C4 (Zhou et al., 2018). Physiological models might also shed light on the functional significance of C3 + CAM. Many C3 + CAM species do not assimilate substantial amounts of carbon via CAM (Herrera, 2008; Winter, 2019), but other benefits, such as recapture of respiratory CO2, and energy and water savings (Martin, 1996; Shameer et al., 2018; Töpfer et al., 2020) or photoprotection (Osmond, 1982; Pieters et al., 2003) under water deficit have been proposed.
THE LANDSCAPE OF CAM PHENOTYPES AND CAM EVOLUTION
The variability of CAM expression was appreciated by the late 19th century, and multiple CAM phenotypes have since been described (recently reviewed by Winter, 2019). These phenotypes are often projected along two axes: CAM mode, i.e. the degree to which CAM is constitutive and/or facultative, and CAM ‘strength’, i.e. the fraction of carbon fixed at night. CAM strength can be measured over short time scales (hours to days) by monitoring gas exchange or changes in titratable acidity (ΔH+) or it can be integrated over longer periods by δ13C values (Box 1). The best-studied CAM plants tend to exhibit either facultative CAM, with moderate to large ΔH+ (e.g. Mesembryanthemum crystallinum), or strong and constitutive CAM (e.g. Kalanchoë daigremontiana) (Winter et al., 2008).
As CAM has been surveyed more broadly and deeply, a complex landscape of CAM phenotypes has emerged, and experiments in the Aizoaceae (Winter, 2019) and Montiaceae (Hancock et al., 2019) demonstrate that both the mode and the strength of CAM can vary considerably among plants that primarily utilize C3 photosynthesis (Fig. 3). CAM can be either constitutive and weak (Calandrinia ptychosperma F.Muell.) or facultative with strong CAM induction [Jordaaniella cuprea (L.Bolus) H.E.K.Hartmann]. Constitutive CAM plants may have further facultative responses [Disphyma crassifolium (L.) L.Bolus] or reduce CAM (e.g. ΔH+) under stress [Titanopsis calcarea (Marloth) Schwantes] (Winter, 2019). Although strongly and constitutively expressed CAM can be identified readily by δ13C values less negative than −20 ‰ (in non-C4 species), plants that obtain one-third or less of their carbon in the dark have isotopic signatures in the range of C3 plants (Winter and Holtum, 2002; Fig. 3 inset).
Fig. 3.
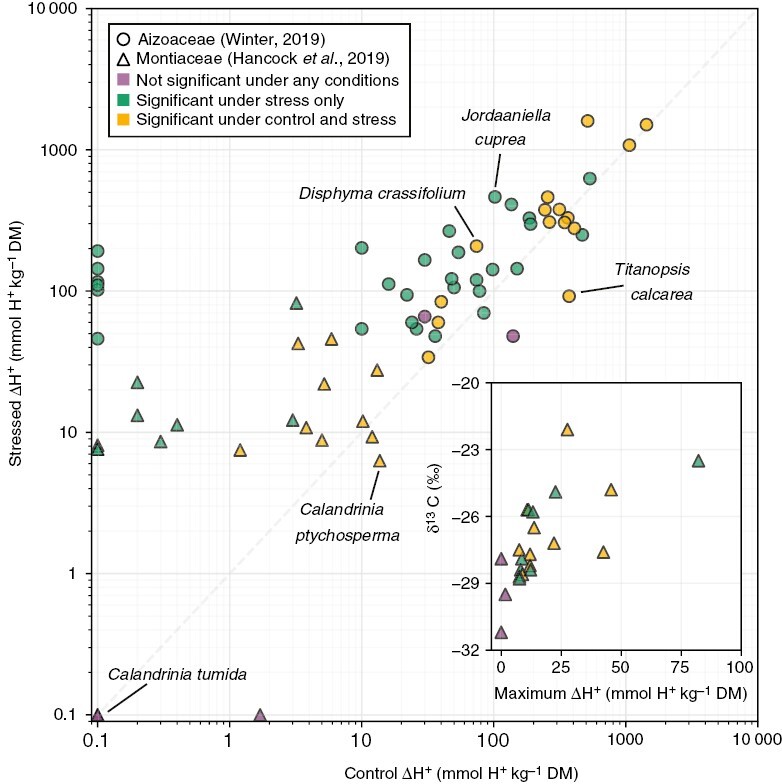
Mean diel ΔH+ in control (well-watered) and stress (drought or drought + salt stress) conditions of 45 Aizoaceae species (circles) (Winter, 2019) and 22 Montiaceae species (triangles) (Hancock et al., 2019). Purple points represent species with no significant ΔH+ in any conditions. Green points represent species with significant ΔH+ only in stress conditions. Yellow points represent species with significant ΔH+ in both control and stress conditions. The inset shows the maximum ΔH+ and mean δ13C values (Hancock et al., 2019) of the same Montiaceae species in the main plot. Note that ΔH+ for Aizoaceae species was calculated by multiplying changes in tissue malate concentrations given by Winter (2019) by a factor of two, assuming that 1 mol malate corresponds to 2 mol H+. Points with ΔH+ < 0.1 were plotted at 0.1 for visualization. Five individual species discussed in the main text are labelled.
Although many labels have been applied to various CAM phenotypes in the past, here we use ‘C3 + CAM’ (sensuEdwards, 2019) to refer to species that use C3 photosynthesis as the principal pathway of carbon gain but also express CAM to various degrees. We use the term ‘strong CAM’ to refer to species that use CAM as the principal pathway of carbon gain; this corresponds to the ‘strong CAM’ category of Edwards (2019), and the ‘CAM plant’ recommendation of Winter et al. (2015). Many species capable of CAM have been demonstrated to induce or upregulate CAM in response to stress (typically drought or salinity stress) in natural or laboratory experiments; this behaviour is known as ‘facultative CAM’. These species do not express CAM in favourable conditions or do so only weakly. Although plants with intermediate δ13C values have been found in many clades and constitute the majority of species in Aizoaceae subfamily Mesembryanthemoideae (most probably owing to intraspecific plasticity in carbon isotopic composition because of seasonal shifts in δ13C) (Winter et al., 1976; Messerschmid et al., 2021; Winter and Smith, 2022), the strong bimodality in δ13C values seen in the majority of clades in which CAM occurs implies that carbon is either mostly fixed with C3 photosynthesis or mostly fixed with CAM (Winter and Holtum, 2002; Winter et al., 2015; Edwards, 2019; Messerschmid et al., 2021; Orlov et al., 2022). The development of a holistic CAM evolutionary model should explain the observations that most clades do not contain a uniform distribution of δ13C values and that C3 + CAM species can occupy distinct regions of the δ13C distribution over long periods of evolutionary time.
It is believed that these phenotypes represent ordered character states, ranging from C3 to C3 + CAM to strong CAM, because strong-CAM lineages are frequently subtended by earlier-branching C3 + CAM lineages in phylogenetic studies of CAM evolution (Hancock and Edwards, 2014), although some have hypothesized that the evolutionary trajectories to facultative and constitutive CAM are different (Yang et al., 2019). However, there are relatively few clades for which we have both sufficiently widespread CAM surveys, including tests of C3 + CAM, and well-resolved phylogenies to be able to infer the relationships between states with confidence. For example, Orchidaceae, which contain numerically a substantial proportion of the planet’s CAM species, and Crassulaceae, which include multiple model CAM species of Kalanchoë, generally have poorly resolved phylogenies at the species level and relatively few surveys of C3 + CAM (but see Silvera et al., 2005, 2014). More detailed studies have demonstrated phylogenetic patterns that support an evolutionary trajectory from C3 to C3 + CAM to strong CAM in multiple clades, e.g. within subtribe Oncidiinae of Orchidaceae (Silvera et al., 2014), Cactaceae (Edwards and Donoghue, 2006), Clusia (Luján et al., 2022) and Agavoideae (Heyduk et al., 2022).
Further exploration might change our understanding of CAM evolution dramatically, as in the Portulacineae (Fig. 2), where tests of C3 + CAM and new phylogenomic studies have revealed lineages with varying CAM phenotypes and very few, if any, purely C3 Portulacineae species outside Montiaceae. Given the young age of many CAM clades [e.g. Ruschioideae, ~7 (3.4–12.6) Ma; Klak et al., 2017a] and that the evolution of CAM has been associated with increased diversification rates in multiple clades (Givnish et al., 2014, 2015; Horn et al., 2014; Silvestro et al., 2014), it has been challenging to identify well-resolved and well-sampled clades with known C3, C3 + CAM and strong-CAM species. Erycina (Oncidiinae: Orchidaceae) has emerged as a candidate model for CAM evolution with the publication of a whole-chloroplast genome of CAM Erycina pusilla (L.) N.H.Williams & M.W.Chase (Pan et al., 2012) and comparative transcriptomic research between E. pusilla and C3Erycina crista-galli (Rchb.f.) N.H.Williams & M.W.Chase (Heyduk et al., 2019a). The Oncidiinae might have multiple transitions from C3 to strong CAM if C3 + CAM is indeed absent in the currently recognized ‘C3’ members, but the clades perhaps currently best situated for studying transitions to and between CAM phenotypes are Clusia (Clusiaceae) and the Agavoideae (Asparagaceae). The Neotropical woody genus Clusia contains >300 species with multiple recognized C3, C3 + CAM and strong-CAM taxa (Lüttge, 2007; Pachon et al., 2022). A recent study of the evolution of CAM physiology and morphology in Clusia found correlations between CAM activity and both leaf morphology and dry season severity (Luján et al., 2022). The authors reconstructed a phylogeny of dozens of Clusia taxa with multiple photosynthetic phenotypes and, depending on model choice, ancestral state reconstructions supported either one origin of strong CAM and several reversions or multiple origins of strong CAM (Luján et al., 2022). Research has confirmed C3, C3 + CAM and strong-CAM species in the Agavoideae and hypothesized multiple origins of CAM over the past 25 Ma (Heyduk et al., 2022). Yucca also produces natural C3 + CAM hybrids (Yucca gloriosa L.) arising from C3 (Yucca filamentosa L.) and strong-CAM (Yucca aloifolia L.) populations (Rentsch and Leebens‐Mack, 2012; Heyduk et al., 2021) that offer a unique means to study the genetic components of CAM.
Although the growing number of C3 + CAM observations continue to amend the hypothesized timing and phylogenetic placement of CAM origins (Box 2), their diversity offers an unrivalled opportunity to study the evolution of convergent ecophysiology. The evolutionary trajectories from C3 to CAM have been debated over recent years. Some have argued that CAM requires relatively few evolutionary changes because all enzymes and biochemical pathways used in CAM exist in C3 plants (Bräutigam et al., 2017; Schiller and Bräutigam, 2021), whereas others have argued that substantial metabolic reprogramming is required to evolve CAM (Winter and Smith, 2022), not least because malate typically accumulates during the daytime in C3 plants but during the nighttime in CAM plants. Others have argued that C3 + CAM can (and has) evolved readily in a diversity of C3 lineages, but that strong CAM requires more anatomical specialization and is less evolutionarily labile (Edwards, 2019). Whatever the path(s) to CAM, comparative studies of C3 + CAM species, and particularly those that use CAM facultatively in response to stress, are poised to shed light on C3-to-CAM transitions. Paired whole-transcriptome and physiological (e.g. gas exchange, ΔH+ and metabolomes) datasets from CAM-induction experiments are available for Talinum (Talinaceae) (Brilhaus et al., 2016), Beschorneria, Agave (including Manfreda and Polianthes) and Yucca (Agavoideae) (Heyduk et al., 2022), Dendrobium (Orchidaceae) (Zou et al., 2018), Sedum (Crassulaceae) (Wai et al., 2019), Portulaca (Portulacaceae) (Ferrari et al., 2020; Gilman et al., 2022; Moreno-Villena et al., 2022), Mesembryanthemum (Aizoaceae) (Cushman et al., 2008) and Isoëtes (Yang and Liu, 2015). These studies have revealed substantial similarities between CAM induction across clades and regardless of life history (annual or perennial) or habit (epiphytic or terrestrial), but also key differences, e.g. varied peak transcription of CAM-specific isoforms of PEPC from mid-afternoon to late dark period. These CAM-induction experiments of the past two decades have been key for identifying and profiling core CAM elements (Winter and Holtum, 2014), and future multi-species comparisons will be essential in describing the regulation of CAM and how CAM is induced. No direct comparisons of regulatory elements (e.g. transcription factors or cis-elements) have been made across C3 + CAM species that could explain how CAM is incorporated into stress responses or how facultative CAM becomes canalized. Most -omics research has focused on large, diverse CAM clades (e.g. Agavoideae, Crassulaceae, Orchidaceae and Portulacineae), but it might be more fruitful to develop model systems around relatively species-poor CAM origins, which afford relevant C3 comparisons in more closely related species. At the time of publication, publicly available genomes exist for ≥23 species capable of CAM, most of reference quality (Table 2); two orchids from genera that contain species with CAM have whole-genome sequences but have not been assessed for C3 + CAM [Cymbidium sinense (Andrews) Willd. (Yang et al., 2021) and Dendrobium huoshanense Z.Z.Tang & S.J.Cheng (Han et al., 2020)], and a genome sequence is available for Mikania micrantha Kunth (Asteraceae), which has been suggested to be CAM, based on gene expression (Liu et al., 2020) (Supplementary Data Table S2).
Table 2.
CAM species with publicly available whole-genome sequences and associated assembly statistics; BUSCO, benchmarking universal single-copy orthologue (Manni et al., 2021).
| Taxon1 | CAM type2 | Genome size | Scaffold N50 | Scaffold L50 | Complete Embryophyta BUSCOs (%) |
|---|---|---|---|---|---|
|
Isoëtaceae
Isoëtes taiwanensis De Vol (Wickell et al., 2021) |
Aquatic strong CAM | 1.65 Gb | 17.4 Mb | Not reported | 94.53 |
|
Asphodelaceae
Aloe vera (L.) Burm.F. (Jaiswal et al., 2021) |
Strong CAM (Winter and Holtum, 2002) | 12.93 Gb | 14.6 kb | Not reported | 74.6 |
|
Orchidaceeae
Cymbidium crassifolium Herb. (Fan et al., 2023; reported as Cymbidium mannii Rchb.f.) |
Strong CAM | 2.88 Gb | 22.7 Mb | Not reported | 97.0 |
| Dendrobium nobile Lindl. (Xu et al., 2022) | C3 + CAM (Qiu et al., 2015) | 1.16 Gb | 64.5 Mb | 8 | 96.2 |
| Dendrobium officinale Kimura & Migo (Yan et al., 2015) | C3 + CAM (Zou et al., 2018; reported as D. catenum Lindl.) | 1.35 Gb | 76.5 kb | 4697 | Not reported |
| Dendrobium chrysotoxum Lindl. (Zhang et al., 2021) | C3 + CAM (Qiu et al., 2015) | 1.37 Gb | 67.8 Mb | 8 | 90.3 |
| Phalaenopsis equestris (Schauer) Rchb.f. (Cai et al., 2015) | Strong CAM (Zhang et al., 2016) | 1.16 Gb | 359 kb | 523 | Not reported |
| Vanilla planifolia Andrews (Hasing et al., 2020) | Strong CAM (Silvera et al., 2010a) | 1.48 Gb | 42.0 Mb | 13 | 93.9 |
|
Bromeliaceae
Aechmea fasciata (Lindl.) Baker (Li et al., 2022) |
Strong CAM (Crayn et al., 2015) | 359 Mb | 4.68 Mb | 38 | 93.4 |
| Ananas comosus (L.) Merr. V3 (Ming et al., 2015) | Strong CAM | 526 Mb | 11.8 Mb | 13 | 89.3 |
|
Crassulaceae
Kalanchoë fedtschenkoi Raym.-Hamet & H.Perrier v1.1 (Yang et al., 2017) |
Strong CAM | 256.35 Mb | 2.5 Mb | 28 | 89.5 |
| Kalanchoë laxiflora Baker v3.1 (Yang et al., 2017) | Strong CAM | 262 Mb | 12.5 Mb | 8 | 93.2 |
| Sedum album L. (Wai et al., 2019) | C3 + CAM | 302 Mb | 93 kb | 868 | 97 |
|
Vitaceae
Cissus rotundifolia Vahl (Xin et al., 2022) |
Strong CAM (Nelson and Sage, 2008) | 350.69 Mb | 27.6 Mb | 5 | 92.4 |
|
Euphorbiaceae
Jatropha curcas L. (Ha et al., 2019) |
C3 + CAM (Winter and Holtum, 2015) | 414 Mb | 1.5 Mb | Not reported | 82.5 |
|
Aizoaceae
Mesembryanthemum crystallinum L. (Shen et al., 2022) |
Facultative CAM | 377.97 Mb | 40.5 Mb | 5 | 98.0 |
|
Cactaceae
Carnegiea gigantea (Engelm.) Britton & Rose (Copetti et al., 2017) |
Strong CAM (Nobel and Hartsock, 1986) | 1.30 Gb | 61.5 kb | 4575 | 91 |
| Lophocereus schottii (Engelm.) Britton & Rose (Copetti et al., 2017) | Strong CAM (Mooney et al., 1974) | 1.47 Gb | 9.3 kb | 21 671 | 74 |
| Pachycereus pringlei (S.Watson) Britton & Rose (Copetti et al., 2017) | Strong CAM (Mooney et al., 1974) | 1.41 Gb | 5.4 kb | 32 562 | 61 |
| Pereskia horrida DC. (Copetti et al., 2017; reported as P. humboltii Britton & Rose) | C3 + CAM (Martin and Wallace, 2000) | 980 Mb | 4.4 kb | 28 266 | 52 |
| Stenocereus thurberi (Engelm.) Buxb. (Copetti et al., 2017) | Strong CAM (Huber et al., 2018) | 1.42 Gb | 10.5 kb | 20 352 | 71 |
| Selenicereus undatus (Haw.) D.R.Hunt (reported as Hylocereus undatus (Haw.) D.R.Hunt) (Chen et al., 2021) | Strong CAM (Wang et al., 2019b) | 1.41 Gb | 127.2 Mb | 670 | 93.83 |
|
Portulacaceae
Portulaca amilis Speg. (Gilman et al., 2022) |
C4 + CAM | 403.89 Mb | 42.6 Mb | 5 | 96.9 |
| Portulaca oleracea L. (Wang et al., 2023) | C4 + CAM | 1.13 Gb | 35.1 Mb | 11 | 98.0 |
1Genome assembly version 1.0 unless otherwise noted.
2Evidence of CAM is provided under the Taxon column unless provided here.
3Eukaryota BUSCOs were used to benchmark Isoëtes taiwanensis (Wickell et al., 2021) and Hylocereus undatus (Chen et al., 2021).
ATYPICAL CAM
Most of our understanding of CAM comes from terrestrial xeric and epiphytic plants, which experience regular water limitation, and CAM species tend to have very high water-use efficiency relative to C3 and C4 species (Winter et al., 2005). However, CAM is also found in dozens of aquatic plant species (Keeley, 1998) and has been observed in more diverse forms and unexpected lineages. These examples, which fall outside the common CAM phenotypes, further highlight the diversity of species capable of CAM and its functional significance.
Aquatic CAM and other photosynthetic pathways
The apparent paradox of xeric adaptations in submerged and aquatic plants can be resolved by recognition that the pressures of water loss and CO2 limitation represent two sides of the same coin for terrestrial species: water limitation leads to stomatal closure, thereby restricting CO2 uptake. Seasonal, or vernal, pools may contain numerous aquatic CAM species from genera including Isoëtes, Crassula and Sagittaria. Vernal pools often exhibit large fluctuations in CO2 concentration because CO2 is depleted during the day by the photosynthetic activity of non-CAM vegetation, solar warming of the shallow water, and CO2 diffusion limitations from the air; at night, heterotrophic respiration by plants and animals increases the concentration of dissolved CO2 in the water dramatically (Raven and Spicer, 1996; Keeley, 1998). These diel trends in dissolved CO2 create a niche for CAM plants, which can capture dissolved CO2 when it is abundant at night and use it during the day when pools become CO2 depleted. CAM activity decreases or ceases entirely in tissues of vernal CAM plants that become emergent because CO2 diffusion in air is orders of magnitude higher than in water, further supporting the functional significance of CAM in CO2-limited conditions (Keeley, 1998).
In addition to vernal pools, aquatic CAM plants can be found in high-elevation oligotrophic lakes. In such nutrient-poor systems, Isoëtes can absorb CO2 directly through their extensive root systems, with diffusion through the well-developed air canals carrying CO2 to the shoot, where it can be fixed in the dark via CAM in leaves; roots may thereby account for the majority of CO2 uptake in species such as Isoëtes andicola (Amstutz) L.D.Gómez (previously Stylites andicola Amstutz) (Keeley et al., 1984).
Although most species with carbon-concentrating mechanisms (CCMs) are hypothesized to have evolved and diversified following the Oligocene atmospheric CO2 decline (Arakaki et al., 2011; Edwards and Ogburn, 2012), the selective pressures to evolve aquatic CAM are less coupled to atmospheric CO2 levels; it is possible that CAM is ancient in lineages such as in the lycophyte Isoëtes, an early vascular plant clade with a stem age estimated to be 370 Ma (Wood et al., 2020). Aquatic and terrestrial CAM species have mostly been treated separately, but the recent publication of an Isoëtes genome (Table 2) and transcriptomic data (Wickell et al., 2021) will facilitate a fuller understanding of CAM regulation using modern comparative methods.
Since the near-simultaneous reports of CAM in the submerged plants Isoëtes howellii Engelm. (Keeley, 1981) and Schoenoplectus subterminalis (Torr.) Soják (previously Scirpus subterminalis Torr.) (Beer and Wetzel, 1981) (the latter has not been revisited or considered a CAM plant since), aquatic CAM has been confirmed in all aquatic species of Isoëtes tested (Keeley, 1998) and in Crassula aquatica Schönland (Crassulaceae) (Keeley and Morton, 1982) and Crassula helmsii (Kirk) Cockayne (Newman and Raven, 1995), Littorella uniflora (L.) Asch. (Plantaginaceae) (Madsen, 1987; Keeley and Morton, 1982), Sagittaria (Alismataceae) (Keeley, 1996, 1998), and Vallisneria and Ottelia (both Hydrocharitaceae) (Helder and van Harmelen, 1982; Webb et al., 1988; Zhang et al., 2014). Many aquatic CAM macrophytes are considered highly invasive outside their native ranges (Klavsen et al., 2011), but whether and how CAM might influence invasiveness has not been studied in detail.
CAM-like phenomena (particularly nocturnal acid or malate accumulation) have been reported for a wider diversity of aquatic or submerged species, including algae (reviewed by Keeley, 1998), but many of these designations have been questioned (Supplementary Data Table S2). Submerged plants might use multiple CCMs or accumulate acid that is not maintained as malic acid through the dark period or does not ultimately enter the Calvin–Benson–Bassham cycle (Keeley, 1999). Certain aquatic lineages use a dissolved bicarbonate-based CCM in addition to fixing dissolved CO2 directly (Keeley, 1998), and there are several known submerged C4 lineages, a small number of which use a form of single-cell C4 photosynthesis without Kranz anatomy (Bowes, 2011). Similar to aquatic CAM, aquatic C4 (with or without Kranz anatomy) can be induced by low CO2 (or bicarbonate) (Keeley, 1998), meaning that detection and delineation between CCMs requires extensive field sampling and laboratory experiments that document gas exchange and diurnal changes in malic acid (Box 1). For example, although Eleocharis maculosa (Vahl) Roem. & Schult. (Cyperaceae) can show very small ΔH+, radiolabel pulse–chase experiments have demonstrated that carbon in nocturnally produced malate does not flow into the Calvin–Benson–Bassham cycle in the following light period, as it does in bona fide aquatic CAM species (Keeley, 1999). However, careful experiments with Hydrilla verticillata (L.f.) Royle (Hydrocharitaceae) (Holaday and Bowes, 1990; Rao et al., 2006) demonstrated that H. verticillata has low-CO2-induced C4 and probably weak CAM (see ‘Terrestrial C4 + CAM’, below). Likewise, Ottelia alismoides (L.) Pers. (Hydrocharitaceae) has been shown to be a constitutive submerged C4 plant with inducible CAM (Zhang et al., 2014; Shao et al., 2017). The diversity of photosynthetic pathways in aquatic plants, in addition to their ability to transition between them and to use multiple CCMs simultaneously, presents a more complicated photosynthetic landscape than in terrestrial plants, and many classifications as ‘CAM’ should be evaluated carefully or investigated further.
Terrestrial C4 + CAM
The combination of CCMs is not restricted to aquatic plants. CAM has been demonstrated clearly in two succulent terrestrial C4 clades (hereafter ‘C4 + CAM’): Portulaca (Portulacaceae) and Trianthema (Aizoaceae). Laboratory measurements of gas exchange and ΔH+ revealed facultative CAM in all studied species of Portulaca (Koch and Kennedy, 1980; Guralnick et al., 2002; Holtum et al., 2017; Gilman et al., 2022), including those with C3–C4 intermediacy (Winter et al., 2019), and low-level constitutive CAM has been demonstrated in C4Trianthema portulacastrum L. (Winter et al., 2021a).
Portulaca is the most studied of the terrestrial C4 + CAM clades, and all research has supported the hypothesis that CAM is ancestral to Portulaca and probably evolved deep within (or in an ancestor of) the Portulacineae (Christin et al., 2014; Goolsby et al., 2018). Given the substantial variation in C4 characters (i.e. leaf anatomy, biochemical subtypes and species-specific gene use; Ocampo et al., 2013; Voznesenskaya et al., 2017; Gilman et al., 2022), in addition to C3–C4 intermediacy in some species, it is likely that C4 evolved convergently in three facultative CAM Portulaca lineages: the Pilosa + Umbraticola, Oleracea + Cryptopetala and opposite-leaved clades. Spatially explicit analyses of gene expression in Portulaca oleracea L. demonstrated that C4 and CAM cycles are integrated, with CAM-generated malate produced in the mesophyll probably being decarboxylated by the C4 cycle in the bundle sheath during the day (Moreno-Villena et al., 2022).
The precise origins (or perhaps single origin) of CAM in Aizoaceae are not known, but the presence of CAM in most subfamilies suggests that CAM evolved very early during the diversification of extant Aizoaceae or is ancestral to it (see ‘The phylogenetic diversity of CAM’, below). Without further surveys of CAM in the Sesuvioideae, we cannot infer whether C4 or CAM evolved first in Trianthema; future comparisons of multiple C4 + CAM taxa will help to resolve long-standing questions of C4 and CAM compatibility within single tissues (Sage, 2002).
Very few C4 species have been investigated for CAM activity, and it is possible that there are many more examples of C4 + CAM photosynthesis yet to be discovered. C4 + CAM has also been reported in five succulent members of Amaranthaceae and the succulent grass Spinifex littoreus (Burm.f.) Merr. (Poaceae), all of which are halophytes (Supplementary Data Table S2). Multiple lines of evidence point to C4 + CAM in five species of the tribe Salsoleae (Amaranthaceae): Halothamnus subaphyllus (C.A.Mey.) Botsch. [previously Aellenia subaphylla (C.A.Mey.) Aellen], Haloxylon ammodendron (C.A.Mey.) Bunge ex Fenzl [previously Haloxylon aphyllum (Minkw.) Iljin], Horaninovia ulicina Fisch. & C.A.Mey., Salsola praecox (Litv.) Litv. and Xylosalsola richteri (Moq.) Akhani & Roalson [previously Salsola richteri (Moq.) Karel ex Litv.] (Zalenskiï and Glagoleva, 1981). All five species exhibited small ΔH+ in the field, and gas-exchange measurements and 14C-radiolabel pulse–chase experiments showed slight dark CO2 assimilation and formation of malate in all but Salsola praecox, which was not measured; furthermore, label from nocturnally formed malate was incorporated into sugars in the light in Haloxylon ammodendron (Zalenskiï and Glagoleva, 1981). However, these taxa have not been revisited for CAM-specific research; further laboratory studies are needed to confirm CAM in these taxa and in the C4 grass Spinifex littoreus, which was recently reported to show CAM-cycling, i.e. small ΔH+ in the absence of any net dark CO2 uptake (Ho et al., 2019). If CAM is confirmed in Spinifex or in any members of the Salsoleae, this would represent the first known evolution of CAM in a C4 lineage, because all Portulaca are hypothesized to have evolved C4 + CAM from C3 + CAM ancestors.
CAM-like physiology
In addition to C3 + CAM, aquatic CAM and C4 + CAM, photosynthetic physiology that mimics CAM and unexpected CAM-inducing stimuli have been discovered. In a process reminiscent of CAM-cycling, plants with ‘alarm photosynthesis’ recapture respired CO2 behind closed stomata into calcium oxalate crystals at night, which are then degraded to release CO2 for photosynthesis during the day (Tooulakou et al., 2016). This physiology is induced by CO2 starvation, including that caused by drought, and has been documented in C3, C4 and CAM species (Tooulakou et al., 2016). Furthermore, calcium oxalate crystals in alarm photosynthesis show less discrimination against 13C, which suggests that calcium oxalate crystal biosynthesis might involve the same carboxylating enzyme used in CAM (PEPC) to form oxaloacetate, which could be converted to oxalate by oxaloacetate acetylhydrolase.
Alarm photosynthesis should not be considered CAM, but the fungal-induced CAM reported in Camellia oleifera C.Abel (Theaceae) (Yuan et al., 2012) might change our definition of a ‘CAM plant’. Following up on the observation of rare succulent small leaves (microphylls) in natural populations of C. oleifera, Yuan et al. (2012) demonstrated that infection by the fungus Exobasidium vexans Massee (Exobasidiaceae) caused the development of succulent tissue and appeared to be correlated with the induction of a CAM cycle. More research is needed to assess the capacity for CAM further in non-infected and infected individuals, and we do not include Camellia as a CAM clade here (Supplementary Data Table S2). But if this study is confirmed, it might help to identify direct mechanistic links between succulent development and CAM expression that have interested botanists for centuries.
THE PHYLOGENETIC DIVERSITY OF CAM PLANTS
CAM plants can be found from hot semi-deserts to rainforest canopies and high-elevation lakes and are as diverse phylogenetically as they are ecologically. The growing list of CAM taxa can both expand and reduce the number of evolutionary origins of CAM. Since the last survey list of genera containing species capable of CAM (Smith and Winter, 1996), CAM has been demonstrated in an additional 134 genera from 18 families. Five of these families are new and were the subjects of detailed studies of at most a few taxa: Araceae (Holtum et al., 2007), Basellaceae (Holtum et al., 2018), Halophytaceae (this study; Supplementary Data Table S1), Urticaceae (Winter et al., 2021b) and Zygophyllaceae (Mok et al., 2023). Many of the other new genera are the result of extensive surveys to fill in gaps in photosynthetic types in species-rich clades, including Aizoaceae, Bromeliaceae, Crassulaceae and Orchidaceae.
Below, we update the estimated number of taxa with CAM and hypothesize the number of CAM origins (i.e. the evolution of the ability to perform CAM) through an extensive assessment of CAM reports and recent phylogenies of groups with CAM. We want to emphasize several limitations of this analysis. First, we discuss primarily ‘CAM genera’, but there are few genera with exhaustive searches for CAM; more often, evidence of CAM has been documented in one or perhaps a handful of species within a CAM genus, and we found direct evidence for CAM in 370 vascular plant genera scattered across 38 families (Fig. 2; Table 1). Second, in our estimates of origins, we treat each CAM genus as containing or representing a single origin of CAM unless its phylogenetic relationship to other CAM genera within a clade suggests that CAM evolved before the origin of the genus. We generally do not assume that a CAM genus has more than one origin of CAM unless evidence shows multiple origins (e.g. Euphorbia and Peperomia), although it is possible and very likely in many large genera. Third, we use genera as the primary units of analysis only for convenience, because they are typically the most specific clade name available to refer to a region of the phylogeny in the absence of an established phylogenetic nomenclature. But we do not consider genera to represent equivalent units across the broad history of vascular plants, and the finding that there are currently 370 reported CAM genera conveys limited information about the diversity, phylogenetic distribution and evolutionary history of CAM; rather, it is a consequence of how various taxonomists have delineated taxa over many years. For example, the numbers of CAM genera in Mesembryanthemoideae (Aizoaceae) and Euphorbiaceae have decreased as a result of taxonomic revision, but the number of species reported to use CAM has increased in both taxa as a result of additional experimental surveys.
Non-seed plants
CAM has been found in the lycophyte genus Isoëtes (Isoëtaceae) and the fern order Polypodiales. The genus Isoëtes contains ~140 species, all of which have been either demonstrated or assumed to use CAM when submerged, although they may not use CAM when terrestrial (Keeley, 1983). As discussed above (see ‘Aquatic CAM and other photosynthetic pathways’), the CO2 limitations of plants in aquatic habitats are less coupled to atmospheric CO2 than terrestrial plants, which implies that CAM might have been adaptive for aquatic plants long before plants in terrestrial habitats. At 45–60 Ma, the Isoëtes crown age is much younger than the stem age of ~370 Ma (Wood et al., 2020), but probably still represents one of the earliest origins of CAM. Isoëtes has been considered a ‘living fossil’ because of morphological similarities between some extant and extinct species dating back to the Carboniferous (Pigg, 2001), a period estimated to have had relatively low atmospheric CO2 (Foster et al., 2017). Gene and genome duplications have been recognized as key events facilitating the evolution of CAM and C4 (Heyduk et al., 2019b), and a recent genomic and transcriptomic study of Isoëtes taiwanensis De Vol found evidence of a whole-genome duplication event ~200 Ma in Isoëtes (Wickell et al., 2021). Isoëtes taiwanensis also uses the bacterial-type PEPC for CAM (Wickell et al., 2021), and the lateral transfer of this gene pre-dates the whole-genome duplication event. Therefore, if CAM is indeed ancestral to all extant Isoëtes, it is likely to have preceded the Oligocene CO2 decline and could conceivably be of Carboniferous origin if large-scale duplication events were not needed.
All CAM-exhibiting ferns are epiphytic and belong to the species-rich families Polypodiaceae and Pteridaceae (Table 1). As in other diverse groups, many clades lack either broad CAM surveys or robust phylogenies (or both) at the resolution needed to infer CAM origins with confidence. CAM has been studied in detail in multiple species of Australasian Pyrrosia (including Drymoglossum) (Wong and Hew, 1976; Winter et al., 1983; Griffiths et al., 1989), but not in species from Africa or mainland Asia. Given recent phylogeographic hypotheses (Wei et al., 2017), CAM appears to have evolved once in Pyrrosia as the lineage spread from mainland Asia throughout the Pacific islands, Australia and New Zealand. However, if CAM is found to be more widespread in Pyrrosia, it might be that CAM evolved in the ancestor of Pyrrosia and Platycerium in the late Eocene or early Oligocene. Unlike the situation in other diverse clades of CAM epiphytes, such as the orchids or bromeliads, strong CAM appears to have evolved only once in ferns, in the genus Pyrrosia, and most investigated ferns show only very low levels of CAM activity. Given that such low-level CAM is difficult to detect in the field, it is likely that more CAM fern lineages will be found, particularly in the mostly epiphytic Polypodiaceae. To our knowledge, no transcriptomic or genomic data are available for CAM ferns.
Acrogymnosperms
Just two acrogymnosperm lineages are known to use CAM: Welwitschia mirabilis Hook.f. (Welwitschiaceae) and Dioon edule Lindl. (Cycadaceae).
Welwitschia mirabilis is the only extant species in the order Welwitschiales and is native to the extremely harsh Namib Desert, where individuals can live for >1000 years. Although CAM is an adaptation to water limitation, few species with CAM tend to inhabit areas with such low and unpredictable precipitation (average annual precipitation is often <100 mm: Schulze et al., 1976). Given that Welwitschia are among the weakest CAM plants known, their ability to survive in the Namib Desert is likely to be the result of multiple adaptations, including a very deep tap root and high cuticular resistances (Winter and Schramm, 1986; von Willert et al., 2005). Uniquely among the gnetophytes, Welwitschia also shows evidence of a lineage-specific whole-genome duplication event (Li et al., 2015).
In contrast, Dioon edule inhabits seasonally dry forests of Mexico and Central America; it is the only cycad species that has been examined for CAM in detail (Vovides et al., 2002). CAM has been associated with Dioon seedling survival throughout the long dry seasons following germination (Yáñez-Espinosa et al., 2014). Other members of Dioon might also use CAM, because D. edule exhibits only very weak CAM activity that might be difficult to detect outside the dry season. With a crown age of 56 (40–75) Ma, CAM could be old in Dioon if it is present throughout the genus; conversely, most speciation events in the history of Dioon are within the last 20 Ma, and D. edule is estimated to have diverged only 5–11 Ma (Gutiérrez-Ortega et al., 2017).
Angiosperms
Amborella–Nymphaeales–Austrobaileyales grade and Magnoliideae
Amongst the Amborella–Nymphaeales–Austrobaileyales grade and Magnoliideae clade of angiosperms, CAM is known only from Peperomia (Piperaceae), a large genus of ~1600 species. As with many extremely diverse clades, our understanding of relationships within Peperomia is generally poor at the species level, although CAM has been assessed using gas exchange, titratable acidity, enzyme activity and carbon isotopes (or a combination of these data) for dozens of species across the major subgenera (Holthe et al., 1992). Most Peperomia species with CAM have been demonstrated to be C3 + CAM, with few exhibiting strong CAM (Holthe et al., 1992; Holtum and Winter, 2005). It is possible that many purported C3Peperomia species are capable of CAM, because most have not been tested thoroughly for C3 + CAM. Assuming that most current C3 species are correctly assigned and that CAM is lost only rarely, based on recent Peperomia phylogenies (Frenzke et al., 2016; Lim et al., 2019), we estimate that CAM has evolved at least five times in Peperomia and perhaps a dozen times if reversions from CAM to C3 do not occur. We expect the true number of origins to be somewhere in between and that some ‘C3’ species will be found to be C3 + CAM. It is noteworthy that the link between epiphytism and CAM does not appear especially strong in Peperomia, given the distribution of habits described by Frenzke et al. (2016).
Monocots
Monocots contain xeric, tropical epiphytic and aquatic CAM species, in addition to species that perform CAM in their chloroplast-containing roots (leafless orchids; Winter et al., 1985) and C4 + CAM in Ottelia (Hydrocharitaceae; Zhang et al., 2014). Within monocots, CAM species are heavily concentrated in the Orchidaceae and Bromeliaceae, which we estimate to be two of the three most CAM-rich plant families (Fig. 4; Supplementary Data Tables S3 and S4). Extensive surveys of carbon isotope ratios have been conducted in these clades (e.g. Medina et al., 1977; Silvera et al., 2010a; Crayn et al., 2015; Torres-Morales et al., 2020), although comparatively few studies have tested for C3 + CAM in living material (Medina and Troughton, 1974; Pierce et al., 2002; Silvera et al., 2005; Beltrán et al., 2013). These surveys suggest that strong CAM has evolved independently in five bromeliad subfamilies and, possibly, might have arisen more than once within subfamilies Bromelioideae, Puyoideae and Tillandsioideae (Crayn et al., 2004, 2015; Givnish et al., 2014). The specific number of origins of strong CAM in Orchidaceae is less clear owing to poor resolution along the backbone of subfamily Epidendroideae, but it is almost certain that CAM has evolved independently in the Vanilloideae and the Epidendroideae, and probably several times within the latter.
Fig. 4.
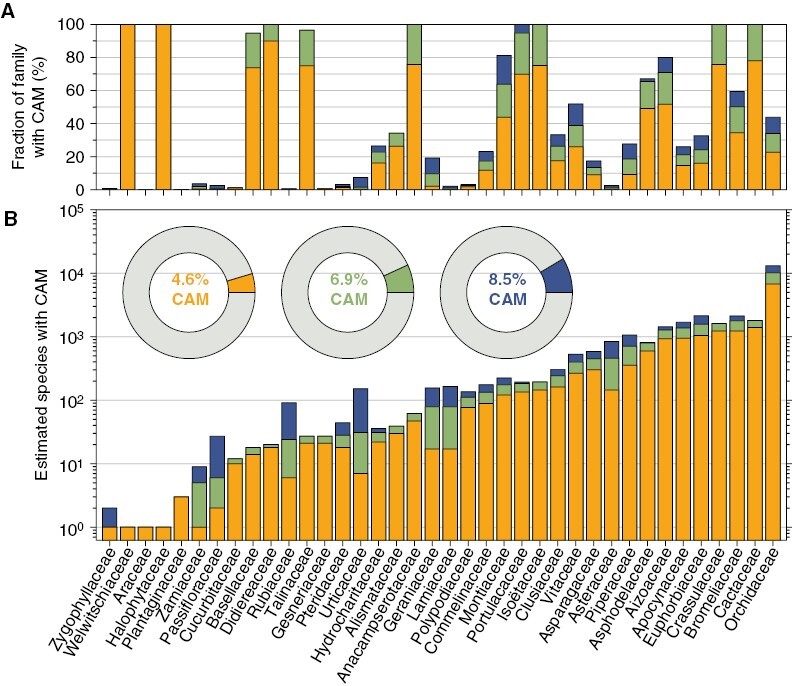
Estimated CAM species diversity in vascular plants by family. The proportional (A) and absolute (B) species diversity capable of CAM is shown in each family with known CAM lineages. Orange, green and blue bars and pie charts show lower bounds, expected and upper bounds of CAM species diversity, respectively, as defined in the main text. CAM species diversity was calculated using the list of CAM genera and assumptions about the phylogenetic placement of CAM origins (Table 1), extent of CAM surveys, and links between CAM and other plant traits. Numbers of species in each genus were taken from POWO (2023), which lists 349 036 accepted names for non-hybrid vascular plant species.
Despite the difficulty of placing CAM origins precisely, comparative phylogenetic studies in Orchidaceae and Bromeliaceae have uncovered correlations between CAM, diversification, and plant morphology and habit. Mapping the occurrence of strong CAM onto recent phylogenies has revealed significant correlations between strong CAM and epiphytism in orchids (Silvera et al., 2009), but not in bromeliads as a whole (Givnish et al., 2014), given that three of the five bromeliad clades in which CAM is found are exclusively terrestrial (Hechtioideae, the xeric clade of Pitcairnioideae, and Puyoideae), and in a fourth (Bromelioideae) the CAM terrestrial species were resolved as ancestral to CAM epiphytes (Crayn et al., 2004, 2015). Under some assumptions, strong CAM was found to be associated with higher diversification rates in both families (Givnish et al., 2014, 2015; Silvestro et al., 2014), but these conclusions should be regarded as tentative, pending denser taxon sampling to improve phylogenetic resolution and studies of living material to confirm the presence or absence of C3 + CAM. The role of CAM as a ‘key innovation’ in the diversification of epiphytic orchids has also been challenged recently in studies where strong CAM was associated with lineages with a higher extinction rate (Hu et al., 2022; see also Zotz et al., 2023). The horticultural and traditional medicinal value of orchids has spurred the generation of many orchid genome assemblies (Table 2). Comparisons between C3 + CAM and CAM genomes, and with C3 orchid genomes (e.g. Apostasia; Zhang et al., 2017), should be fruitful for understanding transitions to and between CAM phenotypes.
In addition to the orchids, CAM is present in arid-adapted lineages of the Asparagales: the Agavoideae and Nolinoideae of Asparagaceae (multiple origins) and Asphodeloideae of Asphodelaceae (probable single origin). As noted above, detailed studies have recently updated our understanding of the evolution of CAM in the Agavoideae; it is now believed that CAM evolved separately in: (1) the ancestor of Agave (including Manfreda and Polianthes), Beschorneria and Furcraea; (2) in Hesperaloe; and (3) in Yucca section sarcocarpa (Heyduk et al., 2022). Like Isoëtes, the Agavoideae are noteworthy for their use of the bacterial type of PEPC in CAM (Heyduk et al., 2022). In contrast to the dense transcriptomic, anatomical and physiological data now available for many Agavoideae subclades, the Nolinoideae and Asphodelaceae have received less attention. CAM is known from only two of 23 genera of the Nolinoideae (Beaucarnea and Sansevieria), which is a diverse clade inhabiting both New and Old World semi-arid ecosystems. Taxonomy of the Nolinoideae has been unstable, but will be aided by recent molecular phylogenies (Meng et al., 2021; Ji et al., 2023). Likewise, Asphodelaceae systematics have improved over the last decade (Manning et al., 2014), supporting a single origin of CAM in the ancestor of subfamily Alooideae and its sister clade Bulbine, because CAM has been found throughout the clade (Table 1). However, CAM-related -omics and physiological data are generally lacking across the clade, although the only genome (Aloe vera L.; Table 2) has been studied in the context of drought tolerance (Jaiswal et al., 2021).
Both aquatic and terrestrial CAM are found in the order Alismatales: aquatic CAM occurs in the genera Sagittaria (Alismataceae), Ottelia and Vallisneria (both Hydrocharitaceae), whereas terrestrial CAM has been reported in the monotypic genus Zamioculcas (Araceae). Each of these four genera is likely to represent an independent origin of CAM, based on recent phylogenies of the Alismatales (e.g. Chen et al., 2022). Although CAM is known from only a single species in Araceae [Zamioculcas zamiifolia (G.Lodd.) Engl.], this large family of >4000 recognized species (POWO, 2023), including many tropical climbers and epiphytes, merits screening for other taxa capable of CAM. Three genera with CAM have been identified in the Commelinaceae: Callisia (including Tripogandra), Cyanotis and Tradescantia. These taxa form a monophyletic group with Gibasis (Jung et al., 2021), which has not been surveyed for CAM to our knowledge. Finally, we note the recent report of small CAM-type acid fluctuations in natural populations of Spinifex littoreus (Ho et al., 2019), the first report of a CAM feature in grasses (Poaceae) (see ‘C4 + CAM’, above) (Supplementary Data Table S2); however, further studies are needed to confirm CAM in this species.
Saxifragales and Vitales
The early-diverging eudicot order Saxifragales contains the Crassulaceae, for which crassulacean acid metabolism is named. CAM has been found in every genus surveyed, spanning all three subfamilies, and it has been assumed that all members of Crassulaceae are capable of CAM (e.g. Pilon-Smits et al., 1996). C3 + CAM is likely to be ancestral in Crassulaceae, with at least one independent evolution of strong CAM in each subfamily, and very weak CAM is found in multiple lineages that have radiated in more temperate environments (Lösch, 1984). Phylogenomic data have not been applied to resolve the large and diverse clades within Crassulaceae, but a recent phylogeny of subfamily Sempervivoideae has dated crown Crassulaceae to 65–100 Ma (Messerschmid et al., 2020), considerably before CO2 began to decrease to near-modern levels. Although there are few phylogenomic studies in the Crassulaceae, multiple whole-genome sequences have been produced (Table 2), and the genus Kalanchoë has become a model for CAM genomics and functional genetics (e.g. Yang et al., 2017; Boxall et al., 2020). Similar to the Agavoideae, hybrids between Crassulaceae species with different CAM phenotypes have been found to exhibit intermediate photosynthetic physiology and leaf thickness (Teeri and Gurevitch, 1984).
In the Vitales, CAM has been found in Cissus and Cyphostemma, succulent vines and lianas of subfamily Vitoideae, each nested within C3 clades and therefore representing two independent origins of CAM. Stem succulence has evolved separately in the southern African and Madagascan lineages of Cyphostemma (Hearn et al., 2018), but only the southern African clade has been assessed for CAM.
Fabids
CAM is currently known from eight genera distributed throughout the Cucurbitales, Zygophyllales and Malpighiales. Many are caudiciform [e.g. Adenia (Passifloraceae), Seyrigia and Xerosicyos (both Cucurbitaceae)], with Xerosicyos forming large caudices that produce succulent deciduous vines. There is only a single report of CAM in Adenia [Mooney et al. (1977) found δ13C = −18.3 ‰], hence further investigations into this clade would be highly desirable. Likewise, the only report of CAM in Oxalis is that of Kluge and Ting (1978), who noted apparent diurnal fluctuations in acidity in Oxalis carnosa (no authority), but it is not clear which of three possible currently recognized taxa this sample represents, and more recent studies of Oxalis have not been able to confirm CAM activity (Supplementary Data Table S2). These Oxalis species are part of a very diverse South American radiation that includes other leaf and stem succulent species from xeric habitats, which would warrant re-evaluation for CAM. In Urticaceae, Pilea peperomioides Diels was recently discovered to be capable of CAM (Winter et al., 2021b) and belongs to a species-rich genus (>600 species) containing other succulents and epiphytes. CAM was recently observed in the succulent, photosynthetic stems of Bulnesia retama (Gillies ex Hook. & Arn.) Griseb. (Zygophyllaceae), a shrub native to semi-arid habitats of South America (Mok et al., 2023). This represents the first confirmed species capable of CAM in Zygophyllaceae, although other succulent shrubs in the family have been suggested to be, or discussed in the context of, CAM (Supplementary Data Table S2). We expect CAM to be more widespread than currently recognized in these fabid clades, and preliminary evidence suggests the possibility of C3 + CAM for at least one additional genus, in that Rundel et al. (1999) reported a δ13C value of −21.3 ‰ (converted from their absolute Δ isotope notation) for Forsskaolea (Urticaceae), a small genus of fleshy-leaved shrubs that inhabit arid lands from the Mediterranean to India and South Africa.
Strong CAM has also been found in each of the four subgenera of Euphorbia (Euphorbiaceae), a genus of ~2000 species exhibiting extreme diversity in growth form, from thin-leaved perennials to cactiform and large tree species; nearly half of all Euphorbia are succulent xeric species. Phylogenetic studies within Euphorbia have shown that the evolution of CCM (either C4 or CAM) was associated with increased diversification (Horn et al., 2014). It appears that strong CAM has evolved more than a dozen times, but the distribution of C3 + CAM is entirely unknown, meaning that Euphorbia could represent anywhere between 1 and 12 origins of CAM biochemistry (Horn et al., 2014). It is noteworthy that the species-rich clade of C3–C4 and C4 species is nested within Euphorbia subgenus Chamaesyce, which contains multiple origins of strong CAM. To our knowledge, Chamaesyce C4 species have not been tested for CAM, but we note its similarity to both Portulaca and Trianthema in being a C4 clade that is phylogenetically sister to or nested within a diverse, CAM-evolving lineage.
Malvids
Two genera of the Geraniaceae, Monsonia (originally reported as Sarcocaulon) and Pelargonium, contain C3 + CAM species. Geranium pratense L. was included in earlier lists of CAM species (Bennet-Clark, 1933; Szarek and Ting, 1977; Kluge and Ting, 1978) based on reports from Kraus (1883), but CAM activity in this taxon could not be confirmed by Thomas and Beevers (1949), who noted the leaves were high in acidity but did not show day–night fluctuations (Supplementary Data Table S2). Based on the relatively distant relationship between Monsonia and Pelargonium (García-Aloy et al., 2017), CAM presumably evolved independently in each lineage, and probably multiple times within the latter (Jones et al., 2003). In one of the earliest phylogenetic studies of C3 + CAM evolution, Jones et al. (2003) found a distribution of C3 + CAM and C3 species in Pelargonium consistent with multiple transitions to, or reversions from, C3 + CAM. Changes in life form, dispersal type and the evolution of CAM in Monsonia are hypothesized to be key innovations that facilitated their diversity throughout the succulent Karoo and Cape region of South Africa (García-Aloy et al., 2017). With their diverse life forms and distribution of C3 + CAM, the Geraniaceae presents an excellent clade for future studies of CAM evolution and, possibl,y reversion to C3.
Caryophyllales
The Caryophyllales contains an extraordinary diversity of CAM plants distributed across xeric ecosystems of the Americas, southern Africa and Australia, and extending into the Middle East and central Asian steppe. With ~1880 species, the majority of which are assumed to use CAM to varying degrees, the Aizoaceae are one of the largest CAM families (Fig. 4B; Supplementary Data Tables S3 and S4). The Aizoaceae also contain C4 + CAM (i.e. Trianthema portulacastrum) and the model facultative CAM plant Mesembryanthemum crystallinum that was the subject of many of the earliest molecular genetic studies of CAM (Cushman et al., 1989, 2008). The Aizoaceae are one of the fastest-radiating lineages of eukaryotes (Klak et al., 2004, 2017a; Valente et al., 2014), and resolution within the hyperdiverse subfamily Ruschioideae remains particularly poor, but the phylogenetic structure between and within the other four subfamilies is stable (Klak et al., 2017b). Tests for C3 + CAM across the clade are also expanding (e.g. Winter, 2019) and suggest that CAM might be ancestral, because it is found in the Sesuvioideae, the sister clade to all other Aizoaceae. CAM has been found in all but one of five subfamilies, although a recent broad investigation of facultative CAM found that several species might truly be non-CAM plants, indicating another potential evolutionary loss of CAM (Winter, 2019; Fig. 3).
The most species-rich single origin of CAM might be the Portulacineae, a clade of >2200 species (the majority of which are cacti) distributed among seven families: Cactaceae, Portulacaceae, Anacampserotaceae, Talinaceae, Didiereaceae, Halophytaceae, Basellaceae and Montiaceae. Increased phylogenetic resolution from a variety of data types (Moore et al., 2018; Wang et al., 2019b), systematic CAM surveys (e.g. Hancock et al., 2019) and an ancestral gene duplication event resulting in a shared CAM-specific PEPC (Christin et al., 2014) have led to hypotheses that CAM is ancestral in the Portulacineae (Goolsby et al., 2018). Similar to the Agavoideae, the Portulacineae demonstrate how decades of traditional and modern ecophysiology data (gas exchange, titratable acidity and transcriptomics) can be placed in a phylogenetic context to understand the evolution of CAM. Although C3 + CAM is inferred to be ancestral to the clade, strong CAM has evolved repeatedly: in Didiereaceae (Winter, 1979), Anacampseros (Guralnick et al., 2008) and multiple times in cacti (Edwards and Donoghue, 2006). Although Cactaceae are one of the more well-studied CAM clades, CAM has been confirmed in only a minority of cacti (representing 54 of the ~150 accepted genera; POWO, 2023), and strong CAM is generally assumed for all others. Within cacti, C3 + CAM is not restricted to Pereskia sensu lato (the leafy clades that form a grade leading to the core cacti) (Edwards and Donoghue, 2006) and can be found in genera including Maihuenia, Pereskiopsis and Quiabentia (Nobel and Hartsock, 1986; Martin and Wallace, 2000). The Montiaceae contains multiple CAM lineages, with some very weak CAM and apparently C3 lineages nested deeply within the clade that provide the most compelling evidence to date of reversions to C3 (e.g. Calandrinia tumida Syeda; Hancock et al., 2019; Fig. 3). With its wide range of CAM phenotypes and possible reversions to C3, the Portulacineae will continue to be an important clade for the study of CAM evolution.
Aspects of CAM activity have also been shown in five C4 genera (Halothamnus, Haloxylon, Horaninovia, Salsola and Xylosalsola) of the Amaranthaceae (see ‘C4 + CAM’, above), but further study is needed to confirm CAM in these taxa. The phylogenetic backbone of the Salsoleae tribe, which encompasses these genera, is not resolved, and Salsola is currently polyphyletic; furthermore, tests for CAM have been rare because this clade was already known to possess C4 photosynthesis. The Salsoleae share many features with Euphorbia and the Portulacineae, including halophytism, multiple evolutions of stem photosynthesis, and highly modified leaf morphologies that combine Kranz anatomy and hydrenchyma in succulent C4 leaves and branches.
Campanulids
Given the species richness and ecological diversity of clades such as Asteraceae, CAM has been reported from relatively few campanulid lineages. All Asteraceae genera with confirmed CAM species are restricted to the tribe Senecioneae, which is a large clade (~3000 species) with only partially resolved relationships that contains succulent, halophytic and stem-photosynthetic species. CAM has been studied in most detail in succulent members of Senecio sensu lato that have been segregated recently (Ozerova et al., 2017; Cicuzza et al., 2018) across the genera Caputia, Curio, Delairea, Kleinia and Senecio (Fioretto and Alfani, 1988). Based on the most recent comprehensive phylogenetic studies of Senecioneae (Pelser et al., 2007; Ozerova et al., 2017), CAM has probably evolved separately in the ‘Gynuroid clade’ (containing Baculellum, Caputia, Crassothonna, Curio, Delairea, Kleinia and Othonna) and in the ‘Faujasia–Bethencourtia clade’ [containing Jacobaea aquatica (Hill) G.Gaertn., B.Mey & Scherb (previously Senecio aquaticus Hill) and Delairea odorata Lem. (previously Senecio scandens DC.)], and possibly twice within the latter. Further studies are needed to confirm CAM in Delairea odorata and Jacobaea aquatica, which only exhibited small and consistent fluctuations in malic acid or net-positive dark-period CO2 uptake in the experiments of Fioretto and Alfani (1988) (Supplementary Data Table S2); however, Delairea odorata was considered ‘CAM-cycling’ by Sternberg et al. (1984) (reported as Senecio mikanioides Otto ex Walp.). Nocturnal malate accumulation has been reported for the succulent halophyte Tripolium pannonicum (Jacq.) Dobrocz. (previously Aster tripolium L.) under salt treatment, but with net CO2 uptake occurring entirely during the light period (Ganzmann and von Willert, 1972), and this species merits re-examination in view of other reports of low-level CAM activity in certain halophytes. The possible occurrence of CAM has also been noted in the (non-succulent) scrambling vine Mikania micrantha Kunth (Liu et al., 2020), based on the expression of genes potentially involved in the CAM pathway and small day–night changes in malate content (Supplementary Data Table S2), but measurements of gas exchange would be desirable to confirm operation of the complete CAM cycle. Information on photosynthetic metabolism exists for only ~1 % of Asteraceae, and therefore the extent and consequences of photosynthetic evolution in this clade have been little explored outside model clades, such as Flaveria (Siniscalchi et al., 2021).
Lamiids
The most diverse group of CAM plants in the Lamiids are in the Apocynaceae, which we estimate to contain a minimum of four independent origins of CAM. In subfamily Apocynoideae, only one species is known to be C3 + CAM: Pachypodium namaquanum (Wyley ex Harv.) Welw., which exhibited low rates of nocturnal CO2 uptake in stems after leaf-shedding and day–night fluctuations in malic acid in leaves during drought (von Willert et al., 1980, 1992). More detailed studies of C3 + CAM in Pachypodium, a genus of heavily spined trees and shrubs with water-storing trunks, are warranted, because reported isotope ratios are C3-like (Mooney et al., 1977; Rundel et al., 1999). Within subfamily Asclepiadoideae, CAM has probably arisen at least three times across three tribes containing succulent-leaved vines and stem-succulent cactiform species. In Marsdenieae, CAM is prevalent in a clade comprising two large genera of succulent epiphytic vines, Dischidia (~80 species) and Hoya (350–450 species), which have diversified in tropical and subtropical Asia, New Guinea, Australia and the western Pacific (Wanntorp et al., 2014; Liede-Schumann et al., 2022). In Ceropegieae, CAM has been reported in Ceropegia and in the ten genera of stem-succulent stapeliads (core Stapeliinae) tested to date (Apteranthes, Boucerosia, Caralluma, Caudanthera, Duvalia, Huernia, Hoodia, Orbea, Quaqua and Stapelia). Based on comparative morphology and life form (Endress et al., 2018), it seems likely that most, if not all, of the remaining 26 genera traditionally recognized in this clade will also possess CAM. Recent systematic studies, however, have shown the large genus Ceropegia to be highly polyphyletic, with Brachystelma and the core Stapeliinae nested within (Bruyns et al., 2017). More extensive sampling for CAM activity among the semi-succulent, thick-stemmed species of Ceropegia sensu stricto would be desirable. Finally, in tribe Asclepiadeae, the CAM genera Folotsia and Sarcostemma have now been subsumed within an expanded Cynanchum (Khanum et al., 2016), which includes the dominant succulent vine Cynanchum viminale (L.) L. [previously Sarcostemma viminale (L.) R.Br.] that spreads rampantly in the absence of browsing by megaherbivores (Coverdale et al., 2021).
Within the Gentianales, CAM has also been found in several species of epiphytic ‘ant-plants’ in the Rubiaceae. These distinctive plants form the subtribe Hydnophytinae within tribe Psychotrieae and are united by the synapomorphy of enlarged woody tubers derived from the hypocotyl that become hollow throughout development and house ant colonies in mutualistic relationships (Huxley, 1980; Chomicki and Renner, 2016). Among the five genera composing this clade, CAM has so far been reported in thicker-leaved species of Hydnophytum (one species), Myrmecodia (one species) and Squamellaria (two species) (Winter et al., 1983; Tsen and Holtum, 2012; Chomicki and Renner, 2016), but the full extent of CAM occurrence within this subtribe has not yet been explored.
Finally, CAM has been observed in three families of the Lamiales: Gesneriaceae, Lamiaceae and Plantaginaceae. In the Gesneriaceae, CAM probably evolved twice: once in a small clade of resurrection plants (Haberlea and Ramonda) and separately in the succulent epiphytic lineage Codonanthopsis. Other fleshy-leaved and epiphytic lineages of Gesneriaceae have been investigated for CAM (i.e. Aeschynanthus, Columnea and Streptocarpus) but shown to be C3 (Guralnick et al., 1986), although further investigations in this relatively large family (~3800 spp.) would be worthwhile. Two genera from different subfamilies of the Lamiaceae contain CAM lineages: Marrubium (subfamily Lamioideae) and Coleus (subfamily Nepetoideae). The genus Marrubium is nested within the paraphyletic Ballota, which has not been assessed for CAM. Taxonomic uncertainty has recently been resolved in Coleus and Plectranthus (Paton et al., 2019), with all CAM species of Plectranthus (Herppich and Herppich, 1996) now treated under Coleus (Winter et al., 2021c). The aquatic lineage Littorella (Plantaginaceae) also expresses CAM (Keeley and Morton, 1982) and has an ‘isoëtid’ growth form (stiff rosette of leaves, large root biomass and continuous root-to-leaf air channels) that facilitates CO2 diffusion directly from soils.
SPECIES DIVERSITY, CAM ORIGINS AND IMPLICATIONS, AND WHERE TO FIND NEW CAM LINEAGES
Using our list of CAM genera (Table 1), lists of species from Kew’s Plants of the World Online (POWO) (2023), and by placing known CAM and non-CAM species in their phylogenetic context, we estimated the number of species capable of CAM on a genus-by-genus basis (Supplementary Data Table S3). Genera with multiple taxa investigated for CAM were binned into all (100 %), most (75 %), half (50 %), some (25 %), few (5 %) or rare (1 %) species estimated to be capable of CAM based on the proportions of CAM and non-CAM taxa reported and their phylogenetic distribution. For example, all species in all genera of Cactaceae were considered to use CAM, because every species studied thus far has been reported to use CAM to some degree; furthermore, these taxa are well distributed throughout the Cactaceae phylogeny. Combining the list of C3 and CAM Peperomia from Holthe et al. (1992) and a recent phylogeny by Frenzke et al. (2016) showed that two major subclades have not been surveyed for CAM, one contained exclusively C3 taxa, one exclusively CAM taxa, and five contained mixed photosynthetic types (including the largest, Micropiper, with ~800 species). Given this phylogenetic distribution of photosynthetic types, we estimated that CAM evolved several times in Peperomia and that roughly half of Peperomia species are capable of CAM. We generally did not assume that CAM evolved along a tip branch (except in monotypic genera, e.g. Welwitschia); that is, despite only single records of CAM so far, we categorized genera including Jatropha and Pilea as having few CAM species (5 %). Finally, to estimate lower and upper bounds on these counts, we moved every genus either down or up a bin, respectively; genera binned as ‘few’ were recategorized as ‘rare’ (1 %) when estimating lower bounds, and genera binned as ‘all’ were not altered when estimating upper bounds. The upper bounds on Australian Calandrinia and on Clusia were reduced to 95 % (rather than 100 %), because multiple taxa have been demonstrated not to express detectable CAM (Hancock et al., 2019; Pachon et al., 2022).
After removing synonymous and hybrid species and using the above assumptions, we estimated the total number of plant species capable of CAM to be 6.9 % (4.6–8.5 %) of all vascular plants (Fig. 4B; Supplementary Data Tables S3 and S4). We stress that the absolute species diversity implied by these proportions differs considerably by authority and should therefore be treated with caution. For example, POWO (2023) and Akeroyd and Synge (1992), the latter of which was used by Winter and Smith (1996), differ in their estimates of vascular plant diversity by nearly 100 000 species, and some upper estimates of seed plant diversity alone are ~450 000 species (Govaerts, 2003). Our proportional estimates, however, are consistent with previous estimates of total CAM species diversity (e.g. 6 % of vascular plant species by Winter and Smith, 1996) and of specific clades. Our estimate of 34 % (23–43 %) of orchids being capable of CAM (Fig. 4A; Supplementary Data Table S4), taking into account the detection of low-level CAM activity in many species, is congruent with estimates by Winter and Smith (1996) (36 %) and Silvera et al. (2010b) (30 %). In an isotopic survey of nearly 60 % of all bromeliad species, Crayn et al. (2015) found 43 % to be CAM; our estimated 50 % (35–59 %) (Fig. 4A; Supplementary Data Table S4) is consistent with their results but skews higher owing to our consideration of C3 + CAM taxa.
By placing reports of CAM species in their phylogenetic contexts and with the caveats listed above, we estimate that the ability to perform CAM has evolved a minimum of 66 times and, possibly, as many as 114 times (Table 1). Although it is probable that some clades will see multiple CAM origins collapsed into fewer origins after further sampling for C3 + CAM, large swaths of the vascular plant phylogeny have not been investigated for CAM activity beyond isotopic surveys. Although it is unlikely that many more strong (constitutive)-CAM lineages will be discovered, the prevalence of C3 + CAM, even among CAM clades that have received substantial attention, could be much higher. It is tempting to use lists of known CAM species, as presented in Table 1, in large-scale evolutionary or ecological studies, but we caution against the treatment of this list as complete for most clades. As an exercise to show the often poor overlap of phylogenetic trees and CAM phenotypic data, we attempted to estimate the number of CAM origins in the Caryophyllales using the topology from Hinchliff and Smith (2014); we coded species not confirmed to be CAM as ‘non-CAM’ (i.e. species from genera not included in Table 1) and assumed an all rates different (ARD) model of trait evolution. Results from 100 stochastic character maps created using the package ‘phytools’ (Revell, 2012) in R v.4.1.2 (R Core Team, 2021) found an average of 9.43 CAM gains and 64.13 CAM losses in the Caryophyllales alone. This number of CAM origins is high but not implausible based on our assessment of the Caryophyllales; however, the extreme number of reversions (and underlying transition rates) is likely to be inflated by lack of CAM information in many genera. In our example, the paucity of information in the core Ruschioideae of Aizoaceae forces reversions on very short branches; a problem likely to affect estimates of CAM transitions in other recently diversifying clades, such as the Epidendroideae (Orchidaceae), Bromeliaceae and Peperomia (Piperaceae).
Estimating the number and phylogenetic placement of CAM origins can inform the ecological pressures leading to CAM evolution (Box 2). Phylogenetically informed studies of CAM genomics and physiology will generate and provide support for hypotheses of CAM evolution and place CAM origins in geographical, chronological and therefore ecological contexts. Like C4, CAM has evolved in dozens of lineages convergently, as a response to lower atmospheric CO2 concentrations established after the Oligocene (Edwards and Ogburn, 2012; Sage et al., 2012). C4 has a minimum of 61 independent origins across angiosperms, resulting in ~8100 extant C4 species (Sage et al., 2011; Sage 2016). However, CAM is also understood as an adaptation to water limitation (Winter et al., 2005), which has been a major stressor since plants colonized terrestrial ecosystems. Most C4 clades date to the Oligocene or later (Christin et al., 2011), and although many of the most species-rich CAM lineages have diversified in the past 30 Myr, phylogenetic reconstructions of some CAM origins appear to pre-date the Oligocene CO2 decline. For example, in addition to possible ancient CAM origins in the non-seed plant clades, the Portulacineae have a crown age estimated between 40 and 50 Ma (Arakaki et al., 2011), which would imply that C3 + CAM evolved while atmospheric CO2 concentrations were ~1000 ppm (Rae et al., 2021). And although the Epidendroideae represent a recent radiation of CAM orchids, Vanilla has an estimated crown age of ~42 Ma (Givnish et al., 2015); if CAM is found in other members of Vanilleae (crown age ~60 Ma) or Vanilloideae (crown age ~78 Ma), these clades might be among the oldest CAM lineages. CAM probably also evolved close to the base of the Crassulaceae phylogeny, with an origin in the Cretaceous (Sage et al., 2023). Low atmospheric CO2 is likely to be a major promoter of CAM evolution (Sage et al., 2023), but because CAM is inherently flexible (allowing less energetically costly C3 photosynthesis to be used when water and CO2 are plentiful) and used in highly dissimilar environments, it is plausible that CAM could have evolved despite high atmospheric CO2, especially if the increased succulence significantly reduces mesophyll CO2 conductance (Nelson and Sage, 2008; Edwards, 2019). Furthering our understanding of CAM evolution requires continued systematic surveys of CAM (along with phylogenies of these groups), which should seek both to fill gaps around transitions in CAM phenotypes and to expand sampling into new areas of plant diversity to find new CAM lineages and confirm (or reject) assumptions of lack of CAM activity.
The most obvious places to start searching for new CAM taxa are in clades closely related to known CAM lineages. Establishing the true placement of C3-to-CAM transitions on phylogenies has been difficult thus far, but will be essential in understanding the earliest steps in CAM evolution. Strong candidates include succulent, xeric and epiphytic species, which have driven many past CAM surveys. Halophytic and succulent C4 species have been largely neglected by CAM researchers, but reports of CAM in Amaranthaceae (Zalenskiï and Glagoleva, 1981), Poaceae (Ho et al., 2019) and Aizoaceae (Winter et al., 2021a) warrant re-evaluation and greater scrutiny of these clades. Proceeding along these routes would continue recent trends in CAM exploration, but we also suggest that the CAM community should cast a much wider net. A growing body of research suggests that major morphological shifts (e.g. increased leaf thickness and succulence) might not be needed for C3 + CAM (Edwards, 2019), and therefore the occurrence of C3 + CAM and low-level CAM activity might be more common throughout vascular plants than previously assumed.
Finally, although we have emphasized how much more there is to discover about the distribution and evolution of CAM, we want to end by highlighting how much knowledge, insight and opportunity this current ‘draft’ list contains. The last attempts at comprehensive lists of taxa with CAM were more than two decades ago, and there has since been enormous progress on multiple fronts: further resolution of the phylogeny of land plants, greater taxonomic stability, and increasingly sophisticated methods for analysing character evolution; additional surveys for CAM in previously unexplored clades; and a new ability, via the revolution in sequencing technology, to probe genetic/regulatory elements of CAM expression. We are hopeful that this updated list will inspire a new era of comparative studies of CAM biology. The repeated evolution of CAM photosynthesis has played a crucial role in the diversification and ecological success of land plants, and it presents an unparalleled opportunity to investigate questions of convergence, parallelism, adaptation and the evolutionary assembly of complex traits.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Table S1: list of genera containing species capable of CAM photosynthesis. Table S2: lineages reported or suspected to use CAM, but for which further corroborative evidence is required. Table S3: estimated CAM species diversity in each genus containing CAM species. Table S4: estimated CAM species diversity in each family containing CAM species.
ACKNOWLEDGEMENTS
The authors would like the thank the editorial staff and anonymous reviewers for their constructive feedback on this manuscript.
Contributor Information
Ian S Gilman, Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT, USA.
J Andrew C Smith, Department of Biology, University of Oxford, Oxford, UK.
Joseph A M Holtum, College of Science and Engineering, James Cook University, Townsville, Queensland, Australia.
Rowan F Sage, Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, ON, Canada.
Katia Silvera, Smithsonian Tropical Research Institute, Balboa, Ancón, Panama; Department of Botany & Plant Sciences, University of California, Riverside, CA, USA.
Klaus Winter, Smithsonian Tropical Research Institute, Balboa, Ancón, Panama.
Erika J Edwards, Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT, USA.
FUNDING
This research was funded by the National Science Foundation (IOS-1754662 to E.J.E.). J.A.C.S. acknowledges support from the Oxford Martin School, University of Oxford. K.W. acknowledges support from the Smithsonian Tropical Research Institute.
DATA AVAILABILITY
The data used to infer family-level statistics of CAM species diversity are available online at: https://github.com/isgilman/CAM-diversity
LITERATURE CITED
- Akeroyd J, Synge H.. 1992. Higher plant diversity. In: Groombridge B, ed. Global diversity: status of the earth’s living resources. London: Chapman & Hall, 64–87. [Google Scholar]
- Angiosperm Phylogeny Group. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Arakaki M, Christin P-A, Nyffeler R, et al. 2011. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proceedings of the National Academy of Sciences of the United States of America 108: 8379–8384. doi: 10.1073/pnas.1100628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo MK, Medina E, Ziegler H.. 1990. Distribution and δ13C values of Portulacaceae species of the High Andes in Northern Chile. Botanica Acta 103: 291–295. doi: 10.1111/j.1438-8677.1990.tb00163.x. [DOI] [Google Scholar]
- Barbour MM. 2017. Understanding regulation of leaf internal carbon and water transport using online stable isotope techniques. New Phytologist 213: 83–88. [DOI] [PubMed] [Google Scholar]
- Barfuss MHJ, Till W, Leme EMC, et al. 2016. Taxonomic revision of Bromeliaceae subfam. Tillandsioideae based on a multi-locus DNA sequence phylogeny and morphology. Phytotaxa 279: 1–97. [Google Scholar]
- Beer S, Wetzel RG.. 1981. Photosynthetic carbon metabolism in the submerged aquatic angiosperm Scirpus subterminalis. Plant Science Letters 21: 199–207. doi: 10.1016/0304-4211(81)90089-4. [DOI] [Google Scholar]
- Beltrán JD, Lasso E, Madriñán S, Virgo A, Winter K.. 2013. Juvenile tank-bromeliads lacking tanks: do they engage in CAM photosynthesis? Photosynthetica 51: 55–62. doi: 10.1007/s11099-012-0077-8. [DOI] [Google Scholar]
- Bender MM. 1971. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry 10: 1239–1244. doi: 10.1016/s0031-9422(00)84324-1. [DOI] [Google Scholar]
- Bender MM, Rouhani I, Vines HM, Black CC Jr. 1973. 13C/12C ratio changes in crassulacean acid metabolism plants. Plant Physiology 52: 427–430. doi: 10.1104/pp.52.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet-Clark TA. 1933. The rôle of the organic acids in plant metabolism. Part I. New Phytologist 32: 37–71. doi: 10.1111/j.1469-8137.1933.tb07003.x. [DOI] [Google Scholar]
- Black CC, Williams S.. 1976. Plants exhibiting characteristics common to crassulacean acid metabolism. In: Burris RH, Black CC, eds. CO2 metabolism and plant productivity. Baltimore: University Park Press, 407–424. [Google Scholar]
- Bone RE, Smith JAC, Arrigo N, Buerki S.. 2015. A macro-ecological perspective on crassulacean acid metabolism (CAM) photosynthesis evolution in Afro-Madagascan drylands: Eulophiinae orchids as a case study. New Phytologist 208: 469–481. doi: 10.1111/nph.13572. [DOI] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Weston DJ, et al. 2014. Engineering crassulacean acid metabolism to improve water-use efficiency. Trends in Plant Science 19: 327–338. doi: 10.1016/j.tplants.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G. 2011. Aquatic single-cell C4 photosynthesis. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms. Dordrecht: Springer, 63–80. [Google Scholar]
- Boxall SF, Kadu N, Dever LV, et al. 2020. Kalanchoë PPC1 is essential for crassulacean acid metabolism and the regulation of core circadian clock and guard cell signaling genes. The Plant Cell 32: 1136–1160. doi: 10.1105/tpc.19.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Schlüter U, Eisenhut M, Gowik U.. 2017. On the evolutionary origin of CAM photosynthesis. Plant Physiology 174: 473–477. doi: 10.1104/pp.17.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilhaus D, Bräutigam A, Mettler-Altmann T, Winter K, Weber APM.. 2016. Reversible burst of transcriptional changes during induction of crassulacean acid metabolism in Talinum triangulare. Plant Physiology 170: 102–122. doi: 10.1104/pp.15.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyns PV, Klak C, Hanáček P.. 2017. A revised, phylogenetically-based concept of Ceropegia (Apocynaceae). South African Journal of Botany 112: 399–436. doi: 10.1016/j.sajb.2017.06.021. [DOI] [Google Scholar]
- Cai J, Liu X, Vanneste K, et al. 2015. The genome sequence of the orchid Phalaenopsis equestris. Nature Genetics 47: 65–72. doi: 10.1038/ng.3149. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Hyvönen J, Schneider H.. 2020. Exploring phylogeny of the microsoroid ferns (Polypodiaceae) based on six plastid DNA markers. Molecular Phylogenetics and Evolution 143: 106665. doi: 10.1016/j.ympev.2019.106665. [DOI] [PubMed] [Google Scholar]
- Chen J, Xie F, Cui Y, et al. 2021. A chromosome-scale genome sequence of pitaya (Hylocereus undatus) provides novel insights into the genome evolution and regulation of betalain biosynthesis. Horticulture Research 8: 164. doi: 10.1038/s41438-021-00612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-Y, Lu B, Morales-Briones DF, et al. 2022. Phylogenomic analyses of Alismatales shed light into adaptations to aquatic environments. Molecular Biology and Evolution 39: msac079. doi: 10.1093/molbev/msac079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomicki G, Renner SS.. 2016. Evolutionary relationships and biogeography of the ant-epiphytic genus Squamellaria (Rubiaceae: Psychotrieae) and their taxonomic implications. PLoS One 11: e0151317. doi: 10.1371/journal.pone.0151317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Arakaki M, Osborne CP, et al. 2014. Shared origins of a key enzyme during the evolution of C4 and CAM metabolism. Journal of Experimental Botany 65: 3609–3621. doi: 10.1093/jxb/eru087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Sage RF, Arakaki M, Edwards EJ.. 2011. C4 eudicots are not younger than C4 monocots. Journal of Experimental Botany 62: 3171–3181. doi: 10.1093/jxb/err041. [DOI] [PubMed] [Google Scholar]
- Cicuzza D, Stheli DS, Nyffeler R, Eggli U.. 2018. Morphology and anatomy support a reclassification of the African succulent taxa of Senecio S.L. (Asteraceae: Senecioneae). Haseltonia 23: 11–26. [Google Scholar]
- Clavel J, Morlon H.. 2017. Accelerated body size evolution during cold climatic periods in the Cenozoic. Proceedings of the National Academy of Sciences of the United States of America 114: 4183–4188. doi: 10.1073/pnas.1606868114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W, Ting IP, Sternberg LO.. 1979. Relationships between stomatal behavior and internal carbon dioxide concentration in crassulacean acid metabolism plants. Plant Physiology 63: 1029–1032. doi: 10.1104/pp.63.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copetti D, Búrquez A, Bustamante E, et al. 2017. Extensive gene tree discordance and hemiplasy shaped the genomes of North American columnar cacti. Proceedings of the National Academy of Sciences of the United States of America 114: 12003–12008. doi: 10.1073/pnas.1706367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho LM. 1969. Novas observações sôbre a ocorrência do ‘Efeito de De Saussure’ e suas relações com a suculência, a temperatura folhear e os movimentos estomáticos. Boletim da Faculdade de Filosofia, Ciências e Letras, Universidade de São Paulo No. 331, Botânica 24: 77–102. [Google Scholar]
- Coverdale TC, O’Connell RD, Hutchinson MC, et al. 2021. Large herbivores suppress liana infestation in an African savanna. Proceedings of the National Academy of Sciences of the United States of America 118: e2101676118. doi: 10.1073/pnas.2101676118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crayn DM, Winter K, Smith JAC.. 2004. Multiple origins of crassulacean acid metabolism and the epiphytic habit in the Neotropical family Bromeliaceae. Proceedings of the National Academy of Sciences of the United States of America 101: 3703–3708. doi: 10.1073/pnas.0400366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crayn DM, Winter K, Schulte K, Smith JAC.. 2015. Photosynthetic pathways in Bromeliaceae: phylogenetic and ecological significance of CAM and C3 based on carbon isotope ratios for 1893 species. Botanical Journal of the Linnean Society 178: 169–221. doi: 10.1111/boj.12275. [DOI] [Google Scholar]
- Cushman JC, Meyer G, Michalowski CB, Schmitt JM, Bohnert HJ.. 1989. Salt stress leads to differential expression of two isogenes of phosphoenolpyruvate carboxylase during crassulacean acid metabolism induction in the common ice plant. The Plant Cell 1: 715–725. doi: 10.1105/tpc.1.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman JC, Tillett RL, Wood JA, Branco JM, Schlauch KA.. 2008. Large-scale mRNA expression profiling in the common ice plant, Mesembryanthemum crystallinum, performing C3 photosynthesis and crassulacean acid metabolism (CAM). Journal of Experimental Botany 59: 1875–1894. doi: 10.1093/jxb/ern008. [DOI] [PubMed] [Google Scholar]
- Earnshaw MJ, Winter K, Ziegler H, et al. 1987. Altitudinal changes in the incidence of crassulacean acid metabolism in vascular epiphytes and related life forms in Papua New Guinea. Oecologia 73: 566–572. doi: 10.1007/BF00379417. [DOI] [PubMed] [Google Scholar]
- Edwards EJ. 2019. Evolutionary trajectories, accessibility and other metaphors: the case of C4 and CAM photosynthesis. New Phytologist 223: 1742–1755. doi: 10.1111/nph.15851. [DOI] [PubMed] [Google Scholar]
- Edwards EJ. 2023. Reconciling continuous and discrete models of C4 and CAM evolution. Annals of Botany, this volume: mcad125. doi: 10.1093/aob/mcad125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Donoghue MJ.. 2006. Pereskia and the origin of the cactus life‐form. The American Naturalist 167: 777–793. doi: 10.1086/504605. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Ogburn RM.. 2012. Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. International Journal of Plant Sciences 173: 724–733. doi: 10.1086/666098. [DOI] [Google Scholar]
- Endress ME, Meve U, Middleton DJ, Liede-Schumann S.. 2018. Apocynaceae. In: Kadereit JW, Bittrich V, eds. The families and genera of vascular plants, Vol. 15, Flowering plants – Eudicots – Apiales, Gentianales (except Rubiaceae). Cham: Springer Nature, 207–411. [Google Scholar]
- Fan W, He Z-S, Zhe M, et al. 2023. High-quality Cymbidium mannii genome and multifaceted regulation of crassulacean acid metabolism in epiphytes. Plant Communications 4: 100564. doi: 10.1016/j.xplc.2023.100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, O’Leary MH, Berry JA.. 1982. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Australian Journal of Plant Physiology 9: 121–137. doi: 10.1071/pp9820121. [DOI] [Google Scholar]
- Ferrari RC, Bittencourt PP, Rodrigues MA, et al. 2020. C4 and crassulacean acid metabolism within a single leaf: deciphering key components behind a rare photosynthetic adaptation. New Phytologist 225: 1699–1714. doi: 10.1111/nph.16265. [DOI] [PubMed] [Google Scholar]
- Fioretto A, Alfani A.. 1988. Anatomy of succulence and CAM in 15 species of Senecio. Botanical Gazette 149: 142–152. doi: 10.1086/337701. [DOI] [Google Scholar]
- Foster GL, Royer DL, Lunt DJ.. 2017. Future climate forcing potentially without precedent in the last 420 million years. Nature Communications 8: 14845. doi: 10.1038/ncomms14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzke L, Goetghebeur P, Neinhuis C, Samain M-S, Wanke S.. 2016. Evolution of epiphytism and fruit traits act unevenly on the diversification of the species-rich genus Peperomia (Piperaceae). Frontiers in Plant Science 7: 1145. doi: 10.3389/fpls.2016.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzmann RJ, von Willert DJ.. 1972. Nachweis eines diurnalen Säurerhythmus beim Halophyten Aster tripolium. Naturwissenschaften 59: 422–423. doi: 10.1007/bf00623140.4564192 [DOI] [Google Scholar]
- García-Aloy S, Sanmartín I, Kadereit G, et al. 2017. Opposite trends in the genus Monsonia (Geraniaceae): specialization in the African deserts and range expansions throughout eastern Africa. Scientific Reports 7: 9872. doi: 10.1038/s41598-017-09834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman IS, Moreno-Villena JJ, Lewis ZR, Goolsby EW, Edwards EJ.. 2022. Gene co-expression reveals the modularity and integration of C4 and CAM in Portulaca. Plant Physiology 189: 735–753. doi: 10.1093/plphys/kiac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish TJ, Barfuss M, Van Ee B, et al. 2014. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution 71: 55–78. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Spalink D, Ames M, et al. 2015. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proceedings of the Royal Society B: Biological Sciences 282: 20151553. doi: 10.1098/rspb.2015.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goolsby EW, Moore AJ, Hancock LP, De Vos JM, Edwards EJ.. 2018. Molecular evolution of key metabolic genes during transitions to C4 and CAM photosynthesis. American Journal of Botany 105: 602–613. [DOI] [PubMed] [Google Scholar]
- Govaerts R. 2003. How many species of seed plants are there? – a response. Taxon 52: 583–584. doi: 10.2307/3647457. [DOI] [Google Scholar]
- Gregory FG, Spear I, Thimann KV.. 1954. The interrelation between CO2 metabolism and photoperiodism in Kalanchoë. Plant Physiology 29: 220–229. doi: 10.1104/pp.29.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H, Cousins AB, Badger MR, von Caemmerer S.. 2007. Discrimination in the dark. Resolving the interplay between metabolic and physical constraints to phosphoenolpyruvate carboxylase activity during the crassulacean acid metabolism cycle. Plant Physiology 143: 1055–1067. doi: 10.1104/pp.106.088302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H, Ong BL, Avadhani PN, Goh CJ.. 1989. Recycling of respiratory CO2 during crassulacean acid metabolism: alleviation of photoinhibition in Pyrrosia piloselloides. Planta 179: 115–122. doi: 10.1007/BF00395778. [DOI] [PubMed] [Google Scholar]
- Griffiths H, Smith JAC.. 1983. Photosynthetic pathways in the Bromeliaceae of Trinidad: relations between life-forms, habitat preference and the occurrence of CAM. Oecologia 60: 176–184. doi: 10.1007/BF00379519. [DOI] [PubMed] [Google Scholar]
- Guo J, Xu W, Hu Y, et al. 2020. Phylotranscriptomics in Cucurbitaceae reveal multiple whole-genome duplications and key morphological and molecular innovations. Molecular Plant 13: 1117–1133. doi: 10.1016/j.molp.2020.05.011. [DOI] [PubMed] [Google Scholar]
- Guralnick LJ, Cline A, Smith M, Sage RF.. 2008. Evolutionary physiology: the extent of C4 and CAM photosynthesis in the genera Anacampseros and Grahamia of the Portulacaceae. Journal of Experimental Botany 59: 1735–1742. doi: 10.1093/jxb/ern081. [DOI] [PubMed] [Google Scholar]
- Guralnick LJ, Edwards G, Ku MSB, Hockema B, Franceschi VR.. 2002. Photosynthetic and anatomical characteristics in the C4–crassulacean acid metabolism-cycling plant, Portulaca grandiflora. Functional Plant Biology 29: 763–773. doi: 10.1071/PP01176. [DOI] [PubMed] [Google Scholar]
- Guralnick LJ, Ting IP, Lord EM.. 1986. Crassulacean acid metabolism in the Gesneriaceae. American Journal of Botany 73: 336–345. doi: 10.1002/j.1537-2197.1986.tb12046.x. [DOI] [Google Scholar]
- Gutiérrez-Ortega JS, Salinas-Rodríguez MM, Martínez JF, et al. 2017. The phylogeography of the cycad genus Dioon (Zamiaceae) clarifies its Cenozoic expansion and diversification in the Mexican transition zone. Annals of Botany 121: 535–548. doi: 10.1093/aob/mcx165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J, Shim S, Lee T, et al. 2019. Genome sequence of Jatropha curcas L., a non-edible biodiesel plant, provides a resource to improve seed-related traits. Plant Biotechnology Journal 17: 517–530. doi: 10.1111/pbi.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Yi J, Dai J, et al. 2020. A chromosome-level genome assembly of Dendrobium huoshanense using long reads and Hi-C data. Genome Biology and Evolution 12: 2486–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock L, Edwards EJ.. 2014. Phylogeny and the inference of evolutionary trajectories. Journal of Experimental Botany 65: 3491–3498. doi: 10.1093/jxb/eru118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock LP, Holtum JAM, Edwards EJ.. 2019. The evolution of CAM photosynthesis in Australian Calandrinia reveals lability in C3+CAM phenotypes and a possible constraint to the evolution of strong CAM. Integrative and Comparative Biology 59: 517–534. doi: 10.1093/icb/icz089. [DOI] [PubMed] [Google Scholar]
- Hasing T, Tang H, Brym M, Khazi F, Huang T, Chambers AH.. 2020. A phased Vanilla planifolia genome enables genetic improvement of flavour and production. Nature Food 1: 811–819. doi: 10.1038/s43016-020-00197-2. [DOI] [PubMed] [Google Scholar]
- Hatch M, Slack C.. 1966. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochemical Journal 101: 103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn DJ, Evans M, Wolf B, McGinty M, Wen J.. 2018. Dispersal is associated with morphological innovation, but not increased diversification, in Cyphyostemma (Vitaceae). Journal of Systematics and Evolution 56: 340–359. [Google Scholar]
- Helder RL, van Harmelen M.. 1982. Carbon assimilation pattern in the submerged leaves of the aquatic angiosperm: Vallisneria spiralis L. Acta Botanica Neerlandica 31: 281–295. [Google Scholar]
- Herppich WB, Herppich M.. 1996. Ecophysiological investigations on plants of the genus Plectranthus (fam. Lamiaceae) native to Yemen and southern Africa. Flora 191: 401–408. doi: 10.1016/s0367-2530(17)33117-1. [DOI] [Google Scholar]
- Herrera A. 2008. Crassulacean acid metabolism and fitness under water deficit stress: if not for carbon gain, what is facultative CAM good for? Annals of Botany 103: 645–653. doi: 10.1093/aob/mcn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, Hwang M, Albert V, et al. 2019a. Altered gene regulatory networks are associated with the transition from C3 to crassulacean acid metabolism in Erycina (Oncidiinae: Orchidaceae). Frontiers in Plant Science 9: 2000. doi: 10.3389/fpls.2018.02000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, McKain MR, Lalani F, Leebens-Mack J.. 2016. Evolution of a CAM anatomy predates the origins of crassulacean acid metabolism in the Agavoideae (Asparagaceae). Molecular Phylogenetics and Evolution 105: 102–113. doi: 10.1016/j.ympev.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Heyduk K, Moreno-Villena JJ, Gilman IS, Christin P-A, Edwards EJ.. 2019b. The genetics of convergent evolution: insights from plant photosynthesis. Nature Reviews Genetics 20: 485–493. doi: 10.1038/s41576-019-0107-5. [DOI] [PubMed] [Google Scholar]
- Heyduk K, McAssey EV, Leebens-Mack J. 2022. Differential timing of gene expression and recruitment in independent origins of CAM in the Agavoideae (Asparagaceae). New Phytologist 235: 2111–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyne B. 1815. On the deoxidation of the leaves of Cotyledon calycina. The Transactions of the Linnean Society of London 11: 213–215. [Google Scholar]
- Hinchliff CE, Smith SA.. 2014. Some limitations of public sequence data for phylogenetic inference (in plants). PLoS One 9: e98986. doi: 10.1371/journal.pone.0098986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C-L, Chiang J-M, Lin T-C, Martin CE.. 2019. First report of C4/CAM-cycling photosynthetic pathway in a succulent grass, Spinifex littoreus (Brum. f.) Merr., in coastal regions of Taiwan. Flora 254: 194–202. doi: 10.1016/j.flora.2018.08.005. [DOI] [Google Scholar]
- Holaday AS, Bowes G.. 1990. C4 acid metabolism and dark CO2 fixation in a submersed aquatic macrophyte (Hydrilla verticillata). Plant Physiology 65: 331–335. doi: 10.1104/pp.65.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthe PA, Patel A, Ting IP.. 1992. The occurrence of CAM in Peperomia. Selbyana 13: 77–87. [Google Scholar]
- Holtum JAM, Aranda J, Virgo A, Gehrig HH, Winter K.. 2004. δ13C values and crassulacean acid metabolism in Clusia species from Panama. Trees 18: 658–668. doi: 10.1007/s00468-004-0342-y. [DOI] [Google Scholar]
- Holtum JAM, Winter K. 2005. Carbon isotope composition of canopy leaves in a tropical forest in Panama throughout a seasonal cycle. Trees 19: 545–551. [Google Scholar]
- Holtum JAM, Winter K, Weeks MA, Sexton TR. 2007. Crassulacean acid metabolism in the ZZ plant, Zamioculcas zamiifolia (Araceae). American Journal of Botany 94: 1670–1676. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K.. 2017. Optional use of CAM photosynthesis in two C4 species, Portulaca cyclophylla and Portulaca digyna. Journal of Plant Physiology 214: 91–96. doi: 10.1016/j.jplph.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, Winter K.. 2018. Crassulacean acid metabolism in the Basellaceae (Caryophyllales). Plant Biology 20: 409–414. doi: 10.1111/plb.12698. [DOI] [PubMed] [Google Scholar]
- Horn JW, Xi Z, Riina R, et al. 2014. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 68: 3485–3504. doi: 10.1111/evo.12534. [DOI] [PubMed] [Google Scholar]
- Hu A-Q, Gale SW, Liu Z-J, Fischer GA, Saunders RMK.. 2022. Diversification slowdown in the Cirrhopetalum alliance (Bulbophyllum, Orchidaceae): insights from the evolutionary dynamics of crassulacean acid metabolism. Frontiers in Plant Science 13: 794171. doi: 10.3389/fpls.2022.794171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J, Dettman DL, Williams DG, Hultine KR. 2018. Gas exchange characteristics of giant cacti species varying in stem morphology and life history strategy. American Journal of Botany 105: 1688–1702. [DOI] [PubMed] [Google Scholar]
- Huxley C. 1980. Symbiosis between ants and epiphytes. Biological Reviews 55: 321–340. doi: 10.1111/j.1469-185x.1980.tb00696.x. [DOI] [Google Scholar]
- Jaiswal SK, Mahajan S, Chakraborty A, Kumar S, Sharma VK.. 2021. The genome sequence of Aloe vera reveals adaptive evolution of drought tolerance mechanisms. iScience 24: 102079. doi: 10.1016/j.isci.2021.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Landis JB, Yang J, et al. 2023. Phylogeny and evolution of Asparagaceae subfamily Nolinoideae: new insights from plastid phylogenomics. Annals of Botany 131: 301–312. doi: 10.1093/aob/mcac144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CS, Cardon ZG, Czaja AD.. 2003. A phylogenetic view of low-level CAM in Pelargonium (Geraniaceae). American Journal of Botany 90: 135–142. doi: 10.3732/ajb.90.1.135. [DOI] [PubMed] [Google Scholar]
- Jung J, Kim C, Kim J-H.. 2021. Insights into phylogenetic relationships and genome evolution of subfamily Commelinoideae (Commelinaceae Mirb.) inferred from complete chloroplast genomes. BMC Genomics 22: 231. doi: 10.1186/s12864-021-07541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley JE. 1981. Isoëtes howellii: a submerged aquatic CAM plant? American Journal of Botany 68: 420–424. doi: 10.1002/j.1537-2197.1981.tb06380.x. [DOI] [Google Scholar]
- Keeley JE. 1983. Lack of diurnal acid metabolism in two terrestrial Isoëtes species. Photosynthetica 17: 93–94. [Google Scholar]
- Keeley JE. 1996. Aquatic CAM photosynthesis. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 281–295. [Google Scholar]
- Keeley JE. 1998. CAM photosynthesis in submerged aquatic plants. Botanical Review 64: 121–175. doi: 10.1007/bf02856581. [DOI] [Google Scholar]
- Keeley JE. 1999. Photosynthetic pathway diversity in a seasonal pool community. Functional Ecology 13: 106–118. doi: 10.1046/j.1365-2435.1999.00294.x. [DOI] [Google Scholar]
- Keeley JE, Morton BA.. 1982. Distribution of diurnal acid metabolism in submerged aquatic plants outside the genus Isoëtes. Photosynthetica 16: 546–553. [Google Scholar]
- Keeley JE, Osmond CB, Raven JA.. 1984. Stylites, a vascular land plant without stomata absorbs CO2 via its roots. Nature 310: 694–695. doi: 10.1038/310694a0. [DOI] [Google Scholar]
- van de Kerke SJ, Shrestha B, Ruhlman TA, et al. 2019. Plastome based phylogenetics and younger crown node age in Pelargonium. Molecular Phylogenetics and Evolution 137: 33–43. [DOI] [PubMed] [Google Scholar]
- Khanum R, Surveswaran S, Meve U, Liede-Schumann S.. 2016. Cynanchum (Apocynaceae: Asclepiadoideae): a pantropical Asclepiadoid genus revisited. Taxon 65: 467–486. doi: 10.12705/653.3. [DOI] [Google Scholar]
- Klak C, Hanáček P, Bruyns PV.. 2017b. Disentangling the Aizooideae: new generic concepts and a new subfamily in Aizoaceae. Taxon 66: 1147–1170. [Google Scholar]
- Klak C, Hanáček P, Bruyns PV.. 2017a. Out of southern Africa: origin, biogeography and age of the Aizooideae (Aizoaceae). Molecular Phylogenetics and Evolution 109: 203–216. doi: 10.1016/j.ympev.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Klak C, Reeves G, Hedderson TAJ.. 2004. Unmatched tempo of evolution in southern African semi-desert ice plants. Nature 427: 63–65. doi: 10.1038/nature02243. [DOI] [PubMed] [Google Scholar]
- Klavsen SK, Madsen TV, Maberly SC.. 2011. Crassulacean acid metabolism in the context of other carbon-concentrating mechanisms in freshwater plants: a review. Photosynthesis Research 109: 269–279. doi: 10.1007/s11120-011-9630-8. [DOI] [PubMed] [Google Scholar]
- Kluge M, Brulfert J, Lipp J, Ravelomanana D, Ziegler H.. 1993. A comparative study by δ13C‐analysis of crassulacean acid metabolism (CAM) in Kalanchoë (Crassulaceae) species of Africa and Madagascar. Plant Biology 106: 320–324. [Google Scholar]
- Kluge M, Brulfert J, Rauh W, Ravelomanana D, Ziegler H.. 1995. Ecophysiological studies on the vegetation of Madagascar: a δ13C and δD survey for incidence of crassulacean acid metabolism (CAM) among orchids from montane forests and succulents from the xerophytic thorn-bush. Isotopes in Environmental and Health Studies 31: 191–210. doi: 10.1080/10256019508234018. [DOI] [Google Scholar]
- Kluge M, Ting IP.. 1978. Crassulacean acid metabolism: analysis of an ecological adaptation. Berlin: Springer. [Google Scholar]
- Kluge M, Vinson B, Ziegler H.. 1998. Ecophysiological studies on orchids of Madagascar: incidence and plasticity of crassulacean acid metabolism in species of the genus Angraecum Bory. Plant Ecology 135: 43–57. [Google Scholar]
- Koch K, Kennedy RA.. 1980. Characteristics of crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. Plant Physiology 65: 193–197. doi: 10.1104/pp.65.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus G. 1883. Über Stoffwechsel bei den Crassulaceen. Abhandlungen der Naturforschenden Gesellschaft zu Halle 16: 393–410. [Google Scholar]
- Lee C-K, Fuse S, Poopath M, Pooma R, Tamura MN.. 2021. Phylogenetics and infrafamilial classification of Commelinaceae (Commelinales). Botanical Journal of the Linnean Society 198: 117–130. doi: 10.1093/botlinnean/boab047. [DOI] [Google Scholar]
- Li Z, Baniaga AE, Sessa EB, et al. 2015. Early genome duplications in conifers and other seed plants. Science Advances 1: e1501084. doi: 10.1126/sciadv.1501084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang J, Zhang X, et al. 2022. The genome of Aechmea fasciata provides insights into the evolution of tank epiphytic habits and ethylene-induced flowering. Communications Biology 5: 920. doi: 10.1038/s42003-022-03918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liede-Schumann S, Reuss SJ, Meve U, et al. 2022. Phylogeny of Marsdenieae (Apocynaceae, Asclepiadoideae) based on chloroplast and nuclear loci, with a conspectus of the genera. Taxon 71: 833–875. [Google Scholar]
- Lim JY, Marshall CR, Zimmer EA, Wagner WL.. 2019. Multiple colonizations of the Pacific by Peperomia (Piperaceae): complex patterns of long-distance dispersal and parallel radiations on the Hawaiian Islands. Journal of Biogeography 46: 2651–2662. doi: 10.1111/jbi.13717. [DOI] [Google Scholar]
- Liu B, Yan J, Li W, et al. 2020. Mikania micrantha genome provides insights into the molecular mechanism of rapid growth. Nature Communications 11: 340. doi: 10.1038/s41467-019-13926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lösch R. 1984. Species-specific responses to temperature in acid metabolism and gas exchange performance of Macaronesian Sempervivoideae. In: Margaris NS, Arianoustow-Farragitaki M, Oechel WC, eds. Tasks for vegetation science. The Hague: Dr. W. Junk, 117–126. [Google Scholar]
- Luján M, Oleas NH, Winter K.. 2022. Evolutionary history of CAM photosynthesis in Neotropical Clusia: insights from genomics, anatomy, physiology and climate. Botanical Journal of the Linnean Society 199: 538–556. doi: 10.1093/botlinnean/boab075. [DOI] [Google Scholar]
- Lüttge U. ed. 2007. Clusia: a woody Neotropical genus of remarkable plasticity and diversity. Berlin: Springer. [Google Scholar]
- Madsen TV. 1987. Sources of inorganic carbon acquired through CAM in Littorella uniflora (L.) Aschers. Journal of Experimental Botany 38: 367–377. doi: 10.1093/jxb/38.3.367. [DOI] [Google Scholar]
- Manni M, Berkeley MR, Seppy M, Simão FA, Zdobnov EM.. 2021. BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Molecular Biology and Evolution 38: 4647–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J, Boatwright JS, Daru BH, Maurin O, van der Bank M. 2014. A molecular phylogeny and generic classification of Asphodelaceae subfamily Alooideae: a final resolution of the prickly issue of polyphyly in the Alooids? Systematic Botany 39: 55–74. [Google Scholar]
- Martin CE. 1996. Putative causes and consequences of recycling CO2 via crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 192–203. [Google Scholar]
- Martin CE, Wallace RS.. 2000. Photosynthetic pathway variation in leafy members of two subfamilies of the Cactaceae. International Journal of Plant Sciences 161: 639–650. doi: 10.1086/314285. [DOI] [Google Scholar]
- Mayer A. 1875. Über die Bedeutung der organischen Säuren in den Pflanzen. Die Landwirtschaftlichen Versuchsstationen 18: 410–452. [Google Scholar]
- Medina E, Delgado M, Troughton JH, Medina JD.. 1977. Physiological ecology of CO2 fixation in Bromeliaceae. Flora 166: 137–152. doi: 10.1016/s0367-2530(17)32126-6. [DOI] [Google Scholar]
- Medina E, Troughton JH.. 1974. Dark CO2 fixation and the carbon isotope ratio in Bromeliaceae. Plant Science Letters 2: 357–362. doi: 10.1016/0304-4211(74)90044-3. [DOI] [Google Scholar]
- Meng R, Luo L-Y, Zhang J-Y, Zhang D-G, Nie Z-L, Meng Y.. 2021. The deep evolutionary relationships of the morphologically heterogeneous Nolinoideae (Asparagaceae) revealed by transcriptome data. Frontiers in Plant Science 11: 584981. doi: 10.3389/fpls.2020.584981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmid TFE, Klein JT, Kadereit G, Kadereit JW.. 2020. Linnaeus’s folly – phylogeny, evolution and classification of Sedum (Crassulaceae) and Crassulaceae subfamily Sempervivoideae. Taxon 69: 892–926. [Google Scholar]
- Messerschmid TFE, Wehling J, Bobon N, et al. 2021. Carbon isotope composition of plant photosynthetic tissues reflects a crassulacean acid metabolism (CAM) continuum in the majority of CAM lineages. Perspectives in Plant Ecology, Evolution and Systematics 51: 125619. [Google Scholar]
- Ming R, VanBuren R, Wai CM, et al. 2015. The pineapple genome and the evolution of CAM photosynthesis. Nature Genetics 47: 1435–1442. doi: 10.1038/ng.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok D, Leung A, Searles P, Sage TL, Sage RF.. 2023. CAM photosynthesis in Bulnesia retama (Zygophyllaceae), a non-succulent desert shrub from South America. Annals of Botany, this volume: mcad114. doi: 10.1093/aob/mcad114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney HA, Bullock SH, Ehleringer JR.. 1989. Carbon isotope ratios of plants of a tropical dry forest in Mexico. Functional Ecology 3: 137–142. doi: 10.2307/2389294. [DOI] [Google Scholar]
- Mooney H, Troughton JH, Berry JA.. 1974. Arid climates and photosynthetic systems. In: Carnegie Institution of Washington Year Book. Washington: Carnegie Institution of Washington, 793–805. [Google Scholar]
- Mooney HA, Troughton JH, Berry JA.. 1977. Carbon isotope ratio measurements of succulent plants in southern Africa. Oecologia 30: 295–305. doi: 10.1007/BF00399762. [DOI] [PubMed] [Google Scholar]
- Moore AJ, De Vos JM, Hancock LP, Goolsby E, Edwards EJ.. 2018. Targeted enrichment of large gene families for phylogenetic inference: phylogeny and molecular evolution of photosynthesis genes in the Portullugo clade (Caryophyllales). Systematic Biology 67: 367–383. doi: 10.1093/sysbio/syx078. [DOI] [PubMed] [Google Scholar]
- Moreno-Villena JJ, Zhou H, Gilman IS, Tausta SL, Cheung CYM, Edwards EJ.. 2022. Spatial resolution of an integrated C4+CAM photosynthetic metabolism. Science Advances 8: eabn2349. doi:2021.11.25.470062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neales TF, Patterson AA, Hartney VJ.. 1968. Physiological adaptation to drought in the carbon assimilation and water loss of xerophytes. Nature 219: 469–472. doi: 10.1038/219469a0. [DOI] [Google Scholar]
- Nelson EA, Sage RF.. 2008. Functional constraints of CAM leaf anatomy: tight cell packing is associated with increased CAM function across a gradient of CAM expression. Journal of Experimental Botany 59: 1841–1850. doi: 10.1093/jxb/erm346. [DOI] [PubMed] [Google Scholar]
- Newman JR, Raven JA.. 1995. Photosynthetic carbon assimilation by Crassula helmsii. Oecologia 101: 494–499. doi: 10.1007/BF00329429. [DOI] [PubMed] [Google Scholar]
- Niechayev NA, Pereira PN, Cushman JC.. 2019. Understanding trait diversity associated with crassulacean acid metabolism (CAM). Current Opinion in Plant Biology 49: 74–85. doi: 10.1016/j.pbi.2019.06.004. [DOI] [PubMed] [Google Scholar]
- Nishida K. 1963. Studies on stomatal movement of crassulacean plants in relation to the acid metabolism. Physiologia Plantarum 16: 281–298. [Google Scholar]
- Nobel PS, Hartsock TL.. 1986. Leaf and stem CO2 uptake in the three subfamilies of the Cactaceae. Plant Physiology 80: 913–917. doi: 10.1104/pp.80.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuernbergk EL. 1961. Endogener Rhythmus und CO2-Stoffwechsel bei Pflanzen mit diurnalem Säurerhythmus. Planta 56: 28–70. doi: 10.1007/bf01894844. [DOI] [Google Scholar]
- Ocampo G, Koteyeva NK, Voznesenskaya EV, et al. 2013. Evolution of leaf anatomy and photosynthetic pathways in Portulacaceae. American Journal of Botany 100: 2388–2402. doi: 10.3732/ajb.1300094. [DOI] [PubMed] [Google Scholar]
- O’Leary MH. 1981. Carbon isotope fractionation in plants. Phytochemistry 20: 553–567. doi: 10.1016/0031-9422(81)85134-5. [DOI] [Google Scholar]
- Orlov NM, Viktorova VA, Eskov AK.. 2022. CAM (crassulacean acid metabolism) photosynthesis in vascular epiphytes. Biology Bulletin Reviews 12: 527–543. doi: 10.1134/s2079086422050073. [DOI] [Google Scholar]
- Osmond CB. 1978. Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology 29: 379–414. doi: 10.1146/annurev.pp.29.060178.002115. [DOI] [Google Scholar]
- Osmond CB. 1982. Carbon cycling and stability of the photosynthetic apparatus in CAM. In: Ting IP, Gibbs M, eds. Crassulacean acid metabolism. Rockville: American Society of Plant Physiologists, 112–127. [Google Scholar]
- Osmond CB, Allaway WG, Sutton BG, et al. 1973. Carbon isotope discrimination in photosynthesis of CAM plants. Nature 246: 41–42. doi: 10.1038/246041a0. [DOI] [Google Scholar]
- Osmond CB, Ziegler H, Stichler W, Trimborn P.. 1975. Carbon isotope discrimination in alpine succulent plants supposed to be capable of crassulacean acid metabolism (CAM). Oecologia 18: 209–217. doi: 10.1007/BF00345423. [DOI] [PubMed] [Google Scholar]
- Ozerova LV, Schanzer IA, Timonin AC.. 2017. Curio alliance (Asteraceae: Senecioneae) revisited. Wulfenia 24: 29–52. [Google Scholar]
- Pachon P, Winter K, Lasso E.. 2022. Updating the occurrence of crassulacean acid metabolism (CAM) in the genus Clusia through carbon isotope analysis of species from Colombia. Photosynthetica 60: 304–322. doi: 10.32615/ps.2022.018. [DOI] [Google Scholar]
- Pan I-C, Liao D-C, Wu F-H, et al. 2012. Complete chloroplast genome sequence of an orchid model plant candidate: Erycina pusilla apply in tropical Oncidium breeding. PLoS One 7: e34738. doi: 10.1371/journal.pone.0034738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton AJ, Mwanyambo M, Govaerts RHA, et al. 2019. Nomenclatural changes in Coleus and Plectranthus (Lamiaceae): a tale of more than two genera. PhytoKeys 129: 1–158. doi: 10.3897/phytokeys.129.34988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelser PB, Nordenstam B, Kadereit JW, Watson LE.. 2007. An ITS phylogeny of tribe Senecioneae (Asteraceae) and a new delimitation of Senecio L. Taxon 56: 1077–1104. doi: 10.2307/25065905. [DOI] [Google Scholar]
- Pierce S, Winter K, Griffiths H.. 2002. Carbon isotope ratio and the extent of daily CAM use by Bromeliaceae. New Phytologist 156: 75–83. doi: 10.1046/j.1469-8137.2002.00489.x. [DOI] [Google Scholar]
- Pieters AJ, Tezara W, Herrera A.. 2003. Operation of the xanthophyll cycle and degradation of D1 protein in the inducible CAM plant, Talinum triangulare, under water deficit. Annals of Botany 92: 393–399. doi: 10.1093/aob/mcg153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigg KB. 2001. Isoetalean lycopsid evolution: from the Devonian to the present. American Fern Journal 91: 99–114. doi: 10.1640/0002-8444(2001)091[0099:ileftd]2.0.co;2. [DOI] [Google Scholar]
- Pilon-Smits EAH, 't Hart H, Meesterburrie JAN, Naber P, Kreuler R, van Brederode J. 1991. Variation in crassulacean acid metabolism within the genus Sedum: carbon isotope composition and water status dependent phosphoenolpyruvate carboxylase activity. Journal of Plant Physiology 137: 342–346. [Google Scholar]
- Pilon-Smits EAH, 't Hart H, Maas JW, Meesterburrie JAN, Kreuler R, van Brederode J. 1992. The evolution of crassulacean acid metabolism in Aeonium inferred from carbon isotope composition and enzyme activities. Oecologia 91: 548–553. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, ‘t Hart H, van Brederode J.. 1996. Evolutionary aspects of crassulacean acid metabolism in the Crassulaceae. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 349–359. [Google Scholar]
- POWO. 2023. Plants of the world online. Kew: Royal Botanic Gardens. http://www.plantsoftheworldonline.org/ (January 2023, date last accessed). [Google Scholar]
- Pucher GW, Leavenworth CS, Ginter WD, Vickery HB.. 1947. Studies in the metabolism of crassulacean plants: changes in the composition of Bryophyllum calycinum during growth. Plant Physiology 22: 1–19. doi: 10.1104/pp.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucher GW, Vickery HB.. 1942. On the identity of the so called crassulacean malic acid with isocitric acid. Journal of Biological Chemistry 145: 525–532. doi: 10.1016/s0021-9258(18)51292-8. [DOI] [Google Scholar]
- Qiu S, Sultana S, Liu ZD, Yin LY, Wang CY.. 2015. Identification of obligate C3 photosynthesis in Dendrobium. Photosynthetica 53: 168–176. doi: 10.1007/s11099-015-0110-9. [DOI] [Google Scholar]
- R Core Team. 2021. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.r-project.org [Google Scholar]
- Rae JWB, Zhang YG, Liu X, Foster GL, Stoll HM, Whiteford RDM.. 2021. Atmospheric CO2 over the past 66 million years from marine archives. Annual Review of Earth and Planetary Sciences 49: 609–641. [Google Scholar]
- Rao S, Reiskind J, Bowes G.. 2006. Light regulation of the photosynthetic phosphoenolpyruvate carboxylase (PEPC) in Hydrilla verticillata. Plant and Cell Physiology 47: 1206–1216. doi: 10.1093/pcp/pcj091. [DOI] [PubMed] [Google Scholar]
- Raven JA, Spicer RA.. 1996. The evolution of crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 360–388. [Google Scholar]
- Rentsch JD, Leebens-Mack J.. 2012. Homoploid hybrid origin of Yucca gloriosa: intersectional hybrid speciation in Yucca (Agavoideae, Asparagaceae). Ecology and Evolution 2: 2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. doi: 10.1111/j.2041-210x.2011.00169.x. [DOI] [Google Scholar]
- Rundel PW, Esler KJ, Cowling RM.. 1999. Ecological and phylogenetic patterns of carbon isotope discrimination in the winter-rainfall flora of the Richtersveld, South Africa. Plant Ecology 142: 133–148. [Google Scholar]
- Rundel PW, Rundel JA, Ziegler H, Stichler W.. 1979. Carbon isotope ratios of central Mexican Crassulaceae in natural and greenhouse environments. Oecologia 38: 45–50. doi: 10.1007/BF00347823. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2002. Are crassulacean acid metabolism and C4 photosynthesis incompatible? Functional Plant Biology 29: 775–785. doi: 10.1071/PP01217. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2016. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame. Journal of Experimental Botany 67: 4039–4056. doi: 10.1093/jxb/erw156. [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin P-A, Edwards EJ.. 2011. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62: 3155–3169. doi: 10.1093/jxb/err048. [DOI] [PubMed] [Google Scholar]
- Sage RF, Gilman IS, Smith JAC, Silvera K, Edwards EJ.. 2023. Atmospheric CO2 decline and the timing of CAM plant evolution. Annals of Botany, this volume: mcad122. doi: 10.1093/aob/mcad122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F.. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63: 19–47. doi: 10.1146/annurev-arplant-042811-105511. [DOI] [PubMed] [Google Scholar]
- de Saussure T. 1804. Recherches chimiques sur la végétation. Paris: Chez la V.e Nyon. [Google Scholar]
- Sayed OH. 2001. Crassulacean acid metabolism 1975–2000, a check list. Photosynthetica 39: 339–352. doi: 10.1023/a:1020292623960. [DOI] [Google Scholar]
- Schiller K, Bräutigam A.. 2021. Engineering of crassulacean acid metabolism. Annual Review of Plant Biology 72: 77–103. doi: 10.1146/annurev-arplant-071720-104814. [DOI] [PubMed] [Google Scholar]
- Schuettpelz E, Chen C-W, Kessler M, et al. 2016. A revised generic classification of vittarioid ferns (Pteridaceae) based on molecular, micromorphological, and geographic data. Taxon 65: 708–722. doi: 10.12705/654.2. [DOI] [Google Scholar]
- Schulze E-D, Ziegler H, Stichler W.. 1976. Environmental control of crassulacean acid metabolism in Welwitschia mirabilis Hook. Fil. in its range of natural distribution in the Namib desert. Oecologia 24: 323–334. doi: 10.1007/BF00381138. [DOI] [PubMed] [Google Scholar]
- Shameer S, Baghalian K, Cheung CYM, Ratcliffe RG, Sweetlove LJ.. 2018. Computational analysis of the productivity potential of CAM. Nature Plants 4: 165–171. doi: 10.1038/s41477-018-0112-2. [DOI] [PubMed] [Google Scholar]
- Shao H, Gontero B, Maberly SC, et al. 2017. Responses of Ottelia alismoides, an aquatic plant with three CCMs, to variable CO2 and light. Journal of Experimental Botany 68: 3985–3995. doi: 10.1093/jxb/erx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Li N, Wang Y, et al. 2022. High-quality ice plant reference genome analysis provides insights into genome evolution and allows exploration of genes involved in the transition from C3 to CAM pathways. Plant Biotechnology Journal 20: 2107–2122. doi: 10.1111/pbi.13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC.. 2010b. Evolution along the crassulacean acid metabolism continuum. Functional Plant Biology 37: 995–916. doi: 10.1071/fp10084. [DOI] [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K.. 2009. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiology 149: 1838–1847. doi: 10.1104/pp.108.132555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K.. 2010a. The incidence of crassulacean acid metabolism in Orchidaceae derived from carbon isotope ratios: a checklist of the flora of Panama and Costa Rica. Botanical Journal of the Linnean Society 163: 194–222. doi: 10.1111/j.1095-8339.2010.01058.x. [DOI] [Google Scholar]
- Silvera K, Santiago LS, Winter K.. 2005. Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Functional Plant Biology 32: 397–407. doi: 10.1071/FP04179. [DOI] [PubMed] [Google Scholar]
- Silvera K, Winter K, Rodriguez BL, Albion RL, Cushman JC.. 2014. Multiple isoforms of phosphoenolpyruvate carboxylase in the Orchidaceae (subtribe Oncidiinae): implications for the evolution of crassulacean acid metabolism. Journal of Experimental Botany 65: 3623–3636. doi: 10.1093/jxb/eru234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro D, Zizka G, Schulte K.. 2014. Disentangling the effects of key innovations on the diversification of Bromelioideae (Bromeliaceae). Evolution 68: 163–175. doi: 10.1111/evo.12236. [DOI] [PubMed] [Google Scholar]
- Siniscalchi CM, Edwards RD, Gomez JL, Moore ER, Mandel JR.. 2021. Photosynthesis metabolism in the Compositae: current knowledge and future directions. Taxon 70: 339–350. doi: 10.1002/tax.12426. [DOI] [Google Scholar]
- Smith BN, Epstein S.. 1971. Two categories of 13C/12C ratios for higher plants. Plant Physiology 47: 380–384. doi: 10.1104/pp.47.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GF, Figueiredo E.. 2020. Asphodelaceae. In: Eggli U, Nyffeler R, eds. Monocotyledons. Illustrated handbook of succulent plants. Berlin: Springer, 475–481. [Google Scholar]
- Smith JAC, Winter K.. 1996. Taxonomic distribution of crassulacean acid metabolism. In: Winter K, Smith JAC. eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 427–436. [Google Scholar]
- Sternberg LO, Deniro MJ, Ting IP.. 1984. Carbon, hydrogen, and oxygen isotope ratios of cellulose from plants having intermediary photosynthetic modes. Plant Physiology 74: 104–107. doi: 10.1104/pp.74.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek SR. 1979. The occurrence of crassulacean acid metabolism: a supplementary list during 1976 to 1979. Photosynthetica 13: 467–473. [Google Scholar]
- Szarek SR, Ting IP.. 1977. The occurrence of crassulacean acid metabolism among plants. Photosynthetica 11: 330–342. [Google Scholar]
- Szecowka M, Heise R, Tohge T, et al. 2013. Metabolic fluxes in an illuminated Arabidopsis rosette. The Plant Cell 25: 694–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takawira-Nyenya R, Mucina L, Cardinal-McTeague WM, Thiele KR.. 2018. Sansevieria (Asparagaceae, Nolinoideae) is an herbaceous clade within Dracaena: inference from non-coding plastid and nuclear DNA sequence data. Phytotaxa 376: 254–276. doi: 10.11646/phytotaxa.376.6.2. [DOI] [Google Scholar]
- Teeri JA, Gurevitch J.. 1984. Environmental and genetic control of crassulacean acid metabolism in two crassulacean species and an F1 hybrid with differing biomass δ13C values. Plant, Cell & Environment 7: 589–596. [Google Scholar]
- Teeri JA, Tonsor SJ, Turner M.. 1981. Leaf thickness and carbon isotope composition in the Crassulaceae. Oecologia 50: 367–369. doi: 10.1007/BF00344977. [DOI] [PubMed] [Google Scholar]
- Tenhunen JD, Tenhunen LC, Ziegler H, Stichler W, Lange OL.. 1982. Variation in carbon isotope ratios of Sempervivoideae species from different habitats of Teneriffe in the spring. Oecologia 55: 217–224. doi: 10.1007/BF00384490. [DOI] [PubMed] [Google Scholar]
- Thomas M. 1949. Physiological studies on acid metabolism in green plants. I. CO2 fixation and CO2 liberation in crassulacean acid metabolism. New Phytologist 48: 390–420. doi: 10.1111/j.1469-8137.1949.tb05133.x. [DOI] [Google Scholar]
- Thomas M, Beevers H.. 1949. Physiological studies on acid metabolism in green plants. II. Evidence of CO2 fixation in Bryophyllum and the study of diurnal variation of acidity in this genus. New Phytologist 48: 421–447. doi: 10.1111/j.1469-8137.1949.tb05134.x. [DOI] [Google Scholar]
- Thurlow J, Bonner J.. 1948. Fixation of atmospheric CO2 in the dark by leaves of Bryophyllum. Archives of Biochemistry 19: 509–511. [PubMed] [Google Scholar]
- Tooulakou G, Giannopoulos A, Nikolopoulos D, et al. 2016. Alarm photosynthesis: calcium oxalate crystals as an internal CO2 source in plants. Plant Physiology 171: 2577–2585. doi: 10.1104/pp.16.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer N, Braam T, Shameer S, Ratcliffe RG, Sweetlove LJ.. 2020. Alternative Crassulacean acid metabolism modes provide environment-specific water-saving benefits in a leaf metabolic model. The Plant Cell 32: 3689–3705. doi: 10.1105/tpc.20.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Morales G, Lasso E, Silvera K, Turner BL, Winter K.. 2020. Occurrence of crassulacean acid metabolism in Colombian orchids determined by leaf carbon isotope ratios. Botanical Journal of the Linnean Society 193: 431–477. doi: 10.1093/botlinnean/boaa027. [DOI] [Google Scholar]
- Tsen EWJ, Holtum JAM.. 2012. Crassulacean acid metabolism (CAM) in an epiphytic ant-plant, Myrmecodia beccarii Hook.f. (Rubiaceae). Photosynthesis Research 113: 311–320. doi: 10.1007/s11120-012-9732-y. [DOI] [PubMed] [Google Scholar]
- Valente LM, Britton AW, Powell MP, Papadopulos AST, Burgoyne PM, Savolainen V.. 2014. Correlates of hyperdiversity in southern African ice plants (Aizoaceae). Botanical Journal of the Linnean Society 174: 110–129. doi: 10.1111/boj.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JC, Lerman JC.. 1969. Groningen radiocarbon dates VIII. Radiocarbon 11: 351–390. doi: 10.1017/s0033822200011279. [DOI] [Google Scholar]
- Vovides AP, Etherington JR, Dresser PQ, Groenhof A, Iglesias C, Ramirez JF.. 2002. CAM-cycling in the cycad Dioon edule Lindl. in its natural tropical deciduous forest habitat in central Veracruz, Mexico. Botanical Journal of the Linnean Society 138: 155–162. doi: 10.1046/j.1095-8339.2002.138002155.x. [DOI] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Edwards GE, Ocampo G.. 2017. Unique photosynthetic phenotypes in Portulaca (Portulacaceae): C3-C4 intermediates and NAD-ME C4 species with Pilosoid-type Kranz anatomy. Journal of Experimental Botany 68: 225–239. doi: 10.1093/jxb/erw393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai CM, Weise SE, Ozersky P, Mockler TC, Michael TP, VanBuren R.. 2019. Time of day and network reprogramming during drought induced CAM photosynthesis in Sedum album. PLoS Genetics 15: e1008209. doi: 10.1371/journal.pgen.1008209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma X, Yan G, et al. 2023. Gene duplications facilitate C4-CAM compatibility in common purslane. Plant Physiology: kiad451. doi: 10.1093/plphys/kiad451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang X, Ma Y, Qing Y, Wang H, Huang X.. 2019a. The highly drought-tolerant pitaya (Hylocereus undatus) is a non-facultative CAM plant under both well-watered and drought conditions. The Journal of Horticultural Science and Biotechnology 94: 643–652. doi: 10.1080/14620316.2019.1595747. [DOI] [Google Scholar]
- Wang N, Yang Y, Moore MJ, et al. 2019b. Evolution of Portulacineae marked by gene tree conflict and gene family expansion associated with adaptation to harsh environments. Molecular Biology and Evolution 36: 112–126. doi: 10.1093/molbev/msy200. [DOI] [PubMed] [Google Scholar]
- Wanntorp L, Grudinski M, Forster PI, Muellner-Riehl AN, Grimm GW.. 2014. Wax plants (Hoya, Apocynaceae) evolution: epiphytism drives successful radiation. Taxon 63: 89–102. doi: 10.12705/631.3. [DOI] [Google Scholar]
- Warburg O. 1886. Über die Bedeutung der organischen Säuren für den Lebensprozess der Pflanzen (speziell der sog. Fettpflanzen). Untersuchungen aus dem Botanischen Institut zu Tübingen 2: 53–150. [Google Scholar]
- Warren DM, Wilkins MB.. 1961. An endogenous rhythm in the rate of dark-fixation of carbon dioxide in leaves of Bryophyllum fedtschenkoi. Nature 191: 686–688. doi: 10.1038/191686a0. [DOI] [Google Scholar]
- Webb DR, Rattray MR, Brown JMA.. 1988. A preliminary survey for crassulacean acid metabolism (CAM) in submerged aquatic macrophytes in New Zealand. New Zealand Journal of Marine and Freshwater Research 22: 231–235. doi: 10.1080/00288330.1988.9516295. [DOI] [Google Scholar]
- Wei X, Qi Y, Zhang X, et al. 2017. Phylogeny, historical biogeography and characters evolution of the drought resistant fern Pyrrosia Mirbel (Polypodiaceae) inferred from plastid and nuclear markers. Scientific Reports 7: 12757. doi: 10.1038/s41598-017-12839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Lu L, Nie Z, et al. 2018. A new phylogenetic tribal classification of the grape family (Vitaceae). Journal of Systematics and Evolution 56: 262–272. doi: 10.1111/jse.12427. [DOI] [Google Scholar]
- Wickell D, Kuo L-Y, Yang H-P, et al. 2021. Underwater CAM photosynthesis elucidated by Isoëtes genome. Nature Communications 12: 6348. doi: 10.1038/s41467-021-26644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MB. 1959. An endogenous rhythm in the rate of carbon dioxide output of Bryophyllum. I. Some preliminary experiments. Journal of Experimental Botany 10: 377–390. doi: 10.1093/jxb/10.3.377. [DOI] [Google Scholar]
- von Willert DJ, Brinckmann E, Scheitler B, Schulze E-D, Thomas DA, Treichel S.. 1980. Ökophysiologische Untersuchungen an Pflanzen der Namib-Wüste. Naturwissenschaften 67: 21–28. doi: 10.1007/bf00424499. [DOI] [Google Scholar]
- von Willert DJ, Eller BM, Werger MJA, Brinckmann E, Ihlenfeldt H-D.. 1992. Life strategies of succulents in deserts. With special reference to the Namib desert. Cambridge: Cambridge University Press. [Google Scholar]
- von Willert DJ, Armbrüster N, Drees T, Zaborowski M. 2005. Welwitschia mirabilis: CAM or not CAM — what is the answer? Functional Plant Biology 32: 389–395. [DOI] [PubMed] [Google Scholar]
- Winter K. 1979. δ13C values of some succulent plants from Madagascar. Oecologia 40: 103–112. doi: 10.1007/BF00388814. [DOI] [PubMed] [Google Scholar]
- Winter K. 1981. C4 plants of high biomass in arid regions of Asia – occurrence of C4 photosynthesis in Chenopodiaceae and Polygonaceae from the Middle East and USSR. Oecologia 48: 100–106. doi: 10.1007/BF00346994. [DOI] [PubMed] [Google Scholar]
- Winter K. 2019. Ecophysiology of constitutive and facultative CAM photosynthesis. Journal of Experimental Botany 70: 6495–6508. doi: 10.1093/jxb/erz002. [DOI] [PubMed] [Google Scholar]
- Winter K, Aranda J, Holtum JAM.. 2005. Carbon isotope composition and water-use efficiency in plants with crassulacean acid metabolism. Functional Plant Biology 32: 381–388. doi: 10.1071/FP04123. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Holtum JAM.. 2008. On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. Journal of Experimental Botany 59: 1829–1840. doi: 10.1093/jxb/ern080. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Virgo A, Ceballos J, Holtum JAM.. 2021a. Does the C4 plant Trianthema portulacastrum (Aizoaceae) exhibit weakly expressed crassulacean acid metabolism (CAM)? Functional Plant Biology 48: 655–665. doi: 10.1071/FP20247. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Virgo A, Smith JAC.. 2021b. Low-level CAM photosynthesis in a succulent-leaved member of the Urticaceae, Pilea peperomioides. Functional Plant Biology 48: 683–690. doi: 10.1071/FP20151. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM.. 2002. How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiology 129: 1843–1851. doi: 10.1104/pp.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM.. 2014. Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. Journal of Experimental Botany 65: 3425–3441. doi: 10.1093/jxb/eru063. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. 2015. Cryptic crassulacean acid metabolism (CAM) in Jatropha curcas. Functional Plant Biology 42: 711–717. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM, Smith JAC.. 2015. Crassulacean acid metabolism: a continuous or discrete trait? New Phytologist 208: 73–78. doi: 10.1111/nph.13446. [DOI] [PubMed] [Google Scholar]
- Winter K, Medina E, Garcia V, Mayoral ML, Muniz R.. 1985. Crassulacean acid metabolism in roots of a leafless orchid, Campylocentrum tyrridion Garay & Dunsterv. Journal of Plant Physiology 118: 73–78. [DOI] [PubMed] [Google Scholar]
- Winter K, Sage RF, Edwards EJ, Virgo A, Holtum JAM.. 2019. Facultative crassulacean acid metabolism in a C3–C4 intermediate. Journal of Experimental Botany 70: 6571–6579. doi: 10.1093/jxb/erz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Schramm MJ.. 1986. Analysis of stomatal and nonstomatal components in the environmental control of CO2 exchange in leaves of Welwitschia mirabilis. Plant Physiology 82: 173–178. doi: 10.1104/pp.82.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Smith JAC, eds. 1996. Crassulacean acid metabolism: biochemistry, ecophysiology, and evolution. Berlin: Springer. [Google Scholar]
- Winter K, Smith JAC.. 2022. CAM photosynthesis: the acid test. New Phytologist 233: 599–609. doi: 10.1111/nph.17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, von Willert DJ.. 1972. NaCl-induzierter Crassulaceensäurestoffwechsel bei Mesembryanthemum crystallinum. Zeitschrift für Pflanzenphysiologie 67: 166–170. doi: 10.1016/s0044-328x(72)80131-4. [DOI] [Google Scholar]
- Winter K, Troughton JH, Evenari M, Läuchli A, Lüttge U.. 1976. Mineral ion composition and occurrence of CAM-like diurnal malate fluctuations in plants of coastal and desert habitats of Israel and the Sinai. Oecologia 25: 125–143. doi: 10.1007/BF00368849. [DOI] [PubMed] [Google Scholar]
- Winter K, Virgo A, Garcia M, Aranda J, Holtum JAM.. 2021c. Constitutive and facultative crassulacean acid metabolism (CAM) in Cuban oregano, Coleus amboinicus (Lamiaceae). Functional Plant Biology 48: 647–654. doi: 10.1071/FP20127. [DOI] [PubMed] [Google Scholar]
- Winter K, Wallace BJ, Stocker GC, Roksandic Z.. 1983. Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia 57: 129–141. doi: 10.1007/BF00379570. [DOI] [PubMed] [Google Scholar]
- Wong SC, Hew CS.. 1976. Diffusive resistance, titratable acidity, and CO2 fixation in two tropical epiphytic ferns. American Fern Journal 66: 121–124. doi: 10.2307/1546463. [DOI] [Google Scholar]
- Wood D, Besnard G, Beerling DJ, Osborne CP, Christin P-A.. 2020. Phylogenomics indicates the ‘living fossil’ Isoëtes diversified in the Cenozoic. PLoS One 15: e0227525. doi: 10.1371/journal.pone.0227525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Wang Y, Li Q, et al. 2022. A genome for Cissus illustrates features underlying its evolutionary success in dry savannas. Horticulture Research 9: uhac208. doi: 10.1093/hr/uhac208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Niu S-C, Li K-L, et al. 2022. Chromosome-scale assembly of the Dendrobium nobile genome provides insights into the molecular mechanism of the biosynthesis of the medicinal active ingredient of Dendrobium. Frontiers in Genetics 13: 844622. doi: 10.3389/fgene.2022.844622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Wang Xiao, Liu H, et al. 2015. The genome of Dendrobium officinale illuminates the biology of the important traditional Chinese orchid berb. Molecular Plant 8: 922–934. [DOI] [PubMed] [Google Scholar]
- Yáñez-Espinosa L, Flores J, Millán PSR, Méndez GR.. 2014. Influence of germination date on Dioon edule (Zamiaceae) seedling tolerance to water stress. Journal of Plant Research 127: 413–422. [DOI] [PubMed] [Google Scholar]
- Yang F, Gao J, Wei Y, et al. 2021. The genome of Cymbidium sinense revealed the evolution of orchid traits. Plant Biotechnology Journal 19: 2501–2516. doi: 10.1111/pbi.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hu R, Yin H, et al. 2017. The Kalanchoë genome provides insights into convergent evolution and building blocks of crassulacean acid metabolism. Nature Communications 8: 1899. doi: 10.1038/s41467-017-01491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Liu X.. 2015. Comparing photosynthetic characteristics of Isoëtes sinensis Palmer under submerged and terrestrial conditions. Scientific Reports 5: 17783. doi: 10.1038/srep17783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Liu D, Tschaplinski TJ, Tuskan GA.. 2019. Comparative genomics can provide new insights into the evolutionary mechanisms and gene function in CAM plants. Journal of Experimental Botany 70: 6539–6547. doi: 10.1093/jxb/erz408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Xu F, Wang S-D, et al. 2012. A single leaf of Camellia oleifera has two types of carbon assimilation pathway, C3 and crassulacean acid metabolism. Tree Physiology 32: 188–199. doi: 10.1093/treephys/tps002. [DOI] [PubMed] [Google Scholar]
- Zalenskiï OV, Glagoleva T.. 1981. Pathway of carbon metabolism in halophytic desert species from Chenopodiaceae. Photosynthetica 15: 244–255. [Google Scholar]
- Zhang L, Chen F, Zhang G-Q, et al. 2016. Origin and mechanism of crassulacean acid metabolism in orchids as implied by comparative transcriptomics and genomics of the carbon fixation pathway. The Plant Journal 86: 175–185. doi: 10.1111/tpj.13159. [DOI] [PubMed] [Google Scholar]
- Zhang G-Q, Liu K-W, Li Z, et al. 2017. The Apostasia genome and the evolution of orchids. Nature 549: 379–383. doi: 10.1038/nature23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yin L, Jiang H-S, Li W, Gontero B, Maberly SC.. 2014. Biochemical and biophysical CO2 concentrating mechanisms in two species of freshwater macrophyte within the genus Ottelia (Hydrocharitaceae). Photosynthesis Research 121: 285–297. doi: 10.1007/s11120-013-9950-y. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang G-Q, Zhang D, et al. 2021. Chromosome-scale assembly of the Dendrobium chrysotoxum genome enhances the understanding of orchid evolution. Horticulture Research 8: 183. doi: 10.1038/s41438-021-00621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Helliker BR, Huber M, Dicks A, Akçay E.. 2018. C4 photosynthesis and climate through the lens of optimality. Proceedings of the National Academy of Sciences of the United States of America 115: 12057–12062. doi: 10.1073/pnas.1718988115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H, Batanouny KH, Sankhla N, Vyas OP, Stichler W.. 1981. The photosynthetic pathway types of some desert plants from India, Saudi Arabia, Egypt, and Iraq. Oecologia 48: 93–99. doi: 10.1007/BF00346993. [DOI] [PubMed] [Google Scholar]
- Zotz G, Ziegler H.. 1997. The occurrence of crassulacean acid metabolism among vascular epiphytes from Central Panama. New Phytologist 137: 223–229. doi: 10.1046/j.1469-8137.1997.00800.x. [DOI] [PubMed] [Google Scholar]
- Zotz G, Andrade JL, Einzmann HJR.. 2023. CAM plants: their importance in epiphyte communities and prospects with climate change. Annals of Botany. doi: 10.1093/aob/mcac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L-H, Wan X, Deng H, Zheng B-Q, Li B-J, Wang Y.. 2018. RNA-seq transcriptomic profiling of crassulacean acid metabolism pathway in Dendrobium catenatum. Scientific Data 5: 180252. doi: 10.1038/sdata.2018.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to infer family-level statistics of CAM species diversity are available online at: https://github.com/isgilman/CAM-diversity


