Abstract
Objective
Transradial approach (TRA) is increasingly used as a viable alternative to the traditional transfemoral approach (TFA) in neuroendovascular therapy (NET) owing to its potential anatomical benefits and lower puncture-site complication rates. However, the real-world challenges of implementing TRA-NET have not been thoroughly studied, particularly those related to guide catheter (GC) placement. In this study, we aimed to explore the feasibility and challenges of TRA-NET, with a specific focus on GC placement.
Methods
This retrospective observational study included patients who underwent NET at our institution between December 2019 and May 2022. Procedural success was defined as the successful placement of a GC in the target vessel. Cases in which a Simmons-shaped GC was used or the approach was changed to TFA were classified as difficult. Safety was assessed based on the rate of severe puncture-site complications requiring either blood transfusion or surgical intervention.
Results
Among the 310 patients who underwent NET during the study period, 222 (71.6%) with a median age of 74 years were selected for TRA-NET. The target vessel was in the left anterior circulation (LtAC) in 101 (45.5%) patients, and 8-F GCs were the most frequently used (40.1%). TRA-NET achieved a 95.0% success rate, with a switch to TFA required in 5.0% of the cases. Procedural challenges occurred in 42 (18.9%) patients, primarily in those with LtAC lesions. Specifically, a type III aortic arch (p <0.0001) and age ≥80 years (p = 0.01) were significantly associated with procedural difficulties. Radial artery evaluation was confirmed in 66 cases (29.7%), revealing one instance (1.5%) of radial artery occlusion. No severe puncture-site complications were observed.
Conclusion
TRA-NET may provide substantial therapeutic benefits without significant limitations in device use. However, it may be challenging, particularly in older patients and those with a type III aortic arch with LtAC lesions. Consequently, careful selection of the approach route is imperative.
Keywords: radial access, neuroendovascular therapy, case series
Introduction
Transradial approach (TRA) for neuroendovascular therapy (NET), first described in 2004,1) has been increasingly used as an alternative to transfemoral approach (TFA), which has traditionally been the most common approach.2) TRA-NET may offer specific anatomical benefits, such as in patients with a bovine aortic arch,3) and has been associated with high patient satisfaction in terms of pain management and postoperative rest.4) Additionally, TRA-NET has been linked to a lower rate of puncture-site complications than that in TFA.5)
Recent studies have primarily focused on procedure success rates and the low incidence of complications,2,6) whereas the practical factors that may impede TRA-NET in real-world clinical settings have not been sufficiently studied. Therefore, in this study, we explored the feasibility of TRA-NET in challenging cases of guide catheter (GC) placement.
Material and Methods
Study design
This was a retrospective observational study that included patients who underwent TRA-NET at our hospital or affiliated institutions between December 2019, when we first introduced TRA-NET, and May 2022. The study was approved by the institutional ethics committee (approval number: 2023-04), and the need for informed consent was waived owing to the retrospective study design.
Radial artery suitability was assessed according to the arterial pulse quality based on our accumulated clinical experience. When the vessel was palpable, TRA was selected as the first-line treatment approach. In contrast, transfemoral approach (TFA) was selected for patients with a normal aortic arch variant, undergoing artificial dialysis, those with proximal carotid artery lesions, and those requiring consecutive days of intervention. Ultimately, the selection of the access site was determined based on several factors: the data gathered during the diagnostic angiography (detailed further in the “Procedures” section), information regarding the approach route inclusive of the aortic arch, and the surgeon’s personal experience. Data were extracted from the medical records and included age, sex, disease, target vessel laterality, and type of GC used. All data used in this study were deidentified.
Procedures
All procedures were performed or supervised by three NET specialists with a minimum of 50 diagnostic angiography procedures performed via TRA.
In scheduled cases, lidocaine/propitocaine cream was applied to the patient’s right forearm at least 1 h in advance. Conventional or distal right radial artery access was established using palpation or ultrasound guidance. In cases of posterior circulation lesions, radial access ipsilateral to the preferred vertebral artery was selected. A “radial cocktail,” composed of 2.5 mg of verapamil, 1 mg of isosorbide dinitrate, and 2000 IU of heparin, was administered after sheath insertion to prevent radial artery spasms. Straight or angle-shaped GCs were commonly used. The sheathless technique was used with 8-F GCs (including those equipped with a balloon). The GC was advanced to the target vessel over a 4- or 5-F 125-cm Simmons C diagnostic catheter (Medikit, Tokyo, Japan). In all cases, except mechanical thrombectomy cases, diagnostic angiography was initially performed either during the preoperative examination for scheduled cases or at the onset of treatment for emergency cases. If the diagnostic catheter could not be smoothly navigated distal to the target vessel, alternative strategies were used: either a Simmons-shaped GC or TFA was used. The Simmons-shaped GC can be used in two ways. First, the Axcelguide Stiff-J (Medikit) can be placed in the common carotid artery as the main GC, and second, the Slimguide 6-F 128-cm Stiff-J-1 (Medikit) can be used as an inner catheter to navigate a non-Simmons-shaped 8-F GC. This latter method facilitates the adjustment of GC positions, offering the versatility of using various types of catheters, including balloon GCs. In mechanical thrombectomy, we opted for a non-Simmons-shaped GC, except in cases involving the left anterior circulation (LtAC). The approach route was carefully chosen to avoid unnecessary prolongation of the procedure time. This decision was made based on preoperative data regarding the approach route and the surgeon’s experience, especially given that guiding the GC into the LtAC can occasionally prove to be challenging. The method for guiding and positioning the Simmons-shaped GC was based on the report by Hanaoka et al.,7) and the pull-back method was employed. Hemostasis at the puncture site was achieved with a TR band (Terumo, Tokyo, Japan) or Prelude SYNC DISTAL radial compression device (Merit Medical, South Jordan, UT, USA) using the patented hemostasis method.8)
Outcome measures
The primary outcome was the procedural success rate, and the secondary outcome was the procedural difficulty rate. Procedural success was defined as successful GC placement in the target vessel. We measured the puncture-to-recanalization time in mechanical thrombectomy, a procedure that requires minimizing the procedural time, as a metric to evaluate the guidance of a GC to the target vessel in NET. Cases in which a Simmons-shaped GC was used or the approach was changed to TFA were classified as difficult. We also examined the incidence of severe puncture-site complications, such as hematoma and pseudoaneurysm, necessitating either a blood transfusion or surgical intervention. Since the occurrence of radial artery occlusion (RAO) was not routinely evaluated, this study evaluated RAO only in patients who had undergone scheduled aneurysm treatment or carotid artery stenting and whose puncture sites were assessed by ultrasonography or palpation.
Statistical analysis
Continuous variables are presented as medians and interquartile ranges, and categorical variables as percentages. Fisher’s exact test was used for statistical comparisons of categorical variables, and the Mann–Whitney U test was used to compare continuous variables. Regarding the patient sample, all except one had LtAC lesions. Accordingly, statistical analyses were predominantly focused on patients with LtAC lesions. Clinical factors potentially influencing the outcome were statistically evaluated using univariate analysis. Furthermore, in cases without LtAC lesions or type III aortic arch, few procedural difficulties were observed. Those cases were examples of “perfect separation” or “quasi-complete separation” phenomenon, and multivariate analysis could not be performed. A p-value less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using EZR10 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).9)
Results
Baseline characteristics
A total of 310 patients underwent NET during the study period. Among them, 222 (71.6%) patients were treated using TRA, with the snuff-box approach used in 139 (62.6%) patients. Additionally, left-sided TRA was performed in 14 patients, all with posterior circulation lesions. The median age was 74 (62–81) years, and the proportion of men was 59.0%. Comorbidities included hypertension (46.8%), dyslipidemia (20.4%), diabetes mellitus (10.6%), ischemic stroke (23.1%), and intracranial hemorrhage (6.9%). The causative diseases included acute ischemic stroke (26.1%), cerebral aneurysms (25.2%), carotid stenosis (22.1%), and other conditions (27.0%). Of the cases studied, 93 (41.9%) were classified as emergency cases, necessitating prompt intervention, whereas the remaining 129 (58.1%) were scheduled cases, allowing for a more detailed anatomical assessment before the procedure. The target vessel locations were predominantly LtAC (45.9%), followed by the right anterior circulation (41.4%) and the posterior circulation (12.6%). The most frequently used GCs were 8-F GCs (40.1%, including 21.2% of cases with balloon GCs), followed by 7-F GCs (24.8%). The patient characteristics are shown in Table 1, with the data for patients who received TFA-NET also provided for reference.
Table 1. Patient characteristics by initial access site for treatment.
| Characteristics | Radial access*(n = 222) | Femoral access*(n = 77) |
|---|---|---|
| Number of male patients (%) | 131 (59.0) | 36 (46.8) |
| Median Age in years (range) | 74 (62–81) | 75 (62–84) |
| Puncture site (%) | ||
| Conventional radial artery | 83 (37.4) | N/A |
| Snuff box | 139 (62.6) | N/A |
| Diseases and procedure (%) | ||
| Aneurysm | ||
| Coil embolization | 55 (25.2) | 21 (27.3) |
| Flow diverter stent | 0 | 1 (1.3) |
| Carotid artery stenting or angioplasty | 49 (22.1) | 11 (14.3) |
| Mechanical thrombectomy for AIS | 58 (26.1) | 26 (33.8) |
| Others | ||
| MMA embolization for CSDH | 33 (14.9) | 4 (5.2) |
| Embolization for tumor | 19 (8.6) | 1 (1.3) |
| AVM or dAVF embolization (TAE) | 5 (2.3) | 4 (5.2) |
| Symptomatic vasospasm after SAH | 3 (0.9) | 9 (11.7) |
| Target vessels (%) | ||
| Right anterior circulation | 92 (41.4) | 20 (26.0) |
| Left anterior circulation | 102 (45.9) | 52 (67.5) |
| Posterior circulation | 28 (12.6) | 5 (6.5) |
| Guide catheters (%) | ||
| 9F balloon GC | 0 | 14 (18.2) |
| 8F | 89 (40.0) | 37 (48.1) |
| Straight GC/ Ballon GC | 42 (18.9)/47 (21.2) | 27 (35.1)/10 (13.0) |
| 7F | 55 (24.8) | 7 (9.1) |
| 6F | 55 (24.8) | 12 (15.6) |
| 5F | 23 (10.4) | 4 (5.2) |
| 4F diagnostic catheter | 0 | 3 (3.9) |
*The first approach selected.
AIS: acute ischemic stroke; AVF: arteriovenous fistula; AVM: arteriovenous malformation; CSDH: chronic subdural hematoma; GC: guide catheter; MMA: middle meningeal artery; SAH: subarachnoid arachnoid hemorrhage; TAE: transarterial embolization
Outcomes
The procedural success rate was 95.0% (211 patients), with conversion to TFA required in 11 patients (5.0%). The median puncture-to-recanalization time in mechanical thrombectomy was 51 (27–88) minutes. On the other hand, it was 53 (37–70) minutes in TFA cases, which did not show a significant difference (p = 0.81).
Procedural difficulties, including the switch to TFA, were encountered in 42 patients (18.9%). Out of these, 12 underwent mechanical thrombectomy. Among them, the Axcelguide Stiff-J was used in four patients, and the Slimguide 6-F Stiff-J-1 was used in seven patients. These decisions were made based on preoperative data regarding the tortuosity of the approach route, anticipating challenges the operator might face in navigating a non-Simmons-shaped GC. Nonetheless, two cases wherein the Axcelguide Stiff-J (Simmons-shaped GC as the main GC) was used and three cases wherein the Slimguide 6-F Stiff-J-1 (Simmons-shaped GC as an inner catheter) was used required a switch to TFA. Moreover, two cases involving the Axcelguide also faced TFA failures. In another situation, a non-Simmons-shaped GC was initially attempted, but due to challenges in navigation, the operator swiftly changed the approach route. Figure 1 provides a flowchart illustrating the GC choices and outcomes for all 42 cases. From the beginning of the procedure, the Axcelguide Stiff-J was used in 20 cases as the main GC, a decision determined preoperatively. In two cases in this subset, catheterization failure occurred, with subsequent attempts to rectify the issue through TFA proving unsuccessful. In another 16 cases, the Slimguide 6-F Stiff-J-1 was used as an inner catheter from the outset of the treatment, resulting in four failures. Within this group, it was deemed necessary to employ a non-Simmons-shaped GC, such as a balloon GC, for the procedure. However, navigating a regular inner catheter within the GC presented substantial challenges, prompting the use of the Slimguide 6-F Stiff-J-1 instead. Furthermore, in five cases, the use of a non-Simmons-shaped GC alone was insufficient. Among these, three cases encountered issues such as catheter kinking or unstable catheter positioning, which necessitated a switch to TFA for successful navigation. In the remaining two cases, the diagnostic catheter could be positioned properly, but the GC could not be navigated coaxially. In these specific instances, a smaller than 8-F GC was used, which made it impossible to use the Slimguide 6-F Stiff-J-1 as an inner catheter. Separately, one case required a switch to TFA following diagnostic angiography (Fig. 1). Notably, all but one of these cases had LtAC lesions. Within this LtAC lesions group, difficulties were predominantly encountered due to the presence of a type III aortic arch (p <0.01). Another significant factor associated with procedural difficulties was age older than 80 years (p = 0.01) (Table 2).
Fig. 1. Flowchart of cases of procedural difficulty showing initial guide catheter choices and outcomes. This flowchart categorizes the cases encountered in the TRA-neurointervention group based on the initial guide catheter selection and subsequent steps: use of a Simmons-shaped guide catheter as the main guide catheter, use of a Simmons-shaped guide catheter as an inner catheter, or use of a non-Simmons-shaped guide catheter alone, with the transition to TFA after diagnostic angiography at treatment initiation. GC: guide catheter; MC: microcatheter; TFA: transfemoral approach; TRA: transradial approach.
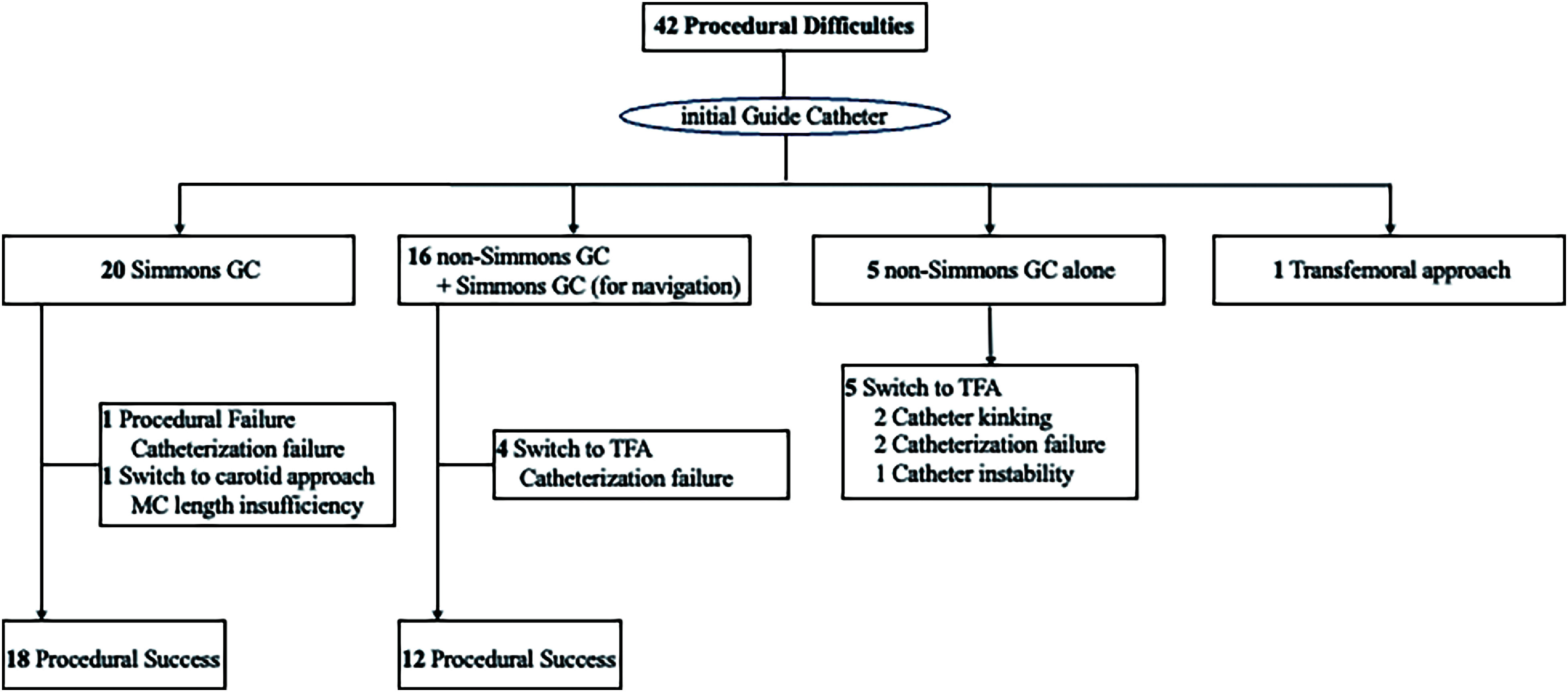
Table 2. Univariate analysis of procedural difficulties in patients with left anterior circulation lesions.
| Variable | Difficulty (+) (n = 41) |
Difficulty (−) (n = 61) |
p value |
|---|---|---|---|
| Age ≥80 (%) | 21 (51.2) | 15 (24.6) | 0.01 |
| Type III aortic arch (%) | 40 (97.6) | 8 (13.1) | <0.01 |
| Sex (male) (%) | 26 (63.4) | 37 (60.7) | 1 |
| Disease and treatment procedure (%) | 0.95 | ||
| Aneurysm | 7 (17.1) | 9 (14.8) | |
| Carotid artery stenting or angioplasty | 10 (24.4) | 14 (23.0) | |
| Mechanical thrombectomy for AIS | 12 (29.3) | 13 (21.3) | |
| Others | |||
| MMA embolization for CSDH | 7 (17.1) | 14 (23.0) | |
| Embolization for tumor | 5 (12.2) | 9 (14.8) | |
| AVM or dAVF embolization (TAE) | 0 | 1 (1.6) | |
| Symptomatic vasospasm after SAH | 0 | 1 (1.6) |
AIS: acute ischemic stroke; AVF: arteriovenous fistula; AVM: arteriovenous malformation; CSDH: chronic subdural hematoma; MMA: middle meningeal artery; SAH: subarachnoid arachnoid hemorrhage; TAE: transarterial embolization
Radial artery evaluation was confirmed in 66 cases (29.7%), revealing one instance (1.5%) of RAO. In this case, a petite older woman underwent carotid artery stenting using an 8-F balloon GC without a sheath, performed under local anesthesia. Although the patient was asymptomatic, RAO was confirmed early after treatment. No severe puncture-site complications were observed.
Illustrative cases
Case 1
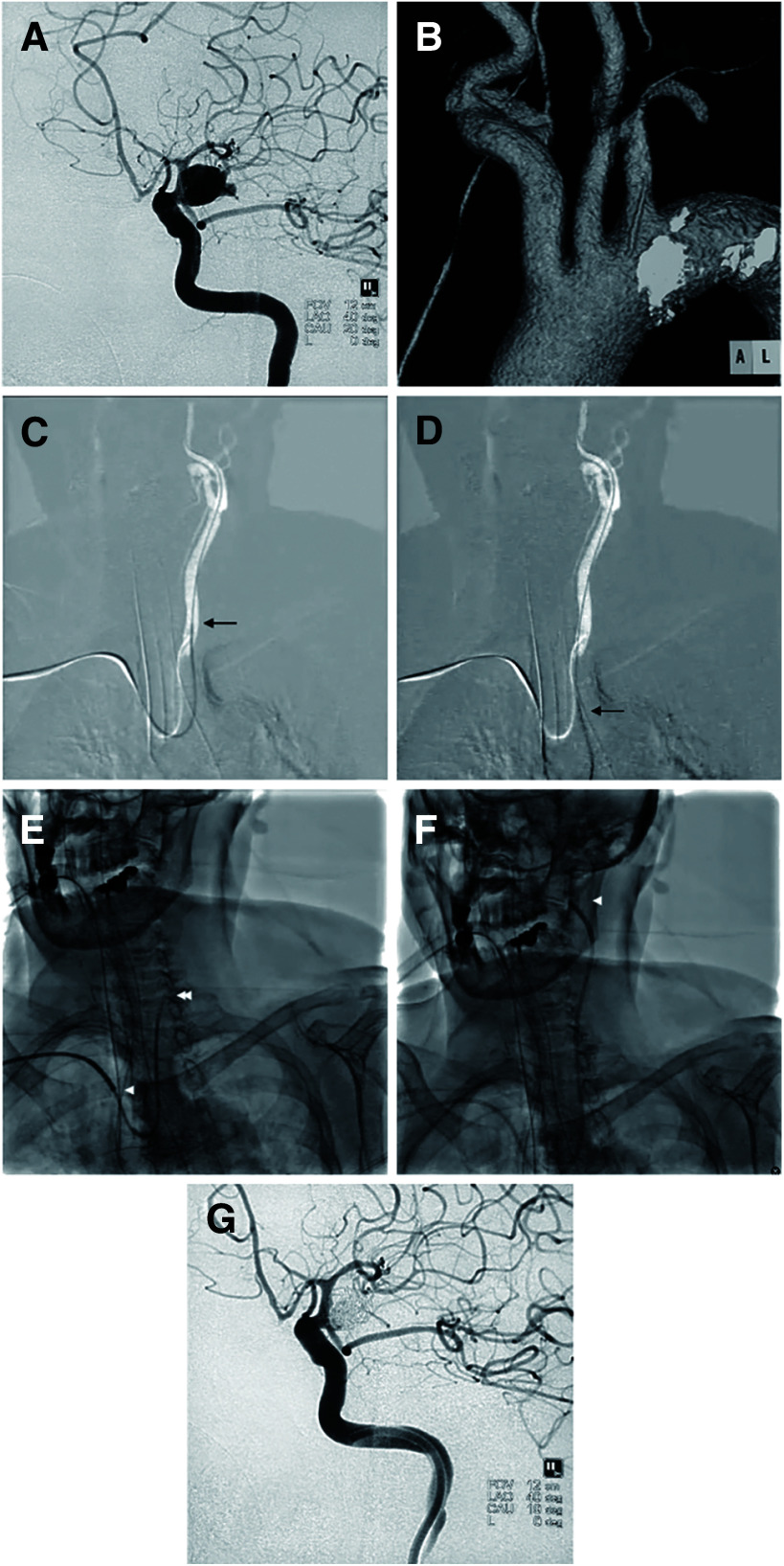
An 87-year-old female patient with a type III aortic arch presented with a subarachnoid hemorrhage (classified as Hunt & Kosnik grade 1), which resulted from the rupture of a posterior communicating artery aneurysm (Fig. 2A and 2B). An 8-F Fubuki catheter (ASAHI Intecc, Aichi, Japan) was selected as the main GC. However, during the diagnostic angiography, the 5-F diagnostic catheter encountered navigational difficulties in reaching the left internal carotid artery (ICA), primarily due to its tendency to herniate easily into the aortic arch (Fig. 2C and 2D). Consequently, a decision was made to use a Slimguide 6-F Stiff-J-1 as an inner catheter to facilitate navigation (Fig. 2E). This strategy proved successful, enabling the Fubuki catheter to be navigated to the distal cervical portion of the ICA (Fig. 2F) and allowing for the successful completion of coil embolization (Fig. 2G).
Fig. 2. Case showing the use of a Simmons-shaped guide catheter as an inner catheter. (A) Left ruptured posterior communicating artery aneurysm; (B) type III aortic arch; (C) navigation of the diagnostic catheter (arrow); (D) diagnostic catheter herniation into the aortic arch (arrow); (E) use of a Simmons-shaped guide catheter (double arrowhead) as an inner catheter, housed within a non-Simmons-shaped guide catheter (arrowhead) as the main guide catheter; (F) advancement of the main guide catheter to the cervical portion of the left internal carotid artery (arrowhead), facilitated by the Simmons-shaped guide catheter; and (G) post-coil embolization.
Case 2
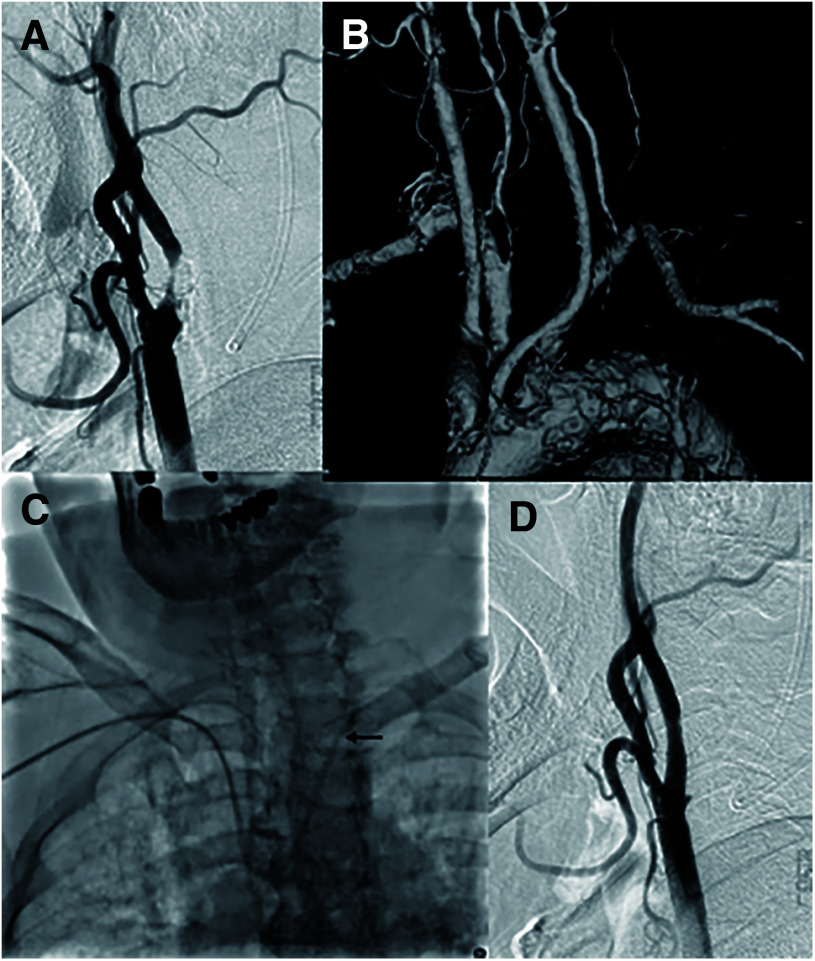
An 85-year-old man with a type III aortic arch experienced a transient ischemic attack attributable to severe stenosis in the left carotid artery (Fig. 3A and 3B). During preoperative diagnostic angiography, the diagnostic catheter was prone to herniation into the aortic arch, prompting the selection of an Axcelguide 6F Stiff-J-1 as the main GC (Fig. 3C). Consequently, the GC was successfully positioned within the left common carotid artery, and carotid artery stenting was successfully completed (Fig. 3D).
Fig. 3. Case showing the use of a Simmons-shaped guide catheter as the main guide catheter. (A) Left carotid artery severe stenosis, (B) type III aortic arch, (C) use of a Simmons-shaped guide catheter (double arrowhead) as the main guide catheter, and (D) post-carotid artery stenting.
Discussion
This case series suggests that TRA-NET is a feasible treatment method for various diseases, including those requiring large GCs. However, procedural challenges may arise when dealing with diseases of the LtAC, particularly in older patients or those with a type III aortic arch.
Feasibility of TRA-NET
In a large case series of procedures performed via TRA, including cerebral angiography, Goldman et al.6) reported a success rate of 92.1% and a crossover rate of 7.9%. Similarly, in their meta-analysis on aneurysm treatment via TRA, Alkhars et al.10) documented a success rate of 93.5%. In a recent systematic review, Joshi et al.2) recorded a procedural success rate of approximately 95.23% for TRA-NET with a crossover rate of 4.77%. These findings are similar to the outcomes observed in the present study.
A significant difference between the current and previous studies is the size of the GCs used. In most prior studies, TRA-NET was performed using GCs ≤7F.3,10,11) Contrarily, in our study, 8-F GCs were the most commonly used, followed by 7-F GCs, demonstrating the feasibility of the procedure even with larger GCs. Larger GCs are particularly useful and frequently used in NET due to their versatility. They allow the use of a distal access catheter or large-bore aspiration catheter, facilitating the treatment of cerebral aneurysms12) and mechanical thrombectomy, respectively.13,14) Larger GCs are also frequently employed in carotid artery stenting procedures.15,16)
While using large GCs raises safety concerns due to the small size of the radial artery,3) Hanaoka et al.7) reported a RAO rate of 1.5% in their study of TRA-NET using a 6-F guide sheath (which has an outer diameter equivalent to that of a sheathless 8-F GC). This rate aligns with findings from previous studies and our study. In this study, the RAO patient was a petite, older woman. Factors such as old age, female, low height, and body weight are associated with the radial artery diameter and may be involved in RAO.8) For patients with these risk factors, considering a smaller device or a different approach might be advisable. However, our study did not identify any severe puncture-site complications. Therefore, we propose that TRA-NET using devices frequently used for TFA-NET in clinical practice is feasible.
Factors associated with procedural difficulty in TRA-NET
The available evidence identifies several factors that contribute to the complexity of TRA-NET procedures, including vessel tortuosity, radial artery loops, aortic arch anatomy, and the branch vessel angle.17,18) However, these studies primarily focused on cannulation of the target vessel during diagnostic angiography. Furthermore, evaluations were conducted for cannulation of the common carotid artery without considering placement into the ICA. Owing to their size and rigidity, GCs can alter the course of vessels. NET often requires a more distal GC placement than that in diagnostic angiography. Thus, these factors may not accurately reflect the challenges encountered with NET. From this viewpoint, we believe that our study is valuable in that it provides insight into the factors that contribute to the complexity of GC placement in NET and aids in the appropriate selection of the approach route. Specifically, in mechanical thrombectomy cases where minimizing the procedure time is crucial, using TFA could still be advantageous, particularly in cases involving LtAC, elderly patients, and patients with a type III aortic arch. Consideration of the previously reported factors complicating TFA-NET and those identified in our study for TRA-NET may facilitate safer and more reliable NET procedures.
Limitations
Although the results are promising, our study had some limitations. Given the retrospective design, there was potential for selection bias. Additionally, our results do not account for issues such as catheter kinking. Finally, since the occurrence of RAO was not systematically evaluated in our study, the actual incidence might have been underestimated. Ideally, patients would have been monitored for RAO for safety reasons. However, given the absence of severe puncture-site complications, it is probable that any cases of RAO, if they occurred, were asymptomatic. To validate our findings, additional prospective studies with larger sample sizes and collection of additional baseline and outcome data are required.
Conclusions
TRA-NET is an effective treatment method that allows unrestricted device use, accommodates various additional treatments, and yields substantial therapeutic benefits. However, this approach can present challenges, particularly in older patients and those with a type III aortic arch with target vessels in the LtAC. Consequently, careful selection of the approach route is imperative.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1).Eskioglu E, Burry MV, Mericle RA. Transradial approach for neuroendovascular surgery of intracranial vascular lesions. J Neurosurg 2004; 101: 767–769. [DOI] [PubMed] [Google Scholar]
- 2).Joshi KC, Beer-Furlan A, Crowley RW, et al. Transradial approach for neurointerventions: a systematic review of the literature. J Neurointerv Surg 2020; 12: 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Snelling BM, Sur S, Shah SS, et al. Transradial approach for complex anterior and posterior circulation interventions: technical nuances and feasibility of using current devices. Oper Neurosurg (Hagerstown) 2019; 17: 293–302. [DOI] [PubMed] [Google Scholar]
- 4).Khanna O, Sweid A, Mouchtouris N, et al. Radial artery catheterization for neuroendovascular procedures. Stroke 2019; 50: 2587–2590. [DOI] [PubMed] [Google Scholar]
- 5).Ghaith AK, El Naamani K, Mualem W, et al. Transradial versus transfemoral approaches in diagnostic and therapeutic neuroendovascular interventions: a meta-analysis of current literature. World Neurosurg 2022; 164: e694–e705. [DOI] [PubMed] [Google Scholar]
- 6).Goldman DT, Bageac D, Mills A, et al. Transradial approach for neuroendovascular procedures: a single-center review of safety and feasibility. AJNR Am J Neuroradiol 2021; 42: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Hanaoka Y, Koyama JI, Yamazaki D, et al. Transradial approach as the primary vascular access with a 6-Fr Simmons guiding sheath for anterior circulation interventions: a single-center case series of 130 consecutive patients. World Neurosurg 2020; 138: e597–e606. [DOI] [PubMed] [Google Scholar]
- 8).Pancholy S, Coppola J, Patel T, et al. Prevention of radial artery occlusion - patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv 2008; 72: 335–340. [DOI] [PubMed] [Google Scholar]
- 9).Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Alkhars H, Haq W, Al-tayeb A, et al. Feasibility and safety of transradial aneurysm embolization: a systematic review and meta-analysis. World Neurosurg 2022; 165: e110–e127. [DOI] [PubMed] [Google Scholar]
- 11).Crockett MT, Selkirk GD, Chiu AH, et al. Arterial access site complications in transradial neurointerventions: single center review of 750 consecutive cases. Clin Neuroradiol 2020; 30: 639–642. [DOI] [PubMed] [Google Scholar]
- 12).Lin LM, Colby GP, Huang J, et al. Ultra-distal large-bore intracranial access using the hyperflexible navien distal intracranial catheter for the treatment of cerebrovascular pathologies: a technical note. J Neurointerv Surg 2014; 6: 301–307. [DOI] [PubMed] [Google Scholar]
- 13).Alawieh A, Chatterjee AR, Vargas J, et al. Lessons learned over more than 500 stroke thrombectomies using ADAPT with increasing aspiration catheter size. Neurosurgery 2020; 86: 61–70. [DOI] [PubMed] [Google Scholar]
- 14).Shallwani H, Shakir HJ, Rangel-Castilla L, et al. Safety and efficacy of the Sofia (6F) PLUS distal access reperfusion catheter in the endovascular treatment of acute ischemic stroke. Neurosurgery 2018; 82: 312–321. [DOI] [PubMed] [Google Scholar]
- 15).Iwata T. Initial experience of a novel sheath guide specifically designed for transradial approach for carotid artery stenting. World Neurosurg 2019; 130: e760–e764. [DOI] [PubMed] [Google Scholar]
- 16).Cappuzzo JM, Monteiro A, Waqas M, et al. Carotid artery stenting using the walrus balloon guide catheter with flow reversal for proximal embolic protection: technical description and single-center case series. Oper Neurosurg (Hagerstown) 2023; 24: 11–16. [DOI] [PubMed] [Google Scholar]
- 17).Khan NR, Peterson J, Dornbos III D, et al. Predicting the degree of difficulty of the trans-radial approach in cerebral angiography. J Neurointerv Surg 2021; 13: 552–558. [DOI] [PubMed] [Google Scholar]
- 18).Yaser Arafath M, Bhatia V, Kumar A, et al. Adapting to transradial approach in cerebral angiography: factors influencing successful cannulation. Neuroradiol J 2023; 36: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.