Abstract
Neurological disorders are a major global challenge, which counts for a substantial slice of disease burden around the globe. In these, the challenging landscape of central nervous system (CNS) diseases, including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and neuro-AIDS, demands innovative and novel therapeutic approaches. Curcumin, a versatile natural compound with antioxidant and anti-inflammatory properties, shows great potential as a CNS adjuvant therapy. However, its limited bioavailability and suboptimal permeability to the blood–brain barrier (BBB) hamper the therapeutic efficacy of curcumin. This review explores how nanocarrier facilitates curcumin delivery, which has shown therapeutic efficacy for various non-CNS diseases, for example, cancers, and can also revolutionize the treatment outcomes in patients with CNS diseases. Toward this, intranasal administration of curcumin as a non-invasive CNS drug delivery route can also aid its therapeutic outcomes as an adjuvant therapy for CNS diseases. Intranasal delivery of nanocarriers with curcumin improves the bioavailability of curcumin and its BBB permeability, which is instrumental in promoting its therapeutic potential. Furthermore, curcumin’s inhibitory effect on efflux transporters will help to enhance the BBB and cellular permeability of various CNS drugs. The therapeutic potential of curcumin as an adjuvant has the potential to yield synergistic effects with CNS drugs and will help to reduce CNS drug doses and improve their safety profile. Taken together, this approach holds a promise for reshaping CNS disease management by maximizing curcumin’s and other drugs’ therapeutic benefits.
Keywords: Nanocarriers, curcumin, CNS diseases, neurodegenerative disorders, intranasal delivery, blood–brain barrier
Impact Statement
Neurological disorders present a global challenge, comprising a significant portion of disease burden worldwide. The complex nature of central nervous system (CNS) diseases demands innovative therapeutic approaches. Curcumin’s potential as an adjuvant therapy for CNS diseases is hindered by its limited bioavailability and blood–brain barrier (BBB) permeability. Nanocarrier-mediated curcumin delivery holds promise in overcoming these challenges. This review highlights how nanocarriers can enhance curcumin’s therapeutic efficacy by improving its bioavailability and BBB permeability. This approach has the potential to reshape CNS disease management, offering synergistic effects with existing drugs and improving safety profiles. Moreover, exploring intranasal curcumin delivery and its utilization as an adjuvant therapy offers novel possibilities for effective CNS disease treatment.
Introduction
Curcumin, a naturally occurring biologically active polyphenolic compound found in the spice turmeric (Curcuma longa), has a rich medicinal history dating back centuries in traditional medicine. 1 It has been utilized in Ayurvedic and Chinese medicine for its therapeutic properties in various conditions, including inflammation, pain, digestive disorders, and skin diseases.1,2 Sourced primarily from the rhizomes of the turmeric plant, curcumin belongs to a class of compounds known as curcuminoids. Curcumin is a molecule that exhibits significant biological activities due to its unique composition (Figure 1), which includes a diarylheptanoid, a β-diketone, and an α,β-unsaturated β-diketone. These properties make curcumin valuable for its antioxidant, anti-inflammatory, and anticancer effects. Structurally, curcumin is a symmetrical compound, also known as diferuloyl methane, and its IUPAC name is (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione. It can be represented by the chemical formula C21H20O6 and has a molecular weight of 368.38. Its structure comprises three distinct components: two aromatic ring systems that incorporate o-methoxy phenolic groups, linked together by a seven-carbon chain containing an α,β-unsaturated β-diketone segment. 3 Curcumin is a lipophilic compound and therefore for optimal absorption, it is commonly ingested with lipid-containing meals. 4 Upon absorption, curcumin binds to multiple cellular targets, including transcription factors, enzymes, and receptors, resulting in its diverse biological effects. 4 Curcumin’s interactions with nuclear factor-kappa B (NF-κB) and cyclooxygenase-2 (COX-2) regulate cellular inflammation. 5 Curcumin exhibits potent antioxidant characteristics by neutralizing free radicals and mitigating reactive oxygen species (ROS)-associated cellular damage in various diseases.6,7
Figure 1.
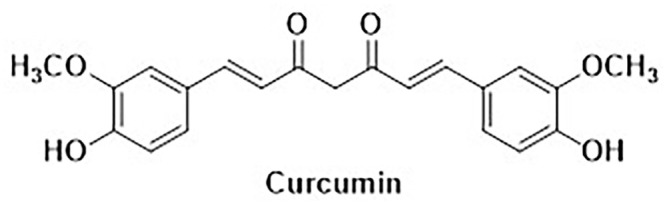
Chemical structure of curcumin.
The conjugated structure of curcumin, marked by multiple double bonds, reinforces its antioxidant capacity by facilitating oxidation and reduction reactions.3,6 Moreover, its abundance of hydroxyl and ketone groups allows interactions with various proteins and enzymes, influencing their activity and stability. 6 The planar structure of curcumin enhances its interactions with other planar molecules, enabling further engagement with receptors and enzymes and alteration in their activity/stability.6,8 These molecular features collectively are responsible for curcumin’s potential as a therapeutic agent to treat a wide range of diseases, including cancer, cardiovascular disorders, and neurodegenerative conditions. 8
Therapeutic spectrum of curcumin
Curcumin has demonstrated promising effects in various disease conditions. In cancer, curcumin has been shown to inhibit the growth of different types of cancer cells, including breast, 9 lung, 10 and colon cancer. 11 Its abilities to induce cell death and hinder tumor angiogenesis hold promise for cancer therapy. In rheumatoid arthritis, curcumin’s anti-inflammatory and antioxidant effects have been shown to alleviate joint inflammation and oxidative stress by modulating proinflammatory cytokines like TNF-α and IL-1β, and increasing anti-inflammatory cytokines such as IL-10.12,13 Furthermore, curcumin has been demonstrated as beneficial effects on inflammatory bowel disease, ameliorating symptoms in conditions like Crohn’s disease and ulcerative colitis through its anti-inflammatory actions in the gut.14,15 In diabetes, curcumin has shown potential in improving insulin sensitivity, reducing blood sugar levels, and lowering the risk of diabetes development by addressing inflammation and oxidative stress.16,17
Besides, curcumin exhibits therapeutic potential in reducing human immunodeficiency virus (HIV)-associated inflammation and cellular damage by modulating autophagy via PI3 K/AKT/IKK/NF-κB signaling. 18 Curcumin has been shown to counter gp120-induced neuronal apoptosis, safeguard synaptic plasticity, reduce ROS levels and microglia-induced inflammation, and accelerate the degradation of Tat protein.19–21 It also inhibits Tat-mediated LTR transactivation and HIV-1 virus production. 21 These findings suggest curcumin’s potential for mitigating HIV-related inflammation and neurotoxicity, warrants further therapeutic exploration in HIV treatment. Furthermore, curcumin’s role in cardiovascular health has been attributed to its ability to reduce inflammation and oxidative stress, as well as its capacity to lower the levels of circulating cholesterol and blood pressure.22–24 These findings support the potential of curcumin as a versatile therapeutic agent for various disease conditions.
Curcumin’s neurotherapeutic applications
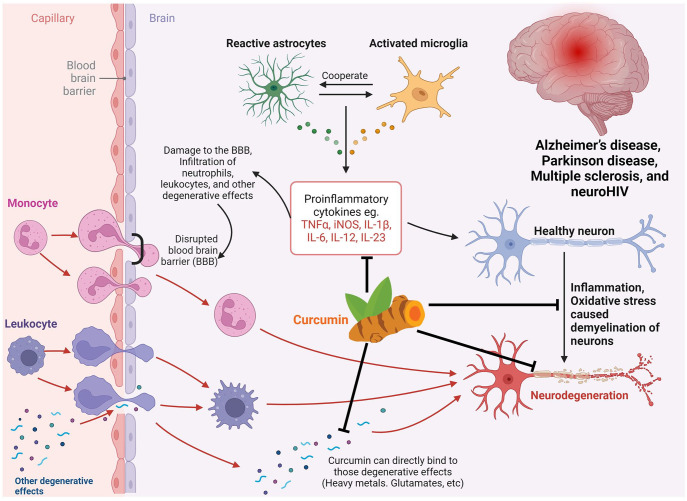
The therapeutic potential of curcumin shown in (Figure 2) in various brain disorders has been extensively investigated due to its anti-inflammatory, antioxidant, and neuroprotective properties.25–27 In Alzheimer’s disease (AD), curcumin has been shown to inhibit the formation of amyloid plaques and improve cognitive function.28,29 The underlying mechanisms include modulation in the activity of enzymes involved in amyloid metabolism and reduction of neuroinflammation via inhibition of neuronal NF-κB signaling. 30 Similarly, in Parkinson’s disease (PD), curcumin’s anti-inflammatory and antioxidant actions have been studied in the context of Lewy body formation and motor function improvement. 31 Curcumin’s ability to enhance the activity of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase, and catalase, in brain cells leads to a reduction in oxidative stress and neuroprotection. 32
Figure 2.
Diverse mechanisms of neuroprotection conferred by curcumin in CNS diseases.
This figure highlights curcumin’s anti-inflammatory effects, antioxidant properties, reduction of degeneration, and support for neuronal regeneration to maintain neuronal health against CNS diseases.
Moreover, curcumin’s anti-inflammatory effects have been explored in depression, traumatic brain injury (TBI), and multiple sclerosis (MS).33,34 In depression, curcumin’s ability to inhibit proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), are important for its antidepressant activity. 35 In TBI, curcumin has been shown to attenuate neuroinflammation and reduce neuronal damage through the inhibition of the p38/MAPK signaling pathway and regulation of pro-/anti-inflammatory mediators. 33 Similarly, curcumin’s anti-inflammatory actions involving the suppression of immune cell activation and reduction of oxidative stress have been suggested as potential mechanisms for protecting from myelin and neuron damage in MS. 34
Curcumin also holds a therapeutic promise in suppressing HIV-associated neurocognitive disorders (HAND) and NeuroAIDS by mitigating neuroinflammation and oxidative stress. Previous studies showed that curcumin reduces gp120-induced inflammation, modulates autophagy, and alters various inflammatory signaling pathways. 18 Nanoparticles comprising curcumin display pain-reducing properties by inhibiting the expression of the P2X3 receptor. 36 Moreover, it regulates HSP70 expression to counteract neuronal apoptosis induced by the gp120 V3 loop. 37 Curcumin also offers protection against synaptic plasticity impairment 20 and reduces HIV-1-mediated apoptosis by curbing ROS production. 19 In addition, curcumin targets the Tat protein and hinders the transactivation and replication of the virus. 21 Recent studies have linked curcumin’s antioxidant properties with potential benefits in schizophrenia, where oxidative stress has been implicated in the pathogenesis of the disorder. 38 Curcumin’s ability to scavenge free radicals and upregulate antioxidant defenses, such as glutathione (GSH), may play a role in alleviating symptoms of schizophrenia.
Challenges in delivering therapeutic drugs to the central nervous system
Neurological disorders are the leading cause of disability-adjusted life-years globally and the second leading cause of death worldwide. 39 Among them, neurodegenerative diseases (NDDs) pose the most difficult management and are characterized by a gradual decline in neurological function and neuronal cell death. NDDs, including AD, PD, amyotrophic lateral sclerosis (ALS), Huntington’s disease, and prion diseases, present a growing health concern worldwide. 39 These diseases share mechanisms involving abnormal accumulation of protein aggregates, which is responsible for selective neuronal damage and degeneration in specific regions of the central nervous system (CNS). 40 Factors such as neuroinflammation, aging, oxidative damage, and protein deposits disrupt neuronal communication, resulting in long-term cognitive and motor dysfunction. 41 Efforts to slow down or halt NDD progression through anti-inflammatory drugs, amyloid-targeting agents, and small molecules have limited success in alleviating the symptoms and improving the overall quality of life in patients with these pathologies. Most brain diseases currently lack effective treatment options, and future studies are required to find novel druggable targets and develop effective therapeutic strategies. 42 Current drugs for NDDs only slow down the progression of the disease but do not reverse its course. 43
While systemic drug administration is convenient and has high feasibility for long-term brain disorder treatment, its therapeutic effect is limited by the suboptimal BBB permeability, which prevents most macromolecular and 98% of small molecule drugs from entering the brain to maintain CNS homeostasis. 44 Researchers have explored strategies to augment BBB permeability, such as changing osmotic pressure and using microbubble fixed-point ultrasound, to improve drug penetration across BBB.45,46 However, these approaches may simultaneously increase the entry of toxic substances into the CNS. 47 Another innovative strategy is to enhance the penetration abilities of therapeutic substances while preserving BBB integrity. The BBB structure or permeability can change under pathological conditions, and exploiting these changes for designing a drug delivery system is a current focus of research. 48
The effective treatment of brain diseases remains challenging due to complex underlying mechanisms and limited therapeutic options. While advances in targeted delivery systems offer hope for more efficient drug delivery to the brain, significant challenges persist. These challenges include suboptimal BBB permeability, fine-tuning secondary targeting within the brain, designing systems for specific disease microenvironments, and achieving optimal therapeutic effects through modulation of the disease microenvironment.
Overcoming limited CNS delivery: harnessing novel strategies for enhanced therapeutic efficacy
Nanomedicine-based drug delivery systems have emerged as a promising strategy to surmount the limitation with suboptimal BBB permeability. 47 These systems offer the potential to enhance the pharmacokinetic profile of therapeutic drugs, optimizing drug concentration within the brain and augmenting therapeutic outcomes. 49 Nanocarriers, such as liposomes, micelles, inorganic nanoparticles, hybrid nanoparticles, and exosomes, have garnered substantial interest in preclinical studies for their capacity to traverse the BBB and transport drugs to the CNS, thereby increasing drug availability at the target site.47,49 This nanotechnology-driven approach holds the promise of reducing nonspecific accumulation while enabling targeted delivery, thereby enhancing therapeutic precision and efficacy. 49 Recent advancements in targeting technology have led to investigations into the secondary targeting effects of nanomedicines beyond BBB permeability, including the potential to target specific cells or even organelles at the subcellular level. 50 These multifaceted approaches offer the potential for controlled, on-demand drug release tailored to the specific disease microenvironment.
In the pursuit of more effective CNS disease treatments, adjuvant therapy using nutraceuticals has garnered attention, particularly due to its ability to enhance therapeutic responses.51,52 Among these, curcumin has emerged as a promising candidate for CNS disorders due to its therapeutic activity against them as discussed in the previous section.6,8 Importantly, curcumin’s role as a modulator of the multidrug resistance protein P-glycoprotein (Pgp), a key efflux transporter at the BBB, offers a unique avenue for addressing CNS drug delivery limitations. 53 Pgp plays a pivotal role in extruding various compounds from the brain, thus limiting their accumulation and potential neurotoxicity. Curcumin’s ability to suppress Pgp expression suggests its potential to overcome drug efflux barriers at the BBB. Thus, the utilization of curcumin not only enhances the BBB permeability of drugs for the treatment of CNS diseases, but it will also enhance target cellular concentration by inhibiting cellular Pgp expression. Furthermore, the utilization of novel nanocarrier-based delivery systems for curcumin holds promise for overcoming limited CNS delivery of curcumin and enhancing its therapeutic efficacy. Thus, the diverse activities of curcumin, coupled with its potential to modulate Pgp-mediated efflux, make it an ideal candidate for addressing CNS drug delivery challenges in the treatment of various CNS diseases.
Optimizing curcumin for neurological health: innovations to improve bioavailability and CNS delivery
Despite its remarkable efficacy and safety profile, curcumin has not yet been authorized as a drug due to its poor gut absorption, rapid metabolism, and systemic elimination, leading to its limited bioavailability. The hydrophobic nature of curcumin, characterized by a logP of approximately 3.2, renders it practically insoluble in water at >30 nM. 3 As a result, curcumin exhibits a short half-life, with studies reporting a mere 10-min half-life at a pH 7.4. 54 In mouse models, both intravenous and oral administration of curcumin resulted in rapid declines in its plasma concentrations within hours. 4 Even with oral administration of a significant dose (1.0 g/kg body weight), plasma levels peak at 0.22 μg/mL after one hour and fall below detectable levels by six hours. 4 Similar patterns were observed with intraperitoneal administration. These findings underscore the formidable challenge of sustaining therapeutic levels of curcumin in the bloodstream for a meaningful duration. The limitations in curcumin’s bioavailability and pharmacokinetics have direct implications for achieving effective CNS delivery. Furthermore, due to its relatively large structure and hydrophobic nature, curcumin has suboptimal BBB permeability. 3 The intricate interplay between curcumin’s limited pharmacokinetic profile and the complexities of CNS delivery necessitates innovative approaches to enhance its solubility, stability, and retention in the bloodstream, thereby enabling its efficient transport across the BBB. Addressing these limitations through innovative formulation approaches and targeted delivery strategies is crucial for harnessing curcumin’s full therapeutic potential in the context of CNS-related pathologies.
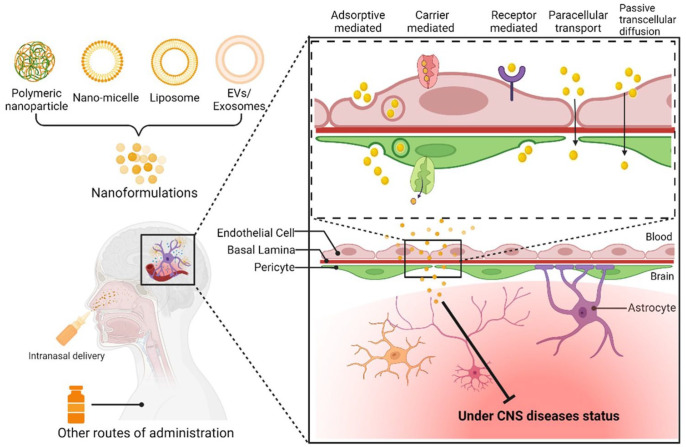
In recent decades, extensive research efforts have focused on addressing the challenges associated with reduced curcumin bioavailability. Nanoparticles, micelles, and liposomes have emerged as promising solutions to enhance the aqueous dispersibility of hydrophobic drugs like curcumin, which inherently suffer from low solubility in their native forms. 55 By encapsulating curcumin within nanoformulations, researchers have harnessed several advantages. These nanocarriers, with sizes typically ranging from 1 to 100 nm offer a high surface area-to-volume ratio. 56 This unique feature contributes to elevating both the solubility and dissolution rate of drugs. Moreover, the reduced particle size extends the drug’s presence in the systemic circulation, facilitating targeted drug delivery and enabling efficient transport across the BBB. Studies have demonstrated that nano-curcumin exhibits significantly higher bioavailability compared to conventional formulations, potentially up to ninefold higher in vivo. 57 The smaller aggregation size of nano-curcumin enables better tissue penetration. These nanocarriers, designed to migrate and home in various tissues, minimize the risk of invasiveness while offering enhanced therapeutic potential.
Researchers have utilized diverse nanocarriers (Figure 3) for delivering nano-curcumin, including chitosan, magnetic nanocomposites, polymer nanocomposites, and montmorillonite. 56 Importantly, curcumin’s safety profile is well-established, with the US Food and Drug Administration (FDA) classifying it as “generally recognized as safe.” 58 Nano-curcumin’s safety and tolerability have been highlighted in human studies, with reported adverse effects being generally mild and manageable. In the context of neurological diseases, nano-curcumin has emerged as a promising avenue for potential therapeutic intervention for AD, PD, HD, MS, epilepsy, and ALS. These investigations have shed light on the potential efficacy of nano-curcumin in clinical applications, offering renewed hope for addressing complex neurological challenges.
Figure 3.
Approaches for targeted curcumin delivery in CNS disease therapy.
The figure show diverse strategies to enhance curcumin’s delivery to the CNS, including polymeric nanoparticles, nanomicelle, liposomal encapsulation, and extracellular vesicles (EVs)/exosomes. The technology of conjugation with brain-targeting ligands has been used in this field to promote the target delivery of curcumin in the CNS.
Therapeutic breakthroughs with nano-curcumin in preclinical studies
Nano-curcumin’s diverse applications outside the CNS
Table 1 presents a comprehensive overview of therapeutic outcomes from diverse curcumin formulations that were tested in various animal models that target distinct disease conditions. These studies collectively emphasize the multifaceted potential of curcumin as a therapeutic agent across a broad spectrum of health concerns. Nanoformulations of curcumin have demonstrated significant potential in various cancer models. Curcumin-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles exhibited improved solubility, anticancer activity, and reduced hypoxic microenvironment in breast and lung cancer cells. 59 ZnO-PBA-Curcumin nanoparticles induced apoptotic cell death in breast cancer cells via oxidative stress and mitochondrial damage. 60 Silver nanoparticle-loaded cellulose hydrogel with curcumin demonstrated potent antimicrobial activity against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida auris in chronic wounds. 61 Infectious diseases were tackled through innovative strategies like mannosylated chitosan nanoparticles targeting leishmaniasis, and PVA/Chi/ZnO-Cur patches aiding wound healing.62,63 Both studies underscore curcumin’s potential in targeted drug delivery and antimicrobial activity.
Table 1.
Applications of nano-curcumin in preclinical studies: non-CNS.
| CUR formulation | Animal – study model | Disease condition | Major findings | Ref |
|---|---|---|---|---|
| PLGA-NP | C57BL/6 mice – cerebral malaria | Cerebral malaria | ↑ therapeutic index | Dende et al. 99 |
| PLGA-NP | Swiss male albino mice malaria model | Malaria | ↑ antiplasmodial activity and safety | Busari et al. 100 |
| PLGA-NPs in hydrogel | C57/BL6 mice – Psoriatic skin preparation | Psoriasis | ↑ anti-psoriasis activity | Sun et al. 101 |
| Mannosylated chitosan NPs | Rat model of leishmaniasis | Leishmaniasis | ↑ targeting to macrophages | Chaubey et al. 62 |
| PVP nano-CUR | BALB/c mice | Oral candidiasis | ↑ antifungal effect ↓ candida colonies |
Anwar et al. 102 |
| Solid lipid NPs | BALB/c mice Rat model of asthma |
Asthma | ↑ PK parameters: ↓ airway hyperresponsiveness, inflammation, and T-helper-2-type cytokines expression |
Wang et al. 103 |
| Polyphosphazene nano-CUR | Mice with acute lung injury | Acute lung injury | ↓ ALI inflammation, cytokines, ROS | Su et al. 104 |
| Nano-CUR | Rats exposed to inhaled paraquat | Acute lung injury | ↑ lung function, antioxidant and anti-inflammatory activity | Ghasemi et al. 105 |
| Gal-POPC/Cur and Gal-DOTAP/siPTTG1 liposomes | Nude mice with human Huh-7 xenografts | Hepatocellular carcinoma | ↑ tumor inhibition, Caspase-3 ↓ Bcl-2 gene expression, HCC treatment |
Kim et al. 106 |
| Nano-liposomes | Zebrafish | Cancer | ↑ CUR and TET solubility, efficacy, and safety. Strong inhibitory effect on cancer cells. |
Song et al. 107 |
| PLGA NPs | Orthotopic mouse model of cervical cancer | Cervical cancer | ↓ cell growth ↑ apoptosis and cell cycle arrest |
Zaman et al. 108 |
| PLGA-DSPE-PEG hybrid NPs | RG2 tumor model (rats) | Glioblastoma | ↓ tumor volume | Orunoglu 69 |
| PLGA NPs | MDA-MB-231 and A549 cell lines | Breast and lung cancer | 10-fold ↑ in solubility threefold ↑ in anticancer activity | Khan et al. 59 |
| HSA-NPs | Breast cancer cell lines | Chemotherapy-resistant cancer | ↓ CUR solubility, stability, and anticancer effects | Matloubi and Hassan 109 |
| ZnO-PBA-NPs | Ehrlich ascites carcinoma tumor-bearing mice | Breast cancer | ↑ Targeted delivery ↓ tumor growth without systemic toxicity |
Kundu 60 |
| Ag NPs | MM-138, FM-55, and MCF-7 cell lines | Melanoma and breast cancer | ↑ anticancer activities | Ali et al. 110 |
| Chitosan/Hyaluronic Acid NPs | Glioblastoma cell culture | Glioblastoma | ↑ Efficient drug delivery, controlled release, cell killing, NGF-driven nerve growth. | Sabourian et al. 111 |
| PVA/Chi/ZnO patch | Wistar albino rats model of wound | Wound healing | ↑ antimicrobial activity, sustained drug release ↑ biocompatibility |
Niranjan et al. 63 |
| C-alginate – nanomicelle | Rats | Colorectal wound healing | ↑ GI wound healing through collagen induction ↓ bacterial activity |
Zhang and Zhang 112 |
| Gelatin/CUR nanofiber membrane | BALB/c mice | Cartilage formation | promote thicker, homogenized cartilage. | Kang et al. 113 |
| CUR/gelatin – nanofibrous mats | Rat skin wound model | Acute wounds | ↑ wound healing, persistent inhibition of inflammatory response ↑ regenerative process. | Dai et al. 114 |
| PVA/Chi/CUR patch | Wistar rats | Epidermal wounds | ↑ cell proliferation, antibacterial activity against major bacterial strains ↑ wound healing |
Niranjan et al. 73 |
| CUR/TiO2—chitosan scaffolds | MRSA-infected wound healing | Infected wounds | ↑ antibacterial activity against Gram +ve and Gram −ve bacteria ↑ wound healing |
Marulasiddeshwara et al. 115 |
| Cellulose nano crystals loaded chitosan films with CUR/Ag NPs | Rabbit model of Skin irritation, Rat model of Wound. | Skin irritation, Wound healing | zero skin irritation ↑ wound healing |
Bajpai et al. 74 |
| Ag NPs-loaded bacterial cellulose hydrogel | Antimicrobial test against P. aeruginosa, S. aureus, and C. auris | Chronic wounds | ↑ cytocompatibility and antimicrobial activity against P. aeruginosa, S. aureus, and C. auris | Gupta et al. 61 |
| CUR-silica NPs | Antimicrobial test against P. aeruginosa, S. aureus | Multidrug resistant bacterial infections, Chronic wound infection | ↑ antimicrobial activity against P. aeruginosa, S. aureus in planktonic and biofilm forms No cytotoxicity |
Mirzahosseinipour et al. 116 |
| Nanostructured lipid carriers | Streptozotocin induced diabetic rat model | Chronic wound | ↑ wound closure ↑ antioxidant enzyme activity |
Mirzahosseinipour et al. 117 |
| CUR-silica NPs | BALB/c mice burn model | Burn wounds, infection | ⊥ in vitro growth of MRSA and P. aeruginosa
↑ wound healing |
Krausz et al. 118 |
| HAp with curcuminoids and 5-fluorouracil nanocomposite | SKOV-3 and HepG2 model cell lines | Cytotoxicity | ↑ intake of biologically active compounds in HAp. | Nguyen et al. 119 |
| Nano-CUR | Rat (Wistar) | Varicocele | ↑ sperm motility ↓ abnormal morphology |
Sadraei et al. 120 |
| CUR and Resveratrol Nanoemulsion | Rat (Albino) | Protein-deficient diet (PDD)-induced hyperammonemia | ↓ ammonia levels ↑ liver and brain function |
Nasr et al. 121 |
| PLGA-NPs | RIN-m5F cells; Sprague Dawley Rats | Type 1 diabetes mellitus | ↑ oral bioavailability ↓ glucose levels ↓ inflammation, and apoptosis in pancreatic islets ↑ beta cell function |
Ganugula et al. 122 |
| CUR-loaded pluronic nanomicelles | Rat model of streptozotocin-induced diabetes | Diabetes | ↑ solubility and bioavailability, optimal redox balance, alleviation of streptozotocin-induced β-cell damage. | El-Far et al. 123 |
CNS: central nervous system; CUR: curcumin; PLGA: poly(lactic-co-glycolic acid); NP: nano particle; ↑: increased/improved; ↓: decreased/reduced; PVP: polyvinylpyrrolidone; ROS: reactive oxygen species; Ag: silver; ⊥: inhibition; PK: pharmacokinetic; ALI: acute lung injury; HCC: hepatocellular carcinoma; TET: tetrandrine; DSPE- PEG : 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol); RG-2: rat glioma 2; MDA-MB-231: human breast cancer cell line; HAS: human serum albumin; PBA: phenyl boronic acid; NGF: nerve growth factor; GI: gastrointestinal; MRSA: methicillin-resistant staphylococcus aureus; HAp: hydroxyapatite; SKOV-3: human ovarian cancer cell line.
Nano-curcumin’s potential for CNS applications
Several studies highlight the versatility and potential of nano-curcumin formulations in addressing various aspects of neurological disorders, ranging from anti-inflammatory effects and antioxidative properties to enhanced drug delivery and targeted action within the CNS (Table 2). In the context of subarachnoid hemorrhage-induced early brain injury, studies have explored the potential of PLGA nanoparticles loaded with curcumin. These nanoparticles have demonstrated a significant reduction in the expression of NF-κB (p65) in a rat model of double hemorrhage, indicating their ability to mitigate neuroinflammation, a common feature in brain injury scenarios. 64 Moreover, another study using the same PLGA nanoparticle formulation observed improved neurological function in a rat model of subarachnoid hemorrhage-induced early brain injury. 65 In the realm of AD, novel nanosystems have emerged as potential therapeutic interventions. T807/TPP-RBC-NPs loaded with curcumin have been investigated for their antioxidative effects in AD. 66 By specifically targeting neuronal mitochondria, these nanosystems hold promise in alleviating AD symptoms and addressing the underlying oxidative stress. Furthermore, a self-nanomicellizing solid dispersion formulation of curcumin demonstrated cognitive function improvement and enhanced cellular uptake in a transgenic AD mouse model. 67 Nano-curcumin formulations have also been explored in the context of PD. Curcumin-loaded polysorbate 80-modified cerasome nanoparticles have exhibited enhanced delivery to brain cell nuclei via BBB opening and ultrasound-mediated microbubble destruction. 68 This approach has shown improvement in motor behaviors and dopamine levels in a mouse model of PD. In highly aggressive brain cancer glioblastoma, various nano-curcumin formulations have revealed potential therapeutic effects. Curcumin-loaded PLGA-1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-Poly(ethylene glycol) hybrid nanoparticles reduce tumor volume when administered intratumorally in an RG2 tumor model. 69 These nanoparticles present a promising strategy for targeting and treating glioblastoma. In addition, PLGA nanoparticles loaded with Aβ generation inhibitor S1 (PQVGHL peptide) and curcumin improved spatial memory, reduced amyloid-beta, and enhanced antioxidant activity in a transgenic AD mouse model. 70
Table 2.
Applications of nano-curcumin in preclinical studies: CNS
| CUR formulation | Animal-study model | Disease condition | Major findings | Ref |
|---|---|---|---|---|
| PLGA-NP | SD rats – SAH-induced EBI | Brain injury | ↓ bio-expression of NF-κB (p65) | Chang et al. 64 |
| PLGA-NP | SD rats – SAH-induced EBI | Brain injury | ↑ neurological function | Zhang et al. 65 |
| T807/TPP-RBC-NPs | Rat primary brain microvascular endothelial cells and primary astrocytes; ICR mice and SD rats | AD | ↓ AD symptoms via antioxidative effects. | Gao et al. 66 |
| self-nanomicellizing solid dispersion | Transgenic AD (APPSwe/PS1deE9) mice | AD | ↑ cognitive functions, ↑ cellular uptake exhibits safety | Zhang et al. 67 |
| CS-BSA NPs | Brain microvascular endothelial cell line, hCMEC/D3; RAW 264.7 cells | AD | ↑ BBB penetration ↑ microglia activation ↑ Aβ peptide phagocytosis ⊥ inflammatory signaling |
Yang et al. 124 |
| Polymeric NPs (NanoCurc™) | Athymic mice | AD | ↑ CUR bioavailability, protects against ROS-mediated insults ↓ H2O2 levels ↓ caspase activities ↑ GSH concentrations |
Ray et al. 125 |
| Lipid-core nanocapsules | Aged female mice | AD | ↑ neuroprotection against Aβ1-42-induced cognitive deficit ↑ inflammatory cytokine |
Giacomeli et al. 126 |
| AmyloLipid nanovesicles | SD rats | Brain delivery | ↑ brain targeting | Sintov 127 |
| PLGA-NPs | Transgenic AD mice | AD | ↑ spatial memory ↓ Aβ, ROS, TNF-α, IL-6 ↑ SOD and synapse numbers |
Huang et al. 70 |
| PLGA-NPs | Transgenic AD mice | AD | ↓ Aβ load ↑ memory deficiency |
Huo et al. 128 |
| Polysorbate 80-modified cerasome NPs | MPTP-induced PD mice | PD | ↑ delivery to brain nuclei via BBB opening and UTMD ↑ motor behaviors, DA levels, and TH expression |
Zhang et al. 68 |
| Glyceryl monooleate NPs | PD mouse model | PD | ↑ inhibition of αS protein aggregation ↓ rotenone-induced toxicity, oxidative stress, and apoptosis |
Kundu et al. 129 |
| Lactoferrin NPs | SK-N-SH cell line | PD | ↑ intracellular drug uptake sustained retention ↑ neuroprotection |
Bollimpelli et al. 130 |
CNS: central nervous system; CUR: curcumin; PLGA: poly(lactic-co-glycolic acid); NP: nano particle; SD: rat – Sprague Dawley Rat; ↓: decreased/reduced; ↑: increased/improved; AD: Alzheimer’s disease: ⊥: inhibition; ROS: reactive oxygen species; BBB: blood–brain barrier; GSH: glutathione; ROS: reactive oxygen species; TNF-α: tumor necrosis factor-alpha; IL-6: interleukin-6; SOD: superoxide dismutase; PD: Parkinson’s disease; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; EBI: early brain injury; SAH: subarachnoid hemorrhage; T807: 7-(6-nitropyridin-3-yl)−5H-pyrido[4,3-b]indole; TPP: triphenylphosphine; RBC: Red blood cell; CS-BSA: chitosan-bovine serum albumin; RAW 264.7 cells- macrophage cell line; UTMD: ultrasound-targeted microbubble destruction; DA: dopamine; TH: tyrosine hydroxylase; SK-N-SH: human neuroblastoma cell line.
Nano-curcumin in clinical trials: a promising adjunctive approach
Table 3 compiles various clinical trials investigating the effects of different curcumin formulations on various health conditions. Curcumin has garnered attention for its potential therapeutic properties, and these clinical trials aim to shed light on its effectiveness in improving various health outcomes. One intriguing study focuses on the use of nano-curcumin in COVID-19 patients. In a double-blind, placebo-controlled trial involving 60 hospitalized COVID-19 patients, nano-curcumin supplementation led to significant reductions in key inflammatory markers, including C-reactive protein (CRP), IL-6, and IL-1β. 71 Notably, nano-curcumin treatment demonstrated the potential to modulate immune response, which could be crucial in managing the hyperinflammatory state associated with severe COVID-19 infection. In patients with metabolic syndrome, a condition marked by a cluster of risk factors for cardiovascular disease, nanomicelle curcumin supplementation proved beneficial. 72 This study, involving 50 patients with metabolic syndrome, highlighted the role of curcumin in improving serum triglyceride levels. This finding is significant as elevated triglyceride levels are the hallmark of metabolic syndrome and it is associated with an increased risk of atherosclerotic cardiovascular disease. The clinical trial involving nanomicelle curcumin therapy in metabolic syndrome patients suggests an improvement in serum triglyceride profile, indicating its potential as a complementary therapeutic option with other lipid-lowering drugs. 72 Wound healing and dermatological applications were addressed by various formulations such as PVA/Chi/Cur patches and cellulose nano crystals loaded with chitosan films.73,74 These studies demonstrated enhanced wound healing properties, antimicrobial activity, and potential for skin regeneration.
Table 3.
Summary of clinical trials exploring nano-curcumin.
| CUR formulation | Disease condition | Major findings | Ref |
|---|---|---|---|
| Nano-CUR | COVID-19 | ↓ IL-6 and IL-1β mRNA expression and cytokine secretion | Valizadeh et al. 71 |
| Nanomicelle curcumin | Metabolic Syndrome (MetS) | ↑ serum TG levels | Bateni et al. 72 |
| Nano-CUR | Depression in patients with diabetic polyneuropathy | ↓ depression and anxiety scores | Asadi et al. 75 |
| CUR Nanomicelles | COVID-19 | ↑ immune response | Hassaniazad et al. 131 |
| Nano-CUR supplementation | Sepsis | ↓ WBCs, neutrophils, platelets, ESR, and IL-8 ↑ total lymphocyte count |
Naeini et al. 132 |
| Nano-CUR supplementation | Mild-to-moderate hospitalized COVID-19 patients | ↓ mRNA expression of IFN-γ and TNF-α differences in IFN-γ, IL-1β, and IL-6 expression and serum levels of IL-1β |
Asadirad et al. 133 |
| Nano-CUR supplementation | Obese and overweight patients with migraine | ↓ MCP-1 serum levels ↓ in headache attack frequencies, severity, and duration |
Sedighiyan et al. 134 |
| Nano-CUR supplementation | Critically ill patients with sepsis | ↑ inflammatory markers, endothelial function, oxidative stress ↓ SOFA score and ventilation duration. |
Karimi et al. 135 |
| Nano-CUR | Radiation-induced skin reactions in breast cancer patients | ↑ radiation-induced skin toxicity ↓ patient-reported pain. |
Talakesh et al. 136 |
| Nano-CUR | Oral lichen planus (OLP) | No significant difference | Kia et al. 137 |
| Nano-CUR and CoQ10 | Migraine | Synergistic effect on clinical features of migraine. | Parohan et al. 138 |
| Nano-CUR supplementation | Migraine | ↑ IL-4 gene expression and serum levels | Djalali et al. 139 |
| Nano-CUR supplementation | Migraine | ↑ adiponectin ↓ headache frequency/severity/ duration in migraines |
Sedighiyan et al. 140 |
| Nano-CUR | Migraine | ↓ IL-17 levels/expression | Djalali et al. 141 |
| Nano-CUR | Diabetic Sensorimotor Polyneuropathy (DSPN) in Type 2 diabetes mellitus | ↓ HbA1c, FBS, total neuropathy score, reflex score, and temperature | Asadi et al. 142 |
| Nano-CUR | Hemodialysis | ↓ inflammation, hs-CRP levels, and adhesion molecules (ICAM-1, VCAM-1) | Vafadar et al. 143 |
| Nano-CUR | Diabetes on hemodialysis | ↓ fasting glucose, insulin levels, lipid levels, hs-CRP, and oxidative stress markers ↑ TAC and nitrite levels, ↑Improved metabolic profile. |
Shafabakhsh et al. 144 |
| Nano-CUR | Type 2 diabetes with mild to moderate coronary artery disease | ↓ inflammation (hs-CRP) and lipid metabolism disruption (LipoPr (a)) | Dastani et al. 145 |
| ω-3 fatty acids, Nano-CUR | Episodic migraine | ↓ serum levels and gene expression of VCAM Combination showed pronounced effect |
Abdolahi et al. 76 |
| ω-3 fatty acids and nano-CUR | Migraine | ↓ attack frequency synergistically and serum IL-1β levels | Honarvar et al. 146 |
| CUR Nanomicelles | Coronary heart disease | ↑lipid profile, oxidative stress factors and inflammatory markers | Helli et al. 147 |
| ω-3 Fatty Acids, Nano-CUR | Migraine | ↓ COX-2/iNOS gene expression ↓serum levels ↓ frequency, severity, and duration of headaches |
Abdolahi et al. 148 |
| ω-3 Fatty Acids, Nano-CUR | Migraine | ↓ IL-6 gene expression ↓ serum IL-6 and hs-CRP levels |
Abdolahi et al. 149 |
| ω-3 Fatty Acids, Nano-CUR | Migraine | ↓ TNF-α gene expression ↓ serum TNF-α levels |
Abdolahi et al. 150 |
| ω-3 Fatty Acids, Nano-CUR | Migraine | ↓ ICAM-1 serum levels and attack frequency | Soveyd et al. 151 |
| Theracurmin (colloidal CUR NPs) | Oral bioavailability study | 27-fold ↑ bioavailability | Sasaki et al. 152 |
| CUR Mouthwash (0.1% w/v) and CUR-Nanocapsule (SinaCurcumin®40) | Radiotherapy-induced oral mucositis | ↓ severity and pain of radiation-induced oral mucositis with higher ulcer-free rates than placebo. | Ramezani et al. 153 |
| 1% and 2% CUR Nanomicelle Gel | Recurrent Aphthous Stomatitis (RAS) | 1% Curcumin nanomicelle gel:↑ efficacy in pain reduction 2% Curcumin gel: ↑ reduction in lesion size and overall healing | Bakhshi et al. 154 |
| CUR-Containing Nanomicelles | COVID-19 | ↓ IFN-γ and IL-17 levels ↑ IL-4 and TGF-β levels, and accelerated recovery in COVID-19 patients |
Hassaniazad et al. 155 |
| Nano-CUR capsule | Knee osteoarthritis | ↑ overall symptoms, pain, stiffness, and physical activity | Hashemzadeh et al. 156 |
| Nano-CUR | Cystic fibrosis | ↓ hs-CRP and fecal calprotectin levels ↑ IL-10 levels ↑ improved quality of life ↓ Pseudomonas colonies ↑ weight |
Talebi et al. 157 |
| Nano-CUR supplementation | Mild and moderate acute pancreatitis | ↓ GI ward length of stay ↓ need for analgesics ↑appetite score |
Chegini et al. 158 |
| Nano-micellar CUR | Benign prostatic hyperplasia | ↑ International Prostate Symptoms Score | Karami et al. 159 |
| Nano-CUR oral soft gels | COVID-19 (moderate-severe) | ↑chest CT scores, oxygen saturation levels, and hospitalization duration | Sadeghizadeh et al. 160 |
| Nano-CUR capsule | Oral leukoplakia | ↓ lesion size, number of lesions, and disease staging ↑ serum SOD levels. |
Deb et al. 161 |
| Nano-CUR supplementation | Metabolic syndrome | ↑ IL-10 and BDNF levels ↓ in IL-6 levels. |
Osali 162 |
| Nano-CUR supplementation | Non-alcoholic fatty liver disease | ↑ glucose indices, including fasting blood sugar and HbA1c. | Jazayeri-Tehrani et al. 77 |
CNS: central nervous system; CUR: curcumin; PLGA: poly(lactic-co-glycolic acid); NP: nano particle; ↓: decreased/reduced; IL-6: interleukin-6; ↑: increased/improved; WBC: white blood cells; FBS: including fasting blood sugar; CRP: C-reactive protein; SOD: superoxide dismutase; TG: triglyceride; ESR: erythrocyte sedimentation rate; MCP-1: monocyte chemoattractant protein-1; SOFA: sequential organ failure assessment; ICAM-1: intercellular adhesion molecule 1; VCAM1: vascular cell adhesion molecule 1; TAC: total antioxidant capacity; CT: computed tomography; BDNF: brain-derived neurotrophic factor; GI: Gastrointestinal.
Curcumin’s potential also extends to addressing mental health concerns. A study involving 80 patients with diabetes and depression showed that nano-curcumin supplementation effectively reduced depression and anxiety scores in patients with diabetic polyneuropathy. 75 This suggests that curcumin’s anti-inflammatory and neuroprotective properties may contribute to alleviating mental health symptoms in diabetic individuals. In the realm of chronic conditions, the trials explore curcumin’s impact on migraines and studies investigated a combination of omega-3 fatty acids and nano-curcumin in 80 episodic migraine patients. The combination therapy not only reduced the frequency of attack but also downregulated the expression of proinflammatory genes, indicating curcumin’s anti-migraine effects. 76 Liver health is another area of interest, with studies focusing on non-alcoholic fatty liver disease (NAFLD). In a randomized, double-blind, placebo-controlled trial involving overweight/obese patients with NAFLD, nano-curcumin supplementation significantly improved glucose indices, including fasting blood sugar (FBS) and glycated hemoglobin (HbA1c). 77 These findings suggest a potential role for curcumin in managing metabolic parameters associated with NAFLD. The trials collectively demonstrate the promising potential of nano-curcumin as an adjunct therapy across various health conditions. Its ability to modulate inflammatory responses, improve metabolic markers, and alleviate symptoms in conditions like migraines and fatty liver disease indicates a versatile therapeutic role. From COVID-19 to metabolic syndrome, migraines, and liver diseases, nanoformulated curcumin demonstrates promising effects on inflammatory markers, metabolic profiles, and symptom relief. As research in this field advances, curcumin’s role as a complementary therapy may become more defined, offering a natural and accessible option for improving various treatment outcomes.
Extracellular vesicles-mediated curcumin delivery: advancing treatments
The use of extracellular vesicles (EVs), natural nanoparticles that are secreted from our cells into biofluids, as nanocarriers in non-neuronal conditions is also being investigated. EVs have been found to improve the delivery of therapeutic intervention, as well as, they serve as biomarkers in liver diseases. In a recent review, Wang et al. 78 found that EVs can serve as cautionary biomarkers, diagnostic and prognostic tools, and a possible mode for treating liver failure by encouraging hepatocyte regeneration and proliferation through various pathways. However, in the case of sepsis, Homma et al. 79 noted that in a sheep model of sepsis, EVs derived from bone marrow mesenchymal stem cells were not capable of lessening the “severity of multiorgan dysfunction” associated with sepsis. Osteoporosis is another condition in which the use of EVs as treatment being considered. In a recent review, He et al. 80 reported that, in mouse models, EVs derived from bone marrow can increase bone mass, enhance the microarchitecture of the bone matrix, and promote bone strength.
EVs loaded with curcumin are also being investigated in non-neuronal conditions, such as rheumatoid arthritis and hyperhomocysteinemia. In the case of rheumatoid arthritis, He et al. 81 found that loading curcumin onto EVs/exosomes helped stabilize curcumin. The curcumin-loaded exosomes (Curc-Exos) were found to aid in decreasing the production of anti-apoptotic proteins, such as IAP1 and IAP2. Curc-Exos was also noted to have anti-inflammatory properties, as it decreased inflammatory mediators, such as IL-6, TNF-α, MMP1, and PGE2. These findings indicated that Curc-Exos should be considered further as a treatment option for rheumatoid arthritis. Regarding hyperhomocysteinemia, Kalani et al. 82 used hyperhomocysteinemia in mouse models as a representation of a disrupted BBB. The results showed that cells treated with Curc-Exos had decreased oxidative stress and endothelial cell layer permeability.
EVs/exosomes are of particular interest to deliver curcumin for the treatment of CNS and other neuronal conditions, especially because EVs can cross the BBB. 83 In the case of PD, Upadhya and Shetty 84 showed that EVs can release pathologic miRNAs and/or proteins, which can cause the progression of the disease state. Liu et al. 85 investigated the use of curcumin as a part of the rabies virus glycoprotein (RVG) peptide–modified exosome (EXO) curcumin/phenylboronic acid-poly(2-(dimethylamino)ethyl acrylate) nanoparticle/small interfering RNA targeting SNCA (REXO-C/ANP/S). This delivery system can cross the BBB and deliver the drug to the neurons involved in the pathologic process. The study found that REXO-C/ANP/S serves as a “nano scavenger” to aid in diminishing alpha-synuclein aggregates. The study also found reduced motor deficits in the mouse models. A recent study by Mohabat et al. 86 investigated the use of curcumin-loaded exosomes derived from human endometrial stem cells (hEnSCs EXOs-Cur) and reported that hEnSCs EXOs-Cur can penetrate the BBB, reduce alpha-synuclein aggregates, and lessen neural cell death. These studies suggest that the anti-inflammatory and antioxidant effects of curcumin can attenuate the pathologic processes associated with PD.
In the AD model, Wang et al. 87 noted that curcumin-loaded exosomes effectively crossed the BBB via transcytosis and increased its bioavailability in the target tissues. The curcumin-loaded exosomes reduced neuronal death and Tau phosphorylation by activating the AKT/GSK-3β pathway. Similarly, Fernandes et al. 88 proposed a new concept for the delivery of curcumin in AD. Using a zebrafish model, they investigated the use of an exosome-like liposome to deliver curcumin to the target tissue. The study found that this novel delivery system shared the benefits of exosomes in crossing the BBB.
In the case of ischemic injuries, He et al. 89 used exosomes derived from macrophages and loaded with curcumin, which decreased the ROS generation in the regions with ischemic damage. The decrease in the accumulation of ROS aids in reducing damage to the BBB and neuronal apoptosis. Furthermore, Tian et al. 90 found that curcumin-loaded exosomes also initiated a suppression of the inflammatory processes in the ischemic brain.
Taken together, these studies show that EV-based curcumin formulations have therapeutic potential for CNS diseases such as AD, PD, ischemic injuries, and rheumatoid arthritis. The therapeutic outcomes of EV-curcumin formulations largely occur by overcoming curcumin limitations of low bioavailability and suboptimal BBB permeability.
Exploring the potential of intranasal curcumin delivery for efficient treatment of neurological disorders
The emergence of intranasal (IN) administration has introduced a paradigm shift in targeted drug delivery to the CNS, presenting a novel and non-invasive approach for effective therapeutic intervention. This approach capitalizes on the distinctive histological attributes of the nasal cavity, facilitating direct access of approx. 50% of the therapeutic agents to the brain. 91 A key advantage of IN delivery lies in its ability to significantly circumvent liver metabolism, systemic circulation, and BBB permeability, leading to high bioavailability in the CNS.91,92 Pharmacokinetic investigations have revealed that despite potential bioavailability reductions attributed to the nasal epithelium, drug concentrations within various CNS regions post-IN administration can exhibit up to a 10-fold increment compared to systemic injection.93,94 Strikingly, dose escalation of IN-administered drugs elicits proportionate enhancements in drug concentration across diverse CNS territories. The non-invasive nature of IN administration, complemented by user-friendly delivery devices such as sprays or atomizers, not only fosters patient acceptance but also accommodates frequent dosing. Clinical trials corroborate the feasibility of repeated IN delivery within brief intervals, even daily, highlighting the adaptability of this approach for versatile treatment regimens.95,96
Leveraging the potential of IN drug delivery, contemporary research has harnessed its capabilities to surmount therapeutic challenges associated with curcumin. IN delivery of curcumin emerges as a promising strategy to enhance its brain bioavailability. Multiple studies have explored the efficacy of IN curcumin delivery employing diverse formulations and carriers, striving to maximize its therapeutic potential for neurological disorders. Strategies such as mucoadhesive microemulsion systems (MMESs) have demonstrated heightened brain uptake relative to intravenous administration, with implications for targeted brain delivery. 97 Microemulsions (MEs) incorporating docosahexaenoic acid (DHA)-rich oil have showcased superior brain penetration, potentially mediated by DHA-induced BBB transport. Furthermore, the development of thermosensitive hydrogels and innovative nanoparticles exhibits promise in augmenting curcumin’s brain delivery, reinforcing the potential of IN administration as a transformative avenue for curcumin’s therapeutic application in CNS disorders. 98 These strategies circumvent the constraints associated with curcumin’s physicochemical attributes, paving the way for enhanced treatments of neurological conditions.
Conclusions
The intricate nature of CNS diseases, often accompanied by a spectrum of comorbidities, requires a comprehensive therapeutic approach. Curcumin’s multifaceted actions as an antioxidant, anti-inflammatory, and immunomodulatory make it an appealing adjuvant therapy by targeting various inflammatory signaling pathways implicated in the pathogenesis of CNS disorders. Innovative strategies, such as combining curcumin with existing medications, show promise in enhancing therapeutic outcomes in CNS diseases. Collaborative efforts to optimize dosing regimens and identify optimal drug combinations and effective delivery systems can reshape the treatment landscape for these conditions. To this end, nanoformulations, especially using natural nanoparticle EVs for curcumin delivery, hold great potential for improving curcumin’s bioavailability and facilitating its passage through the BBB. Furthermore, IN delivery methods of curcumin nanoformulations offer opportunities to enhance direct brain targeting while minimizing the peripheral side effects in the other parts of the body. Thus, the combined efforts in unraveling curcumin’s therapeutic potential and harnessing innovative delivery systems including EVs as nanocarriers and IN method hold the promise of improving the outcomes and management of CNS diseases.
Acknowledgments
Figures were created and edited on BioRender.
Footnotes
Authors’ contributions: SG contributed to the initial drafting of the manuscript, review of literature, preparation of tables, and overall editing. LZ contributed to the initial drafting of the manuscript and prepared the figure. SS contributed to the initial drafting of the manuscript. BS and UPS provided critical overall manuscript editing and revision. SK contributed to conceptualization, funding, overall supervision, and supported review development and overall editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding from the NIH grants AG081140 and MH125670 to SK.
ORCID iD: Santosh Kumar  https://orcid.org/0000-0001-7846-5674
https://orcid.org/0000-0001-7846-5674
References
- 1. Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019;24:2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mirzaei H, Shakeri A, Rashidi B, Jalili A, Banikazemi Z, Sahebkar A. Phytosomal curcumin: a review of pharmacokinetic, experimental and clinical studies. Biomed Pharmacother 2017;85:102–12 [DOI] [PubMed] [Google Scholar]
- 3. Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules 2014;19:20091–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm 2007;4:807–18 [DOI] [PubMed] [Google Scholar]
- 5. Buhrmann C, Mobasheri A, Busch F, Aldinger C, Stahlmann R, Montaseri A, Shakibaei M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem 2011;286:28556–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel SS, Acharya A, Ray RS, Agrawal R, Raghuwanshi R, Jain P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit Rev Food Sci Nutr 2020;60:887–939 [DOI] [PubMed] [Google Scholar]
- 7. Larasati YA, Yoneda-Kato N, Nakamae I, Yokoyama T, Meiyanto E, Kato JY. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci Rep 2018;8:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liczbiński P, Michałowicz J, Bukowska B. Molecular mechanism of curcumin action in signaling pathways: review of the latest research. Phytother Res 2020;34:1992–2005 [DOI] [PubMed] [Google Scholar]
- 9. Wang L, Wang C, Tao Z, Zhao L, Zhu Z, Wu W, He Y, Chen H, Zheng B, Huang X, Yu Y, Yang L, Liang G, Cui R, Chen T. Curcumin derivative WZ35 inhibits tumor cell growth via ROS-YAP-JNK signaling pathway in breast cancer. J Exp Clin Cancer Res 2019;38:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao L, Shao T, Zheng W, Ding J. Curcumin suppresses tumor growth of gemcitabine-resistant non-small cell lung cancer by regulating lncRNA-MEG3 and PTEN signaling. Clin Transl Oncol 2021;23:1386–93 [DOI] [PubMed] [Google Scholar]
- 11. Marjaneh RM, Rahmani F, Hassanian SM, Rezaei N, Hashemzehi M, Bahrami A, Ariakia F, Fiuji H, Sahebkar A, Avan A, Khazaei M. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J Cell Physiol 2018;233:6785–98 [DOI] [PubMed] [Google Scholar]
- 12. Amalraj A, Varma K, Jacob J, Divya C, Kunnumakkara AB, Stohs SJ, Gopi S. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: a randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. J Med Food 2017;20:1022–30 [DOI] [PubMed] [Google Scholar]
- 13. Makuch S, Wiecek K, Wozniak M. The immunomodulatory and anti-inflammatory effect of curcumin on immune cell populations, cytokines, and in vivo models of rheumatoid arthritis. Pharmaceuticals 2021;14:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryan JJ, Hanes DA, Bradley RD, Contractor N. Effect of a nutrition support formula in adults with inflammatory bowel disease: a pilot study. Glob Adv Health Med 2019;8:2164956119867251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goulart RA, Barbalho SM, Lima VM, Souza GA, Matias JN, Araújo AC, Rubira CJ, Buchaim RL, Buchaim DV, Carvalho ACA, Guiguer ÉL. Effects of the use of curcumin on ulcerative colitis and Crohn’s disease: a systematic review. J Med Food 2021;24:675–85 [DOI] [PubMed] [Google Scholar]
- 16. Thota RN, Acharya SH, Garg ML. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: a randomised controlled trial. Lipids Health Dis 2019;18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altobelli E, Angeletti PM, Marziliano C, Mastrodomenico M, Giuliani AR, Petrocelli R. Potential therapeutic effects of curcumin on glycemic and lipid profile in uncomplicated type 2 diabetes-a meta-analysis of randomized controlled trial. Nutrients 2021;13:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen G, Liu S, Pan R, Li G, Tang H, Jiang M, Xing Y, Jin F, Lin L, Dong J. Curcumin attenuates gp120-induced microglial inflammation by inhibiting autophagy via the PI3K pathway. Cell Mol Neurobiol 2018;38:1465–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo L, Xing Y, Pan R, Jiang M, Gong Z, Lin L, Wang J, Xiong G, Dong J. Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. PLoS ONE 2013;8:e70565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen LL, Jiang ML, Liu SS, Cai MC, Hong ZQ, Lin LQ, Xing YY, Chen GL, Pan R, Yang LJ, Xu Y, Dong J. Curcumin improves synaptic plasticity impairment induced by HIV-1gp120 V3 loop. Neural Regen Res 2015;10:925–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ali A, Banerjea AC. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci Rep 2016;6:27539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahmadabady S, Beheshti F, Shahidpour F, Khordad E, Hosseini M. A protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide in rats. Biochem Biophys Rep 2021;25:100908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sangouni AA, Taghdir M, Mirahmadi J, Sepandi M, Parastouei K. Effects of curcumin and/or coenzyme Q10 supplementation on metabolic control in subjects with metabolic syndrome: a randomized clinical trial. Nutr J 2022;21:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. du Preez R, Pahl J, Arora M, Ravi Kumar MNV, Brown L, Panchal SK. Low-dose curcumin nanoparticles normalise blood pressure in male wistar rats with diet-induced metabolic syndrome. Nutrients 2019;11:1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-kappaB pathways in BV2 cells. Mol Immunol 2019;116:29–37 [DOI] [PubMed] [Google Scholar]
- 26. Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 2017;87:223–9 [DOI] [PubMed] [Google Scholar]
- 27. He HJ, Xiong X, Zhou S, Zhang XR, Zhao X, Chen L, Xie CL. Neuroprotective effects of curcumin via autophagy induction in 6-hydroxydopamine Parkinson’s models. Neurochem Int 2022;155:105297. [DOI] [PubMed] [Google Scholar]
- 28. Khurshid B, Rehman AU, Muhammad S, Wadood A, Anwar J. Toward the noninvasive diagnosis of Alzheimer’s disease: molecular basis for the specificity of curcumin for fibrillar amyloid-beta. ACS Omega 2022;7:22032–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenfield SA, Cole GM, Coen CW, Frautschy S, Singh RP, Mekkittikul M, Garcia-Ratés S, Morrill P, Hollings O, Passmore M, Hasan S, Carty N, Bison S, Piccoli L, Carletti R, Tacconi S, Chalidou A, Pedercini M, Kroecher T, Astner H, Gerrard PA. A novel process driving Alzheimer’s disease validated in a mouse model: therapeutic potential. Alzheimers Dement 2022;8:e12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deng Y, Lu X, Wang L, Li T, Ding Y, Cao H, Zhang Y, Guo X, Yu G. Curcumin inhibits the AKT/NF-kappaB signaling via CpG demethylation of the promoter and restoration of NEP in the N2a cell line. AAPS J 2014;16:649–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma N, Nehru B. Curcumin affords neuroprotection and inhibits alpha-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018;26:349–60 [DOI] [PubMed] [Google Scholar]
- 32. Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, Huang X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019;14:e0216711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li G, Duan L, Yang F, Yang L, Deng Y, Yu Y, Xu Y, Zhang Y. Curcumin suppress inflammatory response in traumatic brain injury via p38/MAPK signaling pathway. Phytother Res 2022;36:1326–37 [DOI] [PubMed] [Google Scholar]
- 34. ELBini-Dhouib I, Neili NE, Marzouki S, Sahraoui G, Ben Achour W, Zouaghi S, BenAhmed M, Doghri R, Srairi-Abid N. Dual mechanism of action of curcumin in experimental models of multiple sclerosis. Int J Mol Sci 2022;23:8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang WY, Guo YJ, Han WX, Yang MQ, Wen LP, Wang KY, Jiang P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int Immunopharmacol 2019;67:138–44 [DOI] [PubMed] [Google Scholar]
- 36. Zhao S, Yang J, Han X, Gong Y, Rao S, Wu B, Yi Z, Zou L, Jia T, Li L, Yuan H, Shi L, Zhang C, Gao Y, Li G, Liu S, Xu H, Liu H, Liang S. Effects of nanoparticle-encapsulated curcumin on HIV-gp120-associated neuropathic pain induced by the P2X(3) receptor in dorsal root ganglia. Brain Res Bull 2017;135:53–61 [DOI] [PubMed] [Google Scholar]
- 37. Xia C, Cai Y, Li S, Yang J, Xiao G. Curcumin increases HSP70 expression in primary rat cortical neuronal apoptosis induced by gp120 V3 loop peptide. Neurochem Res 2015;40:1996–2005 [DOI] [PubMed] [Google Scholar]
- 38. Moghaddam AH, Maboudi K, Bavaghar B, Sangdehi SRM, Zare M. Neuroprotective effects of curcumin-loaded nanophytosome on ketamine-induced schizophrenia-like behaviors and oxidative damage in male mice. Neurosci Lett 2021;765:136249. [DOI] [PubMed] [Google Scholar]
- 39. Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol 2020;19:255–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 2006;75:333–66 [DOI] [PubMed] [Google Scholar]
- 41. Monaco A, Fraldi A. Protein aggregation and dysfunction of autophagy-lysosomal pathway: a vicious cycle in lysosomal storage diseases. Front Mol Neurosci 2020;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement 2021;7:e12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Athar T, Al Balushi K, Khan SA. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol Biol Rep 2021;48:5629–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev 2019;99:21–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dong Y, Li J, Li P, Yu J. Ultrasound microbubbles enhance the activity of vancomycin against Staphylococcus epidermidis biofilms in vivo. J Ultrasound Med 2018;37:1379–87 [DOI] [PubMed] [Google Scholar]
- 46. Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Discov 2021;20:362–83 [DOI] [PubMed] [Google Scholar]
- 47. Ding S, Khan AI, Cai X, Song Y, Lyu Z, Du D, Dutta P, Lin Y. Overcoming blood-brain barrier transport: advances in nanoparticle-based drug delivery strategies. Mater Today 2020;37:112–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pandit R, Chen L, Götz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev 2020;165–166:1–14 [DOI] [PubMed] [Google Scholar]
- 49. Pinheiro RGR, Coutinho AJ, Pinheiro M, Neves AR. Nanoparticles for targeted brain drug delivery: what do we know? Int J Mol Sci 2021;22:11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alotaibi BS, Buabeid M, Ibrahim NA, Kharaba ZJ, Ijaz M, Noreen S, Murtaza G. Potential of nanocarrier-based drug delivery systems for brain targeting: a current review of literature. Int J Nanomedicine 2021;16:7517–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dell’Acqua G, Richards A, Thornton MJ. The potential role of nutraceuticals as an adjuvant in breast cancer patients to prevent hair loss induced by endocrine therapy. Nutrients 2020;12:3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reed D, Raina K, Agarwal R. Nutraceuticals in prostate cancer therapeutic strategies and their neo-adjuvant use in diverse populations. NPJ Precis Oncol 2018;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lopes-Rodrigues V, Sousa E, Vasconcelos MH. Curcumin as a modulator of P-glycoprotein in cancer: challenges and perspectives. Pharmaceuticals 2016;9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jamwal R. Bioavailable curcumin formulations: a review of pharmacokinetic studies in healthy volunteers. J Integr Med 2018;16:367–74 [DOI] [PubMed] [Google Scholar]
- 55. Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res 2011;4:1158–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karthikeyan A, Senthil N, Min T. Nanocurcumin: a promising candidate for therapeutic applications. Front Pharmacol 2020;11:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci 2009;37:223–30 [DOI] [PubMed] [Google Scholar]
- 58. Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, Sestito S, Rapposelli S, Neffe-Skocińska K, Zielińska D, Salehi B, Setzer WN, Dosoky NS, Taheri Y, El Beyrouthy M, Martorell M, Ostrander EA, Suleria HAR, Cho WC, Maroyi A, Martins N. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol 2020;11:01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Khan MN, Haggag YA, Lane ME, McCarron PA, Tambuwala MM. Polymeric nano-encapsulation of curcumin enhances its anti-cancer activity in breast (MDA-MB231) and lung (A549) cancer cells through reduction in expression of HIF-1alpha and nuclear p65 (Rel A). Curr Drug Deliv 2018;15:286–95 [DOI] [PubMed] [Google Scholar]
- 60. Kundu M, Sadhukhan P, Ghosh N, Chatterjee S, Manna P, Das J, Sil PC. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J Adv Res 2019;18:161–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gupta A, Briffa SM, Swingler S, Gibson H, Kannappan V, Adamus G, Kowalczuk M, Martin C, Radecka I. Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromolecules 2020;21:1802–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chaubey P, Patel RR, Mishra B. Development and optimization of curcumin-loaded mannosylated chitosan nanoparticles using response surface methodology in the treatment of visceral leishmaniasis. Expert Opin Drug Deliv 2014;11:1163–81 [DOI] [PubMed] [Google Scholar]
- 63. Niranjan R, Kaushik M, Prakash J, Venkataprasanna KS, Prema D, Christy A, Pannerselvam B, Devanand Venkatasubbu G. Chitosan based wound dressing patch loaded with curcumin tagged ZnO nanoparticles for potential wound healing application. Inorg Chem Commun 2023;154:110885 [Google Scholar]
- 64. Chang CZ, Wu SC, Lin CL, Kwan AL. Curcumin, encapsulated in nano-sized PLGA, down-regulates nuclear factor kappaB (p65) and subarachnoid hemorrhage induced early brain injury in a rat model. Brain Res 2015;1608:215–24 [DOI] [PubMed] [Google Scholar]
- 65. Zhang ZY, Jiang M, Fang J, Yang MF, Zhang S, Yin YX, Li DW, Mao LL, Fu XY, Hou YJ, Fu XT, Fan CD, Sun BL. Enhanced therapeutic potential of nano-curcumin against subarachnoid hemorrhage-induced blood-brain barrier disruption through inhibition of inflammatory response and oxidative stress. Mol Neurobiol 2017;54:1–14 [DOI] [PubMed] [Google Scholar]
- 66. Gao C, Wang Y, Sun J, Han Y, Gong W, Li Y, Feng Y, Wang H, Yang M, Li Z, Yang Y, Gao C. Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimer’s disease mice. Acta Biomater 2020;108:285–99 [DOI] [PubMed] [Google Scholar]
- 67. Parikh A, Kathawala K, Li J, Chen C, Shan Z, Cao X, Zhou XF, Garg S. Curcumin-loaded self-nanomicellizing solid dispersion system: part II: in vivo safety and efficacy assessment against behavior deficit in Alzheimer disease. Drug Deliv Transl Res 2018;8:1406–20 [DOI] [PubMed] [Google Scholar]
- 68. Zhang N, Yan F, Liang X, Wu M, Shen Y, Chen M, Xu Y, Zou G, Jiang P, Tang C, Zheng H, Dai Z. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy. Theranostics 2018;8:2264–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Orunoglu M, Kaffashi A, Pehlivan SB, Sahin S, Soylemezoglu F, Oguz KK, Mut M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater Sci Eng C Mater Biol Appl 2017;78:32–8 [DOI] [PubMed] [Google Scholar]
- 70. Huang N, Lu S, Liu XG, Zhu J, Wang YJ, Liu RT. PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Abeta generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer’s disease mice. Oncotarget 2017;8:81001–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Valizadeh H, Abdolmohammadi-Vahid S, Danshina S, Ziya Gencer M, Ammari A, Sadeghi A, Roshangar L, Aslani S, Esmaeilzadeh A, Ghaebi M, Valizadeh S, Ahmadi M. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol 2020;89:107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bateni Z, Rahimi HR, Hedayati M, Afsharian S, Goudarzi R, Sohrab G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: a randomized, double-blind clinical trial. Phytother Res 2021;35:3945–53 [DOI] [PubMed] [Google Scholar]
- 73. Niranjan R, Kaushik M, Prakash J, Venkataprasanna KS, Arpana C, Balashanmugam P, Venkatasubbu GD. Enhanced wound healing by PVA/Chitosan/curcumin patches: in vitro and in vivo study. Colloids Surf B Biointerfaces 2019;182:110339. [DOI] [PubMed] [Google Scholar]
- 74. Bajpai SK, Ahuja S, Chand N, Bajpai M. Nano cellulose dispersed chitosan film with Ag NPs/Curcumin: an in vivo study on albino rats for wound dressing. Int J Biol Macromol 2017;104:1012–9 [DOI] [PubMed] [Google Scholar]
- 75. Asadi S, Gholami MS, Siassi F, Qorbani M, Sotoudeh G. Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res 2020;34:896–903 [DOI] [PubMed] [Google Scholar]
- 76. Abdolahi M, Karimi E, Sarraf P, Tafakhori A, Siri G, Salehinia F, Sedighiyan M, Asanjarani B, Badeli M, Abdollahi H, Yoosefi N, Yousefi A, Rad AS, Djalali M. The omega-3 and nano-curcumin effects on vascular cell adhesion molecule (VCAM) in episodic migraine patients: a randomized clinical trial. BMC Res Notes 2021;14:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jazayeri-Tehrani SA, Rezayat SM, Mansouri S, Qorbani M, Alavian SM, Daneshi-Maskooni M, Hosseinzadeh-Attar MJ. Nano-curcumin improves glucose indices, lipids, inflammation, and nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutr Metab 2019;16:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lu W, Tang H, Li S, Bai L, Chen Y. Extracellular vesicles as potential biomarkers and treatment options for liver failure: a systematic review up to March 2022. Front Immunol 2023;14:1116518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Homma K, Bazhanov N, Hashimoto K, Shimizu M, Heathman T, Hao Q, Nawgiri R, Muthukumarana V, Lee JW, Prough DS, Enkhbaatar P. Mesenchymal stem cell-derived exosomes for treatment of sepsis. Front Immunol 2023;14:1136964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. He X, Wang Y, Liu Z, Weng Y, Chen S, Pan Q, Li Y, Wang H, Lin S, Yu H. Osteoporosis treatment using stem cell-derived exosomes: a systematic review and meta-analysis of preclinical studies. Stem Cell Res Ther 2023;14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. He X, Zhang C, Amirsaadat S, Jalil AT, Kadhim MM, Abasi M, Pilehvar Y. Curcumin-loaded mesenchymal stem cell-derived exosomes efficiently attenuate proliferation and inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes. Appl Biochem Biotechnol 2023;195:51–67 [DOI] [PubMed] [Google Scholar]
- 82. Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci 2014;107:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhou L, Kodidela S, Godse S, Thomas-Gooch S, Kumar A, Raji B, Zhi K, Kochat H, Kumar S. Targeted drug delivery to the central nervous system using extracellular vesicles. Pharmaceuticals 2022;15:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Upadhya R, Shetty AK. Extracellular vesicles for the diagnosis and treatment of Parkinson’s disease. Aging Dis 2021;12:1438–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu L, Li Y, Peng H, Liu R, Ji W, Shi Z, Shen J, Ma G, Zhang X. Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing alpha-synuclein and immune activation of Parkinson’s disease. Sci Adv 2020;6:eaba3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mobahat M, Sadroddiny E, Nooshabadi VT, Ebrahimi-Barough S, Goodarzi A, Malekshahi ZV, Ai J. Curcumin-loaded human endometrial stem cells derived exosomes as an effective carrier to suppress alpha-synuclein aggregates in 6OHDA-induced Parkinson’s disease mouse model. Cell Tissue Bank 2023;24:75–91 [DOI] [PubMed] [Google Scholar]
- 87. Wang H, Sui H, Zheng Y, Jiang Y, Shi Y, Liang J, Zhao L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3beta pathway. Nanoscale 2019;11:7481–96 [DOI] [PubMed] [Google Scholar]
- 88. Fernandes M, Lopes I, Magalhaes L, Sarria MP, Machado R, Sousa JC, Botelho C, Teixeira J, Gomes AC. Novel concept of exosome-like liposomes for the treatment of Alzheimer’s disease. J Control Release 2021;336:130–43 [DOI] [PubMed] [Google Scholar]
- 89. He R, Jiang Y, Shi Y, Liang J, Zhao L. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater Sci Eng C Mater Biol Appl 2020;117:111314. [DOI] [PubMed] [Google Scholar]
- 90. Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018;150:137–49 [DOI] [PubMed] [Google Scholar]
- 91. Crowe TP, Hsu WH. Evaluation of recent intranasal drug delivery systems to the central nervous system. Pharmaceutics 2022;14:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kumar A, Zhou L, Godse S, Sinha N, Ma D, Parmar K, Kumar S. Intranasal delivery of darunavir improves brain drug concentrations in mice for effective HIV treatment. Biochem Biophys Rep 2023;33:101408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kozlovskaya L, Abou-Kaoud M, Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release 2014;189:133–40 [DOI] [PubMed] [Google Scholar]
- 94. Kumar NN, Lochhead JJ, Pizzo ME, Nehra G, Boroumand S, Greene G, Thorne RG. Delivery of immunoglobulin G antibodies to the rat nervous system following intranasal administration: distribution, dose-response, and mechanisms of delivery. J Control Release 2018;286:467–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Craft S, Raman R, Chow TW, Rafii MS, Sun CK, Rissman RA, Donohue MC, Brewer JB, Jenkins C, Harless K, Gessert D, Aisen PS. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol 2020;77:1099–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Detyniecki K, Van Ess PJ, Sequeira DJ, Wheless JW, Meng TC, Pullman WE. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters-a randomized, double-blind, placebo-controlled trial. Epilepsia 2019;60:1797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shinde RL, Devarajan PV. Docosahexaenoic acid-mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv 2017;24:152–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen X, Zhi F, Jia X, Zhang X, Ambardekar R, Meng Z, Paradkar AR, Hu Y, Yang Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J Pharm Pharmacol 2013;65:807–16 [DOI] [PubMed] [Google Scholar]
- 99. Dende C, Meena J, Nagarajan P, Nagaraj VA, Panda AK, Padmanaban G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in experimental cerebral malaria. Sci Rep 2017;7:10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Busari ZA, Dauda KA, Morenikeji OA, Afolayan F, Oyeyemi OT, Meena J, Sahu D, Panda AK. Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticles. Front Pharmacol 2017;8:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sun L, Liu Z, Wang L, Cun D, Tong HHY, Yan R, Chen X, Wang R, Zheng Y. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J Control Release 2017;254:44–54 [DOI] [PubMed] [Google Scholar]
- 102. Anwar SK, Elmonaem SNA, Moussa E, Aboulela AG, Essawy MM. Curcumin nanoparticles: the topical antimycotic suspension treating oral candidiasis. Odontology 2023;111:350–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang W, Zhu R, Xie Q, Li A, Xiao Y, Li K, Liu H, Cui D, Chen Y, Wang S. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomedicine 2012;7:3667–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Su X, Jing X, Jiang W, Li M, Liu K, Teng M, Ma Y, Wang D, Meng L, Zhang Y, Ji W. Polyphosphazene nanodrugs for targeting delivery and inflammation responsive release of curcumin to treat acute lung injury by effectively inhibiting cytokine storms. Colloids Surf B Biointerfaces 2023;229:113446. [DOI] [PubMed] [Google Scholar]
- 105. Ghasemi SZ, Beigoli S, Behrouz S, Gholamnezhad Z, Mohammadian Roshan N, Boskabady MH. Evaluation of nano-curcumin against inhaled paraquat-induced lung injury in rats. Pharmacol Rep 2023;75:671–81 [DOI] [PubMed] [Google Scholar]
- 106. Kim M, Kim S-B, Kim J, Kim K-S, Kim D-E. Co-delivery of curcumin and PTTG1 siRNA by galactose receptor-targeted liposomes for enhanced anti-tumor effects in hepatocellular carcinoma. J Drug Deliv Sci Technol 2023;86:104692 [Google Scholar]
- 107. Song JW, Liu YS, Guo YR, Zhong WX, Guo YP, Guo L. Nano-liposomes double loaded with curcumin and tetrandrine: preparation, characterization, hepatotoxicity and anti-tumor effects. Int J Mol Sci 2022;23:6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zaman MS, Chauhan N, Yallapu MM, Gara RK, Maher DM, Kumari S, Sikander M, Khan S, Zafar N, Jaggi M, Chauhan SC. Curcumin nanoformulation for cervical cancer treatment. Sci Rep 2016;6:20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Matloubi Z, Hassan Z. HSA-curcumin nanoparticles: a promising substitution for curcumin as a cancer chemoprevention and therapy. Daru 2020;28:209–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ali I, Ahmed SBM, Elhaj BM, Ali HS, Alsubaie A, Almalki ASA. Enhanced anticancer activities of curcumin-loaded green gum acacia-based silver nanoparticles against melanoma and breast cancer cells. Applied Nanoscience 2021;11:2679–87 [Google Scholar]
- 111. Sabourian P, Ji J, Lotocki V, Moquin A, Hanna R, Frounchi M, Maysinger D, Kakkar A. Facile design of autogenous stimuli-responsive chitosan/hyaluronic acid nanoparticles for efficient small molecules to protein delivery. J Mater Chem B 2020;8:7275–87 [DOI] [PubMed] [Google Scholar]
- 112. Zhang Z, Zhang X. Curcumin loading on alginate nano-micelle for anti-infection and colonic wound healing. J Biomed Nanotechnol 2021;17:1160–9 [DOI] [PubMed] [Google Scholar]
- 113. Kang BK, Yu Z, Chen W, Jiang T, Shim YH, Gao J, Zhou G, Cao D. Using gelatin/curcumin nano-fiber membranes as scaffolds in a subcutaneous model for tissue engineered cartilages. Cell Tissue Bank 2021;22:443–51 [DOI] [PubMed] [Google Scholar]
- 114. Dai X, Liu J, Zheng H, Wichmann J, Hopfner U, Sudhop S, Prein C, Shen Y, Machens H-G, Schilling AF. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater 2017;9:e368–168 [Google Scholar]
- 115. Marulasiddeshwara R, Jyothi MS, Soontarapa K, Keri RS, Velmurugan R. Nonwoven fabric supported, chitosan membrane anchored with curcumin/TiO(2) complex: scaffolds for MRSA infected wound skin reconstruction. Int J Biol Macromol 2020;144:85–93 [DOI] [PubMed] [Google Scholar]
- 116. Mirzahosseinipour M, Khorsandi K, Hosseinzadeh R, Ghazaeian M, Shahidi FK. Antimicrobial photodynamic and wound healing activity of curcumin encapsulated in silica nanoparticles. Photodiagnosis Photodyn Ther 2020;29:101639. [DOI] [PubMed] [Google Scholar]
- 117. Lee HJ, Jeong M, Na YG, Kim SJ, Lee HK, Cho CW. An EGF- and curcumin-co-encapsulated nanostructured lipid carrier accelerates chronic-wound healing in diabetic rats. Molecules 2020;25:4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Krausz AE, Adler BL, Cabral V, Navati M, Doerner J, Charafeddine RA, Chandra D, Liang H, Gunther L, Clendaniel A, Harper S, Friedman JM, Nosanchuk JD, Friedman AJ. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine 2015;11:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nguyen TP, Wilczewski S, Lewandowski J, Majkowska-Pilip A, Żelechowska-Matysiak K, Nieciecka D, Studziński W, Olusegun SJ, Syczewski M, Giersig M, Dinh TMT, Krysiński P, Osial M. 5-fluorouracil and curcuminoids extract from Curcuma longa L. loaded into nanohydroxyapatite as a drug delivery carrier for SKOV-3 and Hepg2 cancer cells treatment. Ceramics International 2023;49:25775–87 [Google Scholar]
- 120. Sadraei MR, Tavalaee M, Forouzanfar M, Nasr-Esfahani MH. Effect of curcumin, and nano-curcumin on sperm function in varicocele rat model. Andrologia 2022;54:e14282 [DOI] [PubMed] [Google Scholar]
- 121. Nasr M, Ahmed-Farid OAH, Ahmed RF. Curcumin-resveratrol nano-formulation counteracting hyperammonemia in rats. Metab Brain Dis 2023;38:1365–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ganugula R, Arora M, Jaisamut P, Wiwattanapatapee R, Jørgensen HG, Venkatpurwar VP, Zhou B, Rodrigues Hoffmann A, Basu R, Guo S, Majeti NVRK. Nano-curcumin safely prevents streptozotocin-induced inflammation and apoptosis in pancreatic beta cells for effective management of type 1 diabetes mellitus. Br J Pharmacol 2017;174:2074–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. El-Far YM, Zakaria MM, Gabr MM, El Gayar AM, Eissa LA, El-Sherbiny IM. Nanoformulated natural therapeutics for management of streptozotocin-induced diabetes: potential use of curcumin nanoformulation. Nanomedicine 2017;12:1689–711 [DOI] [PubMed] [Google Scholar]
- 124. Yang R, Zheng Y, Wang Q, Zhao L. Curcumin-loaded chitosan-bovine serum albumin nanoparticles potentially enhanced Abeta 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res Lett 2018;13:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ray B, Bisht S, Maitra A, Maitra A, Lahiri DK. Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc) in the neuronal cell culture and animal model: implications for Alzheimer’s disease. J Alzheimers Dis 2011;23:61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Giacomeli R, Izoton JC, Dos Santos RB, Boeira SP, Jesse CR, Haas SE. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by beta-amyloid 1-42 peptide in aged female mice. Brain Res 2019;1721:146325. [DOI] [PubMed] [Google Scholar]
- 127. Sintov AC. AmyloLipid nanovesicles: a self-assembled lipid-modified starch hybrid system constructed for direct nose-to-brain delivery of curcumin. Int J Pharm 2020;588:119725. [DOI] [PubMed] [Google Scholar]
- 128. Huo X, Zhang Y, Jin X, Li Y, Zhang L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid beta aggregation in Alzheimer’s disease. J Photochem Photobiol B 2019;190:98–102 [DOI] [PubMed] [Google Scholar]
- 129. Kundu P, Das M, Tripathy K, Sahoo SK. Delivery of dual drug loaded lipid based nanoparticles across the blood-brain barrier impart enhanced neuroprotection in a rotenone induced mouse model of Parkinson’s disease. ACS Chem Neurosci 2016;7:1658–70 [DOI] [PubMed] [Google Scholar]
- 130. Bollimpelli VS, Kumar P, Kumari S, Kondapi AK. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem Int 2016;95:37–45 [DOI] [PubMed] [Google Scholar]
- 131. Hassaniazad M, Inchehsablagh BR, Kamali H, Tousi A, Eftekhar E, Jaafari MR, Fathalipour M, Nikoofal-Sahlabadi S, Gouklani H, Alizade H, Nikpoor AR. The clinical effect of nano micelles containing curcumin as a therapeutic supplement in patients with COVID-19 and the immune responses balance changes following treatment: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Naeini F, Tutunchi H, Razmi H, Mahmoodpoor A, Vajdi M, Sefidmooye Azar P, Najifipour F, Tarighat-Esfanjani A, Karimi A. Does nano-curcumin supplementation improve hematological indices in critically ill patients with sepsis? A randomized controlled clinical trial. J Food Biochem 2022;46:e14093 [DOI] [PubMed] [Google Scholar]
- 133. Asadirad A, Nashibi R, Khodadadi A, Ghadiri AA, Sadeghi M, Aminian A, Dehnavi S. Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial. Phytother Res 2022;36:1023–31 [DOI] [PubMed] [Google Scholar]
- 134. Sedighiyan M, Abdolahi M, Jafari E, Vahabi Z, Sohrabi Athar S, Hadavi S, Narimani Zamanabadi M, Yekaninejad MS, Djalali M. The effects of nano-curcumin supplementation on adipokines levels in obese and overweight patients with migraine: a double blind clinical trial study. BMC Res Notes 2022;15:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Karimi A, Naeini F, Niazkar HR, Tutunchi H, Musazadeh V, Mahmoodpoor A, Asghariazar V, Mobasseri M, Tarighat-Esfanjani A. Nano-curcumin supplementation in critically ill patients with sepsis: a randomized clinical trial investigating the inflammatory biomarkers, oxidative stress indices, endothelial function, clinical outcomes and nutritional status. Food Funct 2022;13:6596–612 [DOI] [PubMed] [Google Scholar]
- 136. Talakesh T, Tabatabaee N, Atoof F, Aliasgharzadeh A, Sarvizade M, Farhood B, Najafi M. Effect of nano-curcumin on radiotherapy-induced skin reaction in breast cancer patients: a randomized, triple-blind, placebo-controlled trial. Curr Radiopharm 2022;15:332–40 [DOI] [PubMed] [Google Scholar]
- 137. Kia SJ, Basirat M, Mortezaie T, Moosavi MS. Comparison of oral nano-curcumin with oral prednisolone on oral lichen planus: a randomized double-blinded clinical trial. BMC Complement Med Ther 2020;20:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Parohan M, Sarraf P, Javanbakht MH, Foroushani AR, Ranji-Burachaloo S, Djalali M. The synergistic effects of nano-curcumin and coenzyme Q10 supplementation in migraine prophylaxis: a randomized, placebo-controlled, double-blind trial. Nutr Neurosci 2021;24:317–26 [DOI] [PubMed] [Google Scholar]
- 139. Djalali M, Abdolahi M, Hosseini R, Miraghajani M, Mohammadi H, Djalali M. The effects of nano-curcumin supplementation on Th2/tregulatory axis in migraine patients: a randomized, double-blind, placebo-controlled trial. Int J Neurosci 2023;133:169–75 [DOI] [PubMed] [Google Scholar]
- 140. Sedighiyan M, Jafari E, Athar SS, Yekaninejad MS, Alvandi E, Abdolahi M, Djalali M. The effects of nano-curcumin supplementation on leptin and adiponectin in migraine patients: a double-blind clinical trial study from gene expression to clinical symptoms. Endocr Metab Immune Disord Drug Targets 2023;23:711–20 [DOI] [PubMed] [Google Scholar]
- 141. Djalali M, Abdolahi M, Hosseini R, Miraghajani M, Mohammadi H, Djalali M. The effects of nano-curcumin supplementation on Th1/Th17 balance in migraine patients: a randomized controlled clinical trial. Complement Ther Clin Pract 2020;41:101256. [DOI] [PubMed] [Google Scholar]
- 142. Asadi S, Gholami MS, Siassi F, Qorbani M, Khamoshian K, Sotoudeh G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled clinical trial. Complement Ther Med 2019;43:253–60 [DOI] [PubMed] [Google Scholar]
- 143. Vafadar Afshar G, Rasmi Y, Yaghmaei P, Khadem-Ansari MH, Makhdomii K, Rasooli J. The effects of nano-curcumin supplementation on serum level of hs-CRP, adhesion molecules, and lipid profiles in hemodialysis patients, a randomized controlled clinical trial. Iran J Kidney Dis 2020;14:52–61 [PubMed] [Google Scholar]
- 144. Shafabakhsh R, Asemi Z, Reiner Z, Soleimani A, Aghadavod E, Bahmani F. The effects of nano-curcumin on metabolic status in patients with diabetes on hemodialysis, a randomized, double blind, placebo-controlled trial. Iran J Kidney Dis 2020;14:290–9 [PubMed] [Google Scholar]
- 145. Dastani M, Rahimi HR, Askari VR, Jaafari MR, Jarahi L, Yadollahi A, Rahimi VB. Three months of combination therapy with nano-curcumin reduces the inflammation and lipoprotein (a) in type 2 diabetic patients with mild to moderate coronary artery disease: evidence of a randomized, double-blinded, placebo-controlled clinical trial. Biofactors 2023;49:108–18 [DOI] [PubMed] [Google Scholar]
- 146. Honarvar NM, Soveid N, Abdolahi M, Djalali M, Hatami M, Karzar NH. Anti-neuroinflammatory properties of n-3 fatty acids and nano-curcumin on migraine patients from cellular to clinical insight: a randomized, double-blind and placebo-controlled trial. Endocr Metab Immune Disord Drug Targets 2021;21:365–73 [DOI] [PubMed] [Google Scholar]
- 147. Helli B, Gerami H, Kavianpour M, Heybar H, Hosseini SK, Haghighian HK. Curcumin nanomicelle improves lipid profile, stress oxidative factors and inflammatory markers in patients undergoing coronary elective angioplasty; a randomized clinical trial. Endocr Metab Immune Disord Drug Targets 2021;21:2090–8 [DOI] [PubMed] [Google Scholar]
- 148. Abdolahi M, Jafarieh A, Sarraf P, Sedighiyan M, Yousefi A, Tafakhori A, Abdollahi H, Salehinia F, Djalali M. The neuromodulatory effects of omega-3 fatty acids and nano-curcumin on the COX-2/ iNOS network in migraines: a clinical trial study from gene expression to clinical symptoms. Endocr Metab Immune Disord Drug Targets 2019;19:874–84 [DOI] [PubMed] [Google Scholar]
- 149. Abdolahi M, Sarraf P, Javanbakht MH, Honarvar NM, Hatami M, Soveyd N, Tafakhori A, Sedighiyan M, Djalali M, Jafarieh A, Masoudian Y, Djalali M. A novel combination of omega-3 fatty acids and nano-curcumin modulates interleukin-6 gene expression and high sensitivity C-reactive protein serum levels in patients with migraine: a randomized clinical trial study. CNS Neurol Disord Drug Targets 2018;17:430–8 [DOI] [PubMed] [Google Scholar]
- 150. Abdolahi M, Tafakhori A, Togha M, Okhovat AA, Siassi F, Eshraghian MR, Sedighiyan M, Djalali M, Mohammadzadeh Honarvar N, Djalali M. The synergistic effects of omega-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-alpha gene expression and serum level in migraine patients. Immunogenetics 2017;69:371–8 [DOI] [PubMed] [Google Scholar]
- 151. Soveyd N, Abdolahi M, Djalali M, Hatami M, Tafakhori A, Sarraf P, Honarvar NM. The combined effects of omega -3 fatty acids and nano-curcumin supplementation on intercellular adhesion molecule-1 (ICAM-1) gene expression and serum levels in migraine patients. CNS Neurol Disord Drug Targets 2018;16:1120–6 [DOI] [PubMed] [Google Scholar]
- 152. Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull 2011;34:660–5 [DOI] [PubMed] [Google Scholar]
- 153. Ramezani V, Ghadirian S, Shabani M, Boroumand MA, Daneshvar R, Saghafi F. Efficacy of curcumin for amelioration of radiotherapy-induced oral mucositis: a preliminary randomized controlled clinical trial. BMC Cancer 2023;23:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bakhshi M, Mahboubi A, Jaafari MR, Ebrahimi F, Tofangchiha M, Alizadeh A. Comparative efficacy of 1% curcumin nanomicelle gel and 2% curcumin gel for treatment of recurrent aphthous stomatitis: a double-blind randomized clinical trial. J Evid Based Dent Pract 2022;22:101708. [DOI] [PubMed] [Google Scholar]
- 155. Hassaniazad M, Eftekhar E, Inchehsablagh BR, Kamali H, Tousi A, Jaafari MR, Rafat M, Fathalipour M, Nikoofal-Sahlabadi S, Gouklani H, Alizade H, Nikpoor AR. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother Res 2021;35:6417–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hashemzadeh K, Davoudian N, Jaafari MR, Mirfeizi Z. The effect of nanocurcumin in improvement of knee osteoarthritis: a randomized clinical trial. Curr Rheumatol Rev 2020;16:158–64 [DOI] [PubMed] [Google Scholar]
- 157. Talebi S, Day AS, Safarian M, Sayedi SJ, Jaafari MR, Abbasi Z, Barghchi H, Kianifar HR. Adjunctive nano-curcumin therapy improves inflammatory and clinical indices in children with cystic fibrosis: a randomized clinical trial. Food Sci Nutr 2023;11:3348–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chegini M, Sadeghi A, Zaeri F, Zamani M, Hekmatdoost A. Nano-curcumin supplementation in patients with mild and moderate acute pancreatitis: a randomized, placebo-controlled trial. Phytother Res. Epub ahead of print 25 July 2023. DOI: 10.1002/ptr.7958. [DOI] [PubMed] [Google Scholar]
- 159. Karami AA, Zameni H, Salehi M, Mirhashemi SM. The nano-micellar curcumin improves International Prostate Symptoms Score (IPSS) in patients with benign prostatic hyperplasia: a randomized clinical trial. World J Urol 2023;41:2465–71 [DOI] [PubMed] [Google Scholar]
- 160. Sadeghizadeh M, Asadollahi E, Jahangiri B, Yadollahzadeh M, Mohajeri M, Afsharpad M, Najafi F, Rezaie N, Eskandari M, Tavakoli-Ardakani M, Feizabadi F, Masjedi MR. Promising clinical outcomes of nano-curcumin treatment as an adjunct therapy in hospitalized COVID-19 patients: a randomized, double-blinded, placebo-controlled trial. Phytother Res 2023;37:3631–44 [DOI] [PubMed] [Google Scholar]
- 161. Deb S, Bhargava D, Bansal P, Kanuru V. Role of nano curcumin on superoxide dismutase levels in leukoplakia. J Oral Maxillofac Pathol 2022;26:21–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Osali A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol Metab Syndr 2020;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]