Abstract
Arthritogenic alphaviruses are mosquito-borne viruses that cause a debilitating rheumatic disease characterized by fever, headache, rash, myalgia, and polyarthralgia with the potential to evolve into a severe and very prolonged illness. Although these viruses have been geographically restricted by vector hosts and reservoirs, recent epidemics have revealed the risks of their spread worldwide. In this review, we aim to discuss the protective and pathological roles of macrophages during the development of arthritis caused by alphaviruses. The progression to the chronic phase of the disease is related to the extension of viral replication and the maintenance of articular inflammation, in which the cellular infiltrate is predominantly composed of macrophages. We explore the possible implications of macrophage polarization to M1/M2 activation phenotypes, drawing a parallel between alphavirus arthritis and rheumatoid arthritis (RA), a chronic inflammatory disease that also affects articular tissues. In RA, it is well established that M1 macrophages contribute to tissue damage and inflammation, while M2 macrophages have a role in cartilage repair, so modulating the M1/M2 macrophage ratio is being considered as a strategy in the treatment of this disease. In the case of alphavirus-induced arthritis, the picture is more complex, as proinflammatory factors derived from M1 macrophages contribute to the antiviral response but cause tissue damage, while M2 macrophages may contribute to tissue repair but impair viral clearance.
Keywords: Alphaviruses, arthritis, inflammation, macrophage, tissue damage and repair, antiviral response
Impact Statement
Arthritogenic alphaviruses may cause an incapacitating and long-lasting articular disease. The increasing number of outbreaks affecting millions of people worldwide makes these pathogens a major public health concern. Macrophages are known to play a central role in alphavirus-induced disease, but a comprehensive analysis of the inflammatory mediators produced by these cells during infection is lacking. Here, we bring new insights to this field, summarizing the stimuli to which macrophages are submitted in different phases of the disease. We also highlight the pathological and protective roles that M1 and M2 macrophage activation phenotypes can play in the onset, maintenance, or control of the disease. Modulating macrophage polarization is currently considered a strategy to treat rheumatoid arthritis, a chronic articular disease that shares several aspects with alphavirus-induced arthritis. Here, we call attention to the complexity and potential therapeutic aspects of modulating macrophage polarization during infection by alphaviruses.
Introduction
Alphavirus is a genus of the Togaviridae family, a group of enveloped, positive sense, single-stranded RNA arboviruses transmitted to humans through the bite of mosquitoes (mainly Aedes sp., Haemagogus sp., and Anopheles sp.) in a sylvatic and urban cycle involving vertebrate reservoirs. 1 These viruses are subgrouped into encephalitic and arthritogenic, according to the symptoms caused by the infection. The arthritogenic group comprises the Barmah Forest virus (BFV), Chikungunya virus (CHIKV), Mayaro virus (MAYV), O’nyong-Nyong virus (ONNV), Semliki Forest virus (SFV), Sindbis virus (SINV), and Ross River virus (RRV).2,3 Infections by these viruses are clearly spreading worldwide, 4 as shown in Figure 1, even though the number of cases reported is probably underestimated due to insufficient surveillance and lack of laboratory diagnostic tools in endemic countries, where a diagnosis is often based only on clinical symptoms that are similar to those caused by other arboviruses.5–8
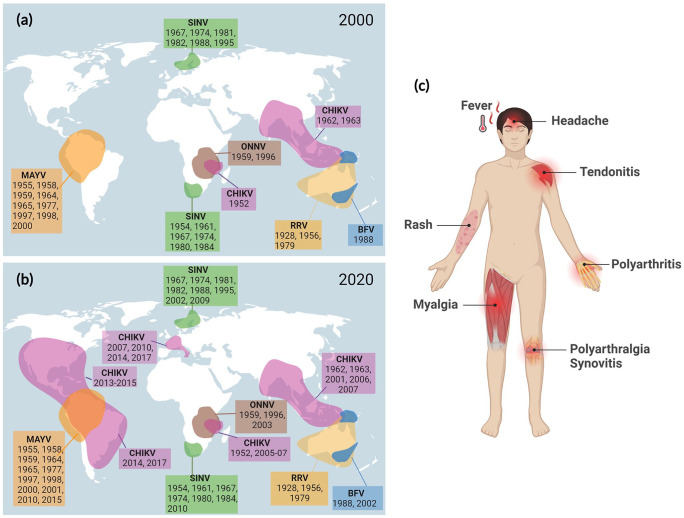
Figure 1.
Geographical distribution of arthritogenic alphaviruses with the years of the main outbreaks (a) until 2000 and (b) between 2001 and 2020. Figure adapted from Zaid A et al. 4 (c) Schematic illustration of the main clinical manifestations of arthritogenic alphavirus infections.
Source: Figure created using BioRender.com.
BFV: Barmah Forest virus; CHIKV: Chikungunya virus; MAYV: Mayaro virus; ONNV: O’nyong-nyong virus; RRV: Ross River virus; SINV: Sindbis virus.
The potential of alphaviruses to expand their geographical distribution is exemplified by the two global CHIKV epidemics. 7 Before the 2000s, CHIKV circulated mainly in sub-Saharan Africa and Southeast Asian regions, causing small local outbreaks. In 2004, the virus spread to the Comoros and La Reunion islands, infecting hundreds of thousands of people between 2005 and 2006, 9 and reached Asia and Europe, causing more than 6 million cases of infection. 7 The second major epidemic occurred in 2013, starting in the Caribbean and spreading throughout the American continent, causing more than 2 million cases of infection in 50 countries. 10 Between 2013 and 2022, Brazil was the most affected country in the Americas, with 1.5 million cases (45% of the confirmed cases), reaching, in 2022, 98.8% of all the cases in the American continent. 11 More recently, MAYV appeared as another potential candidate for spreading, broadening its endemic area as it adapted to infect Aedes aegypti mosquitoes.8,12,13
After inoculation by mosquito bites, arthritogenic alphaviruses disseminate through the microvasculature to the lymphoid tissues, liver, and spleen, then reach the muscles and joints.14,15 Viral replication in these tissues triggers an inflammatory response, resulting in symptoms that characterize the acute phase of the disease, including fever, rash, headache, myalgia, arthralgia, and arthritis (Figure 1). 15 Arthritis is the most prevalent clinical sign among the symptoms, and although it may be resolved in a few days after infection, in some individuals, the disease evolves into a severe and disabling condition, with movement restriction and persistent swelling and pain, which may persist for weeks, months, or even years.15–17 The progression to the chronic phase of the disease is associated with the extension of viral replication and the maintenance of articular inflammation, with the infiltration of immune cells in muscles, joints, and associated tissues.17,18 Experiments in animal models have demonstrated that the cellular infiltrate is composed mainly of mononuclear cells, comprising macrophages, monocytes, lymphocytes, and natural killer cells,19,20 with macrophages being the predominant cells.19,21 Although most of the studies on arthritogenic alphaviruses focus on the muscles and joints, macrophage infiltration along with tissue damage has already been reported in the brain and the liver of humans and animal models,22,23 highlighting other potential pathological roles of macrophages in alphavirus-induced disease. This review aims to discuss the protective and pathogenic roles of the macrophage present in infected tissues during alphavirus-induced arthritis.
Macrophage plasticity and their activation phenotypes
Macrophages are highly plastic cells that can differentiate into distinct activation phenotypes depending on the stimuli they receive from the environment. 24 Th1 lymphocyte–derived cytokines, such as interferon gamma (IFN-γ), induce macrophage polarization to a proinflammatory phenotype, termed M1 or “classically activated,” which is known to play roles in the inflammation onset and maintenance and are usually proinjurious.24,25 However, anti-inflammatory cytokines derived from Th2 lymphocytes, such as IL-13, IL-4, and IL-10, favor polarization to a tissue repair profile, referred to as M2 or “alternatively activated.”25,26 It is currently known that the M2 phenotype consists of a heterogeneous population of anti-inflammatory and tissue modeling macrophages, which have been classified into specific subtypes that display different functions depending on the differentiation stimuli.24,25 Among these subtypes, resolution-promoting macrophages (Mres) specifically act on tissue regeneration and homeostasis.25,26
Several studies have demonstrated that arthritogenic alphaviruses can establish a productive and persistent infection in macrophages.14,27,28 Therefore, during alphavirus-induced arthritis, macrophages are not only exposed to the inflammatory environment stimuli but are also targets for viral infection, adding another layer of complexity to their polarization phenotype. Virus replication results in macrophage overactivation with the production of several inflammatory mediators that lead to tissue damage. 29 For instance, in vitro studies with CHIKV and RRV have shown that macrophage infection induces the production of IL-1β, IL-6, TNF, and IFN-γ.30,31 This is consistent with the detection of increased serum levels of IL-1β and IL-6 in CHIKV-infected patients who developed more severe disease. 32 TNF and IL-6 are also secreted after MAYV and SINV infection in cell lines and primary cultures of human and mouse macrophages.33,34 These proinflammatory features observed during macrophage infection in vitro suggest an M1-like activation pattern. However, understanding macrophage polarization in vivo is much more complex as these cells are exposed to multiple stimuli and to changes in the cytokine profile as the disease progresses. Therefore, overlapping phenotypes may coexist.
Macrophage infiltration in the pathogenesis of alphavirus infection
Macrophage infiltration in the muscles and joints has a central role in the pathogenesis of alphavirus-induced disease by promoting tissue inflammation and damage. 29 Several studies have shown that the macrophage-recruiting chemokines CCL2 and macrophage inhibitory factor (MIF), key molecules in the regulation of immune cell infiltration, are produced during alphavirus infection both in animal models and in cultured macrophages.29,33,35,36 Thus, in addition to being chemoattracted by CCL2 and MIF, macrophages are also the primary source of these chemokines,37,38 thus amplifying the recruitment of the inflammatory infiltrate. Accordingly, the administration of bindarit, an inhibitor of CCL2 production, to CHIKV-infected mice reduced macrophage infiltration in the joints and bone loss.18,39 In addition, in MIF-deficient mice infected with RRV, inflammation, cellular infiltration, and muscle damage were mitigated, while the reconstitution of MIF levels exacerbated the disease’s severity. 40 Together, these results reinforce the proinjurious features of the macrophages in the alphavirus-induced disease.
The correlation between macrophage infiltration and muscle pathology is also supported by the observation that the administration of macrophage-toxic agents (such as silica) in mice prior to RRV infection abrogated the muscle damage observed during the disease. 41 The observations that macrophage-depleted mice displayed lower levels of the inflammatory cytokines TNF and IFN-γ and that either macrophage depletion or direct inhibition of these inflammatory mediators attenuated the severity of RRV-induced arthritis and myositis29,41 imply that macrophages contribute to tissue damage primarily through the production of proinflammatory cytokines.
In addition to the macrophage contribution to the development of arthritis and myositis during alphavirus infection, the role of these cells in the antiviral response has also been reported. For example, macrophage-depleted mice displayed prolonged viremia upon CHIKV infection. 21 Furthermore, the depletion of inflammatory monocytes expressing the CCL2 receptor (CCR2) promoted more severe disease and higher viral loads in mouse models of CHIKV and RRV infection. 42 Collectively, these data suggest that macrophage polarization to a proinflammatory profile in the acute phase of alphavirus-induced disease contributes to the pathogenesis but may also have a role in restricting viral replication.
Paradoxically, macrophages can also act as viral reservoirs in the late stages of alphavirus-induced arthritis, thus assuming distinct roles in the early or late stages of the disease. Studies using a nonhuman primate model of CHIKV infection detected viral RNA and antigens in macrophages even after 3 months of viral inoculation. 14 Furthermore, a cohort study of CHIKV-infected patients detected the presence of CHIKV RNA and proteins in the synovial macrophages of a patient 18 months after the first symptoms of the disease, suggesting that macrophages’ persistent infection may contribute to the development of the chronic phase of CHIKV-induced arthritis. 43
Taken together, these results indicate that macrophages’ contribution to alphavirus pathogenesis is closely intertwined with their activation phenotype, which may be influenced by changes in the tissue microenvironment as the disease progresses.
Macrophage roles in tissue repair and inflammation control
Although the inflammatory mediators released by macrophages during alphavirus infection contribute to the onset of arthritis, evidence supports that these cells also play a role in inflammation control and tissue repair.29,44,45 Despite some differences in the cytokine profile observed in humans and animal models, the initial stage of alphavirus-induced diseases is usually characterized by an increase in the serum levels of proinflammatory cytokines, including TNF, IL-6, and IFN-γ,36,46–48 thus favoring M1 macrophage polarization. Although the serum levels of some proinflammatory cytokines remain elevated as the disease progresses, anti-inflammatory cytokines, such as IL-4, IL-10, and IL-13, are found at higher levels than those observed in the acute phase.36,46–49
This pattern of a proinflammatory profile in the initial stages of the infection followed by an anti-inflammatory response is also illustrated in a study with CHIKV-infected patients, which found elevated IL-10 levels along with an increased ratio of peripheral regulatory T cells (Treg) over effector T cells (Teff) as the disease progressed to its resolution. 50 These changes in the cytokine profile and T cell populations in the late stages of the disease seem to have important roles in suppressing the inflammatory process. Treg cells can potentially promote M2 macrophage polarization through the secretion of anti-inflammatory cytokines. 51 IL-10 can induce macrophage Mres phenotype and act as a potent inhibitor of the Th1 inflammatory response.25,52
In the case of RRV-induced myositis, the inflammatory monocyte population in the muscle tissue is replaced by macrophages expressing CX3CR1 as the disease progresses to its resolution. 44 This receptor plays a role in tissue repair and is typical of M2 macrophages. 53 Accordingly, CX3CR1-deficient mice infected with RRV showed more severe disease, with increased muscle tissue fibrosis. 44 Furthermore, the depletion of the inflammatory monocytes using immune-modifying particles (IMPs) ameliorated the disease signs and increased the number of CX3CR1 macrophages in the muscle tissues and the proportion of regenerating myofibers. This reinforces the role of CX3CR1+ macrophages in tissue homeostasis in the late stages of alphavirus-induced disease. 44
In mice lacking CCR2 infected with CHIKV, the monocyte/macrophage infiltrate is replaced by a neutrophil infiltrate, leading to more severe and prolonged arthritis. 45 In addition, macrophage-derived anti-inflammatory mediators, such as IL-10, Ym1, and arginase-1 (arg-1), were downregulated in CCR2-deficient mice compared to wild-type mice during CHIKV infection, 45 underlining the importance of the anti-inflammatory macrophages in tissue repair and inflammation control during CHIKV infection. However, genetic ablation of arg-1 in macrophages and neutrophils in mice increased viral clearance in the later stages of the disease, 54 suggesting that the anti-inflammatory properties of arg-1 expression may impair viral clearance. Overall, these studies highlight the dual role of anti-inflammatory mediators during alphavirus-induced disease, which can be beneficial in some situations, such as tissue repair, and detrimental in others, such as viral clearance.
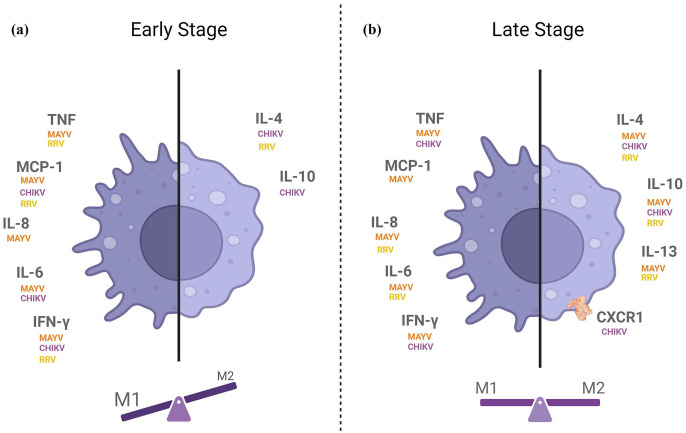
Although classifying macrophages into polarization profiles may help explain some inflammatory response patterns, it is common sense in the scientific community that this classification is a hypersimplification of the phenotypes that macrophages can assume. In the case of alphavirus infection, even though M1 stimuli are prominent over M2 in the disease onset, overlapping phenotypes of macrophages might be present at different ratios during the early or late stages of the disease (Figure 2).
Figure 2.
Profile of cytokine production during the (a) early and (b) late stages of alphavirus-induced arthritis, with the respective virus for which each cytokine was detected. Proinflammatory cytokines (TNF, CCL2, IL-8, IL-6, and IFN-γ) are represented on the left of each panel, and anti-inflammatory cytokines (IL-4, IL-13, and IL-10) and the M2 macrophage receptor CXCR1 are represented on the right of each panel. The balance scale illustrates the contribution of the macrophage phenotype in each disease stage.
Source: Figure created using BioRender.com.
MAYV: Mayaro virus; CHIKV: Chikungunya virus; RRV: Ross River virus.
While knowledge about macrophage polarization during alphavirus infection remains limited, a better understanding of this phenomenon has been attained within the context of rheumatoid arthritis (RA), a disease that shares similar features with alphavirus-induced arthritis. Diverse macrophage populations have demonstrated contrasting, yet pivotal, roles in shaping the progression of RA. The increase in the M1/M2 macrophage ratio contributes to the development of RA:55,56 while M1 macrophage overactivation causes tissue damage and bone loss, 55 M2 macrophages have a role in cartilage repair and inflammation control. 26 Modulating the ratio of M1/M2 macrophages is already being studied as a therapeutical strategy to treat RA. However, in the case of alphavirus infections, further studies are necessary to better understand the contribution of macrophage populations to the disease.
In conclusion, although macrophages can act as pathogenic effectors during alphavirus infection, their activation phenotype may affect their role in disease progression. Therefore, modulating macrophage activation phenotype may be a potential therapeutic strategy to treat alphavirus-induced arthritis.
Acknowledgments
The authors thank Dr Lorena de Oliveira Fernandes Siqueira for her help in figure design.
Footnotes
Authors’ contributions: All authors participated in the interpretation of the studies, design of the figures, conceptualization, and writing of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financed by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), grant numbers E-26/201.316/2016 and E-26/201.173/2021; and Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant number 312650/2021-3.
ORCID iD: Andrea T Da Poian  https://orcid.org/0000-0002-3969-704X
https://orcid.org/0000-0002-3969-704X
References
- 1. Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol 2004;2:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zacks MA, Paessler S. Encephalitic alphaviruses. Vet Microbiol 2010;140:281–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levi LI, Vignuzzi M. Arthritogenic alphaviruses: a worldwide emerging threat? Microorganisms 2019;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaid A, Burt FJ, Liu X, Poo YS, Zandi K, Suhrbier A, Weaver SC, Texeira MM, Mahalingam S. Arthritogenic alphaviruses: epidemiological and clinical perspective on emerging arboviruses. Lancet Infect Dis 2021;21:e123–33 [DOI] [PubMed] [Google Scholar]
- 5. Storm N, Weyer J, Markotter W, Kemp A, Leman PA, Dermaux-Msimang V, Nel LH, Paweska JT. Human cases of Sindbis fever in South Africa, 2006–2010. Epidemiol Infect 2014;142:234–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montalvo Zurbia-Flores G, Reyes-Sandoval A, Kim YC. Chikungunya virus: priority pathogen or passing trend? Vaccines 2023;11:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silva LA, Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest 2017;127:737–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Acosta-Ampudia Y, Monsalve DM, Rodríguez Y, Pacheco Y, Anaya J-M, Ramírez-Santana C. Mayaro: an emerging viral threat? Emerg Microbes Infect 2018;7:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Renault P, Solet JL, Sissoko D, Balleydier E, Larrieu S, Filleul L, Lassalle C, Thiria J, Rachou E, de Valk H, Ilef D, Ledrans M, Quatresous I, Quenel P, Pierre V. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005-2006. Am J Trop Med Hyg 2007;77:727–31 [PubMed] [Google Scholar]
- 10. Zeller H, Van Bortel W, Sudre B. Chikungunya: its history in Africa and Asia and its spread to new regions in 2013-2014. J Infect Dis 2016;214:S436–S440 [DOI] [PubMed] [Google Scholar]
- 11. Pan American Health Organization. Cases of Chikungunya Virus Disease. 2023, https://www3.paho.org/data/index.php/en/mnu-topics/chikv-en/550-chikv-weekly-en.html (accessed on 1 September 2023)
- 12. Maia LMS, Bezerra MCF, Costa MCS, Souza EM, Oliveira MEB, Ribeiro ALM, Miyazaki RD, Slhessarenko RD. Natural vertical infection by dengue virus serotype 4, Zika virus and Mayaro virus in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus. Med Vet Entomol 2019;33:437–42 [DOI] [PubMed] [Google Scholar]
- 13. Diagne CT, Bengue M, Choumet V, Hamel R, Pompon J, Missé D. Mayaro virus pathogenesis and transmission mechanisms. Pathog Basel Switz 2020;9:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, de Lamballerie X, Suhrbier A, Cherel Y, Le Grand R, Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest 2010;120:894–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assunção-Miranda I, Cruz-Oliveira C, Da Poian AT. Molecular mechanisms involved in the pathogenesis of alphavirus-induced arthritis. Biomed Res Int 2013;2013:973516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sane J, Kurkela S, Desdouits M, Kalimo H, Mazalrey S, Lokki M-L, Vaheri A, Helve T, Törnwall J, Huerre M, Butler-Browne G, Ceccaldi P-E, Gessain A, Vapalahti O. Prolonged myalgia in Sindbis virus infection: case description and in vitro infection of myotubes and myoblasts. J Infect Dis 2012;206:407–14 [DOI] [PubMed] [Google Scholar]
- 17. Runowska M, Majewski D, Niklas K, Puszczewicz M. Chikungunya virus: a rheumatologist’s perspective. Clin Exp Rheumatol 2018;36:494–501 [PubMed] [Google Scholar]
- 18. Rulli NE, Guglielmotti A, Mangano G, Rolph MS, Apicella C, Zaid A, Suhrbier A, Mahalingam S. Amelioration of alphavirus-induced arthritis and myositis in a mouse model by treatment with bindarit, an inhibitor of monocyte chemotactic proteins. Arthritis Rheum 2009;60:2513–23 [DOI] [PubMed] [Google Scholar]
- 19. Morrison TE, Whitmore AC, Shabman RS, Lidbury BA, Mahalingam S, Heise MT. Characterization of Ross river virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J Virol 2006;80:737–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrison TE, Oko L, Montgomery SA, Whitmore AC, Lotstein AR, Gunn BM, Elmore SA, Heise MT. A mouse model of chikungunya virus–induced musculoskeletal inflammatory disease. Am J Pathol 2011;178:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, Schroder WA, Higgs S, Suhrbier A. Chikungunya virus arthritis in adult wild-type mice. J Virol 2010;84:8021–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corrêa DG, Freddi TAL, Werner H, Lopes FPPL, Moreira MEL, de Almeida Di Maio Ferreira FCP, de Andrade Lopes JM, Rueda-Lopes FC, da Cruz LCH., Jr. Brain MR imaging of patients with perinatal chikungunya virus infection. AJNR Am J Neuroradiol 2020;41:174–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chua HH, Abdul Rashid K, Law WC, Hamizah A, Chem YK, Khairul AH, Chua KB. A fatal case of chikungunya virus infection with liver involvement. Med J Malaysia 2010;65:83–4 [PubMed] [Google Scholar]
- 24. Lichtnekert J, Kawakami T, Parks WC, Duffield JS. Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol 2013;13:555–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ariel A, Serhan CN. New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front Immunol 2012;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dai M, Sui B, Xue Y, Liu X, Sun J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 2018;180:91–103 [DOI] [PubMed] [Google Scholar]
- 27. Linn ML, Aaskov JG, Suhrbier A. Antibody-dependent enhancement and persistence in macrophages of an arbovirus associated with arthritis. J Gen Virol 1996;77(Pt3):407–11 [DOI] [PubMed] [Google Scholar]
- 28. Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, Sol-Foulon N, Le Roux K, Prevost MC, Fsihi H, Frenkiel MP, Blanchet F, Afonso PV, Ceccaldi PE, Ozden S, Gessain A, Schuffenecker I, Verhasselt B, Zamborlini A, Saïb A, Rey FA, Arenzana-Seisdedos F, Desprès P, Michault A, Albert ML, Schwartz O. Characterization of reemerging chikungunya virus. Plos Pathog 2007;3:e89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lidbury BA, Rulli NE, Suhrbier A, Smith PN, McColl SR, Cunningham AL, Tarkowski A, van Rooijen N, Fraser RJ, Mahalingam S. Macrophage-derived proinflammatory factors contribute to the development of arthritis and myositis after infection with an arthrogenic alphavirus. J Infect Dis 2008;197:1585–93 [DOI] [PubMed] [Google Scholar]
- 30. Guerrero-Arguero I, Høj TR, Tass ES, Berges BK, Robison RA. A comparison of Chikungunya virus infection, progression, and cytokine profiles in human PMA-differentiated U937 and murine RAW264.7 monocyte derived macrophages. PLoS ONE 2020;15:e0230328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Danillo Lucas Alves E, Benedito Antonio Lopes da F. Characterization of the immune response following in vitro mayaro and chikungunya viruses (Alphavirus, Togaviridae) infection of mononuclear cells. Virus Res 2018;256:166–73 [DOI] [PubMed] [Google Scholar]
- 32. Ng LFP, Chow A, Sun Y-J, Kwek DJC, Lim P-L, Dimatatac F, Ng L-C, Ooi E-E, Choo K-H, Her Z, Kourilsky P, Leo Y-S. IL-1β, IL-6, and RANTES as biomarkers of chikungunya severity. Plos ONE 2009;4:e4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Assunção-Miranda I, Bozza MT, Da Poian AT. Pro-inflammatory response resulting from sindbis virus infection of human macrophages: implications for the pathogenesis of viral arthritis. J Med Virol 2010;82:164–74 [DOI] [PubMed] [Google Scholar]
- 34. Cavalheiro MG, Costa LS, Campos HS, Alves LS, Assunção-Miranda I, Poian AT. Macrophages as target cells for Mayaro virus infection: involvement of reactive oxygen species in the inflammatory response during virus replication. An Acad Bras Cienc 2016;88:1485–99 [DOI] [PubMed] [Google Scholar]
- 35. Kelvin AA, Banner D, Silvi G, Moro ML, Spataro N, Gaibani P, Cavrini F, Pierro A, Rossini G, Cameron MJ, Bermejo-Martin JF, Paquette SG, Xu L, Danesh A, Farooqui A, Borghetto I, Kelvin DJ, Sambri V, Rubino S. Inflammatory cytokine expression is associated with chikungunya virus resolution and symptom severity. Plos Negl Trop Dis 2011;5:e1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaaitanya IK, Muruganandam N, Sundaram SG, Kawalekar O, Sugunan AP, Manimunda SP, Ghosal SR, Muthumani K, Vijayachari P. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol 2011;24:265–71 [DOI] [PubMed] [Google Scholar]
- 37. Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 1994;179:1895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin Y, Gong J, Zhang M, Xue W, Barnes PF. Production of monocyte chemoattractant protein 1 in tuberculosis patients. Infect Immun 1998;66:2319–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen W, Foo S-S, Taylor A, Lulla A, Merits A, Hueston L, Forwood MR, Walsh NC, Sims NA, Herrero LJ, Mahalingam S. Bindarit, an inhibitor of monocyte chemotactic protein synthesis, protects against bone loss induced by chikungunya virus infection. J Virol 2015;89:581–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Herrero LJ, Nelson M, Srikiatkhachorn A, Gu R, Anantapreecha S, Fingerle-Rowson G, Bucala R, Morand E, Santos LL, Mahalingam S. Critical role for macrophage migration inhibitory factor (MIF) in Ross River virus-induced arthritis and myositis. Proc Natl Acad Sci 2011;108:12048–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lidbury BA, Simeonovic C, Maxwell GE, Marshall ID, Hapel AJ. Macrophage-induced muscle pathology results in morbidity and mortality for Ross River virus-infected mice. J Infect Dis 2000;181:27–34 [DOI] [PubMed] [Google Scholar]
- 42. Haist KC, Burrack KS, Davenport BJ, Morrison TE. Inflammatory monocytes mediate control of acute alphavirus infection in mice. PLoS Pathog 2017;13:e1006748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoarau J-J, Jaffar Bandjee M-C, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, Tallet F, Moiton MP, Gauzère BA, Bruniquet S, Jaffar Bandjee Z, Morbidelli P, Martigny G, Jolivet M, Gay F, Grandadam M, Tolou H, Vieillard V, Debré P, Autran B, Gasque P. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol Baltim Md 1950 2010;184:5914–27 [DOI] [PubMed] [Google Scholar]
- 44. Zaid A, Tharmarajah K, Mostafavi H, Freitas JR, Sheng K-C, Foo S-S, Chen W, Vider J, Liu X, West NP, Herrero LJ, Taylor A, Mackay LK, Getts DR, King NJC, Mahalingam S. Modulation of monocyte-driven myositis in alphavirus infection reveals a role for CX3CR1+ macrophages in tissue repair. Mbio 2020;11:e03353–10319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poo YS, Nakaya H, Gardner J, Larcher T, Schroder WA, Le TT, Major LD, Suhrbier A. CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J Virol 2014;88:6862–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Santos FM, Dias RS, de Oliveira MD, Costa ICTA, Fernandes LS, Pessoa CR, da Matta SLP, Costa VV, Souza DG, da Silva CC, de Paula SO. Animal model of arthritis and myositis induced by the Mayaro virus. Plos Negl Trop Dis 2019;13:e0007375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Santiago FW, Halsey ES, Siles C, Vilcarromero S, Guevara C, Silvas JA, Ramal C, Ampuero JS, Aguilar PV. Long-term arthralgia after mayaro virus infection correlates with sustained pro-inflammatory cytokine response. Plos Negl Trop Dis 2015;9:e0004104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Venugopalan A, Ghorpade RP, Chopra A. Cytokines in acute chikungunya. Plos ONE 2014;9:e111305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tappe D, Pérez-Girón JV, Gómez-Medina S, Günther S, Muñoz-Fontela C, Schmidt-Chanasit J. Increased proinflammatory cytokine levels in prolonged arthralgia in Ross river virus infection. Emerg Infect Dis 2017;23:702–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kulkarni SP, Ganu M, Jayawant P, Thanapati S, Ganu A, Tripathy AS. Regulatory T cells and IL-10 as modulators of chikungunya disease outcome: a preliminary study. Eur J Clin Microbiol Infect Dis 2017;36:2475–81 [DOI] [PubMed] [Google Scholar]
- 51. Hou X-X, Wang X-Q, Zhou W-J, Li D-J. Regulatory T cells induce polarization of pro-repair macrophages by secreting sFGL2 into the endometriotic milieu. Commun Biol 2021;4:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: the role of T regulatory cells. Immunology 2006;117:433–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ishida Y, Kimura A, Nosaka M, Kuninaka Y, Hemmi H, Sasaki I, Kaisho T, Mukaida N, Kondo T. Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of fibrocyte and M2 macrophage migration. Sci Rep 2017;7:16833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stoermer KA, Burrack A, Oko L, Montgomery SA, Borst LB, Gill RG, Morrison TE. Genetic ablation of arginase 1 in macrophages and neutrophils enhances clearance of an arthritogenic alphavirus. J Immunol Baltim Md 1950 2012;189:4047–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu W, Li X, Fang S, Zhang X, Wang Y, Zhang T, Li Z, Xu Y, Qu S, Liu C, Gao F, Pan H, Wang G, Li H, Sun B. Anti-citrullinated protein antibodies induce macrophage subset disequilibrium in RA patients. Inflammation 2015;38:2067–75 [DOI] [PubMed] [Google Scholar]
- 56. Fukui S, Iwamoto N, Takatani A, Igawa T, Shimizu T, Umeda M, Nishino A, Horai Y, Hirai Y, Koga T, Kawashiri S, Tamai M, Ichinose K, Nakamura H, Origuchi T, Masuyama R, Kosai K, Yanagihara K, Kawakami A. M1 and M2 monocytes in rheumatoid arthritis: a contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol 2018;8:1958. [DOI] [PMC free article] [PubMed] [Google Scholar]