Abstract
Objective
Cerebral venous sinus thrombosis (CVST) is one of the rare and severe complications of coronavirus disease 2019 (COVID-19) vaccines. CVST has also been reported to develop into dural arteriovenous fistula; however, there were no reports of dural arteriovenous fistula associated with COVID-19 vaccine-induced cerebral venous sinus thrombosis. Here, we describe a rare case of a transverse–sigmoid sinus dural arteriovenous fistula followed by CVST due to COVID-19 vaccination.
Case Presentation
A 70-year-old patient presented with headache five days after receiving a second dose of COVID-19 vaccine. MRI showed a CVST in the superior sagittal sinus, left transverse sinus, and left sigmoid sinus. His headache improved after the administration of anticoagulant therapy. Six months later, a similar headache recurred, and cerebral angiography demonstrated a dural arteriovenous fistula in the left transverse sigmoid sinus and convexity dural arteriovenous fistulas in the left parietal cortex. The patient was treated twice with two sessions of transarterial embolization, and the shunts were completely occluded. His symptoms improved, and he was discharged with a modified Rankin Scale score of 0.
Conclusion
Dural arteriovenous fistula can develop after CVST in association with COVID-19 vaccination.
Keywords: COVID-19, vaccine, dural arteriovenous fistula, cerebral venous sinus thrombosis
Introduction
Cerebral venous sinus thrombosis (CVST) has been reported to be one of the rare and severe complications of coronavirus disease 2019 (COVID-19) vaccines.1,2) Some reports indicate that it occurs less than once in 100000 doses after Oxford-AstraZeneca vaccine (AZD1222 [ChAdOx1]), an adenovirus vector vaccine, while others indicate that it occurs six times in 7 million doses after Johnson & Johnson COVID-19 vaccine (JNJ-78436735 [Ad26.COV2.S]), an adenovirus vector vaccine.3,4) Moreover, CVST after COVID-19 vaccination has been reported to occur more frequently with the adenovirus vector vaccine than with the messenger RNA (mRNA) vaccine.5) Schultz et al. reported that the incidence of CVST after adenovirus vector vaccination was approximately 10 times higher than that after mRNA vaccination.5)
Dural arteriovenous fistulas (DAVFs) are rare pathological shunts between arteries and dural veins. They were detected at an annual rate of 0.16 per 100000 individuals in a Scottish study.6) Majority of DAVFs are generally acquired idiopathic lesions.7) However, DAVF may also occur due to the following acquired causes: CVST, coagulation abnormalities, trauma, hormonal abnormalities, phlebitis, and venous hypertension.8,9)
CVST has also been reported to develop into DAVF; however, there were no reports of DAVF associated with COVID-19 vaccine-induced CVST. Here, we describe a rare case of transverse sigmoid sinus DAVF and convexity DAVF after CVST due to the mRNA COVID-19 vaccine.
Case Presentation
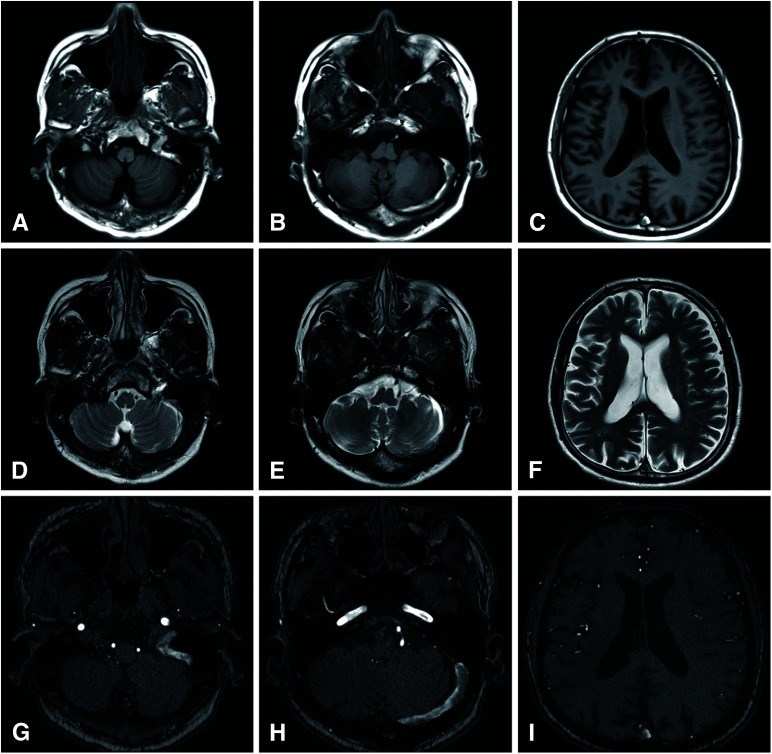
A 70-year-old patient with a medical history of diabetes mellitus received the Pfizer/BioNTech COVID-19 vaccine (BNT162b2) twice. Five days after the second vaccination, the patient presented with headache, and MRI T1- and T2-weighted imaging demonstrated abnormal signal intensity in the superior sagittal sinus (SSS), left transverse sinus, and left sigmoid sinus (Fig. 1A–1F). MRA revealed no abnormal vascular shunts (Fig. 1G–1I). The initial laboratory investigations, including the levels of platelets (25.9 × 104/μL), protein C activity, protein S activity, antiplatelet factor IV antibody, serum anti-proteinase 3 antineutrophil cytoplasmic antibody, myeloperoxidase-antineutrophil cytoplasmic antibodies, and cardiolipin Immunoglobulin G (IgG) were within normal limits, except for D-dimer (7.7 μg/mL). The patient was diagnosed with CVST in the SSS, left transverse sinus, and left sigmoid sinus and was hospitalized. The patient’s symptoms improved with anticoagulant therapy. Thus, the patient was discharged after 3 weeks of hospitalization. MRI was performed six months after the onset of CVST, suggesting residual thrombi in the SSS and transverse–sigmoid sinus. Subsequently, the patient became asymptomatic.
Fig. 1. MRI at diagnosis of CVST. T1-weighted image (A–C) and T2 weighted image (D–F) show the CVST in left transverse–sigmoid sinus and SSS. Time-of-flight MRA revealed no evidences of dural arteriovenous fistula (G–I). CVST: cerebral venous sinus thrombosis; SSS: superior sagittal sinus.
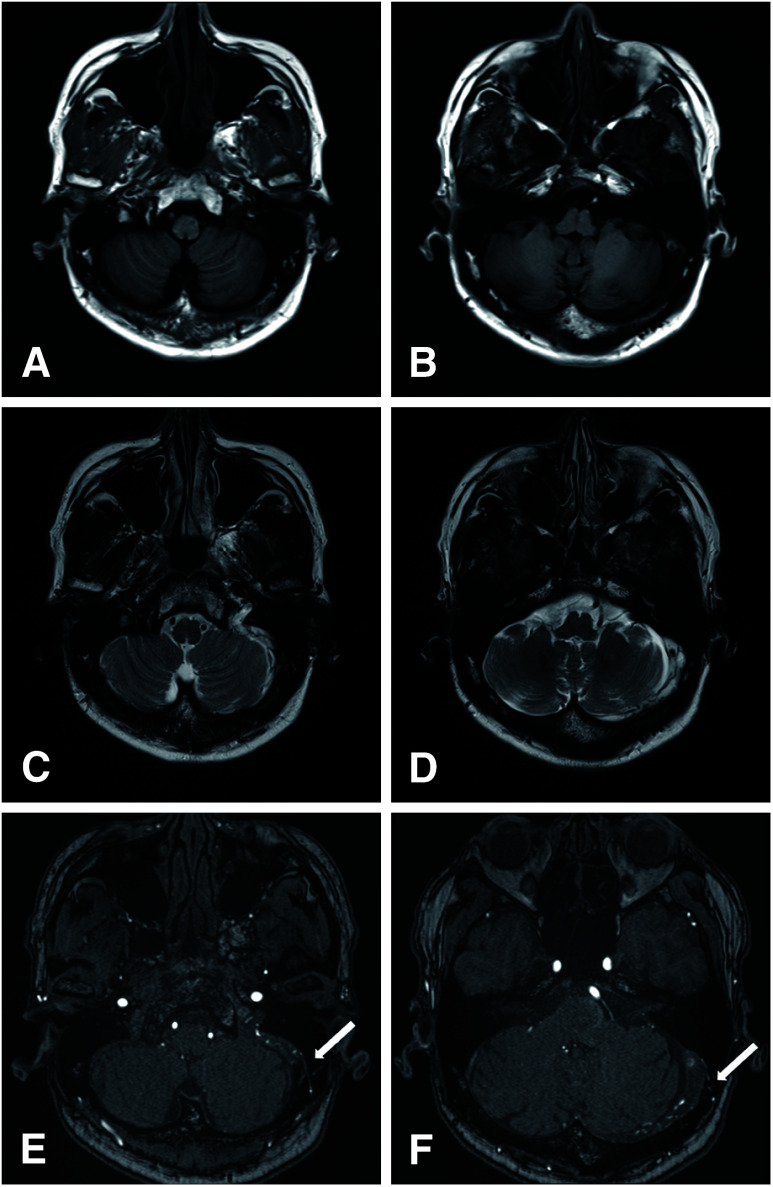
Eight months after the patient’s second COVID-19 vaccination, the patient visited the previous physician again because of recurrent headache. MRI revealed a residual thrombus in the left transverse sigmoid sinus (Fig. 2A–2D) and DAVF in the left transverse sigmoid sinus, which had not been observed at the time of CVST onset (Fig. 2E and 2F). DSA revealed a DAVF in the left transverse sigmoid sinus, which was fed by the right and left occipital arteries (OAs) (Fig. 3A and 3B, black arrowhead; Fig. 3C and 3D, black arrow), left middle meningeal artery (MMA) (Fig. 3B, white arrowhead), and left ascending pharyngeal artery (Fig. 3B, large black arrow). The left sigmoid sinus was occluded (Fig. 3E, large white arrow), and blood flow drained into the cortical vein (Fig. 3F, large black arrowhead). The DAVF was diagnosed as Borden type II and Cognard type 2a + b. DSA revealed that the left MMA was partially shunted into the cortical vein, forming a convexity DAVF (Fig. 3B, white arrow). However, we decided to follow this convexity DAVF and consider whether to treat this convexity DAVF after a follow-up angiography, since there was a possibility that some of these arteries were not feeders but just arteries on the surface of the brain.
Fig. 2. MRI at diagnosis of DAVF. T1-weighted image (A and B) and T2-weighted image (C and D) show some thromboses in some part of left transverse–sigmoid sinus. Time-of-flight MRA reveals some feeders from OA to the left transverse sigmoid sinus (E and F, white arrow). DAVF: dural arteriovenous fistula; OA: occipital artery.
Fig. 3. DSA before and after transarterial embolizations. Left external carotid artery angiography demonstrates multiple arterial feeders to the transverse sinus. Arterial feeders include the OA (black arrowhead), ascending pharyngeal artery (large black arrow), and MMA (white arrowhead) (A and B). Furthermore, some feeders from MMA shunts directly to the cortical vein (B, white arrow). Right external carotid artery angiography demonstrates multiple arterial feeders from OA to the transverse sinus (C and D, black arrow). The proximal of left sigmoid sinus is occluded (E, large white arrow). The main outflow routes were through the confluence to the right transverse sinus and through the cortical vein to the SSS (F, large black arrowhead). Left external carotid artery angiography demonstrates no residual fistulas after endovascular treatments (G and H). MMA: middle meningeal artery; OA: occipital artery; SSS: superior sagittal sinus.
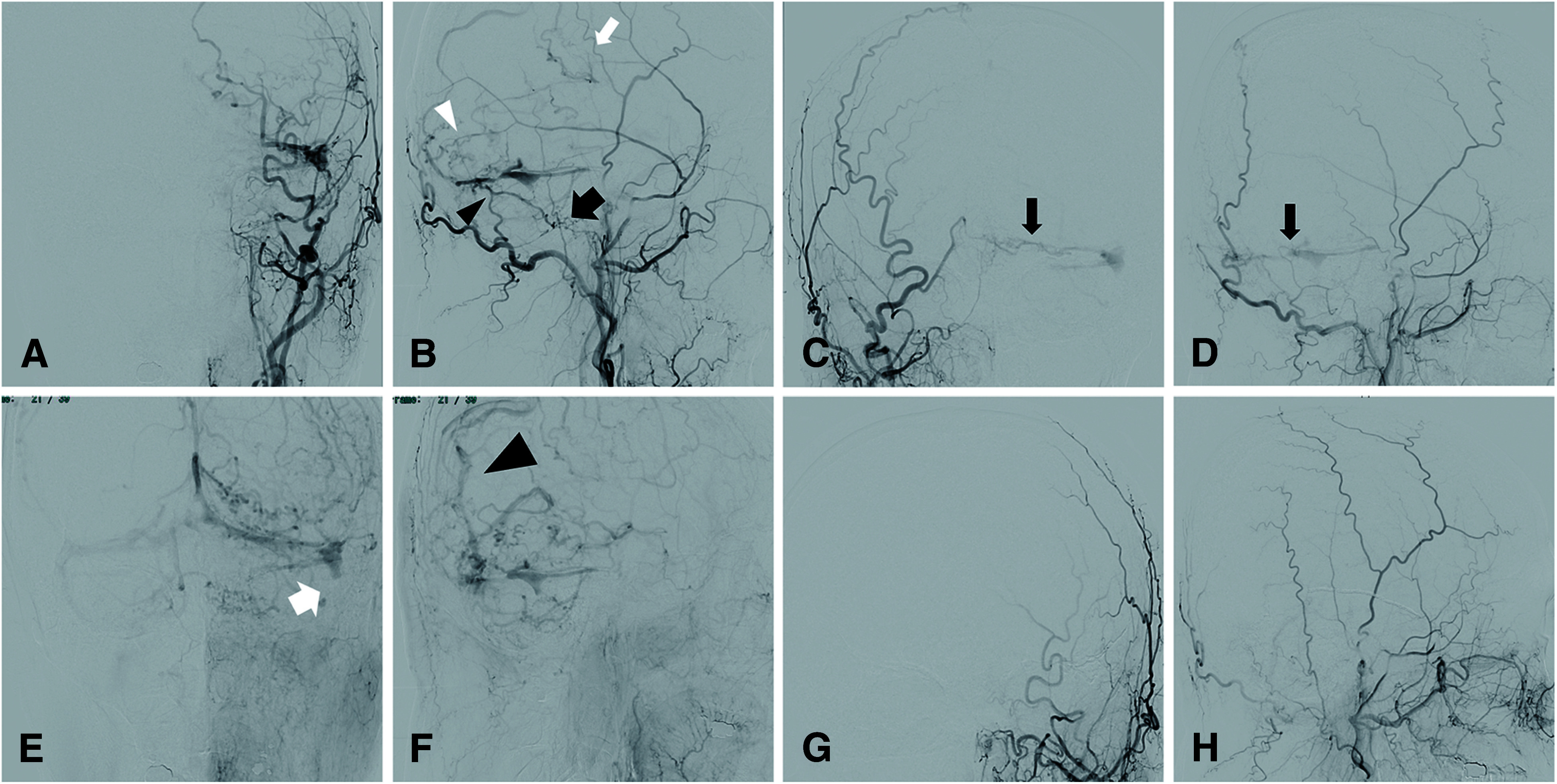
First, transarterial embolization (TAE) was performed for DAVF. The DAVF in the left transverse–sigmoid sinus was treated. Endovascular occlusion of the right OA was performed with N-butyl cyanoacrylate (NBCA), left OA, and left MMA using Onyx18. Two months later, follow-up angiography showed two convexity DAVFs whose feeders were posterior convexity branches of the left MMA and whose drainers were cortical veins of parietal lobe. So, we decided to perform a second endovascular occlusion of the convexity DAVF. Two posterior convexity branches of the left MMA were embolized using several coils and NBCA. Complete occlusion was achieved (Fig. 3G and 3H), and TAE-related complications did not occur. His symptoms improved, and he was discharged with a modified Rankin Scale score of 0.
Discussion
We encountered a case of DAVF following CVST associated with a COVID-19 vaccine that was treated with endovascular therapy. Two types of COVID-19 vaccines have been currently developed: mRNA vaccines, such as Moderna (mRNA-1273) and Pfizer/BioNTech (BNT162b2) vaccines, and adenovirus vector vaccines, such as Oxford-AstraZeneca (AZD1222 [ChAdOx1]) and Johnson & Johnson COVID-19 (JNJ-78436735 [Ad26.COV2.S]) vaccines.10) The mechanism of CVST has been reported to differ between adenovirus vector and mRNA vaccines. CVST after adenovirus vector vaccination was reported to occur 4–28 days after vaccination and is a thrombotic disorder characterized by thrombocytopenia. The mechanism of CVST after adenoviral vector vaccination is similar to that of heparin-induced thrombocytopenia, which is thrombosis with thrombocytopenia.11,12) Laboratory findings of CVST after adenovirus vector vaccination were characterized by thrombocytopenia, positive anti-platelet factor IV antibodies, and high D-dimer levels. However, CVST has been reported to occur within 24 h to 10 days after mRNA vaccination. However, the mechanism underlying this remains unclear. It has been suggested that mRNA binds to receptors associated with the inflammatory cascade before the translation of spike protein, thereby causing thrombosis.13,14) Laboratory findings of CVST after mRNA vaccination were characterized by normal platelet counts and negative antiplatelet factor IV antibodies. In the present case, CVST occurred five days after mRNA vaccination, and the blood samples indicated normal platelet count and negative antiplatelet factor IV antibodies, which is consistent with the findings of previous reports.
Several studies have reported that CVST can cause DAVF. Kuiper et al. reported that among 178 patients with DAVF, four (2.2%) had CVST before the development of DAVF.9) The mechanism of DAVF after CVST has been suggested to be due to a thrombus in the venous sinus that may become organized and develop an arteriovenous shunt, which may lead to DAVF, or DAVF that may develop as a reflux channel in response to elevated venous pressure.15) In addition, the following two factors have been reported as conditions for DAVF after CVST: partial residual thrombus without complete recanalization and new vascular occlusion or thrombus formation.16,17) Ferro et al. reported that DAVF secondary to CVST tends to develop in the SSS area and that non-sinus-type DAVF often develop in the circumflex area near the SSS.18) Furthermore, CVST can lead to multiple DAVFs or DAVF and pial AVF simultaneously.19,20) In the present case, there was a partial residual thrombus in the left transverse sigmoid sinus after CVST, in which a DAVF also occurred. This DAVF was also complicated by convexity DAVF, which is consistent with the characteristics of DAVF after CVST. The incidence of DAVF after CVST during an average follow-up of 78 months is approximately 1%.18) The incidence was recently reported to be 27.8%, which could be attributed to the improved resolution provided by MRI/MRA studies and addition of new sequences to diagnostic images.17) There is a possibility that the incidence of DAVF after CVST may be higher than that previously reported, suggesting that careful follow-up is necessary even when the outcome of CVST after vaccination is favorable.
Conclusion
We encountered a case of CVST 5 days after mRNA vaccination against COVID-19, followed by transverse–sigmoid DAVF and convexity DAVF. Patients with CVST may develop secondary DAVF; therefore, they should be followed up with careful imaging studies.
Acknowledgments
We thank Naoyuki Kakuta (Department of Neurology, Higashiyamato Hospital), Ikuo Kobayashi, and Tsunehiro Hatashita (Department of Neurosurgery, Higashiyamato Hospital) for their assistance with the treatment and collection of patient data.
Disclosure Statement
Kazutaka Sumita received honoraria from Stryker. The other authors declare no conflicts of interest.
References
- 1).Rizk JG, Gupta A, Sardar P, et al. Clinical characteristics and pharmacological management of COVID-19 vaccine-induced immune thrombotic thrombocytopenia with cerebral venous sinus thrombosis: a review. JAMA Cardiol 2021; 6: 1451–1460. [DOI] [PubMed] [Google Scholar]
- 2).Casolla B, Cordonnier C. Cerebral venous sinus thrombosis associated with COVID-19 vaccine-induced thrombocytopenia: improvement in mortality rate over time. Eur J Neurol 2022; 29: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med 2021; 384: 2254–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021; 325: 2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Schulz JB, Berlit P, Diener HC, et al. COVID-19 vaccine-associated cerebral venous thrombosis in Germany. Ann Neurol 2021; 90: 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke 2003; 34: 1163–1169. [DOI] [PubMed] [Google Scholar]
- 7).Reynolds MR, Lanzino G, Zipfel GJ. Intracranial dural arteriovenous fistulae. Stroke 2017; 48:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Suh DC, Lee JH, Kim SJ, et al. New concept in cavernous sinus dural arteriovenous fistula: correlation with presenting symptom and venous drainage patterns. Stroke 2005; 36: 1134–1139. [DOI] [PubMed] [Google Scholar]
- 9).Kuiper L, Sánchez van Kammen M, Coert BA, et al. Association between dural AVFs and cerebral venous thrombosis. AJNR Am J Neuroradiol 2022; 43: 1722–1729. [DOI] [PubMed] [Google Scholar]
- 10).Calina D, Docea AO, Petrakis D, et al. Towards effective COVID-19 vaccines: updates, perspectives and challenges (Review). Int J Mol Med 2020; 46: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Choi PY. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 385: e11. [DOI] [PubMed] [Google Scholar]
- 12).Kawano H, Hashimoto Y, Hirano T. Cerebral vein/venous sinus thrombosis with thrombocytopenia syndrome after COVID-19 vaccination. Rinsho Shinkeigaku 2021; 61: 594–601. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 13).Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol 2021; 224: 108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Hamada Y, Goto K, Inoue T, et al. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery 1997; 40: 452–456; discussion, 456–458. [DOI] [PubMed] [Google Scholar]
- 16).Kusaka N, Sugiu K, Katsumata A, et al. The importance of venous hypertension in the formation of dural arteriovenous fistulas: a case report of multiple fistulas remote from sinus thrombosis. Neuroradiology 2001; 43: 980–984. [DOI] [PubMed] [Google Scholar]
- 17).Kubo M, Kuwayama N. Dural arteriovenous fistulas: dural arteriovenous fistula after cerebral venous sinus thrombosis ‒ what remains still unrevealed? – No Kekkannai Chiryo 2020; 5: 65–71. (in Japanese) [Google Scholar]
- 18).Ferro JM, Lopes MG, Rosas MJ, et al. Long-term prognosis of cerebral vein and dural sinus thrombosis. Results of the VENOPORT study. Cerebrovasc Dis 2002; 13: 272–278. [DOI] [PubMed] [Google Scholar]
- 19).Barnwell SL, Halbach VV, Dowd CF, et al. Multiple dural arteriovenous fistulas of the cranium and spine. AJNR Am J Neuroradiol 1991; 12: 441–445. [PMC free article] [PubMed] [Google Scholar]
- 20).Vilela P, Terbrugge K, Willinsky R. Association of distinct intracranial pial and dural arteriovenous shunts. Neuroradiology 2001; 43: 770–777. [DOI] [PubMed] [Google Scholar]